Abstract
Intravenous therapy is a common practice among many specialties. Intravenous therapy extravasation is a potential complication to such therapy. Hospitals without a dedicated wound care team trained in these interventions will often default to plastic surgical consultation, making an understanding of available interventions essential to the initial evaluation and management of these injuries. The goal of this article was to provide plastic surgeons and health care providers with a general overview of the acute management of intravenous infiltration and extravasation injuries. Though the decision for surgical versus nonsurgical management is often a clear one for plastic surgeons, local interventions, and therapies are often indicated and under-utilized in the immediate postinfiltration period. Thorough knowledge of these interventions should be a basic requirement in the armamentarium of plastic surgery consultants.
INTRODUCTION
Originating in the mid 1600s in Germany, intravenous infusion has progressed considerably from the once used feather quills to greater than a million chemotherapeutic infusions per day.1,2 Intravenous therapy (IVT) is prevalent in every medical specialty, with a diversity of applications including anesthesia, rehydration, transfusion, drug delivery, parenteral nutrition, central venous pressure monitoring, dialysis, contrast imaging, and countless other important applications.3 With such substantial use, infiltration and extravasation injuries to the soft tissue are commonplace, occurring in up to 6% of patients.4
Iatrogenic complications of IVT include infection, phlebitis, infiltration, extravasation, soft-tissue necrosis, and compartment syndrome.3,5 Severity often correlates with the characteristics of the injected solution. Though the terms are often used interchangeably, strictly speaking, infiltration refers to leakage of nonvesicant (nonirritating) solution into the extravascular space,6,7 while extravasation involves a vesicant (irritating) solution.6
Various agents induce soft-tissue injury through distinct mechanisms. Vasoactive agents induce vasoconstriction causing ischemia and subsequent necrosis.7 Electrolyte-rich solutions alter the electrical potential and subsequent tone in pre- and postcapillary sphincters leading to ischemia.7 Hyperosmolar solutions cause a fluid shift from vascular and cellular compartments into the surrounding tissues leading to elevated compartment pressures and potentially compartment syndrome.7,8 Finally, cytotoxic solutions are directly toxic to the surrounding tissues.
Plastic surgeons and trainees are often on the forefront of management of these injuries.5 Treatment in the inpatient setting is not an emphasized part of the standard plastic surgery curriculum, and consequently, many trainees remain unaware of interventions outside the realm of antibiotic ointments, debridement, and soft-tissue reconstruction. This is despite compelling data that shows a benefit with certain early interventions. Adding to the confusion with the standard of care is the variation and inconsistency among many hospital-specific guidelines,9,10 as many treatments have only an anecdotal basis. As an essential component of the wound management team in many settings, plastic surgeons should be knowledgeable and involved in the development of guidelines to minimize iatrogenic morbidity. The objective of this article was to provide an evidence-based review of extravasation injuries relevant to plastic surgeons and trainees.
MANAGEMENT
Recognition and Classification
Immediate symptoms of IVT extravasation may include blanching of the skin, discomfort, edema, changes in IV flow, and fluid leakage (Table 1).3 Early recognition is important. There are multiple classifications of extravasations. The United States Department of Health and Human Services, National Cancer institute, and National Institute of Health published the Common Terminology Criteria for Adverse Events (Version 4.0 of May 2009), which includes a devised 5-point grading system for extravasation (Table 2).11,12
Table 1.

Table 2.
Common Terminology Criteria for Adverse Events: Grading System for IV Extravasation Injuries12

The peripheral intravenous infiltration and extravasation scale is a more recently developed scale, dependent upon the proportion of appendage affected rather than exclusively the length of the infiltrated area. This ratio is determined by measuring the maximum dimension of edema (width or length) and dividing by the length of the appendage (axilla to longest finger or groin to longest toe).13 There are 3 grades (Table 3): mild (< 30% affected), moderate (30–60% affected), and severe (> 60% affected), with suggested interventions based on the severity of limb involvement.
Table 3.
The Peripheral Intravenous Infiltration and Extravasation Scale used in Pediatric Infiltration and Extravasation Injuries (Adapted from Scale Originally by Children’s Medical Center of Dallas)13
Nonpharmacologic Management
Following identification, IVT should immediately be discontinued while keeping the catheter in place, allowing for potential aspiration of the infiltrated agent with 3–5 mL of blood using a 10 mL syringe.14,15 A reversal agent, if available, may also be administered via the catheter, followed by removal. Maneuvers to reduce edema, such as limb elevation, should be instituted.
Thermal compresses are commonly utilized in the acute period, though their efficacy outside of increasing patient comfort remains anecdotal with no controlled studies.16,17 They are typically administered for 20 minutes 4 times per day for 1–2 days.15 In theory, cold compresses elicit local vasoconstriction, which can reduce pain, local inflammation, and the further spread of the infused agent—useful for infiltration by hyperosmolar solutions such as Total Parenteral Nutrition (TPN). Cold compresses are contraindicated for cases of infiltration by vasopressors, vinca alkaloids, and epipodophyllotoxins (etoposide) due to evidence of subsequent further deterioration.9,11,15 In contrast, warm compresses cause local vasodilation and are preferred for vasopressors, vinca alkaloids, phenytoin, and contrast media.14 The local vasodilation is thought to increase blood flow, thereby augmenting drug disposal. Regardless of the lacking evidence, compresses are soothing to the patient and are recommended for that reason.
Support for manual extraction methods is sparse and anecdotal. Some reported interventions, such as the squeeze maneuver or the multiple puncture procedure, involve using an 18-gauge needle to make 5–8 fenestrations at the peri-insertion area and subsequent compression proximal to the extravasation to liberate the infiltrated vesicant in adult infiltrations.18,19 Early subcutaneous washout has been documented to be quite successful especially within 6 hours of the extravasation injury. In this method, isotonic saline is infiltrated through a liposuction cannula. The fluid is then removed by careful suction through the cannula. This procedure is repeated until 300–500 mL has been removed in total.20 The success of washout may be due to a prompt reduction of the infiltrated agent’s concentration.
In cases of soft-tissue ischemia or necrosis, the area of injury should be protected with a nonadherent dressing, which provides a protective barrier. Topical antimicrobials or a wound gel are also routinely used to promote a moist wound environment. Reassessments are paramount by a plastic surgery or wound team to be sure no further serious injury is noted.
Pharmacologic Management
The propensity for damage and the subsequent management of an infiltration insult is highly dependent upon the physiochemical characteristics of the extravasated agent.21 Those most commonly associated with extravasations will be described below.
Vasopressors
Extravasation by vasopressors (vasopressin, norepinephrine, epinephrine, dopamine, and dobutamine) causes local vasoconstriction, resulting in tissue ischemia and necrosis6 (Figs. 1, 2). Though ordinarily administered via large central veins,22 peripheral infusion of vasopressors is sometimes performed to hasten the placement of IV access.22
Fig. 1.
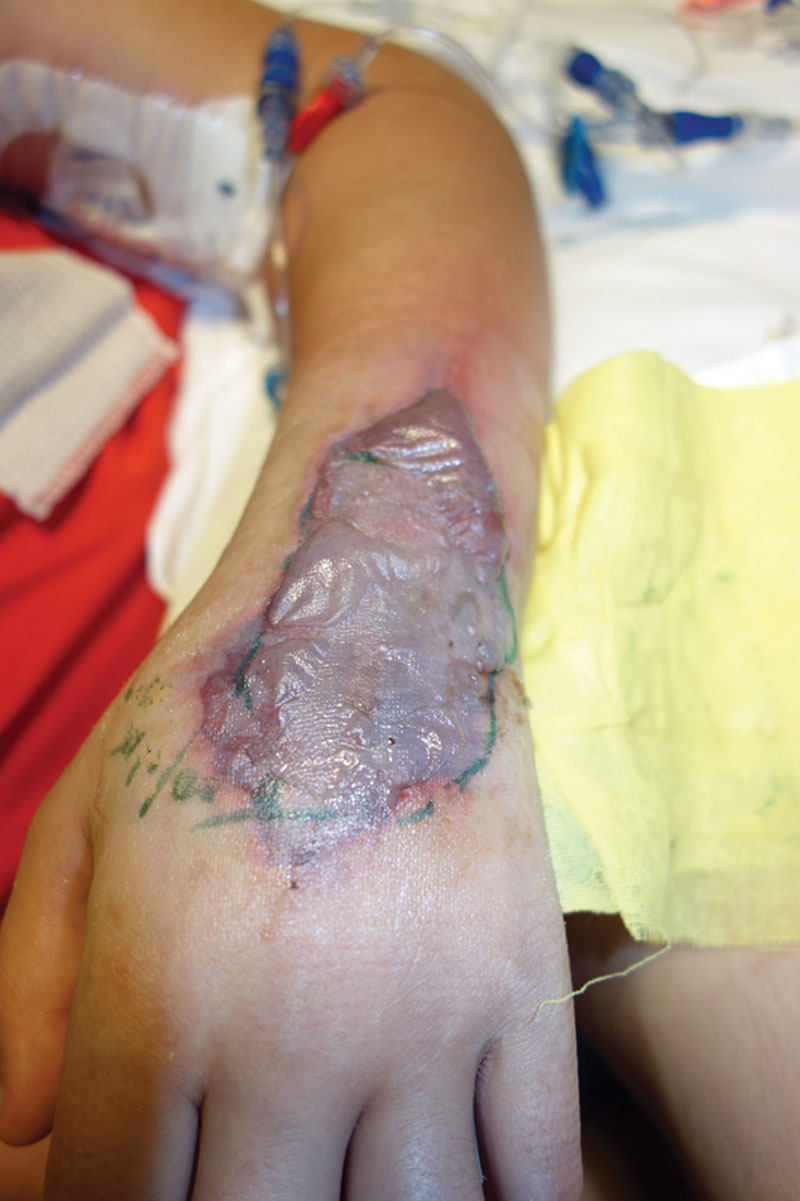
A 11-year-old female with dopamine extravasation 5 days post infusion. Treated with warm compress and phentolamine.
Fig. 2.
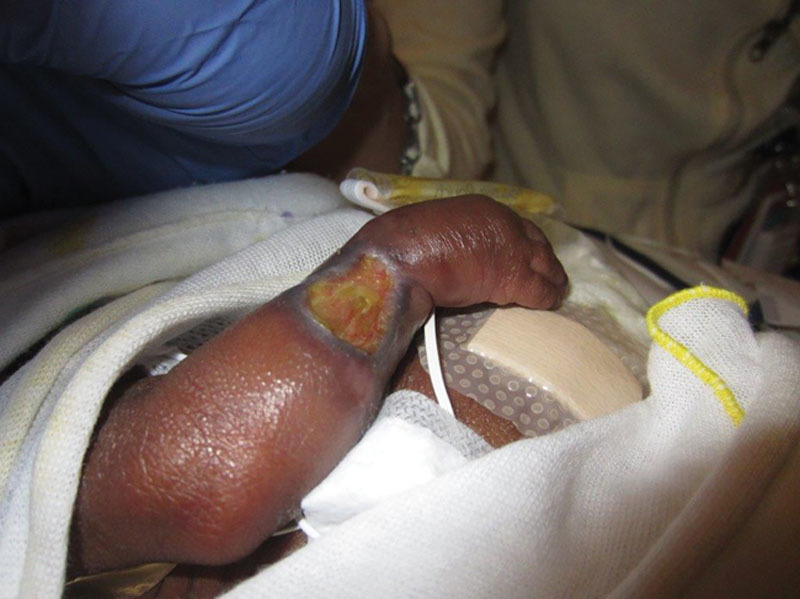
A 32-week premature infant with a dopamine extravasation 2 months post infusion.
Vasoconstriction occurs via an α-1–mediated response, with norepinephrine being the most commonly reported cause of vasopressor-mediated infiltration.14 Dopamine and epinephrine (β-1, β-2, and α-1 agonist) also function on α-1 receptors. Phentolamine may serve as a local therapy/reversal agent in these cases23,24 (Figs. 2, 3). In a study of 734 patients receiving peripheral intravenous vasopressors, there was a 2% incidence of infiltration.25 In all cases, local phentolamine was injected in addition to application of a nitroglycerin paste, and no patients experienced irreversible tissue necrosis. In contrast, vasopressin exerts its vasoactive properties via the V1 receptor, and there are currently no reports of successful intervention with a direct reversal agent. Phentolamine, terbutaline, or topical nitroglycerin may provide some protective effect in vasopressin infiltrations via their inherent vasodilatory effects.14
Fig. 3.
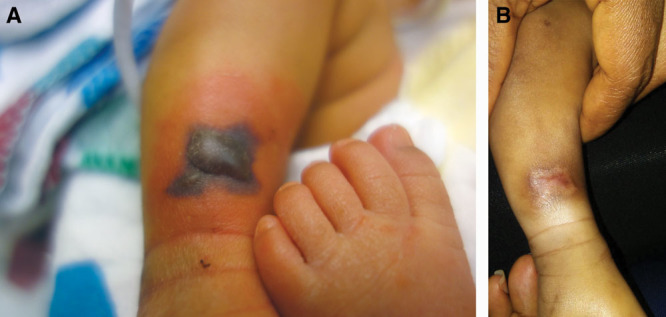
A, A 5-day old full-term neonate with an R lower leg IV extravasation; dopamine and PPN were infusing. Treated initially with phentolamine and elevation. B, 30-day Status post healing by secondary intention, with residual cosmetic skin changes.
Phentolamine is a reversible alpha-adrenergic receptor antagonist.14 For neonates, the provider should perform a 1:10 dilution of the commercial 5 mg/mL phentolamine to a concentration of 0.5 mg/mL, followed by 5 injections of 0.2 mL of phentolamine into the area of extravasation.19 For infants, children, and adults, 5 mg of phentolamine reconstituted with 5 mL of normal saline (1 mg/mL) or a 1:5 dilution should be performed of the commercially available 5 mg/mL phentolamine to attain a final concentration of 1 mg/mL phentolamine. The solution should be injected in 0.5–1 mL aliquots 5 times around the injection site using a 25-gauge or 27-gauge needle.25 Phentolamine’s effects are time dependent with no beneficial outcome observed after 13 hours of the injury14 (Fig. 4). Nitroglycerin can be given to augment phentolamine-mediated vasodilation. Topical application of 2.5 cm of 2% nitroglycerin paste with reapplication every 8 hours has been shown to initiate reperfusion.14
Fig. 4.
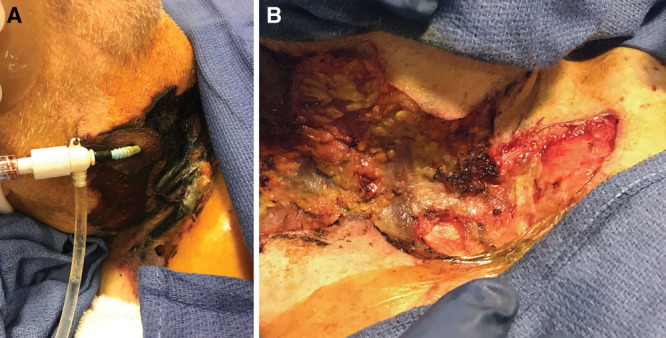
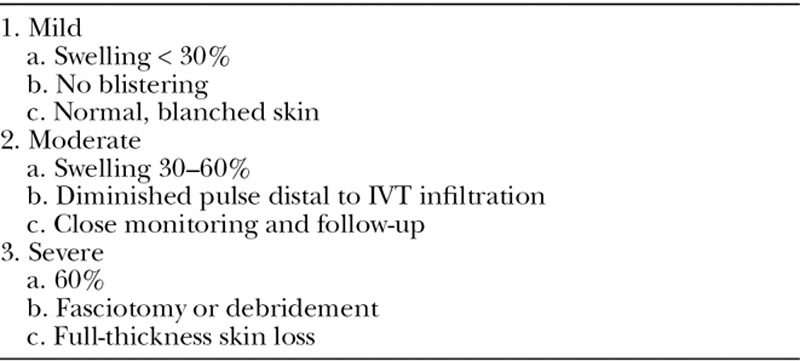
A, Severe epinephrine extravasation injury to the neck. Therapies including subcutaneous phentolamine and topical nitroglycerin paste were utilized after identification. Nevertheless, substantial irreversible tissue ischemia occurred, possibly due to a delay in identification. B, Debridement of necrotic skin and subcutaneous tissue was performed.
Osmotically Active Agents
Infiltration of hypertonic solutions [TPN, peripheral parenteral nutrition (PPN), dextrose > 10%, sodium bicarbonate, potassium, and calcium 26) causes an imbalance in the equilibrium between the intracellular and extracellular compartments, causing fluid shifts, cellular dysfunction, and cell damage/death. Accumulation of fluid further compromises the tissue compartment through hypoperfusion and subsequent tissue necrosis14 (Fig. 5). A retrospective study on 352 patients receiving PPN found a 40% incidence of phlebitis or infiltration. Those receiving PPN with osmolarity greater than 1,000 mOsm/L had a 17% incidence versus 7% incidence in patients receiving PPN with an osmolarity less than 1,000 mOsm/L.27 In these cases, the patient must be evaluated and monitored for compartment syndrome, with a low threshold to obtain a compartment pressure or perform fasciotomies if clinically suspicious (positive if greater than 30 mm Hg).28
Fig. 5.
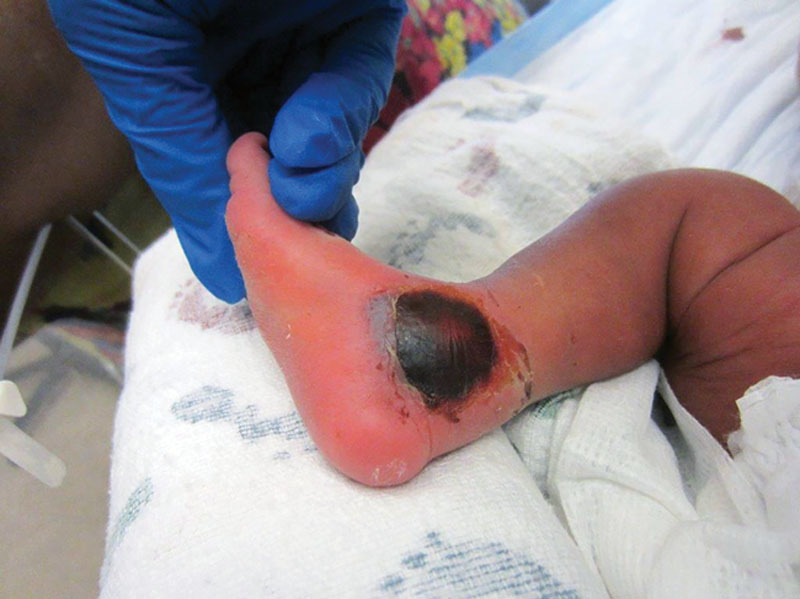
A 3-month-old infant with a TPN infiltrate 1 week post infusion. Treated with hyaluronidase and elevation.
Hyaluronidase is often utilized for treating hypertonic extravasations, though not Food and Drug Administration (FDA) approved for this indication.14 Although there are conflicting data regarding its efficacy, most case studies report that hyaluronidase augments dispersion of the extravasated agent.29 However, no randomized clinical studies have been performed to date.30 Hyaluronidase functions via depolymerization of glycosaminoglycans such as chondroitin sulfate and hyaluronic acid, which is thought to increase tissue permeability and subsequently aid in dispersion of the infiltrated agent (technique described below under cytotoxic drugs section). Local injection of hyaluronidase and saline washout is the current principle modality to treat severe TPN-associated extravasations; however, this is based on limited studies14 (Fig. 5). Hyaluronidase was shown to be successful in the treatment of 2 cases of TPN extravasations with improvement in 6–12 hours.31
Contrast Media
Contrast media extravasation carries an incidence of 0.1–0.9%32,33 and is usually minor and self-resolving; however, it can escalate to more serious complications.32,34 Extravasated contrast media is toxic to the surrounding tissues and is associated with an inflammatory response that peaks between 24 and 48 hours.32 The mechanism of toxicity is similar to that of osmotically active agents, as its hypertonicity results in fluid shifts.32
There is no evidence-based consensus on management; however, most commonly, the limb is elevated and treated with a warm compress.33 The efficacy of this maneuver is not proven.16,32 The attempted aspiration of contrast media, liposuction, or injection of local therapies such as hyaluronidase or corticosteroids has no evidential support for the treatment of a contrast media extravasation.32 As in all extravasation injuries, monitoring of the patient for progression of symptoms, such as progressive neurologic or vascular complications, is appropriate and necessary.33
Cytotoxic Drugs
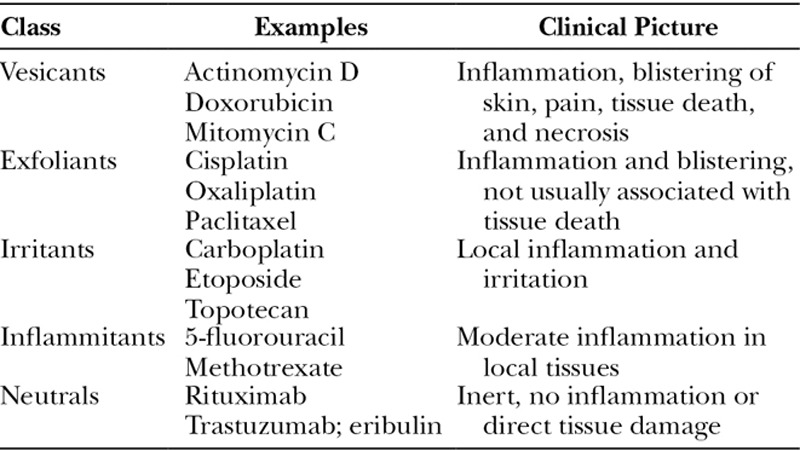
Cytotoxic drugs are grouped into 5 categories: irritants, inflammitants, exfoliants, vesicants, and neutrals (Table 4),11 with each causing characteristic clinical pictures of soft-tissue damage. Below, we will review the described pharmacologic interventions for these chemotherapeutic extravasations.
Table 4.
Hyaluronidase
Hyaluronidase degrades glycosaminoglycans and hyaluronic acid, with subsequent increases in tissue permeability and dispersion of extravasated agent.11,14 Hyaluronidase has been used for extravasation by etoposide, taxanes (ie, paclitaxel, docetaxel, and cabazitaxel), and vinca alkaloids.11 Although hyaluronidase is recognized as therapeutic for extravasation, there is much inconsistency with regard to proper technique and dosage. Additionally, there is a dearth of controlled, nonanecdotal studies proving its efficacy.
Rat studies have shown hyaluronidase to be effective at reducing the wound size and healing time in vinorelbine-induced extravasations.35 In 1994, hyaluronidase was also effective at preventing necrosis in 7 patients with accidental vinca alkaloid extravasation.36 Despite the poor evidence-based strength of recommendation, it is worth noting that the manufacturers of vinblastine indicate the use of hyaluronidase if extravasation occurs.37,38
Recent guidelines, especially for chemotherapeutic extravasations in adults, recommend a dosage of 150 units of hyaluronidase.30 However, dosage varies among institutions. For Amphadase (Amphastar Pharmaceuticals, Rancho Cucamonga, Calif.), Hydase (Akorn, Lake Forest, Ill.), and Hylenex (Baxter Healthcare Corporation, Deerfield, Ill.), vials contain 150 units/mL, which do not need further dilution.30 Vitrase (ISTA Pharmaceuticals, Irvine, Calif.) contains 2 mL of 200 units hyaluronidase, which should be further diluted in a 3:1 ratio of vitrase to normal saline (0.75 mL of vitrase solution with 0.25 mL of normal saline). Hyaluronidase (150 unit in 1 mL) should be administered through five 0.2 mL subcutaneous injections into the extravasation site.
For pediatric patients, it has been shown that the administration of 15 units of hyaluronidase is effective to resolve the extravasation.19 This dilution is achieved with 0.1 mL (150 unit/mL) hyaluronidase solution with 0.9 mL of normal saline, yielding the desired 15 units/mL for injection. The administration should be done immediately, with benefit seen when given within 3 hours of insult. In pediatric patients or infants, a 27–30 gauge needle is used to inject five 0.2 mL injections of hyaluronidase around the infiltration site in a circular pattern.19,30,39
Dexrazoxane Hydrochloride
Dexrazoxane [Zinecard (Pfizer, New York City), Savene and Totect (Clinigen Group Plc, Burton-on-Trent, United Kingdom)] is an FDA-approved local therapy for anthracycline (daunorubicin, doxorubicin) extravasation,11 which commonly results in severe tissue damage. Its mode of action is not well understood.11,40 Two prospective clinical trials, TT01 and TT02, were conducted to determine the efficacy and tolerability of treatment with dexrazoxane [Savene (Clinigen Group Plc, Burton-on-Trent, United Kingdom)]. These studies, unlike other extravasation studies, used fluorescence biopsies to objectify the diagnosis of extravasation.41 Fifty-four patients were confirmed to have anthracycline extravasation. The 54 patients underwent dexrazoxane treatment: 1,000 mg/m2 within 6 hours of insult, 1,000 mg/m2 24 hours after initial treatment, and 500 mg/m2 48 hours after initial treatment.41 Of the 54 patients receiving treatment, only 1 patient required a surgical intervention, an efficacy rate of 98%.41,42 In addition, over two-thirds the patients had no resultant delay in chemotherapeutic treatment secondary to soft-tissue wounds.41,42
Indication for dexrazoxane administration is extravasation of more than 5 mL of an anthracycline drug.9 It is contraindicated in the following patient populations: less than 18 years old, hepatic or renal insufficiency, recent inoculation with a live vaccine, and patients receiving phenytoin.9,43 It may also be teratogenic and should be avoided during pregnancy.44
The treatment consists of a 3-day course of IV dexrazoxane.45 The first dose of 1,000 mg/m2 is ideally administered as soon as possible, followed by 1,000 mg/m2 on day 2, and 500 mg/m2 on day 3.15,43,45
Concomitant administration of dimethyl sulfoxide (DMSO) is not recommended,15,43,46 as this has been associated with an increased occurrence of wounds.43
DMSO
DMSO is a topical organosulfur solvent that increases skin permeability and, therefore, augments absorption and subsequent dispersion of the extravasated agent,11,30,47 and functioning as a free-radical scavenger.11 Ninety-nine percentage of DMSO is applied topically and may improve outcomes for smaller anthracycline extravasations. It was shown to be effective in resolving anthracycline extravasation in a prospective pilot trial of 20 patients. No patient required surgical intervention or had lesion formation.48 However, there is a lack of evidence using objective fluorescence biopsy data, and thus routine use in anthracycline extravasation remains controversial.47 However, DMSO in conjunction with cold compresses remains the most common acute therapy minor anthracycline extravasation, particularly if dexrazoxane is not available.11
DMSO should be implemented within the first 10–25 minutes of extravasation,9 while minimizing exposure to unaffected skin, followed by hydrocortisone if erythema is present,9 and then a cold compress. This process should be repeated every 2 hours for the first day, after which the frequency is reduced.9
Sodium Thiosulfate
Sodium thiosulfate functions as an additional target for alkylation and can hypothetically neutralize certain vesicants, specifically mechlorethamine (nitrogen mustard, no longer commercially available) or concentrated cisplatin.49–51 However, while many providers report the use of sodium thiosulfate, no randomized clinical trials have been conducted on its efficacy.51
When administered, sodium thiosulfate is injected subcutaneously in a pinwheel fashion around the affected tissue. Four to 8 mL of 10% sodium thiosulfate is mixed with 6 mL of sterile water.50 Two milliliter of sodium thiosulfate solution is injected for every milligram of mechlorethamine or 100 milligrams of cisplatin infiltrated.
Gault Method
The Gault Method of saline washout for extravasation injuries was first described in 1993.26 This method involves early saline washout with subsequent liposuction via a small incision. Forty-four of the 96 patients studied underwent early washout. Eighty-six percentage healed with no soft tissue loss compared with 15% in the late referral group. In areas with less subcutaneous fat, Gault describes injection 1 unit of hyaluronidase. This is followed by making 4 small incisions surrounding the extravasated area. Finally, the extravasation area is flushed with normal saline, resulting in irrigation of subcutaneous tissue and efflux through the previously placed incisions.26
Dressings
In the event of tissue necrosis, the wound should be dressed in a manner to promote a moist, clean, and warm environment, while removing excess exudate.52,53 Eschar and necrotic tissue can impair reepithelialization and strong consideration should be given to debridement once the extent of tissue death has demarcated.54 Plain gauze (wet to dry dressings), while beneficial in debriding the wound bed on removal, can also traumatize viable healthy tissue, be painful, and needs frequent dressing changes to be effective. Nonadherent dressings [ie, Xeroform (Tyco Healthcare/Kendall, Mansfield, Mass.) and Adaptic [Johnson and Johnson, Somerville, N.J.)] can maintain a moist environment in epithelializing wounds and reduce pain with dressing changes, however should be changed daily to prevent them from drying and traumatizing the tissue bed on removal. Hydrocolloid dressings [DuoDERM (Convatec, Bridgewater, N.J.), Exuderm (Medline, Havre de Grace, MD), Comfeel (Coloplast, Minneapolis, Minn.), etc] can absorb mild amounts of exudate, maintain a moist environment, and minimize skin trauma with dressing changes. They have been shown to be of benefit in the neonatal population.55 Wounds with higher degrees of exudate may require more absorptive dressings such as alginates or hydrofibers.
Surgical Management
In the majority of instances, conservative management is successful, as only 1 in 3 vesicants results in ulceration.56 Surgical management may become indicated if soft-tissue necrosis develops. Healing capability is typically impaired due to a lack of granulation tissue and restricted epithelial ingrowth resulting in ulcer progression.56 In patients undergoing chemotherapeutic treatment, open wounds may present an increased infection risk and delays in treatment. A discussion between medical oncologists and the surgical team is critical to determine the optimal treatment course and timeframe for the patient.
Debridement (with or without reconstruction) is indicated if extravasation results in continuous pain (persisting for 1–2 weeks), persistent ulceration, or full-thickness skin necrosis.9,11,14,15,46,57 Fasciotomy is the treatment of choice if compartment syndrome develops.6,41
CONCLUSIONS
Iatrogenic injuries secondary to intravenous extravasation are commonplace in hospitals. In severe cases, limb loss is a potential consequence of poor or delayed management. Nursing algorithms for management of extravasation injuries are usually directed toward consultation of a wound care team immediately after identification, who may implement local interventions in the appropriate time-sensitive manner. Hospitals without a dedicated wound care team trained in these interventions will often default to plastic surgical consultation, making an understanding of available interventions essential to the initial evaluation and management of these injuries. Guidelines for the treatment of extravasation injuries facilitate decision making, given their time-sensitive nature. Appropriate immediate management may reduce further damage, limb loss, and even avoid potential litigation. Although there is no standardized protocol for extravasations injuries, a proposed treatment algorithm is shown in Supplemental Digital Content 1.
The goal of this article was to provide plastic surgeons and trainees with a comprehensive overview of the acute management of intravenous extravasation injuries. Our hope is that this will serve as an effective resource for caregivers treating acute extravasation injuries (See figure, Supplemental Digital Content 1, which displays the treatment algorithm, http://links.lww.com/PRSGO/A724). Though the decision for surgical versus nonsurgical management is often a clear one for plastic surgeons, local interventions, and therapies are often indicated and under-utilized in the immediate postextravasation period. Thorough knowledge of these interventions should be a basic requirement in the armamentarium of plastic surgery consultants.
Footnotes
Published online 19 April 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Feldmann H. [History of injections. Pictures from the history of otorhinolaryngology highlighted by exhibits of the German History of Medicine Museum in Ingolstadt]. Laryngorhinootologie. 2000;79:239–246.. [DOI] [PubMed] [Google Scholar]
- 2.Coyle C, Griffie J, Czaplewski L. Eliminating extravasation events: a multidisciplinary approach. Infusion Nurses Society. 2015;38:43–50.. [DOI] [PubMed] [Google Scholar]
- 3.Dychter S, Gold D, Carson D, et al. Intravenous therapy: a review of complications and economic considerations of peripheral access. Art Science Infusion Nursing. 2012;35:84–91.. [DOI] [PubMed] [Google Scholar]
- 4.Scuderi N, Onesti MG. Antitumor agents: extravasation, management, and surgical treatment. Ann Plast Surg. 1994;32:39–44.. [PubMed] [Google Scholar]
- 5.Lake C, Beercroft CL. Extravasation injuries and accidental intra-arterial injection. Continuing education in anaesthesia. Crit Care Pain. 2012;10:109–112.. [Google Scholar]
- 6.Doellman D, Bowe-Geddes LA, Franlkin M, et al. Infiltration and extravasation: update on prevention and management. J Infus Nurs. 2009;32:203–211.. [DOI] [PubMed] [Google Scholar]
- 7.Schummer W, Schummer C, Bayer O, et al. Extravasation injury in the perioperative setting. Anesth Analg. 2005;100:722–727., table of contents. [DOI] [PubMed] [Google Scholar]
- 8.Edwards JJ, Samuels D, Fu ES. Forearm compartment syndrome from intravenous mannitol extravasation during general anesthesia. Anesth Analg. 2003;96:245–246., table of contents. [DOI] [PubMed] [Google Scholar]
- 9.St Luke’s Cancer Alliance NH. Guidelines for prevention and management of chemotherapy extravasation. 2014;1–18..
- 10.Guide to extravasation management in adult and pediatric patients. University of Kansas Hospital. 2016. Available at http://icmwk.com/wp-content/uploads/2016/02/Extravasations-management-UoKansasH-.pdf. Accessed July 12, 2016.
- 11.Kreidieh FY, Moukadem HA, El Saghir NS. Overview, prevention and management of chemotherapy extravasation. World J Clin Oncol. 2016;7:87–97.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.S. Department of Health and Human Services, National Institute of Health, National Cancer Institute. Common terminology criteria for adverse events (CTAE). 2009.
- 13.Simona R. A pediatric peripheral intravenous infiltration assessment tool. J Infus Nurs. 2012;35:243–248.. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds P, MacLaren R, Mueller S, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617–632.. [DOI] [PubMed] [Google Scholar]
- 15.Wengström Y, Margulies A; European Oncology Nursing Society Task Force. European Oncology Nursing Society extravasation guidelines. Eur J Oncol Nurs. 2008;12:357–361.. [DOI] [PubMed] [Google Scholar]
- 16.ACR manual on contrast media. ACR committee on drugs and contrast media Version 10.2.. 2016. Available at http://www.acr.org/Quality-Safety/Resoruces/Contrast-Manuel. Accessed July 12, 2016.
- 17.Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870–886.. [DOI] [PubMed] [Google Scholar]
- 18.Tsai YS, Cheng SM, Ng SP, et al. Squeeze maneuver: an easy way to manage radiological contrast-medium extravasation. Acta Radiol. 2007;48:605–607.. [DOI] [PubMed] [Google Scholar]
- 19.Thigpen JL. Peripheral intravenous extravasation: nursing procedure for initial treatment. Neonatal Netw. 2007;26:379–384.. [DOI] [PubMed] [Google Scholar]
- 20.Giunta R. Early subcutaneous wash-out in acute extravasations. Ann Oncol. 2004;15:1146; author reply 1147. [DOI] [PubMed] [Google Scholar]
- 21.Al-Benna S, O’Boyle C, Holley J. Extravasation injuries in adults. ISRN Dermatol. 2013;2013:856541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loubani OM, Green RS. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J Crit Care. 2015;30:653.e9–653.17.. [DOI] [PubMed] [Google Scholar]
- 23.Sinclair M, Bailey M, McAree B, et al. Rapid epinephrine ‘reversal’ with phentolamine following autoinjector inoculation. Myels. Vascular Med. 2011;16:215–216.. [DOI] [PubMed] [Google Scholar]
- 24.Mathez C, Favrat B, Staeger P. Management options for accidental injection of epinephrine from an autoinjector: a case report. J Med Case Rep. 2009;3:7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardenas-Garcia J, Schaub KF, Belchikov YG, et al. Safety of peripheral intravenous administration of vasoactive medication. J Hosp Med. 2015;10:581–585.. [DOI] [PubMed] [Google Scholar]
- 26.Gault DT. Extravasation injuries. Br J Plast Surg. 1993;46:91–96.. [DOI] [PubMed] [Google Scholar]
- 27.Dugan S, Le J, Jew RK. Maximum tolerated osmolarity for peripheral administration of parenteral nutrition in pediatric patients. JPEN J Parenter Enteral Nutr. 2014;38:847–851.. [DOI] [PubMed] [Google Scholar]
- 28.Wall CJ, Lynch J, Harris IA, et al. ; Liverpool(Sydney) and Royal Melbourne Hospitals. Clinical practice guidelines for the management of acute limb compartment syndrome following trauma. ANZ J Surg. 2010;80:151–156.. [DOI] [PubMed] [Google Scholar]
- 29.Schaverien MV, Evison D, McCulley SJ. Management of large volume CT contrast medium extravasation injury: technical refinement and literature review. J Plast Reconstr Aesthet Surg. 2008;61:562–565.; discussion 565. [DOI] [PubMed] [Google Scholar]
- 30.Schulmeister L. Vesicant chemotherapy extravasation antidotes and treatments. Clin J Oncol Nurs. 2009;13:395–398.. [DOI] [PubMed] [Google Scholar]
- 31.Gil ME, Mateu J. Treatment of extravasation from parenteral nutrition solution. Ann Pharmacother. 1998;32:51–55.. [DOI] [PubMed] [Google Scholar]
- 32.Nicola R, Shaqdan KW, Aran S, et al. Contrast media extravasation of computed tomography and magnetic resonance imaging: management guidelines for the radiologist. Curr Probl Diagn Radiol. 2016;45:161–164.. [DOI] [PubMed] [Google Scholar]
- 33.Rose TA, Jr, Choi JW. Intravenous imaging contrast media complications: the basics that every clinician needs to know. Am J Med. 2015;128:943–949.. [DOI] [PubMed] [Google Scholar]
- 34.Bellin MF, Jakobsen JA, Tomassin I, et al. ; Contrast Media Safety Committee Of The European Society Of Urogenital Radiology. Contrast medium extravasation injury: guidelines for prevention and management. Eur Radiol. 2002;12:2807–2812.. [DOI] [PubMed] [Google Scholar]
- 35.Zhu QC, Luo RC, Miao JX, et al. Topical dimethyl sulfoxide and intralesional hyaluronidase administration for vinorelbine extravasation-induced rat skin injury. J South Med Univ. 2007;27:9. [PubMed] [Google Scholar]
- 36.Bertelli G, Dini D, Forno GB, et al. Hyaluronidase as an antidote to extravasation of Vinca alkaloids: clinical results. J Cancer Res Clin Oncol. 1994;120:505–506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.APP Pharmaceuticals, LLC. Available at http://medlibrary.org/lib/rx/meds/vinblastine-sulfate-1/page/4/. Accessed July 2016.
- 38.Gonzalez T. Chemotherapy extravasations: prevention, identification, management, and documentation. Clin J Oncol Nurs. 2013;17:61–66.. [DOI] [PubMed] [Google Scholar]
- 39.Beaulieu MJ. Hyaluronidase for extravasation management. Neonatal Netw. 2012;31:413–418.. [DOI] [PubMed] [Google Scholar]
- 40.Conde-Estévez D, Mateu-de Antonio J. Treatment of anthracycline extravasations using dexrazoxane. Clin Transl Oncol. 2014;16:11–17.. [DOI] [PubMed] [Google Scholar]
- 41.Mouridsen HT, Langer SW, Buter J, et al. Treatment of anthracycline extravasation with Savene (dexrazoxane): results from two prospective clinical multicentre studies. Ann Oncol. 2007;18:546–550.. [DOI] [PubMed] [Google Scholar]
- 42.Langer SW. Dexrazoxane for the treatment of chemotherapy-related side effects. Cancer Manag Res. 2014;6:357–363.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Medicines Agency. 2006. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000682/human_med_001047.jsp&mid=WC0b01ac058001d124. Accessed July 2016.
- 44.Jordan K, Behlendorf T, Mueller F, et al. Anthracycline extravasation injuries: management with dexrazoxane. Ther Clin Risk Manag. 2009;5:361–366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassagnol M. Management of chemotherapy extravasations. US Pharm. 2009;34:3–11.. [Google Scholar]
- 46.Fontaine C, Noens L, Pierre P, et al. Savene® (dexrazoxane) use in clinical practice. Support Care Cancer. 2012;20:1109–1112.. [DOI] [PubMed] [Google Scholar]
- 47.Langer SW, Sehested M, Jensen PB. Anthracycline extravasation: a comprehensive review of experimental and clinical treatments. Tumori. 2009;95:273–282.. [DOI] [PubMed] [Google Scholar]
- 48.Olver IN, Aisner J, Hament A, et al. A prospective study of topical dimethyl sulfoxide for treating anthracycline extravasation. J Clin Oncol. 1988;6:1732–1735.. [DOI] [PubMed] [Google Scholar]
- 49.Schulmeister L. Extravasation management. Semin Oncol Nurs. 2007;23:184–190.. [DOI] [PubMed] [Google Scholar]
- 50.Ener RA, Meglathery SB, Styler M. Extravasation of systemic hemato-oncological therapies. Ann Oncol. 2004;15:858–862.. [DOI] [PubMed] [Google Scholar]
- 51.Boulanger J, Ducharme A, Dufour A, et al. ; Comité de l’évolution de la pratique des soins pharmaceutiques (CEPSP); Comité de l’évolution des pratiques en oncologie (CEPO). Management of the extravasation of anti-neoplastic agents. Support Care Cancer. 2015;23:1459–1471.. [DOI] [PubMed] [Google Scholar]
- 52.Okan D, Woo K, Ayello EA, et al. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20:39–53.; quiz 53. [DOI] [PubMed] [Google Scholar]
- 53.Sawatzky-Dickson D, Bodnaryk K. Neonatal intravenous extravasation injuries: evaluation of a wound care protocol. Neonatal Netw. 2006;25:13–19.. [DOI] [PubMed] [Google Scholar]
- 54.Attinger CE, Janis JE, Steinberg J, et al. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117: 72S–109S.. [DOI] [PubMed] [Google Scholar]
- 55.Sung KY, Lee SY. Nonoperative management of extravasation injuries associated with neonatal parenteral nutrition using multiple punctures and a hydrocolloid dressing. Wounds. 2016;28:145–151.. [PubMed] [Google Scholar]
- 56.Schrijvers DL. Extravasation: a dreaded complication of chemotherapy. Ann Oncol. 2003;3:26–30.. [DOI] [PubMed] [Google Scholar]
- 57.Shenaq SM, Abbase EH, Friedman JD. Soft-tissue reconstruction following extravasation of chemotherapeutic agents. Surg Oncol Clin N Am. 1996;5:825–845.. [PubMed] [Google Scholar]


