Abstract
Background:
Multiple symmetric lipomatosis (MSL) is defined as a disorder of nonencapsulated adipose tissue growth. Its prevalence is indicated as 1:25,000 and affects, as stated in the literature, mainly Mediterranean males (male:female ratio of 15:1). Phenotypes are still classified as defined by Donhauser in 1991. We report clinical and phenotypic data of the largest patient cohort investigated in Germany so far.
Methods:
Forty-five patients diagnosed with MSL at the University Hospital Regensburg between 2007 and 2017 were photographed, clinically examined, and blood samples were taken. Based on the photographs (n = 33), 11 independent observers assessed patients using the Donhauser classification. Furthermore, the bodies of all patients were subdivided into 12 body areas, and the viewers had to indicate all MSL-affected areas per patient. Prevalence was calculated, comorbidities were assessed, and blood samples were analyzed.
Results:
According to the established Donhauser classification, less than 50% of the patients could be classified. Therefore, based on the constellations of MSL-affected body areas, a new classification that divides phenotypes of MSL into 5 types (Ia, Ib, Ic, II, and III) was set up and was able to cover 100% of our patients. The male to female ratio was found to be 1:2.5 (male:female). Prevalence of MSL in the catchment area was found to be 1:25,000. Hypercholesterinemia and hypothyroidism were frequent comorbidities, and blood analyses were normal besides a hypercholesterinemia.
Discussion:
The new proposed classification system describes 5 subtypes and allowed to classify all assessed patients. Male to female ratio (1:2.5) contradicted most previous publications.
INTRODUCTION
Multiple symmetric lipomatosis (MSL) (syn.: Launois Bençaude Syndrome, Morbus Madelung, benign symmetric lipomatosis) was first described in 1846 by Brodie and is defined as a symmetric disorder of nonencapsulated adipose tissue growth.1 In 1888, Madelung reported on 3 patients with symmetrical submental depositions of fat, the “Madelung’s collar,” and in 1898, Launois and Bençaude described the phenotype as “symmetrical adenolipomatosis.” Its prevalence is indicated as 1:25,000 and affects, as stated in the literature, mainly Mediterranean males (male: female ratio of 15–30:1).2–6
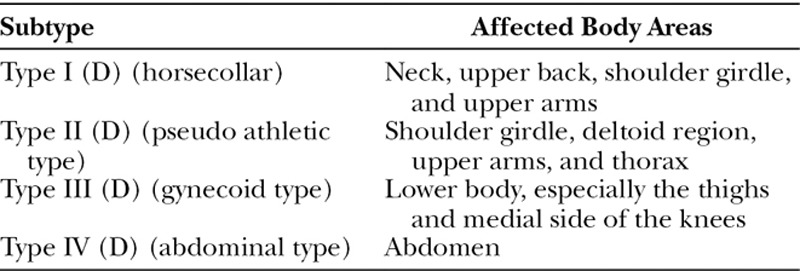
Diagnosis is established through physical examination and anamnesis. There are principally 2 different classifications used in the literature. Enzi et al. (E)1 described 2 types of anatomic distribution of fat deposits. Type 1 (E) is predominantly distributed in the neck (Madelung’s collar), shoulders, supraclavicular triangle, and proximal upper limbs. In type 2 (E), the neck area and upper trunk are not affected; deposits occur in the abdomen and thighs.1 The most common classification was described in 1991 by Donhauser (D) and divides patients into 4 types of MSL,7 as shown in Table 1.
Table 1.
Donhauser Classification (D)7
The pathophysiology of MSL still remains unclear, although several approaches have been described. An association with hyperinsulinemia, hyperlipoproteinemia, hypothyroidism, hyperuricemia, renal tubular acidosis, neuropathy, and myopathy has been described.8–13 Neurological multisystem manifestation is common in these patients but cannot be solely explained by alcohol abuse.14,15 Quite generally, the association between alcohol consumption and MSL is a controversial issue.3,14,16 Reports of sudden death and autonomous nervous affection have expanded the list of possible related diseases and complications.15
There are only a few reports of familiar MSL cases that indicate a monogenetic cause that is inherited in an autosomal dominant manner.17 A mitochondrial disorder, myoclonic epilepsy with ragged red fibers syndrome, is often associated with lipomatosis, although a correlated gene mutation has not been discovered.15
The purpose of the present study was to investigate the largest German cohort of MSL patients (n = 45) treated at the University Hospital Regensburg and to obtain further data on disease classification and epidemiology, as most of the patients could not be reliably classified by current classifications.
METHODS AND MATERIALS
The database of the University Hospital Regensburg contains 64 patients with a suspected diagnosis of MSL recorded between 2011 and 2017. From May to July 2017, all these patients were contacted (either called or sent a written invitation) and invited to show up for clinical reevaluation. Besides anamnesis, age, sex, weight, and height, assessment of comorbidities was prospectively recorded. Blood samples were taken, and the patients were clinically examined and photographed (with patient approval). For patients who could not or refused to show up, the same data were retrospectively collected from the database as present.
Diagnosis was clinically conducted by considering morphological characteristics and anamnesis. Morphological characteristics were defined as disproportionate hypertrophic symmetric fat tissue growth of MSL-typical areas. Anamnestic factors were the sudden occurrence of fat deposits that grew independently of general body fat increase and resistance to conservative therapies, such as diets. Based on the photographic documentation, 11 independent observers were asked to classify the patients according to the Donahuser classification (D). Furthermore, the body of every patient was subdivided into 12 body areas. Chosen areas were neck, collar, shoulder girdle, upper arm, forearm, chest, abdomen, upper and lower back, hips, upper and lower limb. The observers evaluated which body parts were affected by MSL. Based on the frequency of every affected body area, we assessed which combinations of areas were affected most frequently and set up a new classification based on these data.
By marking the postal codes of all patients on a map, a geographical area could be defined. From the local registration office, the population of the defined area was found. By dividing the population by the number of affected patients, we could get an approximate prevalence of MSL in the area.
Ascertained blood values were electrolytes (Na2+, K+, Ca2+), creatinine, liver function reading (aspartate aminotransferase [GOT], Alanine aminotransferase [GPT], gamma-glutamyl transferase [gGT]), bilirubin, insulin, HBA1c, thyroid stimulating hormone (TSH), cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL), and triglycerides were ascertained.
RESULTS
General
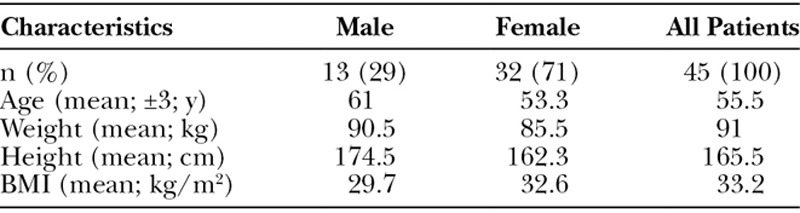
Of the 64 patients invited for reevaluation, only 26 patients appeared to be clinically prospectively reevaluated. Prospectively collected data of these patients were family anamnesis, sudden disease occurrence, weight, height, blood samples, photographs, and comorbidities. Age of first presentation was collected retrospectively. Thirty-eight patients were completely retrospectively reevaluated out of the patient file. Three patients had already died. One patient died of cardiac failure; the causes of death of the other patients are unknown. In total, only 45 patients were correctly diagnosed with MSL following the criteria above. Misdiagnoses were lipedema (n = 3), lipomas (n = 3), lipomatosis dolorosa (n = 4), and obesity (n = 6). Figure 1 shows an overview of the patient flow. Gender distribution was 13 (29%) males and 32 (71%) females (ratio m/f: 1/2.5). The mean age at the date of recording was 55.5 (± 3) years (range, 27–78 years old). The mean weight was 91 kg (range, 53–130 kg), and mean height was 165.5 cm (range, 148–188 cm). The average body mass index was 33.2 kg/m2. The area obtained by marking the postal codes on a regional map, correspond to “Oberpfalz” in Bavaria, Germany, with a population of 1,092,339. Prevalence was therefore calculated to be 1:24,274. (Table 2).
Fig. 1.
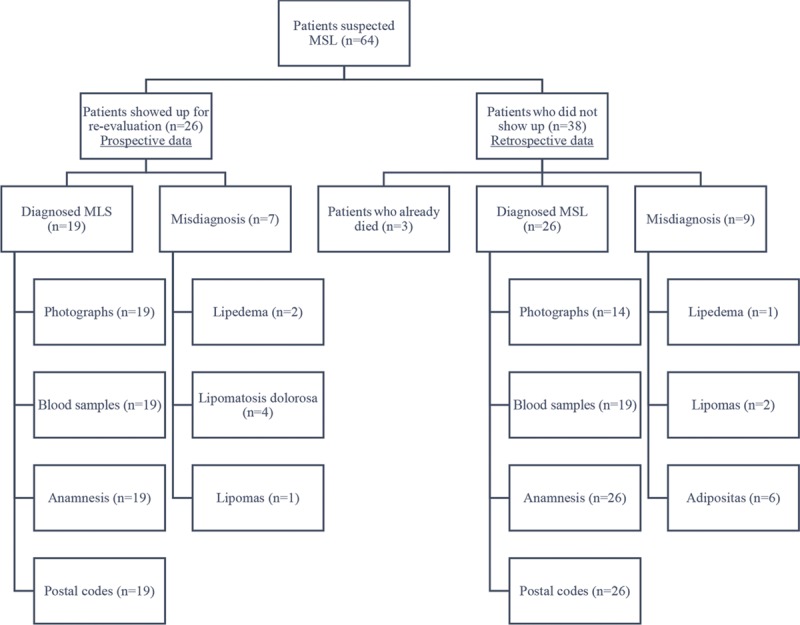
Shows an overview of the patient flow.
Table 2.
General Characteristics of Our Patients
Phenotyping
Photographs of 33 patients were assessed for phenotyping. Fallowing the Donhauser classification criteria, only 45% (n = 15) patients could be classified by the observers (Table 3). Affected body areas were collar in 3% of all patients (n = 1), neck in 64% (n = 21), shoulder girdle was affected in 36% (n = 12), upper arms in 48% (n = 16), forearms were not affected, chest was affected in 33% (n = 11), and abdomen in 36% (n = 12). The upper back was affected in 30% (n = 10) and the lower back in 21% (n = 7). Hips and bottom showed MSL changes in 52% (n = 17), upper legs were affected in 45% (n = 15), and only 3% of lower legs were affected (Fig. 2).
Table 3.
Comparison New Classification to Donhausers Classification (D) and Applicability on Our Patients
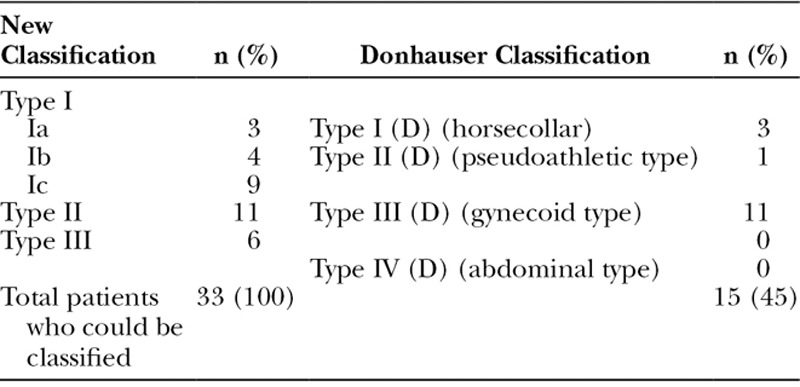
Fig. 2.
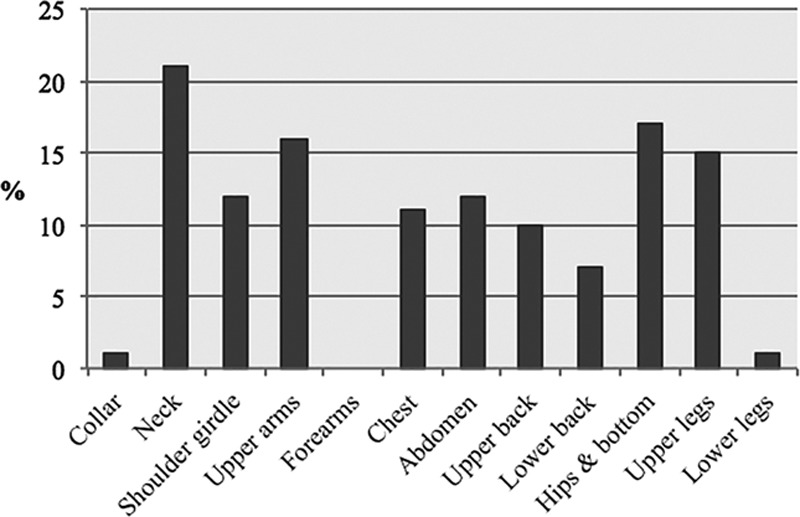
Shows how often the different body areas are affected.
Most patients showed an affection of the 4 following areas: neck (64%), upper arms (48%), hips and bottom (52%), upper legs (45%). Of those with an affected shoulder girdle, 57% of these also had an affected neck. Of those with affected upper arms, 62% of these also had an affected neck. Nearly half of all patients showed an affected chest and abdomen (48%). In contrast, of those patients with affected neck or shoulder girdle, chest and abdomen, 69% and 63%, respectively, were affected in the upper arms. Fifty-six percentage showed an affected upper back. Patients with affected hips and bottom showed an affection of the upper limb in 88% of cases. All patients suffering from affected upper legs showed affection of hip and bottom too.
When focusing on the number of affected areas per patient, only 3 patients (9%) showed only 1 affected area. In all 3 of them, only neck was affected. Twelve patients (36%) showed 2 affected areas. In these cases, 75% showed an affection of the hips/bottom and upper limbs and 25% an affection of neck and shoulder girdle. Seven patients (21%) showed 3 affected areas. Eighty-six percentage had an affection of the neck, shoulder girdle, and upper arms. Forty-three percentage showed in addition to neck and upper arms fat deposits on hips/bottom and 43% on the abdomen. Eleven patients (33%) showed more than 3 affected areas. Most of them showed an affection of neck, upper arms, whole trunk, hips/bottom, and upper limb.
In general, one can say that in most cases either the upper body parts (type I; n = 16; 49%; neck, shoulder girdle, upper arms, and trunk), the lower body parts (type II; n = 11; 33%; hips/bottom and upper legs) or a general distribution, apart from head, forearms, and lower legs are affected (type III; n = 6; 18%; Fig. 3A; Table 3).
Fig. 3.
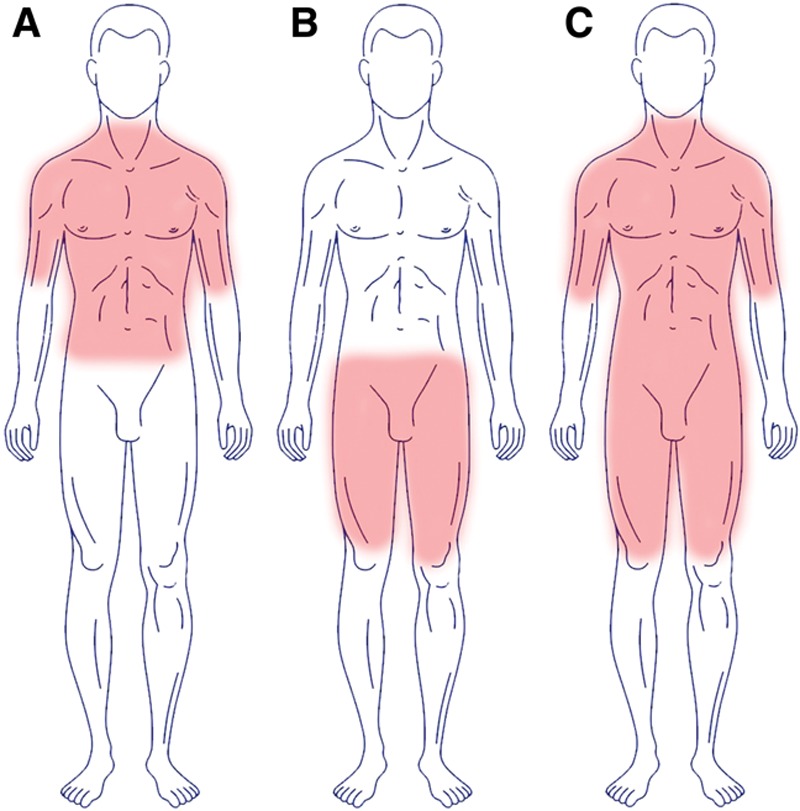
Showing the different phenotypes of the new classification. A, Phenotype I. B, Phenotype II. C, Phenotype III.
The upper body part can be subdivided in type Ia (neck; n = 3; 9%), type Ib (neck + shoulder girdle + upper arms; n = 4; 12%) and type Ic (neck + shoulder girdle + upper arms + trunk; n = 9; 28%; Figs. 4–10; Table 4).
Fig. 4.
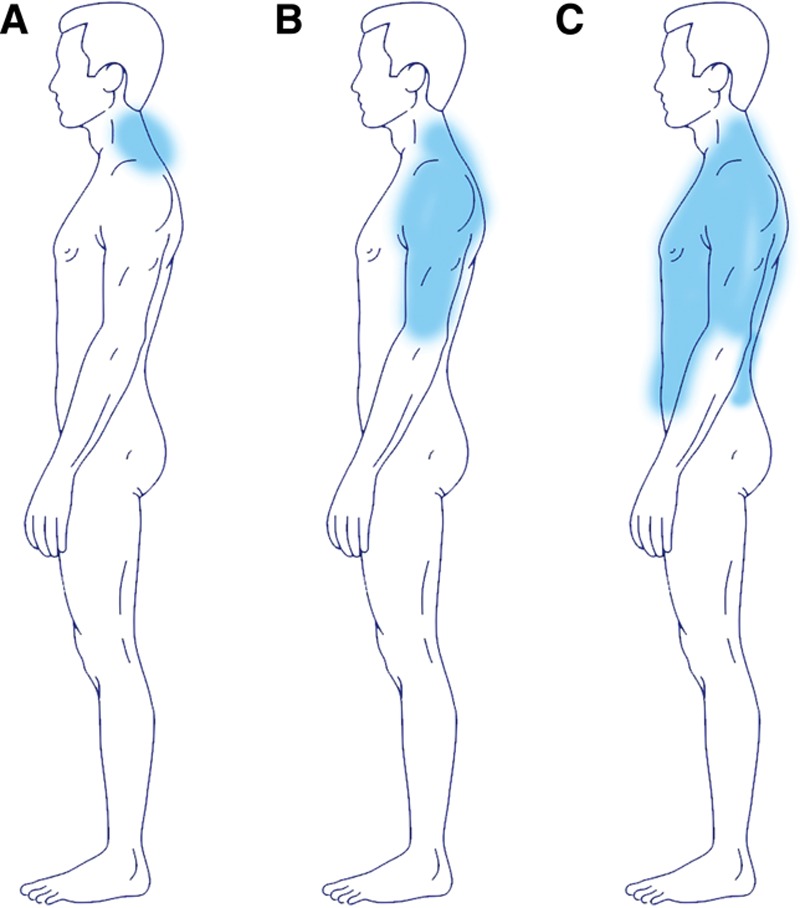
Showing the different subtypes of type I (new classification). A, Ia. B, Ib. C, Ic.
Fig. 10.
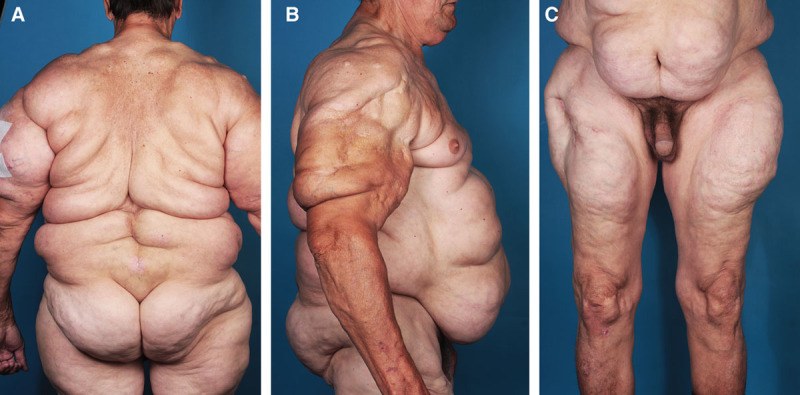
A–C, Patient suffering from MSL type III (new classification).
Table 4.
Overview of Phenotypes According to the New Classification
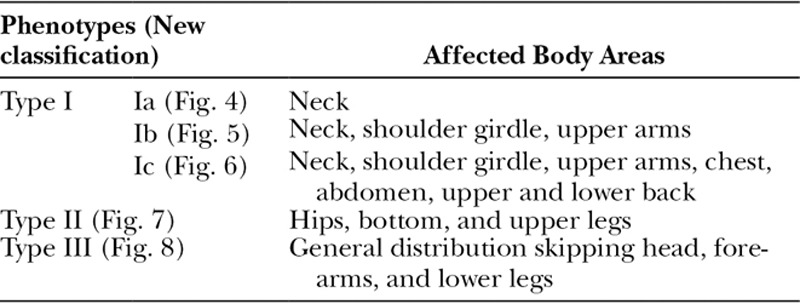
Fig. 5.
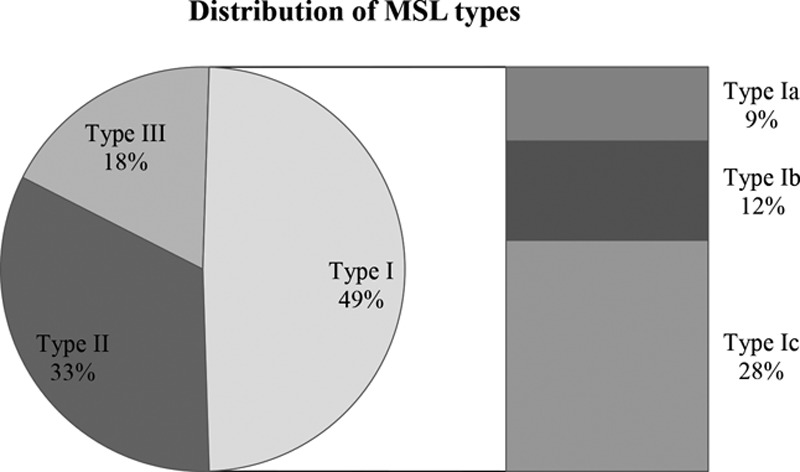
Different phenotypes and subtypes and its distribution in our patients (%; new classification).
Fig. 6.
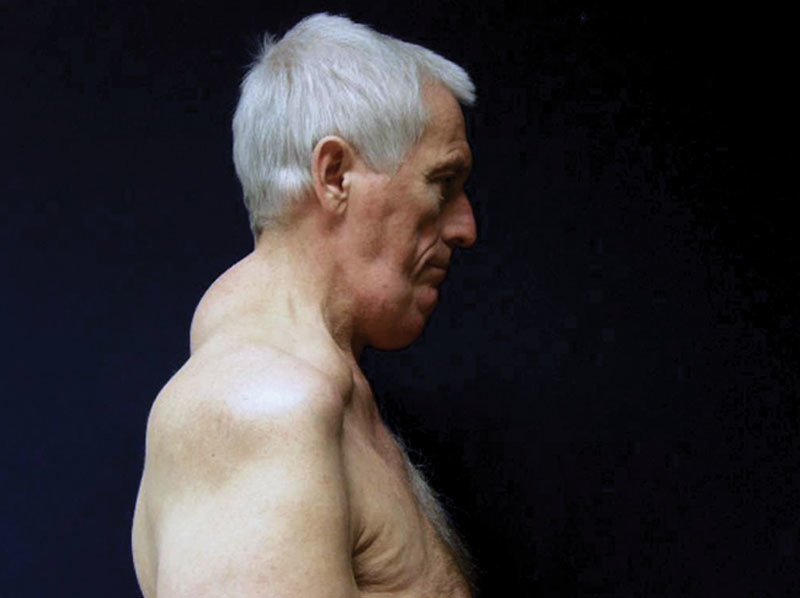
Patient suffering from MSL type Ia (new classification).
Fig. 7.
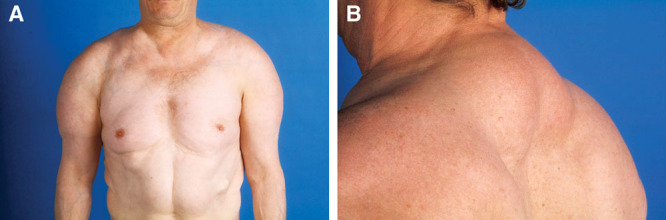
A and B, Patient suffering from MSL type Ib (new classification).
Fig. 8.
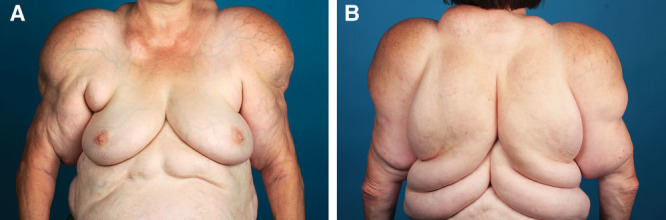
A and B, Patient suffering from MSL type Ic (new classification).
Fig. 9.
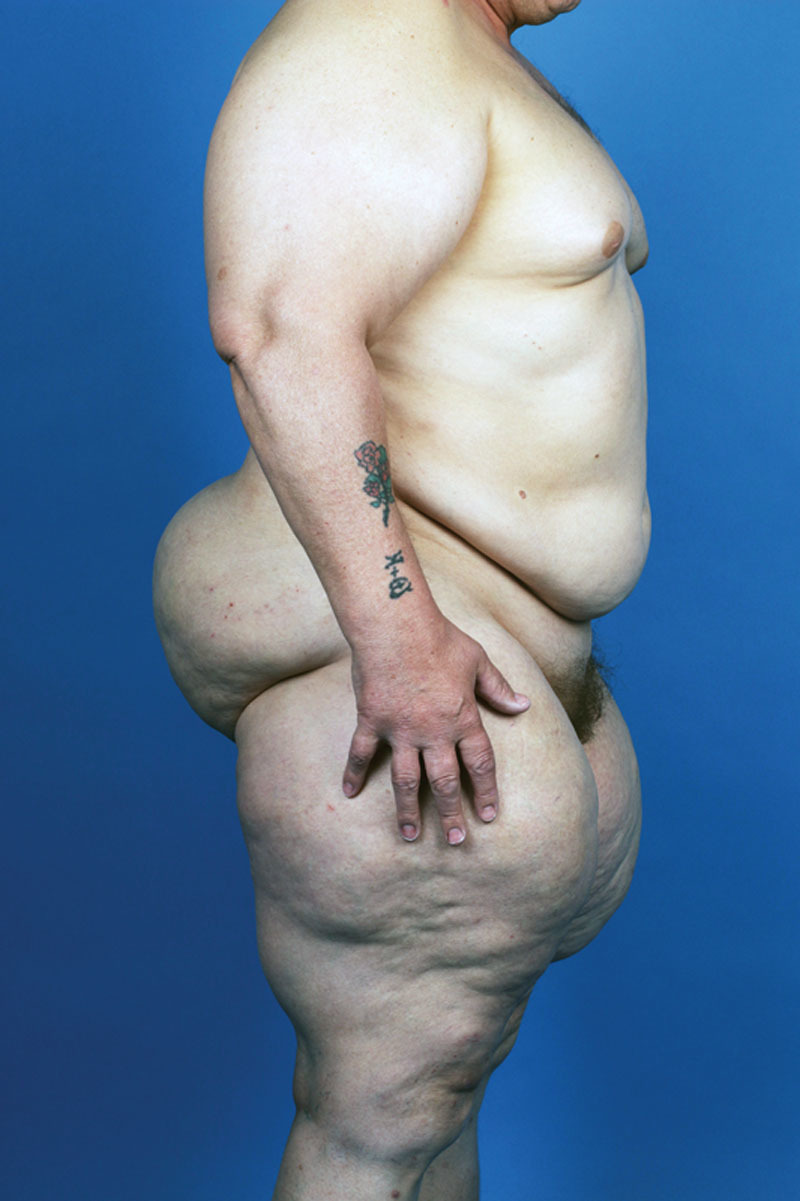
Patient suffering from MSL type II (new classification).
One female patient, presenting a phenotype type Ib, showed fat accumulation in the external labia as well. On demand, the patient stated that growth of the labia appeared delayed to the other, more typical fat accumulations. One female (type II) suffered additionally from a lipedema and lymphedema on both legs.
Comorbidities
Comorbidities were assessed through anamnesis and were obtained from all 45 patients. The most frequent comorbidities were hypercholesterolemia (n = 10) and hypothyreosis (n = 9; Fig. 11). Fourteen patients (37%) had a positive family anamnesis of MSL. Twenty-one percentage (n = 8) of the patients consumed alcohol regularly, and 2 patients admitted to suffering from alcohol abuse.
Fig. 11.
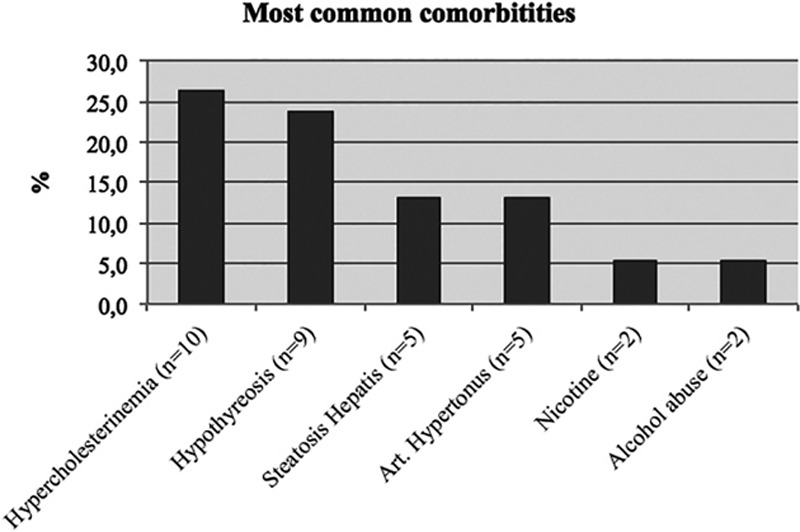
Most common comorbidities in our MSL patients.
Blood Analysis
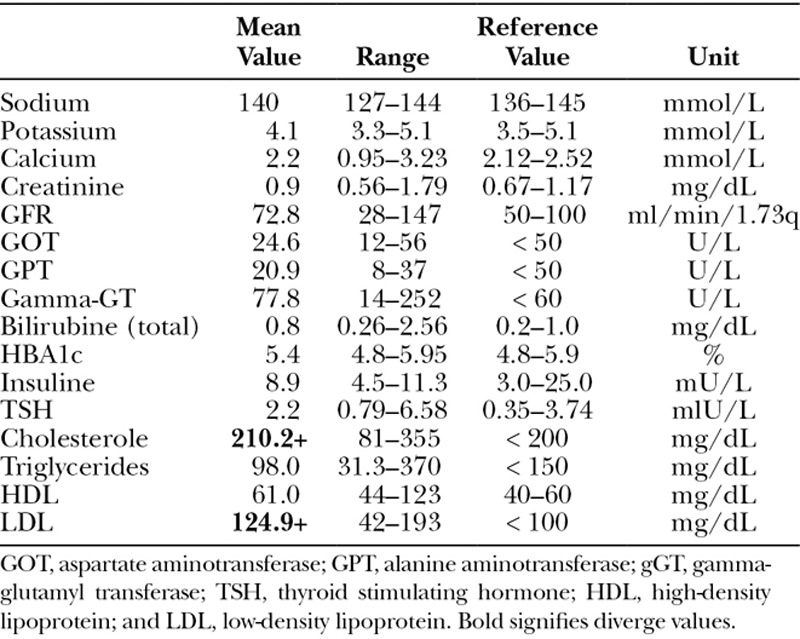
Blood samples were collected and analyzed from 30 patients. The mean values are shown in Table 4. In total, one can say that results of the blood analyses were normal, besides a hypercholesterolemia (Table 5).
Table 5.
Results of Blood Analysis (Mean Values)
DISCUSSION
Of the 45 patients diagnosed with MSL (largest cohort in Germany so far), current phenotype classifications were mostly not applicable. According to current classifications, an affected abdomen does not occur in combination with the upper body part. In contrast, we found that when fat deposits occur in the abdominal area, it is always in combination with other parts of the upper body (new type Ic) or (new type III) a general distribution (46% of our patients). The general distribution type (new type III) that was found in 18% of our patients is not described in any current classification. The Donhauser classification7 is the most common classification, set up in 1991 based on the clinical examination of 1 single patient and descriptive data from the literature of previous case reports. It lacks a general distribution of fat deposits and the combination of upper arms, shoulders, thorax, and abdomen. In 55% (n = 18), the Donhauser classification was not applicable. Therefore, a new classification is needed. We tested the classification on our 45 patients, and all of them corresponded to 1 of the defined types (Table 3). To validate the applicability of this classification, it has to be tested on a much larger number of patients and further studies must be done.
Recent studies have shown the presence of brown adipose tissue in the neck of adults.18,19 Brown fat seems to play a role in the pathogenesis of MSL, as protein-1 mRNA, which is unique to brown fat, was found in resected fat tissue from MSL patients.6,20 As the neck was affected in 64% of our patients and shows up in type Ia–c and type III, it might be possible that proliferation of MSL starts in the neck and spreads toward the lower body while progressing. Therefore, a structured and detailed anamnesis about the disease history of each patient has to be ascertained.
Patients who presented a phenotype type II often showed skin morphology as found in lipedema. Sometimes it is difficult to distinguish lipedema from MSL type II. One criterion is that the hips and bottom are affected, which are not affected in patients suffering from lipedema. Some patients may suffer from both, or from a mixed type.
The prevalence of MSL is indicated as 1:25,000 in the literature2–6 and refers to a clinical report from Italy in 1984.21 In the region of “Oberpfalz,” we found a similar prevalence of 1:24,274. However, the prevalence is considered to be higher, because there are expected to be many unknown cases. Without detailed epidemiological research, this number cannot be projected on a larger population and should be seen as a regional prevalence. Furthermore, we found that contrary to most previous research, women (71%) are affected more often than men (29%; male:female ratio, 1:2.5). Plummer et al.20 also found a contrary male to female ratio of 1:6 in a series of 7 patients. Thus, the exact value of the male:female ratio and its possible determinants of variations is an open issue.
Comorbidities found in our study agree with the comorbidities found in the literature. Hypercholesterinemia and hypothyroidism indicate a possible link to metabolic diseases. Hyperinsulinemia, neuropathy, and myopathy could not be found. However, patients were only interviewed about their comorbidities, without individual examination. Some comorbidities might not have been assessed. Eight patients (21%) consumed alcohol regularly, but less than 24 g ethanol/d, and 2 patients (5.3%) were suffering from alcohol abuse. Compared with the normal population in Bavaria, where 8.7% drink more than 24 g ethanol/d and 4.1% are suffering from alcohol abuse (Land Statistical Office, Germany 2012), a link to alcoholism in MSL could not be found. Blood analyses correspond to data from previous publications. Other than a manifest hypercholesterinemia and some high TSH levels, blood values were found to be more or less normal.
As the only current therapies are liposuctions and surgical lipectomy (with a high level of recurrence),22–26 further research regarding pathogenesis is required. First, a systematic anamnestic study, to assess the exact age and area of first lipomatous occurrence, a possible evolution or progress of affected areas, family occurrence, eating and drinking habits, physical activity, psychological factors, desire for therapy, and postoperative satisfaction is highly desirable. Second, basic research on adipocytes from MLS tissue must be undertaken.
CONCLUSIONS
We set up a new, more precise classification that divides phenotypes of MSL into 5 types (Ic, Ib, Ic, II, and III) based on the largest German cohort. Prevalence of MSL in central Europe must be considered higher than 1:25,000. The male to female ratio was found to be 1:2.5 (m:f). Hypercholesterinemia and hypothyroidism are frequent comorbidities. Blood analyses are normal besides a hypercholesterinemia. A link to alcoholism could not be found. Pathogenesis of MSL remains unclear.
Footnotes
Published online 4 April 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Enzi G, Busetto L, Ceschin E, et al. Multiple symmetric lipomatosis: clinical aspects and outcome in a long-term longitudinal study. Int J Obes Relat Metab Disord. 2002;26:253–261.. [DOI] [PubMed] [Google Scholar]
- 2.Jiménez Aragón F, Morales Puebla JM, Corzón Pereira T. Madelung’s disease. Acta Otorrinolaringol Esp. 2013;64:166–167.. [DOI] [PubMed] [Google Scholar]
- 3.Pinto CI, Carvalho PJ, Correia MM. Madelung’s disease: revision of 59 surgical cases. Aesthetic Plast Surg. 2017;41:359–368.. [DOI] [PubMed] [Google Scholar]
- 4.Prantl L, Schreml J, Gehmert S, et al. Transcription profile in sporadic multiple symmetric lipomatosis reveals differential expression at the level of adipose tissue-derived stem cells. Plast Reconstr Surg. 2016;137:1181–1190.. [DOI] [PubMed] [Google Scholar]
- 5.Ruzicka T, Vieluf D, Landthaler M, et al. Benign symmetric lipomatosis Launois-Bensaude. Report of ten cases and review of the literature. J Am Acad Dermatol. 1987;17:663–674.. [DOI] [PubMed] [Google Scholar]
- 6.Enzi G, Busetto L, Sergi G, et al. Multiple symmetric lipomatosis: a rare disease and its possible links to brown adipose tissue. Nutr Metab Cardiovasc Dis. 2015;25:347–353.. [DOI] [PubMed] [Google Scholar]
- 7.Donhauser G, Vieluf D, Ruzicka T, et al. [Benign symmetric Launois-Bensaude type III lipomatosis and Bureau-Barrière syndrome]. Hautarzt. 1991;42:311–314.. [PubMed] [Google Scholar]
- 8.Dorigo P, Prosdocimi M, Carpenedo F, et al. Multiple symmetric lipomatosis. A defect in adrenergic stimulated lipolysis II. Pharmacol Res Commun. 1980;12:625–638.. [DOI] [PubMed] [Google Scholar]
- 9.Enzi G, Inelmen EM, Baritussio A, et al. Multiple symmetric lipomatosis: a defect in adrenergic-stimulated lipolysis. J Clin Invest. 1977;60:1221–1229.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu W, Dou J, Yang G, et al. The endocrine and metabolic evaluation of benign symmetrical lipomatosis: a case report and literature review. Neuro Endocrinol Lett. 2010;31:446–450.. [PubMed] [Google Scholar]
- 11.Kan Y, Yao P, Xin W, et al. [Recent progress on diagnosis and treatment of benign symmetric lipomatosis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24:105–107.. [PubMed] [Google Scholar]
- 12.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324.. [DOI] [PubMed] [Google Scholar]
- 13.Brea-García B, Cameselle-Teijeiro J, Couto-González I, et al. Madelung’s disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthetic Plast Surg. 2013;37:409–416.. [DOI] [PubMed] [Google Scholar]
- 14.Naumann M, Schalke B, Klopstock T, et al. Neurological multisystem manifestation in multiple symmetric lipomatosis: a clinical and electrophysiological study. Muscle Nerve. 1995;18:693–698.. [DOI] [PubMed] [Google Scholar]
- 15.Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862–866.. [DOI] [PubMed] [Google Scholar]
- 16.Hirose A, Okada Y, Morita E, et al. Benign symmetric lipomatosis associated with alcoholism. Intern Med. 2006;45:1001–1005.. [DOI] [PubMed] [Google Scholar]
- 17.Chalk CH, Mills KR, Jacobs JM, et al. Familial multiple symmetric lipomatosis with peripheral neuropathy. Neurology. 1990;40:1246–1250.. [DOI] [PubMed] [Google Scholar]
- 18.Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805.. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plummer C, Spring PJ, Marotta R, et al. Multiple symmetrical lipomatosis—a mitochondrial disorder of brown fat. Mitochondrion. 2013;13:269–276.. [DOI] [PubMed] [Google Scholar]
- 21.Enzi G. Multiple symmetric lipomatosis: an updated clinical report. Medicine (Baltimore). 1984;63:56–64.. [DOI] [PubMed] [Google Scholar]
- 22.Atiyeh B, Costagliola M, Illouz YG, et al. Functional and therapeutic indications of liposuction: personal experience and review of the literature. Ann Plast Surg. 2015;75:231–245.. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Zhang X, Liu H. [Benign symmetric lipomatosis (Madelung’s disease): four cases report]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29:1919–1921.. [PubMed] [Google Scholar]
- 24.Sharma N, Hunter-Smith DJ, Rizzitelli A, et al. A surgical view on the treatment of Madelung’s disease. Clin Obes. 2015;5:288–290.. [DOI] [PubMed] [Google Scholar]
- 25.Tadisina KK, Mlynek KS, Hwang LK, et al. Syndromic lipomatosis of the head and neck: a review of the literature. Aesthetic Plast Surg. 2015;39:440–448.. [DOI] [PubMed] [Google Scholar]
- 26.Tremp M, Wettstein R, Tchang LA, et al. Power-assisted liposuction (PAL) of multiple symmetric lipomatosis (MSL)—a longitudinal study. Surg Obes Relat Dis. 2015;11:155–160.. [DOI] [PubMed] [Google Scholar]


