Abstract
Background:
Every year millions of individuals acquire scars. A literature review of patient-reported outcome (PRO) instruments identified content limitations in existing scar-specific measures. The aim of this study was to develop a new PRO instrument called SCAR-Q for children and adults with surgical, traumatic, and burn scars.
Methods:
We performed a secondary analysis of the qualitative datasets used in the development of PRO instruments for plastic and reconstructive surgery, that is, BREAST-Q, FACE-Q, BODY-Q, and CLEFT-Q. The keyword “scar*” was used to extract scar-specific text. Data were analyzed to identify concepts of interest and to form a comprehensive item pool. Scales were developed and refined through multiple rounds of cognitive interviews with patients and with input from international clinical experts between July 2015 and December 2016.
Results:
A total of 52 children and 192 adults from the qualitative datasets provided between 1 and 34 scar-specific codes (n = 1,227). The analysis led to the identification of 3 key domains for which scales were developed: scar appearance (eg, size, color, contour), scar symptoms (eg, painful, tight, itchy), and psychosocial impact (eg, feeling self-conscious, bothered by scar). Cognitive interviews with 25 adults and 20 pediatric participants with scars, plus feedback from 27 clinical experts, led to rewording and removal of items, and new items added. These steps ensured content validity for SCAR-Q in a broad range of scars.
Conclusions:
The SCAR-Q is now being field-tested. Once completed, we anticipate SCAR-Q will be used in clinical practice and in clinical trials to test different scar therapies.
INTRODUCTION
Millions of scars from surgical interventions, burns, and trauma occur annually.1 The annual operative burden worldwide is 234 million operations, with 1 in 25 individuals expected to undergo surgery that results in a visible scar.1 Although numerous clinical outcome assessment (COA) tools are used to evaluate scars, such tools represent the perspective of the health care provider.2 Although such COA tools have been used extensively, and are hence well known, they do not measure how a patient feels and functions. To measure the patient perspective, a patient-reported outcome (PRO) instrument is needed.2
PRO instruments are rating scales that measure outcomes that matter to patients by asking them directly.2 Mundy et al.3 reviewed the development and content of PRO instruments designed for surgical and traumatic scars and found 4 scales as follows: Patient and Observer Scar Assessment Scale,4 Bock Quality of Life Questionnaire for Patients with Keloid and Hypertrophic Scarring,5 Patient Scar Assessment Questionnaire,6 and the Patient-Reported Impact of Scars Measure.7 These PRO instruments, designed for both children and adults, focus mainly on the measurement of symptoms and psychological issues. No PRO instrument exists that comprehensively measures scar appearance (eg, size, shape, color, contour). Since treatments aim to specifically improve the appearance of scars, asking patients what they think about how their scar looks seems a fundamental and practical measure of outcome for a scar-specific PRO instrument.
Given content limitations of existing scar-specific PRO instruments, our team created the SCAR-Q. In this article, we describe the various steps taken to develop this new PRO instrument.
METHODS
Our team adheres to recommended methods for PRO instrument development,2,8,9 including specific guidance for pediatric populations.10,11 We follow a mixed methods approach.12 Phase 1 involved a systematic review published elsewhere,3 qualitative research, and expert input.
Qualitative Research
Scale Formation
To identify concepts of interest to patients with scars, a secondary analysis was performed of qualitative datasets used by our team to design the following PRO instruments: BREAST-Q, with modules for different types of breast surgery,14–17 FACE-Q, with modules for aesthetic treatments,18 head and neck cancer,19 skin cancer,20 and children and young adults with craniofacial conditions (ie, ear anomalies, facial paralysis, skeletal conditions, and soft-tissue conditions),21 BODY-Q for weight loss and body contouring22 and CLEFT-Q for cleft lip and/or palate.23
Within each qualitative dataset, the key word “scar*” was used as a search term to identify and extract all quotations where a scar was mentioned. Quotations were pasted into an Excel spreadsheet alongside participants’ age, sex, and the originating sample. Each quotation was examined in turn and coded to label concepts of interest. Two or more levels of coding (domain, theme, and subtheme) were applied. Quotations were also used to create a comprehensive item pool for use in scale development. For example, a participant from the BODY-Q sample who underwent massive weight loss followed by surgery to remove excess skin said the following:
“The scar has actually healed up pretty well for the most part. It’s still a little red in some sections. I was left with right body numbness all the way around from, like from here to here.”
This quotation led to 3 preliminary items that were coded as follows: (1) The scars healed up well: domain = appearance, theme = healing, subtheme = better; (2) The scars are a little red: domain = appearance, theme = color, subtheme = red; and 3) The scars feel numb: domain = symptom, theme = numb.
When all items were developed, a process of constant comparison was used to clean codes and ensure consistency of coding across domains and themes. All items and codes were created by 1 reviewer (A.K.) and independently verified by a second reviewer (N.Z.).
The item pool was used to identify common and unique concerns by originating sample and by pediatric versus adult. The item pool was then used to populate a set of independently functioning scales using a modern psychometric approach called Rasch Measurement Theory, whereby scales are designed to work like “rulers” with the items mapping out a clinical hierarchy.24 In creating the wording for items in scales, we retained the words of patients as much as possible and used the lowest possible grade reading level to maximize comprehension by children as young as 8 years of age. We obtained feedback for different response option approaches, that is, satisfaction, acceptability, agreement, and frequency. Each response option set was limited to 4 labeled options to keep the scales simple and in line with guidelines.25
Scale Review
Ethics approval for the cognitive interview phase was obtained from the Memorial Sloan-Kettering Cancer Center Institutional Review Board and the Research Ethics Board at the Hospital for Sick Children. Cognitive interviews, which involved the think-aloud technique,26,27 were used to refine the scales and to establish content validity.8,9 Potential participants were invited to participate in an interview by a member of the health care team at each site. Patients were eligible if they had a surgical, traumatic, or burn scar, were 8 years of age or older, and were able to read English. The goal was to recruit a varied sample of patients who differed by age, sex, and scar type and location.
Interviews were conducted in a series of rounds, beginning with the adult sample. Conducting interviews in rounds made it possible to continually revise the scales throughout the interview process. Informed consent was obtained from all participants as well as from the parents for the pediatric patients where applicable. Interviews were conducted in the home or hospital or, for teenagers and adults, by phone. Interviews were digitally recorded and transcribed verbatim. Transcripts were analyzed to identify feedback on the instructions, response options, and items and to generate new content and to make the content as easy to understand as possible. Interviews continued until no new concerns or content were identified. Interviews were conducted between July 2015 and October 2015 with adults, and between February and December 2016 with the pediatric participants. The pediatric participants were given a $50 gift card to thank them for their time.
Expert Opinion
To ensure that the scales comprehensively covered all clinically important issues for different types of scars, we obtained expert feedback. An online Research Electronic Data Capture28 survey was designed and experts identified through our team’s professional networks were invited by e-mail in September 2016 to provide feedback and to suggest new content. Reminders were e-mailed 2 and 4 weeks later. Expert input was used to revise scales after the first round of cognitive interviews with children. In addition, as none of the originating samples included patients with burns, focus group sessions were used to obtain feedback on the scales from a burns-perspective from the 7 members of the multidisciplinary pediatric burn team at the Canadian site. Input was used to revise the scales after the second round of cognitive interviews with children.
RESULTS
Concept Elicitation
Table 1 shows the number of participants in the originating samples and the subset of participants from each sample to provide at least 1 scar quote alongside age and sex. A total of 52 pediatric (ie, aged up to 19 years) and 192 adult participants provided between 1 and 34 scar-specific codes each (n = 1,227). Data analysis led to identification of 3 outcome domains as follows: scar appearance (n = 752; 61%); psychosocial impact (n = 339; 28%); and scar symptoms (n = 136; 11%). These domains were relevant to both the pediatric and adult patients (Fig. 1). Each domain had multiple themes and subthemes that are described below.
Table 1.
Demographic Information of Each Qualitative Dataset Used in the Development of SCAR-Q
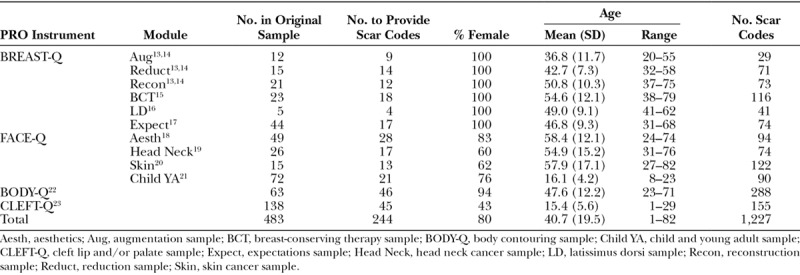
Fig. 1.
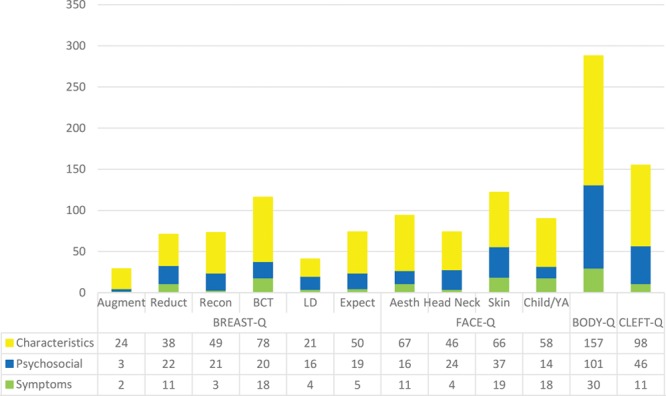
Number of items per domain by originating sample. Aesth, aesthetics; Aug, augmentation sample; BCT, breast-conserving therapy sample; BODY-Q, body contouring sample; Child YA, child and young adult sample; CLEFT-Q, cleft lip and/or palate sample; Expect, expectations sample; Head Neck, head neck cancer sample; LD, latissimus dorsi sample; Recon, reconstruction sample; Reduct, reduction sample; Skin, skin cancer sample.
Scar Appearance
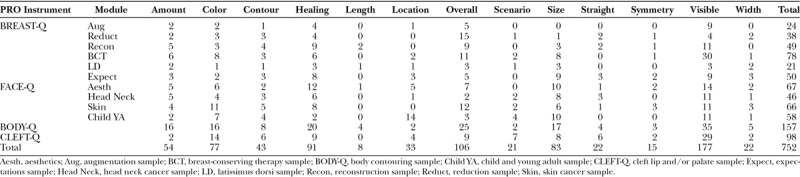
The 752 items (123 pediatric, 629 adult) in this domain related to 13 themes. Table 2 shows the number of codes by theme and originating sample, providing evidence that the themes were relevant to a broad range of scars types and locations. Participants described their scars in terms of size (eg, big, small, length, width), shape (eg, straight/crooked, asymmetric), the amount (eg, lots, a little), color or pigment and how well it blended with their skin color, contour (eg, flat, raised, lumps), how visible or noticeable the scars were, scar location, how much the scars had healed over time (eg, faded, gone, same, worse), and how the scars looked in different scenarios (eg, mirror, photographs, up close, when sunburnt). Participants also used overall statements that were positive (eg, scar looks nice, good, great), negative (eg, scar looks bad, ugly, scary, weird), or evaluative (eg, how happy, pleased, or satisfied with scar appearance).
Table 2.
Number of Codes for Each Scar Appearance Theme by Originating Sample
Psychosocial
The 339 items (46 pediatric, 293 adult) in this domain were mainly psychological (n = 262; 77%). Items in this domain were classified as either positive, neutral, or negative in focus. The neutral (n = 126) and positive (n = 80) items together outnumbered the negative items (n = 132). Most items classified as neutral were about not being bothered or worried about the scar(s), or not caring about the scar(s). Many of the positive comments were from breast cancer and body contouring participants who described accepting the scar(s) (“You just kind of—I just kind of learned to live with it”), or that they preferred the scar(s) to cancer (“I can look at the scars and I can see life.”) or to having excess skin (“I would live with this scar for the rest of my life with not having that belly flap.”). A breast reconstruction patient said the following:
“The fact that I have two scars that go across... thank God I can have two scars that go across and I am not six feet under!”
Negative items were mainly participants saying they were bothered or concerned about their scar(s) and often mentioned feeling self-conscious, or that they hid their scar(s) under clothes or with jewelry or make-up. For example, a girl aged 13 years who had surgery for a birthmark said the following:
“I would try to like draw an eyebrow here so it looks like I have an eyebrow. Also, make-up covers it up really well, my scar.”
Some participants mentioned that they felt anxious or upset when people looked at or asked about their scar(s). Participants bothered by a visible facial scar described being teased, or that they had isolated themselves because of their scar(s). A girl aged 10 years with prominent ears explained that she was nervous about people noticing her ear scar(s):
“I think, if people would notice, and people would be like “uh why’d you do that”, and people would judge. Or maybe somebody saw my ear and they’d be like, ew what happened, or something, or like they saw the scar”.
Symptoms
The 136 symptom items (24 pediatric, 112 adult) included a range of scar-specific symptoms. Symptoms included negative outcomes such as how the scar(s) felt to the touch (eg, rough, hard, bumpy), and how sore, tender, sensitive, uncomfortable, painful, numb, tight, stiff, pulling, itchy, irritated, and swollen the scar was.
Scale Refinement
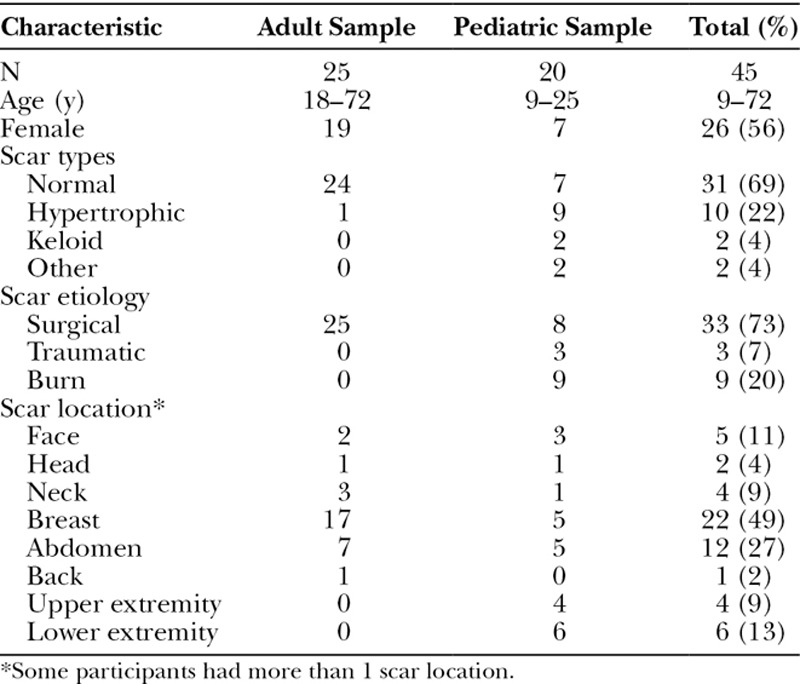
The item pool was used to develop the first draft of 3 scales covering scar appearance (15 items), scar symptoms (12 items), and psychosocial impact (12 items). Table 3 shows characteristics of the 25 adults and 20 pediatric cognitive interview participants. Age ranged from 9 to 72 years, and 56% were female. For scar appearance, based on feedback from 25 adults over 3 rounds of interviews, 3 items were added and 1 item was revised. For the psychosocial impact, 5 items were added, 1 was revised, and 3 were dropped. No changes were required for scar symptoms.
Table 3.
Cognitive Interview Participant Characteristics
The 3 revised scales (44 items) were shown to the pediatric participants in 3 rounds. The Research Electronic Data Capture survey was sent to 64 experts and 21 responded (33% response rate). The focus group session included 6 burn scar experts who were able to confirm the scales contained clinically relevant indications for all common scar revision therapies. The 27 experts were from Australia, Chile, Finland, Ireland, The Netherlands, United Kingdom, United States, and Canada, and included 19 surgeons, 3 physiotherapist, 1 nurse, 2 occupational therapists, 1 researcher, and 1 medical oncologist. Most experts (n = 26) said that scar treatment was an important focus of their profession. For the scar appearance scale, based on feedback from the pediatric sample and experts, 8 items were added, 5 were revised, and 7 were dropped. For scar symptoms, 7 items were added, 3 were revised, and 2 were dropped. Two items were added, 3 were revised, and 4 were dropped from the psychosocial impact scale. Most of the changes aimed to simplify and/or clarify the wording of items. For example, feedback from children suggested we needed to clarify the meaning of the item “My scar feels tingly,” to which we added the clause (pins and needles feeling). Example of concepts missed, suggested by experts, included how much the scar(s) have faded and scar dryness.
Based on feedback from participants and experts, clinically relevant and acceptable response options were chosen for each scale. The appearance scale (19 items) ask respondents to indicate how bothered they are by their scar(s) (not at all, a little, quite a bit, very much) in terms of characteristics such as size, width, color, contour, and so on. The scar symptoms scale (17 items) uses the same response options for a series of statements (eg, “My scar feels tender when I touch it” or “My scar feels sore”). The psychosocial impact scale (12 items) asks respondents to answer a series of statements (eg, “I feel self-conscious about my scar” or “I get upset when people see my scar”) in terms of never/sometimes/often/always. For the time frame, the appearance scale asks respondents to think of how their scar looks now, whereas scar symptoms and psychosocial impact are based on the past week. Instructions clarify that anyone who has more than 1 scar should answer thinking of about the scar that bothers them the most. The mean Flesch-Kincaid readability statistics for the appearance, symptoms, and psychosocial impact scales are 1.6, 2.5, and 2.7, respectively.
DISCUSSION
The SCAR-Q is a new PRO instrument designed to measure outcomes specific to any type of scar. Based on the large combined qualitative sample representing a broad spectrum of patients, we found that how the scar looked was a common concern for both adults and children. Scar appearance is also a major focus of COAs, such as the Vancouver Scar Scale,29 but is not the main focus of the existing scar PRO instruments, which focus more on symptoms and psychosocial impact.4–7 The SCAR-Q will be useful in research where appearance is an important outcome (eg, to evaluate scar therapies, compare techniques of surgical site closure, examine the impact of minimally invasive surgical incisions). The SCAR-Q could be used alongside the current gold standard COAs, to incorporate the patient perspective alongside that of the clinician. As new therapies to improve scar appearance are rapidly emerging (such as laser treatment and biologic agents), the SCAR-Q will be a valuable tool in clinical trials to evaluate the impact of treatment from the patient’s perspective.
In terms of psychosocial impact, while more participants in the combined qualitative sample made neutral or positive comments that indicated they were not concerned or bothered by their scar(s), a large subgroup of participants described feeling self-conscious, unattractive, or embarrassed about their scar(s). Scars were sometimes the reason participants isolated themselves because of being teased (eg, children with cleft lip scar), and participants mentioned not liking it when people looked at or asked about their scar(s). These psychosocial concepts are in line with the concepts included in the other published scar-specific PRO instrument to measure impact of scars from the patient perspective.5–7
The SCAR-Q is the first PRO instrument designed to comprehensively evaluate appearance of all scar types from the perspective of adults and children as young as 8 years of age. Although most of the items in our item pool were from adults, the data we had from children covered similar concerns. Cognitive interviews made it possible for us to confirm content validity across age and to ensure the scale content was easy for children to read and understand. The field-tested data we collect will be used to determine if SCAR-Q items work the same for adults and children. Specifically, we will examine Differential Item Function (DIF), which measures whether items are answered differently by subgroups in a sample.30 DIF will be examined to determine if the SCAR-Q works the same for children and adults, and by scar type. Items that show DIF can be dropped or kept in with adjustments made to the scoring to account for the differences.
A strength of our study was the large and heterogeneous sample of participants in the combined qualitative dataset used to identify the key domains and to develop the SCAR-Q. Although the combined qualitative sample included a broad range of surgical procedures that resulted in a heterogeneous range of scars and scar symptoms, a limitation was that no burn scars and only few traumatic scars were included. In addition, the pediatric qualitative sample only included facial scars, and there were just fewer data in the combined qualitative datasets for children compared with adults. A likely reason why the symptom domain had the fewest items in the item pool was that most participants had scars that had healed. However, these limitations were counterbalanced by the large and varied sample of participants, both adult and pediatric, who took part in the cognitive interviews. Their input, plus that of a broad range of experts, enabled us to establish content validity for a diverse range of adult and pediatric patients with surgical, traumatic, and burns scars.
CONCLUSIONS
Scar appearance is important to patients, but not well addressed in current PRO instruments designed for scars. The SCAR-Q is now being tested internationally. Once finalized, we anticipate the SCAR-Q will be used in clinical practice with patients and in research to test different scar therapies.
Footnotes
Published online 24 April 2018.
Disclosure: SCAR-Q is owned by Memorial Sloan-Kettering Cancer Center and McMaster University. Drs Klassen and Pusic are co-developers of the SCAR-Q and, as such, could potentially receive a share of any license revenues as royalties based on their institutions inventor sharing policy. The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for from the Memorial Sloan-Kettering Cancer Center Philanthropy Research Fund.
REFERENCES
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144.. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Fed Regist. 2009;74:1–43.. [Google Scholar]
- 3.Mundy LR, Miller HC, Klassen AF, et al. Patient-reported outcome instruments for surgical and traumatic scars: a systematic review of their development, content, and psychometric validation. Aesthetic Plast Surg. 2016;40:792–800.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:1960–1965.; discussion 1966. [DOI] [PubMed] [Google Scholar]
- 5.Bock O, Schmid-Ott G, Malewski P, et al. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433–438.. [DOI] [PubMed] [Google Scholar]
- 6.Durani P, McGrouther DA, Ferguson MW. The patient scar assessment questionnaire: a reliable and valid patient-reported outcomes measure for linear scars. Plast Reconstr Surg. 2009;123:1481–1489.. [DOI] [PubMed] [Google Scholar]
- 7.Brown BC, McKenna SP, Solomon M, et al. The patient-reported impact of scars measure: development and validation. Plast Reconstr Surg. 2010;125:1439–1449.. [DOI] [PubMed] [Google Scholar]
- 8.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14:967–977.. [DOI] [PubMed] [Google Scholar]
- 9.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14:978–988.. [DOI] [PubMed] [Google Scholar]
- 10.Matza LS, Patrick DL, Riley AW, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health. 2013;16:461–479.. [DOI] [PubMed] [Google Scholar]
- 11.Bevans KB, Riley AW, Moon J, et al. Conceptual and methodological advances in child-reported outcomes measurement. Expert Rev Pharmacoecon Outcomes Res. 2010;10:385–396.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong Riff KW, Tsangaris E, Goodacre T, et al. International multiphase mixed methods study protocol to develop a cross-cultural patient-reported outcome instrument for children and young adults with cleft lip and/or palate (CLEFT-Q). BMJ Open. 2017;7:e015467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klassen AF, Pusic AL, Scott A, et al. Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health. 2009;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353.. [DOI] [PubMed] [Google Scholar]
- 15.Scott AM, McCarthy CM, Klassen AF, et al. Development of a new BREAST-Q module: The Breast-Conserving Therapy (BCT) module. Qual Life Res. 2010;19:112–113.. [Google Scholar]
- 16.Browne JP, Jeevan R, Pusic AL, et al. Measuring the patient perspective on latissimus dorsi donor site outcomes following breast reconstruction. J Plast Reconstr Aesthet Surg. 2018 Mar;71(3):336–343.. [DOI] [PubMed] [Google Scholar]
- 17.Snell L, McCarthy C, Klassen A, et al. Clarifying the expectations of patients undergoing implant breast reconstruction: a qualitative study. Plast Reconstr Surg. 2010;126:1825–1830.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klassen AF, Cano SJ, Scott A, et al. Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010;26:303–309.. [DOI] [PubMed] [Google Scholar]
- 19.Albornoz CR, Pusic AL, Reavey P, et al. Measuring health-related quality of life outcomes in head and neck reconstruction. Clin Plast Surg. 2013;40:341–349.. [DOI] [PubMed] [Google Scholar]
- 20.Lee EH, Klassen AF, Lawson JL, et al. Patient experiences and outcomes following facial skin cancer surgery: a qualitative study. Australas J Dermatol. 2016;57:e100–e104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longmire NM, Wong Riff KWY, O’Hara JL, et al. Development of a new module of the FACE-Q for children and young adults with diverse conditions associated with visible and/or functional facial differences. Facial Plast Surg. 2017Oct;33(5):499–508.. [DOI] [PubMed] [Google Scholar]
- 22.Klassen AF, Cano SJ, Scott A, et al. Satisfaction and quality-of-life issues in body contouring surgery patients: a qualitative study. Obes Surg. 2012;22:1527–1534.. [DOI] [PubMed] [Google Scholar]
- 23.Wong Riff KWY, Tsangaris E, Goodacre TEE, et al. What matters to patients with cleft lip and/or palate: an international qualitative study informing the development of the CLEFT-Q. Cleft Palate Craniofac J. Revisions requested. [DOI] [PubMed]
- 24.Rasch G. Probabilistic Models for Some Intelligence and Attainment Tests. 1960Copenhagen, Denmark: Danish Institute for Education Research. [Google Scholar]
- 25.Khadka J, Gothwal VK, McAlinden C, et al. The importance of rating scales in measuring patient-reported outcomes. Health Qual Life Outcomes. 2012;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willis GB. Analysis of the Cognitive Interview in Questionnaire Design: Understanding Qualitative Research. 2015Toronto, ON: Oxford University Press. [Google Scholar]
- 27.Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. 2005New York, N.Y.: Sage Publications. [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan T, Smith J, Kermode J, et al. Rating the burn scar. J Burn Care Rehabil. 1990;11:256–260.. [DOI] [PubMed] [Google Scholar]
- 30.Andrich D. Rasch Models for Measurement. Sage University Papers Series Quantitative Application in the Social Sciences. 1988Beverly Hills, Calif.: Sage Publications. [Google Scholar]


