Supplemental Digital Content is available in the text.
Summary:
Fenestrated acellular dermal matrix (ADM) has improved patient outcomes in both direct-to-implant and 2-stage tissue expander/implant breast reconstruction. This technical alteration utilizes optimal fenestration overlap to enhance the breast reconstruction experience. We present a novel, surgeon-designed shaped fenestrated ADM, placed in the recently repopularized prepectoral pocket for anterior coverage of implants in direct-to-implant and 2-stage breast reconstruction. A retrospective review of 10 patients (18 breasts) who underwent direct-to-implant or 2-stage breast reconstruction utilizing fenestrated shaped ADM in the prepectoral plane at a major academic institution in 2016 was conducted. Sixteen breasts (88.9%) underwent direct-to-implant reconstruction, and 2 breasts (11.1%) received tissue expanders. All reconstructions were performed using FlexHD Pliable ADM with surgeon-designed shape and fenestrations. The average implant size was 544.4 cc (±137.2 cc). The average intraoperative tissue expander fill volume measured 450 cc (90% of tissue expander size). The single expander case utilized 1 office fill (day 21) for full expansion. Major complications requiring reoperation within 90 days postoperatively were observed in 22.2% (4 breasts) of reconstructions. Three breasts (16.7%) due to partial mastectomy flap necrosis, 1 breast (5.5%) explantation due to infection. There was no seroma or capsular contracture. Prepectoral reconstruction with shaped fenestrated ADM is safe with high intraoperative fill volumes and facilitates more direct-to-implant reconstructions. Patients undergo fewer postoperative expansions, experience less time to full expansion, and subjectively report less pain. Patients benefit from improved cosmetic outcomes with better shape and no functional loss or animation deformity.
INTRODUCTION
Breast reconstruction following mastectomy is overwhelmingly implant-based with acellular dermal matrix (ADM) as support at the inferolateral portion of the breast. The addition of fenestrations to the ADM improves overall breast shape and facilitates fluid egress, reducing the number of drains and consequently the incidence of seroma.1–3 With less restriction at the lower pole, the fenestrated ADM permits greater on-table tissue expander fill during immediate breast reconstruction and allows more direct-to-implant cases, even following skin-sparing mastectomy. Fewer postoperative visits are required, improving the overall reconstructive experience. Furthermore, resources are redirected, which translates into institutional savings.4
ADM fenestration has been shown to decrease capsular contracture.5 With additional studies also citing decreased capsular contracture rates with ADM use in breast reconstruction, there has been a recent resurgence in implant-based prepectoral breast reconstruction.6–16 Synthetic mesh products and xenograft and allograft ADM have been used to provide either anterior or circumferential coverage of the implant or tissue expander.17
Prepectoral or subcutaneous breast reconstruction was performed as early as the 1970s with multiple studies demonstrating high complication rates, especially capsular contracture.18–22 It is suggested that using ADM as anterior implant coverage in this plane attenuates the morbid complication profile, leading to a resurrection of this technique. This is the first study presenting a novel surgeon-designed shaped fenestrated ADM, placed in a prepectoral pocket for anterior coverage of implants in direct-to-implant and 2-stage breast reconstruction.
MATERIALS AND METHODS
A retrospective review of 10 patients who underwent direct-to-implant or 2-stage breast reconstruction using fenestrated shaped ADM in the prepectoral plane at a single institution in 2016 was completed (IRB # 2017–3317). Patient demographics are depicted in Table 1. Complications were identified as major if they occurred within 90 days of the initial reconstructive procedure and required reoperation. The patient undergoing staged reconstruction (preference for larger breasts) was followed for 30 days following implant exchange.
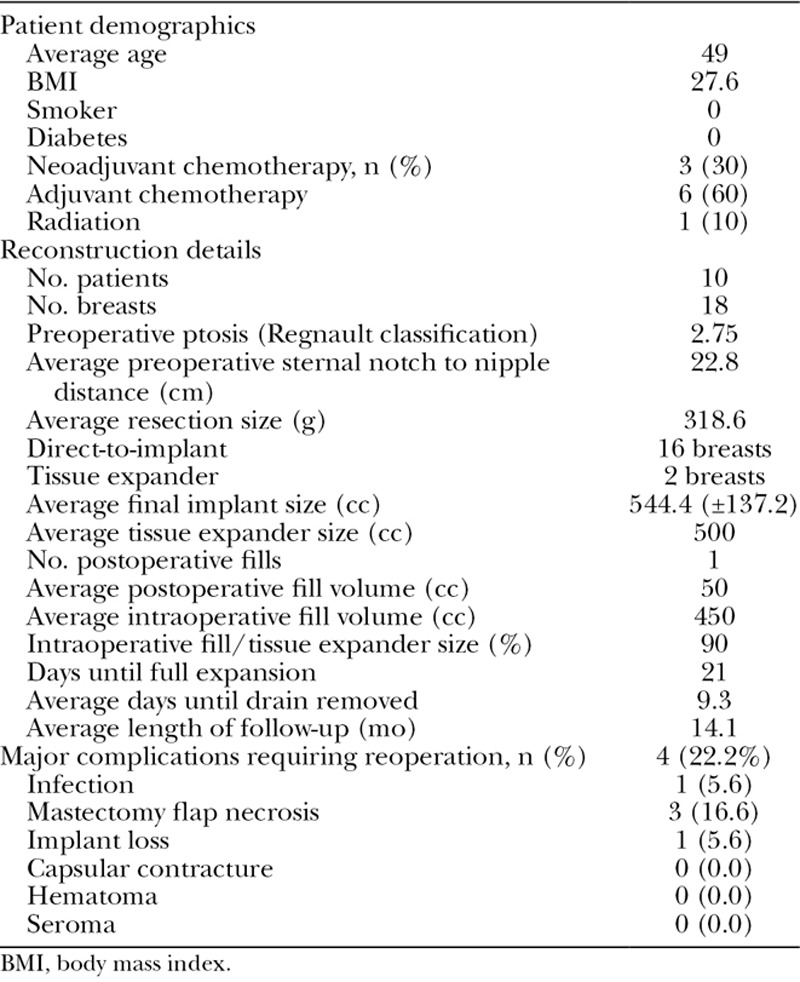
Table 1.
Patient Demographics, Reconstruction Details, and Major Complications
For cases requiring smaller implants (≤ 450 cc), 1 piece of FlexHD Pliable MAX ADM (Musculoskeletal Transplant Foundation, Edison, N.J.; 8 × 16 cm) was sewn to either a 6 × 16 cm piece of FlexHD Pliable ADM (Musculoskeletal Transplant Foundation) or another FlexHD Pliable MAX to achieve full anterior coverage of the implant. For larger (> 450 cc) implants, a piece of 16 × 20 cm FlexHD Pliable ADM was tailored and fenestrated as previously described by the authors to cover the anterior surface of the implant1 (Fig. 1; see figure, Supplemental Digital Content 1, which displays preservation of the fenestrations are demonstrated during a second-stage exchange procedure, http://links.lww.com/PRSGO/A690). This design includes overlap of the fenestrations in adjacent rows to ideally expand the medial, lateral, and inferior portion of the ADM over the implant or expander. This overlap is critical to achieving ideal reduction in the material’s Young’s Modulus in the desired areas, facilitating expansion of the graft in a predictable fashion.1 The ADM is sewn to the pectoralis major muscle fascia superiorly and medially, followed by the serratus anterior muscle laterally and the inframammary fold inferiorly. A single drain is placed between the ADM and the mastectomy skin flap.
Fig. 1.
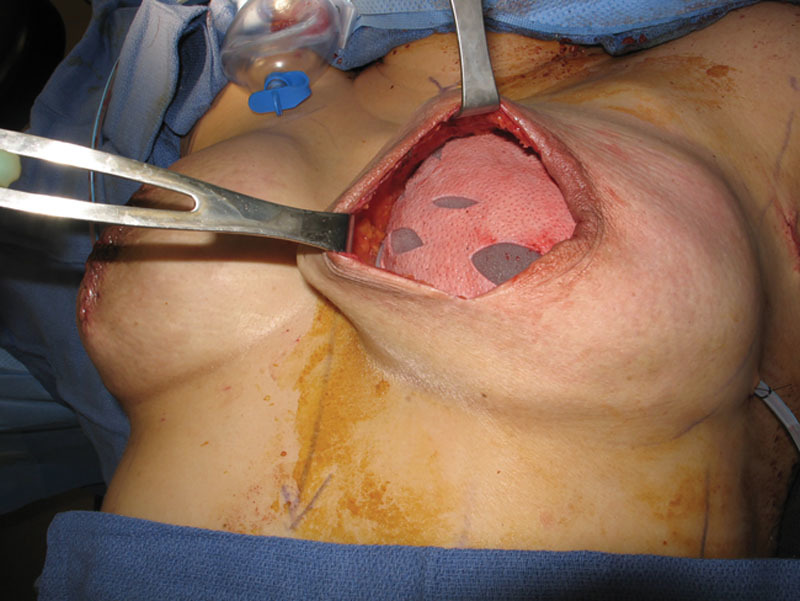
Shaped fenestrated ADM covering the anterior surface of a tissue expander inflated to 90% of its nominal fill volume.
RESULTS
All reconstructions were performed immediately following mastectomy. There were 14 (77.8%) skin-sparing mastectomies and 4 (22.2%) nipple-sparing mastectomies in 10 patients. Reconstructive details are depicted in Table 1.
One patient experienced cellulitis, which resolved without sequelae following the administration of intravenous antibiotics. Four breasts (22.2%) experienced major complications (Table 1).
Three breasts in 2 patients underwent secondary procedures. Capsulorrhaphy was performed on 2 breasts and an average of 146.7 cc (±30.6 cc) of fat was grafted for superior pole camouflaging in 3 breasts.
DISCUSSION
The authors have introduced a novel design that incorporates shaped, fenestrated human ADM for immediate breast reconstruction in the prepectoral plane. This is the first study to demonstrate the feasibility of a fenestrated ADM design for full anterior implant coverage in this plane. The unique fenestrated design improves the reconstructed breast shape as it facilitates the recreation of the natural position of the breast, allows for more rapid expansion, and makes direct-to-implant reconstruction more feasible. Our results show that breast reconstruction in the prepectoral plane with shaped fenestrated ADM is a safe operation with a similar complication profile to that which has been reported for partial submuscular reconstruction (15.4%).23 Furthermore, the present study’s complication rate is similar to the reported complication rates in the largest series of subcutaneous reconstructions where 9–29% of breasts experienced complications with 19% requiring reoperation.9,11,16,24 This is despite the fact that we perform immediate prepectoral or subcutaneous reconstruction using shaped fenestrated ADM in patients with large breasts with thin flaps following skin-sparing mastectomy for later stage breast cancers. The present study’s 5.5% explantation rate is comparable with the published 6.5% explantation rate in our previous partial subpectoral fenestrated ADM reconstruction series data.2,23 No seroma or capsular contracture were noted in our patients, which is consistent with our previous experience using fenestrated ADM for partial subpectoral reconstruction. The absence of seromas in our patients can be explained by the fact that the fenestrations not only improve fluid egress, but also facilitate ideal effacement of the ADM with the implant and the mastectomy flaps. This reduces potential “dead-space,” which is further minimized by the increased fill volumes and direct-to-implant reconstructions that can be accommodated.
Based on the early encouraging data, we propose that the indications for prepectoral reconstruction not be exclusively reserved for patients with small or medium breasts or only those who have undergone nipple-sparing mastectomy. Nearly 90% of our cases were direct-to-implant and in those cases the average size of the implant was 544.4 cc [Figs. 2, 3; see figure, Supplemental Digital Content 2, which displays additional (lateral) preoperative view of the patient depicted in Fig. 2, http://links.lww.com/PRSGO/A691; see figure, Supplemental Digital Content 3, which displays additional (lateral) postoperative view of the patient depicted in Fig. 3, http://links.lww.com/PRSGO/A692].
Fig. 2.
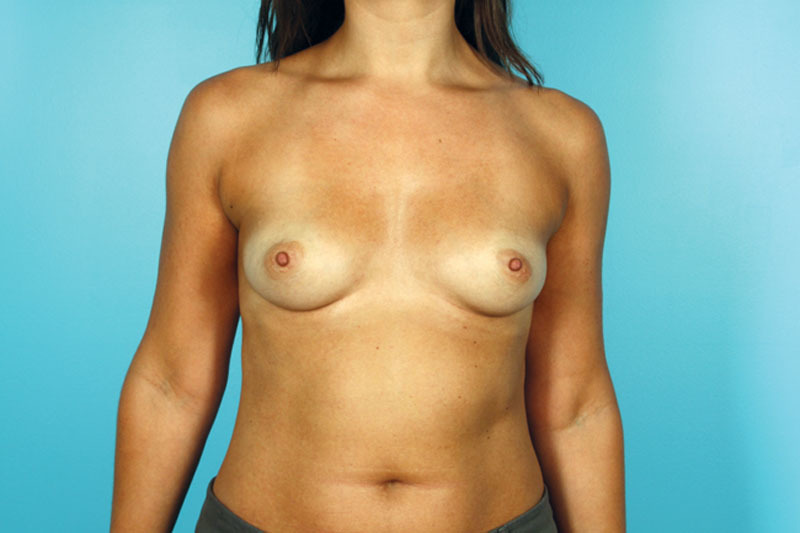
Thirty-year-old female with right breast cancer. Preoperative images.
Fig. 3.
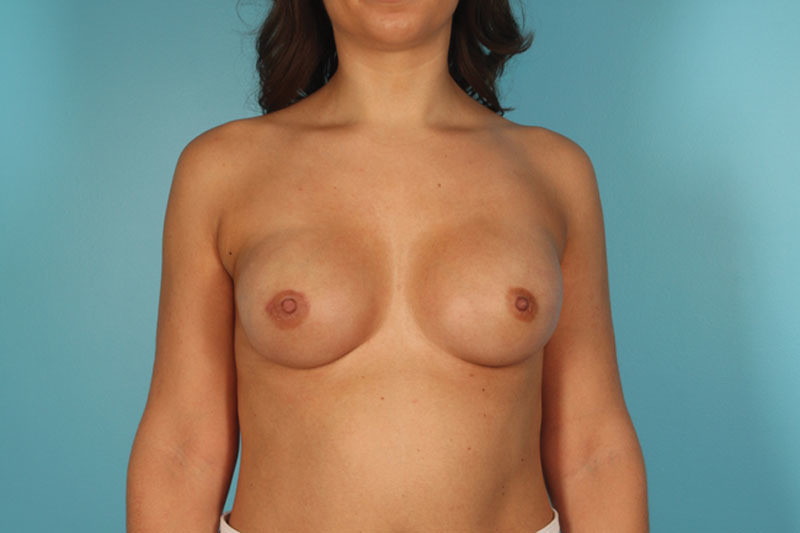
Fourteen weeks following a bilateral nipple-sparing mastectomy with direct-to-implant reconstruction using two 8 × 16 cm sheets of Flex HD Pliable MAX (prefenestrated) and prepectoral placement of 415 cc Allergan Natrelle Inspira SRF implants.
The use of ADM in prepectoral reconstruction can be costly, especially when the implant is wrapped in its entirety.6 The marginal benefit of providing full implant coverage is not clear and seems to only result in unjustified increases in institutional cost. The ADM fenestrations allow the product to expand, yet still cover the implant anteriorly, offering comparable support.
The protection ADM affords against capsular contracture ultimately saves time and money and anecdotally results in improved patient comfort and satisfaction. A recent study retrospectively identified patients that underwent prepectoral expander-based reconstruction following nipple-sparing mastectomy via an inframammary fold incision. The combined baker grade III and IV capsular contracture rate was 7.6%, which is higher than in studies that have used ADM.20,24
The benefits of shaped fenestrated ADM in subcutaneous breast reconstruction add to the established functional advantages of prepectoral reconstruction, which include the absence of animation deformity, faster recovery, and shorter procedural time.
Supplementary Material
Footnotes
Published online 4 April 2018.
Drs. Paydar, Wirth, and Mowlds have a royalty and consultancy agreement with the Musculoskeletal Transplant Foundation Biologics (Edison, N.J.). No financial support or funding was provided in the preparation of this article.
Disclosure: Drs. Paydar, Wirth, and Mowlds have a royalty and consultancy agreement with the Musculoskeletal Transplant Foundation Biologics (Edison, N.J.). The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Wirth GA, Mowlds DS, Guidotti P, et al. Acellular dermal matrix fenestrations and their effect on breast shape. Eur J Plast Surg. 2015;38:267–272.. [Google Scholar]
- 2.Martin JB, Moore R, Paydar KZ, et al. Use of fenestrations in acellular dermal allograft in two-stage tissue expander/implant breast reconstruction. Plast Reconstr Surg. 2014;134:901–904.. [DOI] [PubMed] [Google Scholar]
- 3.Palaia DA, Arthur KS, Cahan AC, et al. Incidence of seromas and infections using fenestrated versus nonfenestrated acellular dermal matrix in breast reconstructions. Plast Reconstr Surg Glob Open. 2015;3:e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan NM, Fischer JP, Basta MN, et al. Is single-stage prosthetic reconstruction cost effective? A cost-utility analysis for the use of direct-to-implant breast reconstruction relative to expander-implant reconstruction in postmastectomy patients. Plast Reconstr Surg. 2016;138:537–547.. [DOI] [PubMed] [Google Scholar]
- 5.Mowlds DS, Salibian AA, Scholz T, et al. Capsular contracture in implant-based breast reconstruction: examining the role of acellular dermal matrix fenestrations. Plast Reconstr Surg. 2015;136:629–635.. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A, Lakhiani C, Saint-Cyr M. Treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast Reconstr Surg. 2013;132:519–529.. [DOI] [PubMed] [Google Scholar]
- 7.Casella D, Bernini M, Bencini L, et al. TiLoop® bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg. 2014;37:599–604.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167.. [DOI] [PubMed] [Google Scholar]
- 9.Becker H, Lind JG, 2nd, Hopkins EG. Immediate implant-based prepectoral breast reconstruction using a vertical incision. Plast Reconstr Surg Glob Open. 2015;3:e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open. 2016;3:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs RK, Hedges K. An alternative technique for immediate direct-to-implant breast reconstruction—a case series. Plast Reconstr Surg Glob Open. 2016;4:e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg. 2016;137:1702–1705.. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal FM, Bhatnagar A, Vidya R. Host integration of an acellular dermal matrix: Braxon mesh in breast reconstruction. Clin Breast Cancer. 2016;16:e209–e211.. [DOI] [PubMed] [Google Scholar]
- 14.Engel H, Huang JJ, Lin CY, et al. Subcutaneous tissue expansion and subsequent subpectoral implantation for breast reconstruction in Asian patients: safety and outcome. Ann Plast Surg. 2013;70:135–143.. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77–e86.. [DOI] [PubMed] [Google Scholar]
- 16.Schnarrs RH, Carman CM, Tobin C, et al. Complication rates with human acellular dermal matrices: retrospective review of 211 consecutive breast reconstructions. Plast Reconstr Surg Glob Open. 2016;4:e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salibian AA, Frey JD, Choi M, et al. Subcutaneous implant-based breast reconstruction with acellular dermal matrix/mesh: a systematic review. Plast Reconstr Surg Glob Open. 2016;4:e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthrie RH., Jr. Breast reconstruction after radical mastectomy. Plast Reconstr Surg. 1976;57:14–22.. [DOI] [PubMed] [Google Scholar]
- 19.Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. 1981;67:312–317.. [DOI] [PubMed] [Google Scholar]
- 20.Salibian AH, Harness JK, Mowlds DS. Staged suprapectoral expander/implant reconstruction without acellular dermal matrix following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:30–39.. [DOI] [PubMed] [Google Scholar]
- 21.Artz JS, Dinner MI, Sampliner J. Breast reconstruction with a subcutaneous tissue expander followed with a polyurethane-covered silicone breast implant. Ann Plast Surg. 1988;20:517–521.. [DOI] [PubMed] [Google Scholar]
- 22.Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69:195–208.. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41.. [DOI] [PubMed] [Google Scholar]
- 24.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg. 2017;139:287–294.. [DOI] [PubMed] [Google Scholar]


