Abstract
Background:
Historically, external ear melanomas have been treated aggressively, due to early perceptions suggesting they had poor prognosis and aggressive biological behavior. More recent evidence has not supported these notions.
Methods:
We completed a complete review of the literature involving malignant melanoma of the external ear. We then completed a quantitative analysis on seventy-three cases from 8 reports that contained case-level data, assessing factors that influence recurrence, and assessing characteristics of the melanomas based on histological subtype. Baseline and outcomes data for all 20 studies were then compiled but not statistically evaluated.
Results:
In our subanalysis, patients who had recurrence were significantly more likely to have had wedge resection versus wide-local excision, and those with no recurrence were more likely to have undergone wide local excision. Nodular tumors had significantly greater thickness. Overall, conservative excisions provided excellent outcomes.
Conclusions:
Conservative treatment for external ear melanoma produces satisfactory outcomes. There is no evidence to support the use of radical amputation and little evidence to support the removal of cartilage or perichondrium. Sentinel lymph node biopsy is warranted only with positive nodes. There is no role for elective neck dissection. The roles for chemo/radiation therapy are unclear and guidelines for other cutaneous melanomas should be followed.
INTRODUCTION
It has been estimated that melanoma of the ear accounts for 7–13% of all cutaneous melanomas of the head and neck.1,2 Presentation can range from subtle macules and patches to ulcerated nodules (Fig. 1). Surgical excision is the primary treatment of ear melanoma. These malignancies present a variety of challenges from a surgical standpoint, as balance should be achieved between sufficient surgical aggressiveness and preservation of function and aesthetics of the ear.
Fig. 1.
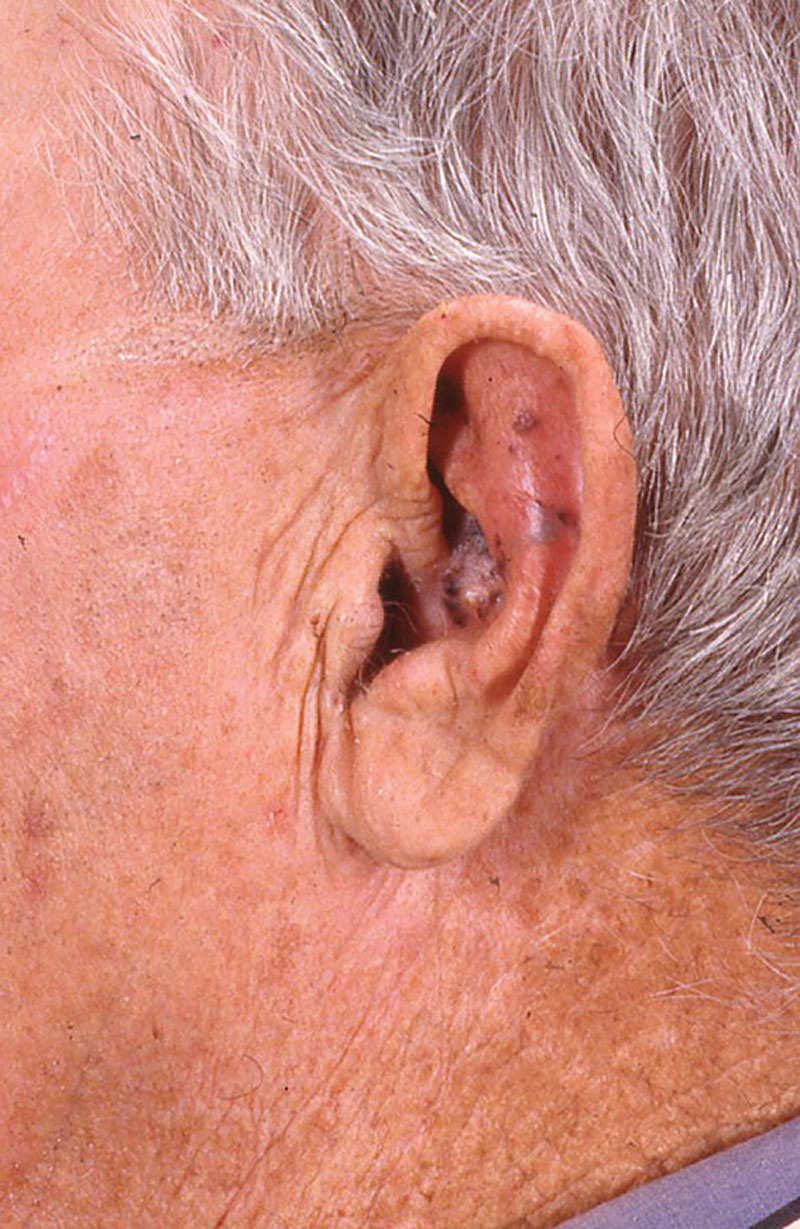
External ear melanoma at conchal bowl.
Early literature suggested that melanomas of the ear were more aggressive than other melanomas, possessing unique biological behavior, which led to recommendations of radical surgical management, often consisting of total amputation of the ear.3–6 Subsequent studies have failed to demonstrate a worse or unique behavior for melanoma of the ear compared with other sites.7,8 Overaggressive treatment can negatively impact cosmetic and functional outcomes. Notably, radical management such as total or partial amputation of the ear could render a patient unable to wear eye glasses or some hearing aids.
Various current cutaneous melanoma treatment guidelines recommend excision with 1, 2, or even 3 cm margins depending on the tumor depth.9–11 Historically, the margins included skin and cartilage in through and through resection; however, there is currently considerable variation in clinical practice concerning the extent of resection performed. More recently, a number of reports have suggested that a more conservative approach can achieve acceptable survival and recurrence rates, a theme common among surgical management of melanomas of other sites.1,12,13 Lymph node management in external ear melanoma is another challenge, as it has been noted that the lymphatic drainage of the ear is unpredictable and variable among patients.14–16
Due to the rarity of ear melanoma, most reports in the literature concerning its surgical management consist of small, single institution case series and case reports, limiting the clinical application of the results and recommendations. Consequently, there is currently a lack of consensus regarding best surgical management. To better understand the epidemiology of these tumors and to aid in the determination of their optimal management, we have performed a complete review of the literature concerning the surgical management of melanomas of the external ear.
METHODS
The terms “ear,” “melanoma,” and “external” were searched in various combinations on the Pubmed database, resulting in 209 articles. Results were limited to human studies, and no restriction was put on publication date. A title screen was performed, and all articles pertaining to surgical management of primary melanoma of the external ear were included. Articles in languages other than English and articles regarding melanoma of the auditory canal or middle ear were excluded (Fig. 2). Twenty-one studies were included in the overall review; however, not all of these articles contained data that could be extracted for individual cases; thus, only 73 cases from 8 reports were included in our subanalyses.
Fig. 2.
Article selection flow chart.
Statistical Analysis
Complete data concerning tumor characteristics, surgical procedure, margins, and clinical outcomes were available in only 73 cases. The cases were first separated by the extent of excision (amputation, partial amputation, excision/skin graft with radical neck dissection and parotidectomy, wedge excision, wedge excision with radical neck dissection and parotidectomy, and wide local excision) per the author’s description of the procedure, and histology, tumor depth, and outcome were determined for these groups (Table 1). Because the sample sizes were insufficient, no statistical analysis was performed for these comparisons. The 73 cases were then separated into 2 groups: those with recurrence and those with no recurrence. The baseline characteristics of these 2 groups were then compared using chi-square tests of independence or Fisher’s exact tests, as appropriate, to identify factors influencing the presence of recurrence (Table 2). An independent groups t test was used to examine differences between those with and without recurrence. Differences among the different histological subtypes were assessed using exact tests (categorical variables) and 1-way analysis of variance with Tukey post hoc comparisons for tumor depth (Table 3). These comparisons of histological subtypes excluded the 2 cases with undetermined/other subtypes.
Table 1.
Melanoma Type, Depth, Outcomes Based on Surgical Procedure in the 73 Patients with Complete Data
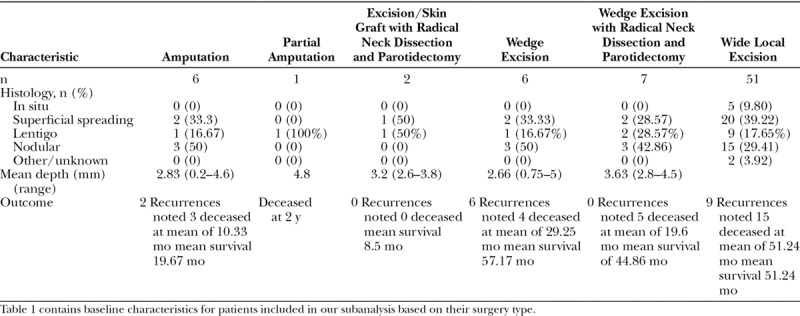
Table 2.
Type of Surgical Intervention and Melanoma Type in 73 Patients with Complete Data: Recurrence Versus No Recurrence
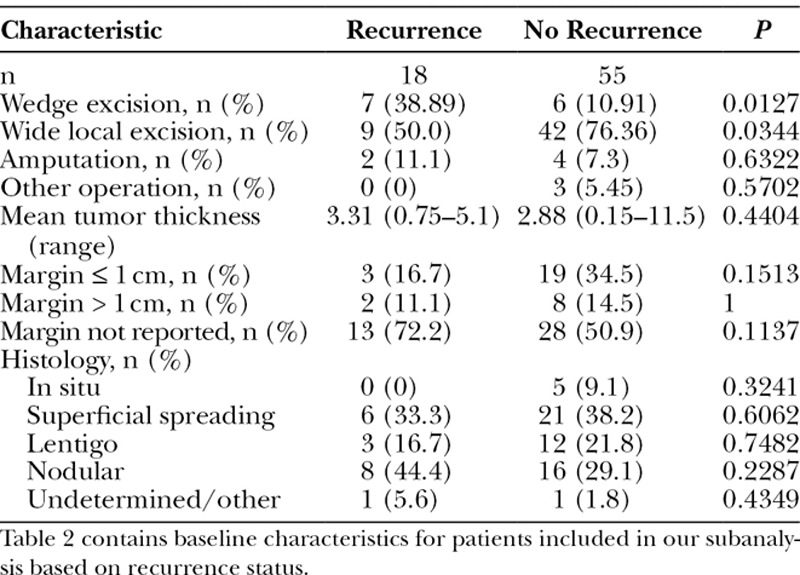
Table 3.
Baseline Characteristics and Melanoma Histological Subtype in 73 Patients with Complete Data
RESULTS
Subanalysis of 73 Cases in Literature
Wide local excision was the most commonly performed procedure, regardless of histological subtype. The remaining types of procedures did not seem to show any relationship with histological subtype, although we did not perform statistical analysis on these comparisons secondary to the small sample sizes within the surgical subtype groups. The mean depth of tumor did not have a significant impact on which subtype of procedure was performed (Table 1).
Of those who had wedge excision, a greater percentage had recurrence (7/13 = 53.8%) as compared with those who did not have wedge excision (11/60 = 18.3%), P = 0.0127 (Table 2). Of those who had wide local excision, a smaller percentage had recurrence (9/51 = 17.6%) as compared with those who did not have wide local excision (9/22 = 40.9%), P = 0.0344 (Table 2). Rate of amputation did not significantly vary between the 2 groups. Mean tumor thickness was greater in the recurrence group, although this did not reach statistical significance. In addition, margin size and histological subtype did not significantly vary between the 2 groups (Table 2).
Nodular tumors were found to be significantly thicker than superficial spreading and lentigo melanomas (P = 0.006; Table 3). Histological subtype did not have a significant impact on margins taken or type of surgery performed (Table 3).
Outcomes and Epidemiology
Following analysis of these 73 cases, all studies in the literature involving melanoma of the external ear melanoma were reviewed, and relevant data were extracted regarding epidemiology and baseline characteristics and outcomes (see table, Supplemental Digital Content 1, which displays epidemiology data, http://links.lww.com/PRSGO/A760; see table, Supplemental Digital Content 2, which displays outcomes data, http://links.lww.com/PRSGO/A761). Supplemental Digital Contents 1 and 2 were not subject to statistical analysis, as case-level data were not available for the majority of studies; thus, these tables serve as synopses of all studies we reviewed.
Our review rendered a composite of 947 cases of melanoma of the external ear that could be included. Males made up the overwhelming majority of cases, and most tumors were located on the helix of the ear. Superficial spreading melanoma comprised the most common histological subtype. Prior nevus was present in 15–69.2% of cases when reported (Supplemental Digital Content 1). Cartilage involvement was specifically noted in only 8 of the 947 cases, and all these cases were reported in the same report.4 The prevalence of ulceration ranged from 10.5 to 58.6% (Supplemental Digital Content 1). Overall survival ranged from 62.5 to 100%, and 5-year survival ranged from 43 to 96% (Supplemental Digital Content 2). Local recurrence in case series ranged from 0% to 27% (Supplemental Digital Content 2).
LITERATURE REVIEW
Prognosis
Early reports of ear melanoma indicated a more aggressive behavior and worse prognosis compared with other cutaneous sites.5 Subsequent literature has indicated that when controlled for other prognostic variables, location on the ear is not associated with a worse prognosis.7,8 As with other anatomic sites, increasing tumor thickness and ulceration are negative prognostic indicators in melanoma of the ear.1,8,12,13,17–20 In addition to tumor characteristics, lymph node involvement is a poor prognostic indicator in melanomas of the ear.1,13
Surgical Management: Excision of Primary Tumor
The optimal surgical management of melanoma of the external ear has yet to be established, as the available evidence consists of generally small, retrospective studies, case series, and case reports. Surgical options range from total amputation of the affected ear with radical neck dissection to conservative wedge resection to Mohs micrographic surgery.
The earliest reports of melanoma of the external ear appeared in the mid 1900s.3,5,21 Poor outcomes in these reports prompted radical recommendations for surgical management of melanoma of the external ear, which included total amputation of the ear, parotidectomy, and prophylactic dissection of the neck (Fig. 3). These recommendations were not founded in high quality evidence, but rather in scarce personal experience among surgeons. One early report stated that while superficial stage I melanomas of the ear could be treated without amputation, producing a low rate of regional metastasis (7%), any infiltrative, large superficial, or posteriorly located melanoma of the ear should be managed with a total amputation of the ear and a dissection of all regional lymph nodes, including parotidectomy.21 These aggressive recommendations dominated the literature for over a decade until larger series were reported.
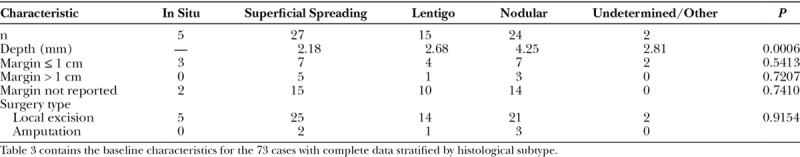
Fig. 3.
Partial ear amputation.
The first large series of ear melanomas was reported in 1980, which included 102 patients.1 This report marked a shift away from the tendency to treat melanomas in a radical fashion, based on the finding that the thickness of the tumor and regional lymph node involvement correlated with survival, while the type of surgical resection did not. Since then, multiple other studies of ear melanoma have similarly found no relationship between surgical approach and survival.1,12,13,19,20
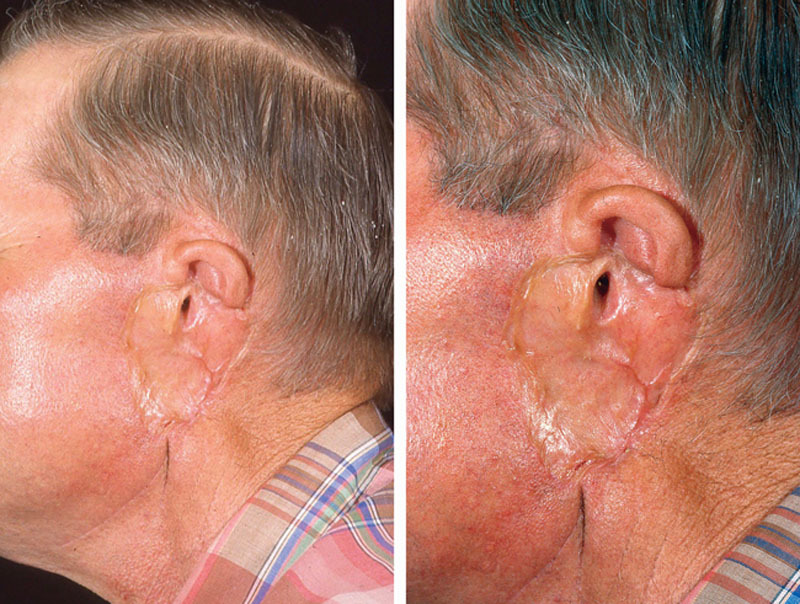
Several groups have shown satisfactory outcomes with regard to survival and recurrence using conservative surgical therapy such as wedge resection or cartilage-sparing wide local excision of the tumor (Fig. 4–6).22–24 Thus, more conservative surgical therapy has predominated in recent years. It is noted that in our subanalysis, the recurrence of melanoma was significantly more prevalent in those patients who underwent wedge resection. This does not necessarily indicate that the procedure itself influenced the outcome, but rather, it may reflect a bias in the cases selected for different procedures, or a utilization of different margins in different surgical approaches. Similarly, we found that the wide local excision was performed less often in those patients who had recurrence.
Fig. 4.
External ear melanoma with wedge excision.
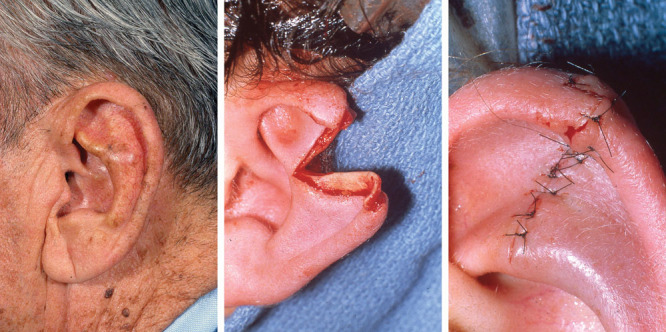
Fig. 6.
Coverage of surgical defect with skin graft.
Fig. 5.
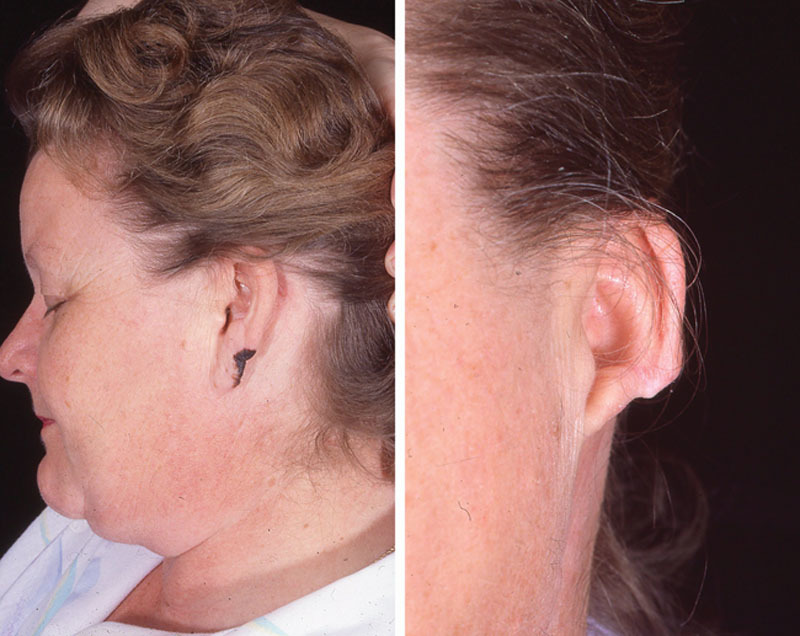
Melanoma at ear lobe with partial resection.
Some authors have proposed that the use of wide local excision with margins of 1 cm is adequate for the management of most ear melanomas.13,17,19 Although recommendations for cutaneous melanoma in general suggest margins of 1 cm for tumors up to 1 mm thick, margins of 1–2 cm for tumors between 1 and 2 mm, and margins of 2 cm for tumors thicker than 2 mm [National Comprehensive Cancer Network (NCCN) 17], these guidelines have not been specifically evaluated in the context of melanoma of the external ear. In addition, margins of this size are more difficult to achieve on the ear while preserving structure and contour compared with other head and neck melanomas.
Removal of Cartilage and Perichondrium
Removal of cartilage and/or the perichondrium during excision of malignant melanoma of the ear remains controversial. In a small series of 12 patients, Sartore et al.23 found that no patients had malignant invasion of the cartilage, a finding that was also reported in earlier studies.19,22 Cole et al.12 showed that cartilage-sparing procures were not related to higher rates of local recurrence, and McCarty et al.24 reported 0% local recurrence in 18 patients treated with cartilage-sparing resections, both providing further evidence that cartilage-sparing procedures produce safe and acceptable outcomes. In a histological analysis of 52 patients, Craig et al.25 also reported 0% involvement of the cartilage and suggested that the perichondrium may serve as a barrier to the tumor and may not need to be removed for satisfactory surgical removal of ear melanomas. Only 1 report in our review described involvement of the cartilage in melanoma of the ear.4 If, as the preponderance of evidence suggests, removal of cartilage is not necessary to preserve survival and control recurrence in melanoma of the external ear, reconstruction could provide more aesthetically and functionally pleasing results. The overwhelming majority of evidence suggests that removal of the cartilage and perichondrium is not necessary, and preservation of these structures may lead to superior reconstruction with more aesthetically and functionally pleasing results (Fig. 7).
Fig. 7.
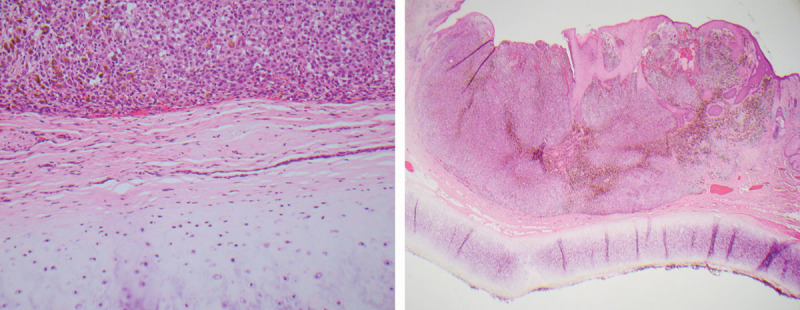
Malignant melanoma cells approaching but not invading perichondrium or cartilage of ear.
Lymph Node Biopsy and Dissection
Appropriate management of regional lymph nodes has been another area of debate in the treatment of melanoma of the external ear. It has been demonstrated that positive local and regional lymph node involvement is associated with poor outcomes.1,13,26 Early literature endorsed “prophylactic” neck dissection and/or parotidectomy3,5,21; however, subsequent reports have failed to show survival or recurrence advantages for patients undergoing elective lymph node dissection.1,27
The role of sentinel lymph node biopsy (SLNB) has since been studied as a means to address lymph node management in external ear melanoma. One retrospective study reviewed 41 patients with ear melanoma undergoing SLNB and found a significantly lower rate of positivity (10%) compared with other cutaneous sites (23%), despite similarity in other tumor prognostic features.28 This could prompt speculation as to whether the SLNB procedure is less reliable on the ear, and, indeed, there have been prior concerns regarding the unpredictability of lymph node drainage of head and neck melanomas,29–32 and of ear melanomas specifically.14,16,33 Nonetheless, another group has demonstrated that SLNB is both feasible and useful in melanoma of the head and neck, including melanoma of the ear. Using the same threshold for performing SLNB as that employed at other sites, Erman et al.34 reported on 353 patients undergoing SLNB for head and neck melanoma, including 59 melanomas on the ear. An SLN was identified in 99.7% of cases, with rates of positivity (23% for ear cases) and prognostic value similar to those expected at other cutaneous sites.34 Morbidity was low, with no permanent cranial nerve or significant vascular injuries, and the authors concluded that SLNB should be performed in head and neck melanoma for the same indications as patients with truncal or extremity melanoma, and that comparable accuracy, safety, and prognostic value can be achieved.34 It is noted that the experience of the surgeon, specific protocol utilized for SLNB, and laboratory handling of the SLN specimens may all influence the yield, accuracy, utility, and safety of this procedure.
Although completion lymph node dissection has traditionally been performed after a positive SLNB, 2 recent prospective randomized trials have shown no survival benefit for completion lymph node dissection compared with nodal observation.35,36 The larger of these trials included 241 melanomas of the head and neck,36 although data specific to ear melanoma was not presented.
Imaging Studies
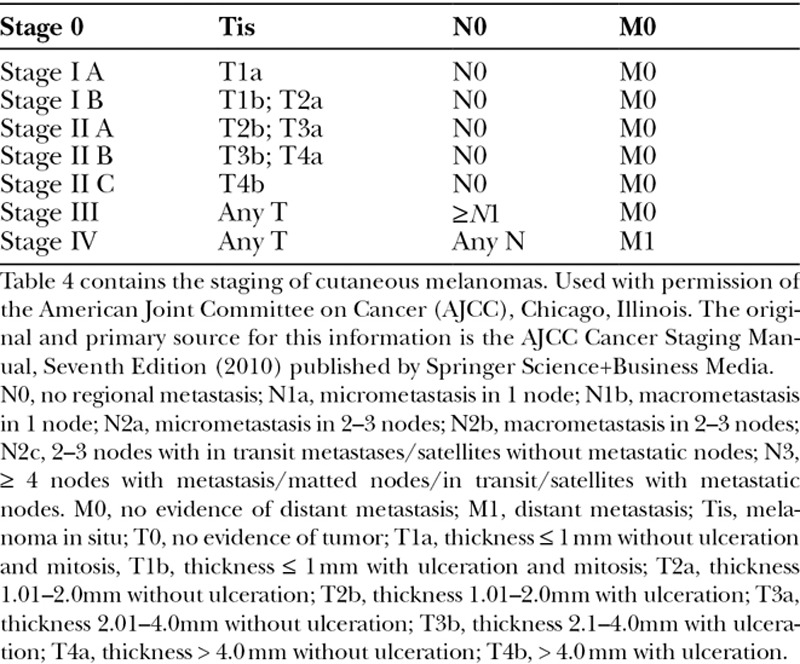
Preoperative lymph node evaluation with computed tomography (CT) has not been studied in external ear melanomas, however, clinical recommendations may be drawn from studies involving all cutaneous melanomas. CT and other imaging modalities were shown by Yancovitz et al.37 to render false-positive rates of greater than 90% and did not impact surgical management or lead to upstaging. Further, it has been demonstrated that SLNB is more sensitive than CT scans in the identification of metastasis.38 A meta-analysis of 74 studies (10,528 patients) in 2010 found that PET-CT has a positive predictive value of only 33%, and thus is not warranted in the initial evaluation of cutaneous melanomas without further clinical evidence for metastasis.39 The current NCCN practice guidelines for melanoma do not recommend using CT or other imaging modalities for stage 0, IA, IB, or II melanomas. Melanoma staging can be seen in Table 4. CT is recommended for stage III melanomas in some circumstances to assess for distant metastasis, or to further evaluate specific signs and symptoms at any stage.9
Table 4.
Melanoma Staging
Nonsurgical Therapies
The role of chemotherapy, immunostimulatory, and radiation therapy in the treatment of cutaneous melanoma has greatly expanded in recent years, particularly with respect to stage III and IV melanoma. We find no literature regarding particular considerations for the use of these agents in melanoma of the ear, and, accordingly, utilization of the same guidelines employed at other sites is advisable until more site-specific data are available. For cutaneous melanomas in general, the NCCN states that interferon alfa, along with observation, or participation in clinical trials, may be considered for state II B and II C melanomas with negative lymph nodes. For stage III melanomas, high dose pegylated interferon, biochemotherapy, or high dose ipilimumab may be considered at the discretion of the provider along with the patient’s wishes.9
With regard to radiation therapy, there is, again, no specific recommendation for melanoma of the external ear. For primary melanoma in general, the NCCN notes that adjuvant radiation is rarely needed following adequate excision, but may be considered for deep desmoplastic/neurotropic melanoma with narrow margins. Although there has been some evidence that radiation therapy may improve local control, the risks of radiation must be weighed against the benefits.9
There are several limitations to this review. First, most studies reviewed were small, and all were single-institution series. This makes it somewhat difficult to draw conclusions regarding specific surgical treatments. We also had relied on the studies’ name or description of the procedures when comparing 1 type of management versus another. Further, we were unable to analyze a large number of studies quantitatively secondary to the heterogeneous nature in which data were reported in the various studies. Despite these limitations, we feel that we have summarized the available literature to make evidence-based decisions regarding surgical management of melanoma of the external ear.
CONCLUSIONS
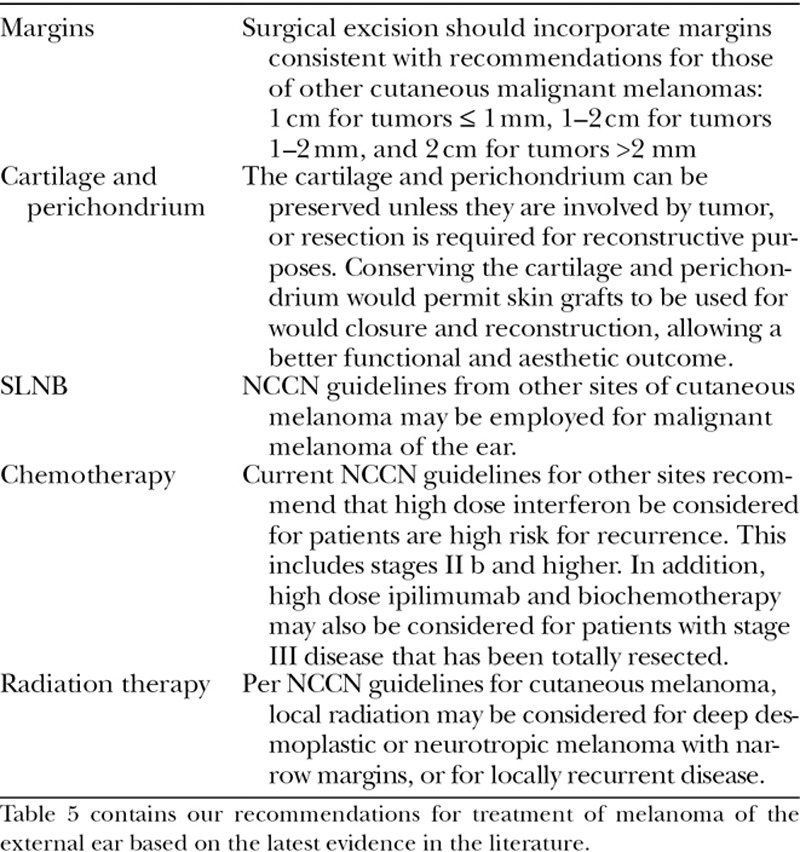
We provide current evidence-based recommendations aimed to optimize management of melanoma of the external ear (Table 5): (1) conservative management of ear melanomas can be attempted, using standard margins employed at other sites. Larger margins and more radical excisions have failed to show survival or recurrence benefit in multiple studies. (2) Assuming that negative margins can be achieved, the perichondrium and underlying cartilage should be preserved unless resection is required for reconstructive purposes, as there is a lack of recent evidence to suggest that its removal provides survival or recurrence benefit. (3) SLNB should be performed using the same guidelines applied at other cutaneous sites. (4) Consideration of chemotherapy and radiation therapy should follow the same guidelines applied at other cutaneous sites.
Table 5.
Current Evidence-based Recommendations
In the interest of developing additional guidelines, it would be useful if future studies regarding surgical management of external ear melanomas could provide the following data in their reports: tumor thickness, histological subtype, location, lymph node status (clinically and on SLNB if performed), cartilage involvement, ulceration status, exact surgical margins, specific surgical procedure performed and whether perichondrium and cartilage were removed, extent of lymph node dissection, and clinical outcomes data. In the absence of guidelines specific to melanoma of the ear, our review points to evidence that these melanomas do not require surgical management more aggressive than other cutaneous sites; thus our recommendations are similar to the NCCN melanoma guidelines. We feel that the data and evidence provided will ultimately allow surgeons to better understand the characteristics of melanoma of the ear, and to further personalize surgical approach based on the characteristics of each case.
Footnotes
Published online 13 April 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg. 1980;140:518–521.. [DOI] [PubMed] [Google Scholar]
- 2.Urist MM, Balch CM, Soong SJ, et al. Head and neck melanoma in 534 clinical stage I patients. A prognostic factors analysis and results of surgical treatment. Ann Surg. 1984;200:769–775.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sylven B, Hamberger CA. Malignant melanoma of the external ear. Report of 36 cases treated between 1928-1944. Ann Otol Rhinol Laryngol. 1950;59:631–647.. [DOI] [PubMed] [Google Scholar]
- 4.Benmeir P, Baruchin A, Weinberg A, et al. Rare sites of melanoma: melanoma of the external ear. J Craniomaxillofac Surg. 1995;23:50–53.. [DOI] [PubMed] [Google Scholar]
- 5.Ward NO, Acquarelli MJ. Malignant melanoma of the external ear. Cancer. 1968;21:226–233.. [DOI] [PubMed] [Google Scholar]
- 6.Wanebo HJ, Cooper PH, Young DV, et al. Prognostic factors in head and neck melanoma. Effect of lesion location. Cancer. 1988;62:831–837.. [DOI] [PubMed] [Google Scholar]
- 7.Helsing P, Robsahm TE, Vos L, et al. Cutaneous head and neck melanoma (CHNM): a population-based study of the prognostic impact of tumor location. J Am Acad Dermatol. 2016;75:975–982.e2.. [DOI] [PubMed] [Google Scholar]
- 8.Augenstein AC, Capello ZJ, Little JA, et al. The importance of ulceration of cutaneous melanoma of the head and neck: a comparison of ear (pinna) and nonear sites. Laryngoscope. 2012;122:2468–2472.. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Melanoma (Version 1.2017). Available at https://www.nccn.org/professionals/physician_gls/PDF/melanoma.pdf. Accessed May 6, 2017.
- 10.Bichakjian CK, Halpern AC, Johnson TM, et al. ; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032–1047.. [DOI] [PubMed] [Google Scholar]
- 11.Marsden JR, Newton-Bishop JA, Burrows L, et al. ; British Association of Dermatologists Clinical Standards Unit. Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol. 2010;163:238–256.. [DOI] [PubMed] [Google Scholar]
- 12.Cole DJ, Mackay GJ, Walker BF, et al. Melanoma of the external ear. J Surg Oncol. 1992;50:110–114.. [DOI] [PubMed] [Google Scholar]
- 13.Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for melanoma of the external ear. Ann Surg Oncol. 2003;10:689–696.. [DOI] [PubMed] [Google Scholar]
- 14.Cole MD, Jakowatz J, Evans GR. Evaluation of nodal patterns for melanoma of the ear. Plast Reconstr Surg. 2003;112:50–56.. [DOI] [PubMed] [Google Scholar]
- 15.Shpitzer T, Gutman H, Barnea Y, et al. Sentinel node-guided evaluation of drainage patterns for melanoma of the helix of the ear. Melanoma Res. 2007;17:365–369.. [DOI] [PubMed] [Google Scholar]
- 16.Peach HS, van der Ploeg AP, Haydu LE, et al. The unpredictability of lymphatic drainage from the ear in melanoma patients, and its implications for management. Ann Surg Oncol. 2013;20:1707–1713.. [DOI] [PubMed] [Google Scholar]
- 17.Hudson DA, Krige JE, Strover RM, et al. Malignant melanoma of the external ear. Br J Plast Surg. 1990;43:608–611.. [DOI] [PubMed] [Google Scholar]
- 18.Davidsson A, Hellquist HB, Villman K, et al. Malignant melanoma of the ear. J Laryngol Otol. 1993;107:798–802.. [DOI] [PubMed] [Google Scholar]
- 19.Bono A, Bartoli C, Maurichi A, et al. Melanoma of the external ear. Tumori. 1997;83:814–817.. [DOI] [PubMed] [Google Scholar]
- 20.Jahn V, Breuninger H, Garbe C, et al. Melanoma of the ear: prognostic factors and surgical strategies. Br J Dermatol. 2006;154:310–318.. [DOI] [PubMed] [Google Scholar]
- 21.Pack GT, Conley J, Oropeza R. Melanoma of the external ear. Arch Otolaryngol. 1970;92:106–113.. [DOI] [PubMed] [Google Scholar]
- 22.Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg. 2001;107:20–24.. [DOI] [PubMed] [Google Scholar]
- 23.Sartore L, Giatsidis G, Reho F, et al. Ear melanoma: influence of perichondrium involvement in evaluating surgical strategy. Eur Arch Otorhinolaryngol. 2012;269:1685–1690.. [DOI] [PubMed] [Google Scholar]
- 24.McCarty MA, Lentsch EJ, Cerrati EW, et al. Melanoma of the ear: results of a cartilage-sparing approach to resection. Eur Arch Otorhinolaryngol. 2013;270:2963–2967.. [DOI] [PubMed] [Google Scholar]
- 25.Craig ES, Nagarajan P, Lee ES, et al. The perichondrium in auricular melanomas: implications for surgical management. Otolaryngol Head Neck Surg. 2013;148:431–435.. [DOI] [PubMed] [Google Scholar]
- 26.Jones TS, Jones EL, Gao D, et al. Management of external ear melanoma: the same or something different? Am J Surg. 2013;206:307–313.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravin AG, Pickett N, Johnson JL, et al. Melanoma of the ear: treatment and survival probabilities based on 199 patients. Ann Plast Surg. 2006;57:70–76.. [DOI] [PubMed] [Google Scholar]
- 28.Harrison C, Mikhail M, Potter M, et al. Sentinel lymph node biopsy for external ear melanoma: a 17 year experience with long term survival data. J Plast Reconstr Aesthet Surg. 2017;70:1301–1303.. [DOI] [PubMed] [Google Scholar]
- 29.Wells KE, Cruse CW, Daniels S, et al. The use of lymphoscintigraphy in melanoma of the head and neck. Plast Reconstr Surg. 1994;93:757–761.. [DOI] [PubMed] [Google Scholar]
- 30.Fincher TR, O’Brien JC, McCarty TM, et al. Patterns of drainage and recurrence following sentinel lymph node biopsy for cutaneous melanoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2004;130:844–848.. [DOI] [PubMed] [Google Scholar]
- 31.Chen SL, Iddings DM, Scheri RP, et al. Lymphatic mapping and sentinel node analysis: current concepts and applications. CA Cancer J Clin. 2006;56:292–309.; quiz 316. [DOI] [PubMed] [Google Scholar]
- 32.Eicher SA, Clayman GL, Myers JN, et al. A prospective study of intraoperative lymphatic mapping for head and neck cutaneous melanoma. Arch Otolaryngol Head Neck Surg. 2002;128:241–246.. [DOI] [PubMed] [Google Scholar]
- 33.Wey PD, De La Cruz C, Goydos JS, et al. Sentinel lymph node mapping in melanoma of the ear. Ann Plast Surg. 1998;40:506–509.. [DOI] [PubMed] [Google Scholar]
- 34.Erman AB, Collar RM, Griffith KA, et al. Sentinel lymph node biopsy is accurate and prognostic in head and neck melanoma. Cancer. 2012;118:1040–1047.. [DOI] [PubMed] [Google Scholar]
- 35.Leiter U, Stadler R, Mauch C, et al. ; German Dermatologic Cooperative Oncology Group (DeCOG). Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757–767.. [DOI] [PubMed] [Google Scholar]
- 36.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376:2211–2222.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007;110:1107–1114.. [DOI] [PubMed] [Google Scholar]
- 38.Kell MR, Ridge JA, Joseph N, et al. PET CT imaging in patients undergoing sentinel node biopsy for melanoma. Eur J Surg Oncol. 2007;33:911–913.. [DOI] [PubMed] [Google Scholar]
- 39.Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129–142.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cascinelli N, Morabito A, Santinami M, et al. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351:793–796.. [DOI] [PubMed] [Google Scholar]
- 41.Cohen BJ, Melisi J, Cohen MH. Ear preservation in the surgical treatment of auricular melanoma. Head Neck. 1990;12:346–351.. [DOI] [PubMed] [Google Scholar]
- 42.Anderson PJ, Rao GS. Clinical photographs. Malignant melanoma of the ear in a child. Otolaryngol Head Neck Surg. 1999;120:135. [DOI] [PubMed] [Google Scholar]
- 43.Kruse-Lösler B, Presser D, Metze D, et al. Surgical reconstruction after subtotal ear resection in malignant melanoma of the ear. J Eur Acad Dermatol Venereol. 2006;20:190–196.. [DOI] [PubMed] [Google Scholar]
- 44.Fisher SR. Cutaneous malignant melanoma of the head and neck. Laryngoscope. 1989;99:822–836.. [DOI] [PubMed] [Google Scholar]


