Abstract
Tobacco use is an established risk factor for the development of several cancers; however, far less work has been done to understand the effects of continued smoking on cancer treatment outcomes, and structured tobacco cessation efforts are not well incorporated into the standard care for patients with cancer. In this Review we discuss the known biological effects of smoking on cancer cell biology and emphasise the clinical effects of continued smoking in patients with cancer treated with chemotherapy or radiotherapy. Although evidence supports the need for inclusion of dedicated tobacco cessation efforts for patients with cancer, clinicians should consider the methods used to provide evidence-based tobacco cessation support and the available resources to deliver and maintain consistent tobacco cessation support. We also address the variables to consider in the design and implementation of a sustainable tobacco cessation programme.
Introduction
Tobacco is a well established cause of at least 13 cancers;1 however, until recently there have been no large evidence-based assessments of the effects of smoking on cancer treatment outcomes. The 2014 Surgeon General’s Report1 is the “first large evidence review to report a causal association between tobacco use and adverse clinical outcomes for patients with cancer”. These findings provide the evidence base to effectively change clinical practice by justifying the need to address tobacco use in patients with cancer (panel 1).
Panel 1. Conclusions of the 2014 Surgeon General’s Report1.
Smoking causes adverse health outcomes in patients with cancer and survivors
In cancer patients and survivors, the evidence is sufficient to infer a causal relationship between cigarette smoking and adverse health outcomes. Quitting smoking improves the prognosis of cancer patients.
In cancer patients and survivors, the evidence is sufficient to infer a causal relationship between cigarette smoking and increased all-cause mortality and cancer-specific mortality.
In cancer patients and survivors, the evidence is sufficient to infer a causal relationship between cigarette smoking and increased risk for second primary cancers known to be caused by cigarette smoking, such as lung cancer.
In cancer patients and survivors, the evidence is suggestive but not sufficient to infer a causal relationship between cigarette smoking and the risk of recurrence, poorer response to treatment, and increased treatment-related toxicity.
Although the evidence-base is now sufficient to infer a causal relation between tobacco use and adverse clinical outcomes, there are substantial deficits in how we address tobacco use in patients with cancer. First, there are no standard recommendations for the accurate assessment of tobacco use in patients with cancer, which limits the ability of clinicians to accurately identify patients who are at risk from continued tobacco use and to infer relationships between tobacco use and cancer treatment outcomes.2 Second, many oncologists do not regularly provide tobacco cessation assistance to patients with cancer who smoke and most do not feel adequately trained to deliver evidence-based tobacco cessation support.3,4 Third, no dedicated attempt has been made to fully address the dynamic effects of tobacco use or tobacco cessation on cancer treatment outcomes. Although smoking adversely affects cancer treatment and the effects of former smoking on outcomes are less damaging1 we do not yet know the optimum period for, or magnitude of benefits from, quitting smoking that would have most benefit for patients with cancer. Fourth, provision of tobacco cessation support in clinical cancer care and clinical research needs efficient design and delivery.
The aim of this Review is to provide a concise overview of the biological and clinical effects of smoking on cancer treatment with emphasis on patients who are treated with chemotherapy and radiotherapy. We provide specific and practical guidance for implementation of changes to improve cancer treatment outcomes for patients through tobacco cessation support in cancer care and research.
The biological effect of tobacco and tobacco products on cancer
Cigarette smoke and cell signalling in cancer
Tremendous work and evidence supports the carcinogenic effects of tobacco for various cancers. Detailed reviews are presented in the 2014 Sugeon General’s Report on the carcinogenic properties of tobacco.1 Far less work has been done, however, to understand the biological effects of tobacco on existing cancer cells.
Cigarette smoke is a complex mixture of 7000 different aerosolised chemicals and tobacco components, including more than 60 known carcinogens in gaseous or particulate phases.4 The gaseous phase is associated with chronic pulmonary disease and lung toxicity, whereas the particulate phase is most frequently associated with cancer.5 The Federal Trade Commission has made attempts to standardise smoke conditions for use in the research setting, but there is substantial variation in the chemical composition of cigarette smoke including cigarette brand, composition, and cigarette usage behaviour.6 As a result, evaluation of the biological effects of cigarette smoke on cancer cells is complicated and largely unexplored at this time.
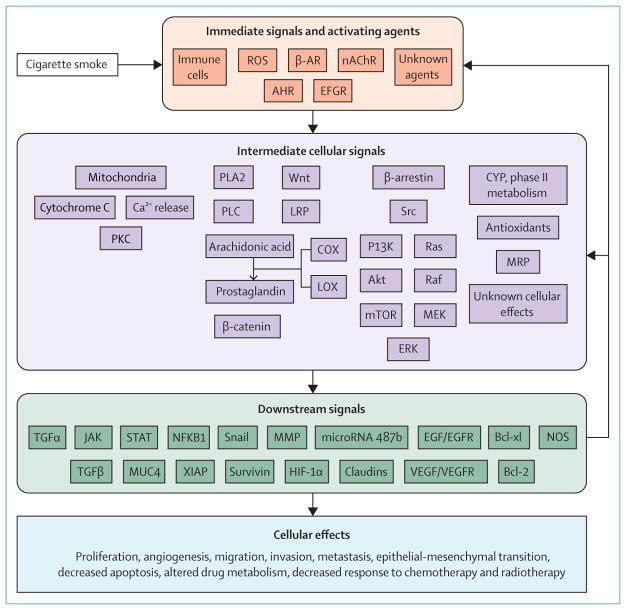
A summary of the known effects of cigarette smoke and constituents of cigarette smoke on cancer cells is shown in the figure. Immediate effects stimulate the immune system and activate cellular receptors leading to activation of intermediate signalling pathways such as Src, Wnt, mitochondrial, arachidonic acid, and other unknown pathways including activation of phase I and phase II drug metabolising enzymes. Intermediate signalling leads to a broad range of downstream signals that promote tumour growth and decrease the response to cytotoxic treatments. Importantly, many downstream signals generate feedback mechanisms that continue to promote a tumorigenic environment. Although some of the effects of cigarette smoke on cancer cells are known (figure), substantial work is needed to clarify the effects and cross-signalling that occurs with exposure to the thousands of compounds in cigarette smoke. Furthermore, the well-established carcinogenic effects of cigarette smoke on non-cancer cells1 are mostly untested with respect to oncogenic mutations across a range of cancer disease sites. The effects of cigarette smoke on tumour biology are far from fully understood.
Figure. The biological effects of cigarette smoke on cancer cell biology.
ROS=reactive oxygen species. β-AR=β-adrenergic receptor. nAChR=nicotinic acetylcholine receptor. AHR=aryl hydrocarbon receptor. EGFR=epidermal growth factor receptor. PKC=protein kinase C. PLA2=phospholipase A2. PLC=phosphoinositide phospholipase C. LRP=lipoprotein receptor-related protein. COX=cyclo-oxygenase. LOX=lipoxygenase. MEK=mitogen activated kinase kinase. ERK=extracellular signal related kinase. CYP=cytochrome P450. MRP=multidrug resistance-associated protein. TGF=transforming growth factor. STAT=signal transducer and activator of transcription. JAK=janus kinase. MUC4=mucin 4, cell surface associated. NFKB1=nuclear factor of kappa light polypeptide gene enhancer in B-cells 1. XIAP=x-linked inhibitor of apoptosis. MMP=matrix metalloproteinase. Bcl-xl=B-cell lymphoma extra large. HIF-1α=hypoxia inducible factor 1-α. EGF=epidermal growth factor. EGFR=epidermal growth factor receptor. VEGF=vascular endothelial growth factor. VEGFR=vascular endothelial growth factor receptor. NOS=nitric oxide. synthase. Bcl-2=B-cell lymphoma 2.
Exposure of cancer cells to cigarette smoke increases proliferation and tumorigenesis through the activation of many cellular signalling pathways. The components of cigarette smoke benzo(a)pyrene and polycyclic aromatic hydrocarbons can act as ligands and bind directly to the aryl hydrocarbon receptor, a transcription factor, leading to expression of CYP450 enzymes, multidrug resistance-associated proteins, ABCG2 and adrenomedullin7–10 that in turn increase tumour growth and potential clonal expansion of cancer stem cells. Exposure to cigarette smoke downregulates DKK1 and leads to activation of Wnt signalling.11 Cigarette smoke also downregulates microRNA-487b, which mediates cell cycle arrest and senescence in lung cancer cells, by targeting WNT5A, a non-conical Wnt ligand, and components PRC1 and PRC2 of the polycomb repressive complex.12 Furthermore, cigarette smoke activates the arachidonic acid cascade through β1-adrenergic receptors and β2-adrenergic receptors and the subsequent expression of COX2, 5-lipoxygenase, VEGF, and matrix metalloproteinases.13–16 Additionally, cigarette smoke modulates canonical ligand-dependent EGFR signalling through reactive oxygen species induced autocrine shedding of the EGFR ligands HBEGF, AREG, and TGF-α.17–19 Oxidative stress due to cigarette smoke induces non-canonical EGFR autophosphorylation at Src-dependent phos phorylation sites leading to recruitment of Src to EGFR, which triggers the Ras/Raf/MEK/ERK and PI3K/AKT signalling cascades and contributes to tyrosine-kinase inhibitor resistance in tyrosine-kinase inhibitor sensitive lung cancer cells.20–21
Evidence shows that cigarette smoke contributes to the ability of cancer cells to evade several cell death pathways by altering SMAD3–SMAD4 complex formation, which upregulates Bcl-2 and reduces TGF-β induced apoptosis in lung cancer cells.22 Exposure to cigarette smoke induces cisplatin resistance in bladder cancer cells by reducing the expression of mitochondrial matrix protein AK3, altering mitochondrial membrane potential, increasing levels of intracellular reactive oxygen species, and elevating Bcl-xl and Bcl-2 expression.23 Studies have also suggested that cigarette smoke might be involved in modulating the NOS pathway and autophagic response in head and neck cancer cells.24
Several studies have also examined the role cigarette smoke has in mediating invasion and metastasis of cancer cells. Long-term exposure of breast cancer cells to cigarette smoke enhances the invasion and metastatic potential of cells.25 Exposure of non-small-cell lung carcinoma lines to cigarette smoke increased the expression of MTA1, which is involved in mediating epithelial to mesenchymal transition.26 Cigarette smoke has also been shown to reduce activity of the Na+/K+-ATPase27 leading to disruption of tight junctions and altered cell polarity that might be involved in early epithelial to mesenchymal transition events.
Effects of nicotinic acetylcholine receptor activation on cancer cells
Nicotine is the primary addictive component of tobacco.1 Several recent reviews have discussed the tumour-promoting activity of nicotine and of the nicotine-derived 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N′-nitrosonornicotine.28–31 There are several excellent reviews on the effects of these tobacco products to stimulate cell proliferation, migration, invasion, angiogenesis, and resistance to cell death pathways such as apoptosis.28–31
Overall, cigarette smoke is distributed throughout the lungs and metabolites of cigarette smoke are present in virtually all peripheral tissues. Nicotine and its metabolites are distributed in almost all tissues of the body. In peripheral tissues, these substances activate systemically expressed nicotinic acetylcholine receptors (nAChRs) and β-adrenergic receptors that in turn activate several pathways, including the Ras/Raf/MEK/MAPK and PI3K/Akt oncogenic pathways causing substantial cross-activation of these pathways leading to a tumour-promoting phenotype. In relation to chemotherapy and radiotherapy, nicotine and activation of nAChRs have been shown to decrease the therapeutic response to chemotherapy and radiotherapy both in vitro and in vivo.32–34 Collectively, these data suggest that activation of systemic nAChRs might be one mechanism associated with cigarette smoke-induced tumour promotion.
The preclinical data showing the tumour-promoting activities of nicotine are a subject of discussion among some oncologists; however, we are not aware of any studies or reports on the safety of nicotine-replacement therapy in patients with cancer with respect to mortality, toxicity, or cancer recurrence. Nicotine-replacement therapy is evidence-based, and increases the effectiveness of smoking cessation35 advocated for use in patients with cancer by both ASCO36 and AACR.37 Although there is little evidence on the potential effects of nicotine-replacement therapy on cancer treatment outcomes at this time there is now strong evidence that smoking causes adverse outcomes in patients with cancer. Unless evidence suggests that nicotine-replacement therapy has any adverse effects in patients with cancer we advise clinicians to consider nicotine-replacement therapy as an effective means to promote tobacco cessation for these patients.
The clinical effects of smoking on patients with cancer
The findings described in the 2014 Surgeon General’s Report warrant a paradigm shift in the standard clinical care for patients with cancer.1 On the basis of evidence in the 2014 Surgeon General’s Report, smoking by patients with cancer should now be viewed as a cause of increased overall mortality and cancer-specific mortality and a cause of second primary cancer. A full review of the clinical effects of smoking on patients with cancer is beyond the scope of this Review and broad ranging data have been provided in the 2014 Surgeon General’s Report.1 For our purposes, large studies reporting one or more clinical effects of current smoking in patients treated with chemotherapy and radiotherapy are highlighted.
The diversity of studies, treatments, disease sites, disease stage, and definitions of tobacco use do not allow an overall comparative analysis to estimate the magnitude of the associations between smoking and outcome. However, 22 (81%) of 27 studies show one or more significant negative associations between current smoking and treatment outcome (table).38–64 Two studies42,60 show near significant negative associations and the results of three studies show no association.40,43,63 No studies reported a positive effect of smoking on outcome, but it is important to note that a recent meta-analysis of smaller studies suggests that smoking decreases the risk of radiation-induced pneumonitis.65 Lung cancer studies represented the largest disease site (ten), prostate cancer (six), head and neck cancer (four), and breast cancer (three) with one study each of cervical cancer, Hodgkin’s disease, colon cancer, and male cancer patients.
Table.
Effects of current smoking on mortality, recurrence, cancer treatment toxicity, and second primary cancer in patients treated with chemotherapy or radiotherapy
| Patients | Treatments | Study period | Outcome | |
|---|---|---|---|---|
| Lung cancer | ||||
|
| ||||
| Florescu et al (2008)38 | 731 (NSCLC stage IIIB–IV, previous therapy unsuccessful) | Chemotherapy, erlotinib (BR21 trial) | NA | Mortality (vs never smoked) Former: HR 1·22, p<0·001 Current: HR 1·89, p<0·001 |
| Herbst et al (2005)39 | 1079 (NSCLC stage IIIB–IV) | Chemotherapy, erlotinib (TRIBUTE trial) | 2001–02 | Median survival Chemotherapy + erlotinib: Current: 8·4 months Former: 10·0 months Never: 22·5 months p=0·01 Chemotherapy: Current: 9·1 months Former: 10·9 months Never: 10·1 months |
| Holgersson et al (2012)*40 | 1146 (NSCLC) | Radiotherapy | 1990–2000 | Mortality (vs never smoking) Former: HR 1·10 (95% CI 0·82–1·45) Current: HR 1·06 (95% CI 0·80–1·41) |
| Kawaguchi et al (2012)41 | 2966 (NSCLC stage IIIB–IV, ≥70 years of age) | Chemotherapy | NA | Mortality (vs never smoking) Former: 70–74 years: HR 0·91 (95% CI 0·80–1·04) 75–79 years: HR 1·06 (95% CI 0·92–1·22) 80+ years: HR 1·08 (95% CI 0·90–1·29) Never: 70–74 years: HR 0·78 (95% CI 0·66–0·93) 75–79 years: HR 0·80 (95% CI 0·66–0·97) ≥80 years: HR 0·99 (95% CI 0·80–1·21) |
| Lee et al (2012)42 | 670 (NSCLC stage IIIB–IV) | Erlotinib (TOPICAL trial) | NA | Cancer-specific mortality (vs never smoking) Former: HR 1·02 (95% CI 0·79–1·32) Current: HR 1·61 (95% CI 0·91–2·86) |
| Li et al (2011)43 | 1214 (NSCLC stage IIIB–IV) | Chemotherapy | NA | Mortality (vs never smoking) Former: HR 1·10 (95% CI 0·79–1·54) Current: HR 1·05 (95% CI 0·63–1·75) |
| Pirker et al (2012)44 | 1125 (NSCLC stage IIIB–IV) | Chemotherapy, cetuximab (phase III FLEX study) | NA | Mortality (vs current smoking): HR 1.0 (referent) Former: HR 0·86 (95% CI 0·73–1·02) Never: HR 0·72 (95% CI 0·58–0·88) |
| Richardson et al (1993)45 | 540 (SCLC) | National Cancer Institute intramural trials | 1973–89 | Second primary cancer (vs general population) Current smoking after diagnosis: RR 32 (95% CI 12–69) Quit smoking after diagnosis: RR 11 (95% CI 4·4–23) |
| Tsao et al (2006)*46 | 1379 (NSCLC stage III–IV) | Chemotherapy | NA | Mortality (vs never smoking) Former: OR 1·47, p=0·003 Current: OR 1·55, p=0·0004 |
| Tucker et al (1997)47 | 611 (SCLC survivors) | Chemotherapy, radiotherapy | 2 years | Second primary cancer (vs general population) Current smoking after diagnosis: RR 17 (95% CI 11–26) Quit smoking at diagnosis: RR 9·9 (95% CI 5·3–17) Quit smoking before diagnosis: RR 9·4 (95% CI 4·7–17) |
|
| ||||
| Head and neck cancer | ||||
|
| ||||
| Fortin et al (2009)*48 | 1871 | Radiotherapy, chemotherapy, systemic therapy | 1989–2006 | Mortality (vs never smoking) Former: HR 1·23 (p value unavailable) Current: HR 1·35, p=0·0005 |
| Gillison et al (2012)49 | 502 (stage III–IV) | Radiotherapy, chemotherapy, (RTOG9003 and RTOG0129) | NA | Mortality (vs all other patients) Overall: HR 2·34 (95% CI 1·56–3·50) Cancer-specific mortality Radiotherapy treated patients: HR 2·19 (95% CI 1·48–3·25) Chemotherapy treated patients: HR 2·73 (95% CI 1·37–5·45) |
| Khuri et al (2006)50 | 1190 (stage I–II) | Radiotherapy, systemic therapy (no chemotherapy), isoretinoin (phase III trial) | NA | Mortality Current vs former: RR 1·60 (95% CI 1·23–2·07) Current vs never: RR 2·51 (95% CI 1·54–4·10) Recurrence Current vs former: RR 1·12 (95% CI 0·76–1·65) Current vs never: RR 1·37 (95% CI 0·76–2·46) |
| Meyer et al (2008)51 | 540 | Radiotherapy, β-carotene | 1994–2000 | Mortality (vs all other patients) Current: HR 2·26 (95% CI 1·29–3·97) |
|
| ||||
| Prostate cancer | ||||
|
| ||||
| Alsadius et al (2011)52 | 836 | Radiotherapy | 1993–2006 | Rectal toxicity (vs never smoking): Current: Abdominal cramps: RR 9·0, p=0·004 Urgency: RR 2·65, p<0·001 Diarrhoea: RR 2·67, p=0·017 Incomplete emptying: RR2·57, p=0·003 Sudden emptying: RR 4·6, p=0·003 |
| Bittner et al (2008)53 | 1354 | Radiotherapy, ADT | 1993–2004 | Mortality (current vs never smoking) Current: Prostate cancer mortality: NS Non-prostate cancer mortality Heart disease: RR 3·05, p=0·007 Non-prostate: RR 5·52, p=0·002 Other: RR 4·09, p=0·002 |
| Boorjian et al (2007)54 | 9780 | Systemic therapy, radiotherapy, ADT (CaPSURE study) | NA | Bladder cancer (vs all other patients) No radiotherapy (all): HR 1·0 (referent) Yes radiotherapy (all): HR 1·59 (95% CI 0·97–2·6) Current (all): HR 2·08 (95% CI 1·09–3·97) Current + radiotherapy: HR 3·65 (95% CI 1·45–9·16) |
| Merrick et al (2006)55 | 938 (stage T1b–T3a) | Radiotherapy, ADT | 1995–2002 | Mortality (vs never smoked) Former: RR 2·15, p=0·007 Current: RR 4·27, p<0·001 Cancer-specific mortality (vs never smoked) Former: p=0·19 Current: p=0·48 Recurrence (vs never smoked) Former: RR 0·95, p=0·43 Current: RR 2·10, p=0·04 |
| Pickles et al (2004)56 | 601 | Radiotherapy | 1994–97 | Mortality, 6-year (vs all other patients) Current: RR 2·38, p=0·009 Cancer-specific mortality (vs all other patients Current: RR 2·89, p=0·08 Recurrence (vs all other patients) Current: OR 1·68 (95% CI 1·11–2·56) |
| Taira et al (2011)57 | 1656 | Radiotherapy, ADT | NA | Mortality (vs never smoking) Former: HR 1·43, p=0·017 Current: HR 2·86, p<0·001 |
|
| ||||
| Breast cancer | ||||
|
| ||||
| Gold et al (2006)58 | 2198 (stage I–IIIA) | Tamoxifen (WHEAL study) | 1995–2000 | Vasomotor toxicity (vs never smoking) Former: OR 1·40 (95% CI 1·10–1·78) Current: OR 2·12 (95% CI 1·19–3·78) |
| Li et al (2009)59 | 1091 (ER positive) | Systemic therapy, radiotherapy, chemotherapy | 1990–2007 | Lung cancer (vs never smoking) Former smoker at diagnosis: OR 1·2 (95% CI 0·8–1·7) Current smoker at diagnosis: OR 1·8 (95% CI 1·1–3·2) Former smoker at most recent follow-up: OR 1·2 (95% CI 0·8–1·7) Current smoker at most recent follow-up: OR 2·2 (95% CI 1·2–4·0) |
| Obedian et al (2000)*60 | 1029 (early stage) | Systemic therapy, radiotherapy, | 1970–90 | Lung cancer (vs never smoking) Former: RR 7·01 Current: RR 8·96, p=0·06 |
|
| ||||
| Other cancers | ||||
|
| ||||
| Eifel et al (2002)*61 | 3489 (stage I–II cervical cancer) | Radiotherapy | 1960–94 | Pelvic complications (vs never smoking) Former: HR 1·40 (95% CI 0·81–2·41) Current: <1 PPD: HR 1·25 (95% CI 0·88–1·79) >1 PPD: HR 2·43 (95% CI 1·95–3·04) |
| Gilbert et al (2003)*62 | 592 (Hodgkin’s disease) | Radiotherapy | 1965–94 | Lung cancer (vs never smoking) Former: RR 6·8 (95% CI 2·8–19·5) Current: RR 24·0 (95% CI 10·3–68) |
| McCleary et al (2010)63 | 1045 (stage III colon cancer) | Systemic therapy, chemotherapy (CALGB 89803 trial) | ·· | Mortality (vs never smoking) Former: HR 1·17 (95% CI 0·87–1·57) Current: HR 1·38 (95% CI 0·87–2·18) Recurrence Former: HR 1·15 (95% CI 0·89–1·48) Current: HR 0·90 (95% CI 0·58–1·41) |
| Park et al (2007)64 | 14 181 (male head and neck, lung, esophageal, laryngeal, oral, kidney, bladder, pancreas, liver, gallbladder, and prostate cancer) | Systemic therapy, radiotherapy, chemotherapy (NHIC study) | 1996–2002 | Second primary cancer (vs never smoking) All cancers: Former: RR 0·87 (95% CI 0·56–1·35) Current: RR 1·13 (95% CI 0·77–1·67) Smoking related: Former: RR 1·03 (95% CI 0·46–2·31) Current: RR 2·02 (95% CI 1·02–4·03) |
95% CI given when available.
Patients treated with surgery, chemotherapy, or radiotherapy included.
NA=not available. ADT=androgen deprivation therapy. RR=relative risk. CaPSURE=Cancer of the Prostate Strategic Urologic Research Endeavor. NHIC=national health insurance corporation. NSCLC=non-small cell lung cancer. HR=hazard ratio. RTOG=Radiation Therapy Oncology Group. NS=not statistically significant. PPD=pack per day. SCLC=small-cell lung cancer. WHEAL=Women’s Healthy Eating and Living Study. ER=oestrogen receptor.
Studies in which tobacco information was captured through a non-standardised assessment or through patients’ chart reviews.
The effect of current smoking on mortality in patients with cancer was reported in 15 studies with 12 (80%) showing that smoking increases overall mortality38,39,44,46,48–51,53,55–57 and three showing no association (table).40,43,63 Several other studies show that mortality is increased in patients who are current smokers compared with patients who are former smokers and those who were never smokers combined. The relative risk (RR) of mortality was increased for patients with head and neck cancer who were current smokers (2·34, 95% CI 1·56–3·50) compared with all other patients in a report on 504 patients with stage III–IV head and neck cancer treated in one of two phase 3 randomised trials.49 Khuri and colleagues50 showed increased risk of mortality in patients who were current smokers compared with those who were former smokers (RR 1·6, 95% CI 1·23–2·07) and those who had never smoked (2·51, 95% CI 1·54–4·10).50 In patients with prostate cancer who are current smokers, mortality is increased compared with those who are former smokers55,57 and in comparison with patients who are former smokers or who have never smoked combined.56 The effects of smoking on mortality are perhaps best shown in a series of 1354 patients with prostate cancer who received radiotherapy with or without androgen deprivation therapy, and in whom current smoking increased the risk of death due to heart disease (relative risk [RR] 3·05, p=0·007), non-prostate cancer related mortality (RR 5·52, p=0·002), and other causes (RR 4·09, p=0·002) with no significant effect on prostate cancer mortality.53 However, deaths not caused by prostate cancer accounted for 92% of overall mortality. The authors of the study note that high rates of cancer control are possible, but that implementation of healthy lifestyle programmes including smoking cessation is important to consider in prostate cancer management.
Current smoking also increases the risk of recurrence and cancer-specific mortality.49,55,56 Increased recurrence and cancer-related mortality are probably closely linked to changes in tumour biology leading to a decreased response to cytotoxic therapy (figure). Smoking can alter cancer-drug metabolism that might lead to changes in therapeutic efficacy or toxicity.66 As cigarette smoke is potently tumorigenic, several studies have shown that current smoking significantly increases the risk of the patient developing a second primary cancer,45,47,54,59,62 particularly second tobacco-related primary cancers.64 Notably, some studies suggest that smoking confers an additive or synergistic risk of development of a second primary cancer when combined with chemotherapy or radiotherapy.54 As a result, patients who are smokers are at increased risk of mortality from recurrence and progression of both primary and secondary cancers.
Quitting smoking seems to improve outcomes in patients with cancer. Those with lung cancer who quit smoking at, or after, diagnosis have a reduced risk of developing a second cancer compared with patients who continue smoking.45,47 Patients who quit smoking after diagnosis also have reduced toxicity associated with cancer treatment.52 Data indicate that patients who smoke less have reduced toxicity due to radiotherapy compared with those who are current heavy smokers.61 Substantial work is needed to clarify the time-dependent and dose-dependent benefits of smoking cessation. However, in 205 patients with head or neck cancer receiving radiotherapy, 43% of patients who smoked and were treated in the morning had grade 3 or higher mucositis compared with 76% of patients who smoked who were treated in the afternoon (p=0·04), suggesting that some of the effects of smoking on toxicity might be reduced within hours.67
Addressing tobacco use in patients
Clinical guidelines and clinical practice
Recent statements from the American Society of Clinical Oncology (ASCO)36 and American Association for Cancer Research (AACR)37 provide strong support and guidance (panel 2).
Panel 2. AACR guidelines for addressing tobacco use in patients with cancer37.
“Patients with cancer from all clinical settings, participants in therapeutic cancer clinical trials, and cancer screening patients who use tobacco or have recently quit (past 30 days) should be provided with evidence-based tobacco cessation assistance. Ideally, that assistance capacity should be within or associated with the oncology practice. Even if the assistance is provided through an external service, the cancer patient’s oncology service provider should assume responsibility for ensuring that the patient receives appropriate care. That capacity can also be supplemented by telephone cessation quitlines in all 50 states that can be reached via a common toll-free telephone number (800-QUIT-NOW). Tobacco use should be comprehensively and repeatedly documented for all patients so that the confounding effects of tobacco on cancer treatment, disease progression, comorbid events, and survival can be evaluated in all oncology clinical trials, from registration to survival endpoints, and in all clinical cancer settings.”
AACR=American Association for Cancer Research
The Tobacco Cessation Guide for Oncology Providers was recently released by ASCO68 to provide guidelines for tobacco cessation support in patients with cancer based on the 5-As model of tobacco cessation from the 2008 Public Health Service (PHS) guidelines.35 In brief, ASCO recommends that clinicians should ask patients about tobacco use at every visit, advise patients of the benefits of tobacco cessation, assess the patient for willingness to quit, assist patients in quitting tobacco use with evidence-based support approaches, and arrange follow-up. Unfortunately, two large recent surveys show that although roughly 90% of oncologists ask patients about tobacco use and about 80% advise patients, only about 40% of oncologists regularly provide assistance to patients to quit smoking.3,4 In view of the recent evidence from the 2014 Surgeon General’s Report, this unfortunate lack of tobacco cessation support for patients with cancer can now be viewed as a deficit in addressing evidence-based medicine.
There are several important considerations in the design and implementation of a sustainable clinical tobacco cessation programme. Variables in a tobacco cessation programme can be integrated into the design and implementation of a structured tobacco-use assessment and cessation approach for patients with cancer (panel 3). Clinicians should consider how tobacco cessation can be delivered, including methods of screening patients for tobacco use, methods of tobacco cessation support, which health-care providers will deliver tobacco-cessation support, and in what setting support can be delivered. Clinicians could further consider a partnership with institutional and community resources. All patients should be screened and we advise that all at-risk patients should receive tobacco cessation support, but this needs participation and support from physicians, clinical staff, and administrators to facilitate the development of a structured tobacco cessation programme to merge with ongoing clinical efforts. This strategy would allow clinicians to then implement tobacco cessation support and tailor a tobacco cessation plan for each patient. We also suggest that clinicians move away from a so-called one size fits all approach and that building a sustainable treatment strategy will need the design of an efficient and clinically effective tobacco cessation programme that integrates well with other aspects of clinical cancer treatment.
Panel 3. Conceptual design of a tobacco cessation programme for patients with cancer: creation of a tobacco-cessation support approach.
Method of screening
Electronic
Paper
By physicians or support sta3
Biochemical testing
Method of tobacco cessation support
Behavioural counselling
Pharmacotherapy
Delivery of tobacco cessation support
By phone, web, or in-person
In-person in a cancer clinic
In-person in a separate clinic
Providers for tobacco cessation support
Physicians
Non-physician health-care professionals
Dedicated tobacco cessation specialists
Obtaining institutional support
Screening for all patients
Treatment or referral of all patients for evidence-based tobacco cessation support
Support for referring physicians, clinical staff, and administrators
Financial support for tobacco cessation programme
Incorporating tobacco-use assessment and tobacco cessation into standard clinical practice
Communication among administrators, clinicians, cessation providers, and patients
Considerations for implementing the 5-As in patients with cancer
Ask about tobacco use repeatedly at initial consultation, treatment, and follow-up
-
Assess willingness to quit
Recommend an immediate quit or choose a quit date in the near future
Prepare patient
-
Advise patient to stop smoking
Cite adverse effects of tobacco on cancer treatment outcomes
Remind patient of adverse health effects of tobacco on non-cancer outcomes such as heart disease, stroke, wound healing, pulmonary disease, etc
Tailor to needs of the patient; consider the treatment plan, diagnosis, and expected outcome
-
Assist patient
Motivational interviews, behavioural counselling, and evidence-based pharmacotherapy
Direct tobacco cessation assistance
Referral to a tobacco cessation specialist
-
Arrange follow-up for patients
By phone or in-person
Consider cancer treatment plan and urgency of cancer treatment
Consistent communication among patients, clinicians, and referring physicians
Behavioural counselling, pharmacotherapy, and electronic cigarettes
Methods of tobacco cessation support for the general population have been discussed extensively, and are systematically reviewed and defined in the 2008 Public Health Service Guidelines.35 The core elements of tobacco cessation support for patients rely on repeatedly addressing tobacco use through motivational and behavioural counselling, accompanied by pharmacotherapy to promote behavioural change. Patient counselling sessions that consist of brief interventions and counselling sessions that involve more detailed and intense counselling are effective, although more intense counselling is generally associated with high tobacco cessation rates. Pharmacotherapy is a proven method to improve the efficacy of tobacco cessation programmes, and many patients will need several attempts to successfully quit smoking. Nicotine-replacement therapy is the most common pharmacotherapy used in the USA in the form of lozenges, nasal sprays, gum, inhalers, and longacting patches. The proper use of these medications is important to promote effective pharmacological replacement. For example, nicotine gum should be chewed slowly to allow absorption of nicotine in the appropriate dose and timeframe. Varenicline, bupropion, and cytisine have also been used with efficacious results, although cytisine is not currently used for tobacco cessation in the USA.69
The use of electronic cigarettes is increasing among the general population and in patients with cancer. There are several brands and designs of these products designed to deliver nicotine through a device that may resemble the shape and feel of a cigarette. However, these products are not well regulated and the true nicotine content and nicotine delivery rate vary substantially.70 Electronic cigarettes have serious implications for worldwide tobacco use as a potential substitute for combustible tobacco, but could also be potentially addictive for non-smokers who begin to use them. The results of a trial of electronic cigarettes versus nicotine patches in aiding tobacco cessation were recently published,71 suggesting that electronic cigarettes have the potential to help patients to quit smoking,71 but the authors of the study noted that the power of the study and analyses were limited, yielding quit rates lower than those commonly associated with a structured tobacco cessation support programme using PHS guidelines.35
A recent publication from the Tobacco Control Committee of IASLC has provided guidance for clinicians regarding e-cigarette use in patients with cancer.72 Currently, there are no clear data supporting the use of electronic cigarettes as an aid to tobacco cessation in patients with cancer. The effects of electronic cigarettes on cancer-cell biology, treatment efficacy, therapy-related toxicity, and patient survival have not been evaluated and there are no published data to confirm that electronic cigarettes are more or less toxic for patients with cancer than are tobacco products. However, the broad adverse effects of smoking on cancer treatment are now well documented.1 Clinicians are urged to consider the recommendations from IASLC72 for patients with cancer (panel 4).
Panel 4. IASLC guidance on e-cigarettes for patients with cancer72.
“There are currently no evidence-based guidelines to support the recommendation of e-cigarettes. Whereas evidence-based cessation strategies should be used wherever possible, clinicians should consider the strong need for cancer patients to stop smoking as soon as possible to promote the most effective outcomes of cancer therapy. In the absence of sufficient evidence that e-cigarettes are effective and safe for treating nicotine dependence in cancer patients, the International Association for the Study of Lung Cancer advises against recommending their use at this time. However, this recommendation may change if new data become available. The International Association for the Study of Lung Cancer does recommend that research be done to evaluate the safety and efficacy of e-cigarettes as a cessation treatment in cancer patients to help guide clinical practice. For individual patients who are either using or planning to use e-cigarettes despite advice not to do so, they should be offered evidence-based stop smoking treatments while monitoring for any adverse effect of e-cigarette use.”
IASLC=International Association for the Study of Lung Cancer
Assessment of tobacco use in patients with cancer
An estimated 30–50% of patients with cancer are smokers at the time of cancer diagnosis, with higher rates of smoking among patients with disease sites for traditionally tobacco-related cancers such as head and neck or lung cancer and lower rates of smoking among patients with disease sites for traditionally non-tobacco-related cancer such as breast or prostate cancer.73 There are no current standardised national or international recommendations for the assessment of tobacco use in patients with cancer, thus these estimates probably reflect some bias in the method of assessment. Guidance has been provided for the inclusion of specific questions to assess a patient’s history of tobacco use and their current tobacco use status in patients with cancer.74–75 We recommend that patients should be asked questions that specifically define their current tobacco use status (current, former, never), their history of tobacco use, amount and frequency of tobacco use, the degree of tobacco dependence, and current use of tobacco cessation medications. In clinical practice, however, patients might be reluctant to answer a broad base of questions about previous tobacco use and might be reluctant to accurately report tobacco use patterns in the presence of family members or friends. Studies also show that patients can be stigmatised due to tobacco use and specific cancer diagnoses such as lung cancer.76 A recent study77 of tobacco use screening questions in nearly 12 000 patients with cancer shows that just a few questions at diagnosis and in follow-up can yield effective referral rates to dedicated tobacco cessation programmes. Moreover, repetition of assessments monthly at follow-up seemed to result in few delayed referrals to a dedicated tobacco cessation programme. This recent study has yet to be duplicated, but suggests that clinically efficient systems can be developed to accurately screen patients for tobacco use and refer patients to dedicated tobacco cessation support programmes.
Consideration of the intensity of tobacco cessation support
Several notable programmes in the USA have developed rigorous tobacco cessation support services, including programmes at the MD Anderson Cancer Center, Mayo Clinic, and the Memorial Sloan Kettering Cancer Center, with commendable quit rates seen in patients with cancer. These programmes generally incorporate intensive assessments, behavioural counselling, pharmacotherapy, and follow-up to achieve quit rates of greater than 30%.78,79 Importantly, these successful programmes have removed clinical tobacco cessation support from the duties of the treating oncologist and placed that responsibility within a centralised and dedicated programme designed specifically to treat tobacco dependence. These effective specialised and intensive programmes have highly trained individuals and are integrated into existing clinical cancer care programmes.
Many institutions might not be able to provide resources for dedicated tobacco cessation support. Clinicians could take on the role of providing tobacco cessation support directly or identifying available external resources. This approach, however, should not deter clinicians from providing evidence-based tobacco cessation support. For example, behavioural counselling even for a few minutes can significantly increase quit rates in smokers.35 In general, more intensive tobacco cessation support yields higher quit rates, as evidenced in the general population by numerous publications.1,35 The results of a meta-analysis80 of tobacco cessation in patients admitted to hospital showed that high-intensity, long-term behavioural inter ventions initiated during hospital stay increased the rate of smoking cessation whereas low-intensity, short-duration interventions did not. In another meta-analysis81 of smoking cessation interventions compared with usual care in patients with cancer, the results did not show a significant difference with either short-term or long-term follow-up (pooled odds ratio [OR] 1·54, 95% CI 0·909–2·64, and pooled OR 1·31, 95% CI 0·93–1·84, respectively). However, studies using combination therapy (pharmacological and non-pharmacological) improved quit rates (pooled OR 1·4, 95% CI 1·06–1·87) and perioperative interventions also showed a strong effect favouring the perioperative period compared with the clinic (pooled OR 2·31, 95% CI 1·32–4·07).81 Despite heterogeneous patient populations and study designs, data support the recommendation that all patients should have access to structured tobacco cessation support beginning at the time of cancer diagnosis. Clinicians who refer patients to external tobacco cessation support programmes should ideally receive updates on tobacco cessation activities from patients and note progress by patients to reduce and ultimately eliminate their use of tobacco.
For patients with cancer treated in a community environment, rather than in a comprehensive cancer treatment centre or other large institution, tobacco cessation programmes might be even more difficult to access. In the USA, the state quitlines are resources that are available by calling 1-800-QUIT-NOW, whereby individuals are automatically connected to tobacco cessation support resources in any state. Unfortunately, many international community oncology practices might not have access to universally available tobacco cessation resources. Community oncologists could also consider obtaining continuing medical education credits in tobacco cessation support, which is offered on-line or in-person. Large group-oncology practices could consider pooling resources to establish a specialist tobacco cessation unit within their management group, local hospital, or in the community for a direct referral resource. Panel 3 and the ASCO toolkit68 provide information to consider in the development and implementation of community-based tobacco cessation support resources for patients with cancer.
Discussion of tobacco use and tobacco cessation with patients
We recommend that clinicians explain empathetically to patients why tobacco cessation is important for their treatment and survival. The 2014 Surgeon General’s Report concludes that continued smoking causes adverse outcomes in patients with cancer, specifically, increased overall mortality, cancer-specific mortality, and second primary cancer.1 Evidence suggests that smoking increases the risk of cancer recurrence and treatment toxicity. However, the clinical toxicity and expected outcomes of cancer treatment varies substantially according to the diagnosis. For example, breast cancer treatment is very different from treatment for head and neck cancer, but patients with either cancer are at an increased risk of mortality, comorbid disease, poor surgical wound healing, adverse effects from systemic therapy, risk of second primary cancer, and poor cosmetic outcome. Patients with breast cancer can tolerate treatment with few adverse effects, are generally able to maintain nutritional requirements, can usually continue daily activities such as a regular work schedule, and might not feel any stigma associated with their diagnosis. By contrast, patients with head and neck cancer might have severe adverse effects from treatment, which causes deficits in maintaining nutrition, absence from work, substantial augmentation of pain, and patients might experience stigmatisation from a seemingly tobacco-related cancer diagnosis. Psychiatric comorbidities (anxiety, depression) and substance use (alcohol) might also differ in patient populations. Clinicians can personalise and tailor the approach to tobacco use and cessation in patients with cancer for whom different clinical outcomes are anticipated.
Search strategy and selection criteria.
A comprehensive review of the literature is given in the 2014 Surgeon General’s Report.1 We used the same general criteria used to identify studies evaluating the effect of current smoking at cancer diagnosis on mortality, toxicity, and risk of second primary cancer in patients treated with chemotherapy and radiotherapy. Published manuscripts that reported the effect of current smoking on one or more of mortality, recurrence, toxicity, second primary cancer were identified. PubMed was searched for studies using the search terms “smoking” and “cancer” with additional search terms according to disease site (appendix). A total of 622 works published between 1990–2012 that reported on the effects of smoking on one or more clinical outcomes in patients with cancer (mortality, toxicity, second primary cancer) were identified. Papers that did not clearly define current smoking, report on any clinical effects of current smoking (as opposed to ever smoking), or specify chemotherapy or radiotherapy were excluded. Studies with fewer than 500 patients were also excluded, a total of 27 studies, reporting on more than 54 000 patients met the criteria (table).
Clinicians might also wish to consider how the message is delivered. So-called gain-framed statements are based on presentation of the benefits and positive aspects associated with a treatment or change in behaviour, and loss-framed statements present the negative aspects of treatment or behavioural change. For example, a statement such as, “quitting smoking will improve your chances of survival” is a gain-framed approach whereas a statement such as, “if you don’t quit smoking, you may have a poor cancer outcome” is a loss-framed approach. Messaging is largely untested in patients with cancer with respect to tobacco use, but loss-framed messaging is a common tool to motivate people in the general population to stop smoking.82 Though gain-framed versus loss-framed messaging has not been explored in relation to tobacco use in patients with cancer, gain-framed messaging might be important to consider in the screening and treatment of these patients.83
Clinicians might feel that patients with cancer with either early stage or curable disease are the ideal groups on whom smoking cessation efforts should be focussed. The 2014 Surgeon Gerneral’s Report reviews a wealth of studies, showing that smoking increases mortality in patients with both early and advanced or metastatic cancer.1 Several studies show that smoking increases the risk of hospital admission, infection, pulmonary complications, wound infections, and decreases patients’ quality of life. The studies presented in the 2014 Surgeon General’s Report might cover several disease sites and modalities of cancer treatment, but these data also suggest that smoking affects clinical outcomes in the care of patients with advanced and metastatic cancer. We are not aware of any studies that provide a comprehensive assessment of the benefits of tobacco cessation across disease sites, disease stage, and stage of treatment. However, clinicians should consider the potential benefits of smoking cessation even in patients with metastatic and recurrent cancers possibly to improve survival, but also possibly to reduce complications in cancer care at the end of life.
Design of tobacco cessation support in clinical practice and research
All patients should receive tobacco cessation support to improve therapeutic outcomes, but clinicians could also consider the integration of tobacco use assessment and tobacco cessation into research programmes.37 Screening patients using paper-based systems that capture subjective tobacco use responses might be useful to encourage referrals for tobacco cessation, but the integration of tobacco use assessments and tobacco cessation support using computerised systems (eg, through the electronic medical record) can substantially increase efficiency in identification of forms of tobacco use, referral of patients to dedicated tobacco cessation support programmes, and tracking of the efficacy of tobacco cessation in patients. Examples of annotated design systems have been recommended and implemented in clinical practice for patients with cancer.74,77 We also suggest that clinicians consider the imple mentation of assessment of tobacco use and tobacco cessation approaches that promote effective tobacco cessation, yet also maintain efficient clinical workflow for other aspects of cancer care. An understanding that smoking cessation is a crucial part of the overall effectiveness of cancer treatment is important for patients.
Societal influences on tobacco cessation support
An often overlooked variable to consider is the social and medical environment in which tobacco cessation is approached. The WHO Framework Convention on Tobacco Control (FCTC) is an effort started in 2003 to reduce the international health burden attributed to tobacco. A summary of the FCTC is provided in a recent review,84 in which the efforts of the tobacco industry to manipulate worldwide tobacco control are highlighted. In some countries, tobacco is increasingly regulated, resulting in a decrease in the prevalence of tobacco use and changes in social norms with smoking no longer seen as socially acceptable but as unacceptable.1 The prevalence of tobacco use, however, is increasing in low-income and middle-income countries. Approaches to tobacco cessation in specific countries, regions, cities, and even clinics might necessitate knowledge of the effect of the tobacco industry on social and medical resources. Reimbursement might be important in medical systems in which there is third party billing. High rates of tobacco use by physicians in some high-income and low-income or middle-income countries might deter implementation of tobacco cessation programmes as standard clinical care for patients with cancer.85 In low-income and middle-income countries, tobacco cessation is only now becoming a priority in the general population, and has yet to be seen as the main target in patients with cancer or other chronic illness. Integration of tobacco cessation into tuberculosis and HIV care has been called for as a priority on the basis of a parallel set of adverse medical and survival outcomes.86,87
Oncology clinicians should champion tobacco cessation as a method to improve clinical cancer treatment outcomes for patients. Clinicians are also encouraged to partner with institutional and community resources to develop a unified approach to the assessment of tobacco use and cessation for patients with cancer at diagnosis, during treatment, and throughout follow-up.
Conclusions
The evidence presented in the 2014 Surgeon General’s Report warrants a substantial change in evidence-based cancer care. Smoking causes adverse outcomes in patients with cancer, in part through the activation of tumorigenic pathways and in part through alterations in physiology that lead to complications associated with cancer treatment and continued development of comorbid disease. There are currently no cancer treatments shown to produce superior results in patients with cancer who smoke compared with those who do not smoke and there are no biomarkers to predict adverse outcomes in patients with cancer who continue to smoke. Tobacco use by patients with cancer should be accurately identified and all at-risk patients should be offered structured tobacco cessation support. On the basis of the findings in the 2014 Surgeon General’s Report, addressing tobacco use in patients with cancer has the potential to substantially improve overall cancer treatment outcomes through reduced treatment-related toxicity, treatment failure, and comorbid disease and improved survival.
Supplementary Material
Acknowledgments
This work was supported in part by the American Cancer Society (MRSG-11-031-01-CCE to GWW) and the National Cancer Institute (P30-CA16672 to ERG).
Footnotes
Contributors
All authors contributed equally to the conceptualisation, design, writing, and final approval of the Review.
Declaration of interests
We declare no competing interests.
References
- 1.US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Land SR. Methodologic barriers to addressing critical questions about tobacco and cancer prognosis. J Clin Oncol. 2012;30:2030–32. doi: 10.1200/JCO.2012.41.7402. [DOI] [PubMed] [Google Scholar]
- 3.Warren GW, Marshall JR, Cummings KM, et al. Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol. 2013;8:543–8. doi: 10.1097/JTO.0b013e318288dc96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: a survey of American Society of Clinical Oncology members. J Oncol Pract. 2013;9:258–62. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorne D, Adamson J. A review of in vitro cigarette smoke exposure systems. Exp Toxicol Pathol. 2013;65:1183–93. doi: 10.1016/j.etp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Shields PG. Tobacco smoking, harm reduction, and biomarkers. J Natl Cancer Inst. 2002;94:1435–44. doi: 10.1093/jnci/94.19.1435. [DOI] [PubMed] [Google Scholar]
- 7.Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biocim Biophys Acta. 2013;1836:197–210. doi: 10.1016/j.bbcan.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Portal-Nuñez S, Shankavaram UT, Rao M, et al. Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice. Cancer Res. 2012;72:5790–800. doi: 10.1158/0008-5472.CAN-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uppstad H, Osnes GH, Cole KJ, Phillips DH, Haugen A, Mollerup S. Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer. 2011;71:264–70. doi: 10.1016/j.lungcan.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Mathur A, Zhang Y, et al. Mithramycin represses basal and cigarette smoke-induced expression of ABCG2 and inhibits stem cell signaling in lung and esophageal cancer cells. Cancer Res. 2012;72:4178–92. doi: 10.1158/0008-5472.CAN-11-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain M, Rao M, Humphries AE, et al. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res. 2009;69:3570–78. doi: 10.1158/0008-5472.CAN-08-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi S, Xu H, Shan J, et al. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J Clin Invest. 2013;123:1241–61. doi: 10.1172/JCI61271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P, Wu WK, Wong HP, Zhang ST, Yu L, Cho CH. Chloroform extract of cigarette smoke induces proliferation of human esophageal squamous-cell carcinoma cells: modulation by beta-adrenoceptors. Drug Chem Toxicol. 2009;32:175–81. doi: 10.1080/01480540902875253. [DOI] [PubMed] [Google Scholar]
- 14.Liu ES, Shin VY, Ye YN, Luo JC, Wu WK, Cho CH. Cyclooxygenase-2 in cancer cells and macrophages induces colon cancer cell growth by cigarette smoke extract. Eur J Pharmacol. 2005;518:47–55. doi: 10.1016/j.ejphar.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–34. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 16.Ye YN, Wu WK, Shin VY, Cho CH. A mechanistic study of colon cancer growth promoted by cigarette smoke extract. Eur J Pharmacol. 2005;519:52–57. doi: 10.1016/j.ejphar.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Newland N, Richter A. Agents associated with lung inflammation induce similar responses in NCI-H292 lung epithelial cells. Toxicol In Vitro. 2008;22:1782–88. doi: 10.1016/j.tiv.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem. 2003;278:26202–07. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- 19.Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L420–27. doi: 10.1152/ajplung.00019.2004. [DOI] [PubMed] [Google Scholar]
- 20.Filosto S, Baston DS, Chung S, Becker CR, Goldkorn T. Src mediates cigarette smoke-induced resistance to tyrosine kinase inhibitors in NSCLC cells. Mol Cancer Ther. 2013;12:1579–90. doi: 10.1158/1535-7163.MCT-12-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filosto S, Becker CR, Goldkorn T. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther. 2012;11:795–804. doi: 10.1158/1535-7163.MCT-11-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samanta D, Gonzalez AL, Nagathihalli N, Ye F, Carbone DP, Datta PK. Smoking attenuates transforming growth factor-β-mediated tumor suppression function through downregulation of Smad3 in lung cancer. Cancer Prev Res (Phila) 2012;5:453–63. doi: 10.1158/1940-6207.CAPR-11-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang X, Ravi R, Pham V, Bedi A, Chatterjee A, Sidransky D. Adenylate kinase 3 sensitizes cells to cigarette smoke condensate vapor induced cisplatin resistance. PLoS One. 2011;6:e20806. doi: 10.1371/journal.pone.0020806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratovitski EA. ΔNp63α/IRF6 interplay activates NOS2 transcription and induces autophagy upon tobacco exposure. Arch Biochem Biophys. 2011;506:208–15. doi: 10.1016/j.abb.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Di Cello F, Flowers VL, Li H, et al. Cigarette smoke induces epithelial to mesenchymal transition and increases the metastatic ability of breast cancer cells. Mol Cancer. 2013;12:90. doi: 10.1186/1476-4598-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Mao XY, Fan CF, Zheng HC. MTA1 expression correlates significantly with cigarette smoke in non-small cell lung cancer. Virchows Arch. 2011;459:415–22. doi: 10.1007/s00428-011-1141-7. [DOI] [PubMed] [Google Scholar]
- 27.Huynh TP, Mah V, Sampson VB, et al. Na,K-ATPase is a target of cigarette smoke and reduced expression predicts poor patient outcome of smokers with lung cancer. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1150–58. doi: 10.1152/ajplung.00384.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14–231. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren GW, Singh AK. Nicotine and lung cancer. J Carcinog. 2013;12:1. doi: 10.4103/1477-3163.106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuller HM. Regulatory role of the α7nAChR in cancer. Curr Drug Targets. 2012;13:680–87. doi: 10.2174/138945012800398883. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momi N, Ponnusamy MP, Kaur S, et al. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene. 2013;32:1384–95. doi: 10.1038/onc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci USA. 2006;103:6332–37. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren GW, Romano MA, Kudrimoti MR, et al. Nicotinic modulation of therapeutic response in vitro and in vivo. Int J Cancer. 2012;131:2519–27. doi: 10.1002/ijc.27556. [DOI] [PubMed] [Google Scholar]
- 35.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update US Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53:1217–22. [PubMed] [Google Scholar]
- 36.Hanna N, Mulshine J, Wollins DS, Tyne C, Dresler C. Tobacco cessation and control a decade later: American society of clinical oncology policy statement update. J Clin Oncol. 2013;31:3147–57. doi: 10.1200/JCO.2013.48.8932. [DOI] [PubMed] [Google Scholar]
- 37.Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS the AACR Subcommittee on Tobacco and Cancer. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19:1941–48. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florescu M, Hasan B, Seymour L, Ding K, Shepherd FA the National Cancer Institute of Canada Clinical Trials Group. A clinical prognostic index for patients treated with erlotinib in National Cancer Institute of Canada Clinical Trials Group study BR.21. J Thorac Oncol. 2008;3:590–98. doi: 10.1097/JTO.0b013e3181729299. [DOI] [PubMed] [Google Scholar]
- 39.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–99. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 40.Holgersson G, Hoye E, Bergqvist M, et al. Swedish Lung Cancer Radiation Study Group: predictive value of age at diagnosis for radiotherapy response in patients with non-small cell lung cancer. Acta Oncol. 2012;51:759–67. doi: 10.3109/0284186X.2012.681064. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi T, Tamiya A, Tamura A, et al. Chemotherapy is beneficial for elderly patients with advanced non-small-cell lung cancer: analysis of patients aged 70–74, 75–79, and 80 or Older in Japan. Clin Lung Cancer. 2012;13:442–47. doi: 10.1016/j.cllc.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Lee SM, Khan I, Upadhyay S, et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2012;13:1161–70. doi: 10.1016/S1470-2045(12)70412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li YH, Shieh SH, Chen CY. The influence of health behaviors on survival in lung cancer patients in Taiwan. Jpn J Clin Oncol. 2011;41:365–72. doi: 10.1093/jjco/hyq188. [DOI] [PubMed] [Google Scholar]
- 44.Pirker R, Pereira JR, Szczesna A, et al. Prognostic factors in patients with advanced non-small cell lung cancer: data from the phase III FLEX study. Lung Cancer. 2012;77:376–82. doi: 10.1016/j.lungcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Richardson GE, Tucker MA, Venzon DJ, et al. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann Intern Med. 1993;119:383–90. doi: 10.7326/0003-4819-119-5-199309010-00006. [DOI] [PubMed] [Google Scholar]
- 46.Tsao AS, Liu D, Lee JJ, Spitz M, Hong WK. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2006;106:2428–36. doi: 10.1002/cncr.21884. [DOI] [PubMed] [Google Scholar]
- 47.Tucker MA, Murray N, Shaw EG, et al. Second primary cancers related to smoking and treatment of small-cell lung cancer. Lung Cancer Working Cadre. J Natl Cancer Inst. 1997;89:1782–88. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 48.Fortin A, Wang CS, Vigneault E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2009;74:1062–69. doi: 10.1016/j.ijrobp.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–11. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–50. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 51.Meyer F, Bairati I, Fortin A, et al. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int J Cancer. 2008;122:1679–83. doi: 10.1002/ijc.23200. [DOI] [PubMed] [Google Scholar]
- 52.Alsadius D, Hedelin M, Johansson KA, et al. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol. 2011;101:495–501. doi: 10.1016/j.radonc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Bittner N, Merrick GS, Galbreath RW, et al. Primary causes of death after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2008;72:433–40. doi: 10.1016/j.ijrobp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Boorjian S, Cowan JE, Konety BR, et al. Bladder cancer incidence and risk factors in men with prostate cancer: results from Cancer of the Prostate Strategic Urologic Research Endeavor. J Urol. 2007;177:883–87. doi: 10.1016/j.juro.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 55.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Allen ZA, Adamovich E. Androgen-deprivation therapy does not impact cause-specific or overall survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2006;65:669–77. doi: 10.1016/j.ijrobp.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 56.Pickles T, Liu M, Berthelet E, Kim-Sing C, Kwan W, Tyldesley S. The effect of smoking on outcome following external radiation for localized prostate cancer. J Urol. 2004;171:1543–46. doi: 10.1097/01.ju.0000118292.25214.a4. [DOI] [PubMed] [Google Scholar]
- 57.Taira AV, Merrick GS, Butler WM, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:1336–42. doi: 10.1016/j.ijrobp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Gold EB, Flatt SW, Pierce JP, et al. Dietary factors and vasomotor symptoms in breast cancer survivors: the WHEL Study. Menopause. 2006;13:423–33. doi: 10.1097/01.gme.0000185754.85328.44. [DOI] [PubMed] [Google Scholar]
- 59.Li CI, Daling JR, Porter PL, Tang MT, Malone KE. Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor-positive invasive breast cancer. J Clin Oncol. 2009;27:5312–18. doi: 10.1200/JCO.2009.23.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obedian E, Fischer DB, Haffty BG. Second malignancies after treatment of early-stage breast cancer: lumpectomy and radiation therapy versus mastectomy. J Clin Oncol. 2000;18:2406–12. doi: 10.1200/JCO.2000.18.12.2406. [DOI] [PubMed] [Google Scholar]
- 61.Eifel PJ, Jhingran A, Bodurka DC, Levenback C, Thames H. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651–57. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert ES, Stovall M, Gospodarowicz M, et al. Lung cancer after treatment for Hodgkin’s disease: focus on radiation effects. Radiat Res. 2003;159:161–73. doi: 10.1667/0033-7587(2003)159[0161:lcatfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.McCleary NJ, Niedzwiecki D, Hollis D, et al. Impact of smoking on patients with stage III colon cancer: results from Cancer and Leukemia Group B 89803. Cancer. 2010;116:957–66. doi: 10.1002/cncr.24866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SM, Lim MK, Jung KW, et al. Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National Health Insurance Corporation Study. J Clin Oncol. 2007;25:4835–43. doi: 10.1200/JCO.2006.10.3416. [DOI] [PubMed] [Google Scholar]
- 65.Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51:875–983. doi: 10.3109/0284186X.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petros WP, Younis IR, Ford JN, Weed SA. Effects of tobacco smoking & nicotine on cancer treatment. Pharmacotherapy. 2012;32:920–31. doi: 10.1002/j.1875-9114.2012.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bjarnason GA, Mackenzie RG, Nabid A, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3) Int J Radiat Oncol Biol Phys. 2009;73:166–72. doi: 10.1016/j.ijrobp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 68.American Society of Clinical Oncology. [accessed Feb 21, 2014];Tobacco cessation guide for oncology providers. http://www.asco.org/sites/default/files/tobacco_cessation_guide.pdf.
- 69.Prochaska JJ, Das S, Benowitz NL. Cytisine, the world’s oldest smoking cessation aid. BMJ. 2013;347:f5198. doi: 10.1136/bmj.f5198. [DOI] [PubMed] [Google Scholar]
- 70.Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109:500–07. doi: 10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- 71.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–37. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 72.Cummings KM, Dresler CM, Field JK, et al. E-cigarettes and cancer patients. J Thorac Oncol. 2014;9:438–41. doi: 10.1097/JTO.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gritz ER, Lam CY, Vidrine DJ, Fingeret MC. Cancer prevention: tobacco dependence and its treatment. In: Devita VT, Lawrence T, Rosenberg SA, editors. cancer: principles and practices in oncology. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 529–42. [Google Scholar]
- 74.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14:2287–93. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 75.Dresler CM, Gritz ER. Smoking, smoking cessation and the oncologist. Lung Cancer. 2001;34:315–23. doi: 10.1016/s0169-5002(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 76.Cataldo JK, Slaughter R, Jahan TM, Pongquan VL, Hwang WJ. Measuring stigma in people with lung cancer: psychometric testing of the cataldo lung cancer stigma scale. Oncol Nurs Forum. 2011;38:E46–54. doi: 10.1188/11.ONF.E46-E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warren GW, Marshall JR, Cummings KM, et al. Automated tobacco assessment and cessation support for cancer patients. Cancer. 2014;120:562–69. doi: 10.1002/cncr.28440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ostroff JS, Burkhalter JE, Cinciripini PM, et al. Randomized trial of a pre-surgical scheduled reduced smoking intervention for patients newly diagnosed with cancer. Health Psychology. 2014;33:737–47. doi: 10.1037/a0033186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garces YI, Schroeder DR, Nirelli LM, Croghan IT, Foote RL, Hurt RD. Tobacco use outcomes among patients with head and neck carcinoma treated for nicotine dependence. Cancer. 2004;101:116–24. doi: 10.1002/cncr.20350. [DOI] [PubMed] [Google Scholar]
- 80.Rigotti NA, Clar C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database Syst Rev. 2012;5:CD001837. doi: 10.1002/14651858.CD001837.pub2. [DOI] [PubMed] [Google Scholar]
- 81.Nayan S, Gupta MK, Strychowsky JE, Sommer DD. Smoking cessation interventions and cessation rates in the oncology population: an updated systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2013;149:200–11. doi: 10.1177/0194599813490886. [DOI] [PubMed] [Google Scholar]
- 82.Toll BA, Rojewski AM, Duncan LR, et al. “Quitting smoking will benefit your health”: the evolution of clinician messaging to encourage tobacco cessation. Clin Cancer Res. 2014;20:301–09. doi: 10.1158/1078-0432.CCR-13-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Consedine NS, Horton D, Magai C, Kukafka R. Breast screening in response to gain, loss, and empowerment framed messages among diverse, low-income women. J Health Care Poor Underserved. 2007;18:550–66. doi: 10.1353/hpu.2007.0057. [DOI] [PubMed] [Google Scholar]
- 84.Yach D. The origins, development, effects, and future of the WHO Framework Convention on Tobacco Control: a personal perspective. Lancet. 2014;383:1771–79. doi: 10.1016/S0140-6736(13)62155-8. [DOI] [PubMed] [Google Scholar]
- 85.Smith DR, Leggat PA. An international review of tobacco smoking in the medical profession: 1974–2004. BMC Public Health. 2007;7:115. doi: 10.1186/1471-2458-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Novotny TE. Smoking cessation and tuberculosis: connecting the DOTS. IntJ Tuberc Lung Dis. 2008;12:1103. [PubMed] [Google Scholar]
- 87.Niaura R, Chander G, Hutton H, Stanton C. Interventions to address chronic disease and HIV: strategies to promote smoking cessation among HIV-infected individuals. Curr HIV/AIDS Rep. 2012;9:375–84. doi: 10.1007/s11904-012-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.