Abstract
CKD and ESRD are associated with abnormalities in bone and mineral metabolism collectively known as chronic kidney disease-bone mineral disorder (CKD-MBD). Patients with CKD and ESRD have skeletal abnormalities characterized by hyperparathyroidism, mixed uremic osteodystrophy, osteomalacia and adynamic bone disease. Such patients also frequently have enhanced vascular and ectopic calcification. Hyperparathyroidism and mixed uremic osteodystrophy are the commonest manifestations of CKD-MBD, and are due to phosphate retention, reduced concentrations of 1, 25 dihydroxyvitamin D and intestinal calcium absorption, and negative calcium balance. Treatment with 1-hydroxylated vitamin D analogs is useful in treating secondary hyperparathyroidism and mixed uremic osteodystrophy in CKD/ESRD.
The spectrum chronic kidney disease-mineral bone disorder (CKD-MBD)
Skeletal abnormalities in patients with chronic kidney disease (CKD)
Alterations in skeletal, cardiovascular, and neurological function occur in CKD and end-stage renal disease (ESRD). Abnormalities in bone and mineral metabolism result not only in changes in the skeleton but also alterations in vascular and soft tissue calcification, the entire syndrome being referred to as chronic kidney disease-mineral and bone disorder (CKD-MBD)1. The role of abnormalities in the vitamin D endocrine system is most clearly defined in the pathogenesis of bone disease in CKD/ESRD2–4. The salutary effects of vitamin D analogs are most apparent in the treatment of disorders of CKD/ESRD-associated bone disease, and are of unknown value in the treatment of vascular and soft tissue calcification. When assessed by bone histomorphometry and the rate of bone mineralization, renal osteodystrophy comprises several groups including secondary hyperparathyroidism of varying severity, mixed uremic osteodystrophy, osteomalacia, and adynamic bone disease1, 5–11. Hyperparathyroidism is the most frequent type of renal osteodystrophy and is most responsive to therapy with vitamin D analogs. Although hyperparathyroidism is readily detectable by the time the glomerular filtration rate (GFR) reaches 40–50 mL/minute/1.73m2 in both adults7, 11–13 and children14, 15, histologic changes in bone in the form of abnormal woven osteoid have been described when the GFR has declined to only 80 mL/minute/1.73m2 12. Phosphate retention7, 16–25, a decline in concentrations of the active metabolite of vitamin-D, 1α,25 dihydroxyvitamin-D3 (1α,25(OH)2D)3, 4, 26–33 with an attendant decrease in intestinal calcium absorption34–37, increased fibroblast growth factor 23 concentrations38–43, and diminished acid excretion by the kidney44–46 occur when the GFR has decreased to 30–50 mL/min/1.73m2, and contribute in inter-related ways to the pathogenesis of CKD-MBD. We will briefly review some aspects of vitamin D metabolism (see reviews by Christakos and Pike in this volume, as well as those in other journals47–64) and describe abnormalities that are known to occur in the context of CKD/ESRD. We will subsequently discuss the value of various vitamin-D analogs in the treatment of CKD MBD.
The pathophysiology of the vitamin D endocrine system in CKD/ESRD
The major physiologic role of vitamin D, through the action of its active metabolite 1α,25(OH)2D, is the maintenance of normal calcium and phosphorus balance65–68. Many other biological effects of 1α,25(OH)2D have been described such as the modulation of immune function69, 70, muscle function51, 59, 71, and cell growth and differentiation53, 72, 73 but are not relevant to the pathogenesis of CKD-MBD.
The generation of vitamin D in the skin is impaired in uremia
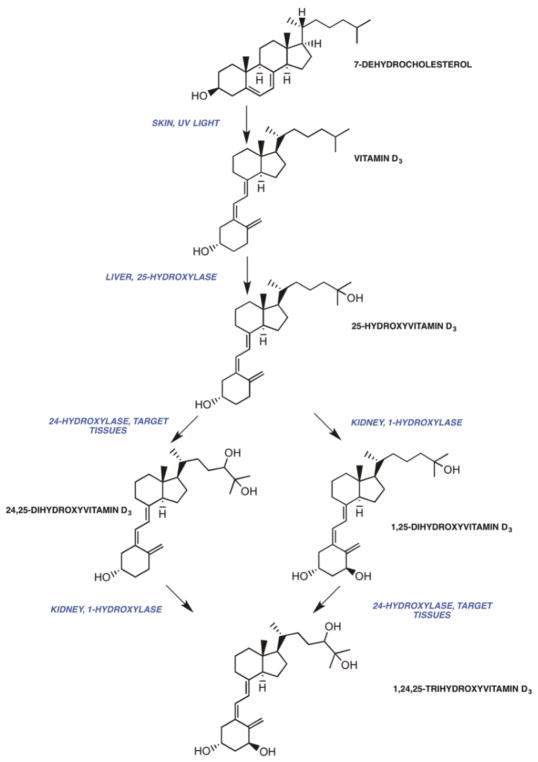
The endogenous form of vitamin D, vitamin D3 (cholecalciferol), is formed in the skin as a result of photolysis of the precursor sterol, 7-dehydrocholesterol (7-DHC)74–82 (Fig. 1). Ultraviolet light cleaves the B-ring of 7-DHC, giving rise to pre-vitamin D3, which undergoes equilibration to vitamin D380–82. Vitamin D3, bound to vitamin D-binding protein (VDBP, or group specific component), to which it preferentially binds relative to its precursor, pre-vitamin D3, exits the skin and enters the circulation81. In plants, a precursor sterol, ergosterol, is converted by photolysis to ergocalciferol or vitamin D283–85. In mammals, vitamin D2 and vitamin D3 have similar metabolic transformations and equivalent bioactivities, and for purposes of this discussion the term vitamin D refers to both vitamin D2 and vitamin D3. The photolytic conversion of 7-DHC to vitamin D3 is impaired in CKD/ESRD in humans86. In uremic white subjects, plasma vitamin D was similar to that seen in normal white subjects but was not detectable in 70% of uremic black subjects studied. Following ultraviolet-B irradiation, the increase in plasma vitamin D was depressed in white dialysis patients when compared to healthy white subjects. 7-DHC content was similar in epidermis from site-matched skin of uremic and normal subjects. The precise reason for the impaired production of vitamin D3 in the skin of uremic subjects is unknown although one might speculate that the accumulation of various pigments might play a role. Low serum concentrations of vitamin D contribute to a decrease in and circulating concentrations of 25-hydroxyvitamin D (25(OH)D).
Figure 1.
The metabolism of vitamin D
25(OH)D concentrations are reduced in CKD/ESRD
Vitamin D is metabolized in the liver microsomes and mitochondria to 25-hydroxyvitamin D3 (25(OH)D) by the vitamin D3-25-hydroxylase87–98 (Fig 1). The vitamin D-25-hydroxylase is only partially inhibited by its product, and increasing amounts of administered vitamin D are associated with increases in 25(OH)D. 25(OH)D is the major circulating vitamin D metabolite65, 78, 94, 99–101, and measurements of its concentration are an excellent index of vitamin D nutritional status102–105. In CKD, 25(OH)D concentrations are frequently diminished and increase upon the administration of vitamin D3106–120. The diminished concentrations of 25(OH)D may be related to a decrease in nutritional intake, a decrease in sun exposure, a reduction in the generation of vitamin D3 from 7-DHC86 or a loss of vitamin D binding protein in the proteinuric patients. Lower levels of 25(OH)D have been associated with increased risk of progression of renal disease and mortality116, 121. 25(OH)D-1-α-hydroxylase enzyme activity is present in tissues other than the kidney122–124 and increased amounts of substrate may result in the generation of 1α,25(OH)2D locally. Therefore, some have advocated the use of vitamin D supplementation in patients with CKD and ESRD. Before 1α,25(OH)2D3 was available for clinical use, 25(OH)D supplementation was used to suppress PTH levels in dialysis patients125. A metaanalysis of observational and randomized controlled trials have shown that vitamin D supplementation results in a small reduction in PTH level in both CKD and dialysis patients113. In a recent randomized controlled trial of 105 dialysis patients comparing two different doses of ergocalciferol with placebo, ergocalciferol did not alter calcium, phosphorous, or PTH levels compared to placebo during a 12-week period126. Given these results, the use of vitamin D supplementation in dialysis patients is not recommended. Vitamin D supplementation can be considered in patients with early stages of CKD where residual 25(OH)D 1-α-hydroxylase enzyme may still be present.
Reduced intestinal calcium absorption and serum 1α,25(OH)2D is present in patients with CKD/ESRD
In CKD and ESRD there is a decrease in the intestinal calcium absorption with concomitant negative calcium balance34–37. Reduced serum concentrations of 1α,25(OH)2D3, 4, 26–33 are present. The central role of 1α,25(OH)2D in maintaining calcium balance in CKD/ESRD is demonstrated by the observations that not only are 1α,25(OH)2D concentrations reduced in CKD/ESRD but that 1α,25(OH)2D readily increases intestinal calcium transport127, 128 and mobilizes calcium from bone129 whereas pharmacological amounts of precursors such as vitamin D itself or intermediary metabolites such as 25(OH)D are required to elicit a biological response in anephric animals and patient27, 129, 130. In the sections that follow we discuss how 1α,25(OH)2D is synthesized, how the processes are regulated, and what perturbations occur in CKD/ESRD that inhibit the formation of 1α,25(OH)2D.
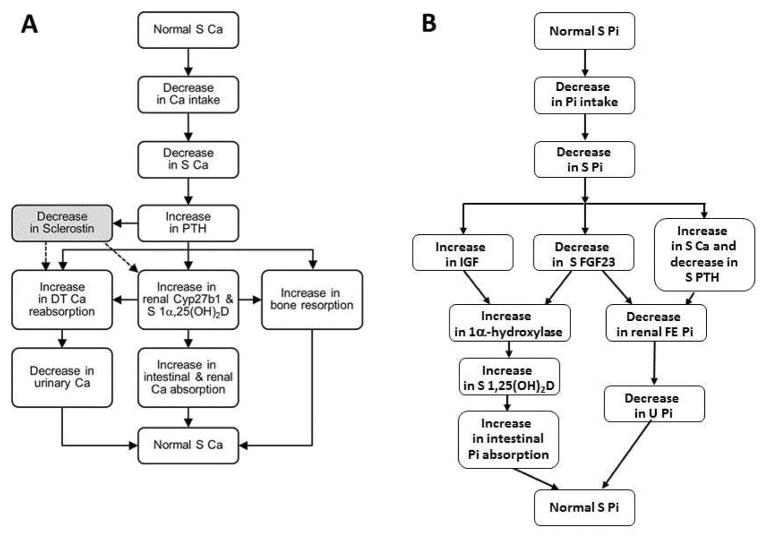
In states of calcium demand, 25(OH)D is metabolized by the renal 25-hydroxyvitamin D-1α-hydroxylase to the biologically active vitamin D metabolite, 1α,25(OH)2D, in the kidney by parathyroid hormone-dependent processes60, 65, 78, 127, 128, 130–139 (Fig 2A). Increased concentrations of 1α,25(OH)2D enhance the expression of genes required for the transport of calcium across the enterocyte140, 141 and in the distal convoluted tubule of the kidney. Calcium is absorbed by the intestine by passive paracellular and active transcellular mechanisms141–143. Active Ca absorption is 1α,25(OH)2D-dependent is transcellular and requires the expenditure of energy144, 145. Several vitamin D dependent proteins, each with a specific function, play a role in the movement of calcium across the apical membrane, the enterocyte cytoplasm and the basal lateral membrane. These include the epithelial calcium or TRPV 5/6 channels (at the apical membrane), calbindin D9K and D28K (within the cell), and the plasma membrane calcium pump (at the basal lateral membrane)146. Deletions of TrpV6 and calbindin D9K genes are not associated with alterations in intestinal Ca transport in vivo in the basal state and following the administration of 1α,25(OH)2D147, 148, although one report suggests that basal Ca transport is normal in TrpV6 knockout mice but adaptations to a low Ca diet are impaired149. Deletion of the Pmca1 in the intestine is associated with reduced growth and bone mineralization, and a failure to up-regulate calcium absorption in response to 1α,25(OH)2D3 thereby establishing the essential role of the pump in transcellular Ca transport150. In states of calcium sufficiency, the 1α,25(OH)2D synthesis is reduced, and the synthesis of 24R,25-dihydroxyvitamin D (24R,25(OH)2D)151–153, a vitamin D metabolite of reduced but uncertain activity, is increased.
Figure 2.
A. Adaptations in response to a decrease in serum calcium concentrations.
B. Adaptations in response to decrease in serum phosphate concentrations.
Serum phosphate concentrations also regulate the synthesis of 1α,25(OH)2D by parathyroid hormone independent mechanisms154. Thus, when serum phosphate concentrations are diminished and in states of phosphorous demand, 25(OH)D is metabolized to 1α,25(OH)2D and the synthesis of 24R,25(OH)2D is reduced64, 133, 154–164 (Fig 2B). The converse occurs in hyperphosphatemic states. A decrease in serum phosphate concentration is associated with an increase in ionized calcium, a decrease in PTH secretion, and a subsequent decrease in renal phosphate excretion. An increase in renal 25-hydroxyvitamin D 1α-hydroxylase activity, increased 1α,25(OH)2D synthesis, and increased phosphorus absorption in the intestine and reabsorption in the kidney occur. In the intestine and kidney, 1α,25(OH)2D regulates the expression of the sodium-phosphate co-transporters IIb, and IIA and IIc, respectively, thereby regulating the efficiency of Pi absorption in enterocytes and proximal tubule cells146, 165–167.
The bioactivity of vitamin D is dependent on the formation of 1α,25(OH)2D. Pharmacological amounts of precursors such as vitamin D itself or intermediary metabolites such as 25(OH)D are required to elicit a biological response in anephric animals and patients27, 129, 130. In such individuals, 1α,25(OH)2D readily increases intestinal calcium transport127, 128 and mobilizes calcium from bone129. The actions of 1α,25(OH)2D3 require the presence of the vitamin D receptor, a steroid hormone receptor, that binds 1α,25(OH)2D3 with high affinity and other vitamin D metabolites with lower affinities168–171. Following binding of the ligand, 1α,25(OH)2D3 to the VDR, a conformational change in the receptor is associated with the recruitment of other steroid hormone receptors such as the RXRα and various co-activator (or co-repressor) proteins to the transcription start side of 1α,25(OH)2D3 regulated genes172–180 (see chapter 1). Several calcium regulating genes are induced or repressed in vitamin D-responsive target tissues such as the intestine, kidney, parathyroid gland and bone67, 71, 181–186.
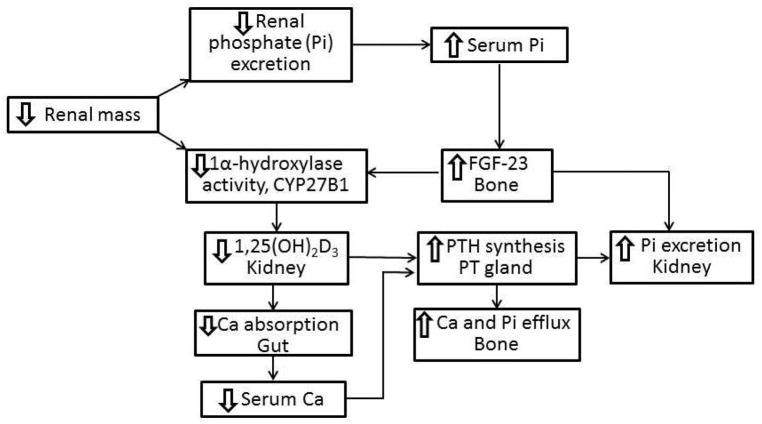
Retention of phosphate and reductions in renal mass are responsible for reduced 1α,25(OH)2D synthesis
As glomerular filtration rate declines (usually less 50 ml/min/SA) hyperphosphatemia develops which is accompanied by hypocalcemia, secondary hyperparathyroidism, a reduction in 1α,25(OH)2D concentrations and an elevation in fibroblast growth factor 23 (FGF23) levels. The retention of phosphate with decreasing GFR21, 25, 187–193 and a reduction in the number of tubular cells2, 4, 26, 106, 194, 195 in which the synthesis of 1α,25(OH)2D occurs are major determinants in evolution of low serum 1α,25(OH)2D concentrations (Fig. 3). Decreased phosphate excretion and increased serum phospate concentrations enhance the production of the phosphaturic factor, FGF2343, 196, which inhibits the production of 1α,25(OH)2D197, 198. Hyperparathyroidism occurs as a result of reduced concentrations of 1α,25(OH)2D and the attendant negative calcium balance from reduced intestinal calcium absorption, and as a result of loss of inhibition of PTH synthesis associated with the low serum concentrations of 1α,25(OH)2D and decreased parathyroid gland VDR concentrations199–209.
Figure 3.
Pathogenesis of secondary hyperparathyroidism in CKD/ESRD
Vitamin D analogs in the treatment of hyperparathyroidism in CKD/ESRD
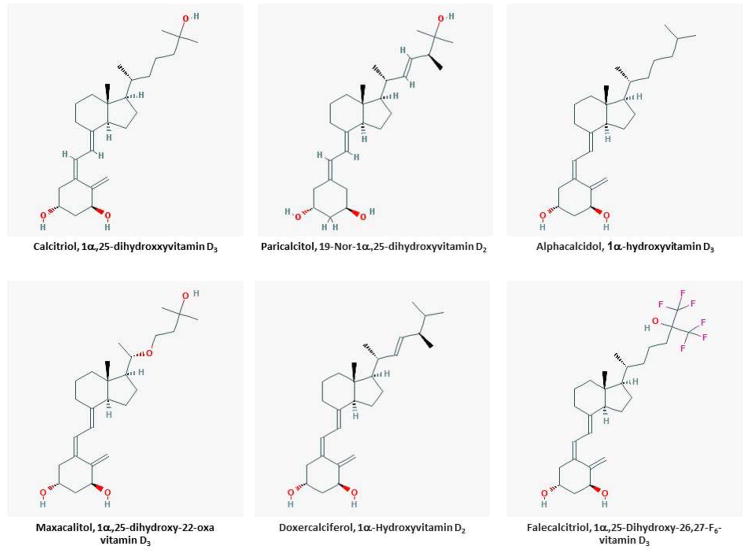
A combined approach aimed at reducing serum phosphate concentrations, improving the negative calcium balance and inhibiting parathyroid hormone secretion is required for the effective treatment of hyperparathyroidism in the context of CKD/ESRD. We favor a stepwise approach of first reducing serum phosphate concentrations with the use of phosphate binders such as sevelamer, calcium salts (e.g. calcium acetate, calcium carbonate), lanthanum carbonate, or various iron preparations (ferric citrate, sucroferric oxyhydride), correcting the negative calcium balance with the use of various 1α-hydroxylated vitamin-D analogs, and finally using calcium sensing receptor agonists such as cinacalcet. In the section that follows we will describe the use of various vitamin-D analogs in the treatment of CKD MBD in the context of CKD/ESRD. The structures of various compounds used in this regard are shown in Figure 4.
Figure 4.
Structures of vitamin D analogs used in the treatment of CKD MBD
Calcitriol (1α,25(OH)2D3)
It is the naturally occurring, active form of vitamin D that is available for use in CKD/ESRD patients. Its primary use is to suppress the PTH gland. An unwanted side effect is increase in calcium and phosphorous levels through its action on the intestine. Both oral and intravenous formulations have been used. Multiple small clinical trials have compared the efficacy of oral vs. intravenous calcitriol in patients with ESRD210–215. A majority of the studies have shown that oral calcitriol is as effective as intravenous calcitriol in reducing PTH concentrations with similar rates of hypercalcemia and hyperphosphatemia. In the most recent meta-analysis of 9 RCT similar findings were noted216. Based on the available data either intravenous or oral formulations are reasonable choices when treating ESRD patients with secondary hyperparathyroidism. The intravenous formulation is more commonly used in the United States whereas the oral formulation is more frequently used in other countries. The intravenous formulation is more expensive but does ensure adherence. These issues need to be taken into account when deciding on what route to choose.
Paricalcitol (1,25(OH)2-19-nor-D2)
This vitamin D2 analog lacks the axis cyclic methylene group on the A ring of the sterol. It was the first analog to be approved for use in patients with CKD. In animal models it is shown to cause equivalent suppression of PTH relative to calcitriol but with less hypercalcemia and hyperphosphatemia217. In uremic rat models, paricalcitol has shown to improve the bone histology218. In placebo-controlled trials of paricalcitol in dialysis patients, 68% of patients treated with paricalcitol achieved 30% reduction in PTH as opposed to 8% in control subjects, and there were few hypercalcemic events recorded in the patients treated with the drug219. Paricalcitol also resulted in a reduction in the bone turnover marker, alkaline phosphatase219. In another placebo-controlled trial, paricalcitol resulted in hypercalcemia mainly in patients who had significant reduction in their PTH concentrations, often with levels less than 100 pg/ml220. In a double blind randomized study comparing calcitriol to paricalcitol in dialysis patients, those treated with paricalcitol had lower PTH levels at the end of the study and achieved target PTH levels faster with fewer episodes of hypercalcemia and lower calcium phosphorous byproducts compared to those treated with calcitriol221. In a large retrospective cohort study of dialysis patients, those treated with paricalcitol were shown to have a survival advantage compared to those treated with calcitriol222 In adjusted models, survival was 16% (95% confidence interval 10–21%) lower in paricalcitol group compared to calcitriol group. The percentage rise in serum calcium and phosphorous was also lower in those who received paricalcitol compared to calcitriol. This survival advantage, however, has not been evaluated in any randomized controlled trials, and the results of this retrospective study should be interpreted with caution.
Doxercalciferol (1α-(OH)D2)
1α-(OH)D2 is converted to the active form, 1α,25(OH)2D2 through the action of 25-hydroxylase in the liver. Clinical studies of doxercalciferol have shown it to be effective in suppressing PTH levels but have also shown a modest rise in serum calcium and phosphorous levels223, 224. An intravenous preparation of doxercalciferol is available, and the drug was found to be effective in suppressing PTH with lower rates of hypercalcemia and hyperphosphatemia than 1α(OH)D2225. In a small clinical study comparing the dosing of doxercalciferol to paricalcitol, dosing doxercalciferol at 60% of the dose of paricalcitol results in similar reduction in PTH level226. In another study comparing doxercalciferol to paricalcitol, doxercalciferol was found to produce a similar reduction in PTH levels and was associated with higher rates of hyperphosphatemia or the Ca X P product227.
Alfacalcidol (1α-(OH)D3)
1α-(OH)D3 is converted to the active form, 1α,25(OH)2D3, in the liver. A study comparing alfacalcidol to calcitriol showed that at doses 1.5 to 2 times that of calcitriol, alfacalcidol is equally effective in suppressing PTH with a similar rate of hypercalcemia and hyperphosphatemia228. In a randomized cross over trial comparing alfacalcidol to paricalcitol in ESRD patients, alfacalcidol was shown to be as effective in suppressing PTH levels with similar rates of hypercalcemia and hyperphosphatemia as paricalcitol229.
Maxacalcitol (22-oxa-1,25(OH)D3)
The structure of maxacalcitol is similar to calcitriol except for the presence of an oxygen between C21 and C23. Maxacalcitol has been shown to result in less hypercalcemia in animal models, but this has not translated into clinical trials230. In humans maxacalcitol has been shown to be effective in suppressing PTH levels231–233, but in a study of 124 dialysis patients treated with maxacalcitol, 41 experienced hypercalcemia232. There is a dose-dependent relationship between the dose of maxacalcitol and risk of hypercalcemia231. Intravenous maxacalcitol is not superior to oral calcitriol234 in as much as decrements in PTH concentrations and the rate of hypercalcemia are similar234.
Falecalcitriol (1,25(OH)2-26,27-F6-D3)
It is a newer analog and is currently only available in Japan. In animal studies it is suggested to be highly potent in inhibiting PTH235. In clinical trials comparing falecalcitriol to alfacalcidol, falecalcitriol has been shown to be more effective than alfacalcidol in reducing PTH with similar rates of hypercalcemia236.
A comparison of the various drugs available for the treatment of hyperparathyroidism in CKD/ESRD is shown in Table 1.
Table 1.
Drugs available for the treatment of secondary hyperparathyroidism in CKD/ESRD
| Drug | Brand Name | Available in US | Oral or IV | Starting doses | Effect on calcium* | Effect on phosphorous* |
|---|---|---|---|---|---|---|
| Calcitriol | Rocaltrol/Calcijex | Yes | Both | 1–2 μg IV thrice weekly | NA | NA |
| Paricalcitol | Zemplar | Yes | Both | 2–4 μg IV thrice weekly | Less hypercalcemia | Less hyperphosphatemia |
| Alfacalcidol | One-Alpha (Canada) | No | Both | Similar to calcitriol | Similar to calcitriol | |
| Doxercalciferol | Hectorol | Yes | Both | 2–4 μg IV thrice weekly | Similar to calcitriol | Similar to calcitriol |
| Maxacalcitol | Oxarol (Japan) | No | IV | Similar to calcitriol | Similar to calcitriol | |
| Falecalcitriol | Hornel (Japan) | No | Oral | Similar to calcitriol | Similar to calcitriol |
compared to calcitriol
Other effects of vitamin D and its analogs
Hypertension and left ventricular hypertrophy
Vitamin D deficiency has been suggested to play a role in both development of cardiac hypertrophy and hypertension. Indeed vitamin D receptor knock out mice have been shown to develop both hypertension and left ventricular hypertrophy (LVH) suggesting that vitamin D may directly affect the myocardium237. Vitamin D may contribute to development of hypertension and LVH through inhibition of renin. Vitamin D receptor null mice are shown to have higher expression of renin and angiotensin II associated with both hypertension and LVH238. Similarly, hypertensive rats treated with vitamin D analogs have less cardiac hypertrophy than rats treated with vehicle239,240. In small clinical trials of dialysis patients, treatment with calcitriol has been shown to attenuate LVH241, 242. In a large randomized placebo-control trial of 227 patients with CKD with mild to moderate LVH, treatment with paricalcitol at a dose of 2 μg/day for 48 weeks did not result in any improvement in left ventricular mass index243.
Albuminuria
In multiple animal models, vitamin D and its analogs have been shown to reduce albuminuria244–248. Similar results have been shown in human clinical trials. Patients with CKD treated with paricalcitol had lower urinary protein or 24 hour albumin excretion rates compared to placebo249, 250. In a larger RCT involving patients with type 2 diabetes those treated with paricalcitol had lower urinary albumin to creatinine ratios compared to placebo-treated subjects, but they also had a lower eGFR251. Whether the reduction in urinary albumin is a true reduction or reflection of hemodynamic changes remains unclear. Similarly whether this reduction translates to better renal outcome long-term is also to be seen. More recent studies however, have failed to show a benefit in albuminuria in patients treated with doxercalciferol252.
Mortality
Low 25(OH)D levels have been associated with increased mortality in patients with CKD and ESRD similar to the general population116, 253–256. Similarly, lower vitamin D levels are associated with increased rate of progression to ESRD116. Many observational studies have suggested that use of active vitamin D in CKD and dialysis patients improves survival257–263. These studies are all confounded by the fact that patients who do not receive vitamin D or who had lower vitamin D levels might have been sicker with attendant higher mortality. This type of bias is not accounted for in standard regression models. Tentori F et al. re-evaluated the data from participants in the DOPPS study to evaluate the association between vitamin D and mortality264. Indeed, patients who were prescribed vitamin D had fewer comorbidities; when adjusted for confounding variables, vitamin D administration was no longer associated with improved mortality264. The lack of mortality benefit with use of vitamin D or its analog was also shown in a large meta-analysis of 76 trials265. Not only did the administration of vitamin D or its analogs not result in mortality benefit, but 1α,25(OH)2D3 administration was associated with higher rate of hypercalcemia and hyperphosphatemia and had a variable effect on reducing PTH levels265. It should be noted that in the various trials included in the meta-analysis265, the degree of suppression of PTH was variable and there were methodological differences in the measurement of PTH.
In conclusion, 1-hydroxylated vitamin D analogs are effective in the treatment of secondary hyperparathyroidism in the context of CKD/ESRD. Further studies are required to assess their benefits in the treatment of heart disease and albuminuria.
Key points.
Secondary hyperparathyroidism and mixed-uremic osteodystrophy are common in chronic kidney disease (CKD) and end-stage renal disease (ESRD).
Reduced concentrations of 1,25-dihydroxyvitamin D, intestinal malabsorption of calcium, and negative calcium balance contribute to secondary hyperparathyroidism in these conditions.
A stepwise approach of reducing serum phosphate concentrations with the use of phosphate binders such as sevelamer, calcium salts (e.g. calcium acetate, calcium carbonate), lanthanum carbonate, or various iron preparations (ferric citrate, sucroferric oxyhydride); correcting negative calcium balance with 1α-hydroxylated vitamin-D analogs; and using calcium sensing receptor agonists such as cinacalcet is generally effective in correcting secondary hyperparathyroidism in CKD and ESRD.
Treatment with 1-hydroxylated vitamin D metabolites or analogs such as calcitriol, alphacalcidol, doxercalciferol, paricalcitol, maxacalcitol and falecalcitriol are effective in the treatment of secondary hyperparathyroidism seen in CKD and ESRD.
References
- 1.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy JT, Kumar R. Behavior of the vitamin D endocrine system in the development of renal osteodystrophy. Semin Nephrol. 1986;6(1):21–30. [PubMed] [Google Scholar]
- 3.McCarthy JT, Kumar R. Renal osteodystrophy. Endocrinol Metab Clin North Am. 1990;19(1):65–93. [PubMed] [Google Scholar]
- 4.McCarthy JT, RK . Role of the Vitamin D System in the Pathogenesis of Renal Osteodystrophy. In: Kumar R, editor. Vitamin D: Basic and Clinical Aspects. Dortrecht: Martinus Nijhoff Publishers; 1984. pp. 611–640. [Google Scholar]
- 5.Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;43(3):558–565. doi: 10.1053/j.ajkd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 6.JWC, DJS, SMO, et al. Bone disease in uremia: a reappraisal. In: AWN, KS, DVH, et al., editors. Vitamin D: Chemical, biochemical and clinical endocrinology of calcium metabolism. Berlin, New York: Walter de Gruyter; 1982. pp. 835–840. [Google Scholar]
- 7.Bricker NS, Slatopolsky E, Reiss E, et al. Caclium, phosphorus, and bone in renal disease and transplantation. Arch Intern Med. 1969;123(5):543–553. [PubMed] [Google Scholar]
- 8.HHM, DAG, SGM Management of renal osteodystrophy with 1,25(OH)2D3. II. Effects on histopathology of bone: evidence for healing of osteomalacia. Mineral Elect Metab. 1979;2:48–55. [Google Scholar]
- 9.London G, Coyne D, Hruska K, et al. The new kidney disease: improving global outcomes (KDIGO) guidelines - expert clinical focus on bone and vascular calcification. Clinical nephrology. 2010;74(6):423–432. [PMC free article] [PubMed] [Google Scholar]
- 10.Malluche HH. Bone disease in renal failure. Introduction Miner Electrolyte Metab. 1991;17(4):209–210. [PubMed] [Google Scholar]
- 11.Malluche HH, Ritz E, Lange HP, et al. Bone histology in incipient and advanced renal failure. Kidney Int. 1976;9(4):355–362. doi: 10.1038/ki.1976.42. [DOI] [PubMed] [Google Scholar]
- 12.Reiss E, Canterbury JM, Kanter A. Circulating parathyroid hormone concentration in chronic renal insufficiency. Arch Intern Med. 1969;124(4):417–422. [PubMed] [Google Scholar]
- 13.Potts JT, Reita RE, Deftos LJ, et al. Secondary hyperparathyroidism in chronic renal disease. Arch Intern Med. 1969;124(4):408–412. [PubMed] [Google Scholar]
- 14.Norman ME, Mazur AT, Borden St, et al. Early diagnosis of juvenile renal osteodystrophy. J Pediatr. 1980;97(2):226–232. doi: 10.1016/s0022-3476(80)80479-3. [DOI] [PubMed] [Google Scholar]
- 15.RB, AM, MN . Histomorphometric classification of juvenile renal osteodystrophy: prevalence of mineralizing defect. In: AWN, KS, DVH, et al., editors. Vitamin D: Chemical, biochemical and clinical endocrinology of calcium metabolism. Berlin: Walter de Gruyter; 1982. pp. 835–840. [Google Scholar]
- 16.Martin DR, Ritter CS, Slatopolsky E, et al. Acute regulation of parathyroid hormone by dietary phosphate. Am J Physiol Endocrinol Metab. 2005;289(4):E729–734. doi: 10.1152/ajpendo.00065.2005. [DOI] [PubMed] [Google Scholar]
- 17.Slatopolsky E, Dusso A, Brown AJ. The role of phosphorus in the development of secondary hyperparathyroidism and parathyroid cell proliferation in chronic renal failure. Am J Med Sci. 1999;317(6):370–376. doi: 10.1097/00000441-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Slatopolsky E. The intact nephron hypothesis: the concept and its implications for phosphate management in CKD-related mineral and bone disorder. Kidney Int Suppl. 2011;(121):S3–8. doi: 10.1038/ki.2011.23. [DOI] [PubMed] [Google Scholar]
- 19.Slatopolsky E, Bricker NS. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney international. 1973;4(2):141–145. doi: 10.1038/ki.1973.92. [DOI] [PubMed] [Google Scholar]
- 20.Slatopolsky E, Bricker NS. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney Int. 1973;4(2):141–145. doi: 10.1038/ki.1973.92. [DOI] [PubMed] [Google Scholar]
- 21.Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl. 1999;73:S14–19. doi: 10.1046/j.1523-1755.1999.07304.x. [DOI] [PubMed] [Google Scholar]
- 22.Slatopolsky E, Brown A, Dusso A. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. Am J Kidney Dis. 2001;37(1 Suppl 2):S54–57. doi: 10.1053/ajkd.2001.20740. [DOI] [PubMed] [Google Scholar]
- 23.Slatopolsky E, Brown A, Dusso A. Calcium, phosphorus and vitamin D disorders in uremia. Contrib Nephrol. 2005;149:261–271. doi: 10.1159/000085687. [DOI] [PubMed] [Google Scholar]
- 24.Slatopolsky E, Caglar S, Gradowska L, et al. On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using “proportional reduction” of dietary phosphorus intake. Kidney Int. 1972;2(3):147–151. doi: 10.1038/ki.1972.84. [DOI] [PubMed] [Google Scholar]
- 25.Slatopolsky E, Delmez JA. Pathogenesis of secondary hyperparathyroidism. Mineral and electrolyte metabolism. 1995;21(1–3):91–96. [PubMed] [Google Scholar]
- 26.McCarthy JT, Kumar R. Renal osteodystrophy. In: Jacobson HR, Striker GE, Klahr S, editors. The Principles and Practice of Nephrology. St. Louis, MO: Mosby-Year Book, Inc; 1995. pp. 1032–1045. [Google Scholar]
- 27.DeLuca HF. The kidney as an endocrine organ involved in the function of vitamin D. Am J Med. 1975;58(1):39–47. doi: 10.1016/0002-9343(75)90531-8. [DOI] [PubMed] [Google Scholar]
- 28.Eisman JA, Hamstra AJ, Kream BE, et al. 1,25-Dihydroxyvitamin D in biological fluids: a simplified and sensitive assay. Science. 1976;193(4257):1021–1023. doi: 10.1126/science.1085035. [DOI] [PubMed] [Google Scholar]
- 29.Hollis BW. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification procedure. Clin Chem. 1986;32(11):2060–2063. [PubMed] [Google Scholar]
- 30.Ishimura E, Nishizawa Y, Inaba M, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999;55(3):1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 31.Mason RS, Lissner D, Wilkinson M, et al. Vitamin D metabolites and their relationship to azotaemic osteodystrophy. Clin Endocrinol (Oxf) 1980;13(4):375–385. doi: 10.1111/j.1365-2265.1980.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 32.Scharla S, Schmidt-Gayk H, Reichel H, et al. A sensitive and simplified radioimmunoassay for 1,25-dihydroxyvitamin D3. Clin Chim Acta. 1984;142(3):325–338. doi: 10.1016/0009-8981(84)90270-5. [DOI] [PubMed] [Google Scholar]
- 33.Yumita S, Suzuki M, Akiba T, et al. Levels of serum 1,25(OH)2D in patients with pre-dialysis chronic renal failure. Tohoku J Exp Med. 1996;180(1):45–56. doi: 10.1620/tjem.180.45. [DOI] [PubMed] [Google Scholar]
- 34.Coburn JW, Koppel MH, Brickman AS, et al. Study of intestinal absorption of calcium in patients with renal failure. Kidney Int. 1973;3(4):264–272. doi: 10.1038/ki.1973.40. [DOI] [PubMed] [Google Scholar]
- 35.Juttmann JR, Hagenouw-Taal JC, Lameyer LD, et al. Intestinal calcium absorption, serum phosphate, and parathyroid hormone in patients with chronic renal failure and osteodystrophy before and during hemodialysis. Calcif Tissue Res. 1978;26(2):119–126. doi: 10.1007/BF02013246. [DOI] [PubMed] [Google Scholar]
- 36.Chanard JM, Drueke T, Zingraff J, et al. Effects of haemodialysis on fractional intestinal absorption of calcium in uraemia. Eur J Clin Invest. 1976;6(3):261–264. doi: 10.1111/j.1365-2362.1976.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 37.Recker RR, Saville PD. Calcium absorption in renal failure: its relationship to blood urea nitrogen, dietary calcium intake, time on dialysis, and other variables. J Lab Clin Med. 1971;78(3):380–388. [PubMed] [Google Scholar]
- 38.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. The Journal of clinical investigation. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pande S, Ritter CS, Rothstein M, et al. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Physiol. 2006;104(1):p23–32. doi: 10.1159/000093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham J, Fraher LJ, Clemens TL, et al. Chronic acidosis with metabolic bone disease. Effect of alkali on bone morphology and vitamin D metabolism. Am J Med. 1982;73(2):199–204. doi: 10.1016/0002-9343(82)90179-6. [DOI] [PubMed] [Google Scholar]
- 45.Eiam-ong S, Kurtzman NA. Metabolic acidosis and bone disease. Mineral & Electrolyte Metabolism. 1994;20(1–2):72–80. [PubMed] [Google Scholar]
- 46.Marone CC, Wong NL, Sutton RA, et al. Acidosis and renal calcium excretion in experimental chronic renal failure. Nephron. 1981;28(6):294–296. doi: 10.1159/000182221. [DOI] [PubMed] [Google Scholar]
- 47.Kumar R. The metabolism of 1,25-dihydroxyvitamin D3. Endocr Rev. 1980;1(3):258–267. doi: 10.1210/edrv-1-3-258. [DOI] [PubMed] [Google Scholar]
- 48.Gray TK, Lowe W, Lester GE. Vitamin D and pregnancy: the maternal-fetal metabolism of vitamin D. Endocr Rev. 1981;2(3):264–274. doi: 10.1210/edrv-2-3-264. [DOI] [PubMed] [Google Scholar]
- 49.Norman AW, Roth J, Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins) Endocr Rev. 1982;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- 50.Brommage R, DeLuca HF. Evidence that 1,25-dihydroxyvitamin D3 is the physiologically active metabolite of vitamin D3. Endocr Rev. 1985;6(4):491–511. doi: 10.1210/edrv-6-4-491. [DOI] [PubMed] [Google Scholar]
- 51.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7(4):434–448. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 52.Christakos S, Gabrielides C, Rhoten WB. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional considerations, and molecular biology. Endocr Rev. 1989;10(1):3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- 53.Bikle DD, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocr Rev. 1993;14(1):3–19. doi: 10.1210/edrv-14-1-3. [DOI] [PubMed] [Google Scholar]
- 54.Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev. 1999;20(2):156–188. doi: 10.1210/edrv.20.2.0359. [DOI] [PubMed] [Google Scholar]
- 55.Gurlek A, Pittelkow MR, Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1alpha,25-dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev. 2002;23(6):763–786. doi: 10.1210/er.2001-0044. [DOI] [PubMed] [Google Scholar]
- 56.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26(5):662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 57.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Girgis CM, Clifton-Bligh RJ, Hamrick MW, et al. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 60.Kumar R. Vitamin D metabolism and mechanisms of calcium transport. J Am Soc Nephrol. 1990;1(1):30–42. doi: 10.1681/ASN.V1130. [DOI] [PubMed] [Google Scholar]
- 61.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 62.Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeLuca HF, Schnoes HK. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- 64.DeLuca HF, Schnoes HK. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- 65.Deluca HF. Historical Overview of Vitamin D. In: Feldman D, Pke JW, Adams JS, editors. Vitamin D. 3. Vol. 1. Boston: Elsevier; 2011. pp. 3–12. [Google Scholar]
- 66.DeLuca HF. Evolution of our understanding of vitamin D. Nutrition reviews. 2008;66(10 Suppl 2):S73–87. doi: 10.1111/j.1753-4887.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 67.Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92(2):77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 68.Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal. 2009;2(75):re4. doi: 10.1126/scisignal.275re4. [DOI] [PubMed] [Google Scholar]
- 69.Griffin MD, Kumar R. Multiple potential clinical benefits for 1alpha,25-dihydroxyvitamin D3 analogs in kidney transplant recipients. The Journal of steroid biochemistry and molecular biology. 2005;97(1–2):213–218. doi: 10.1016/j.jsbmb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 71.Ryan ZC, Craig TA, Folmes CD, et al. 1alpha,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. The Journal of biological chemistry. 2016;291(3):1514–1528. doi: 10.1074/jbc.M115.684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gurlek A, Kumar R. Regulation of osteoblast growth by interactions between transforming growth factor-beta and 1alpha,25-dihydroxyvitamin D3. Crit Rev Eukaryot Gene Expr. 2001;11(4):299–317. [PubMed] [Google Scholar]
- 73.Gurlek A, Pittelkow MR, Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1alpha,25-dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev. 2002;23(6):763–786. doi: 10.1210/er.2001-0044. [DOI] [PubMed] [Google Scholar]
- 74.McCollum EV, Simmonds N, Becker JE, et al. Studies on experimental rickets XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem. 1922;53:293–312. [PubMed] [Google Scholar]
- 75.Windaus A, Schenck F, von Weder F. Uber das antirachitsch wirksame bestrahlungs-produkt aus 7-dehydro-cholesterin. Hoppe-Seylers Z Physiol Chem. 1936;241:100–103. [Google Scholar]
- 76.Steenbock H, Black A. Fat-soluble vitamins. XVII. The induction of gross-promoting and calcifying properties in a ration by exposure to ultraviolet light. J Biol Chem. 1924;61:405–422. [Google Scholar]
- 77.Hess AF, Weinstock M. Anti-rachitic properties imparted to lettuce and to growing wheat by ultraviolet irradiation. Proceedings of the Society of Experimental Biology and Medicine. 1924;22:5–6. [Google Scholar]
- 78.DeLuca HF. The Metabolism, Physiology and Function of Vitamin D. In: Kumar R, editor. Vitamin D. Boston/The Hague/Dordrecht/Lancaster: Martinus Nijhoff Publishing; 1984. [Google Scholar]
- 79.Esvelt RP, Schnoes HK, DeLuca HF. Vitamin D3 from rat skins irradiated in vitro with ultraviolet light. Arch Biochem Biophys. 1978;188(2):282–286. doi: 10.1016/s0003-9861(78)80010-1. [DOI] [PubMed] [Google Scholar]
- 80.Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed Proc. 1978;37(12):2567–2574. [PubMed] [Google Scholar]
- 81.Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 82.Holick MF, Richtand NM, McNeill SC, et al. Isolation and identification of previtamin D3 from the skin of rats exposed to ultraviolet irradiation. Biochemistry. 1979;18(6):1003–1008. doi: 10.1021/bi00573a011. [DOI] [PubMed] [Google Scholar]
- 83.Windaus A, Linsert O, Luttringhaus A, et al. Crystalline-vitamin D 2. Annalen de Chemie. 1932;492:226–241. [Google Scholar]
- 84.Windaus A, Schenck F, FvW Uber das antirachitisch wirksame bestrahlungs-produkt aus 7-dehydro-cholesterin. Hoppe-Selyers Z Physiol Chem. 1936;241:100–103. [Google Scholar]
- 85.Steenbock H, Kletzien SWF, Halpin JG. The reaction of the chicken to irradiated ergosterol and irradiated yeast as contrasted with the natural vitamin of fish liver oils. Journal of Biological Chemistry. 1932;97:249– 264. [Google Scholar]
- 86.Jacob AI, Sallman A, Santiz Z, et al. Defective photoproduction of cholecalciferol in normal and uremic humans. J Nutr. 1984;114(7):1313–1319. doi: 10.1093/jn/114.7.1313. [DOI] [PubMed] [Google Scholar]
- 87.Blunt JW, DeLuca HF, Schnoes HK. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968;7(10):3317–3322. doi: 10.1021/bi00850a001. [DOI] [PubMed] [Google Scholar]
- 88.Suda T, DeLuca HF, Schnoes H, et al. 25-hydroxyergocalciferol: a biologically active metabolite of vitamin D2. Biochem Biophys Res Commun. 1969;35(2):182–185. doi: 10.1016/0006-291x(69)90264-2. [DOI] [PubMed] [Google Scholar]
- 89.Suda T, DeLuca HF, Schnoes HK, et al. The isolation and identification of 25-hydroxyergocalciferol. Biochemistry. 1969;8(9):3515–3520. doi: 10.1021/bi00837a005. [DOI] [PubMed] [Google Scholar]
- 90.Ponchon G, DeLuca HF. The role of the liver in the metabolism of vitamin D. The Journal of clinical investigation. 1969;48(7):1273–1279. doi: 10.1172/JCI106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ponchon G, Kennan AL, DeLuca HF. “Activation” of vitamin D by the liver. The Journal of clinical investigation. 1969;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhattacharyya MH, DeLuca HF. The regulation of rat liver calciferol-25-hydroxylase. The Journal of biological chemistry. 1973;248(9):2969–2973. [PubMed] [Google Scholar]
- 93.Bhattacharyya MH, DeLuca HF. Subcellular location of rat liver calciferol-25-hydroxylase. Arch Biochem Biophys. 1974;160(1):58–62. doi: 10.1016/s0003-9861(74)80008-1. [DOI] [PubMed] [Google Scholar]
- 94.DeLuca HF, Schnoes HK. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- 95.Madhok TC, DeLuca HF. Characteristics of the rat liver microsomal enzyme system converting cholecalciferol into 25-hydroxycholecalciferol. Evidence for the participation of cytochrome p-450. Biochem J. 1979;184(3):491–499. doi: 10.1042/bj1840491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng JB, Levine MA, Bell NH, et al. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(20):7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523(1):30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 98.Zhu JG, Ochalek JT, Kaufmann M, et al. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(39):15650–15655. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeLuca HF, Schnoes HK. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- 100.Haddad JG., Jr Transport of vitamin D metabolites. Clin Orthop Relat Res. 1979;(142):249–261. [PubMed] [Google Scholar]
- 101.Schwartz JB, Lai J, Lizaola B, et al. Variability in free 25(OH) vitamin D levels in clinical populations. The Journal of steroid biochemistry and molecular biology. 2014;144(Pt A):156–158. doi: 10.1016/j.jsbmb.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009;(183):1–420. [PMC free article] [PubMed] [Google Scholar]
- 103.Rosen CJ, Gallagher JC. The 2011 IOM report on vitamin D and calcium requirements for north america: clinical implications for providers treating patients with low bone mineral density. J Clin Densitom. 2011;14(2):79–84. doi: 10.1016/j.jocd.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 104.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 105.Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US); 2011. The National Academies Collection: Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 106.Al-Badr W, Martin KJ. Vitamin D and kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1555–1560. doi: 10.2215/CJN.01150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dusso A, Gonzalez EA, Martin KJ. Vitamin D in chronic kidney disease. Best Pract Res Clin Endocrinol Metab. 2011;25(4):647–655. doi: 10.1016/j.beem.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 108.Echida Y, Mochizuki T, Uchida K, et al. Risk factors for vitamin D deficiency in patients with chronic kidney disease. Intern Med. 2012;51(8):845–850. doi: 10.2169/internalmedicine.51.6897. [DOI] [PubMed] [Google Scholar]
- 109.Elder GJ, Mackun K. 25-Hydroxyvitamin D deficiency and diabetes predict reduced BMD in patients with chronic kidney disease. J Bone Miner Res. 2006;21(11):1778–1784. doi: 10.1359/jbmr.060803. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez EA, Sachdeva A, Oliver DA, et al. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24(5):503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 111.Hari P, Gupta N, Hari S, et al. Vitamin D insufficiency and effect of cholecalciferol in children with chronic kidney disease. Pediatr Nephrol. 2010;25(12):2483–2488. doi: 10.1007/s00467-010-1639-2. [DOI] [PubMed] [Google Scholar]
- 112.Helvig CF, Cuerrier D, Hosfield CM, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78(5):463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 113.Kandula P, Dobre M, Schold JD, et al. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6(1):50–62. doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.LaClair RE, Hellman RN, Karp SL, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45(6):1026–1033. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 115.Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(4):1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 117.Rucker D, Tonelli M, Coles MG, et al. Vitamin D insufficiency and treatment with oral vitamin D3 in northern-dwelling patients with chronic kidney disease. J Nephrol. 2009;22(1):75–82. [PubMed] [Google Scholar]
- 118.Satirapoj B, Limwannata P, Chaiprasert A, et al. Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. BMC Nephrol. 2013;14:206. doi: 10.1186/1471-2369-14-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zehnder D, Landray MJ, Wheeler DC, et al. Cross-sectional analysis of abnormalities of mineral homeostasis, vitamin D and parathyroid hormone in a cohort of pre-dialysis patients. The chronic renal impairment in Birmingham (CRIB) study. Nephron Clin Pract. 2007;107(3):c109–116. doi: 10.1159/000108652. [DOI] [PubMed] [Google Scholar]
- 120.Cheng S, Coyne D. Vitamin D and outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2007;16(2):77–82. doi: 10.1097/MNH.0b013e32802ef494. [DOI] [PubMed] [Google Scholar]
- 121.Mehrotra R, Kermah DA, Salusky IB, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76(9):977–983. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 123.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13(3):621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 124.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 125.Recker R, Schoenfeld P, Letteri J, et al. The efficacy of calcifediol in renal osteodystrophy. Arch Intern Med. 1978;138(Spec No):857–863. [PubMed] [Google Scholar]
- 126.Bhan I, Dobens D, Tamez H, et al. Nutritional vitamin D supplementation in dialysis: a randomized trial. Clin J Am Soc Nephrol. 2015;10(4):611–619. doi: 10.2215/CJN.06910714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Holick MF, Schnoes HK, DeLuca HF. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proceedings of the National Academy of Sciences of the United States of America. 1971;68(4):803–804. doi: 10.1073/pnas.68.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holick MF, Schnoes HK, DeLuca HF, et al. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971;10(14):2799–2804. doi: 10.1021/bi00790a023. [DOI] [PubMed] [Google Scholar]
- 129.Holick MF, Garabedian M, DeLuca HF. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science. 1972;176(4039):1146–1147. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- 130.Reeve L, Tanaka Y, DeLuca HF. Studies on the site of 1,25-dihydroxyvitamin D3 synthesis in vivo. The Journal of biological chemistry. 1983;258(6):3615–3617. [PubMed] [Google Scholar]
- 131.Bilezikian JP, Canfield RE, Jacobs TP, et al. Response of 1alpha,25-dihydroxyvitamin D3 to hypocalcemia in human subjects. The New England journal of medicine. 1978;299(9):437–441. doi: 10.1056/NEJM197808312990902. [DOI] [PubMed] [Google Scholar]
- 132.Boyle IT, Gray RW, DeLuca HF. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proceedings of the National Academy of Sciences of the United States of America. 1971;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DeLuca HF. Regulation of vitamin D metabolism in the kidney. Adv Exp Med Biol. 1977;81:195–209. doi: 10.1007/978-1-4613-4217-5_22. [DOI] [PubMed] [Google Scholar]
- 134.Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiological reviews. 1984;64(2):478–504. doi: 10.1152/physrev.1984.64.2.478. [DOI] [PubMed] [Google Scholar]
- 135.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 136.Garabedian M, Holick MF, Deluca HF, et al. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proceedings of the National Academy of Sciences of the United States of America. 1972;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shultz TD, Fox J, Heath H, 3rd, et al. Do tissues other than the kidney produce 1,25-dihydroxyvitamin D3 in vivo? A reexamination. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(6):1746–1750. doi: 10.1073/pnas.80.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ryan ZC, Ketha H, McNulty MS, et al. Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A. 2013;110(15):6199–6204. doi: 10.1073/pnas.1221255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kumar R, Vallon V. Reduced renal calcium excretion in the absence of sclerostin expression: evidence for a novel calcium-regulating bone kidney axis. J Am Soc Nephrol. 2014;25(10):2159–2168. doi: 10.1681/ASN.2014020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 141.Tebben PJ, Kumar R. The Hormonal Regulation of Calcium Metabolism. In: Alpern RJ, Moe OW, Caplan M, editors. Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology. Vol. 2. New York: Academic Press; 2013. pp. 2273–2330. [Google Scholar]
- 142.Wasserman RH, Corradino RA, Fullmer CS, et al. Some aspects of vitamin D action; calcium absorption and the vitamin D-dependent calcium-binding protein. Vitamins & Hormones. 1974;32:299–324. doi: 10.1016/s0083-6729(08)60017-5. [DOI] [PubMed] [Google Scholar]
- 143.Wasserman RH, Fullmer CS. Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr. 1995;125(7 Suppl):1971S–1979S. doi: 10.1093/jn/125.suppl_7.1971S. [DOI] [PubMed] [Google Scholar]
- 144.Wasserman RH, Smith CA, Brindak ME, et al. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102(3):886–894. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- 145.Wasserman RH, Chandler JS, Meyer SA, et al. Intestinal calcium transport and calcium extrusion processes at the basolateral membrane. Journal of Nutrition. 1992;122(3 Suppl):662–671. doi: 10.1093/jn/122.suppl_3.662. [DOI] [PubMed] [Google Scholar]
- 146.Berndt T, Thompson JR, Kumar R. The Regulation of Calcium, Magnesium, and Phosphate Excretion by the Kidney. In: Skorecki K, Chertow G, Marsden P, et al., editors. Brenner and Rector’s The Kidney. 10. Vol. 1. Elsevier; 2015. pp. 185–203. [Google Scholar]
- 147.Benn BS, Ajibade D, Porta A, et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149(6):3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kutuzova GD, Sundersingh F, Vaughan J, et al. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19655–19659. doi: 10.1073/pnas.0810761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lieben L, Benn BS, Ajibade D, et al. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone. 2010;47(2):301–308. doi: 10.1016/j.bone.2010.04.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ryan ZC, Craig TA, Filoteo AG, et al. Deletion of the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in mice is associated with decreased bone mineral density and impaired responsiveness to 1, 25-dihydroxyvitamin D3. Biochem Biophys Res Commun. 2015;467(1):152–156. doi: 10.1016/j.bbrc.2015.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Holick MF, Schnoes HK, DeLuca HF, et al. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972;11(23):4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- 152.Lam HY, Schnoes HK, DeLuca HF, et al. 24,25-Dihydroxyvitamin D3. Synthesis and biological activity Biochemistry. 1973;12(24):4851–4855. doi: 10.1021/bi00748a007. [DOI] [PubMed] [Google Scholar]
- 153.Tanaka Y, DeLuca HF, Ikekawa N, et al. Determination of stereochemical configuration of the 24-hydroxyl group of 24,25-dihydroxyvitamin D3 and its biological importance. Arch Biochem Biophys. 1975;170(2):620–626. doi: 10.1016/0003-9861(75)90157-5. [DOI] [PubMed] [Google Scholar]
- 154.Tanaka Y, Deluca HF. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- 155.Baxter LA, DeLuca HF. Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. The Journal of biological chemistry. 1976;251(10):3158–3161. [PubMed] [Google Scholar]
- 156.Ribovich ML, DeLuca HF. 1,25-Dihydroxyvitamin D3 metabolism. The effect of dietary calcium and phosphorus. Arch Biochem Biophys. 1978;188(1):164–171. doi: 10.1016/0003-9861(78)90369-7. [DOI] [PubMed] [Google Scholar]
- 157.Dominguez JH, Gray RW, Lemann J., Jr Dietary phosphate deprivation in women and men: effects on mineral and acid balances, parathyroid hormone and the metabolism of 25-OH-vitamin D. The Journal of clinical endocrinology and metabolism. 1976;43(5):1056–1068. doi: 10.1210/jcem-43-5-1056. [DOI] [PubMed] [Google Scholar]
- 158.Gray RW, Wilz DR, Caldas AE, et al. The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. The Journal of clinical endocrinology and metabolism. 1977;45(2):299–306. doi: 10.1210/jcem-45-2-299. [DOI] [PubMed] [Google Scholar]
- 159.Steele TH, Engle JE, Tanaka Y, et al. Phosphatemic action of 1,25-dihydroxyvitamin D3. Am J Physiol. 1975;229(2):489–495. doi: 10.1152/ajplegacy.1975.229.2.489. [DOI] [PubMed] [Google Scholar]
- 160.Gray RW. Control of plasma 1,25-(OH)2-vitamin D concentrations by calcium and phosphorus in the rat: effects of hypophysectomy. Calcif Tissue Int. 1981;33(5):485–488. doi: 10.1007/BF02409478. [DOI] [PubMed] [Google Scholar]
- 161.Gray RW. Effects of age and sex on the regulation of plasma 1,25-(OH)2-D by phosphorus in the rat. Calcif Tissue Int. 1981;33(5):477–484. doi: 10.1007/BF02409477. [DOI] [PubMed] [Google Scholar]
- 162.Gray RW, Garthwaite TL. Activation of renal 1,25-dihydroxyvitamin D3 synthesis by phosphate deprivation: evidence for a role for growth hormone. Endocrinology. 1985;116(1):189–193. doi: 10.1210/endo-116-1-189. [DOI] [PubMed] [Google Scholar]
- 163.Gray RW, Garthwaite TL, Phillips LS. Growth hormone and triiodothyronine permit an increase in plasma 1,25(OH)2D concentrations in response to dietary phosphate deprivation in hypophysectomized rats. Calcif Tissue Int. 1983;35(1):100–106. doi: 10.1007/BF02405013. [DOI] [PubMed] [Google Scholar]
- 164.Gray RW, Haasch ML, Brown CE. Regulation of plasma 1,25-(OH)2-D3 by phosphate: evidence against a role for total or acid-soluble renal phosphate content. Calcif Tissue Int. 1983;35(6):773–777. doi: 10.1007/BF02405122. [DOI] [PubMed] [Google Scholar]
- 165.Kido S, Kaneko I, Tatsumi S, et al. Vitamin D and type II sodium-dependent phosphate cotransporters. Contrib Nephrol. 2013;180:86–97. doi: 10.1159/000346786. [DOI] [PubMed] [Google Scholar]
- 166.Taketani Y, Segawa H, Chikamori M, et al. Regulation of type II renal Na+-dependent inorganic phosphate transporters by 1,25-dihydroxyvitamin D3. Identification of a vitamin D-responsive element in the human NAPi-3 gene. Journal of Biological Chemistry. 1998;273(23):14575–14581. doi: 10.1074/jbc.273.23.14575. [DOI] [PubMed] [Google Scholar]
- 167.Wagner CA, Hernando N, Forster IC, et al. The SLC34 family of sodium-dependent phosphate transporters. Pflugers Arch. 2014;466(1):139–153. doi: 10.1007/s00424-013-1418-6. [DOI] [PubMed] [Google Scholar]
- 168.Baker AR, McDonnell DP, Hughes M, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jehan F, DeLuca HF. Cloning and characterization of the mouse vitamin D receptor promoter. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(19):10138–10143. doi: 10.1073/pnas.94.19.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lu Z, Hanson K, DeLuca HF. Cloning and origin of the two forms of chicken vitamin D receptor. Arch Biochem Biophys. 1997;339(1):99–106. doi: 10.1006/abbi.1996.9864. [DOI] [PubMed] [Google Scholar]
- 171.Brumbaugh PF, Haussler MR. 1Alpha,25-dihydroxyvitamin D3 receptor: competitive binding of vitamin D analogs. Life sciences. 1973;13(12):1737–1746. doi: 10.1016/0024-3205(73)90120-3. [DOI] [PubMed] [Google Scholar]
- 172.Rachez C, Lemon BD, Suldan Z, et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398(6730):824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 173.Ciesielski F, Rochel N, Moras D. Adaptability of the Vitamin D nuclear receptor to the synthetic ligand Gemini: remodelling the LBP with one side chain rotation. The Journal of steroid biochemistry and molecular biology. 2007;103(3–5):235–242. doi: 10.1016/j.jsbmb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 174.Hourai S, Rodrigues LC, Antony P, et al. Structure-based design of a superagonist ligand for the vitamin D nuclear receptor. Chem Biol. 2008;15(4):383–392. doi: 10.1016/j.chembiol.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 175.Rochel N, Hourai S, Perez-Garcia X, et al. Crystal structure of the vitamin D nuclear receptor ligand binding domain in complex with a locked side chain analog of calcitriol. Arch Biochem Biophys. 2007;460(2):172–176. doi: 10.1016/j.abb.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 176.Rochel N, Wurtz JM, Mitschler A, et al. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5(1):173–179. doi: 10.1016/s1097-2765(00)80413-x. [DOI] [PubMed] [Google Scholar]
- 177.Molnar F, Perakyla M, Carlberg C. Vitamin D receptor agonists specifically modulate the volume of the ligand-binding pocket. The Journal of biological chemistry. 2006;281(15):10516–10526. doi: 10.1074/jbc.M513609200. [DOI] [PubMed] [Google Scholar]
- 178.Vaisanen S, Ryhanen S, Saarela JT, et al. Structurally and functionally important amino acids of the agonistic conformation of the human vitamin D receptor. Mol Pharmacol. 2002;62(4):788–794. doi: 10.1124/mol.62.4.788. [DOI] [PubMed] [Google Scholar]
- 179.Yamada S, Shimizu M, Yamamoto K. Structure-function relationships of vitamin D including ligand recognition by the vitamin D receptor. Med Res Rev. 2003;23(1):89–115. doi: 10.1002/med.10023. [DOI] [PubMed] [Google Scholar]
- 180.Yamamoto K, Masuno H, Choi M, et al. Three-dimensional modeling of and ligand docking to vitamin D receptor ligand binding domain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1467–1472. doi: 10.1073/pnas.020522697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Darwish H, DeLuca HF. Vitamin D-regulated gene expression. Crit Rev Eukaryot Gene Expr. 1993;3(2):89–116. [PubMed] [Google Scholar]
- 182.Haussler MR, Jurutka PW, Mizwicki M, et al. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25(4):543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 183.Jurutka PW, Whitfield GK, Hsieh JC, et al. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2(2):203–216. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 184.Lowe KE, Maiyar AC, Norman AW. Vitamin D-mediated gene expression. Crit Rev Eukaryot Gene Expr. 1992;2(1):65–109. [PubMed] [Google Scholar]
- 185.Craig TA, Zhang Y, Magis AT, et al. Detection of 1alpha,25-dihydroxyvitamin D-regulated miRNAs in zebrafish by whole transcriptome sequencing. Zebrafish. 2014;11(3):207–218. doi: 10.1089/zeb.2013.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Craig TA, Zhang Y, McNulty MS, et al. Research resource: whole transcriptome RNA sequencing detects multiple 1alpha,25-dihydroxyvitamin D(3)-sensitive metabolic pathways in developing zebrafish. Molecular endocrinology. 2012;26(9):1630–1642. doi: 10.1210/me.2012-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dusso AS, Arcidiacono MV, Sato T, et al. Molecular basis of parathyroid hyperplasia. J Ren Nutr. 2007;17(1):45–47. doi: 10.1053/j.jrn.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 188.Dusso AS, Sato T, Arcidiacono MV, et al. Pathogenic mechanisms for parathyroid hyperplasia. Kidney Int Suppl. 2006;(102):S8–11. doi: 10.1038/sj.ki.5001595. [DOI] [PubMed] [Google Scholar]
- 189.Slatopolsky E, Gradowska L, Kashemsant C, et al. The control of phosphate excretion in uremia. The Journal of clinical investigation. 1966;45(5):672–677. doi: 10.1172/JCI105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Slatopolsky E, Robson AM, Elkan I, et al. Control of phosphate excretion in uremic man. J Clin Invest. 1968;47(8):1865–1874. doi: 10.1172/JCI105877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Slatopolsky E, Rutherford WE, Hruska K, et al. How important is phosphate in the pathogenesis of renal osteodystrophy? Arch Intern Med. 1978;138(Spec No):848–852. [PubMed] [Google Scholar]
- 192.Slatopolsky E, Rutherford WE, Martin K, et al. The role of phosphate and other factors on the pathogenesis of renal osteodystrophy. Adv Exp Med Biol. 1977;81:467–475. doi: 10.1007/978-1-4613-4217-5_47. [DOI] [PubMed] [Google Scholar]
- 193.Slatopolsky E, Rutherford WE, Martin K, et al. The role of phosphate and other factors on the pathogenesis of renal osteodystrophy. Adv Exp Med Biol. 1977;81:467–475. doi: 10.1007/978-1-4613-4217-5_47. [DOI] [PubMed] [Google Scholar]
- 194.McCarthy JT, Kumar R. Renal osteodystrophy. Endocrinol Metab Clin North Am. 1990;19(1):65–93. [PubMed] [Google Scholar]
- 195.Nigwekar SU, Tamez H, Thadhani RI. Vitamin D and chronic kidney disease-mineral bone disease (CKD-MBD) Bonekey Rep. 2014;3:498. doi: 10.1038/bonekey.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney International. 2003;64(6):2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 197.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. The Journal of clinical investigation. 2008;118(12):3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. The Journal of clinical investigation. 2004;113(4):561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Naveh-Many T, Marx R, Keshet E, et al. Regulation of 1,25-dihydroxyvitamin D3 receptor gene expression by 1,25-dihydroxyvitamin D3 in the parathyroid in vivo. The Journal of clinical investigation. 1990;86(6):1968–1975. doi: 10.1172/JCI114931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Naveh-Many T, Rahamimov R, Livni N, et al. Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. The Journal of clinical investigation. 1995;96(4):1786–1793. doi: 10.1172/JCI118224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Naveh-Many T, Silver J. Regulation of parathyroid hormone gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. The Journal of clinical investigation. 1990;86(4):1313–1319. doi: 10.1172/JCI114840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Silver J, Naveh-Many T, Mayer H, et al. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. The Journal of clinical investigation. 1986;78(5):1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Silver J, Russell J, Sherwood LM. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(12):4270–4273. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brown AJ, Zhong M, Finch J, et al. The roles of calcium and 1,25-dihydroxyvitamin D3 in the regulation of vitamin D receptor expression by rat parathyroid glands. Endocrinology. 1995;136(4):1419–1425. doi: 10.1210/endo.136.4.7895652. [DOI] [PubMed] [Google Scholar]
- 205.Denda M, Finch J, Brown AJ, et al. 1,25-dihydroxyvitamin D3 and 22-oxacalcitriol prevent the decrease in vitamin D receptor content in the parathyroid glands of uremic rats. Kidney Int. 1996;50(1):34–39. doi: 10.1038/ki.1996.283. [DOI] [PubMed] [Google Scholar]
- 206.Dusso AS, Thadhani R, Slatopolsky E. Vitamin D receptor and analogs. Semin Nephrol. 2004;24(1):10–16. doi: 10.1053/j.semnephrol.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 207.Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl. 1999;73:S14–19. doi: 10.1046/j.1523-1755.1999.07304.x. [DOI] [PubMed] [Google Scholar]
- 208.Slatopolsky E, Finch J, Ritter C, et al. Effects of 19-nor-1,25(OH)2D2, a new analogue of calcitriol, on secondary hyperparathyroidism in uremic rats. Am J Kidney Dis. 1998;32(2 Suppl 2):S40–47. doi: 10.1053/ajkd.1998.v32.pm9808142. [DOI] [PubMed] [Google Scholar]
- 209.Takahashi F, Finch JL, Denda M, et al. A new analog of 1,25-(OH)2D3, 19-NOR-1,25-(OH)2D2, suppresses serum PTH and parathyroid gland growth in uremic rats without elevation of intestinal vitamin D receptor content. Am J Kidney Dis. 1997;30(1):105–112. doi: 10.1016/s0272-6386(97)90571-0. [DOI] [PubMed] [Google Scholar]
- 210.Bacchini G, Fabrizi F, Pontoriero G, et al. ‘Pulse oral’ versus intravenous calcitriol therapy in chronic hemodialysis patients. A prospective and randomized study. Nephron. 1997;77(3):267–272. doi: 10.1159/000190286. [DOI] [PubMed] [Google Scholar]
- 211.Fischer ER, Harris DC. Comparison of intermittent oral and intravenous calcitriol in hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol. 1993;40(4):216–220. [PubMed] [Google Scholar]
- 212.Indridason OS, Quarles LD. Comparison of treatments for mild secondary hyperparathyroidism in hemodialysis patients. Durham Renal Osteodystrophy Study Group. Kidney Int. 2000;57(1):282–292. doi: 10.1046/j.1523-1755.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- 213.Liou HH, Chiang SS, Huang TP, et al. Comparative effect of oral or intravenous calcitriol on secondary hyperparathyroidism in chronic hemodialysis patients. Miner Electrolyte Metab. 1994;20(3):97–102. [PubMed] [Google Scholar]
- 214.Mazzaferro S, Pasquali M, Ballanti P, et al. Intravenous versus oral calcitriol therapy in renal osteodystrophy: results of a prospective, pulsed and dose-comparable study. Miner Electrolyte Metab. 1994;20(3):122–129. [PubMed] [Google Scholar]
- 215.Quarles LD, Yohay DA, Carroll BA, et al. Prospective trial of pulse oral versus intravenous calcitriol treatment of hyperparathyroidism in ESRD. Kidney Int. 1994;45(6):1710–1721. doi: 10.1038/ki.1994.223. [DOI] [PubMed] [Google Scholar]
- 216.Haiyang Z, Chenggang X. Comparison of intermittent intravenous and oral calcitriol in the treatment of secondary hyperparathyroidism in chronic hemodialysis patients: a meta-analysis of randomized controlled trials. Clin Nephrol. 2009;71(3):276–285. doi: 10.5414/cnp71276. [DOI] [PubMed] [Google Scholar]
- 217.Slatopolsky E, Finch J, Ritter C, et al. A new analog of calcitriol, 19-nor-1,25-(OH)2D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hypercalcemia. Am J Kidney Dis. 1995;26(5):852–860. doi: 10.1016/0272-6386(95)90455-7. [DOI] [PubMed] [Google Scholar]
- 218.Slatopolsky E, Cozzolino M, Lu Y, et al. Efficacy of 19-Nor-1,25-(OH)2D2 in the prevention and treatment of hyperparathyroid bone disease in experimental uremia. Kidney Int. 2003;63(6):2020–2027. doi: 10.1046/j.1523-1755.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 219.Martin KJ, Gonzalez EA, Gellens M, et al. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9(8):1427–1432. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- 220.Martin KJ, Gonzalez EA, Gellens ME, et al. Therapy of secondary hyperparathyroidism with 19-nor-1alpha,25-dihydroxyvitamin D2. Am J Kidney Dis. 1998;32(2 Suppl 2):S61–66. doi: 10.1053/ajkd.1998.v32.pm9808145. [DOI] [PubMed] [Google Scholar]
- 221.Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63(4):1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 222.Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349(5):446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 223.Frazao JM, Chesney RW, Coburn JW. Intermittent oral 1alpha-hydroxyvitamin D2 is effective and safe for the suppression of secondary hyperparathyroidism in haemodialysis patients. 1alphaD2 Study Group. Nephrol Dial Transplant. 1998;13(Suppl 3):68–72. doi: 10.1093/ndt/13.suppl_3.68. [DOI] [PubMed] [Google Scholar]
- 224.Tan AU, Jr, Levine BS, Mazess RB, et al. Effective suppression of parathyroid hormone by 1 alpha-hydroxy-vitamin D2 in hemodialysis patients with moderate to severe secondary hyperparathyroidism. Kidney Int. 1997;51(1):317–323. doi: 10.1038/ki.1997.39. [DOI] [PubMed] [Google Scholar]
- 225.Maung HM, Elangovan L, Frazao JM, et al. Efficacy and side effects of intermittent intravenous and oral doxercalciferol (1alpha-hydroxyvitamin D(2)) in dialysis patients with secondary hyperparathyroidism: a sequential comparison. Am J Kidney Dis. 2001;37(3):532–543. [PubMed] [Google Scholar]
- 226.Zisman AL, Ghantous W, Schinleber P, et al. Inhibition of parathyroid hormone: a dose equivalency study of paricalcitol and doxercalciferol. Am J Nephrol. 2005;25(6):591–595. doi: 10.1159/000089707. [DOI] [PubMed] [Google Scholar]
- 227.Joist HE, Ahya SN, Giles K, et al. Differential effects of very high doses of doxercalciferol and paricalcitol on serum phosphorus in hemodialysis patients. Clin Nephrol. 2006;65(5):335–341. doi: 10.5414/cnp65335. [DOI] [PubMed] [Google Scholar]
- 228.Kiattisunthorn K, Wutyam K, Indranoi A, et al. Randomized trial comparing pulse calcitriol and alfacalcidol for the treatment of secondary hyperparathyroidism in haemodialysis patients. Nephrology (Carlton) 2011;16(3):277–284. doi: 10.1111/j.1440-1797.2010.01398.x. [DOI] [PubMed] [Google Scholar]
- 229.Hansen D, Rasmussen K, Danielsen H, et al. No difference between alfacalcidol and paricalcitol in the treatment of secondary hyperparathyroidism in hemodialysis patients: a randomized crossover trial. Kidney Int. 2011;80(8):841–850. doi: 10.1038/ki.2011.226. [DOI] [PubMed] [Google Scholar]
- 230.Murayama E, Miyamoto K, Kubodera N, et al. Synthetic studies of vitamin D3 analogues. VIII. Synthesis of 22-oxavitamin D3 analogues. Chem Pharm Bull (Tokyo) 1986;34(10):4410–4413. doi: 10.1248/cpb.34.4410. [DOI] [PubMed] [Google Scholar]
- 231.Akizawa T, Ohashi Y, Akiba T, et al. Dose-response study of 22-oxacalcitriol in patients with secondary hyperparathyroidism. Ther Apher Dial. 2004;8(6):480–491. doi: 10.1111/j.1774-9987.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 232.Akizawa T, Suzuki M, Akiba T, et al. Long-term effect of 1,25-dihydroxy-22-oxavitamin D(3) on secondary hyperparathyroidism in haemodialysis patients. One-year administration study. Nephrol Dial Transplant. 2002;17(Suppl 10):28–36. doi: 10.1093/ndt/17.suppl_10.28. [DOI] [PubMed] [Google Scholar]
- 233.Yasuda M, Akiba T, Nihei H. Multicenter clinical trial of 22-oxa-1,25-dihydroxyvitamin D3 for chronic dialysis patients. Am J Kidney Dis. 2003;41(3 Suppl 1):S108–111. doi: 10.1053/ajkd.2003.50097. [DOI] [PubMed] [Google Scholar]
- 234.Tamura S, Ueki K, Mashimo K, et al. Comparison of the efficacy of an oral calcitriol pulse or intravenous 22-oxacalcitriol therapies in chronic hemodialysis patients. Clin Exp Nephrol. 2005;9(3):238–243. doi: 10.1007/s10157-005-0363-x. [DOI] [PubMed] [Google Scholar]
- 235.Nishizawa Y, Morii H, Ogura Y, et al. Clinical trial of 26,26,26,27,27,27-hexafluoro-1,25-dihydroxyvitamin D3 in uremic patients on hemodialysis: preliminary report. Contrib Nephrol. 1991;90:196–203. doi: 10.1159/000420143. [DOI] [PubMed] [Google Scholar]
- 236.Ito H, Ogata H, Yamamoto M, et al. Comparison of oral falecalcitriol and intravenous calcitriol in hemodialysis patients with secondary hyperparathyroidism: a randomized, crossover trial. Clin Nephrol. 2009;71(6):660–668. doi: 10.5414/cnp71660. [DOI] [PubMed] [Google Scholar]
- 237.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 238.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kong J, Kim GH, Wei M, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol. 2010;177(2):622–631. doi: 10.2353/ajpath.2010.091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bodyak N, Ayus JC, Achinger S, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104(43):16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim HW, Park CW, Shin YS, et al. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102(1):c21–29. doi: 10.1159/000088295. [DOI] [PubMed] [Google Scholar]
- 242.Park CW, Oh YS, Shin YS, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33(1):73–81. doi: 10.1016/s0272-6386(99)70260-x. [DOI] [PubMed] [Google Scholar]
- 243.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 244.Lillevang ST, Rosenkvist J, Andersen CB, et al. Single and combined effects of the vitamin D analogue KH1060 and cyclosporin A on mercuric-chloride-induced autoimmune disease in the BN rat. Clin Exp Immunol. 1992;88(2):301–306. doi: 10.1111/j.1365-2249.1992.tb03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Branisteanu DD, Leenaerts P, van Damme B, et al. Partial prevention of active Heymann nephritis by 1 alpha, 25 dihydroxyvitamin D3. Clin Exp Immunol. 1993;94(3):412–417. doi: 10.1111/j.1365-2249.1993.tb08210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schwarz U, Amann K, Orth SR, et al. Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998;53(6):1696–1705. doi: 10.1046/j.1523-1755.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 247.Migliori M, Giovannini L, Panichi V, et al. Treatment with 1,25-dihydroxyvitamin D3 preserves glomerular slit diaphragm-associated protein expression in experimental glomerulonephritis. Int J Immunopathol Pharmacol. 2005;18(4):779–790. doi: 10.1177/039463200501800422. [DOI] [PubMed] [Google Scholar]
- 248.Zhang Z, Zhang Y, Ning G, et al. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105(41):15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52(2):249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 250.Fishbane S, Chittineni H, Packman M, et al. Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trial. Am J Kidney Dis. 2009;54(4):647–652. doi: 10.1053/j.ajkd.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 251.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 252.Moe SM, Saifullah A, LaClair RE, et al. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):299–306. doi: 10.2215/CJN.07131009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Drechsler C, Pilz S, Obermayer-Pietsch B, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31(18):2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Drechsler C, Verduijn M, Pilz S, et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2011;26(3):1024–1032. doi: 10.1093/ndt/gfq606. [DOI] [PubMed] [Google Scholar]
- 255.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 256.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 257.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 258.Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168(4):397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 259.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70(2):351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 260.Shoben AB, Rudser KD, de Boer IH, et al. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19(8):1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19(1):179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 262.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16(4):1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 263.Wolf M, Betancourt J, Chang Y, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008;19(7):1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tentori F, Albert JM, Young EW, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2009;24(3):963–972. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]
- 265.Palmer SC, McGregor DO, Macaskill P, et al. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147(12):840–853. doi: 10.7326/0003-4819-147-12-200712180-00004. [DOI] [PubMed] [Google Scholar]