Abstract
Embryonic exposure to steroids often leads to long-term phenotypic effects. It has been hypothesized that mothers may be able to create a steroid environment that adjusts the phenotypes of offspring to current environmental conditions. Complicating this hypothesis is the potential for developing embryos to modulate their early endocrine environment. This study utilized the threespined stickleback (Gasterosteus aculeatus) to characterize the early endocrine environment within eggs by measuring four steroids (progesterone, testosterone, estradiol, and cortisol) of maternal origin. We then examined how the concentrations of these four steroids changed over the first 12 days post fertilization (dpf). Progesterone, testosterone, estradiol, and cortisol of maternal origin could be detected within unfertilized eggs and levels of all four steroids declined in the first 3 days following fertilization. While levels of progesterone, testosterone, and estradiol remained low after the initial decline, levels of cortisol rose again by 8 dpf. These results demonstrate that G. aculeatus embryos begin development in the presence of a number of maternal steroids but levels begin to change quickly following fertilization. This suggests that embryonic processes change the early endocrine environment and hence influence the ability of maternal steroids to affect development. With these findings, G. aculeatus becomes an intriguing system in which to study how selection may act on both maternal and embryonic processes to shape the evolutionary consequence of steroid-mediated maternal effects.
For vertebrates, conditions experienced during embryonic development have long-lasting effects on phenotypic traits. Studies have shown that the early endrocine environment has an important influence on development in vertebrates (mammals: (Gerall et al., ’92), birds: (Balthazart and Adkins-Regan, 2002), reptiles: (Lance, ’97), amphibians (Hayes, ’98), andfish: (Devlin and Nagahama, 2002)). More specifically, embryonic steroid exposure can produce organizational effects that are permanent such that the effects remain even after the steroid signal is no longer present (Arnold, 2009; Pheonix et al., 1959). The bulk of what we know about the phenotypic consequences of steroid exposure comes from studies that exogenously manipulate steroid levels and characterize the phenotypic effects. Historically, research has tended to focus on the maladaptive or pathological effects of abnormal steroid exposure (Berenbaum and Beltz, 2011). An offshoot of this research began with the discovery that environ-mental/exogenous chemicals could also produce effects that were similar to abnormal steroid exposure (Colborn et al., ’93). Together these studies highlight just how important regulation of steroid signaling is to embryonic development.
A major component of the steroid environment during development comes from maternally derived steroids. Whether placental or oviparous, all vertebrate embryos develop in the presence of maternally derived steroids (Paitz and Bowden, 2010). In placental vertebrates, embryos are subject to steroids present in maternal circulation (Diczfalusy, ’64) while embryos of oviparous vertebrates are subject to steroids present in the egg at the time of oviposition (Groothuis et al., 2005). Upon the discovery that variation in levels of maternal steroids were related to phenotypic variation in offspring (Schwabl, ’93), researchers began to test the hypothesis (primarily in birds) that females could adaptively adjust offspring phenotypes with differential allocation of maternal steroids (Gil, 2008; Groothuis et al., 2005; Groothuis and Schwabl, 2008; Sheriff and Love, 2013). An assumption for many of the early studies investigating the potentially adaptive consequences of maternal steroid exposure was that the embryo was a passive responder to maternal steroids (Moore and Johnston, 2008; Williams, 2012). Recent work (Paitz et al., 2011; Paitz and Bowden, 2008; von Engelhardt et al., 2009) in conjunction with a re-examination of previous work (Diczfalusy, ’64; Gonzalez et al., ’83; Levitz, ’66; Parsons, ’70), has shown that vertebrate embryos are capable of metabolizing maternally derived steroids and modulating levels of maternal steroids to which they are exposed. In placental vertebrates, steroids are metabolized via a variety of pathways as they pass from maternal circulation into the placenta and subsequently into fetal circulation (Diczfalusy, ’64; Painter and Moore, 2005). The extra embryonic membranes of oviparous vertebrates are also capable of metabolizing steroids of maternal origin (Albergotti et al., 2009; Paitz and Bowden, 2008). A major implication of these data is that the interplay of maternal and embryonic processes dictates the level of maternal steroids to which the embryo is exposed and thus the phenotypic consequences of maternal steroid exposure. So to fully understand the evolutionary consequences of steroid-mediated maternal effects, we must understand both maternal and embryonic processes.
Teleost fishes have long served as a model taxa for investigating the phenotypic consequences of early steroid exposure with a focus on the effect of exogenous steroids on sex determination (Yamamoto, ’69). Although not always framed within the context of steroid-mediated maternal effects, a number of studies have characterized levels of maternally derived steroids in fish eggs. Maternally derived steroids are detectable in unfertilized fish eggs with levels dropping very rapidly following fertilization (progesterone- (coho salmon, Oncorhynchus kisutch, Feist et al., 1996); estradiol- (tilapia, Oreochormis nilotica), Rothbard et al., ’87), (Coho salmon, Oncorhynchus kisutch, Feist et al., ’90), (rainbow trout, Oncorhynchus mykiss, Feist and Schreck, ’96), (tilapia, Oreochormis nilotica, Hines et al., ’99); testosterone- (tilapia, Oreochormis nilotica, Rothbard et al., ’87), (coho salmon, Oncorhynchus kisutch, Feist et al., ’90), (rainbow trout, Oncorhynchus mykiss, Feist and Schreck, ’96), (tilapia, Oreochormis nilotica, Hines et al., ’99), (medaka, Oryzias latipes, Iwamatsu et al., 2006); cortisol- (Japanese flounder, Paralichthys olivaceus, de Jesus et al., ’91), (tilapia, Oreochormis nilotica, Hwang et al., ’92), (Asian seabass, Lates calcarifer, Sampathkumar et al., ’95), (rainbow trout, Oncorhynchus mykiss, Barry et al., ’95), (yellow perch, Perca flavescens, Jentoft et al., 2002), (zebrafish, Danio rerio, Alsop and Vijayan, 2008), (rainbow trout, Oncorhynchus mykiss, Fuzzen et al., 2011)). This decline in steroid concentrations is thought to be due to embryonic steroid metabolism since fish embryos possess a number of metabolic enzymes (Antila, ’84; Hines et al., ’99; Khan et al., ’97a, b; Li et al., 2012; Petkam et al., 2002; Rowell et al., 2002;). While the metabolism of maternal steroids by developing embryos may produce inactive metabolites in some situations, it is possible that the metabolites of maternal steroids may themselves be biologically active or could serve as precursors for steroid production later in development (Paitz and Bowden, 2011). Thus, the metabolism of maternal steroids by no means negates the possibility of steroid-mediated maternal effects. Indeed steroid-mediated maternal effects have been documented in a number of fish species (Auperin and Geslin, 2008; Burton et al., 2011; Eriksen et al., 2006; Eriksen et al., 2007; Li et al., 2010, 2011; McCormick, ’98, ’99). Overall, the work in fishes suggests that, similar to other vertebrates, both maternal and embryonic processes may be subject to selective pressures that shape the evolutionary consequences of steroid-mediated maternal effects.
Accumulating evidence suggests that steroid-mediated maternal effects may influence phenotypic variation in the threespined stickleback (Gasterosteus aculeatus). Subjecting female sticklebacks to simulated predation risk increases the cortisol content within the eggs (Giesing et al., 2011). This maternal predator exposure also influences several traits in her offspring such as egg size (Giesing et al., 2011), metabolic rate (Giesing et al., 2011), learning (Roche et al., 2012), behavior (Giesing et al., 2011), stress response (Mommer and Bell, 2013), and survival (McGhee et al., 2012) suggesting that the increase in egg cortisol levels may be responsible for mediating the effects of maternal stress. G.aculeatus is renowned for geographic variation in behavior and morphology related to environmental factors (Bell and Foster, ’94). These populations also differ in a variety of female life-history traits such as egg size and clutch size (Baker et al., 2008). At this point it is unknown what role maternal effects may play in the production/maintenance of this variation, but taken together, the evolutionary history of G. aculeatus creates a powerful system in which to study the evolutionary potential of steroid-mediated maternal effects.
The first objective of this study was to quantify levels of maternally derived progesterone, testosterone, estradiol, and cortisol in clutches of G. aculeatus eggs. We chose to quantify one steroid from each of the four major classes of vertebrate steroids to provide a more complete picture of the early endocrine environment in this species. While cortisol has received the most attention as a mediator of maternal effects in fish, studies in other oviparous vertebrates suggest that progesterone (Paitz and Casto, 2012), testosterone (reviewed by Groothuis and Schwabl, 2008), and estradiol (Bowden et al., 2000; Williams et al., 2005) may also influence embryonic development. The second objective of this study was to examine how these levels change throughout development. Relative to most fish species that have been used to characterize the endocrine environment during development, there is a relatively brief period of time between fertilization and hatching in G. aculeatus. This rapid development may constrain the opportunity for embryos to modulate maternal steroid levels prior to the initiation of processes like neurogenesis. We predicted that G. aculeatus eggs would contain all four steroids and that these levels would decline during development as has been shown in other fish species. Understanding how the early endocrine environment changes during development is vital to identifying processes that underlie the effect of maternal steroids on offspring development.
MATERIALS AND METHODS
Sample Collection
Adult sticklebacks were collected from a natural population in Navarro Creek, CA, USA. Fish were held on a natural photoperiod at 20°C and fed frozen bloodworms, mysis shrimp, cyclop-eeze, and brine shrimp ad libitum. Males were housed singly in small tanks (36 L × 33 W × 24 H cm, 26 L), supplied with nesting material (algae), sand, and visual access to neighbors. After a male completed his nest, a female was introduced and the pair was allowed to spawn. We retrieved eggs within 12 hr of fertilization and artificially incubated them in a cup with a mesh screen bottom and bubbling airstone positioned under each cup. Eggs that were not developing were removed once detected to prevent fungal growth. For the unfertilized group, eggs were collected by gently squeezing the eggs from gravid females. In total, 36 clutches of eggs were collected and sampled at seven different points of development. The number of clutches collected at each sampling period ranged from three to seven. Whole clutches were sampled as unfertilized eggs or after a specific number of days following fertilization (3,4,6,7,8, or 12 days respectively) such that every sample represented an entire clutch and no females were used more than once. At the time of sampling, eggs or larvae were snap frozen on dry ice and stored at −80°C until steroid quantification.
Steroid Quantification
To quantify steroids, tissues were weighed and homogenized with a glass potter-elvehjem homogenizer. From each homogenate, two 20 mg aliquots were collected. One aliquot was used to quantify cortisol via enzyme-linked immunosorbent assay (ELISA) (Enzo Life Sciences, Plymouth Meeting, PA, USA) and the other aliquot was used to quantify progesterone (PROG), testosterone (T), and estradiol (E2) via radioimmunoassay (RIA). This RIA has previously been used to investigate how levels of PROG, T, and E2 change in bird (Paitz et al., 2011) and reptile (Paitz and Bowden 2009) eggs during development. The RIA aliquot also received a 2000 cpm tracer of PROG, T, and E2 to calculate the recovery of each steroid. The tritiated steroids NET-381 (PROG), NET 553 (T), and NET 517 (E2) were purchased from PerkinElmer (Boston, MA, USA). Each aliquot was added to 1 mL distilled H2O and steroids were extracted twice with 3 mL diethyl ether that was then dried under nitrogen gas (Paitz and Bowden, 2009).
For the quantification of progesterone, testosterone, and estradiol, steroids were fractionated via celite chromatography using hormone-specific ethyl acetate:isooctane ratios (PROG = 2%, T = 20%, and E2 = 40%) (Paitz and Bowden, 2009). Steroid concentrations were measured in competitive binding RIAs with a standard curve that ranged from 3.91 to 1000 pg for PROG and 1.95 to 500 pg for T and E2. Average recovery was 58% for PROG, 63% for T, and 61% for E2. The intra-assay coefficients of variation were 8% for PROG, 10% for T, and 8% for E2. Antibody 20R-PR053w (Fitzgerald Industries, Acton, MA, USA) was used to quantify PROG and had a reported cross reactivity of 50% with prenenolone and less than 1% with all other reported steroids. Antibody 20R-TR018w (Fitzgerald Industries) was used to quantify T and had a reported cross reactivity of 60% with 5alpha-dihydrotestosterone, < 5% with other androgenic steroids, and less than 0.05% with estradiol and all other reported steroids. Antibody 7010 (Biogenesis, Kingston, NH, USA) was used to quantify E2 and had a reported cross reactivity of 14%with estrone, 5% with estriol, and less than 0.01% with all other reported steroids.
For the quantification of cortisol the other 20 mg aliquot of homogenate from each sample was measured in duplicate via competitive ELISA (Giesing et al., 2011). The antibody used in this assay is reported by the manufacturer to have a sensitivity of 56.72 pg/mL and a 27.7% cross reactivity with corticosterone, 4.0% with 11-deoxycortisol, 3.6% with progesterone, and 0.1% or less with testosterone, androstenedione, cortisone, and estradiol. Thawed samples were centrifuged to isolate the supernatant, diluted with assay buffer, and divided in half to be measured as duplicates. Following the manufacturer’s protocol, 96-well ELISA plates were prepared and absorbance read at 405 nm for each sample (Enzo Life Sciences) on a FilterMax F3 microplate reader (Molecular Devices, Sunnyvale, CA, USA) and absorbance data averaged across duplicate samples with Multi-Mode Analysis software (Molecular Devices version 3.4.0.25). Blank-corrected, total-activity-corrected optical densities were converted to “percent bound” for each sample, and converted to cortisol concentration (ng/mL) by applying a four-factor polynomial standard curve derived from each plate’s standards. Sample values were multiplied by their dilution factor to obtain the cortisol concentration of the original, undiluted egg and tissue 20 mg homogenates. Samples that fell above the curve (n = 4) were assigned the value of the most concentrated standard. The intra-assay coefficient of variation was 1.7%.
Statistical Analysis
An ANOVA was used to examine whether steroid concentrations changed during development. Concentrations of all four steroids were log transformed prior to analysis in order to normalize the data. For each steroid, we performed a one-way ANOVA using day of development as a fixed factor. Post hoc comparisons (Tukey’s HSD) were used to compare differences between days of development. All statistical tests were performed in SAS v. 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Maternally derived progesterone, testosterone, estradiol, and cortisol could be detected in unfertilized G. aculeatus eggs. The mean concentration (± SE) in unfertilized eggs was 9.2 ± 2.1 ng/g for PROG, 0.33 ± .03 ng/g for T, 0.62 ± .12 ng/g for E2, and 12.6 ± 1.6 ng/g for cortisol. When examining how these concentrations changed during development, levels of E2 and PROG declined by day three and six respectively and did not change thereafter (Fig. 1). Cortisol levels also declined early in development but increased a few days after hatching (Fig. 1). Levels of testosterone did not change during development. Interestingly, if the analyses are restricted to compare only unfertilized eggs and those 3 dpf, concentrations of all four steroids decline significantly (all P < 0.025)
Figure 1.
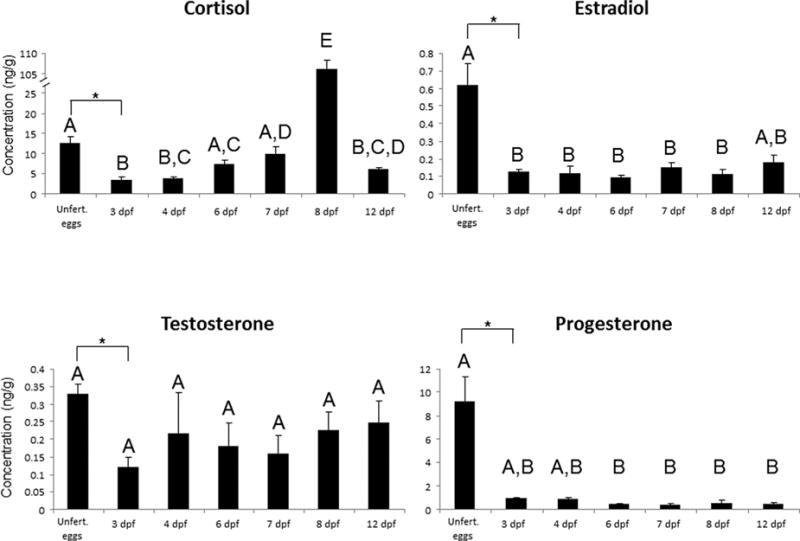
Mean concentrations of steroids (± SE) within G. aculeatus eggs and larvae over the first 12 days following fertilization (dpf). Groups not sharing a letter are significantly different (P < 0.05). Asterisks indicate a significant difference when the analysis is restricted to unfertilized eggs and 3 dpf. Sample sizes: Unfert = 7, 3 dpf = 5, 4 dpf = 5, 6 dpf =5, 7 dpf = 5, 8 dpf = 5, 12 dpf = 4.
DISCUSSION
We demonstrated that G. aculeatus eggs contain maternally derived steroids from all four classes of vertebrate steroids at the time of oviposition. Progesterone, testosterone, estradiol, and cortisol were all detectable in unfertilized eggs with concentrations of PROG and cortisol being relatively high compared to T and E2. When focusing the analysis on the first 72 hr post-fertiliztation, concentrations of all four steroids declined. This decline is consistent with reports from other fish species that show concentrations of steroids within eggs decline following fertilization (progesterone- (Feist et al., ’90); estradiol- (Feist et al., ’90; Feist and Schreck, ’96; Hines et al., ’99; Rothbard et al., ’87); testosterone- (Feist et al., ’90; Feist and Schreck, ’96; Hines et al., ’99; Iwamatsu et al., 2006; Rothbard et al., ’87); cortisol- (Alsop and Vijayan, 2008; Barry et al., ’95; de Jesus et al., ’91; Fuzzen et al., 2011; Hwang et al., ’92; Jentoft et al., 2002; Sampathkumar et al., ’95). While the fate of these maternal steroids remains to be deciphered, metabolism is a likely plausible candidate as numerous studies have demonstrated that fish embryos possess a suite of enzymes capable of metabolizing numerous steroids (Antila, ’84; Hines et al., ’99; Khan et al., ’97a, b; Li et al., 2012; Petkam et al., 2002; Rowell et al., 2002; Yeoh et al., ’96). It has been hypothesized that the embryonic metabolism of modulation of steroid levels buffers the process of gonadal differentiation from the effects of maternal steroids (Feist et al., ’90). Our data are consistent with this hypothesis as gonadal differentiation in G. aculeatus begins around 12 dpf (Lewis et al., 2008), long after the drop in maternal steroid levels. Despite the relatively short period between oviposition and gonadal differentiation, maternal steroids do not appear to be present during this process.
The metabolism of maternal steroids would not necessarily negate the ability of these steroids to influence embryonic development as a number of experiments have demonstrated steroid mediated maternal effects (Auperin and Geslin, 2008; Burton et al., 2011; Eriksen et al., 2006; Eriksen et al., 2007; Li et al., 2010, 2011; McCormick, ’98, ’99). It is possible that these steroids are eliciting an effect prior to metabolism as fish embryos are capable of responding to steroids within 48 hr of fertilization (Li et al., 2012; Zucchi et al., 2012). Conversely, the metabolites of maternal steroids may be capable of influencing offspring phenotypes (Paitz and Bowden, 2011). These scenarios are not mutually exclusive. In addition to metabolism, processes that result in the steroids exiting the egg may account for the observed decline in steroid levels. Passive diffusion and active efflux of steroids may also play a role in the decline of steroid levels. Indeed, ATP-binding cassette (ABC) transporters are capable of transporting a wide range of chemicals from cells and have recently been shown to be active during the early stages of development of zebrafish (Danio rerio) embryos (Fischer et al., 2013). Much more work is needed to determine which mechanisms may be operating to regulate the exposure of embryos to maternal steroids. Deciphering the mechanism(s) underlying the effects of maternal steroids is important to understanding the evolutionary consequences of steroid-mediated maternal effects. If embryos are capable of modulating the effects, then selection could act on both maternal and embryonic processes to shape how maternal steroids influence phenotypic variation. This could create a situation of parent-offspring conflict if the interests of mother and offspring do not match (Muller et al., 2007).
In addition to the observed decline in steroid concentrations early in development, levels of cortisol dramatically increased soon after hatching before falling again. Cortisol levels have also been shown to increase after hatching in zebrafish (Alsop and Vijayan, 2008) and rainbow trout (Auperin and Geslin, 2008; Barry et al., ’95; Fuzzen et al., 2011), which is thought to be the result of the embryonic HPI axis beginning to produce cortisol. At this point, it is unknown whether maternal contributions influence this cortisol production. PROG could potentially serve as a precursor for cortisol production but the fact that PROG does not also increase during this period suggests that PROG is not involved in the pathway leading to this cortisol production. The dynamic nature of cortisol levels in this study highlight the role of the embryo in modulating aspects of the early endocrine environment.
Operating under the premise that G. aculeatus embryos are capable of modulating their exposure to maternal steroids, this species provides a unique opportunity to investigate how selective pressures may shape maternal and embryonic processes related to the effect of maternal steroids on phenotypic variation. The recent evolutionary history of G. aculeatus created a situation where populations inhabiting different post-glacial habitats exhibit a substantial amount of behavioral and morphological variation thought to be tied to population differences in local ecology (Bell and Foster, ’94). Since ecological factors such as population density (Pilz and Smith, 2004), human disturbance (Bertin et al., 2008), predation pressure (Gieising et al., 2011), day length (Schwabl, ’96), maternal condition (Hegyi et al., 2011; Safran et al., 2008), and maternal social status (Muller et al., 2002) have all been shown to influence maternal steroid levels in the eggs of oviparous vertebrates, it is possible that steroid-mediated maternal effects may account for some of the observed population differences in phenotypes in G. aculeatus. Experimental work aimed at elucidating population differences in maternal and embryonic processes related to maternal steroid exposure will facilitate our understanding of the role these maternal effects may play in evolutionary processes. The role of maternal effects in evolutionary processes may take on even greater importance when examining the response of species to rapid environmental change (Bonduriansky et al., 2012). Along the lines of changing environments, characterizing the mechanisms underlying the decline in maternal steroid concentrations has possible implications for studies of endocrine disruption. G. aculeatus has been used as a model system to study how endocrine disrupting chemicals (EDCs) elicit their effects (Bell, 2001; Bell, 2004; Hogan et al., 2008; Pottinger et al., 2002). It has recently been hypothesized that some EDCs may elicit their effects by inhibiting the metabolism of maternal steroids such that the observed effects are due to an increased exposure of endogenous steroids that are present in the egg (Clairardin et al., 2013). By demonstrating that G. aculeatus eggs contain a variety of maternally derived steroids and that levels decline early following fertilization, it suggests that embryos could be disrupted by exposure to various steroids depending on the susceptibility of metabolic pathways to inhibition by EDCs. More work is required to characterize the metabolic fate of maternal steroids in G. aculeatus and determine what effects maternal steroids have on embryonic development.
The data from this study demonstrate that G. aculeatus eggs contain a number of maternal steroids at the time of oviposition that may be capable of influencing embryonic development. Concentrations of these steroids decline rapidly thereby highlighting the dynamic nature of the endocrine environment during development. While the precise role of G. aculeatus embryos in modulating steroid levels remains unknown, it is likely that developing embryos play an active role in regulating the early endocrine environment. Understanding the processes (both maternal and embryonic) involved in steroid-mediated maternal effects (or lack thereof) has important implications for the understanding evolutionary potential of these effects. Additionally, disruption of these processes may also have important consequences for the developing embryo. Overall, G. aculeatus provides a powerful system for addressing these questions related to steroid-mediated maternal effects.
Acknowledgments
We would like to thank Rachel Bowden for the use of space and equipment to quantify steroids. This work was supported by grants from the NIH (R01 GM082937-01) and the NSF (IOS 1121980). All procedures were approved by the IACUC at the University of Illinois (protocol #12118).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
LITERATURE CITED
- Albergotti LC, Hamlin HJ, McCoy MW, Guillette LJ. Endocrine activity of extraembryonic membranes extends beyond placental amniotes. PLoS ONE. 2009;4(5):e5452. doi: 10.1371/journal.pone.0005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2008;294:711–719. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- Antila E. Steroid conversion by oocytes and early embryos of Salmo gairdneri. Ann Zool Fenn. 1984;21:465–471. [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auperin B, Geslin M. Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen Comp Endocrinol. 2008;158:234–239. doi: 10.1016/j.ygcen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Baker JA, Heins DC, Foster SA, King RW. An overview of life-history variation in female threespine stickleback. Behaviour. 2008;145:579–602. [Google Scholar]
- Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. New York: Academic Press; 2002. [Google Scholar]
- Barry TP, Malison JA, Held JA, Parrish JJ. Ontogeny of the cortisol stress-response in larval rainbow-trout. Gen Comp Endocrinol. 1995;97:57–65. doi: 10.1006/gcen.1995.1006. [DOI] [PubMed] [Google Scholar]
- Bell AM. Effects of an endocrine disrupter on courtship and aggressive behaviour of male three-spined stickleback, Gasterosteus aculeatus. Anim Behav. 2001;62:775–780. [Google Scholar]
- Bell AM. An endocrine disrupter increases growth and risky behavior in threespined stickleback (Gasterosteus aculeatus) Horm Behav. 2004;45:108–114. doi: 10.1016/j.yhbeh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Bell MA, Foster SA. Evolutionary biology of the three-spined stickleback. New York: Oxford University Press; 1994. [Google Scholar]
- Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Bertin A, Richard-Yris MA, Houdelier C, et al. Habituation to humans affects yolk steroid levels and offspring phenotype in quail. Horm Behav. 2008;54:396–402. doi: 10.1016/j.yhbeh.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, Crean AJ, Day T. The implications of nongenetic inheritance for evolution in changing environments. Evol Appl. 2012;5:192–201. doi: 10.1111/j.1752-4571.2011.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden RM, Ewert MA, Nelson CE. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc R Soc Lond B Biol Sci. 2000;267:1745–1749. doi: 10.1098/rspb.2000.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton T, Hoogenboom MO, Armstrong JD, Groothuis TGG, Metcalfe NB. Egg hormones in a highly fecund vertebrate: do they influence offspring social structure in competitive conditions? Funct Ecol. 2011;25:1379–1388. [Google Scholar]
- Clairardin SG, Paitz RT, Bowden RM. In ovo inhibition of steroid metabolism by bisphenol A as a potential mechanism of endocrine disruption. Proc Roy Soc Lond B Biol Sci. 2013;280:20131773. doi: 10.1098/rspb.2013.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus EG, Hirano T, Inui Y. Changes in cortisol and thyroid concentrations during early development and metamorphosis in the Japanese flounder, Paralichthys olivaceus. Gen Comp Endocrionol. 1991;82:369–376. doi: 10.1016/0016-6480(91)90312-t. [DOI] [PubMed] [Google Scholar]
- Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. [Google Scholar]
- Diczfalusy E. Endocrine functions of the human fetoplacental unit. Fed Proc. 1964;23:791–798. [PubMed] [Google Scholar]
- Eriksen MS, Bakken M, Espmark A, Braastad BO, Salte R. Prespawning stress in farmed Atlantic salmon, Salmo salar: maternal cortisol exposure and hyperthermia during embryonic development affect offspring survival, growth and incidence of malformations. J Fish Biol. 2006;69:114–129. [Google Scholar]
- Eriksen MS, Espmark A, Braastad BO, Salte R, Bakken M. Long-term effects of maternal cortisol exposure and mild hyperthermia during embryogeny on survival, growth and morphological anomalies in farmed Atlantic salmon (Salmo salar) offspring. J Fish Biol. 2007;70:462–473. [Google Scholar]
- Feist G, Schreck CB. Brain-pituitary-gonadal axis during early development and sexual differentiation in the rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. 1996;102:394–409. doi: 10.1006/gcen.1996.0083. [DOI] [PubMed] [Google Scholar]
- Feist G, Schreck CB, Fitzpatrick MS, Redding JM. Sex steroid profiles of coho salmon (Oncorhynchus kisutch) during early development and sexual differentiation. Gen Comp Endocrinol. 1990;80:299–313. doi: 10.1016/0016-6480(90)90174-k. [DOI] [PubMed] [Google Scholar]
- Fischer S, Kluver N, Burkhardt-Medicke K, et al. Abcb4 acts as multixenobiotic transporter and active barrier against chemical uptake in zebrafish (Danio rerio) embryos. BMC Biol. 2013;11:1–16. doi: 10.1186/1741-7007-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzzen MLM, Alderman SL, Bristow EN, Bernier NJ. Ontogeny of the corticotropin-releasing factor system in rainbow trout and differential effects of hypoxia on the endocrine and cellular stress responses during development. Gen Comp Endocrinol. 2011;170:604–612. doi: 10.1016/j.ygcen.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Gagliano M, McCormick MI. Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia. 2009;160:657–665. doi: 10.1007/s00442-009-1335-8. [DOI] [PubMed] [Google Scholar]
- Gerall AA, Moltz M, Ward I. Sexual differentiation. New York: Plenum; 1992. [Google Scholar]
- Giesing ER, Suski CD, Warner RE, Bell AM. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proc R Soc Lond B Biol Sci. 2011;278:1753–1759. doi: 10.1098/rspb.2010.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D. Hormones in avian eggs: physiology, ecology, and behavior. In: Brockmann HJ, Roper TJ, Naguib M, Wynne-Edwards KE, Barnard C, Mitani JC, editors. Advances In The Study Of Behavior Amsterdam. Academic Press; 2008. pp. 337–398. [Google Scholar]
- Gonzalez CB, Cozza EN, Debedners MEO, Lantos CP, Aragones A. Progesterone and its reductive metabolism in steroidogenic tissues of the developing hen embryo. Gen Comp Endocrinol. 1983;51:384–393. doi: 10.1016/0016-6480(83)90054-0. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Muller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil Trans R Soc B. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB. Sex determination and primary sex differentiation in amphibians: genetic and developmental mechanisms. J Exp Zool. 1998;281:373–399. [PubMed] [Google Scholar]
- Hegyi G, Herenyi M, Szollosi E, et al. Yolk androstenedione, but not testosterone, predicts offspring fate and reflects parental quality. Behav Ecol. 2011;22:29–38. [Google Scholar]
- Hines GA, Boots LR, Wibbels T, Watts SA. Steroid levels and steroid metabolism in relation to early gonadal development in the tilapia Oreochromis niloticus (Teleostei : Cyprinoidei) Gen Comp Endocrinol. 1999;114:235–248. doi: 10.1006/gcen.1998.7244. [DOI] [PubMed] [Google Scholar]
- Hogan NS, Wartman CA, Finley MA, van der Lee JG, van den Heuvel MR. Simultaneous determination of androgenic and estrogenic endpoints in the threespine stickleback (Gasterosteus aculeatus) using quantitative RT-PCR. Aquat Toxicol. 2008;90:269–276. doi: 10.1016/j.aquatox.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Hwang PP, Wu SM, Lin JH, Wu LS. Cortisol content of eggs and larvae of teleosts. Gen Comp Endocrinol. 1992;86:189–196. doi: 10.1016/0016-6480(92)90101-o. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T, Kobayashi H, Sagegami R, Shuo T. Testosterone content of developing eggs and sex reversal in the medaka (Oryzias latipes) Gen Comp Endocrinol. 2006;145:67–74. doi: 10.1016/j.ygcen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jentoft S, Held JA, Malison JA, Barry TP. Ontogeny of the cortisol stress response in yellow perch (Perca flavescens) Fish Physiol Biochem. 2002;26:371–378. [Google Scholar]
- Khan MN, Renaud RL, Leatherland JF. Steroid metabolism by embryonic tissues of Arctic charr, Salvelinus alpinus. Gen Comp Endocrinol. 1997a;105:344–357. doi: 10.1006/gcen.1996.6835. [DOI] [PubMed] [Google Scholar]
- Khan MN, Renaud RL, Leatherland JF. Metabolism of estrogens and androgens by embryonic tissues of Arctic charr, Salvelinus alpinus. Gen Comp Endocrinol. 1997b;107:118–127. doi: 10.1006/gcen.1997.6908. [DOI] [PubMed] [Google Scholar]
- Lance VA. Sex determination in reptiles: An update. Am Zool. 1997;37:504–513. [Google Scholar]
- Levitz M. Conjugation and transfer of fetal-placental steroid hormones. J Clin Endocrinol Metab. 1966;26:773–779. doi: 10.1210/jcem-26-7-773. [DOI] [PubMed] [Google Scholar]
- Lewis ZR, McClellan MC, Postlethwait JH, Cresko WA, Kaplan RH. Female-specific increase in primordial germ cells marks sex differentiation in Threespine Stickleback (Gasterosteus aculeatus) J Morphol. 2008;269:909–921. doi: 10.1002/jmor.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Bureau DP, King WA, Leatherland JF. The actions of in ovo cortisol on egg fertility, embryo development and the expression of growth-related genes in rainbow trout embryos, and the growth performance of juveniles. Mol Reprod Dev. 2010;77:922–931. doi: 10.1002/mrd.21239. [DOI] [PubMed] [Google Scholar]
- Li M, Russell SK, Lumsden JS, Leatherland JF. The influence of oocyte cortisol on the early ontogeny of intelectin and TLR-5, and changes in lysozyme activity in rainbow trout (Oncorhynchus mykiss) embryos. Comp Biochem Physiol B. 2011;160:159–165. doi: 10.1016/j.cbpb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Li M, Christie HL, Leatherland JF. The in vitro metabolism of cortisol by ovarian follicles of rainbow trout (Oncorhynchus mykiss): comparison with ovulated oocytes and pre-hatch embryos. Reproduction. 2012;144:713–722. doi: 10.1530/REP-12-0354. [DOI] [PubMed] [Google Scholar]
- McCormick MI. Behaviorally induced maternal stress in a fish influences progeny quality by a hormonal mechanism. Ecology. 1998;79:1873–1883. [Google Scholar]
- McCormick MI. Experimental test of the effect of maternal hormones on larval quality of a coral reef fish. Oecologia. 1999;118:412–422. doi: 10.1007/s004420050743. [DOI] [PubMed] [Google Scholar]
- McGhee KE, Pintor LM, Suhr EL, Bell AM. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Funct Ecol. 2012;26:932–940. doi: 10.1111/j.1365-2435.2012.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer BC, Bell AM. A test of maternal programming of offspring stress response to predation risk in threespined sticklebacks. Physiol Behav. 2013;122:222–227. doi: 10.1016/j.physbeh.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MC, Johnston GIH. Toward a dynamic model of deposition and utilization of yolk steroids. Integr Comp Biol. 2008;48:411–418. doi: 10.1093/icb/icn079. [DOI] [PubMed] [Google Scholar]
- Muller W, Eising CM, Dijkstra C, Groothuis TGG. Sex differences in yolk hormones depend on maternal social status in Leghorn chickens (Gallus gallus domesticus) Proc R Soc Lond B Biol Sci. 2002;269:2249–2255. doi: 10.1098/rspb.2002.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W, Lessells CM, Korsten P, von Engelhardt N. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am Nat. 2007;169:E84–E96. doi: 10.1086/511962. [DOI] [PubMed] [Google Scholar]
- Painter DL, Moore MC. Steroid hormone metabolism by the chorioallantoic placenta of the mountain spiny lizard (Sceloporus jarrovi) as a possible mechanism for buffering maternal-fetal hormone exchange. Physio Biochem Zool. 2005;78:364–372. doi: 10.1086/430222. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM. A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr Comp Biol. 2008;48:419–427. doi: 10.1093/icb/icn034. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM. Rapid decline in the concentrations of three yolk steroids during development: Is it embryonic regulation? Gen Comp Endocrinol. 2009;161:246–251. doi: 10.1016/j.ygcen.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM. Progesterone metabolites, “xenobioticsensing” nuclear receptors, and the metabolism of maternal steroids. Gen Comp Endocrinol. 2010;166:217–221. doi: 10.1016/j.ygcen.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM. Biological activity of oestradiol sulphate in an oviparous amniote: implications for maternal steroid effects. Proc R Soc Lond B Biol Sci. 2011;278:2005–2010. doi: 10.1098/rspb.2010.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM, Casto JM. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris) Proc R Soc Lond B Biol Sci. 2011;278:99–106. doi: 10.1098/rspb.2010.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz RT, Casto JM. The decline in yolk progesterone concentrations during incubation is dependent on embryonic development in the European starling. Gen Comp Endocrinol. 2012;176:415–419. doi: 10.1016/j.ygcen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Parsons IC. Metabolism of testosterone by early chick embryonic blastoderm. Steroids. 1970;16:59–65. doi: 10.1016/s0039-128x(70)80095-2. [DOI] [PubMed] [Google Scholar]
- Petkam R, Renaud RL, Freitas A, Canario AVM, Leatherland JF. In vitro metabolism of progesterone, androgens and estrogens by rainbow trout embryos. Fish Physiol Biochem. 2002;27:117–128. [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pilz KM, Smith HG. Egg yolk androgen levels increase with breeding density in the European Starling, Sturnus vulgaris. Funct Ecol. 2004;18:58–66. [Google Scholar]
- Pottinger TG, Carrick TR, Yeomans WE. The three-spined stickleback as an environmental sentinel: effects of stressors on whole-body physiological indices. J Fish Biol. 2002;61:207–229. [Google Scholar]
- Roche DP, McGhee KE, Bell AM. Maternal predator-exposure has lifelong consequences for offspring learning in threespined sticklebacks. Biol Lett. 2012;8:932–935. doi: 10.1098/rsbl.2012.0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard S, Moav B, Yaron Z. Changes in steroid concentrations during sexual ontogeny in tilapia. Aquaculture. 1987;61:59–74. [Google Scholar]
- Rowell CB, Watts SA, Wibbels T, Hines GA, Mair G. Androgen and estrogen metabolism during sex differentiation in mono-sex populations of the Nile tilapia, Oreochromis niloticus. Gen Comp Endocrinol. 2002;125:151–162. doi: 10.1006/gcen.2001.7691. [DOI] [PubMed] [Google Scholar]
- Safran RJ, Pilz KM, McGraw KJ, Correa SM, Schwabl H. Are yolk androgens and carotenoids in barn swallow eggs related to parental quality? Behav Ecol Sociobiol. 2008;62:427–438. [Google Scholar]
- Sampathkumar R, Byers RE, Munro AD, Lam TJ. Profile of cortisol during the ontogeny of the asian sea-bass, Lates calcarifer. Aquaculture. 1995;132:349–359. [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Environment modifies the testosterone levels of a female bird and its eggs. J Exp Zool. 1996;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Love OP. Determining the adaptive potential of maternal stress. Ecol Lett. 2013;16:271–280. doi: 10.1111/ele.12042. [DOI] [PubMed] [Google Scholar]
- Sloman KA. Exposure of ova to cortisol pre-fertilisation affects subsequent behaviour and physiology of brown trout. Horm Behav. 2010;58:433–439. doi: 10.1016/j.yhbeh.2010.05.010. [DOI] [PubMed] [Google Scholar]
- von Engelhardt N, Henriksen R, Groothuis TGG. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen Comp Endocrinol. 2009;163:175–183. doi: 10.1016/j.ygcen.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Williams TD. Physiological adaptations for breeding in birds. Princeton: Princeton University Press; 2012. [Google Scholar]
- Williams TD, Ames CE, Kiparissis Y, Wynne-Edwards KE. Laying-sequence-specific variation in yolk oestrogen levels, and relationship to plasma oestrogen in female zebra finches (Taeniopygia guttata) Proc R Soc Lond B Biol Sci. 2005;272:173–177. doi: 10.1098/rspb.2004.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. Sex differentiation. In: Hoar WS, Randall DJ, editors. Fish Physiology. New York: Academic Press; 1969. pp. 117–175. [Google Scholar]
- Yeoh CG, Schreck CB, Fitzpatrick MS, Feist GW. In vivo steroid metabolism in embryonic and newly hatched steelhead trout (Oncorhynchus mykiss) Gen Comp Endocrinol. 1996;102:197–209. doi: 10.1006/gcen.1996.0061. [DOI] [PubMed] [Google Scholar]
- Zucchi S, Castiglioni S, Fent K. Progestins and antiprogestins affect gene expression in early development in zebrafish (Danio rerio) at environmental concentrations. Environ Sci Technol. 2012;46:5183–5192. doi: 10.1021/es300231y. [DOI] [PubMed] [Google Scholar]


