Abstract
With post-transplantation cyclophosphamide (PTCy) as graft-versus-host disease (GVHD) prophylaxis, nonmyeloablative HLA-haploidentical (NMA haplo) and HLA-matched blood or marrow transplantation (BMT) have comparable outcomes. Early discontinuation of immunosuppression may reduce the risk of relapse and improve immune reconstitution, but may increase the risk of GVHD. We conducted a prospective trial of NMA haplo BMT for patients with hematologic malignancies (median age, 61 years), evaluating the safety of early discontinuation of tacrolimus. All patients received T cell-replete bone marrow followed by high-dose PTCy, mycophenolate mofetil, and tacrolimus. Tacrolimus was prespecified to stop without taper at day +90, +60, or +120, contingent on having ≥5% donor T cells, no relapse, and no grade II-IV acute or significant chronic GVHD. Safety stopping rules were based on ≥5% graft failure, ≥10% nonrelapse mortality (NRM), or a ≥20% combined incidence of severe acute and chronic GVHD from the tacrolimus stop date through day +180. Of the 47 patients in the day +90 arm, 23 (49%) stopped tacrolimus as planned. Of the 55 patients in the day +60 arm, 38 (69%) stopped as planned. Safety stopping criteria were not met. In both arms, at day +180, the probability of grade II-IV acute GVHD was <40%, that of grade III-IV acute GVHD was <8%, and that of NRM was <5%. The 1-year probabilities of chronic GVHD and NRM were <15% and <10%, respectively, in both arms. The 1-year GVHD-free relapse-free survival was higher in the day 60 arm. Thus, stopping tacrolimus as early as day +60 is feasible and carries acceptable risks after NMA haplo BMT with PTCy. This approach may facilitate post-transplantation strategies for relapse reduction. © 2018 Published by Elsevier Inc. on behalf of the American Society for Blood and Marrow Transplantation.
Keywords: Tacrolimus, Cyclophosphamide, Haploidentical, Graft-versus-host disease, Immunosuppression, Nonmyeloablative
INTRODUCTION
Modern approaches to graft-versus-host disease (GVHD) prophylaxis, such as high-dose post-transplantation cyclophosphamide (PTCy) [1,2], have reduced transplantation-related toxicities and have expanded the donor pool for patients who lack HLA-matched donors [3–5]. Such approaches have broadened the applicability of allogeneic blood or marrow transplantation (BMT), especially when coupled with nonmyeloablative (NMA) or reduced-intensity conditioning (RIC) regimens [6,7]. NMA, related HLA-haploidentical (haplo) BMT with PTCy has safety, efficacy, and survival outcomes comparable to those of HLA-matched BMT [7–10]. The leading cause of treatment failure with either approach is now relapse, rather than toxicity.
A calcineurin inhibitor plus methotrexate or mycophenolate mofetil is commonly used for GVHD prophylaxis. Prophylactic immunosuppression (IS) must be sufficient to permit engraftment and mitigate excessive GVHD, but extended courses can increase the risks of infection, drug toxicity, and disease relapse [11]. By suppressing T cell activation, calcineurin inhibitors may also blunt the graft-versus-tumor effect [12,13]. Shortening IS may augment the graft-versus-tumor effect, but may increase graft failure and the incidence and severity of GVHD [14–16].
In addition to potentially reducing relapse, shortening the duration of pharmacologic IS may mitigate the risks of opportunistic infections and drug toxicities, such as tacrolimus-associated nephrotoxicity and neurotoxicity. These considerations support curtailing calcineurin inhibitor administration to the shortest duration compatible with acceptably low incidences of graft failure and severe GVHD. Nonetheless, few published studies have evaluated the optimal duration of GVHD prophylaxis for RIC regimens [11,16]. A retrospective analysis of NMA matched related BMT with mycophenolate mofetil and cyclosporine for GVHD prophylaxis suggested 180 days of cyclosporine was optimal [16]. Not only is there is a paucity of prospective data overall, but also we are unaware of any published studies on the minimum required duration of IS after haplo BMT. We present a completed, single-center clinical trial of stopping tacrolimus, without taper, 3 or 4 months earlier than our day 180 standard after NMA haplo BMT with PTCy.
PATIENTS AND METHODS
This single-arm, phase I/II clinical trial was conducted at Johns Hopkins University. The study received Institutional Review Board approval, and all participants provided written informed consent. Between August 2011 and November 2015, 114 patients with hematologic malignancies underwent NMA haplo BMT on this study. The primary objective was to evaluate the feasibility and safety of reduced-duration tacrolimus, stopping tacrolimus without taper before our institution’s day 180 standard.
Eligibility and Treatment
Criteria for BMT were based on institutional standards and included age 0.5 to 75 years, Eastern Cooperative Oncology Group performance status ≤2, left ventricular ejection fraction ≥35%, forced expiratory volume in 1 second and forced vital capacity ≥40% of predicted, and adequate hepatic function. Morphological complete response was required for acute leukemia, and at least partial response was required for aggressive lymphoma. Recipients of previous allogeneic BMT were excluded. To mitigate graft rejection, cytotoxic chemotherapy or ≥4 cycles of a hypomethylating agent were recommended before conditioning.
Donors were first-degree relatives or half-siblings who shared ≥5/10 but <10/10 HLA alleles (HLA-A, -B, -Cw, -DRB1, -DQB1). Donor selection was done following our institutional standards at the time, as described previously [6]. The degree of HLA match, as long as at least 5/10, did not enter into the selection prioritization. Donors were ineligible if the recipient had anti-HLA donor-specific antibodies too strong for desensitization; however, desensitization was permitted [17].
All patients received our standard NMA conditioning consisting of fludarabine (30 mg/m2 i.v. on days −6 to −2, renally adjusted), Cy (14.5 mg/ kg i.v. on days −6 and −5), and total body irradiation (200 cGy on day −1), followed by a T cell-replete bone marrow graft on day 0 [3]. GVHD prophylaxis consisted of high-dose PTCy (50 mg/kg i.v. on days +3 and +4) with mesna, mycophenolate mofetil (on days +5 to +35), and tacrolimus (initiated on day +5) [3]. Tacrolimus was dosed with a target trough of 5 to 15 ng/ mL and discontinued without taper as described below. Filgrastim was administered from day +5 until neutrophil recovery to ≥1000/μL for 3 days. Supportive care was provided as described previously [6]. Management of GVHD was not protocol-specified.
Before transplantation, patients were assigned to stop tacrolimus early if eligible, without taper, after the last dose on day +90, +60, or +120. Tacrolimus was first planned up through day +90 (termed the “day 90 cohort”). If safety was acceptable, day +60 tacrolimus cessation would be studied; otherwise, day +120 tacrolimus cessation would be studied. After the day +90 cohort completed accrual, the day +120 cohort was briefly enrolled (n = 3) while safety data were maturing. On demonstration of acceptable safety in the day +90 cohort, study of day +60 tacrolimus cessation commenced. Stopping tacrolimus up to 5 days after the scheduled stop date was permissible for logistical reasons. Two patients (in the day +60 cohort) stopped 1 day beyond this window, and 1 patient (in the day +90 cohort) stopped 3 days beyond this window.
Eligibility for early tacrolimus cessation was evaluated before the planned stop date and consisted of having ≥5% donor T cells at ~day +56 onward, no relapse, no history of grade II-IV acute GVHD, no chronic GVHD other than oral, and no use of alternative IS due to tacrolimus intolerance. Ongoing grade I acute GVHD was permissible, provided there was no history of higher-grade GVHD. For patients not meeting these criteria, IS was individualized and continued to day +180 in the absence of relapse, graft failure, or GVHD. Patients who experienced tacrolimus toxicity resulting in its earlier-than-scheduled discontinuation, or who were eligible to stop early but did not, were considered unevaluable for the primary endpoint. Of the 114 transplant recipients, 9 were unevaluable for the primary endpoint because of tacrolimus intolerance (n = 7), noncompliance (n = 1), or inadvertent continuation of tacrolimus (n = 1). The remaining 105 patients compose the cohort for analysis.
Statistical Methods
The primary analysis evaluated both the feasibility and safety of reduced-duration tacrolimus. The study was not designed to detect improvements in relapse risk. Feasibility and safety were evaluated continually using Bayesian monitoring rules. The monitoring rules deemed reduced IS “feasible” if ≥33% of patients could stop tacrolimus early as planned. Up to 55 transplants per cohort were planned, to identify at least 15 patients evaluable for safety. The safety of reduced-duration IS was evaluated from the tacrolimus stop date to ~day +180. Safety stopping rules were based on a ≥65% probability of a ≥5% incidence of graft failure, a ≥10% incidence of nonrelapse mortality (NRM), or a ≥20% incidence of GVHD events as next defined in this window. The GVHD stopping rule was initially based on a ≥20% combined incidence of grade II-IV acute and severe chronic GVHD, then was revised to a ≥20% combined incidence of grade III-IV acute and severe chronic GVHD. An interim assessment of the day +60 tacrolimus cohort triggered an accrual pause due to a potential increase in grade II-IV GVHD. There was no apparent increased incidence of grade III-IV GVHD or NRM, and emerging data suggested that grade II limited GVHD after haplo BMT is associated with improved progression-free survival (PFS) [18]. The GVHD stopping rule was modified and study of day +60 tacrolimus cessation continued with Institutional Review Board approval.
Historical data from 212 NMA haplo transplants at Johns Hopkins, using the identical regimen but with tacrolimus until day +180, informed safety risk calculations. The study’s monitoring plan made use of this historical information, appropriately discounted, as a basis for a prior distribution for the risks of monitored events within prespecified intervals between days +1 and +180. Data from the day +90 tacrolimus cohort also informed safety assessments for the day +60 cohort.
Graft failure was defined as ≤5% donor chimerism in peripheral blood and/or bone marrow by ~day +56 without detected bone marrow malignancy. The study evaluated donor chimerism at ~days +28, +56, +84, +112, +180, +270, and +365. Acute GVHD was scored using the modified Keystone criteria [19], and chronic GVHD was evaluated using National Institutes of Health consensus criteria [20].
PFS, overall survival (OS), and GVHD-free relapse-free survival (GRFS) [21] were estimated by the Kaplan-Meier method. Median follow-up was calculated by the reverse Kaplan-Meier method. Cumulative incidences of relapse, NRM, count recovery, and GVHD were estimated using competing-risk methods [22]. Graft failure, treatment of relapse, and death were competing risks for GVHD, and relapse and NRM were mutual competing risks. The database was locked on November 13, 2016. Time-to-event endpoints are measured from day 0 unless noted otherwise.
RESULTS
Overall Outcomes
Patient and transplantation characteristics are summa-rized in Table 1. The 105 evaluable patients included 47 in the day +90 cohort, 55 in the day +60 cohort, and 3 in the day +120 cohort. The median age was 61 years, with approximately one-third (34%) age ≥65 years. Seventy-eight percent of grafts had ≥4 antigen mismatches at HLA class I and II combined, with <5% having <3 antigen mismatches. The most common diagnoses were acute leukemia (51%), myelodysplastic syndrome or myeloproliferative neoplasm (18%), and aggressive non-Hodgkin lymphoma (17%). By refined Disease Risk Index (DRI) grouping, 11% of diseases were low risk, 70% were intermediate risk, and 19% were high risk. The day +90 and day +60 tacrolimus cohorts were similar with respect to median patient age, DRI distribution, degree of HLA antigen mismatch, patient cytomegalovirus serostatus, and CD34+ cell graft dose (Table 1). There were statistically significant differences between the day +90 and day+ 60 arms in other parameters, with younger donors, higher total nucleated cell graft doses, and more patients with acute myelogenous leukemia in the day +60 arm. Overall, 16% of patients, significantly more in the day +60 cohort, received post-transplantation maintenance therapy, consisting chiefly of tyrosine kinase inhibitors or a hypomethylating agent.
Table 1.
Patient Characteristics Overall and by Planned Tacrolimus Duration
| Variable | Planned Tacrolimus Stop Date
|
|||||
|---|---|---|---|---|---|---|
| Day +60, +90, or +120 (n = 105)*,† | Day +90 (n = 47) | Day +60 (n = 55) | ||||
| Patient age, yr | ||||||
| Median (range) | 61 | (13–74) | 61 | (19–73) | 60 | (13–74) |
| ≥65 yr, n (%) | 36 | (34) | 15 | (32) | 19 | (35) |
| Male sex, n (%) | 75 | (71) | 39 | (83) | 34 | (62) |
| Feasibility of reduced IS, n (%) | ||||||
| Feasible to stop early | 63 | (60) | 23 | (49) | 38 | (69) |
| Not feasible | 42 | (40) | 24 | (51) | 17 | (31) |
| Reasons for infeasibility of reduced IS, n (%) | ||||||
| Acute GVHD | 20 | (19) | 10 | (21) | 10 | (18) |
| Grade II | 19 | 9 | 10 | |||
| Grade III-IV | 1 | 1 | 0 | |||
| Graft failure or <5% donor T cells | 10 | (10) | 6 | (13) | 4 | (7) |
| Relapse | 9 | (9) | 6 | (13) | 2 | (4) |
| NRM | 3 | (3) | 2 | (4) | 1 | (2) |
| Diagnosis, n (%) | ||||||
| AML | 45 | (43) | 13 | (28) | 30 | (55) |
| ALL | 8 | (8) | 4 | (9) | 4 | (7) |
| MDS or MPN | 19 | (18) | 10 | (21) | 8 | (15) |
| CMML or CML | 3 | (3) | 1 | (2) | 2 | (4) |
| Aggressive NHL | 13 | (12) | 7 | (15) | 6 | (11) |
| Hodgkin lymphoma | 8 | (8) | 4 | (9) | 4 | (7) |
| Indolent NHL, MCL or CLL | 5 | (5) | 4 | (9) | 1 | (2) |
| Multiple myeloma | 4 | (4) | 4 | (9) | 0 | (0) |
| Refined DRI group, n (%) | ||||||
| Low risk | 12 | (11) | 6 | (13) | 5 | (9) |
| Intermediate risk | 73 | (70) | 33 | (70) | 39 | (71) |
| High risk‡ | 20 | (19) | 8 | (17) | 11 | (20) |
| Patient CMV IgG positive, n (%) | 60 | (57) | 26 | (55) | 32 | (58) |
| CMV mismatch, n (%) | 38 | (36) | 14 | (30) | 22 | (40) |
| Donor age, yr, median (range) | 38 | (17–68) | 40 | (17–68) | 33 | (18–61) |
| Class I and II antigen mismatches,§ | ||||||
| Median (range) | 5 | (1–5) | 5 | (1–5) | 5 | (2–5) |
| 5, n (%) | 59 | (56) | 24 | (51) | 32 | (58) |
| 4, n (%) | 23 | (22) | 11 | (23) | 12 | (22) |
| 3, n (%) | 19 | (18) | 9 | (19) | 10 | (18) |
| 1–2, n (%) | 4 | (4) | 3 | (6) | 1 | (2) |
| Female donor for male patient, n | 25 | (24) | 15 | (32) | 9 | (16) |
| Cell dose infused, median (IQR) | ||||||
| Total nucleated cells ×108/kg | 4.6 | (3.9–5.3) | 4.2 | (3.8–5.1) | 4.9 | (4.2–5.4) |
| CD3+ cells ×107/kg | 4.2 | (3.4–5.4) | 4.0 | (3.4–5.0) | 4.8 | (3.5–5.7) |
| CD34+ cells ×106/kg | 4.1 | (3.5–5.3) | 4.3 | (3.0–5.3) | 4.1 | (3.6–5.2) |
| Post-BMT maintenance therapy, n (%) | 17‖ | (16) | 4 | (9) | 13 | (24) |
AML indicates acute myelogenous leukemia; ALL, acute lymphoblastic leukemia or lymphoma; CLL, chronic lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; DRI, disease risk index; IQR, interquartile range; IS, immunosuppression; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma.
Patients assigned to particular tacrolimus duration, regardless of actual duration.
All percentages are for column-wise comparisons.
None were very high-risk.
Antigen mismatching in any direction; composite of HLA-A, -B, -Cw, -DRB1, and -DQB1.
BCR-ABL tyrosine kinase inhibitor (n = 5 patients), sorafenib (n = 5), azacitidine (n = 5), rituximab (n = 2).
For the group overall, 63 patients (60%) stopped tacrolimus early according to protocol-specified criteria. Ineligibility for shortened IS was most commonly due to acute GVHD, followed by low donor T-cell chimerism or graft failure and early relapse (Table 1).
Regardless of tacrolimus duration, neutrophils recovered at a median of 17 days (90% by day +30), and platelets recovered to ≥20,000/μL at a median of 26 days (88% by day +60). Primary or secondary graft failure occurred in 11% of the evaluable patients, with autologous hematopoietic recovery in most cases. At day +180, the probability of NRM was 3% (90% confidence interval [CI], 0% to 6%), that of grade II-IV acute GVHD was 38% (90% CI, 30% to 46%), and that of grade III-IV acute GVHD was 7% (90% CI, 3% to 11%). The 1-year probability of any chronic GVHD was 11% (90% CI, 6% to 16%), with the majority of cases (8 of 12) being mild.
With a median follow-up of 33 months overall, the 2-year estimates of relapse, PFS, and OS were 52% (90% CI, 43% to 61%), 39% (90% CI, 32% to 49%), and 56% (90% CI, 48% to 65%), respectively. The estimated 1-year GRFS was 41% (90% CI, 34% to 50%).
Safety of Early Tacrolimus Cessation
Safety stopping criteria, based on the probabilities of ≥20% grade III-IV acute or severe chronic GVHD, ≥5% graft failure, and ≥10% NRM in the safety evaluation window, were not met after either day +90 or day +60 tacrolimus discontinuation. Safety, evaluated from the early tacrolimus stop date to ~day +180, is summarized in Table 2. Two of 3 patients also discontinued tacrolimus at day +120 as planned, without safety stopping events.
Table 2.
Safety of Early Tacrolimus Cessation
| Parameter | Day +90 Tacrolimus Cessation (n = 23) | Day +60 Tacrolimus Cessation (n = 38) | ||
|---|---|---|---|---|
| Features before early tacrolimus cessation, n (%) | ||||
| Donor T cell chimerism, n | ||||
| Full donor (≥95%) | 22 | (96) | 33 | (87) |
| ≥50 but <95% | 0 | (0) | 2 | (5) |
| >5 but <50% | 1 | (4) | 3 | (9) |
| Grade I or possible grade I GVHD, n | 3* | (13) | 8* | (21) |
| In patients who developed grade II-IV acute GVHD | 2† | 5† | ||
| In patients with no safety events ‡ | 1 | 3 | ||
| Outcomes from tacrolimus stop date to ~day +180 | ||||
| No safety events,‡ n (%) | 21 | (91) | 34 | (89) |
| NRM, n (%) | 0 | (0) | 0 | (0) |
| Graft failure, n (%) | 0 | (0) | 1 | (3) |
| Grade II-IV acute GVHD, n (%) | 7 | (30)§ | 12 | (32) |
| Maximum grade II acute GVHD | 5‖ | (22) | 9 | (24) |
| With ungradable visceral GVHD | 0 | 2 | ||
| Grade III acute GVHD | 2 | (9) | 3 | (8) |
| Grade IV acute GVHD | 0 | (0) | 0 | (0) |
| Timing of grade II-IV acute GVHD | ||||
| Median days to diagnosis (range) | 18 | (7–42) | 17 | (8–56) |
| Cases diagnosed ≤30 days after stopping tacrolimus, n (%) | 6/7 | (86) | 11/12 | (92) |
| Severe chronic GVHD, n (%) | 0‖ | (0) | 0 | (0) |
One patient in the day +90 group and 6 in the day +60 group had grade I GVHD; in the remainder, GVHD was not distinguishable from drug eruption.
Of the 7 patients combined whose grade I GVHD evolved to grade II or higher after stopping tacrolimus, 6 had maximum grade II GVHD, including 2 with ungradable visceral involvement, and 1 had grade III GVHD.
Safety events defined as ≥5% graft failure, ≥10% NRM, or ≥20% combined incidence of grade III-IV acute and severe chronic GVHD, measured from date of tacrolimus cessation to ~day 180.
Percentages represent proportions rather than cumulative incidences.
One patient developed severe chronic GVHD after day +180 and died from sepsis.
Of the 47 patients in the day +90 cohort, 23 (49%) stopped tacrolimus early per protocol-specified criteria. Before early tacrolimus cessation, all but 1 patient had achieved full-donor T cell chimerism (Table 2). Of the patients stopping tacrolimus early, 14 have relapsed. No patients had NRM, graft failure, or severe chronic GVHD in the safety evaluation window. Of the 23 patients, 21 (91%) had no safety stopping events. Five patients (22%) developed maximum grade II acute GVHD, with 1 case complicated after day 180 by severe chronic GVHD and fatal sepsis. Two patients (9%) developed grade III-IV acute GVHD.
Of the 55 patients in the day +60 cohort, 38 (69%) stopped tacrolimus early as prespecified, including patients with grade I GVHD (Table 2). Before early tacrolimus cessation, 87% of patients had achieved full-donor T cell chimerism. Of the patients who stopped tacrolimus early, 12 have relapsed. No patients had NRM or severe chronic GVHD in the safety evaluation window. Of the 38 patients, 34 (89%) had no safety stopping events as defined above. One patient who had 11% donor T cells before stopping tacrolimus developed secondary graft failure. Nine patients (24%) developed maximum grade II acute GVHD, including 2 with ungradable visceral GVHD, and 3 (8%) developed grade III-IV acute GVHD.
After early tacrolimus cessation, the median time to diagnosis of grade II-IV GVHD in either group was 2.5 weeks, with almost all cases developing within 30 days (Table 2). Treatment of acute GVHD in this setting most commonly consisted of corticosteroids and resumption of tacrolimus, followed by corticosteroids alone. Most cases of acute grade II GVHD after early tacrolimus cessation resolved with initial treatment. Of the 5 patients with grade III-IV acute GVHD, 3 had resolution, and 2 were responding to GVHD treatment but died of relapse.
Outcomes According to Planned Tacrolimus Duration
Safety
Safety outcomes by cohort, regardless of the actual tacrolimus duration, are shown in Figure 1 and Table 3. Outcomes are presented relative to the historical experience with tacrolimus until day +180 (n = 212), without formal comparison. In both the day +90 arm (n = 47) and day +60 arm (n = 55), at day +180, the probability of grade III-IV acute GVHD was <40%, and that of grade III-IV acute GVHD was <8%, similar to historical experience. In the day +60 arm, compared with the day +90 arm, there was a signal for more grade II-IV acute GVHD occurring between approximately days +60 and +90 (Figure 1A); however, there were no evident increases in grade III-IV acute GVHD (Figure 1B) or NRM (Figure 1D). In both arms, the 1-year probability of any chronic GVHD was <15% (12% historically), that of 180-day NRM was <5%, and that of 1-year NRM was <10%.
Figure 1.
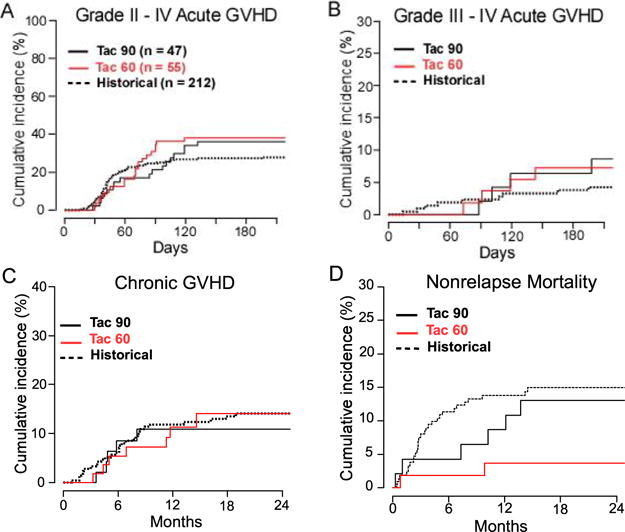
Safety outcomes of NMA, HLA-haploidentical BMT according to planned duration of tacrolimus. Cumulative incidences by competing-risk analysis of (A) grade II-IV acute GVHD, (B) grade III-IV acute GVHD, (C) any chronic GVHD, and (D) NRM. Point estimates and confidence intervals are provided in Table 3. The graphs represent all patients with the planned duration of tacrolimus (Tac), regardless of the actual duration.
Table 3.
Outcomes after NMA HLA-Haploidentical BMT According to Planned Tacrolimus Duration
| Outcome*,† | Planned Tacrolimus Stop Date
|
|||||
|---|---|---|---|---|---|---|
| Day +180 (Historical) (n = 212), % (90% CI) | Day +90 (n = 47),‡ % (90% CI) | Day +60 (n = 55), ‡ % (90% CI) | ||||
| Grade II-IV acute GVHD, CuI | ||||||
| Day +90 | 25 | (20–29) | 21 | (11–31) | 31 | (21–41) |
| Day +180 | 27 | (22–32) | 36 | (24–48) | 38 | (27–49) |
| Grade III-IV acute GVHD, CuI | ||||||
| Day +90 | 2 | (1–4) | 2 | (0–6) | 2 | (0–5) |
| Day +180 | 4 | (2–6) | 6 | (0–12) | 7 | (1–13) |
| Any chronic GVHD, CuI | ||||||
| 1 year | 12 | (8–16) | 11 | (3–19) | 11 | (4–19) |
| NRM, CuI | ||||||
| Day +180 | 11 | (8–15) | 4 | (0–9) | 2 | (0–5) |
| 1 year | 14 | (10–18) | 9 | (2–16) | 4 | (0–8) |
| Relapse, CuI | ||||||
| 1 year | 42 | (36–48) | 52 | (39–64) | 33 | (22–44) |
| 2 year | 51 | (45–57) | 58 | (46–71) | 47 | (34–60) |
| PFS | ||||||
| 1 year | 44 | (39–50) | 40 | (29–53) | 63 | (53–75) |
| 2 year | 35 | (29–41) | 28 | (19–42) | 49 | (38–63) |
| 3 year | 32 | (27–38) | 24 | (15–37) | = | |
| OS | ||||||
| 1 year | 60 | (55–66) | 59 | (48–72) | 78 | (69–88) |
| 2 year | 49 | (43–55) | 41 | (31–55) | 71 | (61–82) |
| 3 year | 41 | (35–48) | 36 | (26–50) | = | |
| GRFS | ||||||
| 1 year | =§ | 27 | (18–40) | 54 | (44–66) | |
| Median follow-up, months | 31 | = | 48 | = | 18 | = |
CuI indicates cumulative incidence.
For patients assigned to particular planned tacrolimus duration, regardless of actual duration.
All outcomes are measured from day 0.
23 patients in the day +90 cohort and 38 in the day +60 cohort stopped tacrolimus early as planned.
1-year GRFS was 45% (95% CI, 40-50) in a larger analysis that included these patients [23].
Efficacy
For descriptive purposes, efficacy outcomes by cohort, regardless of actual tacrolimus duration, are shown in Figure 2 and Table 3. In the day +90 arm, with a median follow-up of 48 months, the 1-year probabilities of relapse, PFS, and OS were 52%, 40%, and 59%, respectively (Figure 2). In the day +60 arm, with a median follow-up of 18 months, the 1-year probabilities of relapse, PFS, and OS were 33%, 63%, and 78%, respectively. Longer-term estimates are provided in Table 3. The estimated 1-year GRFS (Figure 2D) was higher in the day +60 arm (54%; 90% CI, 44% to 66%) compared with the day +90 arm (27%; 90% CI, 18% to 40%).
Figure 2.
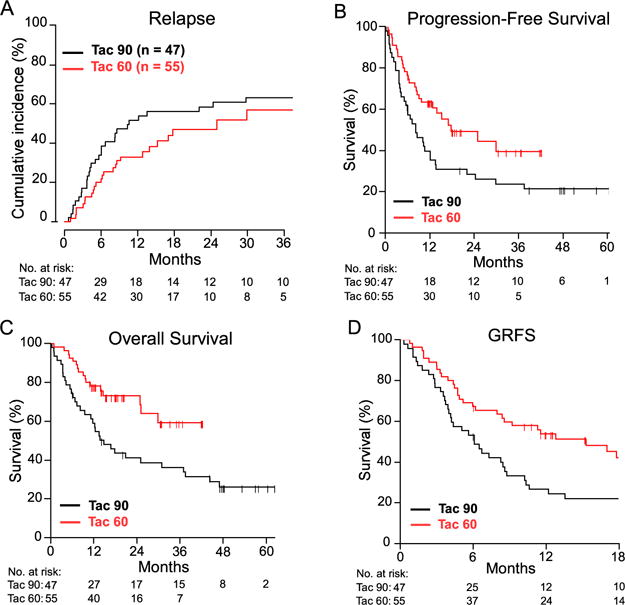
Efficacy of NMA, HLA-haploidentical BMT according to planned duration of tacrolimus. (A) Cumulative incidence of relapse by competing-risk analysis. (B) PFS. (C) OS. (D) GFRS. Point estimates and confidence intervals are provided in Table 3. The median follow-up was 48 months for the day +90 cohort and 18 months for the day +60 cohort.
DISCUSSION
Although the applicability of partially HLA-mismatched BMT has increased significantly, the optimal duration of GVHD prophylaxis has not yet been defined. There are very few published data (and even fewer prospective data) on the minimum acceptable duration of pharmacologic IS for NMA transplants, and such data are almost exclusively limited to HLA-matched BMT [23]. This prospective clinical trial uniquely informs the safety and feasibility of shortened-duration IS in an increasingly adopted platform of NMA haplo BMT. Despite T cell-replete allografting, the observed rates of severe acute GVHD, chronic GVHD, graft failure, and NRM in this study were similar to historical outcomes with tacrolimus until day +180 [23]. In both the day +60 and day +90 tacrolimus cohorts, the probability of grade III-IV acute GVHD was <10%, and there was no signal for excess NRM (<10% probability at 1 year). In the patients who developed acute GVHD after early tacrolimus cessation, overall responsiveness to GVHD treatment did not seem to be affected. The ability to reduce prophylactic IS is attributable to the unique immunomodulatory effects of PTCy [1].
To be relevant, an IS regimen not only must be safe, but also must be applicable to a sufficient number of patients. In this study, more than one-half of patients overall (49% in the day +90 cohort and 69% in the day +60 cohort) could discontinue tacrolimus early as prespecified. The higher feasibility in the latter arm was expected, given the less time to develop events that would render a patient ineligible for early stopping. Thus, our present data suggest that with this particular regimen, complete cessation of tacrolimus well before day +180 (3 to 4 months earlier) not only is associated with acceptable risks, but also is possible for a significant proportion of patients and may have a favorable impact on outcomes.
Importantly, these findings should not be extrapolated to unrelated donors, other GVHD prophylaxis platforms, or peripheral blood grafts. A recent retrospective comparison of bone marrow and peripheral blood BMT with PTCy showed a lower incidence of relapse with peripheral blood grafts, but this did not translate into improved OS [24]. Importantly, these transplantations used standard, long-course IS. The present study evaluated shortened IS exclusively in bone marrow grafts. How shortening IS would affect the outcomes of peripheral blood haplo transplants with PTCy is unknown, but is currently being studied at Johns Hopkins.
Among the strengths of this study are its prospective nature, the uniformity of the regimen, and the consistency of criteria for tacrolimus cessation. The small number and heterogeneity of patients are among its limitations. This single-institution, phase I/II study was not intended to definitively characterize the safety of early tacrolimus discontinuation. The CIs for the estimated toxicity rates after tacrolimus discontinuation are large, particularly for relatively infrequent events, such as severe GVHD and NRM. Particularly in the day +60 cohort, which had substantially shorter follow-up than the day +90 cohort, significant changes in relapse and toxicity outcomes might still occur. Other uncertainties to address include the safety of early IS discontinuation in patients with low donor T-cell chime-rism or ongoing or previous grade I acute GVHD. Of the 11 patients with grade I or possible grade I GVHD before early tacrolimus cessation, 7 developed higher-grade GVHD, although most cases were grade II (Table 2). A larger prospective study is needed to define the optimal duration of IS that balances GVHD risk and relapse risk, as well as the impact of post-transplantation maintenance therapy on these risks after shortened-duration IS.
Relapse remained the main cause of treatment failure in our study cohort. Notably, this study was not designed to detect whether shortening the duration of tacrolimus is associated with reduced relapse risk. A larger, randomized trial is needed to address the question of whether a reduction in IS confers a lower risk of relapse and improved PFS. As such, the comparisons of disease and survival outcomes between tacrolimus cohorts are exploratory only, and formal comparisons are not intended or valid. Although the distributions of patient age and DRI are similar in the 2 study arms, some important factors, such as diagnosis, use of maintenance therapy, and duration of follow-up, vary. Nevertheless, we find the suggestion of improved 1-year GRFS in the day +60 cohort relative to the day +90 cohort encouraging.
Based on these safety outcomes and the immediate need to improve efficacy, Johns Hopkins has adopted reduced-duration tacrolimus, planned until day +60, as its current standard for NMA bone marrow transplantation with PTCy. With this PTCy-based platform, shortened-duration IS may allow for earlier or more effective administration of preemptive therapies for relapse reduction, such as cellular immunotherapies and checkpoint inhibitors [25–27].
Acknowledgments
The authors thank Ms. Judy Baker for data management and the Cell Therapy Laboratory at Johns Hopkins for graft data.
Financial disclosure: This work was supported by the National Institutes of Health Grants K23 CA124465 (to Y.L.K.), P01 CA015396 (to R.J.J.), and P30 CA006973.
Footnotes
Clinicaltrials.gov registration: NCT01342289.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: Y.L.K. and R.J.J. were involved in the study’s conception and design, study conduct, data collection, analysis, and interpretation, and manuscript writing; M.Z. and G.L.R. designed and statistically analyzed the study; and E.J.F. was involved in the study’s conception and design and data collection, analysis, and interpretation. The remaining authors contributed to data collection. All authors approved the final manuscript.
References
- 1.Jones RJ. Haploidentical transplantation: repurposing cyclophosphamide. Biol Blood Marrow Transplant. 2012;18:1771–1772. doi: 10.1016/j.bbmt.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24. doi: 10.1038/nrclinonc.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasamon YL, Ambinder RF, Fuchs EJ, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1:288–292. doi: 10.1182/bloodadvances.2016002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh N, Karmali R, Rocha V, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a Center for International Blood and Marrow Transplant Research analysis. J Clin Oncol. 2016;34:3141–3149. doi: 10.1200/JCO.2015.66.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–3031. doi: 10.1182/blood-2015-01-623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127:938–947. doi: 10.1182/blood-2015-09-671834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura R, Forman SJ. Reduced-intensity conditioning for allogeneic hematopoietic cell transplantation: considerations for evidence-based GVHD prophylaxis. Expert Rev Hematol. 2014;7:407–421. doi: 10.1586/17474086.2014.898561. [DOI] [PubMed] [Google Scholar]
- 12.Staveley-O’Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan KM, Storb R, Buckner CD, et al. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med. 1989;320:828–834. doi: 10.1056/NEJM198903303201303. [DOI] [PubMed] [Google Scholar]
- 15.Mengarelli A, Iori AP, Romano A, et al. One-year cyclosporine prophylaxis reduces the risk of developing extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Haematologica. 2003;88:315–323. [PubMed] [Google Scholar]
- 16.Burroughs L, Mielcarek M, Leisenring W, et al. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81:818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 17.Gladstone DE, Zachary AA, Fuchs EJ, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19:647–652. doi: 10.1016/j.bbmt.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCurdy SR, Kanakry CG, Tsai HL, et al. Grade II acute graft-versus-host disease and higher nucleated cell graft dose improve progression-free survival after HLA-haploidentical transplant with post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2018;24:343–352. doi: 10.1016/j.bbmt.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.McCurdy SR, Kasamon YL, Kanakry CG, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with posttransplantation cyclophosphamide. Haematologica. 2017;102:391–400. doi: 10.3324/haematol.2016.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farhan S, Lee DA, Champlin RE, Ciurea SO. NK cell therapy: targeting disease relapse after hematopoietic stem cell transplantation. Immunotherapy. 2012;4:305–313. doi: 10.2217/imt.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375:143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130:221–228. doi: 10.1182/blood-2017-01-761346. [DOI] [PMC free article] [PubMed] [Google Scholar]


