Abstract
Rexinoids are powerful ligands that bind to retinoid-X-receptors (RXRs) and show great promise as therapeutics for a wide range of diseases, including cancer. However, only one rexinoid, bexarotene (Targretin TM) has been successfully transitioned from the bench to the clinic and used to treat cutaneous T-cell lymphoma (CTCL). Our goal is to develop novel potent rexinoids with a less untoward side effect profile than bexarotene. To this end, we have synthesized a wide array of rexinoids with EC50 values and biological activity similar to bexarotene. In order to determine their suitability for additional downstream analysis, and to identify potential candidate analogs for clinical translation, we treated human CTCL cells in culture and employed microarray technology to assess gene expression profiles. We analyzed twelve rexinoids and found they could be stratified into three distinct categories based on their gene expression: similar to bexarotene, moderately different from bexarotene, and substantially different from bexarotene. Surprisingly, small changes in the structure of the bexarotene parent compound led to marked differences in gene expression profiles. Furthermore, specific analogs diverged markedly from our hypothesis in expression of genes expected to be important for therapeutic promise. However, promoter analysis of genes whose expression was analyzed indicates general regulatory trends along structural frameworks. Our results suggest that certain structural motifs, particularly the basic frameworks found in analog 4 and analog 9, represent important starting points to exploit in generating additional rexinoids for future study and therapeutic applications.
Keywords: Rexinoids, RXR, Gene Expression, Microarrays, Analytics, Cancer
Graphical Abstract
Introduction
Bexarotene is a synthetic pan-agonist for all known isoforms (α, β, γ) of the retinoid X receptor (RXR)—a transcriptional factor that often partners with other nuclear receptor (NR)1 transcription factors to regulate several critical pathways in the body [1]. For example, a few nuclear receptors for which RXR plays a partnering role include the retinoic acid receptor (RAR), the vitamin D receptor (VDR), the thyroid hormone receptor (TR), the liver X receptor (LXR), the constitutive androstane receptor, and the peroxisome proliferator activated receptor (PPAR). Most often, the NRs promote gene expression when associated with a specific molecular ligand, a DNA target site, and any necessary partnering receptor. The specific molecular ligand(s) associates with a NR ligand-binding domain (LBD) in the receptor that mediates a conformational change inducing dimerization, recruitment of co-factors, and finally binding to a corresponding hormone responsive element (HRE) on the DNA. It is remarkable that at least one isoform of human RXR is expressed in every tissue type, and coupled with the fact that RXR impacts so many other pathways, it is understandable that small structural changes in RXR agonists (rexinoids) might give rise to highly differential gene expression [2,3].
While TR, RAR and VDR were all initially believed to homodimerize prior to association with their corresponding HREs, they must partner with RXR to form heterodimers instead [4,5]. An isomer of all trans-retinoic acid (ATRA), 9-cis-retinoic acid (9-cis-RA), is one of several natural rexinoids that binds to RXR and in most cases drives RXR homodimer formation and binding to RXR HREs (RXREs) [6,7]. There are several natural and synthetic “canonical” rexinoids that bind tightly to RXR’s LBD in addition to “noncanonical” rexinoids that bind loosely—such as (R)-Etodolac (an NSAID) and rhein (from rhubarb)—as well as ligands that target alternate surface binding sites on RXR [8]. When partnering with other NRs, the LBD of RXR may be occupied or vacant. For the RXR-VDR heterodimer, the RXR LBD has been reported to be vacant [9]. For the RXR-LXR heterodimer, however, the RXR LBD may be occupied by ligands that in many cases promote the functioning of the heterodimer [10].
From several studies like the above, investigating the occupancy of the RXR LBD in the active RXR-heterodimer assemblies, two primary classifications of RXR-heterodimers—permissive and nonpermissive—emerged. For purely nonpermissive RXR heterodimers, only heteropartner selective ligands will affect transcriptional activity, whereas for permissive RXR heterodimers both rexinoids and heteropartner ligands can promote activity [11]. While the RXR-TR, RXR-VDR and RXR-RAR heterodimers are primarily nonpermissive, where RXR is “silent” in most (but not every) set of conditions for VDR and TR, the RXR-RAR heterodimer can exhibit enhanced activity when treated with both RAR selective agonists and certain rexinoids. In fact, certain antagonist rexinoids have been observed to activate RXR-RAR, and thus the RXR-RAR might reasonably be termed conditionally permissive [12]. The RXR-LXRs, RXR-PPARs, and RXR-FXRs are fully permissive.
The potential of a rexinoid to stimulate permissive RXR-heterodimer activity, or remove RXR from participating in nonpermissive heterodimers—thereby blocking those pathways—has increased the risk associated with rexinoid agonists as clinical therapeutics. For example, the 9-cis-RA rexinoid and other rexinoid agonists have been reported to inhibit the activity of nonpermissive RXR-heterodimers with VDR and TR due to rexinoid-driven formation of competing RXR-RXR homodimers [13–16]. In a similar manner, 1,25-dihydroxyvitamin D3 (1,25D) and T3—the agonist ligands specific for VDR and TR, respectively—have been observed to arrest other pathways where RXR-RXR participates because these ligands stimulate RXR-VDR and RXR-TR formation, removing the RXR protein from a limited reservoir [17]. Additionally, certain rexinoids can stimulate hypertriglyceridemia by activating the permissive RXR-LXR heterodimer. Thus, two important issues to consider in creating novel rexinoids to explore as clinical therapeutics include RXR-heterodimer selectivity and potency.
Bexarotene is a synthetic rexinoid approved for use by the FDA for the efficacy of its antineoplastic properties in the treatment of cutaneous T-cell lymphoma (CTCL). Despite a growing number of reported synthetic rexinoids, only a few have advanced into clinical trials [18,19]. Bexarotene has also been examined as an off-label treatment for other cancers including non-small cell lung cancer (NSCLC) and breast cancer. Additionally, there has been interest in exploring bexarotene and other rexinoids to treat diabetes, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [20–24]. The side effect profile of bexarotene is a serious concern, and as such the development of more potent RXR agonists with more limited side-effect profiles, would be of great benefit.
A more potent rexinoid would enable reduced dosage requirements, possibly mitigating the severity of the known side-effects. Additionally, it is likely, given the hetero- and homodimer binding modalities of RXR, as well as the large number of RXR partnering receptors that an alternative rexinoid may have a wholly different side-effect profile from bexarotene, which could serve to mitigate the side-effect concerns associated with use of this kind of drug.
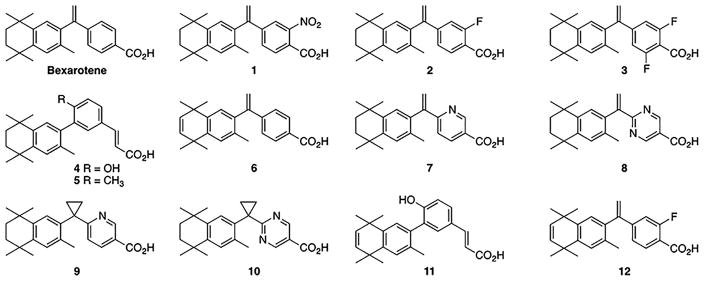
The goal of the present study is to explore the viability of several synthetic bexarotene analogs (Fig 1). Specifically, we probed how our suite of analogs might influence the potency of the desirable and undesirable effects of this drug. To this end, eight rexinoids developed by our group (analogs 1–3, 5, 8, and 10–12) and five previously reported rexinoids (bexarotene and analogs 4, 6, 7, and 9) were used to dose a human cell culture cutaneous T-cell lymphoma cell line (HuT78) to determine promising candidate analogs for further experimentation. Human CTCL cells were treated in culture with an analog or bexarotene and studied using gene expression analysis.
Figure 1.
Bexarotene analog structures. Twelve bexarotene analogs were used to explore differential gene expression in an in-vitro cutaneous T-cell lymphoma cell model. Microarray analysis was employed to determine which among them show promise for further investigation and if any trends emerge in their ability to regulate gene expression.
Analysis of the findings is directed towards cancer-related gene activity, with the goals of both eliminating analogs which behave unpredictably, as well as deciphering which analogs stand apart as the most promising for future experimentation. Rexinoid-mediated gene regulation in HuT78 cells is examined in light of including (1) the hypothesized apoptotic mechanism of the RXR/PPARγ dimer, which is likely the primary source of the antineoplastic activity of rexinoids, (2) potential cell cycle arresting effects, (3) influence on the thyroid hormone axis, (4) dyslipidemic effects, and (5) possible metabolic effects associated with the citric acid cycle.
Our results reveal that the studied analogs sort into 3 distinct groupings, separated by both the number and magnitude of their differences from bexarotene. We analyze these novel molecules by group as well as by promoter composition, and present five analogs which we propose as promising candidates for future study.
Experimental
Compound analysis and instrumentation
All 1H NMR spectra were acquired at 400 MHz or 500 MHz on Bruker or Varian spectrometers. Chemical shifts (δ) are listed in ppm against the non-deuterated solvent peaks as an internal reference. Coupling constants (J) are reported in Hz, and the abbreviations for splitting include: s, single; d, doublet; t, triplet; q, quartet; p, pentet; m, multiplet; br, broad. All 13C NMR spectra were acquired on Bruker instruments at 125.8 MHz or 100.6 MHz. Chemical shifts (δ) are listed in ppm against solvent carbon peaks as an internal reference. High resolution mass spectra were recorded using either a JEOL GCmate(2004), a JEOL LCmate(2002) high resolution mass spectrometer or an ABI Mariner (1999) ESI-TOF mass spectrometer. Melting points were assayed on a Thomas Hoover capillary melting point apparatus. All tested compounds were analyzed for purity by combustion analysis through Columbia Analytical Services (formerly Desert Analytics in Tucson, AZ), except for analog 4 which was purchased from TOCRIS Biosciences and labelled to be ≥ 97% by HPLC according to the company’s specifications. The spectroscopic and characterization data for each compound is provided as well as their 1H-NMR and 13C-NMR spectra. Individual compound spectra and further details relating to compound analysis may be found in the Appendix.
HuT78 treatment and mRNA harvesting
Human CTCL cells (HuT78) cultured in Roswell Park Memorial Institute (RPMI) Medium #1640 + 10% charcoal stripped Fetal Bovine Serum (FBS) + Sodium Pyruvate (NaPyr) + Penicillin/Streptomycin (P/S) were treated with bexarotene or bexarotene analog. After a 24-hour treatment period with either bexarotene or the indicated analog at 100nM, cells were transferred along with media into 15 ml conical tubes and centrifuged for 5 minutes at 300g whereupon 1 mL phosphate-buffered saline (PBS) was added to each and the contents were then aspirated, and 1mL of cold PBS was added to each group. The cells were then progressively vortexed and centrifuged and ultimately treated with Aurum Total RNA Lysis solution (Bio-Rad, Hercules, CA). The RNA yield was quantified via UV spectrophotometry and the RNA quality was estimated using the A260/A280 and A260/A230 ratios. RNA concentrations from each treatment were in the range of 0.40 μg/μl to 0.80 μg/μl, with an average concentration of 0.60 μg/μl.
cDNA generation
RNA from cells treated with Bexarotene and from cells treated with a single analog from above treatments was thawed as a pair and hybridized with 1 μl of reverse transcriptase (RT) primer each (1x Cy3 green and 1x Cy5 red). The tubes containing each treatment were brought to concentration parity using nuclease-free water such that both final 11 μl volumes contained an RNA concentration of 0.2 μg/μl. The tubes were heated, subsequently placed on ice, and then added to a reaction mix composed of Invitrogen SuperScript II first-strand buffer (Invitrogen, Carlsbad, CA), more nuclease-free water, dNTP mix, an RNase inhibitor, and RT enzyme utilizing the Array 350 kit (Genisphere, Hatfield, PA).
The combined contents were incubated and then had their reactions halted with the addition of solution containing 0.5 M sodium hydroxide (NaOH) and 50 mM ethylene-diaminetetraacetic acid (EDTA). The tube contents were then again incubated and the reaction was neutralized with 1 M Tris-HCl to a pH of 7.5. The neutralized solutions were combined and a Qiagen PCR cleanup kit (Qiagen, Germantown, MD) was employed to isolate the cDNA portion. Vacufugation was performed to bring the PCR cleanup results to a usable volume. The resultant 10 μl volume of concentrated cDNA was combined with 2 μl of locked nucleic acid (LNA) dT blocker, 17 μl of nuclease-free water, and 29 μl of a 2X formamide-based hybridization buffer.
Microarray preparation, hybridization, and analysis
Human MI ReadyArrays were procured from Microarrays Inc. (Huntsville, AL). Each array consists of 48,958 individual wells conforming to the Human Exonic Evidence Based Oligonucleotide (HEEBO) standard. Before applying cDNA solutions, each slide was treated with a prehybridization/blocking procedure. This procedure was accomplished using a bovine serum albumen (BSA) ssDNA solution to reduce non-specific binding events. Slides were incubated and washed by gentle rocking in vials with 3 M NaCl/0.3 M sodium citrate (20xSSC), 10% sodium dodecyl sulfate (SDS), and the BSA solution. Each slide was then transferred to a series of separate vials and washed five times by gentle agitation in millipore water. Rinsed and dried slides had the cDNA solution applied to them while warmed. Each was covered, stored in an individual humidified hybridization chamber, and placed in a hybridization oven with gentle agitation.
After incubation, the slides were agitated in a shaker with a series of progressively less concentrated treatments of SSC and SDS. After their final wash, slides were dried and treated for visualization with a solution composed of nuclease-free water, 2X formamide-based hybridization buffer, Cy3 and Cy5 3DNA capture reagents. Slides were then once again incubated in a humidified hybridization chamber. Post-DNA hybridization washes were performed similar to pre-hybridization washes, and then the chips were dried and immediately moved to be scanned by a GenePix 4000B microarray scanner using the Genepix Pro® 7 Microarray Acquisition and Analysis software. Each slide was checked for abnormalities and backgrounding effects and then log-transformed luminosity results were exported to Microsoft Excel.
Gene expression analysis, grouping
Gene expression analysis was performed along several lines of investigation. The expression profile of each analog was culled to a list of only cancer-related study genes for the present investigation (Appendix) and then the analogs were compared to each other using this data sub-set (raw data in [25] ). The trends in these profiles were recorded and the analogs were split into three groupings based upon the magnitude and enumeration of their differences from their parent molecule.
Gene expression was analyzed as absolute fold changes as compared to bexarotene using expression data for the genes outlined in the Appendix, with the dataset described in [25]. The number of genes from the Appendix that fell into three categories were determined. The cutoffs thresholds for these categories are expression levels of |1.2|, |1.8|, and |2.4| fold-change differences from bexarotene (absolute value indicates that fold-changes could be in either direction, thus only magnitude was considered). Each analog was grouped by comparing these changes. Group one contains analogs that elicited the most changes in expression: four or more genes from the study group demonstrating greater than a |1.8|-fold difference from bexarotene. Group two contains analogs for which measured fold changes had lower magnitudes than group one: one or more genes from the study group revealed greater than a |1.8|-fold difference from the parent molecule, and/or five or more total measured differences of |1.2|-fold magnitude or greater. Group three demonstrates the expression most similar to bexarotene: zero to four measured differences of less than |1.8|-fold from bexarotene.
Gene expression analysis, heat maps
The expression profile of each analog was further analyzed using heat maps generated with web-enabled heat mapping software provided by Wishart Research Group through their service at www.heatmapper.ca [26]. Images were generated using single linkage methodology and Euclidian distance measuring.
Principal component analysis
Principal Component Analysis was performed on the data from Table 1 following a methodology prescribed in literature [27]. Bexarotene was kept as its own separate group and the other compounds were grouped according to similarities in the magnitudes of their expression. The ellipses represent the 95% confidence interval.
Table 1.
Chemical compound analysis.
| Compound | cLogP1 | cLogS2 | tPSA3 | MW4 |
|---|---|---|---|---|
| Bexarotene | 4.8 | −6.4 | 37.3 | 348.49 |
| 1 | 3.9 | −6.8 | 83.1 | 393.481 |
| 2 | 4.9 | −6.7 | 37.3 | 366.48 |
| 3 | 5.0 | −7.0 | 37.3 | 384.47 |
| 4 | 5.2 | −6.5 | 57.5 | 364.49 |
| 5 | 5.9 | −7.2 | 37.3 | 362.51 |
| 6 | 4.5 | −6.2 | 37.3 | 346.47 |
| 7 | 3.9 | −5.6 | 50.2 | 349.47 |
| 8 | 3.1 | −4.7 | 63.1 | 350.46 |
| 9 | 4.7 | −4.97 | 50.2 | 363.50 |
| 10 | 4.0 | −4.0 | 63.1 | 364.49 |
| 11 | 4.9 | −6.3 | 57.5 | 362.47 |
| 12 | 4.6 | −6.5 | 37.3 | 364.46 |
Compounds were analyzed using DataWarrior for physical characteristics.
P is [conc]octanol/[conc]water.
S is water solubility [mols/Liter] at pH = 7.5 and 25 °C.
tPSA is topological polar surface area.
MW is molecular weight.
Gene expression analysis, divergence scoring
To further differentiate the analogs from each other, the expression elicited after analog treatment was compared using another metric referred to herein as divergence scoring (DS), a novel intraexperimental decision tool. Several well-characterized genes were selected as a subset of the study group [25] using two criteria, their impact in differentiating the analogs from each other, and the importance of directionality of regulation as understood by the present literature. Divergence in this case means the extent to which the gene expression of each individual gene elicited by the analog was performing contrary to the presumed direction of expression of that gene based on its ontology. For example, we hypothesized that better analogs would increase the apoptotic potential of the treated CTCL cells, and a departure from this expression hypothesis results in a divergence.
For every DS gene on each analog, fold-change differences from the parent molecule were either discarded or aggregated depending on whether the recorded value matched a predictive model ([25]. The predictive model operates on a fundamental design presumption that the child molecule should be an improved version of the parent molecule. To this end, in those instances where the recorded data value did align with the predicted value from the model, the observed difference between the child and parent was discarded [25]. For the instances in which there was a mismatch between the directional expression difference of the analog and bexarotene, and that mismatch did not align with the predictive model, the absolute value of the observed data point was recorded and then aggregated. The aggregate value of recorded mismatches was averaged across the total number of DS genes, and the DS for the given analog was recorded as this value.
Docking analysis
Docking of ligands to the RXR binding domain has been reviewed and was performed in this study with AutoDock 4.2 [28,29]. Ligands were prepared and energy minimized with Avogadro [30]. Coordinates for the protein were taken from the protein data bank structure 1MVC [31], and Arg316 and Ile268 were treated as flexible. Docking was performed with the Lamarckian genetic algorithm using 25 million energy evaluations per dock. A total of 250 docks were performed per compound. Poses were visualized with Chimera [32].
Analysis of putative RXR binding motifs
Names of cancer-related study genes were converted to National Center for Biotechnology Information (NCBI) Reference Sequence Database ID numbers (RefSeq ID) using the ID conversion tool available from DAVID (Database for Annotation, Visualization and Integrated Discovery; [33,34]). Of the study genes, 92 were successfully converted to RefSeq IDs for compatibility with the Pscan software tool. Presumptive promoter regions for all 92 genes were scanned for the presence of nine RXR binding motifs using Pscan [35]. Scanned regions were limited to −450 to +50 base pairs (bp) with respect to the transcription start site. This study focused on identifying enriched RXR motifs using 9 well-defined Position Frequency Matrices (PFMs) from the JASPAR 2016 database. The 9 RXR binding motifs along with their corresponding JASPAR 2016 Matrix ID numbers are as follows: PPARγ::RXRα (MA0065.1), PPARγ::RXRα_v2 (MA0065.2), RXRα::VDR (MA0074.1), NR1H2::RXRα (MA0115.1), RARα::RXRα (MA0159.1), NR1H3::RXRα (MA0494.1), RXRα (MA0512.2), RXRβ (MA0855.1), and RXRγ (MA0856.1). Genes were determined to contain the motif if the computationally-determined matching score was higher than expected when compared to the whole genome promoter set. Consensus sequences for each gene containing the relevant RXR motif were converted to graphical Sequence Logos using the web-based application WebLogo [36,37].
Genes were scored manually based on the presence (1) or absence (0) of each of the 9 RXR motifs. A heat map of RXR motifs per gene was generated using ClustVis and hierarchical cluster analysis based on Euclidian correlation distances and complete linkage methods [27]. Unit variance scaling was applied to each row. Promoter regions for each gene cluster were again analyzed using Pscan to determine which RXR binding motifs were significantly (p≤0.05) enriched in each cluster. Average gene expression per cluster was determined for each of the 12 analogs using Excel. For each analog, two-sample t-tests were used to determine whether average gene expression for each cluster differed significantly from the remaining study genes.
Results
Gene expression analysis
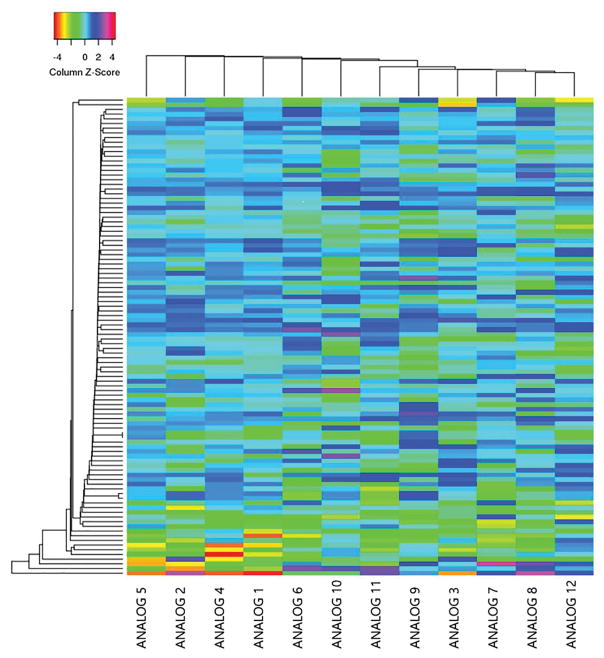
In order to develop a more powerful structure-activity framework (SAR) framework by which to synthesize novel rexinoids, we sought to analyze gene expression of an array of published rexinoids. We wanted to determine which molecules maximized gene expression of pathways advantageous for rexinoid treatment of cancer cells. When treated with bexarotene analogs, HuT-78 cell cultures (derived from human cutaneous T cell lymphoma cells) produced substantially different mRNA expression profiles when compared to those produced by bexarotene (Figs 2, 3a, 3b, and 3c). Our investigation is focused on differential mRNA production as it relates to the treatment outcomes of patients primarily with CTCL, but also NSCLC and breast cancer. We therefore present results germane to the treatment of these three cancers. Of the 12 analogs studied, each produced an mRNA profile which fits into one of 3 general categories.
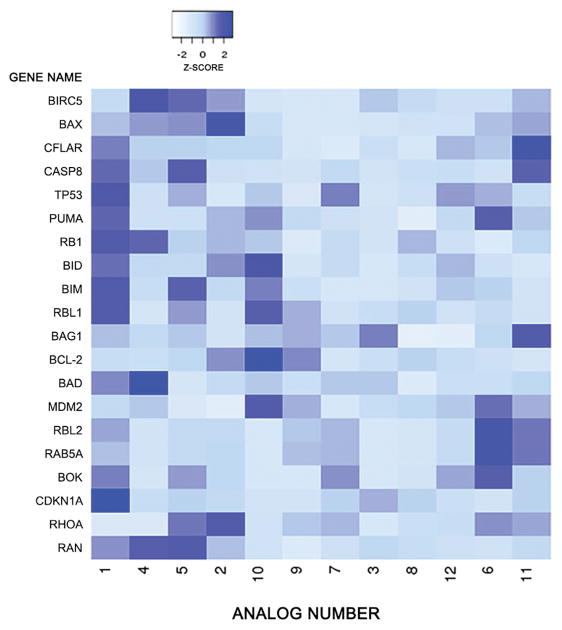
Figure 2.
Heat map of study genes. Heat map analysis of expression of genes from study gene list. Gene expression was compared between analogs using 102 the genes outlined in the Appendix.
Figure 3.
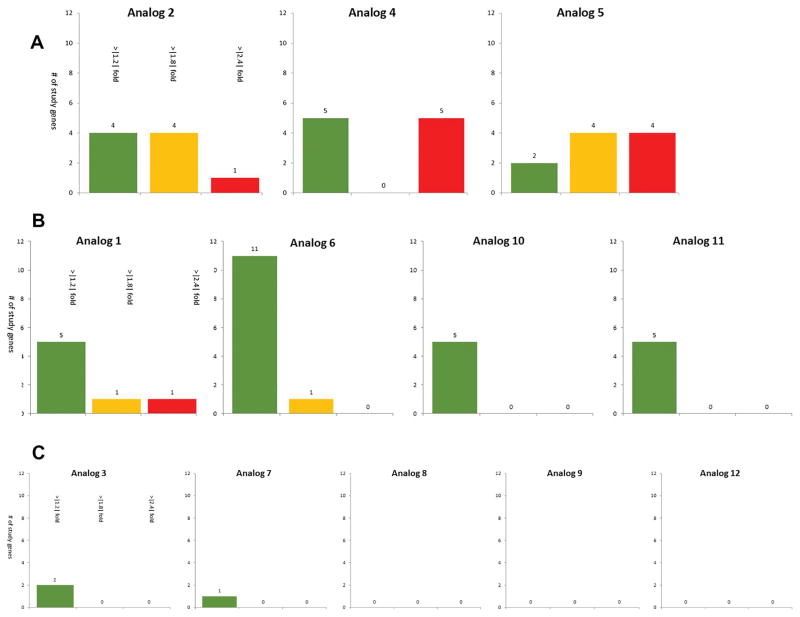
Comparison and grouping of rexinoids via gene expression analysis. Figure 3a. Group 1 analogs versus bexarotene. Group 1 analogs contain those compounds which expressed the most substantial changes from bexarotene. Members of this group have at least 4 study genes with greater than |1.8| fold changes from bexarotene the number of study genes which sort into one of 3 tranches representing increasing magnitudes of fold-changes from bexarotene. Green bars represent the number of genes with a greater than 1.2 but less than 1.8 fold change (absolute) as compared to bexarotene. Yellow bars represent the number of genes with a greater than 1.8 but less than 2.4 fold change (absolute) as compared to bexarotene. Red bars represent the number of genes with a greater than 2.4 fold change (absolute) as compared to bexarotene. Figure 3b. Group 2 analogs versus bexarotene. Group 2 analogs contain those compounds which expressed intermediate changes compared from bexarotene as compared to groups 1 or 3. Members of this group have either least one study genes with a greater than |1.8| fold change from bexarotene, or five or more total changes from bexarotene of a magnitude greater than |1.2| fold. Green bars represent the number of genes with a greater than 1.2 but less than 1.8 fold change (absolute) as compared to bexarotene. Yellow bars represent the number of genes with a greater than 1.8 but less than 2.4 fold change (absolute) as compared to bexarotene. Red bars represent the number of genes with a greater than 2.4 fold change (absolute) as compared to bexarotene. Figure 3c. Group 3 analogs versus bexarotene. Group 3 analogs contain those compounds which expressed the fewest substantial changes from bexarotene. Members of this group have four or fewer |1.8| magnitude fold change differences from bexarotene. Green bars represent the number of genes with a greater than 1.2 but less than 1.8 fold change (absolute) as compared to bexarotene.
The first of these categories, “Group 1,” includes analogs 2, 4, and 5 (Fig 3a). This group shows a trend of similar behavior to bexarotene for the genes in the study group, but each compound has more than a few instances of stark product differences from the parent molecule. Categorically, analogs which fit in to this group have 4 or more genes from the study group gene list (Appendix) which demonstrate greater than a 1.8-fold difference in gene expression from bexarotene.
Group 2 members (analogs 1, 6, 10, and 11 seen in Fig 3b) lack the distinctive drastic differences from bexarotene compared to the Group 1 analogs yet contains species which produce mRNA profiles that are similar to bexarotene and tend to differ only in that they are either slightly more or less active for particular study genes. Categorically, this group consists of analogs for which either: less than 4 genes from the study group show a greater than 1.8-fold difference from bexarotene, in either direction, and/or analogs which have 5 or more total measured differences from bexarotene for the gene study group of greater than a 1.2-fold difference in either direction. Unlike group 1, this second group has a milder fold-difference in gene-specific mRNA production when compared to bexarotene. For the overwhelming majority of the genes of interest, group 2 members’ gene products are within a 1-fold change as compared to their parent molecule (>75% of study genes within a +/− 0.5-fold change). Group 2 members trend towards being only slightly more or slightly less active than bexarotene with respect to the study genes.
Group 3 analogs (3, 7, 8, 9, and 12 seen in Fig 3c) consists of compounds which have 4 or fewer total measured differences from bexarotene at the 1.2-fold level, and further did not contain any members which demonstrated for any study gene greater than a 1.8-fold difference from bexarotene (a constraint which would categorize an analog as a member of group 2).
Outliers and divergence scoring
In order to further differentiate rexinoid characteristics and attractiveness as potential therapeutics, we developed a metric to probe undesirable gene expression, the Divergence Score. This evaluation probes gene expression by each molecule to determine how the expression deviates from expected results. Divergence scores (DS) were assigned to each analog to facilitate comparison to group cohorts on a relative measurement of gene-specific performance predictability. These results are mapped in Fig 4 and a heat map analysis of DS genes is shown in Fig 5. Genes for this scoring method were selected as a subset of the study’s gene list for their well-understood features in current literature and the certainty with which the effects of the directionality of their regulation is understood (Appendix and [25]).
Figure 4.
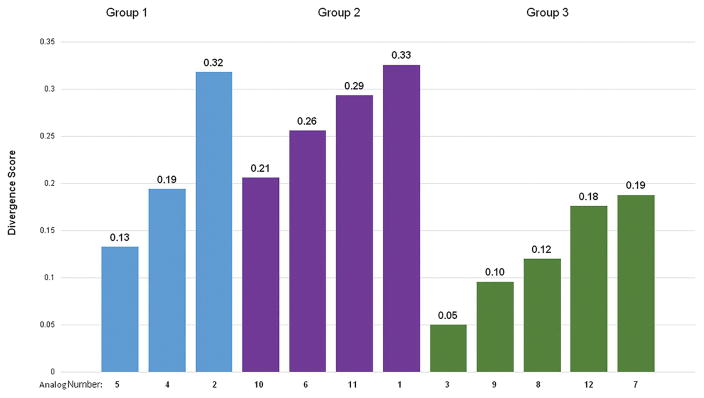
Divergence scores by group. Divergence scoring (DS) first aggregates then averages undesirable expression behavior from each analog across a subset of genes with well understood activity. This allows for a relative metric for comparing the potential of each analog to the others on the basis of their anti-oncogenic potential. Lower DS indicates a more desirable expression profile on average, higher DS indicates a less desirable expression profile on average. Group 1 analogs are in blue, group 2 analogs are in purple, and group 3 analogs are in green.
Figure 5.
Heat map of divergence score genes. Heat map analysis of expression of genes used to calculate divergence score. Whiter hues indicate expression levels closer to parent molecule. Bluer hues indicate expression that differs more drastically from parent molecule.
For group 1, Analog 5 has the most total substantially different gene regulation (Fig 3a), with 8 differentially regulated mRNAs (total measured differences = 10, total measured differences over the 1.8-fold level = 8). Analog 2 has the fewest total substantially different gene regulation (Fig 3a), with 5 differentially regulated mRNAs (total measured differences = 9, total measured differences over the 1.8-fold level = 5), and analog 2 has the highest DS for this group, and the second highest overall DS at 0.32 (analog 4 = 0.19, 5 = 0.13), making it a less attractive study candidate compared to its peers (Fig 4). Analog 5 appears to be the most attractive candidate from Group 1, with both substantially high differences from bexarotene in terms of differential gene product abundance, as well as its comparatively low divergance scoring versus its group peers (DS = 0.13), indicating that, compared to its group members, biological activity of analog 5 is more in line with what we might expect from an anti-oncogenic molecule (Figs 3a and 4).
Among group 2 analogs, analog 6 has the most total measured differences from bexarotene with 12 (Fig 3b). Both it and analog 1 are the only two members of this group to have measurements from the gene study group of greater than a 1.8-fold change from bexarotene. Analogs 11 and 10 are tied for the fewest measured differences, with 5, while analog 1 has the highest DS for this group, and highest overall DS at 0.33, making it a less attractive candidate as compared to its peers (Fig 4) (analog 6 = 0.26, 10 = 0.21, and 11 = 0.29). The low divergence score of analog 10 in this category (DS=0.21) indicates that, compared to its group cohorts, it is the most promising member of this group to be an improvement to the parent molecule (Fig 4).
Within group 3, analog 3 has the most total measured differences from bexarotene with 2, while analogs 8, 9, and 12 are tied for the fewest measured differences with 0 (Fig 3c). Analog 3 has the lowest DS for this group at 0.0499, making it an attractive candidate compared to its peers (Fig 4). No members of this group have any observed differences from the gene study group exceeding a 1.8-fold difference from bexarotene. Both analog 3 and analog 9 have low divergence scores (DS = 0.05, DS = 0.10). For the group of study genes covered by this investigation, these two appear to produce an mRNA expression profile that is most likely to be an improvement to the parent molecule, bexarotene (Figs 3c and 4).
Chemical properties of molecules
We sought to determine if the expression groups could in part be described by the physical nature of the rexinoid molecules (Table 1), covering the solubility of the molecule (cLogP and cLogS), the topological polar surface area (tPSA, a metric often used to approximate permeability characteristics), and molecular weight. Taking into account the characteristics described in Table 1, in general Group 3 analogs tend to have high cLogP, analogs with lower DS generally correlate with high cLogS as well as a higher MW, and Group 2 analogs associate with a higher tPSA. Interestingly, Group 1 analogs, which have the most differential expression from Bexarotene also have the most dissimilar MW from bexarotene and they also cluster the most tightly on the PCA analysis (see below).
In order to determine if the characteristics in Table 1 are contributing to the clustering of the compounds into groups, we undertook Principal Component Analysis (PCA) of the data in Table 1. PCA is a method to find patterns in the data based upon their correlations. The PCA analysis is seen in Fig 6 and was analyzed using the expression grouping method described earlier. The first correlation or principal component in this analysis accounts for over half of the variation of the sample (PC1 is 52.1%), and the second PC accounts for 34.7% of the data, together accounting for 84.8% of the variability. Fig 6 demonstrates that generally the groupings can be explained by the physicality of the compounds (Table 1). Although compound 11 by this analysis may be closer to Group 1 and compound 10 falls within Group 3’s characteristics, in general the compounds are clustering by this analysis in the Groups defined previously by gene expression. Analogs which show the greatest differences from bexarotene at the level of gene expression (those in Group 1) tend to associate with each other closely at the level of their physical properties, as seen in the red ellipse (Fig 6). Group 2 has the most diffuse characteristics (Table 1 and blue ellipse) and this can confound PCA analysis. However, the characteristics are well correlated to the gene expression analysis and will serve as a starting point for future studies.
Figure 6.
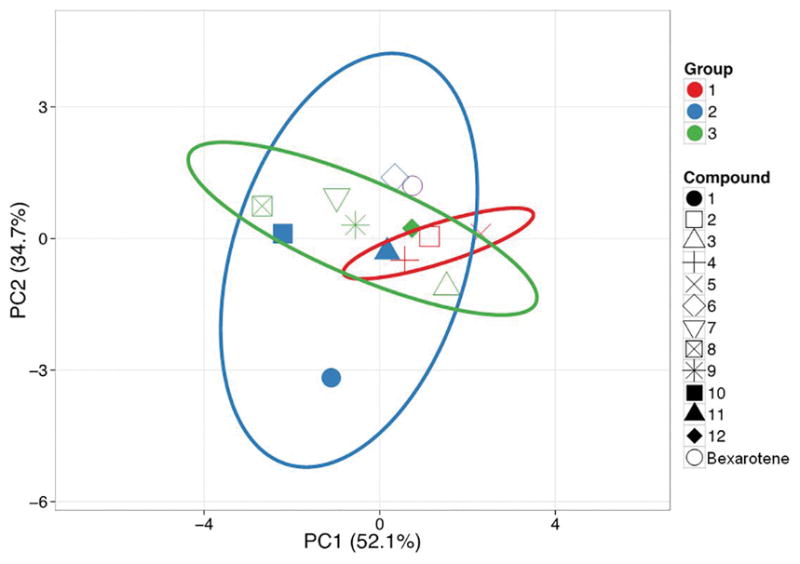
Principal Component Analysis. Unit variance scaling is applied to rows; SVD with imputation is used to calculate principal components. X and Y axis show principal component 1 and principal component 2 that explain 52.1% and 34.7% of the total variance, respectively. Prediction ellipses are such that with probability 0.95, a new observation from the same group will fall inside the ellipse. N = 13 data points.
Putative RXR binding motifs
Because RXR is a promiscuous nuclear receptor, we sought to determine if there was a difference between the RXR homodimers and/or heterodimers that might mediate the response elicited by each analog. To accomplish this, we examined a 500 base pair (bp) presumptive promoter region located at −450 bp through +50 bp relative to the transcription start site for each of the cancer-related study genes (Appendix). The 500 bp regions were scanned for the presence of 9 different RXR homo- and heterodimer binding motifs as defined using 9 well-defined Position Frequency Matrices (PFMs) from the JASPAR 2016 database (Table 2) [38,39]. Approximately 90% of the genes analyzed contained at least one putative RXR motif (Table 2 and [25]).
Table 2.
RXR binding motifs in presumptive promoter regions.
| Matrix ID | Motif Name | # Genes | Consensus Sequence |
|---|---|---|---|
| MA0065.1 | PPARγ::RXRα | 42 |
|
| MA0065.2 | PPARγ::RXRα_v2 | 38 |
|
| MA0074.1 | RXRα::VDR | 45 |
|
| MA0115.1 | NR1H2::RXRα | 37 |
|
| MA0159.1 | RARα::RXRα | 37 |
|
| MA0494.1 | NR1H3::RXRα | 30 |
|
| MA0512.2 | RXRα | 31 |
|
| MA0855.1 | RXRβ | 30 |
|
| MA0856.1 | RXRγ | 33 |
|
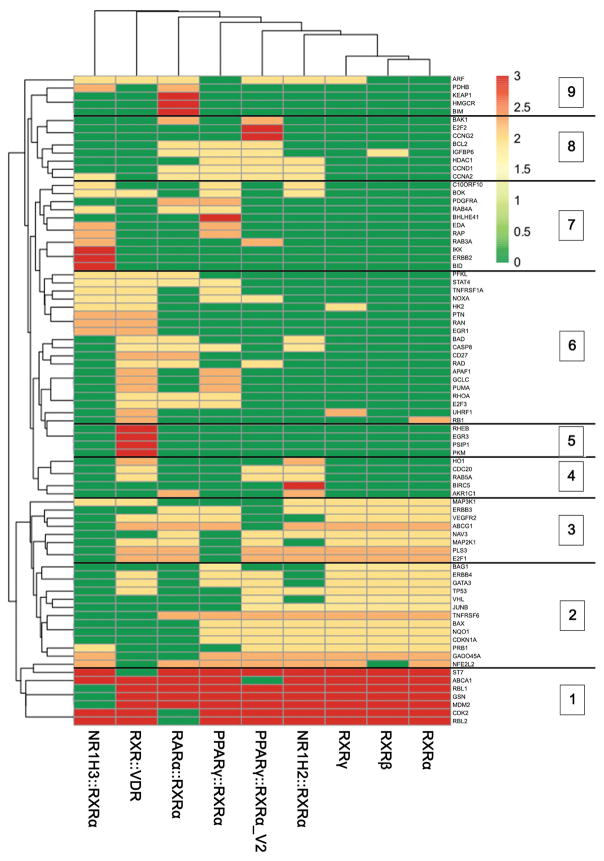
Study genes were clustered based on the absence or presence of each of the 9 binding motifs resulting in 11 distinct gene clusters (Fig 7 and [25]). Each cluster was examined to determine whether their promoters were significantly enriched in any of the RXR motifs (p<0.05, Table 3). Notably, the RXRα::VDR motif was the most frequently observed motif and was significantly enriched in 36% (4/11) of the gene clusters (Table 3). In contrast, the RARα::RXRα motif was only detected in gene promoters associated with cluster 9. Cluster 10 was the only cluster that did not contain any significantly enriched RXR binding motifs (Table 3).
Figure 7.
Gene clustering based on RXR binding motifs. Genes were clustered (Euclidian, complete) based on the presence (1, red or orange) or absence (0, green) of 9 known RXR binding motifs. Unit variance scaling has been applied to each row, resulting in a scale of 0–3. Analysis yielded 11 groups. Clusters 1–9 are shown. Cluster 10 (not shown) contains genes that have no obvious RXR binding motif while cluster 11 (not shown) contains genes containing all 9 motifs. N = 92 study genes that were successfully mapped to REFSEQ ID numbers.
Table 3.
Significantly enriched RXR motifs in gene clusters.
| NR1H3::RXRα | RXRα::VDR | RARα:RXRα | PPARγ::RXRα | PPARγ::RXRα_v | NR1H2::RXRα | RXRγ | RXR β | RXRα | |
|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 0.041 | 0.02 | 0.08 | 0.01 | 0.01 | 0.03 | 0.00 | 0.01 | 0.00 |
| Cluster 2 | 0.99 | 0.89 | 0.97 | 0.00 | 0.10 | 0.11 | 0.01 | 0.00 | 0.01 |
| Cluster 3 | 0.80 | 0.36 | 0.11 | 0.14 | 0.48 | 0.15 | 0.00 | 0.01 | 0.00 |
| Cluster 4 | 0.85 | 0.01 | 0.86 | 0.86 | 0.78 | 0.06 | 0.91 | 0.91 | 0.42 |
| Cluster 5 | 1.00 | 0.00 | 0.86 | 1.00 | 0.86 | 1.00 | 1.00 | 1.00 | 0.98 |
| Cluster 6 | 0.00 | 0.09 | 0.94 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 0.92 |
| Cluster 7 | 0.03 | 0.82 | 0.79 | 0.95 | 0.03 | 0.91 | 0.99 | 0.99 | 0.54 |
| Cluster 8 | 0.99 | 0.99 | 0.11 | 0.12 | 0.03 | 0.50 | 0.85 | 0.73 | 0.56 |
| Cluster 9 | 0.42 | 0.91 | 0.02 | 0.88 | 0.94 | 0.91 | 0.97 | 0.97 | 0.69 |
| Cluster 10 | 0.86 | 0.99 | 0.87 | 1.00 | 0.95 | 1.00 | 1.00 | 1.00 | 0.74 |
| Cluster 11 | 0.17 | 0.01 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Results of Pscan-calculated z-tests are shown for each cluster. Motifs that were significantly enriched (p<0.05) are highlighted.
Average gene expression was determined for each cluster and two-sample t-tests were used to determine whether any of the clusters responded to the 12 bexarotene analogs significantly differently (p≤0.05) than the remaining cancer-related study genes (Table 4, [25]). Approximately 73% (8/11) of the gene clusters demonstrated a significant response following treatment with at least one of the 12 analogs. Of note, analogs 7, 9, 10 and 11 did not induce a significant response in any of the gene clusters (Table 4).
Table 4.
Cluster-specific response to Analogs.
| Analog 1 | Analog2 | Analog 3 | Analog 4 | Analog 5 | Analog 6 | Analog 7 | Analog 8 | Analog 9 | Analog 10 | Analog 11 | Analog 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 0.021 | 0.25 | 0.43 | 0.01 | 0.00 | 0.88 | 0.74 | 0.29 | 0.62 | 0.80 | 0.73 | 0.80 |
| Cluster 2 | 1.00 | 0.71 | 0.54 | 0.62 | 0.87 | 0.33 | 0.31 | 0.04 | 0.63 | 0.34 | 0.40 | 0.47 |
| Cluster 3 | 0.59 | 0.23 | 0.27 | 0.12 | 0.02 | 0.66 | 0.58 | 0.75 | 0.67 | 0.80 | 0.45 | 0.84 |
| Cluster 4 | 0.70 | 0.83 | 0.95 | 0.98 | 0.90 | 0.05 | 0.49 | 0.89 | 0.21 | 0.28 | 0.67 | 0.01 |
| Cluster 5 | 0.02 | 0.92 | 0.27 | 0.03 | 0.07 | 0.41 | 0.61 | 0.43 | 0.95 | 0.64 | 0.47 | 0.30 |
| Cluster 6 | 0.62 | 0.21 | 0.13 | 0.11 | 0.04 | 0.51 | 0.73 | 0.25 | 0.07 | 0.18 | 0.85 | 0.20 |
| Cluster 7 | 0.48 | 0.55 | 0.51 | 0.00 | 0.14 | 0.82 | 0.52 | 0.62 | 0.97 | 0.93 | 0.83 | 0.25 |
| Cluster 8 | 0.19 | 0.28 | 0.51 | 0.64 | 0.50 | 0.45 | 0.76 | 0.37 | 0.52 | 0.49 | 0.51 | 0.46 |
| Cluster 9 | 0.37 | 0.59 | 0.75 | 0.45 | 0.33 | 0.43 | 0.15 | 0.41 | 0.40 | 0.57 | 0.19 | 0.06 |
| Cluster 10 | 0.88 | 0.18 | 0.63 | 0.63 | 0.09 | 0.54 | 0.86 | 0.29 | 0.64 | 0.07 | 0.26 | 0.25 |
| Cluster 11 | 0.09 | 0.00 | 0.01 | 0.40 | 0.32 | 0.52 | 0.57 | 0.63 | 0.08 | 0.40 | 0.20 | 0.40 |
Gene clusters which responded significantly differently (p<0.5) following rexinoid treatment compared to remaining study genes are highlighted.
In order to determine whether there was a difference in the types of RXR motifs that mediate the response to each analog, we identified motifs that were shared between gene clusters demonstrating a significant response to rexinoid treatment (Tables 3–5). The RXR::VDR motif was associated with a significant response to analogs 1–4, 6, and 12, although the direction of the response (e.g. increased vs. decreased gene expression relative to bexarotene treatment) varied between the different clusters. Additional RXR heterodimer binding motifs were identified as possibly mediating the response to analogs 2–6 and 8.
Table 5.
Clusters and motifs associated with significant changes in gene expression for each analog.
| Analog | Clusters | Shared Motifs | Expression relative to Bexarotene | Analog | Clusters | Shared Motifs | Expression relative to Bexarotene |
|---|---|---|---|---|---|---|---|
 1 |
1 5 |
RXRα::VDR | +0.71 +0.58 |
 7 |
- | - | - |
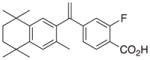 2 |
11 | RXRα::VDR PPARγ::RXRα PPARγ::RXRα_v2 NR1H2::RXRα RXRα RXRγ RXRβ |
+0.38 |
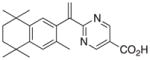 8 |
2 | PPARγ::RXRα RXRγ RXRβ RXRα |
+0.21 |
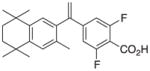 3 |
11 | RXRα::VDR PPARγ::RXRα PPARγ::RXRα_v2 NR1H2::RXRα RXRα RXRγ RXRβ |
+0.17 |
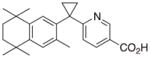 9 |
- | - | - |
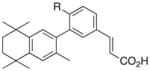 4 R = OH 5 R = CH3 |
1 5 |
RXRα::VDR | −0.10 −0.10 |
 10 |
- | - | - |
| 1 7 |
NR1H3::RXRα PPARγ::RXRα_v2 |
−0.10 +0.02 |
 11 |
- | - | - | |
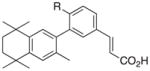 4 R = OH 5 R = CH3 |
1 3 |
RXRγ RXRβ RXRα |
+0.58 +0.38 |
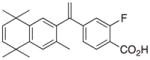 12 |
4 | RXRα::VDR | +0.43 |
| 1 6 |
NR1H3::RXRα | +0.58 −0.83 |
|||||
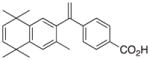 6 |
4 | RXRα::VDR | −0.98 | ||||
Discussion
In the present study, we provide evidence that a family of twelve bexarotene analogs sort into three categories based upon the frequency and magnitude of differential gene expression when compared to bexarotene in a CTCL HuT78 human cell culture. Three of the analogs are previously studied and nine are novel molecules (Fig 1) [40–45]. Within the three identified categories each analog presents a differing degree of promise for use as a therapeutic agent. Utilizing these groupings as well as divergence scoring, we are able to identify which analogs show the most promise for future study. The goal of the present study, and those which may follow, is to discover which bexarotene analogs are the most promising candidates as either more efficacious or less harmful RXR agonists in their application as chemotherapeutic agents.
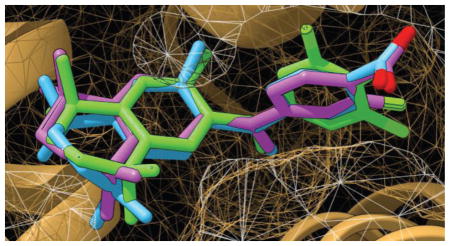
RXRs are type-II nuclear receptors (NR) [46] that are transcriptionally inactive in the absence of an agonist (like bexarotene or a bexarotene-like molecule) due to the presence of corepressor proteins. Bexarotene and to varying degrees, the suite of twelve analogs employed in this study, are thought to mimic the binding activity of an endogenous ligand by binding to RXR and facilitating a protein conformation change that results in receptor activation. The binding activity for type-II NR ligands in the target pocket is thought to release repressor molecules and activate the complex to stimulate transactivation and induce mRNA synthesis. Fig 8 shows the location of this binding pocket near helices 2, 4, and 6 on RXR.
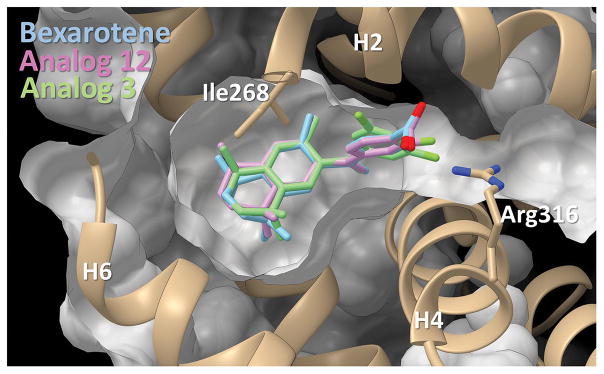
Figure 8.
Bexarotene and analogs 3 and 12 in binding pocket. Pictured is the docking of analog 3 and 12 alongside their parent molecule in pocket. The binding pocket is viewed with helices 2, 4, and 6 as reference points. Small chemical changes to the ligands of interest can produce large differences to both their orientation in three-dimensional space as well as the way in which they dock in the pocket.
Our findings, as well as those of others studying bexarotene and its analogs, demonstrate these molecules have anti-oncogenic properties [47]. Variations in fit for the on-target binding of our suite of study molecules likely induce a range of biological protein conformations for the corepressor-disassociated RXR complex. We hypothesize there is likely to be a bexarotene analog that may optimize this biological activity in its use against CTCL in humans. The current understanding of conformational changes induced by ligand-bound rexinoids suggests that the ligand-binding domain undergoes significant structural rearrangement. Changes to the properties of the ligand may cause this rearrangement to differentially expose residues important to other NR-associated activity, including potential coactivation by other molecules [48,49]. This effect indicates that small changes to the properties of a ligand can exert large influences on the behavior of the NR with which it is associated.
The common side effects of bexarotene include acute pancreatitis, hypertriglyceridemia, hyperlipidemia, central hypothyroidism, and headaches [50–53]. The discovery of a more potent chemotherapeutic agent or one with less severe side effects may mitigate the harm consideration when selecting bexarotene-like treatments as cancer treatment options, thereby increasing the rate of positive outcomes through reductions in dosage requirements or the prevalence of side effects.
To this end, we have produced molecules with promising variations in the chemical attributes of bexarotene that may alter the biological activity of RXR upon ligand binding. While it would be challenging to attribute common structural features that characterize the three different groups of compounds classified in this study, the compound with the lowest divergence score in group 1—analog 5 (Fig 4)—shares a common framework based on the analog 4 framework. Compound 5 differs from 4 by a methyl group substitution for the hydroxyl group of 4.
The low divergence score for analog 5 (Figs 4 and 9, DS = 0.13) provides compelling justification to further investigate this potent rexinoid, but also motivates the development of additional analogs based upon this framework. It is also notable that the compound with the second lowest divergence across all of the analogs, analog 9 (Fig 4), is a compound that has been well-documented to possess potent anti-cancer and anti-tumor activity in the literature [54–60].
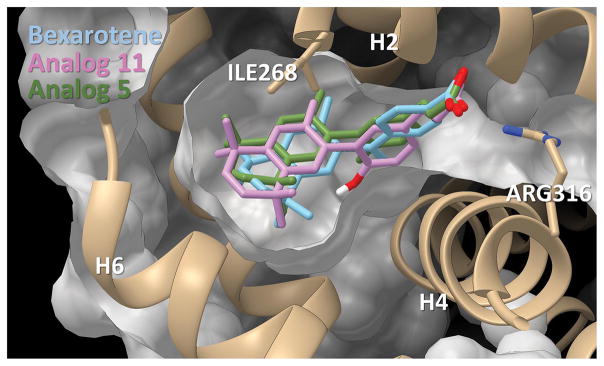
Figure 9.
Bexarotene and analogs 5 and 11 in binding pocket. Pictured is the docking of bexarotene and analogs 5 and 11 in pocket. The binding pocket is viewed with helices 2, 4, and 6 as reference points.
Investigation of the label-approved use of bexarotene for CTCL HuT-78 cell lines, both in the present study and in others, reveals that p53 pathway activated apoptosis appears to be a fundamental contributor to the anti-oncogenic activity of bexarotene [61]. The gene product abundance from BAX, survivin, bcl-2, and other genes are significantly altered by the presence of bexarotene, and to varying degrees several of our analogs show promise in this regard by comparison.
Grouping and divergence scoring methodologies
Analogs were split into three groups based upon differences in the measurements of gene product abundance via microarray analysis using a suite of study genes (Appendix). Results reveal that some of the analogs present distinctly sharp differences from the parent molecule, bexarotene, while other analogs display more mild differences, and still a third group of compounds do not reveal substantial differences from bexarotene in the magnitude or direction of gene regulation. The criteria used to designate these three categories is based on two factors, the enumeration of gene products from the study group which show differences from bexarotene, and the magnitude of these changes for each of the study genes.
Divergence Scoring [25] is useful to identify which are more likely to be suitable candidates in future hypothesis testing, however further analysis can lend more information as to how useful each analog may be compared to its group cohorts. To this end, a relative measure of scoring the predictability of each analog’s performance was designed, wherein each analog is scored relative to the others in its divergence from predicted gene directional movement compared to bexarotene. A subset of genes with well-understood mechanisms was used to construct a pass/fail filter.
Using improved anti-oncogenic activity as a biomarker, genes which performed in a way predicted to be an improvement to bexarotene were scored as a ‘pass’ and removed from this filter, whereas gene expression which functioned in a way that is counterproductive (worse than bexarotene) were aggregated as failures. Sums of the absolute values of deviations were divided across the total number of sub-set study genes for which anti-oncogenic activity is well understood, and the analog was assigned the resulting divergence score. In this way, large deviations from expectation on a gene-by-gene basis weigh more heavily than do small deviations. This methodology allows for a quantitative and less biased approach to the identification of RXR analogs which may possess improved therapeutic efficacy.
Identifying the study group of genes
We identified 102 study genes of interest to our investigation by way of examination of current literature, knowledge of cancer biology, and inferences from well-understood principles of cellular apoptosis, cell-cycle arrest, and other oncogenic cellular activity. Of these 102, 20 were selected (Appendix) for their power in differentiating the analogs as potential candidates for further study.
This sub-group of 20 genes was used for divergence scoring. BAX (BCL-associated apoptosis regulator) and BIRC-5 (survivin) are two examples of well-understood genes used in this subgroup. BAX is a participant in p53-mediated apoptosis (Appendix) and as a member of the BCL2 group of proteins the products of this gene should be analyzed for apoptosis regulation. Survivin, an inhibitor of apoptosis, is an often-studied anti-apoptotic gene known for being able to suppress multiple channels of apoptosis activation [62,63].
One example of the power and versatility provided by this type of analysis concerns the ability to identify which compounds modulate gene expression in a beneficial direction and further to examine how that group of compounds modulate other gene sets in beneficial directions. Taking survivin as an example, it is reasonable to hypothesize that drug candidates down-regulating survivin to a greater extent than bexarotene could potentially be more effective cancer drugs. Current research has identified that the downregulation of survivin is linked to increase rates of apoptosis, a key trait of tumor shrinking chemotherapeutic agents [64]. If we were interested in this specific mechanism, we could evaluate only analogs which outperform bexarotene in this one regard [25]. Divergence scoring provides a means of grading across each analog for 19 other well-understood genes like survivin. We can, using this tool, check whether an analog outperforms bexarotene according to a model of directionality provided by the current literature, and use this information to better inform decisions about future research.
Analogs may act through RXR homodimerization or heterodimerization
Previous research has indicated that small changes in rexinoid structure may lead to highly differential gene expression [3,65]. Consistent with this view, our results suggest that analogs 6 and 12 which have very similar structures, differing only in the presence or absence of a single fluorine, appear to regulate gene expression through activation of RXRα/VDR dimers (Table 5). However, analog 12 which contains a fluorine seems to perform better than bexarotene, while analog 6, which lacks the fluorine residue and regulates gene activity via the same heterodimer complex yet does not perform as well as bexarotene. Further, we find that structures that are quite different may act through the same RXR dimer complex. Analogs 1 and 4 are quite different from one another, yet both appear to act through the RXRα/VDR dimer. The possibility remains that analog 4 decreases gene expression through recruitment of different cofactors or inhibition of receptor conformational changes.
The RXRα/VDR dimer is thought to be non-permissive and therefore incapable of generating a response in the absence of 1,25-dihydroxyvitamin D3 (1,25D3)[66]. However, in the studies presented here, cells were treated only with bexarotene analogs which indicates that the analogs are regulating receptor dynamics independent of vitamin D binding. These findings are consistent with previous reports that indicate that the RXRα/VDR heterodimer may act as a permissive receptor, at least in some cases [67,68]. Conformational changes of the RXRα/VDR dimer induced solely by 9-cis-retinoic acid (9-cis-RA) binding are thought to enhance VDR-DBD stability and may facilitate interactions with co-regulatory proteins such as SRC1-RID [67].
For some analogs, it is unclear which RXR dimer is the most probable candidate to mediate analog activity. For example, analogs 2 and 3 which differ only in the presence of one (analog 2) or two (analog 3) fluorine atoms were both able to significantly induce expression of cluster 11 genes. However, because 7 of the 9 RXR motifs examined in this study were significantly enriched in cluster 11, determination of the functional RXR motif is more challenging and may limit the use of these analogs in cancer treatments. The strong correlation between RXR binding motifs and the response to specific analogs demonstrates that both analog structure and the RXR motifs present in the cis-regulatory region of target genes will likely determine the direction of the response (e.g. enhance or reduce gene expression) to various rexinoids.
Most of the cancer-related study genes contain at least one putative RXR binding motif in the presumptive 500 bp promoter examined in this study [25]. Gene cluster 10 did not respond to any of the analogs tested and it is notable that cluster 10 was the only one that was not enriched in any of the 9 RXR motifs, providing further compelling evidence for the relevance of our in-silico approach. Further, none of the analogs were able to induce a significant effect on expression of genes in clusters 8 and 9. Cluster 8 genes are enriched only in the PPARγ::RXRα_v2 motif while only the RARα::RXRα motif was detected in cluster 9 genes. Together, these data suggest that neither the PPARγ/RXRα dimer nor the RARα/RXRα dimer are sufficient to mediate the actions of the 11 Rexinoids tested in this study. This observation is also consistent with our measured RAR “cross-over” binding studies suggesting that the ability of these analogs to activate the RAR-RXR heterodimer is low (data not shown).
Surprisingly, analogs 7, and 9–11 did not induce a significant change in gene expression for any of the gene clusters compared to bexarotene. There are several possibilities that can explain this. First, it is possible that the structure of these analogs prevents receptor activation or interaction with co-activators. It is also possible that these analogs regulate the expression of genes that were not represented in the cancer-related study genes selected for analysis in this study. Finally, these analogs may simply be too similar to bexarotene in their structure and binding to have differential gene expression versus bexarotene.
The sorting of the 12 investigational analogs into 3 groups, each containing compounds with similar expression profiles, and the further differentiation between the members of these groups using divergence scoring allows us to make some assessments about the likely viability of each analog for future testing. Selecting an analog with a low divergence score suggests that the chosen compound is likely not performing task-critical effects less effectively than the parent molecule. Also important is selecting a diverse representation of molecules in the way that they have substantial performance differences compared to bexarotene.
Given that we have a limited basis to appraise an analog regarding exactly how drastic a difference from bexarotene may be desirable, we assert that it is prudent to not disqualify a candidate because of this metric. Instead, we select analogs representing the best candidate from each group, analog 5 from group 1, containing substantial differences from bexarotene and the best divergence score among its peers—analog 10, the best divergence scoring species from group 2 which represents molecules with mild differences from bexarotene—and lastly analog 3 from group 3.
We are interested in both analogs 3 and 8 together from the third group. These two compounds have attractive DS compared to all analogs as a whole and further, we are interested in analog 8 in particular because analog 8 also shows promise from other research, and its DS compared to all other analogs is below the average score, an attractive quality. The sterol regulatory element-binding protein profile of analog 8 as studied in other research shows promise that it may provide a superior side-effect consideration [69]. Given this consideration, as well as its attractive placement as the third lowest DS in the study (DS = 0.12), we decide to include both species as recommendations for further study.
Utilizing this expression profiling, analogs 3, 5, 8, 10 and 11 hold the most promise for future work, as they have the lowest divergence scores and represent a diverse selection from each of the analog archetype groupings. Studying RXR homodimerization and gene expression in cultured CTCL cells is a quick and effective method to screen for the most promising analogs; however, the next steps must include moving into an animal disease model. There is promising work on a new mouse model of CTCL as well as excellent information about rexinoid treatment of lung cancer and future steps must include identifying in vivo side effect and lipid profiles of candidates, correlating these to the gene expression profiles, and then judiciously treating model organisms of disease to determine whole animal efficacy, with an eye towards clinical trials [70,71].
New rexinoid synthesis should take into account the physical characteristics of the molecules as they map out in Fig 6. Analogs which tend to have physical characteristics that would group them tightly in group 1 are likely to produce more significantly different expression profiles from bexarotene than are analogs that fit into groups 2 or 3.
Supplementary Material
Highlights.
CTCL cells reveal differential gene expression when treated with chemically distinct rexinoids.
Rexinoids can be partitioned into discrete classes employing gene expression profiles.
Minor variations in rexinoid structure lead to unique gene expression signatures.
Divergence Scoring using expression profiles can lead to valuable structural and decision making insights in rexinoid design.
Five of the twelve studied bexarotene analogs show promise for further analysis/drug development.
Acknowledgments
This work was supported by the National Institutes of Health National Cancer Institute (Grant 1 R15 CA139364-01A2) and a Women and Philanthropy Grant, both to CEW, PWJ, and PAM. We wish to thank Julie Furmick for initial microarray experimental work. PAM wishes to thank the Genome Consortium for Active Teaching for initial microarray training and support for this project. Many thanks to Roger Berger, Anthony Falsetti, and Lara Ferry for discussions of data analysis and to Michael Heck for discussions of the gene list. We thank Skysong Innovations (formerly AzTE) for ongoing intellectual property support of our work. Computer time was provided by USF Research Computing, sponsored in part by NSF MRI CHE-1531590 to AvdV.
Footnotes
Abbreviations: 9-cis-RA 9-cis-retinoic acid; ATRA all trans retinoic acid; CTCL cutaneous T cell lymphoma; DS divergence score; FXR; farnesoid X receptor; HRE hormone-responsive element; LBD ligand-binding domain; LXR liver X receptor; NSCLC non-small cell lung cancer; NR nuclear receptor; PCA principal component analysis; PPAR peroxisome proliferator-activated receptor; RA retinoic acid; RAR retinoic acid receptor; RXR retinoid X receptor; RXRE RXR-responsive element; tPSA topological polar surface area; TR thyroid hormone receptor; VDR vitamin D receptor
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boehm MF, Zhang L, Badea BA, White SK, Mais DE, et al. Synthesis and structure-activity relationships of novel retinoid X receptor-selective retinoids. J Med Chem. 1994;37:2930–2941. doi: 10.1021/jm00044a014. [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf DJ, Umesono K, Evans RM. The Retinoids. Raven Press; New York, NY: 1994. pp. 319–349. [Google Scholar]
- 3.Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signaling pathways. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-Z. [DOI] [PubMed] [Google Scholar]
- 4.Forman BM, Yang CR, Au M, Casanova J, Ghysdael J, et al. A domain containing leucine-zipper-like motifs mediate novel in vivo interactions between the thyroid hormone and retinoic acid receptors. Mol Endocrinol. 1989;3:1610–1626. doi: 10.1210/mend-3-10-1610. [DOI] [PubMed] [Google Scholar]
- 5.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XK, Lehmann J, Hoffmann B, Dawson MI, Cameron J, et al. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992;358:587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez M, Alvarez S, de Lera AR. Natural and Structure-based RXR Ligand Scaffolds and Their Functions. Curr Top Med Chem. 2017;17:631–662. doi: 10.2174/1568026616666160617072521. [DOI] [PubMed] [Google Scholar]
- 8.Su Y, Zeng Z, Chen Z, Xu D, Zhang W, et al. Recent Progress in the Design and Discovery of RXR Modulators Targeting Alternate Binding Sites of the Receptor. Curr Top Med Chem. 2017;17:663–675. doi: 10.2174/1568026616666160617092241. [DOI] [PubMed] [Google Scholar]
- 9.Thompson PD, Remus LS, Hsieh JC, Jurutka PW, Whitfield GK, et al. Distinct retinoid X receptor activation function-2 residues mediate transactivation in homodimeric and vitamin D receptor heterodimeric contexts. J Mol Endocrinol. 2001;27:211–227. doi: 10.1677/jme.0.0270211. [DOI] [PubMed] [Google Scholar]
- 10.Svensson S, Ostberg T, Jacobsson M, Norstrom C, Stefansson K, et al. Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. EMBO J. 2003;22:4625–4633. doi: 10.1093/emboj/cdg456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 12.Lala DS, Mukherjee R, Schulman IG, Koch SS, Dardashti LJ, et al. Activation of specific RXR heterodimers by an antagonist of RXR homodimers. Nature. 1996;383:450–453. doi: 10.1038/383450a0. [DOI] [PubMed] [Google Scholar]
- 13.Lemon BD, Freedman LP. Selective effects of ligands on vitamin D3 receptor- and retinoid X receptor-mediated gene activation in vivo. Mol Cell Biol. 1996;16:1006–1016. doi: 10.1128/mcb.16.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald PN, Dowd DR, Nakajima S, Galligan MA, Reeder MC, et al. Retinoid X receptors stimulate and 9-cis retinoic acid inhibits 1,25-dihydroxyvitamin D3-activated expression of the rat osteocalcin gene. Mol Cell Biol. 1993;13:5907–5917. doi: 10.1128/mcb.13.9.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson PD, Jurutka PW, Haussler CA, Whitfield GK, Haussler MR. Heterodimeric DNA binding by the vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. Evidence for allosteric receptor interactions. J Biol Chem. 1998;273:8483–8491. doi: 10.1074/jbc.273.14.8483. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann JM, Zhang XK, Graupner G, Lee MO, Hermann T, et al. Formation of retinoid X receptor homodimers leads to repression of T3 response: hormonal cross talk by ligand-induced squelching. Mol Cell Biol. 1993;13:7698–7707. doi: 10.1128/mcb.13.12.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haussler MR, Whitfield GK, Haussler CA, Sabir MS, Khan Z, et al. 1,25-Dihydroxyvitamin D and Klotho: A Tale of Two Renal Hormones Coming of Age. Vitam Horm. 2016;100:165–230. doi: 10.1016/bs.vh.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Muccio DD, Atigadda VR, Brouillette WJ, Bland KI, Krontiras H, et al. Translation of a Tissue-Selective Rexinoid, UAB30, to the Clinic for Breast Cancer Prevention. Curr Top Med Chem. 2017;17:676–695. doi: 10.2174/1568026616666160617093604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner CE, Jurutka PW, Marshall PA, Heck MC. Retinoid x receptor selective agonists and their synthetic methods. Current Topics in Medicinal Chemistry. 2017;17(6):742–767. doi: 10.2174/1568026616666160617091559. [DOI] [PubMed] [Google Scholar]
- 20.Morishita KI, Kakuta H. Retinoid X Receptor Ligands with Anti-Type 2 Diabetic Activity. Curr Top Med Chem. 2017;17:696–707. doi: 10.2174/1568026616666160617085545. [DOI] [PubMed] [Google Scholar]
- 21.Koster KP, Smith C, Valencia-Olvera AC, Thatcher GRJ, Tai LM, LaDu MJ. Rexinoids as Therapeutics for Alzheimer’s Disease: Role of APOE. Current Topics in Medicinal Chemistry. 2017;17:708–720. doi: 10.2174/1568026616666160617090227. [DOI] [PubMed] [Google Scholar]
- 22.McFarland K, Spalding TA, Hubbard D, Ma JN, Olsson R, et al. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem Neurosci. 2013;4:1430–1438. doi: 10.1021/cn400100f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin RJ, ffrench-Constant C, Edgar JM, Smith KJ. Neuroprotection and repair in multiple sclerosis. Nat Rev Neurol. 2012;8:624–634. doi: 10.1038/nrneurol.2012.200. [DOI] [PubMed] [Google Scholar]
- 24.Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nunez V, et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain. 2015;138:3581–3597. doi: 10.1093/brain/awv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hackney Price J, Hanish B, Wagner CE, Kaneko I, Jurutka PW, Marshall PA. Dataset on the response of Hut78 Cells to Novel Rexinoids. Data in Brief. 2018 doi: 10.1016/j.dib.2018.09.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Vaart A, Lorkowski A, Ma N, Gray GM. Computer Simulations of the Retinoid X Receptor: Conformational Dynamics and Allosteric Networks. Curr Top Med Chem. 2017;17:731–741. doi: 10.2174/1568026616666160617084745. [DOI] [PubMed] [Google Scholar]
- 29.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egea PF, Mitschler A, Moras D. Molecular recognition of agonist ligands by RXRs. Mol Endocrinol. 2002;16:987–997. doi: 10.1210/mend.16.5.0823. [DOI] [PubMed] [Google Scholar]
- 32.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 33.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 34.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zambelli F, Pesole G, Pavesi G. Pscan: finding over-represented transcription factor binding site motifs in sequences from co-regulated or co-expressed genes. Nucleic Acids Res. 2009;37:W247–252. doi: 10.1093/nar/gkp464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider TD, Stephens RM. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathelier A, Fornes O, Arenillas DJ, Chen CY, Denay G, et al. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2016;44:D110–115. doi: 10.1093/nar/gkv1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandelin A, Alkema W, Engstrom P, Wasserman WW, Lenhard B. JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004;32:D91–94. doi: 10.1093/nar/gkh012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner CE, Jurutka PW, Marshall PA, Groy TL, van der Vaart A, et al. Modeling, synthesis and biological evaluation of potential retinoid X receptor (RXR) selective agonists: novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) J Med Chem. 2009;52:5950–5966. doi: 10.1021/jm900496b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furmick JK, Kaneko I, Walsh AN, Yang J, Bhogal JS, et al. Modeling, synthesis and biological evaluation of potential retinoid X receptor-selective agonists: novel halogenated analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) ChemMedChem. 2012;7:1551–1566. doi: 10.1002/cmdc.201200319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez Santin E, Germain P, Quillard F, Khanwalkar H, Rodriguez-Barrios F, et al. Modulating retinoid X receptor with a series of (E)-3-[4-hydroxy-3-(3-alkoxy-5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-y l)phenyl]acrylic acids and their 4-alkoxy isomers. J Med Chem. 2009;52:3150–3158. doi: 10.1021/jm900096q. [DOI] [PubMed] [Google Scholar]
- 43.Jurutka PW, Kaneko I, Yang J, Bhogal JS, Swierski JC, et al. Modeling, synthesis, and biological evaluation of potential retinoid X receptor (RXR) selective agonists: novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) and (E)-3-(3-(1,2,3,4-tetrahydro-1,1,4,4,6-pentamethylnaphthalen-7-yl)-4-hydroxypheny l)acrylic acid (CD3254) J Med Chem. 2013;56:8432–8454. doi: 10.1021/jm4008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Badea BA, Enyeart D, Berger EM, Mais DE, et al. Syntheses of isotopically labeled 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethenyl]benzoic acid (LGD1069), a potent retinoid x receptor-selective ligand. Journal of Labelled Compounds and Radiopharmaceuticals. 1995;36:701–712. doi: 10.1002/jlcr.2580360712. [DOI] [Google Scholar]
- 45.Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, et al. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–3155. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- 46.Klinge CM, Bodenner DL, Desai D, Niles RM, Traish AM. Binding of type II nuclear receptors and estrogen receptor to full and half-site estrogen response elements in vitro. Nucleic Acids Res. 1997;25:1903–1912. doi: 10.1093/nar/25.10.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen WC, Prudente RY, Corpuz MR, Negro-Vilar A, Lamph WW. A selective retinoid X receptor agonist bexarotene (LGD1069, targretin) inhibits angiogenesis and metastasis in solid tumours. Br J Cancer. 2006;94:654–660. doi: 10.1038/sj.bjc.6602995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boerma LJ, Xia G, Qui C, Cox BD, Chalmers MJ, et al. Defining the communication between agonist and coactivator binding in the retinoid X receptor alpha ligand binding domain. J Biol Chem. 2014;289:814–826. doi: 10.1074/jbc.M113.476861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochim Biophys Acta. 2012;1821:57–69. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Lowe MN, Plosker GL. Bexarotene. Am J Clin Dermatol. 2000;1:245–250. doi: 10.2165/00128071-200001040-00006. discussion 251–242. [DOI] [PubMed] [Google Scholar]
- 51.Talpur R, Ward S, Apisarnthanarax N, Breuer-Mcham J, Duvic M. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672–684. doi: 10.1016/j.jaad.2003.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Assaf C, Bagot M, Dummer R, Duvic M, Gniadecki R, et al. Minimizing adverse side-effects of oral bexarotene in cutaneous T-cell lymphoma: an expert opinion. Br J Dermatol. 2006;155:261–266. doi: 10.1111/j.1365-2133.2006.07329.x. [DOI] [PubMed] [Google Scholar]
- 53.Gupta V, Lee M. Central hypothyroidism. Indian J Endocrinol Metab. 2011;15:S99–S106. doi: 10.4103/2230-8210.83337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uray IP, Brown PH. Chemoprevention of hormone receptor-negative breast cancer: new approaches needed. Recent Results Cancer Res. 2011;188:147–162. doi: 10.4103/2230-8210.83337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seo HS, Woo JK, Shin YC, Ko SG. Identification of biomarkers regulated by rexinoids (LGD1069, LG100268 and Ro25-7386) in human breast cells using Affymetrix microarray. Mol Med Rep. 2015;12:800–818. doi: 10.3892/mmr.2015.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uray IP, Rodenberg JM, Bissonnette RP, Brown PH, Mancini MA. Cancer-preventive rexinoid modulates neutral lipid contents of mammary epithelial cells through a peroxisome proliferator-activated receptor gamma-dependent mechanism. Mol Pharmacol. 2012;81:228–238. doi: 10.1124/mol.111.072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiff R, Chamness GC, Brown PH. Advances in breast cancer treatment and prevention: preclinical studies on aromatase inhibitors and new selective estrogen receptor modulators (SERMs) Breast Cancer Res. 2003;5:228–231. doi: 10.1186/bcr626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.le Maire A, Alvarez S, Shankaranarayanan P, Lera AR, Bourguet W, et al. Retinoid receptors and therapeutic applications of RAR/RXR modulators. Curr Top Med Chem. 2012;12:505–527. doi: 10.2174/156802612799436687. [DOI] [PubMed] [Google Scholar]
- 59.Liby K, Rendi M, Suh N, Royce DB, Risingsong R, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. 2006;12:5902–5909. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 60.Liby KT, Sporn MB. Rexinoids for prevention and treatment of cancer: opportunities and challenges. Curr Top Med Chem. 2016 doi: 10.2174/1568026616666160617090702. [DOI] [PubMed] [Google Scholar]
- 61.Nieto-Rementeria N, Perez-Yarza G, Boyano MD, Apraiz A, Izu R, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519–526. doi: 10.1111/j.1365-2133.2008.08931.x. [DOI] [PubMed] [Google Scholar]
- 62.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 63.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 64.Chen XQ, Yang S, Li ZY, Lu HS, Kang MQ, et al. Effects and mechanism of downregulation of survivin expression by RNA interference on proliferation and apoptosis of lung cancer cells. Mol Med Rep. 2012;5:917–922. doi: 10.3892/mmr.2012.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangelsdorf DJ. Vitamin A receptors. Nutr Rev. 1994;52:S32–44. doi: 10.1111/j.1753-4887.1994.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 66.Dawson MI, Xia Z. The retinoid X receptors and their ligands. Biochim Biophys Acta. 2012;1821:21–56. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanchez-Martinez R, Castillo AI, Steinmeyer A, Aranda A. The retinoid X receptor ligand restores defective signalling by the vitamin D receptor. EMBO Rep. 2006;7:1030–1034. doi: 10.1038/sj.embor.7400776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Badea BA, Enyeart D, Berger EM, Mais DE, et al. Syntheses of isotopically labeled 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethenyl]benzoic acid (LGD1069), a potent retinoid x receptor-selective ligand. Journal of Labelled Compounds and Radiopharmaceuticals. 1995;36:701–712. doi: 10.1002/jlcr.2580360712. [DOI] [Google Scholar]
- 69.Marshall PA, Jurutka PW, Wagner CE, van der Vaart A, Kaneko I, et al. Analysis of differential secondary effects of novel rexinoids: select rexinoid X receptor ligands demonstrate differentiated side effect profiles. Pharmacol Res Perspect. 2015;3:e00122. doi: 10.1002/prp2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mishra A, La Perle K, Kwiatkowski S, Sullivan LA, Sams GH, et al. Mechanism, Consequences, and Therapeutic Targeting of Abnormal IL15 Signaling in Cutaneous T-cell Lymphoma. Cancer Discov. 2016;6:986–1005. doi: 10.1158/2159-8290.CD-15-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao M, Royce DB, Risingsong R, Williams CR, Sporn MB, et al. The Rexinoids LG100268 and LG101506 Inhibit Inflammation and Suppress Lung Carcinogenesis in A/J Mice. Cancer Prev Res (Phila) 2016;9:105–114. doi: 10.1158/1940-6207.CAPR-15-0325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.