Summary
Zebrafish embryos are useful to study haematopoietic gene function in vertebrates, although lack of antibodies to zebrafish proteins has limited the purification of specific cell populations. Here, we purified primitive zebrafish erythrocytes using 1, 5-bis{[2-(di-methylamino)ethyl]amino}-4, 8-dihydroxyanthracene-9, 10-dione (DRAQ5™), a DNA-staining fluorescent dye. At 48-h post-fertilization, we sorted small-sized cells from embryos using forward scatter and found that they consisted of DRAQ5high and DRAQ5low populations. DRAQ5high cells contained haemoglobin, lacked myeloperoxidase activity and expressed high levels of embryonic globin (hbae3 and hbbe1.1) mRNA, all characteristics of primitive erythrocytes. Following DRAQ5™ analysis of gata1:dsRed transgenic embryos, we purified primitive DRAQ5high dsRed+ erythrocytes from haematopoietic progenitor cells. Using this method, we identified docking protein 2 (Dok2) as functioning in differentiation of primitive erythrocytes. We conclude that DRAQ5™-based flow cytometry enables purification of primitive zebrafish erythrocytes.
Keywords: zebrafish, primitive erythrocyte, DRAQ5™, flow cytometry
Zebrafish (Danio rerio) is a useful model to study vertebrate haematopoiesis, owing to highly conserved functions of haematopoiesis-related genes. Several zebrafish mutant strains relevant to erythropoiesis-related human diseases, such as sherocytosis (Liao et al, 2000), sideroblastic anaemia (Brownlie et al, 1998) and thalassemia-like disorder (Brownlie et al, 2003), have been generated. Adult transgenic zebrafish in which blood cells express particular oncogenes also serve as models of leukaemogenesis (Langenau et al, 2003) and leukaemia progression (Feng et al, 2007; Feng et al, 2010).
Zebrafish embryos and larvae are also useful models to study vertebrate haematopoiesis. Their small size and large number plus their extra-utero development allow high-throughput genetic screens that have identified numerous genes that function in erythropoiesis (Jing & Zon, 2011), some of which were later confirmed as underlying pathophysiology of human anaemia. Moreover, in zebrafish embryos, genome editing techniques, including TALEN (Transcription activator-like effector nucleases) and CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) (Xiao et al, 2013), are currently being applied to genetic screens of erythropoiesis (Chung et al, 2015) and macrophage formation (Shiau et al, 2015) as well as the establishment of human blood disease models (Lin et al, 2016).
Flow cytometry allows quantitative analysis and isolation of human and mouse erythroid cells due to availability of fluorescence-labelled, erythrocyte-specific antibodies. However, there is a limited number of antibodies available that specifically bind membrane proteins in zebrafish erythrocytes. A previous report shows that flow cytometry can separate erythrocytes from the kidney tissue of adult zebrafish based on light scatter characteristics (Traver et al, 2003). However, its application in zebrafish embryos has not been investigated. Besides antibodies, several DNA-binding fluorochromes are available for flow cytometry. One such fluorochrome, the red fluorescing agent, 1, 5-bis{[2-(di-methylamino) ethyl]amino}-4, 8-dihydroxyanthracene-9, 10-dione (DRAQ5™), is a synthetic anthraquinone (Smith et al, 2000) that rapidly enters living cells and specifically binds DNA with high affinity. DRAQ5™ fluorescence intensity depends on DNA content and chromatin complexity, which affects accessibility to DRAQ5™ (Smith et al, 1999). To expand application of flow cytometry for study of zebrafish erythropoiesis, we have developed an antibody-free DNA staining-based approach to purify zebrafish primitive erythrocytes. Here, we applied it to the identification of docking protein 2 (Dok2) as a novel regulator of zebrafish erythropoiesis.
Methods
Fish maintenance
Adult zebrafish (Danio rerio, India strain) were housed in tanks of recirculated dechlorinated tap water at 26·5°C (water temperature). The transgenic line Tg(gata1:dsRed) (Traver et al, 2003) and Tg(myb:GFP) (North et al, 2007) was obtained from the Aquatic Research Program, Boston Children’s Hospital, MA, USA. Animal care was performed in accordance with institutional and national guidelines and regulations. The study protocol was approved by the institutional review board of the Medical Institute of Bioregulation, Kyushu University.
Cell preparation
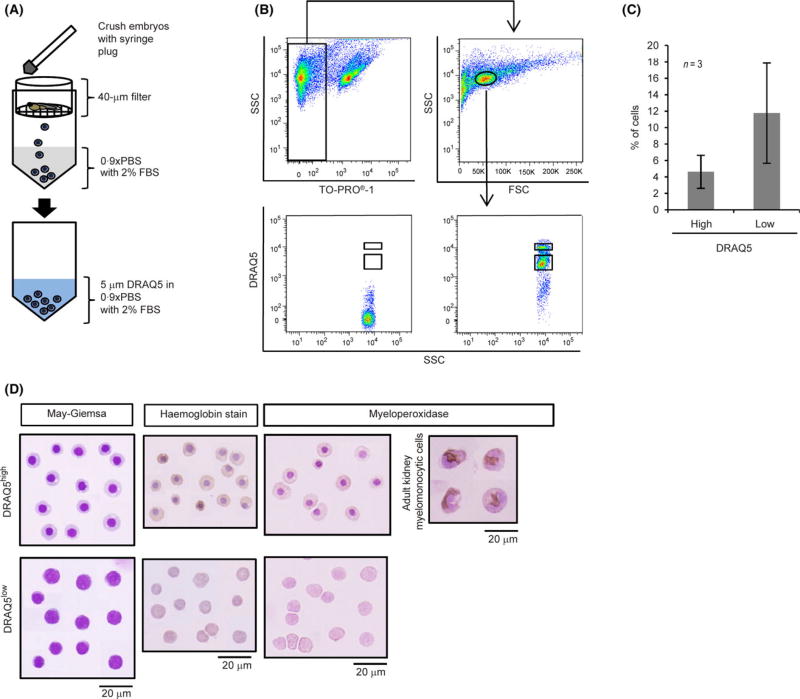
Figure 1A shows a diagram of cell preparation from 48-h post-fertilization (hpf) embryos. Embryos were washed three times with 0·9 × phosphate-buffered saline (PBS) and allowed to settle by gravity after each wash. After removing 0·9 × PBS, the embryos were immersed in 0·9 × PBS containing 2% fetal bovine serum (FBS), crushed with the plunger of 1-ml syringe and passed through a 30-µm nylon filter (Falcon® Cell Strainer, Corning, Durham, NC, USA). The resulting cells were washed three times with 0·9 × PBS containing 2% FBS. Cells were pelleted by centrifugation at 200 × g for 5 min at room temperature and then re-suspended in 0·9 × PBS containing 2% FBS. Cells were stained with trypan blue, and living cells were counted using a haematocytometer.
Fig 1.
DRAQ5high cells are erythroid-like cells containing haemoglobin. (A) Schematic diagram of preparation and staining of 48-h post-fertilization zebrafish cells. (B) Flow cytometric analysis of zebrafish embryonic cells. Dead cells were excluded using TOPRO-1. TOPRO-1 negative cells were then gated according to SSC and FSC. Cells were analysed for DRAQ5™ intensity in FSClow and SSClow fractions, and detected via PerCp-Cy5. DRAQ5high and DRAQ5low cells were gated. (C) Percentage of DRAQ5high and DRAQ5low obtained from three independent flow cytometric analyses and shown as mean±SD. (D) May-Giemsa staining, haemoglobin staining and myeloperoxidase activity of DRAQ5high and DRAQ5low cells. Scale bars: 20 µm for all panels. Orange-red in cytoplasm indicates haemoglobin. Nuclei are stained by haematoxylin and appear as grey. DRAQ5, 1, 5-bis{[2-(di-methylamino)ethyl]amino}-4, 8-dihydroxyanthracene-9, 10-dione; FBS, fetal bovine serum; FSC, forward scatter; PBS, phosphate-buffered saline; SSD, side scatter. [Colour figure can be viewed at wileyonlinelibrary.com]
DRAQ5™ staining and flow cytometric analysis
DRAQ5™ staining was undertaken using the manufacturer’s instruction (BioStatus Ltd., Loughborough, UK). Briefly, 1 × 105 cells were suspended in 1 ml of 5 µmol/l DRAQ5™ in 0·9 × PBS containing 2% FBS and kept in the dark for 15 min at room temperature. Cells were pelleted by centrifugation at 200 × g for 5 min at room temperature and analysed by flow cytometry without washing. Dead cells were excluded by incubation with TO-PRO®-1 iodide (Invitrogen, Eugene, OR, USA) in the case of both wild-type and Tg(gata1:dsRed) lines or SYTOX® Blue (Invitrogen) in the case of the Tg(myb:GFP) line. A filter of Per-Cp-Cy5 was set to detect the emitted fluorescent signal of DRAQ5™. Cells were sorted into RNAlater® (Life Technologies, Carlsbad, CA, USA) for gene expression analysis and into 0·9 × PBS containing 2% FBS for morphological analysis. Flow cytometry and cell sorting were performed using a BD FACSAria (BD Bioscience, San Jose, CA, USA).
May-Grünwald Giemsa staining
Sorted cells were attached onto glass slides (SUPERFROST®, Matsunami Glass Ind., Ltd., Osaka, Japan) using a CytoSpin™4 Cytocentrifuge (Thermo Fisher Scientific, Waltham, MA, USA) at 23 × g for 7 min and then rapidly air-dried. Cells were fixed and stained using May-Grünwald reagent (Muto Pure Chemicals, Tokyo, Japan) for 5 min at room temperature. After a brief wash in running tap water, cells were incubated with phosphate buffer (pH = 6·4) for 2 min and stained with 1:18 diluted Giemsa solution (Muto Pure Chemicals) at room temperature for 30 min. After one wash with running tap water, slides were air-dried and covered with glass coverslips (Matsunami Glass Ind., Ltd., Osaka, Japan) with a drop of MGK-S mounting solution (Matsunami Glass Ind., Ltd.). Cell morphology was assessed using an Olympus CKX41 microscope (Olympus, Tokyo, Japan).
Haemoglobin staining with o-dianisidine
Sorted cells were attached to glass slides as described above. Cells were incubated with o-dianisidine solution (0·62 mg/ml of o-dianisidine in 0·65% H2O2, 43% ethanol in 10 mmol/l sodium acetate) for 10 min at room temperature in a moisture chamber. After 3 washes in tap water, cells were counterstained in Mayer’s Haematoxylin solution (Muto Pure Chemicals) at room temperature for 10 min. After washing slides with running tap water, cells were air-dried and covered with glass coverslips with a drop of MGK-S mounting solution (Matsunami Glass Ind., Ltd.). Cell morphology was assessed as described above.
Analysis of myeloperoxidase activity
Myeloperoxidase activity was assessed following the manufacturer’s instructions (Sigma-Aldrich, St Louis, MO, USA). Briefly, sorted cells were attached onto glass slides (Matsunami Glass Ind., Ltd.) using a CytoSpin™4 Cytocentrifuge (Thermo Fisher Scientific) at 23 × g for 7 min and then rapidly air-dried. Cells attached to glass slides were fixed at room temperature for 30 s in 3·7% formaldehyde in 85·5% ethanol. After washing in running tap water and air-drying, glass slides were placed in freshly prepared peroxidase indicator reagent (Sigma-Aldrich) containing 0·01% H2O2 at 37°C for 30 min in the dark. After washing with running tap water and air-drying, cells were counterstained in acid haematoxylin solution at room temperature for 10 min. Cells on slides were then washed with running tap water, air-dried and covered with glass coverslips with a drop of MGK-S mounting solution (Matsunami Glass Ind., Ltd.). Cell morphology was assessed as described above.
RNA extraction and real-time polymerase chain reaction (PCR)
Total RNA was extracted from sorted cells using an RNA-queous®4PCR Kit (Ambion, Austin, TX), and cDNA was prepared using a High-Capacity RNA-to-cDNA Kit (Life Technologies). Expression of runx1, myb, gata2a, ptprc (cd45), spi1b (pu1), gata1, klf1 (klfd), hbbe3 hbae1, hbbe1.1, hbaa1 and ba1 was assessed by StepOnePlus™ real-time PCR (Life Technologies) with a SYBR Green Gene Expression System (Life Technologies). Primer sets were designed using Primer3 and validated for specific amplification using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). mRNA levels were normalized to eukaryotic translation elongation factor 1 alpha 1, like 1 (eef1a1l1) mRNA (Tang et al, 2007), and relative transcript levels were calculated using a relative standard curve method.
PCR-amplification of zebrafish dok2 cDNA and RNA preparation
dok2 cDNA was prepared from haematopoietic cells isolated from adult zebrafish kidney marrow using PCR. Primers were designed based on a cDNA reference sequence (GenBank accession number XM_001341478.7) and using software Primer Express® version 3.0 (Applied Biosystems, Life Technologies). Primer sequences used for dok2 amplification were: 5′-tttgaattcatggaggaagacattc-3′ and 5′-tttctcgagttcttttgcattacttttac-3′. The amplicon was cloned into the pCS2P+ vector (kindly provided by Dr. Ishitani Tohru, Kyushu University), and the sequence of cloned cDNA was verified by DNA sequencing. The dok2-pCS2P+ vector was then linearized with XhoI endonuclease and subjected to RNA synthesis using an mMESSAGE mMACHINE kit (Ambion, Life Technologies, Vilnius, Lithuania). Synthesized RNA was purified using an RNeasy® Plus Micro kit (Qiagen, Hilden, Germany) prior to injection.
Morpholino and RNA injection
Morpholino antisense oligonucleotides (MO) against dok2 were designed and obtained from Gene Tools (Philomath, OR, USA). The MO sequence was 5′-gcatgttcgccttaccttcccgaac-3′, which binds to the exon 1/intron 1 junction. Oligonucleotides (2·5 ng) were injected into embryos at the one- or two-cell stage using a GD-1 glass capillary needle (Narishige, Tokyo, Japan) and a nitrogen gas-pressurized injection system (Narishige). A random control oligo 25-N obtained from Gene Tools served as a negative control. For rescue experiments, a mixture of the dok2 MO plus dok2 RNA was injected at a ratio (MO:RNA) of 2·5 ng:15 pg. After injection, total RNA was extracted from MO-injected embryos at 24 hpf and subjected to cDNA synthesis. dok2 expression was assessed by real-time PCR as described above. Primers used for real-time PCR were 5′-at ggaggaagacattcgaaagaag-3′ and 5′-tgcggtttgctttctccagcgt-3′, which bind dok2 at exons 1 and 2, respectively.
In vitro differentiation assay
Kidney stromal cells were prepared following a previous report (Bertrand et al, 2007). Briefly, kidney was dissected out from adult zebrafish and bleached in 0·000525% sodium hypochlorite. After washing with 0·9 × PBS, kidney was gently crushed with the plunger of 1-ml syringe. To remove haematopoietic cells, the dissociated cells were passed through a 40-µm nylon filter (Falcon® Cell Strainer, Corning). The remaining cells were cultured in medium [50% L-15, 35% Dulbecco’s modified Eagle medium (DMEM), 15% Ham’s F-12 media, 10% FBS, 2% penicillin/streptomycin (Wako Pure Chemical Industries, Osaka, Japan), 1% L-glutamine, 150 mg/l sodium bicarbonate, and 1·5% HEPES] at 32°C, 5% CO2. To prepare immature primitive erythroid cells (defined as Gata1+Myb+), adult gata1:dsRed Tg zebrafish were crossed with myb:GFP Tg zebrafish. Embryos having dsRed+ and GFP+ were selected. At 30 hpf, embryos were dissociated as described in the cell preparation section. gata1:dsRed+ myb:GFP+ cells were sorted out using flow cytometry and co-cultured with kidney stromal cells for 2 days. The cultured floating cells were analysed for expression of gata1:dsRed and myb:GFP using flow cytometry.
Results
Flow cytometric profile of cells prepared from 48-hpf zebrafish embryos
In zebrafish embryos, blood circulation is established by 24 hpf, and the number of erythrocytes increases after 48 hpf. Thus, we performed flow cytometric analysis of cells prepared from 48-hpf zebrafish embryos and separated cells into four fractions based on forward scatter (FSC) using a blue laser. The designations F1, F2, F3 and F4 were assigned to cells having the lowest, low, intermediate and highest FSC (Figure S1A). The F1 fraction contained cell debris and melanin-containing granules, while F2 contained erythrocyte-like cells, which were round and exhibited a small, condensed nucleus. In F2 we also observed round cells having high nuclear/cytoplasmic ratio, which is characteristic of blast cells. F3 and F4 fractions contained mixtures of cells size larger than cells in F2. Some erythrocyte-like cells were observed in F4, but they were larger than erythrocyte-like cells in F2 (Figure S1B).
To confirm the presence of erythroid cells in the 4 fractions, we conducted the same analysis using gata1:dsRed transgenic zebrafish, in which erythroid cells are marked by dsRed (Figure S1C). We observed dsRed+ erythroid cells in the F2 (7·6 ± 1·8%, n = 4) and F3 (2·3 ± 1·4%, n = 4) fractions (Figure S1D). The number of dsRed+ erythroid cells in the F2 fraction was 3·3-fold higher than that seen in the F3 fraction. Therefore, the rest of our analysis focuses on cells of the F2 fraction.
The DRAQ5high sub-fraction exhibits erythroid, but not myeloid, characteristics
Because DRAQ5™ fluorochrome intensity depends on the accessibility of DRAQ5™ to genomic DNA (Smith et al, 1999), we employed DRAQ5™ to sub-fractionate F2 based on intensity and observed two sub-fractions: (i) low intensity DRAQ5 cells (DRAQ5low, 11·8 ± 6·1%, n = 3) and (ii) high intensity DRAQ5 cells (DRAQ5high, 4·6 ± 2·0%, n = 3) (Fig 1B, C). DRAQ5-negative cells were seen in debris and excluded from analysis. May-Giemsa staining revealed that the DRAQ5low sub-fraction contained round cells with a high nuclear/cytoplasmic ratio, a characteristic of immature blood cells, whereas the DRAQ5high sub-fraction contained round cells with a small and condensed nucleus (Fig 1D).
In zebrafish embryos, primitive erythroid cells, erythroid-myeloid progenitors (EMPs) and primitive myeloid cells (macrophage and neutrophils) are present by 48 hpf (Bennett et al, 2001; Burns et al, 2002; Kalev-Zylinska et al, 2002; Berman et al, 2005; Bertrand et al, 2007; Chen & Zon, 2009). Moreover, staining for haemoglobin as well as assessment of myeloperoxidase activity as a marker of myeloid cells can distinguish erythroid cells from other cell types. Only the DRAQ5high sub-fraction contained haemoglobin-expressing cells, and no cell in either the DRAQ5low or DRAQ5high sub-fraction exhibited myeloperoxidase activity compared to myelomonocytic cells prepared from kidney of adult zebrafish (Fig 1D). We conclude that the DRAQ5high sub-fraction of F2 possesses erythroid, but not myeloid, characteristics.
DRAQ5high cells express high levels of erythroid-specific transcripts
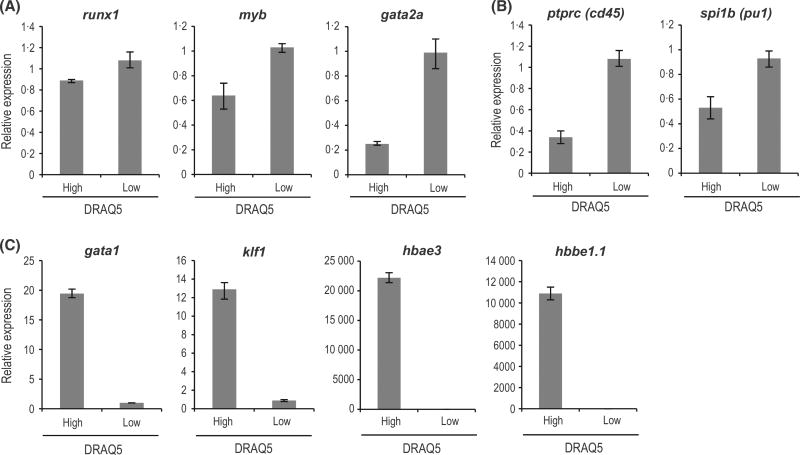
After 24 hpf, zebrafish embryonic erythroid cells express the erythroid transcription factors gata1 and klf1, which are orthologues of human GATA1 and KLF1, respectively. Embryonic α-globin (hbae3) and β-globin (hbbe1.1) mRNAs are reportedly detected at 48 hpf (Ganis et al, 2012). PCR analysis of the DRAQ5low sub-fraction revealed expression of haematopoietic progenitor-regulated transcription factors myb and gata2a (Fig 2A) and myeloid progenitor-specific spi1b and ptprc genes (Fig 2B) at levels higher than in the DRAQ5high sub-fraction. By contrast, the DRAQ5high fraction showed gata1 and klf1 expression at levels higher than the DRAQ5low sub-fraction (Fig 2C). Respective levels of hbae3 and hbbe1.1 mRNAs in the DRAQ5high sub-fraction were approximately 20 000- and 10 000-fold higher than those in the DRAQ5low sub-fraction. These data confirm that DRAQ5high cells are primarily comprised of primitive erythroid cells.
Fig 2.
DRAQ5high cells express high levels of erythropoiesis-related transcripts. (A) Gene expression analysis of DRAQ5high and DRAQ5low cells obtained from 48-h post-fertilization embryos. Expression levels of haematopoietic stem and progenitor-related mRNAs. (B) Expression levels of myeloid-related mRNAs in DRAQ5high and DRAQ5low cells obtained from 48-hpf embryos. (C) Expression levels of erythroid-related mRNAs. DRAQ5, 1, 5-bis{[2-(di-methylamino)ethyl]amino}-4, 8-dihydroxyanthracene-9, 10-dione.
Purification of erythroid cells using DRAQ5™ and gata1:dsRed transgenic zebrafish
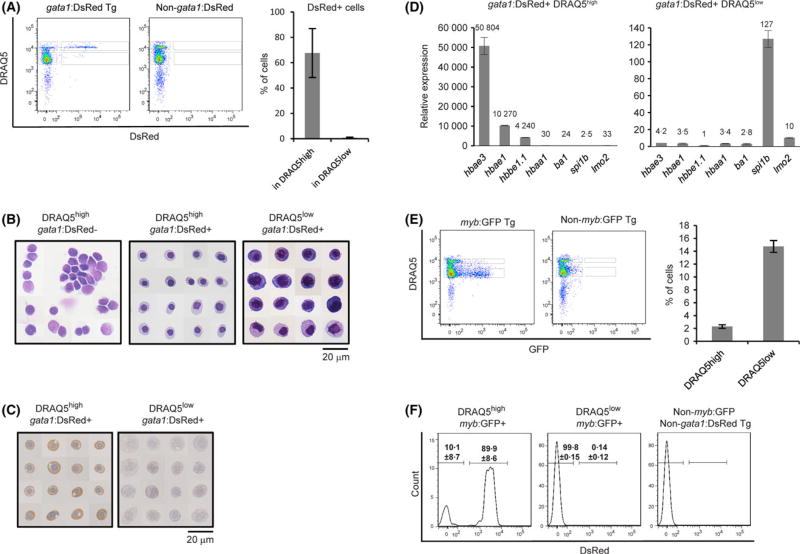
Human GATA1 is an erythroid-specific transcription factor regulating erythropoiesis and haemoglobin synthesis. We employed transgenic zebrafish, in which dsRed is expressed under control of the promoter of gata1a, an orthologue of human GATA1. The DRAQ5high sub-fraction contained dsRed+ erythroid cells (67·7 ± 19·3%) at levels 75-times higher than the DRAQ5low sub-fraction (0·9 ± 0·4%) (Fig 3A). Morphological analysis showed that DRAQ5high gata1:dsRed+ cells were round with small and condensed nuclei, while DRAQ5high gata1:dsRed− cells exhibited larger nuclei with apparently more loosely compacted content and scant cytoplasm. By contrast, DRAQ5low gata1:dsRed+ cells were heterogeneous: some showed a high nucleus/cytoplasm ratio with dark blue cytoplasm, which is indicative of early haematopoietic progenitors (Bertrand et al, 2007), and others exhibited a small, kidney-shaped nucleus with pink-orange cytoplasm (Fig 3B). Moreover, o-dianisidine staining revealed haemoglobin in DRAQ5high gata1:dsRed+ but not in DRAQ5low gata1:dsRed+ cells, indicating that the latter were relatively more mature than DRAQ5low gata1:dsRed+ erythroid cells (Fig 3C). In accord with morphological observations and haemoglobin staining, DRAQ5high gata1:dsRed+ cells expressed hbae3 and hbbe1.1 mRNAs at respective levels 10 000- and 4000-times higher than DRAQ5low gata1:DsRed+ cells (Fig 3D).
Fig 3.
DRAQ5high gata1:dsRed+ cells are mature primitive erythroid cells. (A) The presence of gata1:dsRed+ erythroid cells in DRAQ5high cells. Percentages of DRAQ5high gata1:dsRed+ and DRAQ5low gata1:dsRed+ erythroid cells obtained from three independent experiments. Data is shown as mean±SD. (B) May-Giemsa staining of DRAQ5high gata1:dsRed−, DRAQ5high gata1:dsRed+ and DRAQ5low gata1:dsRed+ cells. (C) Haemoglobin staining of DRAQ5high gata1:dsRed+ and DRAQ5low gata1:dsRed cells. (D) Expression levels of erythroid and myeloid-related mRNAs in DRAQ5high gata1:dsRed+ and DRAQ5low gata1:dsRed cells. (E) Percentages of myb:GFP+ haematopoietic progenitor cells in DRAQ5high and DRAQ5low fractions were calculated from three independent experiments. (F) The presence of erythroid-myeloid progenitor cells was confirmed by analysing gata1:dsRed; myb:GFP transgenic zebrafish embryos. Data is shown as mean ± SD. DRAQ5, 1, 5-bis{[2-(di-methylamino)ethyl] amino}-4, 8-dihydroxyanthracene-9, 10-dione. [Colour figure can be viewed at wileyonlinelibrary.com]
By contrast, DRAQ5low gata1:dsRed+ cells expressed higher levels of mRNAs encoding the myeloid-specific transcription factor Spi1b than did DRAQ5high gata1:dsRed+ cells. Co-expression of gata1:dsRed and spi1b mRNA suggests that DRAQ5low gata1:dsRed+ cells contain EMPs and/or haematopoietic progenitor cells. To confirm this, we first assessed expression level of lmo2 mRNA, which was reportedly expressed in EMPs (Bertrand et al, 2007). DRAQ5high gata1:dsRed+ cells expressed lmo2 mRNAs at respective levels 3·3-times higher than DRAQ5low gata1:dsRed+ cells (Fig 3d). Secondly, we utilized myb:GFP transgenic embryos, in which Myb-expressing haematopoietic progenitor cells are GFP+ (Thompson et al, 1998; Bertrand et al, 2008). The DRAQ5low fraction contained 14·7 ± 0±92% GFP+ cells, whereas the DRAQ5high fraction contained 2±3 ± 0±28% GFP+ cells. The presence of myb:GFP+ progenitors in the DRAQ5high fraction was consistent with our morphological observation of round cells with a high nuclear/cytoplasmic ratio (Fig 3B).
Reportedly, primitive erythroid cells (Thompson et al, 1998), EMPs (Bertrand et al, 2008) and non-EMP haematopoietic progenitor cells (Bertrand et al, 2008) also express Myb. To further characterize the DRAQ5high myb:GFP+ cells, we prepared single cell suspension of 48-hpf gata1:dsRed;myb:GFP Tg embryos by crossing adult gata1:dsRed Tg with myb:GFP Tg zebrafish and analysed the cells by flow cytometry. The DRAQ5high myb:GFP+ fraction contained 89·9 ± 8·6% of DsRed+ cells, suggesting that this fraction mainly contained primitive erythroid cells and EMP, which is consistent with high expression levels of embryonic globin and lmo2 genes (Fig 3D). The DRAQ5low myb:GFP+ fraction contained 0·14 ± 0·12% of DsRed+ cells (n = 3), also implying that myb:GFP+ cells in the DRAQ5low fraction contain non-EMP haematopoietic progenitor cells. Overall, analysis of gata1:dsRed transgenic zebrafish with DRAQ5™ enabled us to distinguish primitive erythroid cells and EMPs from non-EMP haematopoietic progenitor cells by flow cytometry.
Use of DRAQ5™ to identify genes functioning in erythropoiesis
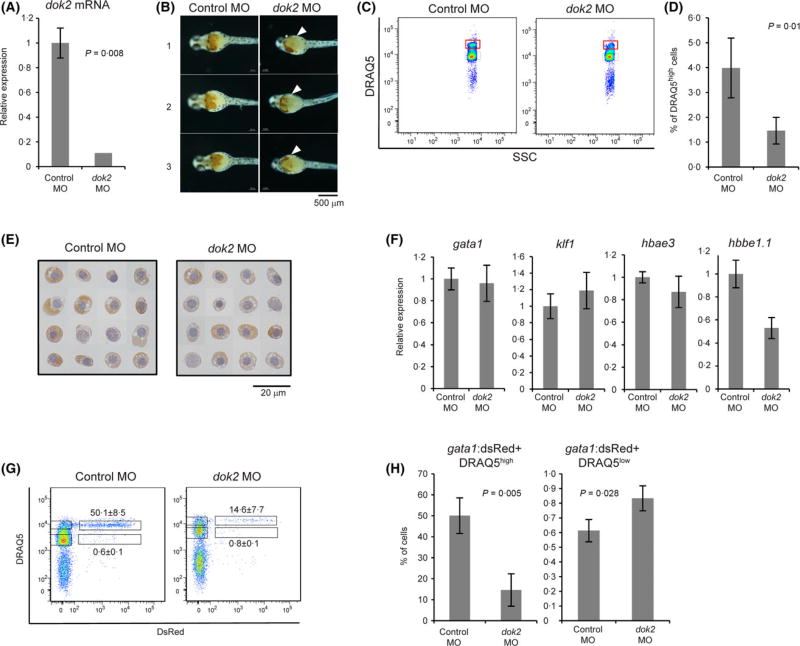
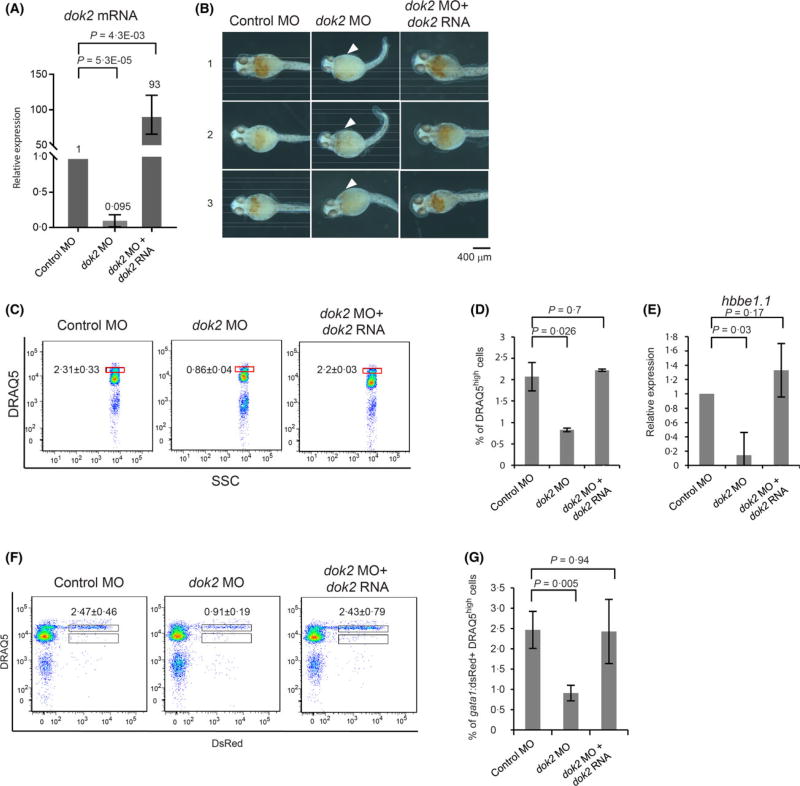
We previously employed global gene transcription analysis to show that treatment of mouse bone marrow-derived haematopoietic cells with Ninjin’yeito, a herbal medicine known as Kampo, accelerated myelopoiesis and suppressed erythropoiesis in vitro. In that study, expression of Dok2 mRNA was suppressed by Ninjin’yeito treatment, suggesting that Dok2 positively regulates erythropoiesis (Inoue et al, 2014). Here, to confirm this possibility, we used morpholinos (MO) to knock down dok2 mRNA to ~10% of levels detected in control MO-injected zebrafish at 24 hpf (Fig 4A). Staining of whole embryos for haemoglobin at 48 hpf showed decreased intensity of haemoglobin (red-orange in Fig 4B) in dok2 MO-injected compared to control MO-injected embryos. When we applied DRAQ5™ plus flow cytometric analysis at 48 hpf, we observed a 2·7-fold decrease in the percentage of erythroid cells in the DRAQ5high fraction (P = 0·01, Fig 4C, D). Although haemoglobin levels were unchanged (Fig 4E), embryonic β-globin (hbbe1.1) mRNA levels in dok2 MO-injected embryos significantly decreased to 52% of that seen in control MO-injected embryos (P = 0·0056, Fig 4F). These phenotypes were rescued by co-injection of dok2 RNA with the dok2 MO (Fig 5A–E).
Fig 4.
Application of DRAQ5™-based flow cytometry to dok2 functional analysis. (A) Knockdown efficiency of dok2 MO in 24-h post-fertilization (hpf) embryos. (B) Haemoglobin staining of dok2 knockdown and control 48-hpf embryos. Haemoglobin-containing cells appear as orange-red. Arrowheads indicate areas where the number of haemoglobin-containing cells decreased after dok2 knockdown. Representative images are obtained from three independent injections of MO and are shown in a ventral view. Scale bar: 500 µm. (C) Flow cytometric analysis of cells prepared from 48-hpf embryos. (D) Percentages of DRAQ5high primitive erythroid-like cells per total cells prepared from 48-hpf embryos were calculated from three independent experiments. Data is shown as means ± SD. (E) Haemoglobin staining of DRAQ5high erythroid-like cells prepared from 48-hpf embryos. Orange-red in cytoplasm indicates haemoglobin. Nuclei are stained by haematoxylin and appear as grey. Scale bar: 20 µm. (F) Expression of erythropoiesis-related genes in DRAQ5high cells sorted from control MO and dok2 MO-injected embryos. (G) Flow cytometric analysis of cells prepared from 48-hpf gata1:dsRed embryos. (H) Shown are percentages of gata1:dsRed+ DRAQ5low hematopoietic progenitors in total DRAQ5low cells and gata1:dsRed+ DRAQ5high primitive erythrocytes in DRAQ5high cells. DRAQ5, 1, 5-bis{[2-(di-methylamino)ethyl]amino}-4, 8-dihydroxyanthracene-9, 10-dione; MO, morpholino antisense oligonucleotide; SSC, side scatter. [Colour figure can be viewed at wileyonlinelibrary.com]
Fig 5.
Rescue of dok2 knockdown phenotypes. (A) Levels of dok2 in zebrafish embryos at 24 h post-fertilization (hpf) injected with both dok2 MO and dok2 RNA (rescue). Data is shown as means±SD of three independent experiments. (B) Haemoglobin staining of control, dok2 knockdown and rescue embryos at 48 hpf. Haemoglobin-containing cells appear as orange-red. Arrowheads indicate areas where the number of haemoglobin-containing cells decreased after dok2 knockdown. Shown are representative images of a ventral view. Scale bar: 400 µm. (C) Flow cytometric analysis of cells prepared from indicated 48-hpf zebrafish embryos. (D) Percentages of DRAQ5high primitive erythroid-like cells per total cells prepared from 48-hpf embryos were calculated from three independent experiments. (E) Expression of hbbe1.1 in DRAQ5high cells sorted from control MO, dok2 MO and dok2 MO plus dok2 RNA (rescue)-injected embryos. (F) Flow cytometric analysis of cells prepared from 48-hpf gata1:dsRed embryos. (G) Shown are percentages of gata1:dsRed+ DRAQ5high primitive erythrocytes in total cells prepared from 48-hpf embryos. Data were calculated from three independent experiments. DRAQ5, 1, 5-bis{[2-(di-methylamino)ethyl]amino}-4, 8-dihydroxyanthracene-9, 10-dione; MO, morpholino antisense oligonucleotide; SSC, side scatter. [Colour figure can be viewed at wileyonlinelibrary.com]
To investigate which stage of erythroid differentiation is altered by dok2 knockdown, we injected dok2 MO into gata1:dsRed transgenic embryos and analysed them by flow cytometry. We observed a 3-fold decrease in the percentage of gata1:dsRed+ erythroid cells in dok2 MO-injected embryos relative to control MO-injected embryos (Fig 4G). When we analysed gata1:dsRed+ cells based on DRAQ5™ intensity, the percentage of haematopoietic progenitor cells (defined as DRAQ5low gata1:dsRed+ cells) slightly increased (Fig 4H). By contrast, the percentage of primitive erythrocytes (defined as DRAQ5high gata1:dsRed+ cells) decreased 3·3-fold (Fig 4H), suggesting inhibition of differentiation. These phenotypes were rescued by co-injection of dok2 RNA with the dok2 MO (Fig 5F–G).
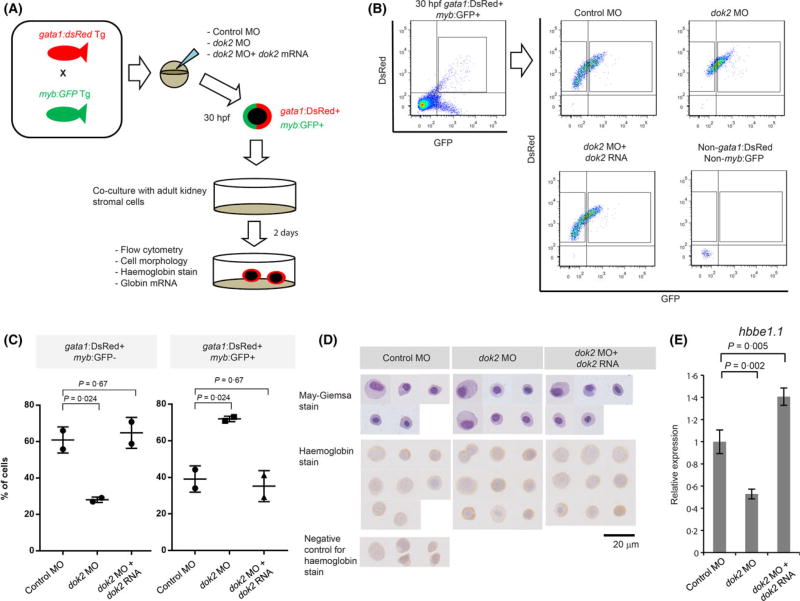
To confirm that Dok2 impairs differentiation of primitive erythrocytes, we isolated gata1:dsRed+ myb:GFP+ cells and performed in vitro differentiation on adult kidney stromal cells (Fig 6A). We observed a 2-fold decrease in the percentage of gata1:dsRed+ myb:GFP− primitive erythrocyte (P = 0·024) and a 1·75-fold increase in the percentage of gata1:dsRed+ myb:GFP+ primitive erythroid cells and EMPs (P = 0·024) in dok2 MO-injected relative to control MO-injected embryos (Fig 6B, C). There was no difference in cell morphology and haemoglobin levels (Fig 6D), whereas embryonic β-globin (hbbe1.1) mRNA level in dok2 MO-injected embryos decreased to 53% of that seen in control MO-injected embryos (P = 0·002, Fig 6E). These phenotypes were rescued by co-injection of dok2 RNA with the dok2 MO (Fig 6B–D). These findings suggest that Dok2 is required for differentiation of primitive erythrocytes.
Fig 6.
In vitro functional analysis of dok2. (A) Diagram shows experimental design. Adult gata1:dsRed Tg fish were crossed with myb:GFP Tg fish. After injecting dok2 MO, only DsRed+ GFP+ embryos were selected. Primitive erythroid cells and erythroid-myeloid progenitors (EMP, defined as gata1:dsRed+ myb:GFP+) were prepared from 30-hpf embryos and cultured on kidney stromal cells for 2 days. (B) Flow cytometric analysis of cultured floating cells. Shown are representative images of two independent experiments. (C) Shown are percentages of gata1:dsRed+ myb:GFP− cells and gata1:dsRed+ myb:GFP+ cells. Data is shown as mean ± SD calculated from two idenpedent experiments. (D) May-Giemsa and Haemoglobin staining of all cultured cells. Scale bar: 20 µm. (E) Expression of embryonic hbbe1.1 globin gene in gata1:dsRed+ myb:GFP− cells sorted from 2-day cultured cells. GFP, green fluorescence protein; MO, morpholino antisense oligonucleotide. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Flow cytometry has been utilized to separate cells of different blood lineages in kidney marrow of adult zebrafish. Based on light-scatter characteristics, adult zebrafish erythrocytes can be distinguished from other blood lineages (Traver et al, 2003). Here, we developed a way to purify primitive mature erythrocytes from zebrafish embryos using flow cytometry. Although gata1:dsRed transgenic zebrafish enable isolation of Gata1-expressing erythroid cells by flow cytometry, our morphological observations revealed that dsRed+ cells are heterogeneous and are comprised of EMP-like cells and primitive mature erythrocytes (Fig 3B). Combining DRAQ5™ analysis with gata1:dsRed transgenic zebrafish, we defined DRAQ5low gata1:dsRed+ cells as EMP-like cells and DRAQ5high gata1: dsRed+ cells as primitive mature erythrocytes.
By 48 hpf, we were able to observe primitive erythrocytes, EMPs and haematopoietic progenitor cells but not myeloid cells, findings compatible with several previous reports (Berman et al, 2005; Brownlie et al, 2003; Chen & Zon, 2009; Bennett et al, 2001; Bertrand et al, 2007; Burns et al, 2002; Kalev-Zylinska et al, 2002). At 12 hpf, gata1-expressing erythroid progenitors differentiate into primitive erythrocytes that enter the circulation around 24 hpf (Berman et al, 2005) and persist until 96 hpf (Chen & Zon, 2009). Primitive erythrocytes express embryonic globin genes (hbae3 and hbbe1.1) (Brownlie et al, 2003). Around 24 hpf, bipotent EMPs are generated and differentiate into gata1+ erythroid (Bennett et al, 2001) and spi1+ myeloid cells (Bertrand et al, 2007). Haematopoietic progenitor cells are generated from dorsal aorta at 33 hpf (Burns et al, 2002; Kalev-Zylinska et al, 2002). However, definitive myelopoiesis starts around 72 hpf in the caudal haematopoietic tissue, as marked by lcp1 expression (Berman et al, 2005). Overall, these findings strongly suggest that our approach is a reliable method to purify and analyse haematopoietic cells in embryonic zebrafish.
Analysis of erythropoiesis in zebrafish embryos was facilitated by use of the DNA-staining fluorochrome DRAQ5™. There are several advantages of DRAQ5™ compared to other DNA-staining fluorochromes. First, it specifically binds at high affinity to DNA, eliminating background signals from RNA and enabling co-staining with RNA-binding fluorochromes. Second, DRAQ5™ can rapidly enter living cells at ambient temperature (Smith et al, 1999) and its application does not require cell fixation or RNase treatment. Thus, damage to zebrafish cells exposed to 37°C temperature is minimized. Moreover, the DRAQ5™ fluorescence emission spectrum does not overlap with spectra of phycoerythrin, Texas Red, Cy3, or GFP. Thus, DRAQ5™ signals can be detected in transgenic zebrafish expressing other fluorescent markers with minimal overlap in spectrum. Importantly, DRAQ5™ undergoes minimal photobleaching (Martin et al, 2005) and is thus stable over long periods of analysis. Overall, we conclude that DRAQ5™ is probably preferable for flow cytometry over other DNA-staining fluorochromes, such as propidium iodide, TOTO™-1, TOTO™-3, Hoechst33258, ethidium bromide and 4′,6-diamidino-2-phenylindole (DAPI).
DRAQ5™ has reportedly been applied to analysis of human peripheral blood (Smith et al, 1999). Enhanced staining potential of DRAQ5™ in monocytes and lymphocytes is related to the accessibility of DRAQ5™ to DNA binding sites in chromatin (Smith et al, 1999). Thus, our observation of DRAQ5high and DRAQ5low populations probably reflects differential chromatin complexity in those groups, although further characterization of nuclei of zebrafish erythroid cells is required.
We also demonstrated the utility of this staining method to analyse gene function in erythropoiesis. Dok2 is an adaptor protein that binds to the intracellular domain of a transmembrane receptor protein tyrosine kinase and inhibits signalling. Dok2 knockout mice show increased incidence of lung adenocarcinoma (Berger et al, 2010) but normal haematopoiesis. Dok1/Dok2 double knockout mice develop myeloproliferative disease but do not exhibit abnormalities in erythropoiesis (Niki et al, 2004). DOK1 and DOK2 reportedly function as inhibitors of signalling pathways in lymphopoiesis (Celis-Gutierrez et al, 2014; Guittard et al, 2009) and myelopoiesis (Mihrshahi et al, 2009; Yasuda et al, 2004). However, the function of DOK2 in erythropoiesis remains unknown. Our data suggest that in zebrafish embryos, Dok2 regulates differentiation of primitive erythrocytes.
Conclusions
Zebrafish primitive erythroid cells can be isolated using DRAQ5™ staining of gata1:dsRed transgenic zebrafish and flow cytometry. Using this method, we identified Dok2 as functioning in the differentiation of primitive erythrocytes.
Supplementary Material
Fig S1. (A) Fractionation of cells obtained from 48-hpf embryos based on FSC. (B) Morphological observation of May-Giemsa stained cells in F1–4. Scale bar is 20 mm. (C) Flow cytometric analysis of 48-hpf embryos of gata1:dsRed transgenic zebrafish. (D) Percentage of gata1:dsRed+ cells in F1–4. Data is from three independent experiments.
Acknowledgments
We thank Kanitta Srinoun, Christian Lawrence, Ayako Takai, Mami Shigeto and Motoko Sumasu for technical support and Dr. Elise Lamar for critical reading of the manuscript. This work was supported by the Subsidy of Expense for Promoting the program for Enhancement of Research University which is under the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Author contributions
Contribution: K.K., Tom.I. and Tor.I. performed experiments, analysed the data and wrote the manuscript; Y.N. and L.I.Z. designed the study and interpreted the data; and D.S. designed the study, interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure
The authors declare no competing financial interest.
Additional Supporting Information may be found in the online version of this article:
References
- Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, Zon LI, Fleming MD, Look AT. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- Berger AH, Niki M, Morotti A, Taylor BS, Socci ND, Viale A, Brennan C, Szoke J, Motoi N, Rothman PB, Teruya-Feldstein J, Gerald WL, Ladanyi M, Pandolfi PP. Identification of DOK genes as lung tumor suppressors. Nature Genetics. 2010;42:216–223. doi: 10.1038/ng.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JN, Kanki JP, Look AT. Zebrafish as a model for myelopoiesis during embryogenesis. Experimental Hematology. 2005;33:997–1006. doi: 10.1016/j.exphem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Kim AD, Teng S, Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135:1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie A, Donovan A, Pratt SJ, Paw BH, Oates AC, Brugnara C, Witkowska HE, Sassa S, Zon LI. Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia. Nature Genetics. 1998;20:244–250. doi: 10.1038/3049. [DOI] [PubMed] [Google Scholar]
- Brownlie A, Hersey C, Oates AC, Paw BH, Falick AM, Witkowska HE, Flint J, Higgs D, Jessen J, Bahary N, Zhu H, Lin S, Zon L. Characterization of embryonic globin genes of the zebrafish. Developmental Biology. 2003;255:48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Burns CE, DeBlasio T, Zhou Y, Zhang J, Zon L, Nimer SD. Isolation and characterization of runxa and runxb, zebrafish members of the runt family of transcriptional regulators. Experimental Hematology. 2002;30:1381–1389. doi: 10.1016/s0301-472x(02)00955-4. [DOI] [PubMed] [Google Scholar]
- Celis-Gutierrez J, Boyron M, Walzer T, Pandolfi PP, Jonjic S, Olive D, Dalod M, Vivier E, Nunes JA. Dok1 and Dok2 proteins regulate natural killer cell development and function. EMBO Journal. 2014;33:1928–1940. doi: 10.15252/embj.201387404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AT, Zon LI. Zebrafish blood stem cells. Journal of Cellular Biochemistry. 2009;108:35–42. doi: 10.1002/jcb.22251. [DOI] [PubMed] [Google Scholar]
- Chung J, Bauer DE, Ghamari A, Nizzi CP, Deck KM, Kingsley PD, Yien YY, Huston NC, Chen CY, Schultz IJ, Dalton AJ, Wittig JG, Palis J, Orkin SH, Lodish HF, Eisenstein RS, Cantor AB, Paw BH. The mTORC1/4E-BP pathway coordinates hemoglobin production with L-leucine availability. Science Signaling. 2015;8:ra34. doi: 10.1126/scisignal.aaa5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Langenau DM, Madge JA, Quinkertz A, Gutierrez A, Neuberg DS, Kanki JP, Look AT. Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. British Journal of Haematology. 2007;138:169–175. doi: 10.1111/j.1365-2141.2007.06625.x. [DOI] [PubMed] [Google Scholar]
- Feng H, Stachura DL, White RM, Gutierrez A, Zhang L, Sanda T, Jette CA, Testa JR, Neuberg DS, Langenau DM, Kutok JL, Zon LI, Traver D, Fleming MD, Kanki JP, Look AT. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell. 2010;18:353–366. doi: 10.1016/j.ccr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis JJ, Hsia N, Trompouki E, de Jong JL, DiBiase A, Lambert JS, Jia Z, Sabo PJ, Weaver M, Sandstrom R, Stamatoyannopoulos JA, Zhou Y, Zon LI. Zebrafish globin switching occurs in two developmental stages and is controlled by the LCR. Developmental Biology. 2012;366:185–194. doi: 10.1016/j.ydbio.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guittard G, Gerard A, Dupuis-Coronas S, Tronchere H, Mortier E, Favre C, Olive D, Zimmermann P, Payrastre B, Nunes JA. Cutting edge: Dok-1 and Dok-2 adaptor molecules are regulated by phosphatidylinositol 5-phosphate production in T cells. Journal of Immunology. 2009;182:3974–3978. doi: 10.4049/jimmunol.0804172. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kulkeaw K, Muennu K, Tanaka Y, Nakanishi Y, Sugiyama D. Herbal drug ninjin’yoeito accelerates myelopoiesis but not erythropoiesis in vitro. Genes to Cells. 2014;19:432–440. doi: 10.1111/gtc.12143. [DOI] [PubMed] [Google Scholar]
- Jing LL, Zon LI. Zebrafish as a model for normal and malignant hematopoiesis. Disease Models & Mechanisms. 2011;4:433–438. doi: 10.1242/dmm.006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, Horsfield JA, Flores MV, Postlethwait JH, Vitas MR, Baas AM, Crosier PS, Crosier KE. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, Lin S, Prochownik E, Trede NS, Zon LI, Look AT. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- Liao EC, Paw BH, Peters LL, Zapata A, Pratt SJ, Do CP, Lieschke G, Zon LI. Hereditary spherocytosis in zebrafish riesling illustrates evolution of erythroid beta-spectrin structure, and function in red cell morphogenesis and membrane stability. Development. 2000;127:5123–5132. doi: 10.1242/dev.127.23.5123. [DOI] [PubMed] [Google Scholar]
- Lin CY, Chiang CY, Tsai HJ. Zebrafish and Medaka: new model organisms for modern biomedical research. Journal of Biomedical Science. 2016;23:19. doi: 10.1186/s12929-016-0236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RM, Leonhardt H, Cardoso MC. DNA labeling in living cells. Cytometry Part A. 2005;67:45–52. doi: 10.1002/cyto.a.20172. [DOI] [PubMed] [Google Scholar]
- Mihrshahi R, Barclay AN, Brown MH. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. Journal of Immunology. 2009;183:4879–4886. doi: 10.4049/jimmunol.0901531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki M, Di Cristofano A, Zhao M, Honda H, Hirai H, Van Aelst L, Cordon-Cardo C, Pandolfi PP. Role of Dok-1 and Dok-2 in leukemia suppression. Journal of Experimental Medicine. 2004;200:1689–1695. doi: 10.1084/jem.20041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, FitzGerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–U1007. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau CE, Kaufman Z, Meireles AM, Talbot WS. Differential requirement for irf8 in formation of embryonic and adult macrophages in zebrafish. PLoS One. 2015;10:e0117513. doi: 10.1371/journal.pone.0117513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Wiltshire M, Davies S, Patterson LH, Hoy T. A novel cell permeant and far red-fluorescing DNA probe, DRAQ5, for blood cell discrimination by flow cytometry. Journal of Immunological Methods. 1999;229:131–139. doi: 10.1016/s0022-1759(99)00116-7. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blunt N, Wiltshire M, Hoy T, Teesdale-Spittle P, Craven MR, Watson JV, Amos WB, Errington RJ, Patterson LH. Characteristics of a novel deep red/infrared fluorescent cell-permeant DNA probe, DRAQ5, in intact human cells analyzed by flow cytometry, confocal and multiphoton microscopy. Cytometry. 2000;40:280–291. doi: 10.1002/1097-0320(20000801)40:4<280::aid-cyto4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochimica et Biophysica Sinica (Shanghai) 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, Oates AC, Fritz A, Gates MA, Amores A, Bahary N, Talbot WS, Her H, Beier DR, Postlethwait JH, Zon LI. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Developmental Biology. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nature Immunology. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- Xiao A, Wang ZX, Hu YY, Wu YD, Luo Z, Yang ZP, Zu Y, Li WY, Huang P, Tong XJ, Zhu ZY, Lin S, Zhang B. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Research. 2013;41:e141. doi: 10.1093/nar/gkt464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Shirakata M, Iwama A, Ishii A, Ebihara Y, Osawa M, Honda K, Shinohara H, Sudo K, Tsuji K, Nakauchi H, Iwakura Y, Hirai H, Oda H, Yamamoto T, Yamanashi Y. Role of Dok-1 and Dok-2 in myeloid homeostasis and suppression of leukemia. Journal of Experimental Medicine. 2004;200:1681–1687. doi: 10.1084/jem.20041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. (A) Fractionation of cells obtained from 48-hpf embryos based on FSC. (B) Morphological observation of May-Giemsa stained cells in F1–4. Scale bar is 20 mm. (C) Flow cytometric analysis of 48-hpf embryos of gata1:dsRed transgenic zebrafish. (D) Percentage of gata1:dsRed+ cells in F1–4. Data is from three independent experiments.