Abstract
Growing number of studies provide strong evidence that the mitochondrial permeability transition pore (PTP), a non-selective channel in the inner mitochondrial membrane, is involved in the pathogenesis of cardiac ischemia–reperfusion and can be targeted to attenuate reperfusion-induced damage to the myocardium. The molecular identity of the PTP remains unknown and cyclophilin D is the only protein commonly accepted as a major regulator of the PTP opening. Therefore, cyclophilin D is an attractive target for pharmacological or genetic therapies to reduce ischemia–reperfusion injury in various animal models and humans. Most animal studies demonstrated cardioprotective effects of PTP inhibition; however, a recent large clinical trial conducted by international groups demonstrated that cyclosporine A, a cyclophilin D inhibitor, failed to protect the heart in patients with myocardial infarction. These studies, among others, raise the question of whether cyclophilin D, which plays an important physiological role in the regulation of cell metabolism and mitochondrial bioenergetics, is a viable target for cardioprotection. This review discusses previous studies to provide comprehensive information on the physiological role of cyclophilin D as well as PTP opening in the cell that can be taken into consideration for the development of new PTP inhibitors.
Keywords: Ischemia–reperfusion injury, Cardioprotection, Mitochondrial permeability transition pores, Cyclophilin D, Oxidative stress
Introduction
Coronary heart diseases, including myocardial infarction (MI) and ischemic heart disease, are the leading cause of mortality and morbidity worldwide accounting for over 370,000 deaths per year in the United States [1]. It is characterized by severe impairment of coronary blood supply, which produces myocardial ischemic injury accompanied by a wide range of clinical symptoms. Indeed, timely restoration of coronary perfusion or reperfusion is the only known effective therapeutic intervention for protecting the heart against ischemic injury. Although reperfusion salvages viable myocardium, it causes additional (up to 50%) damage known as “reperfusion injury” to the myocardium, increasing arrhythmia, myocardial stunning and infarction size, and thus, diminishing myocardial contraction [2]. Despite continuous intensive studies, including a variety of pharmacological and non-pharmacological (conditional) approaches, there is no effective therapy for preventing cardiac ischemia–reperfusion (IR) injury. Therefore, in addition to restoring coronary perfusion, current therapeutic strategies focus on limiting reperfusion injury [3, 4].
IR injury is a multifaceted process that affects many aspects of cardiac metabolism including energy metabolism, contractility, ion homeostasis, redox state, and many others. Mitochondria are the central players in cardiac IR injury where the loss of mitochondrial function leads to cell death through apoptosis and necrosis [3, 5–7]. Importantly, during ischemia, the loss of oxygen compromises mitochondrial ATP production and induces a rise in intracellular Ca2+. Reperfusion after severe ischemia further increases intracellular and mitochondrial Ca2+ accompanied by enhanced generation of reactive oxygen species (ROS). All these factors, along with increased concentration of inorganic phosphate (Pi) and normalization of intracellular pH (pHi) result in changes in inner mitochondrial membrane (IMM) permeability concurrently with the opening of the non-specific, high-conductance mitochondrial permeability transition pore (PTP) (reviewed in [8, 9]). Notably, the PTP remains closed during ischemia due to low pHi (<7.0) but it opens during the first minutes of reperfusion with normalization of pHi [10, 11]. In favor of this conclusion, temporary acidosis at reperfusion [12], and pharmacological inhibition of Na+–H+ exchanger 1 (NHE-1), a plasma membrane antiporter [13–15], inhibited PTP opening and improved cardiac recovery and mitochondrial function after myocardial infarction (IR). However, the PTP presumably opens during anoxia (hypoxia) due to coronary perfusion that keeps pHi at a relatively normal (>7.0) level. The molecular identity and precise function of the PTP complex is not clear; however, cyclophilin D (CypD) has been widely accepted as a major regulator of the PTP opening [16, 17]. The opening of the PTP during reperfusion further compromises cellular energetics and ultimately results in mitochondria-mediated cell death.
Due to its central role in cell death, inhibition of the PTP opening is the most promising therapeutic strategy for protection and restoration of mitochondrial function during cardiac IR. Since CypD is the only known PTP player whose activation stimulates pore opening, pharmacological targeting of CypD can serve as an adequate approach to protecting the heart against IR injury. Indeed, numerous experimental and clinical studies since the 1990s revealed beneficial effects of CypD inhibitors in animal models of cardiac IR (reviewed in [16, 18]) and in small groups of patients with MI undergoing percutaneous coronary intervention (PCI) (reviewed in [19]). However, a recent large multicentre clinical trial that investigated the efficacy of the PTP inhibitor cyclosporine A (CsA) revealed no protective effects of the drug on clinical outcomes in MI patients [20]. Lack of pharmacological efficacy urges for comprehensive reviewing and discussion of the physiological role of CypD as well as PTP opening to clarify whether CypD is a feasible target for cardioprotection. Growing data indicate that CypD, in addition to its role in the mitochondrial permeability transition, may play a pivotal role in regulating overall cell metabolism. Studies including genetic deletion/silencing or pharmacological inhibition of CypD unveiled its contribution to the sarcoplasmic reticulum (SR)–mitochondria interaction in regulating cytosolic Ca2+ signaling [21, 22], and mitochondria-to-nuclei gene expression signaling to direct cell proliferation [23]. Moreover, CypD regulates mitochondrial genome [24], tricarboxylic acid (TCA) cycle [25, 26], respiratory function [24, 26], and oxidative phosphorylation [27, 28]. In addition, CypD is involved in cell metabolism, regulates glucose homeostasis and insulin resistance [26, 29, 30], and participates in the maintenance of immune and inflammatory responses [31, 32].
This review summarizes and discusses previous studies on the physiological role of CypD and PTP opening in cellular metabolism and mitochondrial bioenergetics. We believe that complete elucidation of the molecular identity and identification of the mechanisms of the PTP regulation can provide new therapeutic targets aiming to attenuate mitochondria-mediated cell death. The review discusses recent achievements in revealing the molecular identity of the PTP complex as well as recapitulates potential obstacles in PTP targeting.
Molecular identity of the PTP complex: the update
Although pioneering studies in the 1970s by Haworth and Hunter discovered the existence of the PTP [33], the molecular identity of the pore still remains unclear. By 2004, biochemical and electrophysiological studies obtained by different groups have proposed that adenine nucleotide translocase (ANT) [34–37] and voltage-dependent anion channel (VDAC) or porin [37, 38] can be the core components of the PTP complex. However, several solid genetic studies in 2004–2007 using ANT and VDAC knockout (KO) animal/cell models have demonstrated pore opening in the absence of the aforementioned proteins [39–42], thus suggesting that they are not involved in the PTP assembly. At the same time, a major regulatory role of CypD in PTP formation has been broadly recognized. The roles of ANT and VDAC in PTP induction were reviewed in detail elsewhere [9, 16, 17, 43] and, therefore, we would like to focus on the proteins that were recently proposed as potential PTP core components.
In 2008, Halestrap’s group proposed the mitochondrial phosphate carrier (PiC) as a potential PTP core component [44] basing on the fact that a calcium-triggered conformational change of the PiC, facilitated by CypD, induced pore opening. PiC is localized in the IMM and transports Pi into the matrix of mitochondria. The studies unveiled the interaction of PiC with CypD and ANT to stimulate PTP opening. Notably, the binding of PiC to CypD was CsA-sensitive while the interaction between PiC and ANT was insensitive to CsA treatment suggesting a regulatory role of the ANT in pore opening [44]. Later, studies of the same group have demonstrated that downregulation of PiC expression by 65–80% with siRNA in HeLa cells had no effect on mitochondrial Ca2+ accumulation and PTP opening [45], thus undermining the role of PiC as a core PTP component. However, it was difficult to delineate an important role for the PiC due to incomplete silencing of its expression.
Most recent genetic studies using mice models with cardiac-specific overexpression or downregulation of PiC shed light on whether PiC is involved in the PTP complex [46, 47]. These observations confirmed previous studies that the PiC directly binds to CypD; however, overexpression or knockdown of PiC exhibited a normal PTP response [46], thus suggesting that the PiC does not appear to be a critical PTP component. Notably, mice with inducible and cardiac-specific deletion of the Slc25a3 gene (PiC protein) developed significant cardiomyopathy [47], and cardiac mitochondria isolated from these mice demonstrated a greater Ca2+ retention capacity (CRC), thus assigning a regulatory role to PiC in pore opening.
Spastic paraplegia 7 (SPG7) has been recently suggested as an essential and conserved component of the PTP [48], although many questions regarding the structural role of SPG7 remain unrevealed [49]. SPG7 is an AAA-protease that co-assembles with a homologous protein, AFG3L, and other unidentified proteins creating complexes with a molecular weight of ~900 kDa in the IMM. It has been shown that the PTP is a heterooligomeric complex containing VDAC, SPG7, and CypD [48]. However, like CypD, ablation of SPG7 did not prevent PTP opening at high concentrations of Ca2+ suggesting a regulatory rather than the structural role of SPG7 in PTP induction. Furthermore, it is still not clear whether the complex composed of VDAC, SPG7, and CypD can function as a channel.
One of the potential candidates that are intensively assessed as a PTP core component is FOF1-ATP synthase (complex V). ATP synthase consists of two protein entities (domains), F1 and FO, that comprise 17 different types of subunits accounting for a total of more than 30 subunits in mammalian mitochondria. The catalytic domain F1 situated in the mitochondrial matrix consists of five different subunits (α, β, γ, δ and ε), whereas the membrane domain FO includes the regular subunits c, a, b, d, F6, oligomycin sensitivity-conferring protein (OSCP) and the accessory subunits e, f, g and A6L. The F1 subunits γ, δ and ε constitute the central stalk while the FO subunits b, d, F6 and OSCP form the peripheral stalk of ATP synthase. The subunits a and A6L of the FO domain are the only ATP synthase subunits encoded by the mitochondrial DNA (mtDNA) [50]. These subunits are responsible for stabilization of the ATP synthase structure, particularly, for monomer–monomer interaction during dimerization of two ATP synthase monomers via the FO domain.
The capacity of ATP synthase to form a supercomplex structure (ATP synthasome) through its dimerization, and interaction with PiC and ANT [51, 52], made it an attractive candidate for the PTP complex. Initial studies demonstrate that Pi increased CypD binding to the lateral stalk of ATP synthase and decreased its enzyme activity in bovine heart mitochondria. Dissociation of the CypD-ATP synthase complex by CsA reversed the enzyme activity of ATP synthase [27]. This study left open the question whether the interactions of CypD with the ATP synthase are related to the PTP induction.
In 2013, genetic studies revealed a crucial role of the c-subunit of ATP synthase in Ca2+-induced PTP opening in Hela cells [53]. This observation was further supported by studies where the purified reconstituted c-subunit ring of the FO domain formed a voltage-sensitive channel, and the persistent opening of the channel by Ca2+ led to a rapid and uncontrolled depolarization of the IMM [54]. High matrix Ca2+ enlarged the c-subunit ring which was disconnected from CypD-binding sites in the F1 domain leading to PTP opening. Interestingly, the purified c-subunit added to mitochondria induced CsA-sensitive PTP induction, which was affected by the phosphorylation/dephosphorylation status of the c-subunit [55]. Nevertheless, it remains unclear whether the c-subunit plays a structural or regulatory role in the PTP activity.
Bernardi’s group proposed an interesting model according to which purified dimers of the ATP synthase reconstituted into lipid bilayers exhibited the PTP-like activity [56]. However, subsequent studies from the same group showed that Rho0 cells depleted of mtDNA still displayed the PTP activity [57]. As aforementioned, the subunits a and A6L encoded by mtDNA are important for dimerization of ATP synthase and the PTP activity in Rho0 cells excludes the role of ATP synthase dimers in forming the PTP. Thus, although several studies confirm a regulatory role of the ATP synthase in PTP activity, a structural role of the synthase remains unknown. Taken together, current studies suggest a possible regulatory or essential role of ATP synthase through the following two mechanisms: (1) dimerization of ATP synthase molecules apparently through the contact sites between monomers to form the PTP, and (2) conversion of the c-subunit ring in the FO domain to the non-selective PTP. However, further studies are certainly needed to confirm if the ATP synthase is a core structural component of the PTP.
Overall, despite intensive and numerous studies, the molecular identity of the PTP complex has not yet been established. The majority of proteins initially known as classic core components of the PTP complex actually participate in the regulation of PTP induction (Fig. 1).
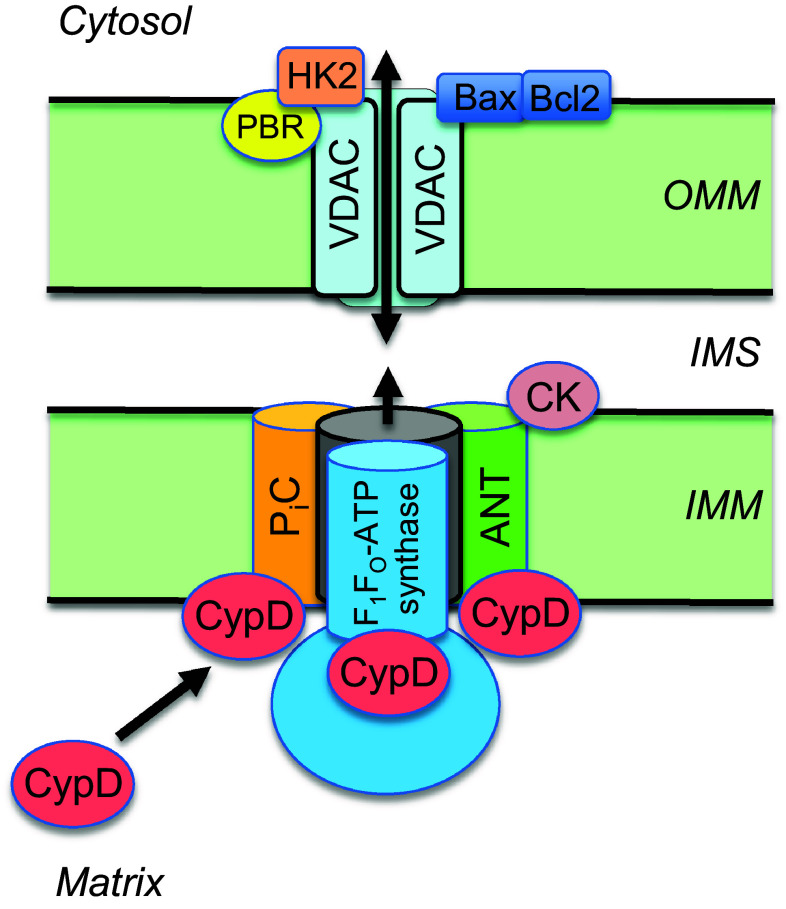
Fig. 1.
A proposed model of the mitochondrial PTP complex. The molecular identity of the PTP is unknown. CypD, a mitochondrial matrix protein, regulates PTP formation through interaction with ANT, FOF1-ATP synthase and PiC in the inner mitochondrial membrane (IMM). Hexokinase II (HK2), peripheral benzodiazepine receptor (PBR) and apoptotic proteins (Bax, Bcl2) through interaction with VDAC on the outer mitochondrial membrane (OMM), in addition to creatine kinase (CK) in the intermembrane space (IMS), contribute to PTP induction. The contribution of the ATP synthasome proteins to PTP formation suggests a crosstalk between pore opening, ATP production and energy transfer
Physiological role of CypD
Mammalian cells contain 17 cyclophilins and all of them are encoded by the nuclear genome, particularly, by the peptidyl-prolyl cis–trans isomerase (PPIase) gene (Ppif). The main isoforms of cyclophilins are CypA, CypB, CypD, CypE, Cyp40 and CypNK that are localized in different subcellular compartments including the cytoplasm, nucleus, sarcoplasmic reticulum and mitochondria [58, 59]. CypD is the only mitochondrial cyclophilin encoded by Ppif that after synthesis in the cytoplasm is translocated to the mitochondria. Upon translocation to the mitochondria, a mitochondrial localization sequence is cleaved leading to the mature form of CypD with a molecular weight of ~21 kDa [60]. PTP-dependent and PTP-independent physiological and pathological functions of CypD are summarized in Fig. 2.
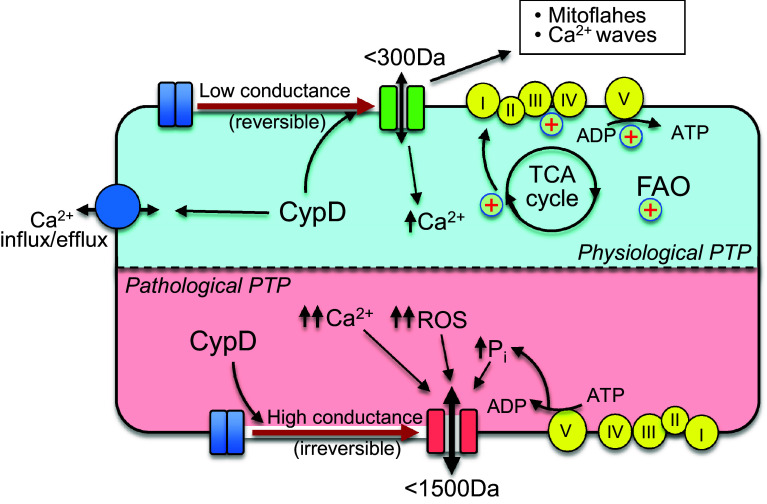
Fig. 2.
Physiological and pathological functions of CypD and PTP opening. Under physiological conditions, CypD can regulate mitochondrial Ca2+ through both PTP-dependent and PTP-independent mechanisms. Mild matrix swelling due to a low-conductance PTP opening can improve mitochondrial bioenergetics including the TCA cycle, ETC activity and oxidative phosphorylation. CypD/low-conductance PTP induction generates mitoflashes and Ca2+ waves through the mitochondrial network. Pathological (high-conductance) PTP opening causes excessive matrix swelling accompanied by rupture of the OMM, loss of ΔΨm and ATP hydrolysis. The mitochondrial alterations initiate cell death through apoptosis and necrosis
Physiological functions of cyclophilins, particularly CypD, have not yet been fully understood. Cyclophilins are the primary binding targets of immunosuppressive agents such as CsA and its derivatives. CypD, like other cyclophilins, catalyzes the cis/trans isomerization of peptidyl-prolyl bonds in proteins [61]. Cyclophilins possess the ability to catalyse the protein folding [62], and CypD contains an N-terminal targeting sequence that participated in translocation of proteins from the cytoplasm to the mitochondria. Previous studies have reported the existence of cyclophilins in the intermembrane space to catalyse folding of newly imported proteins; however, it remains unclear, whether CypD is involved in protein folding in the intermembrane space [60, 63].
CypD as a regulator of mitochondrial Ca2+
Growing data suggest that CypD through a low-conductance PTP opening participates in the regulation of mitochondrial Ca2+ homeostasis. Pioneering studies demonstrated that CsA inhibits Ca2+ release from mitochondria in rat cardiomyocytes [64].
PTP flickering in a low-conductance mode, which is permeable to solutes, mostly ions, with a molecular weight less than 300 Da, induces negligible matrix swelling [65]. Hence, under physiological conditions, PTP flickering associated with periodic transient increases/decreases in permeability of the IMM can act as a fast Ca2+ extrusion pathway. Inhibition of PTP opening by CsA inhibited mitochondrial Ca2+ release under relatively normal conditions suggesting an important role of the PTP in the regulation of mitochondrial and cytoplasmic Ca2+ homeostasis [64, 66]. Apparently, short openings of PTP induces partial dissipation of Ψm and the fast release of accumulated Ca2+ from the matrix thus serving as an emergency mechanism [67]. In addition, mitochondria are excitable organelles that generate and convey electrical and Ca2+ signals. In this aspect, CypD through the opening of the low-conductance PTP can initiate mitochondrial depolarization spikes and generate Ca2+ waves through Ca2+-induced release of Ca2+ from one mitochondrion to another [68]. Conversely, mice lacking CypD exhibited elevated levels of matrix Ca2+ presumably due to reduced physiological opening of the PTP [25].
Several studies questioned the role of CypD to increase mitochondrial Ca2+ efflux through the PTP. Pharmacological or genetic inhibition of CypD had no effect on mitochondrial Ca2+ release in HeLa cells [69]. Studies using CsA in isolated guinea pig cardiac mitochondria reported a negligible role of PTP in Ca2+ efflux compared to that mediated by mitochondrial Na+/Ca2+ exchange [70]. The extent of mitochondrial Ca2+ overload and the amount of CsA should, apparently, be taken into consideration to assess the PTP-induced Ca2+ release. CsA has beneficial effects at a narrow range of concentration (0.2–1.0 µM), whereas at high concentrations (>2 µM), it exerts detrimental effects in isolated cardiomyocytes [71] and cardiac mitochondria [70], and aggravates IR injury [72]. It has been suggested that the toxic effects of CsA depend on the extracellular [Ca2+] [71] and that increased mitochondrial Ca2+ loading could potentially contribute to detrimental effects of CsA at high concentrations [70].
In addition to Ca2+ efflux, several studies demonstrated a possible role of CypD in Ca2+ uptake into the mitochondria. CsA at high concentrations (1–10 μM) inhibited mitochondrial Ca2+ uptake induced by the MCU activator SB202190 in HeLa cells. Interestingly, this effect was not mimicked by another PTP (bongkrekic acid) or calcineurin inhibitors (FK-506 or cypermethrin) suggesting that CsA inhibits Ca2+ influx through the MCU by a mechanism, which is independent of PTP or calcineurin [73]. Several studies demonstrated a crosstalk between endoplasmic reticulum and the mitochondria that modulates Ca2+ uptake and release during cardiac IR injury, which can serve as a target for pharmacotherapy to reduce PTP induction and cell death (Reviewed in [74]). Genetic or pharmacological inhibition of CypD in H9c2 cardiomyoblasts and adult cardiomyocytes reduced the Ca2+ transfer from the SR to the mitochondria through a SR membrane receptor for inositol 1,4,5-trisphosphate receptor type 1 (IP3R1), suggesting a new role of CypD in SR–mitochondria interaction [21]. The authors suggested that inhibition of Ca2+ channeling though the ER–mitochondria interaction can protect cardiomyocytes against hypoxia-reperfusion injury by the reduction of mitochondrial Ca2+ overload. These studies devote a new role for CypD that in addition to inhibiting PTP opening, decreases Ca2+ overload through the reduction of Ca2+ transfer from the SR to the mitochondria. However, it is not clear whether other cyclophilins (e.g. CypA) in the cytoplasm and the SR are involved in the inhibitory effects of CsA on the SR–mitochondria interaction. Indeed, CypA was shown to bind to the Ca2+ storage protein of the endoplasmic reticulum calreticulin [75] suggesting a possible role of cyclophilins in regulating cytosolic Ca2+ signaling through modulation of the mitochondrial and ER Ca2+ uptake. It should be pointed out that several other studies unveiled the capacity of CsA to enhance Ca2+ uptake into the mitochondria. CsA increased mitochondrial Ca2+ accumulation and reduced maximum respiration rate (state 3) of mitochondria [76]. In addition, CsA reduced Ca2+ uptake in the endoplasmic reticulum and mitochondria and thus, inhibited IP3-dependent Ca2+ signals in intact hepatocytes [22]. These studies could designate a possible role of CypD in regulating mitochondrial Ca2+ uptake; however, contradictory data observed in similar studies might be due to the toxic effects of CsA at high concentrations.
Thus, CypD, apart from regulating cell death, presumably plays an important physiological role in the cell through regulation of energy metabolism and Ca2+ homeostasis. Ppif −/− mice developed greater cardiac hypertrophy and myocardial dysfunction in response to pressure overload or sustained exercise stimulation [25]. In addition to a cardiac phenotype, cardiac mitochondria isolated from Ppif −/− mice exhibited elevated levels of matrix Ca2+ and high activity of Ca2+-dependent dehydrogenases due to altered Ca2+ efflux. Importantly, cardiomyocyte-specific transgene expression of CypD in Ppif −/− mice rescued the functional alterations. This study suggests an important physiological function for CypD and PTP to maintain Ca2+ homeostasis in mitochondria and thus, regulate metabolism and timely response to myocardial workload.
CypD and cellular energy metabolism
Several studies provide evidence that CypD is involved in the regulation of energy metabolism in cells. Currently, PTP-independent role of CypD in glucose and fatty acid metabolism are intensively debated. The ability of CypD to regulate insulin production and glucose homeostasis was confirmed recently in CypD KO mice in vivo wherein the glycolytic shift due to CypD deletion was associated with increased number of insulin-producing islet β cells, mild hyperinsulinemia, improved glucose tolerance, and resistance to high-fat diet-induced liver damage and weight gain compared to WT animals [26].
Genetic or pharmacological inhibition of CypD prevented insulin resistance due to increased glucose uptake through insulin-stimulated translocation of GLUT4 to the plasma membrane in skeletal muscle. Mitochondria isolated from the skeletal muscle exhibited low PTP opening associated with improved CRC but without changes in insulin signaling [30]. However, in mouse and human primary hepatocytes, inhibition of CypD had opposite effects and diminished insulin sensitivity. Overexpression of CypD enhanced insulin action associated with changes in ER–mitochondria interaction [29]. Based on these data, CypD apparently participates in insulin resistance and glucose uptake, which can be regulated differently depending on the tissue/cell type. It is not clear whether the observed effects of CypD on insulin resistance and glucose uptake are secondary to PTP inhibition or if they result from a PTP-independent action of CypD.
Recent studies using both loss- and gain-of-function approaches provided strong evidence that CypD is an important positive regulator of mitoflash activity in the heart through modulation of mitoflash frequency [77]. The mitoflashes may include mitochondrial concurrent and sudden ROS burst, ΔΨm dissipation, NADH depletion, transient permeability transition, and matrix alkalization [77, 78]. Since mitoflashes are involved in the regulation of cell metabolism and signaling [79], modulation of mitoflash activity by CypD [77] suggests that it plays a significant role in maintaining cellular metabolism including, among others, heart mitochondrial bioenergetics under physiological and pathological conditions in the heart.
The role of CypD in the regulation of mitochondrial bioenergetics
CypD can regulate ATP synthesis through changes in mitochondrial Ca2+ by a low-conductance PTP induction. Calcium stimulates mitochondrial dehydrogenases thereby increasing NADH and FADH2 resulting in a rise in ATP synthesis [80]. Small or transient changes in the matrix [Ca2+] can be regulated, in addition to other mechanisms, by transient PTP opening. Hearts of Ppif −/− mice showed an increase in the succinate to glutamate ratio suggesting stimulation of the TCA cycle flux in the absence of CypD. In favor of this, the activities of the Ca2+-regulated mitochondrial enzymes pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (αKGD) were significantly enhanced in Ppif −/− hearts with no differences in protein expression levels of these and other two Ca2+-regulated mitochondrial dehydrogenases [25]. These data suggest that the increase in enzymatic activity might be due to high mitochondrial [Ca2+]. CypD depletion had no effect on respiratory function in isolated cardiac mitochondria [81]; however, CypD-null cardiomyocytes revealed reduced mitochondrial maximal respiration rate and reserved capacity [77]. Likewise, significant defects in mitochondrial TCA and fatty acid oxidation (FAO) associated with reduced oxygen consumption were observed in mouse embryonic fibroblasts (MEFs) from CypD KO mice [26]. CypD silencing can reduce ΔΨm and oxygen consumption in HEK293T cells and downregulated mtDNA-encoded transcripts for subunits of ETC complexes [24].
There was no difference between WT or CypD KO mice in the activity of individual ETC complexes in liver [26] and cardiac [82] mitochondria. CypD deficiency had no effect on supercomplex I + III + IV (respirasome) levels but reduced the yield of mitochondria and citrate synthase activity in the mouse heart [82]. On the other hand, CypD−/− hearts showed decreased FAO rates and increased oxidation of glucose. CypD ablation reduced acylcarnitines levels in the heart apparently due to lower expression of carnitine-palmitoyltransferase 1 (CPT1), an enzyme that catalyzes the reaction which converts acyl-CoA to acylcarnitine on the outer mitochondrial membrane, in CypD−/− hearts [81]. In MEFs, unlike the heart, impaired FAO induced by CypD deletion was associated with increased accumulation of acylcarnitines [26]. In this study, liver extracts from CypD KO mice exhibited significantly reduced activity of the mitochondrial trifunctional protein (TFP) compared to its WT counterparts. The TFP is a multienzyme complex of the mitochondrial β-oxidation, a dominant energy-producing metabolic pathway in the heart [26].
Recent studies on the role of CypD in regulating ATP synthase open new avenues in understanding the role of CypD in energy metabolism. CypD has been shown to interact with the ATP synthase through binding to the lateral stalk, and the ATP synthase–CypD interaction could modulate the complex’s enzymatic activity in intact bovine heart mitochondria [27]. Phosphate increased CypD binding and decreased the activity of ATP synthase, whereas CsA, in contrast, decreased CypD binding and upregulated the activity of the enzyme. Interestingly, CsA has no effect on mitochondria isolated from Ppif −/− mice, which exhibited a higher specific activity of the ATP synthase than WT mitochondria, suggesting a regulatory role of CypD in ATP synthesis [27]. Inhibition of the ATP synthase activity by CypD binding may reduce energy dissipation under oxidative stress when the enzyme acts in a reverse mode hydrolyzing ATP. Thus, increased [Pi] stimulates CypD binding to the ATP synthase. Interestingly, genetic ablation or pharmacological inhibition of CypD increased the ATP synthase activity associated with slightly increased respiration rates but did not affect ANT-mediated ADP–ATP exchange rates in intact mitochondria [28]. CypD binding could affect oligomerization of the ATP synthase favoring PTP formation although it may modulate the enzymatic activity independently of the PTP induction. Recent studies unveiled downregulation of ATP6, a mtDNA-encoded subunit of FOF1-ATPase in CypD-silenced cells that was consistent with reduced oxidative phosphorylation [24].
Taken together, CypD presumably participates in regulating both glucose and fatty acid metabolism as well as mitochondrial bioenergetics including TCA cycle, ETC and oxidative phosphorylation in the cell in a tissue/organ-specific manner. However, the physiological role of PTP in mediating the effects of CypD on energy metabolism remains elusive. Since mitochondria are highly sensitive to small changes in matrix volume [83], mitochondrial metabolism can be regulated by a low conductance PTP when volume-mediated increases over the physiological range can stimulate mitochondrial bioenergetics.
Modulation of CypD activity
The mechanisms of activation of CypD and its interaction with the PTP complex to regulate pore opening have not been discovered. The lack of knowledge on the molecular identity of the pore affects understanding the regulatory role of CypD in PTP activation. Genetic studies questioned the role of VDAC, ANT, PiC, and ATP synthase as the core components of the PTP complex although they play a regulatory role in pore formation. Also, the interaction of CypD with VDAC, ANT, PiC, and ATP synthase may not be associated with the PTP induction and can regulate PTP-unrelated functions of these proteins. Hypothetically, regulation of the PTP can occur through several mechanisms of interaction between CypD and a target protein(s) in the IMM. Apparently, activation of CypD and/or target protein(s) is necessary for stimulation of PTP induction. CypD can bind to a target protein in the IMM and induce PTP opening through the interaction of CypD alone (direct binding) or in complex with a matrix protein (indirect binding). Changes in PPIase activity of CypD resulted from posttranslational modifications or other mechanisms can affect its binding to a PTP core protein as well as PTP regulatory proteins including ANT, PiC and/or ATP synthase to modulate PTP induction.
Post-translational modifications of CypD
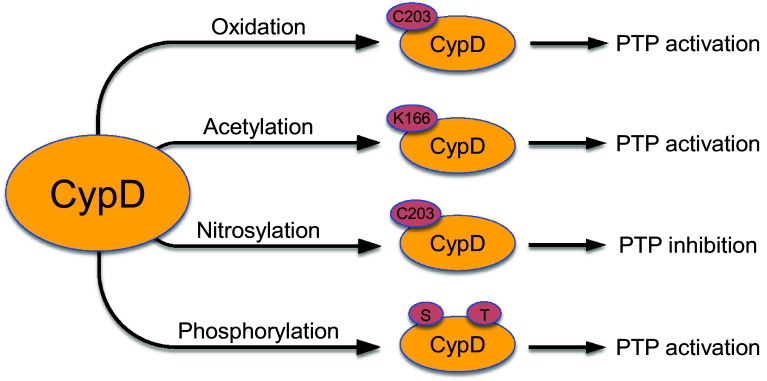
The activity of CypD to stimulate or inhibit PTP induction can be regulated by post-translational modifications (PTMs). Post-translational modifications of CypD by phosphorylation, nitrosylation, oxidation or acetylation have been shown to modulate pore opening (Fig. 3).
Fig. 3.
Main post-translational modifications of CypD. Post-translational modifications of CypD through phosphorylation, nitrosylation, oxidation, and acetylation are shown
Phosphorylation
There are a limited number of studies demonstrating phosphorylation of CypD. Cardioprotective effects of glycogen synthase kinase 3β (GSK-3β) were associated with its translocation from cytosol to mitochondria via the cGMP/PKG pathway where it interacted with CypD, but not VDAC and ANT [84]. However, it is not clear if the beneficial effects were due to GSK-3β-induced phosphorylation of CypD in this study. Active recombinant GSK was shown to directly phosphorylate CypD at Ser/Thr and phospho-CypD promoted depolarization of mitochondria and PTP opening in tumor cells [85]. ERK1/2 inhibition stimulated GSK-3-dependent phosphorylation of CypD, whereas GSK-3 inhibition protected mitochondria from PTP opening. Furthermore, ERK1/2 inhibition increased mitochondrial depolarization in cells overexpressing CypD, and was ineffective in CypD KO cells suggesting a critical role of the ERK1/2-GSK-3-CypD axis in mitochondria-mediated cell death in cancer cells [85]. Conversely, pharmacological inhibition of GSK-3β prevented CypD phosphorylation and inhibited PTP opening in murine tubular epithelial cells [86]. Given the complexity of translocation of protein kinases to mitochondria and their role in phosphorylation/dephosphorylation of matrix proteins, further studies are required to clarify the mechanisms of CypD phosphorylation.
S-Nitrosylation
Protein S-nitrosylation (SNO) has emerged as an important post-translational modification that protects the heart against IR injury [87, 88]. Nitric oxide (NO) can exert opposite effects on the PTP; low NO levels inhibit while high NO stimulate pore opening [89, 90]. Nitrosylation through covalent attachment of NO to a protein’s cysteine residue has been shown to exert cardioprotective effects [91]. However, NO can interact with ROS and produce a highly toxic and more powerful oxidant peroxynitrite (ONOO−) during severe oxidative stress. In vitro, NO and ONOO− initiated permeabilization of mitochondrial membranes, and this effect was inhibited by CsA, indicating the involvement of the PTP complex in the permeabilization. Nitric oxide and ONOO− also attacked ANT, a PTP regulator that binds CypD, to permeabilize ANT-containing proteoliposomes [92]. Neuronal mitochondria contained increased nitration of CypD and ANT on tyrosine consistent with PTP and cell death after cortical injury [93].
Post-translational modification of CypD induced by SNO can regulate PTP activation. Heart homogenates treated with an NO donor, GSNO, exhibited increased SNO of CypD on Cys203 [87]. To further investigate the role of Cys203 in PTP activation, Cys203 of CypD was mutated to a serine residue (C203S) [94]. Mouse embryonic fibroblasts reconstituted with C203S-CypD were resistant to H2O2-induced PTP opening in the presence or absence of GSNO. Likewise, liver mitochondria isolated from CypD−/− mice or mice expressing C203S-CypD were resistant to Ca2+-induced swelling compared to WT mice. Pretreatment with GSNO attenuated H2O2-induced PTP opening in MEFs isolated from WT but not CypD KO mice. These studies suggest a critical role of the Cys203 residue in PTP activation although the contribution of Cys203 nitrosylation to pore induction remains unclear [94]. CypD SNO at Cys203 could prevent protein–protein interactions between CypD and PTP component(s) and thereby, inhibit pore opening [88]. Further studies are required for understanding the precise mechanisms through which CypD SNO regulates the PTP activity.
Oxidation
Oxidation of CypD has implicated in the modulation of the mitochondrial redox state. Hydrogen peroxide-induced oxidation of CypD affected its conformation and enzymatic activity, and oxidized CypD could stimulate PTP induction and cell death [95]. These studies identified Cys157 and Cys203 by site-directed mutagenesis as the only residues that influence the redox conformation of CypD due to the formation of an intramolecular disulfide bridge. It was suggested that CypD might act as a redox sensor and regulate redox environment in mitochondria. Conversely, liver mitochondria expressing the CypD mutant Cys203Ser were resistant to Ca2+-induced PTP opening [94]. Oxidative stress in fibroblasts from patients with X-linked adrenoleukodystrophy induced oxidative modifications in CypD and increased its protein expression levels [96].
Recent studies found that the mitochondrial thioredoxin system, a major redox regulator, stimulated the PPIase activity of CypD in isolated rat heart mitochondria, and the effect was prevented by CsA and the thioredoxin reductase inhibitor auranofin. Hydrogen peroxide induced a concomitant oxidation of thioredoxin and CypD suggesting a link between these two components [97]. These studies show that the thioredoxin system of the mitochondria controls the redox state of CypD through a specific redox signaling whereby the thiol redox changes can modulate CypD. The redox conditions of CypD can regulate the PTP by acting the activity of ANT, PiC, ATP synthase, and other PTP-related proteins containing redox-sensitive thiols.
Acetylation
Post-translational modification of proteins through acetylation/deacetylation plays an important regulatory role in mitochondrial metabolism. Deacetylation of mitochondrial proteins is catalyzed by a major mitochondrial isoform of sirtuins, sirtuin-3 (SIRT3), a NAD+-dependent deacetylase. Several studies suggested a possible role of CypD acetylation in PTP activation. Deacetylation of CypD by SIRT3 diminished its PPIase activity and induced its dissociation from ANT in cancer cells [98]. Conversely, inhibition of the SIRT3 activity by ethanol enhanced the acetylation and activity of CypD associated with increased interaction between CypD and ANT-1 [99]. Wild-type but not a catalytically inactive SIRT3 (SIRT3-H248Y mutant) deacetylated CypD on Lys166, adjacent to the binding site of CsA in 293T cells emphasizing the role of SIRT3 to deacetylate CypD-K166 [100].
Only a few studies investigated the role of CypD acetylation in cardiac diseases. Post-MI heart failure increased acetylation of CypD and reduced SIRT3 expression in rats [101]. Decreased CypD acetylation and increased SIRT3 expression was associated with improved cardiac function and mitochondrial respiration. Likewise, SIRT3 overexpression prevented CypD acetylation, inhibited PTP opening, and reduced cell death induced by IR in H9C2 cells [102]. Cells expressing non-acetylable CypD mutant (CypD-KR) demonstrated less cell death after IR compared to cells expressing intact CypD WT. Ischemic postconditioning could not reduce infarct size and CypD acetylation in SIRT3 KO mice [102], suggesting that deacetylation of CypD plays a pivotal role in cardioprotection against IR. SIRT3 KO mice were hypersensitive to heart stress induced by transverse aortic constriction (TAC) and developed more cardiac hypertrophy and fibrosis compared to WT animals [100]. These data suggest that SIRT3-dependent CypD acetylation is involved in the pathogenesis of cardiac hypertrophy. Lack of data on the cause–effect relationship makes it difficult to underline an exact contribution of CypD acetylation to the development of hypertrophy. Interestingly, CypD KO mice exhibited substantially greater cardiac hypertrophy and fibrosis associated with myocardial dysfunction in response to pressure overload than WT mice [25]. Conversely, cardiomyocyte-specific transgene expression of CypD rescued the pressure overload-induced hypertrophy and cardiac dysfunction in Ppif −/− mice.
Interestingly, CypD per se can participate in modulating protein acetylation in mitochondria by a yet unknown mechanism(s). Analysis of acetylated proteins in cardiac mitochondria isolated from WT and Ppif −/− mice found increased acetylation of proteins involved in FAO and branched-chain amino acid metabolism in Ppif −/− samples [103]. Hyperacetylation of FAO proteins, e.g. l-3-hydroxyacyl-CoA dehydrogenase, in Ppif −/− hearts was associated with reduced enzymatic activity of these proteins compared with the WT mitochondria. These results suggest that ablation of CypD causes substantial changes in the mitochondrial acetylome, which may contribute to altered mitochondrial metabolism in Ppif −/− hearts.
Thus, the role of CypD acetylation in regulating pathological signaling network through the PTP is controversial and desires further studies. Notably, the contribution of CypD to modulate cardiac metabolism and function was demonstrated only in the presence of genetic overexpression or downregulation of SIRT3 and, thus, whether endogenous SIRT3 deacetylates CypD in the mitochondria remains to be elucidated. It is not clear if SIRT3 has or not an additional direct effect on cardiac hypertrophy and IR independent of PTP opening.
Overall, the role of PTMs of CypD in the regulation of PTP opening remains elusive. To date, only four types of PTMs of the CypD molecule have been studied. Phosphorylation, oxidation and acetylation of CypD were associated with PTP activation whereas CypD nitrosylation attenuated the PTP (Fig. 3). Although several studies demonstrated an association between PTMs of CypD and PTP induction, a cause–effect relationship between these events have not been fully investigated. Furthermore, acetylation of CypD was only investigated in cardiomyocytes and cardiac mitochondria, which is an obstacle to understand the role of PTMs of CypD in IR injury and cardioprotection.
Interaction of CypD with other proteins
In addition to post-translational modifications, interaction with proteins in the matrix and IMM can modulate the CypD activity and PTP opening (Fig. 4). Conformational changes in a PTP core component in response to oxidative stress can facilitate CypD binding and thus, pore opening. Numerous studies provided strong evidence that CypD can interact with other PTP regulatory proteins including ANT [104, 105], PiC [44] and the FOF1-ATP synthase subunit OSCP [27] and thus, stimulate PTP induction. Initial studies demonstrated that a Ca2+-triggered conformational change of the ANT was facilitated by CypD which had a high PPIase activity [35]. CsA-sensitive binding of CypD to the ANT stimulated PTP opening in isolated heart and liver mitochondria [104, 105]. Later studies revealed that in vitro oxidative stress antagonized adenine nucleotide binding to the ANT, enhanced CypD–ANT interaction, and stimulated Ca2+-induced PTP opening [106]. Chemical modifications on Cys56, Cys159 and Cys256 of the ANT by oxidative stress or thiol reagents caused a conformational change of the protein [107]. Additionally, two distinct thiol groups in the ANT have been shown to modulate the PTP activity [108]. The thiol groups were suggested to work as a regulator of the binding affinity of ANT for CypD and ADP [109]. Moreover, cross-linking of two matrix facing cysteine residues (Cys56 and Cys159) of ANT, induced by oxidative stress or thiol reagents was shown to enhance CypD-ANT interaction and PTP activity [110]. Thus, thiol oxidation of ANT and/or Ca2+ can facilitate the interaction of CypD with ANT and stimulate pore opening. Although genetic studies questioned the role of ANT as a core PTP component, growing data have implicated the ANT-CypD interaction in the regulation of PTP activity.
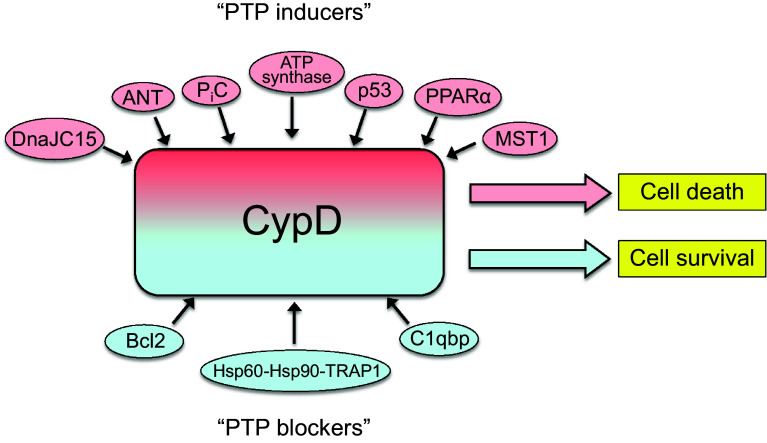
Fig. 4.
Interaction of CypD with mitochondrial proteins. CypD can modulate the PTP opening through interaction with proteins in the matrix and IMM. Interaction of CypD with “PTP inducers” (red background) sensitizes PTP opening and induces cell death whereas the interaction with “PTP blockers” (blue background) inhibits pore opening and prevents cell death
Subsequent studies revealed interaction of CypD with PiC that was stimulated by oxidative stress and thiol reagents thereby increasing PTP opening [44]. Inhibition of ANT with carboxyatractyloside increased the CypD–PiC interaction in liver mitochondria suggesting a crosstalk between the PTP regulatory proteins. Interestingly, CypD binding to ANT and PiC is blocked by CsA but not sanglifehrin A (SfA), a close analog of CsA; the latter even increases CypD interaction with these proteins [44]. Precise mechanisms of opposite effects of CsA and SfA on the binding of CypD with ANT and PiC are not clear. Sanglifehrin A, but not CsA, inhibits the PPIase activity of CypD with no effect on its interaction with ANT and PiC. Unlike CsA, SfA induces dimerization of CypA [111] and CypD [112]. In addition, dependence between CsA concentration and PTP inhibition is linear, whereas between SfA and PTP inhibition is sigmoidal and, therefore, SfA has a negligible inhibitory effect on PTP opening at low concentrations [112]. The interaction between CypD and FOF1-ATP synthase was demonstrated in bovine heart mitochondria [27]. Results of cross-linking studies revealed that CypD modulates the activity of FOF1-ATP synthase by interacting with the lateral stalk of the complex. Phosphate stimulated CyP-D binding to FOF1-ATP synthase. Genetic ablation of CypD or the inhibition of its binding to FOF1-ATP synthase by CsA increased the activity of FOF1-ATP synthase [27, 28].
Numerous proteins in the matrix activated or inhibited PTP induction through interaction with CypD. In brain IR, the tumor suppressor protein p53 translocated to the mitochondrial matrix, where it interacted with CypD and caused PTP opening [113]. Conversely, ablation of p53 or pharmacological inhibition of CypD with CsA induced dissociation of the p53–CypD complex and prevented pore opening that was associated with effective protection from stroke injury [113]. In favor of this, the role of mitochondrial CypD–p53 signaling in PTP-induced cell death was subsequently reported in pancreatic cancer cells [114], neuronal cells [115], and osteoblasts [116]. These findings suggest translocation of the p53–CypD complex to the IMM where it triggers the pore opening through interaction with PTP core protein(s). Indeed, when localized in the mitochondrial matrix, p53 specifically interacted with the FOF1-ATP synthase subunit OSCP in human colorectal carcinoma HCT116 cells [117]. However, many questions regarding the contribution of the p53–CypD interaction to pore opening remain unanswered. Indeed, the p53 inhibitor pifithrin-α prevented loss of ΔΨm and inhibited PTP opening in angiotensin II-treated H9c2 cardiomyoblasts but there was no interaction of p53 with CypD, ANT or VDAC [118]. Calcium-dependent PTP opening was not regulated by p53 [113] raising a concern on how the p53–CypD complex induces Ca2+-independent opening of the PTP [119]. It should be noted that the p53–CypD complex may be involved in PTP-induced cell death through distinct mechanisms in cancer and normal (non-cancer) cells.
Complement 1q-binding protein (C1qbp), a C1q-binding receptor found in subcellular compartments including mitochondria, was involved in PTP induction [120]. Recent gain- and loss-of-function studies revealed that C1qbp KO sensitized MEFs to H2O2-induced PTP opening and cell death whereas overexpression of the protein had opposite effects and protected the cells from oxidative stress [121]. C1qbp was found to bind to CypD suggesting that it appears to act as a PTP inhibitor through CypD, and protect the cells against oxidative stress.
We have recently demonstrated that oxidative stress induced by H2O2 in H9c2 cardioblasts [122] and in vivo IR in rat hearts [123] stimulates translocation of the peroxisome proliferator-activated receptor alpha (PPARα), a transcription factor and a major regulator of FAO, to the mitochondria and increases its interaction with CypD. The AMPK activators metformin and A-769662 as well as the CypD inhibitor SfA prevented protein–protein interaction between CypD and PPARα and improved cell survival and post-ischemic cardiac recovery [122, 123]. Although these studies suggest a possible role of the PPARα–CypD interaction to stimulate PTP opening, many questions on the cause-and-effect relationship between these two events remain to be elucidated. Moreover, it is not clear how and which PTP core component(s) interacts with the PPARα–CypD complex.
Notably, unlike cardiac IR, CypD has opposite effects in tumor cells, where it reduces PTP-induced apoptosis and necrosis [124–126]. Although the mechanisms underlying this effect remains unclear, the effects of CypD were explained to be in part associated with hexokinase II binding to the mitochondria [127]. CypD was found upregulated in human tumors including breast, ovarian, or uterus cancer specifically associated with repressed ANT-1-induced apoptosis [125]. These studies suggest that upregulation of CypD in carcinogenesis inhibits opening of the PTP. Interestingly, interaction of the multi-chaperone complex comprising of heat-shock protein 60 (Hsp60), Hsp90, and tumor necrosis factor receptor-associated protein 1 (TRAP1) with CypD was shown in tumor but not in normal mitochondria. Normal (non-tumor) mitochondria contain low levels of these chaperones which have no physical interaction with CypD. Genetic targeting of Hsp60 by siRNA prevented interaction of the chaperon protein with CypD thereby stimulating PTP opening. These data suggest that the cytoprotective chaperone network containing Hsp60, Hsp90 and TRAP1 antagonizes CypD-mediated cell death in tumors [128, 129].
Interestingly, chaperone proteins and translocation of precursor proteins into mitochondria are both regulated by J-proteins. Human mitochondria consist of two J-proteins with the primary protein import activity, DnaJC19 and DnaJC15 [130]. Overexpression of DnaJC15 resulted in PTP opening and apoptosis, whereas reduced amount of DnaJC15 suppressed PTP in cisplatin-treated normal and cancer cells. Importantly, DnaJC15 interacted with CypD which was associated with its concomitant release from the CypD–TRAP1 complex [131]. Likewise, interaction of mammalian sterile 20-like kinase 1 (MST1) with CypD was involved in gemcitabine-induced pancreatic cancer cell death that was attenuated by MST1 or CypD shRNA silencing, but was aggravated by their overexpression [132]. On the other hand, interaction of CypD with a major anti-apoptotic protein Bcl-2 significantly attenuated cell death and CsA disrupted the CypD–Bcl2 interaction. Overexpression of CypD and its effect through the CypD–Bcl2 complex may be an additional mechanism of suppression of apoptosis in cancer cells [133]. However, it is not clear, how the interaction of CypD with apoptotic proteins modulates the PTP activity in non-tumor cells.
In conclusion, a growing body of studies demonstrate that CypD can interact with several proteins in the matrix and IMM and thus, modulate the PTP activity. However, the precise mechanisms of the cause and effect relationship between protein–protein interactions and PTP opening remain unknown. Apparently, PTP opening is a complex interplay of several CypD-dependent and CypD-independent regulatory mechanisms. Studies on CypD binding to other mitochondrial proteins have been mostly shown in non-cardiac mitochondria and under normal physiological conditions. Therefore, it is difficult to stipulate the contribution of interaction CypD with other proteins in the regulation of PTP to cardiac IR injury and cardioprotection.
CypD as a target for cardioprotection against IR injury
Experimental studies
All clinical and experimental studies elucidating the cardioprotective action of PTP inhibition are mostly focused on targeting CypD which is the only broadly accepted PTP regulator. Among other CypD inhibitors, the immunosuppressive drug CsA has been extensively used to verify cardioprotective effects of PTP inhibition against IR injury. It should be noted that CsA was initially used only for immunosuppression during organ grafting, to suppress rejection after internal organ transplantation through inhibition of cyclophilins (immunophilins) and calcineurin [134, 135]. CsA was first used as an immunosuppressive drug in human kidney transplantation in the late 1970s and thus, opened a new era for the treatment of end-stage organ damage in patients—recipients of cadaveric organs [136, 137]. Notably, persistent treatment of patients undergoing organ transplantations with CsA was associated with side effects, including nephrotoxicity, hypertension, and tremor, among others. In initial animal studies, CsA was used at high doses (>20 mg/kg) and had no beneficial effects on renal ischemic injury [138, 139]. In the late 1980s, first studies showed the beneficial effect of CsA (10 mg/kg) on liver ischemia in dogs [140] and rats [141] preventing necrotic damage and increasing mitosis of hepatocytes.
Discovery of CypD with cyclosporin-sensitive PPIase activity in liver and heart mitochondrial-matrix fractions and its interaction with ANT in the presence of Ca2+ to cause PTP opening [35, 142] motivated further studies on the protective action of CsA against IR injury. First studies in 1991 demonstrated that CsA at low doses (<1 µM) attenuated anoxia-induced injury in isolated cardiomyocytes [143] and protected against IR-induced damage in Langendorff-perfused rat hearts [72]. Since then, many studies using various MI and IR models in mice [144, 145], rats [72, 146, 147], rabbits [148–150], and pigs [151, 152] provided strong evidence that CsA and its analogs reduce infarct size and improve post-ischemic recovery of the heart. Cardioprotective effects of CsA and its analogs were not associated with inhibition of the calcineurin activity since the specific inhibitor of calcineurin, FK-506, had no beneficial effects on cardiac IR [150, 153]. On the other hand, several new CsA analogs such as SfA [112, 147], NIM811 [149, 154] and Debio-025 [155], which do not inhibit the calcineurin activity, protected hearts from IR injury.
Interestingly, a systematic review and meta-analysis of 29 published studies describing the efficacy of CsA using in vivo animal models of acute reperfused MI in 2012 [156] found that CsA had variable effects on infarct size compared with placebo. Notably, not all of the studies exhibited positive results and importantly, large animal studies using a porcine model of reperfused MI [157, 158] reported no beneficial effects thus undermining the potential cardioprotective action of CsA in patients. In addition, a growing body of studies provide strong evidence that spatially distinct subpopulations of cardiac mitochondria known as subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria display functional and structural heterogeneity (Reviewed in [159, 160]). Animal studies elucidating PTP opening and cardioprotective effects of CsA were carried out mostly on SSM. Isolated cardiac IFM demonstrated a higher CRC and were more resistant to high [Ca2+] compared to SSM [161]. On the other hand, our studies on cultured cardiomyocytes revealed increased level of oxidized flavoproteins and higher Ca2+ accumulation in SSM [162]. Therefore, evaluation of both mitochondrial subpopulations of the heart is important for understanding the mechanisms of cardioprotective action of PTP inhibition. Furthermore, cardiac mitochondria demonstrate sex and age differences in the sensitivity to Ca2+ that should be taken into consideration. Female cardiac mitochondria demonstrated lower Ca2+ uptake rates under physiological conditions [163] and were more resistant to high Ca2+ with no differences in CypD levels compared to male mitochondria [164]. Cardiac mitochondria isolated from female rats were more efficient and generated less ROS than male rats [165]. These data are in line with other sex-based differences such as lower expression of apoptotic genes in female rat hearts compared to males at all ages [166]. Based on these studies, females may have better preservation of hearts in response to oxidative stress. Interestingly, SfA prevented IR-induced PTP opening in adult but not in neonatal rats [167] suggesting that lower sensitivity to PTP opening may explain the higher tolerance of neonatal hearts to IR injury.
It should be noted that both genetic [40, 168] and pharmacological inhibition [35] of CypD by CsA and its analogs fails to induce complete inhibition of the PTP, especially at higher Ca2+ when, despite CypD inhibition, the threshold for pore opening is reached. Lack of an effect may be due to inhibition of CypD binding to a PTP core or regulatory protein (e.g. ANT, PiC, ATP synthase) or associated with the CsA-insensitive pore opening at high [Ca2+]. Studies using the mitochondrial 2-deoxy-[3H]-glucose ([3H]-DOG) entrapment technique, which provides the most accurate estimate of PTP numbers in the intact perfused heart, demonstrated that CsA and SfA inhibited the PTP opening by only 30 and 37%, respectively, in rat hearts subjected to global IR [147]. These studies indicate that not all PTPs are induced by CypD, thus suggesting that PTP opening can occur in a CypD-independent manner. Indeed, CyP-D-deficient mitochondria isolated from Ppif −/− mice demonstrated PTP opening suggesting that CypD plays a regulatory role in pore opening, and the PTP can open in the absence of CypD, if the mitochondria have a strong stimulus such as high Ca2+, oxidative and energy stress [40, 41, 109, 168]. These data, as well as other studies demonstrating a wide-range physiological role of CypD, indicate the importance of developing new compounds that can block PTP opening independent of CypD inhibition. Most recently, several groups studied novel compounds that could inhibit pore opening with no effect on CypD. Particularly, N-phenylbenzamides [169], cinnamic anilides [170–172], and isoxazoles [173] have emerged as potent inhibitors of the PTP. CypD-independent effects of these inhibitors have been proven mostly in isolated mitochondria where they were able to inhibit PTP opening in response to Ca2+ overload and oxidative stress. One of the most promising compounds from cinnamic anilides, (E)-3-(4-fluoro-3-hydroxy-phenyl)-N-naphthalen-1-yl-acrylamide 22 inhibited PTP opening and reduced IR injury in the rabbit model of myocardial infarction [170].
Thus, new PTP inhibitors lack potential limitations associated with inhibition of physiological function of all cyclophilins including CypD, thereby avoiding off-target effects of CsA and its analogs. However, further studies are required to elucidate the mechanism, toxicity, and therapeutic efficacy of these compounds. Also, lack of knowledge of the PTP molecular identity is the main obstacle in determining a pore component(s) that can be targeted by new PTP inhibitors.
Clinical studies
Myocardial preservation during the first few minutes of reperfusion, which induce additional severe damage to the ischemic myocardium, is the main therapeutic goal in MI patients. PCI and thrombolytic therapy, which are the primary and broadly accepted therapeutic strategies, do not provide adequate cardioprotection and thus, emphasizes the importance of preclinical and clinical studies in this area [174, 175]. There are many challenges in translating positive results of animal studies into clinical therapy, and one of them is the comparability of animal models with the human condition for understanding the pathogenesis and treatment of diseases [176, 177]. For instance, in animal studies elucidating therapeutic strategies to reduce reperfusion damage to the myocardium, infarction size is mostly more than 30% of the left ventricle. Cardioprotective effects of additional therapeutic interventions at reperfusion occur when the infarct size exceeds 20% [176]. Therefore, the beneficial effects of additional treatment observed in animal models cannot be seen in approximately 75% of MI patients receiving reperfusion therapy who have the infarct size equal or less than 20% of the left ventricle [177]. In addition, animal models mostly use 30–40 min of ischemia followed by reperfusion. However, door-to-balloon times for most MI patients in the United States are substantially longer and can reach 90–120 min [178]. These studies suggest that: (1) animal models of MI for the interpretation of the efficiency of reperfusion therapy do not completely simulate human MI, and (2) inclusion of patients with small infarction to clinical trials can significantly dilute results of clinical outcomes in treatment groups. Large animal models (canine or porcine model of MI) are more clinically relevant in pre-clinical studies.
We will briefly discuss results of clinical studies on targeting CypD for prevention of PTP-mediated reperfusion injury. Initial translational studies on human atrial tissue harvested from patients undergoing cardiac surgery revealed protective effects CsA (0.2 µM) against in vitro simulated IR [179] or lethal hypoxia/reoxygenation [180] injury and improved cell survival and recovery of baseline contractile function. Clinical studies that were initiated in 2008 on small groups of patients with MI demonstrated that administration of an intravenous bolus of CsA (2.5 mg/kg, IV) prior to PCI [181], sternotomy [182], and aortic clamping [183] significantly reduced MI size and improved post-operative recovery. On the other hand, administration of CsA before thrombolysis with streptokinase did not reduce myocardial injury or arrhythmias in patients with acute STEMI [184]. Based on successful preclinical and clinical studies, in 2014 a large multicenter, double-blind, randomized phase III clinical trial Cyclosporine and Prognosis in Acute Myocardial Infarction Patients (CIRCUS) was conducted in 970 patients with an acute STEM undergoing PCI to determine whether CsA (2.5 mg/kg, IV) improves clinical outcomes of the patients after 1 year [185]. However, these studies found that CsA did not improve immediate outcomes (cumulative creatine kinase release, infarction size) and did not prevent long-term adverse left ventricular remodeling in comparison with placebo [20].
Several factors such as the severity of infarction, a quite narrow time window for protection, the vehicle for dissolving of CsA, masking of binding site of CypD for CsA by IR-induced post-translational modification(s), route, dose, timing of administration that would be appropriate for CsA to inhibit CypD, among others, should be discussed to find the factors responsible for the lack of cardioprotection in the CIRCUS trial. Nevertheless, these studies challenge the clinical use of CsA and other CypD inhibitors for cardioprotection. These studies also emphasize the importance of future studies to clarify whether CypD is a feasible target for inhibition that can protect the heart and other organ/tissues from IR injury.
Conclusions and perspectives
Intensive studies during the last 20 years led to discovery of several overviews to be considered for the development of new therapeutic strategies for prevention PTP-mediated IR injury. First, PTP opening at low conductance regulates mitochondrial Ca2+ homeostasis and generates Ca2+ waves through the Ca2+-induced Ca2+ release mechanism. Second, CypD has PTP-independent functions in the cell and participates in the regulation of energy metabolism and mitochondrial bioenergetics. Third, sustained inhibition of CypD exerts detrimental effects on cell/heart function. Fourth, PTP remains closed during the ischemic period, and opens in the first minutes of reperfusion concurring with pHi recovery. Fifth, the extent of PTP opening in response to oxidative stress is different in spatially divergent subpopulations (SSM and IFM) of heart mitochondria due to variability in CRC and ROS production. Sixth, CsA inhibits not only CypD but all cyclophilins in the cell and hence, may have PTP-independent detrimental effects. Seventh, CsA and its analogs (e.g. SfA, NIM811, Debio-025) exert cardioprotective effects at a narrow range of concentrations (in vitro: 0.2–1.0 µM and in vivo: 1–5 mg/kg), and have detrimental effects at high concentrations. Eighth, genetic ablation or pharmacological inhibition of CypD has a little or no effect on the PTP at high mitochondrial Ca2+ levels which reach the threshold for pore opening. Ninth, lack of knowledge on the precise molecular identity of the PTP complex and regulation of pore opening are the major obstacles to the development of new pharmacological compounds targeting the PTP. Importantly, heterogeneity of IR-induced injury on cardiac tissue, cardiomyocyte, and mitochondria levels, where the physiological function of CypD and PTP coincides with pathological pore opening, presumably plays a decisive role in the therapeutic action of CsA and its analogs. It requires precise therapeutic intervention for specific targeting that would only prevent pathological consequences of pore opening. Based on a growing number of studies that demonstrate an essential physiological role of CypD in mitochondrial metabolism and function, it is challenging to consider CypD as a viable and promising target for cardioprotection. A myriad of limitations associated with physiological function of CypD and pharmacological effects of CypD inhibitors such as CsA, urges development of new inhibitors that can prevent PTP opening without targeting CypD. Targeting pathological mitochondrial swelling that develops through both PTP-dependent and PTP-independent mechanisms can be considered as a viable therapeutic strategy in prevention of mitochondria-mediated IR injury in the heart.
Acknowledgements
The authors apologize that they could not cite all important studies in this field due to space restriction. This study was supported by the NHLBI NIH Grants SC1HL118669 (to SJ).
Abbreviations
- ANT
Adenine nucleotide translocase
- CRC
Ca2+ retention capacity
- CsA
Cyclosporine A
- CypD
Cyclophilin D
- ETC
Electron transport chain
- FAO
Fatty acid oxidation
- Hsp60
Heat-shock protein 60
- IFM
Interfibrillar mitochondria
- IMM
Inner mitochondrial membrane
- IR
Ischemia–reperfusion
- KO
Knockout
- ΔΨm
Mitochondrial membrane potential
- MEFs
Mouse embryonic fibroblasts
- MI
Myocardial infarction
- mtDNA
Mitochondrial DNA
- NO
Nitric oxide
- PCI
Percutaneous coronary intervention
- Pi
Inorganic phosphate
- PiC
Phosphate carrier
- PPARα
Peroxisome proliferator-activated receptor alpha
- PPIase
Peptidyl-prolyl cis-trans isomerase
- Ppif
CypD gene
- PTM
Post-translational modification
- PTP
Permeability transition pore
- ROS
Reactive oxygen species
- SfA
Sanglifehrin A
- SPG7
Spastic paraplegia 7
- SSM
Subsarcolemmal mitochondria
- TCA
Tricarboxylic acid
- TRAP1
Tumor necrosis factor receptor-associated protein 1
- VDAC
Voltage-dependent anion channel
- WT
Wild type
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloner RA, Schwartz Longacre L. State of the science of cardioprotection: challenges and opportunities–proceedings of the 2010 NHLBI Workshop on Cardioprotection. J Cardiovasc Pharmacol Ther. 2011;16:223–232. doi: 10.1177/1074248411402501. [DOI] [PubMed] [Google Scholar]
- 5.Ertracht O, Malka A, Atar S, Binah O. The mitochondria as a target for cardioprotection in acute myocardial ischemia. Pharmacol Ther. 2014;142:33–40. doi: 10.1016/j.pharmthera.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walters AM, Porter GA, Jr, Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol. 1999;276:H496–H502. doi: 10.1152/ajpheart.1999.276.2.H496. [DOI] [PubMed] [Google Scholar]
- 12.Kitakaze M, Takashima S, Funaya H, Minamino T, Node K, et al. Temporary acidosis during reperfusion limits myocardial infarct size in dogs. Am J Physiol. 1997;272:H2071–H2078. doi: 10.1152/ajpheart.1997.272.5.H2071. [DOI] [PubMed] [Google Scholar]
- 13.Javadov S, Huang C, Kirshenbaum L, Karmazyn M. NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J Mol Cell Cardiol. 2005;38:135–143. doi: 10.1016/j.yjmcc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Javadov S, Purdham DM, Zeidan A, Karmazyn M. NHE-1 inhibition improves cardiac mitochondrial function through regulation of mitochondrial biogenesis during postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;291:H1722–H1730. doi: 10.1152/ajpheart.00159.2006. [DOI] [PubMed] [Google Scholar]
- 15.Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, et al. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res. 2008;77:416–424. doi: 10.1093/cvr/cvm039. [DOI] [PubMed] [Google Scholar]
- 16.Halestrap AP, Richardson AP. The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury. J Mol Cell Cardiol. 2015;78:129–141. doi: 10.1016/j.yjmcc.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Javadov S, Karmazyn M, Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther. 2009;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- 18.Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Alam MR, Baetz D, Ovize M. Cyclophilin D and myocardial ischemia-reperfusion injury: a fresh perspective. J Mol Cell Cardiol. 2015;78:80–89. doi: 10.1016/j.yjmcc.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 21.Paillard M, Tubbs E, Thiebaut PA, Gomez L, Fauconnier J, et al. Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation. 2013;128:1555–1565. doi: 10.1161/CIRCULATIONAHA.113.001225. [DOI] [PubMed] [Google Scholar]
- 22.Smaili SS, Stellato KA, Burnett P, Thomas AP, Gaspers LD. Cyclosporin A inhibits inositol 1,4,5-trisphosphate-dependent Ca2+ signals by enhancing Ca2+ uptake into the endoplasmic reticulum and mitochondria. J Biol Chem. 2001;276:23329–23340. doi: 10.1074/jbc.M100989200. [DOI] [PubMed] [Google Scholar]
- 23.Tavecchio M, Lisanti S, Lam A, Ghosh JC, Martin NM, et al. Cyclophilin D extramitochondrial signaling controls cell cycle progression and chemokine-directed cell motility. J Biol Chem. 2013;288:5553–5561. doi: 10.1074/jbc.M112.433045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radhakrishnan J, Bazarek S, Chandran B, Gazmuri RJ. Cyclophilin-D: a resident regulator of mitochondrial gene expression. Faseb J. 2015;29:2734–2748. doi: 10.1096/fj.14-263855. [DOI] [PubMed] [Google Scholar]
- 25.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavecchio M, Lisanti S, Bennett MJ, Languino LR, Altieri DC. Deletion of Cyclophilin D impairs beta-oxidation and promotes glucose metabolism. Sci Rep. 2015;5:15981. doi: 10.1038/srep15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, et al. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem. 2009;284:33982–33988. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinopoulos C, Konrad C, Kiss G, Metelkin E, Torocsik B, et al. Modulation of F0F1-ATP synthase activity by cyclophilin D regulates matrix adenine nucleotide levels. Febs J. 2011;278:1112–1125. doi: 10.1111/j.1742-4658.2011.08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tubbs E, Theurey P, Vial G, Bendridi N, Bravard A, et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes. 2014;63:3279–3294. doi: 10.2337/db13-1751. [DOI] [PubMed] [Google Scholar]
- 30.Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab. 2014;3:124–134. doi: 10.1016/j.molmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng D, Tang Y, Kwon H, Zong H, Hawkins M, et al. High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes. 2011;60:2134–2143. doi: 10.2337/db10-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang DZ, Jones AW, Wang WZ, Wang M, Korthuis RJ. Soluble guanylate cyclase activation during ischemic injury in mice protects against postischemic inflammation at the mitochondrial level. Am J Physiol Gastrointest Liver Physiol. 2016;310:G747–G756. doi: 10.1152/ajpgi.00323.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 34.Le Quoc K, Le Quoc D. Involvement of the ADP/ATP carrier in calcium-induced perturbations of the mitochondrial inner membrane permeability: importance of the orientation of the nucleotide binding site. Arch Biochem Biophys. 1988;265:249–257. doi: 10.1016/0003-9861(88)90125-7. [DOI] [PubMed] [Google Scholar]
- 35.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ BioChemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- 37.Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett. 1996;396:189–195. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- 38.Szabo I, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. I. Binary structure and voltage dependence of the pore. FEBS Lett. 1993;330:201–205. doi: 10.1016/0014-5793(93)80273-W. [DOI] [PubMed] [Google Scholar]
- 39.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, et al. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 42.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, et al. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J Mol Cell Cardiol. 2015;78:142–153. doi: 10.1016/j.yjmcc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varanyuwatana P, Halestrap AP. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion. 2012;12:120–125. doi: 10.1016/j.mito.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez-Aguilar M, Douglas DL, Gibson AK, Domeier TL, Molkentin JD, et al. Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J Mol Cell Cardiol. 2014;72:316–325. doi: 10.1016/j.yjmcc.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, et al. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014;21:1209–1217. doi: 10.1038/cdd.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, et al. SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore. Mol Cell. 2015;60:47–62. doi: 10.1016/j.molcel.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernardi P, Forte M. Commentary: SPG7 is an essential and conserved component of the mitochondrial permeability transition pore. Front Physiol. 2015;6:320. doi: 10.3389/fphys.2015.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonckheere AI, Smeitink JA, Rodenburg RJ. Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis. 2012;35:211–225. doi: 10.1007/s10545-011-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko YH, Delannoy M, Hullihen J, Chiu W, Pedersen PL. Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J Biol Chem. 2003;278:12305–12309. doi: 10.1074/jbc.C200703200. [DOI] [PubMed] [Google Scholar]
- 52.Wittig I, Schagger H (2008) Structural organization of mitochondrial ATP synthase. Biochim Biophys Acta 1777: 592–598 [DOI] [PubMed]
- 53.Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12:674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azarashvili T, Odinokova I, Bakunts A, Ternovsky V, Krestinina O, et al. Potential role of subunit c of F0F1-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium. 2014;55:69–77. doi: 10.1016/j.ceca.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masgras I, Rasola A, Bernardi P. Induction of the permeability transition pore in cells depleted of mitochondrial DNA. Biochim Biophys Acta. 2012;1817:1860–1866. doi: 10.1016/j.bbabio.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 58.Lee J, Kim SS. An overview of cyclophilins in human cancers. J Int Med Res. 2010;38:1561–1574. doi: 10.1177/147323001003800501. [DOI] [PubMed] [Google Scholar]
- 59.Gutierrez-Aguilar M, Baines CP. Structural mechanisms of cyclophilin D-dependent control of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2015;1850:2041–2047. doi: 10.1016/j.bbagen.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson N, Khan A, Virji S, Ward JM, Crompton M. Import and processing of heart mitochondrial cyclophilin D. Eur J Biochem. 1999;263:353–359. doi: 10.1046/j.1432-1327.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 61.Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 62.Lodish HF, Kong N. Cyclosporin A inhibits an initial step in folding of transferrin within the endoplasmic reticulum. J Biol Chem. 1991;266:14835–14838. [PubMed] [Google Scholar]
- 63.Tanveer A, Virji S, Andreeva L, Totty NF, Hsuan JJ, et al. Involvement of cyclophilin D in the activation of a mitochondrial pore by Ca2 + and oxidant stress. Eur J Biochem. 1996;238:166–172. doi: 10.1111/j.1432-1033.1996.0166q.x. [DOI] [PubMed] [Google Scholar]
- 64.Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, et al. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol. 1992;262:H1699–H1704. doi: 10.1152/ajpheart.1992.262.6.H1699. [DOI] [PubMed] [Google Scholar]
- 65.Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta. 1998;1366:33–50. doi: 10.1016/S0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 66.Bernardi P, Petronilli V. The permeability transition pore as a mitochondrial calcium release channel: a critical appraisal. J Bioenerg Biomembr. 1996;28:131–138. doi: 10.1007/BF02110643. [DOI] [PubMed] [Google Scholar]
- 67.Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343(2):311–317. doi: 10.1042/bj3430311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/S0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 69.De Marchi E, Bonora M, Giorgi C, Pinton P. The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium. 2014;56:1–13. doi: 10.1016/j.ceca.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei AC, Liu T, Cortassa S, Winslow RL, O’Rourke B. Mitochondrial Ca2 + influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporine A. Biochim Biophys Acta. 2011;1813:1373–1381. doi: 10.1016/j.bbamcr.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olbrich HG, Geerts H, Waldmann U, Mutschler E, Ver Donck L, et al. The effect of cyclosporine on electrically paced isolated rat cardiomyocytes. Transplantation. 1991;51:972–976. doi: 10.1097/00007890-199105000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 73.Montero M, Lobaton CD, Gutierrez-Fernandez S, Moreno A, Alvarez J. Calcineurin-independent inhibition of mitochondrial Ca2 + uptake by cyclosporin A. Br J Pharmacol. 2004;141:263–268. doi: 10.1038/sj.bjp.0705609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res. 2012;94:168–180. doi: 10.1093/cvr/cvs116. [DOI] [PubMed] [Google Scholar]
- 75.Reddy PA, Atreya CD. Identification of simian cyclophilin A as a calreticulin-binding protein in yeast two-hybrid screen and demonstration of cyclophilin A interaction with calreticulin. Int J Biol Macromol. 1999;25:345–351. doi: 10.1016/S0141-8130(99)00053-7. [DOI] [PubMed] [Google Scholar]
- 76.Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987;19:297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- 77.Shang W, Gao H, Lu F, Ma Q, Fang H, et al. Cyclophilin D regulates mitochondrial flashes and metabolism in cardiac myocytes. J Mol Cell Cardiol. 2016;91:63–71. doi: 10.1016/j.yjmcc.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 78.Li K, Zhang W, Fang H, Xie W, Liu J, et al. Superoxide flashes reveal novel properties of mitochondrial reactive oxygen species excitability in cardiomyocytes. Biophys J. 2012;102:1011–1021. doi: 10.1016/j.bpj.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang H, Chen M, Ding Y, Shang W, Xu J, et al. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 2011;21:1295–1304. doi: 10.1038/cr.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarasov AI, Griffiths EJ, Rutter GA. Regulation of ATP production by mitochondrial Ca(2+) Cell Calcium. 2012;52:28–35. doi: 10.1016/j.ceca.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menazza S, Wong R, Nguyen T, Wang G, Gucek M, et al. CypD(-/-) hearts have altered levels of proteins involved in Krebs cycle, branch chain amino acid degradation and pyruvate metabolism. J Mol Cell Cardiol. 2013;56:81–90. doi: 10.1016/j.yjmcc.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jang S, Lewis TS, Powers C, Khuchua Z, Baines CP et al (2016) Elucidating mitochondrial electron transport chain supercomplexes in the heart during ischemia-reperfusion. Antioxid Redox Signal. doi:10.1089/ars.2016.6635 [DOI] [PMC free article] [PubMed]
- 83.Halestrap AP. Regulation of mitochondrial metabolism through changes in matrix volume. Biochem Soc Trans. 1994;22:522–529. doi: 10.1042/bst0220522. [DOI] [PubMed] [Google Scholar]
- 84.Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur J Pharmacol. 2009;604:111–116. doi: 10.1016/j.ejphar.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rasola A, Sciacovelli M, Chiara F, Pantic B, Brusilow WS, et al. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci USA. 2010;107:726–731. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bao H, Ge Y, Zhuang S, Dworkin LD, Liu Z, et al. Inhibition of glycogen synthase kinase-3beta prevents NSAID-induced acute kidney injury. Kidney Int. 2012;81:662–673. doi: 10.1038/ki.2011.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kohr MJ, Sun J, Aponte A, Wang G, Gucek M, et al. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ Res. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun J, Steenbergen C, Murphy E. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 2006;8:1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 90.Pagliaro P, Moro F, Tullio F, Perrelli MG, Penna C. Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxid Redox Signal. 2011;14:833–850. doi: 10.1089/ars.2010.3245. [DOI] [PubMed] [Google Scholar]
- 91.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vieira HL, Belzacq AS, Haouzi D, Bernassola F, Cohen I, et al. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20:4305–4316. doi: 10.1038/sj.onc.1204575. [DOI] [PubMed] [Google Scholar]
- 93.Martin LJ, Adams NA, Pan Y, Price A, Wong M. The mitochondrial permeability transition pore regulates nitric oxide-mediated apoptosis of neurons induced by target deprivation. J Neurosci. 2011;31:359–370. doi: 10.1523/JNEUROSCI.2225-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, et al. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem. 2011;286:40184–40192. doi: 10.1074/jbc.M111.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, et al. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys. 2009;491:39–45. doi: 10.1016/j.abb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 96.Lopez-Erauskin J, Galino J, Bianchi P, Fourcade S, Andreu AL, et al. Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain. 2012;135:3584–3598. doi: 10.1093/brain/aws292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Folda A, Citta A, Scalcon V, Cali T, Zonta F et al (2016) Mitochondrial Thioredoxin System as a Modulator of Cyclophilin D Redox State. Sci Rep 6:23071 [DOI] [PMC free article] [PubMed]
- 98.Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci. 2010;123:894–902. doi: 10.1242/jcs.061846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Shulga N, Pastorino JG. Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3. J Cell Sci. 2010;123:4117–4127. doi: 10.1242/jcs.073502. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging. 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S. Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol Biochem. 2012;29:841–850. doi: 10.1159/000178526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bochaton T, Crola-Da-Silva C, Pillot B, Villedieu C, Ferreras L, et al. Inhibition of myocardial reperfusion injury by ischemic postconditioning requires sirtuin 3-mediated deacetylation of cyclophilin D. J Mol Cell Cardiol. 2015;84:61–69. doi: 10.1016/j.yjmcc.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen TT, Wong R, Menazza S, Sun J, Chen Y, et al. Cyclophilin D modulates mitochondrial acetylome. Circ Res. 2013;113:1308–1319. doi: 10.1161/CIRCRESAHA.113.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336(2):287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 106.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J. 2002;367:541–548. doi: 10.1042/bj20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Majima E, Koike H, Hong YM, Shinohara Y, Terada H. Characterization of cysteine residues of mitochondrial ADP/ATP carrier with the SH-reagents eosin 5-maleimide and N-ethylmaleimide. J Biol Chem. 1993;268:22181–22187. [PubMed] [Google Scholar]
- 108.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 109.Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 110.Halestrap AP, Brennerb C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 111.Kallen J, Sedrani R, Zenke G, Wagner J. Structure of human cyclophilin A in complex with the novel immunosuppressant sanglifehrin A at 1.6 A resolution. J Biol Chem. 2005;280:21965–21971. doi: 10.1074/jbc.M501623200. [DOI] [PubMed] [Google Scholar]
- 112.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 113.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, et al. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen B, Xu M, Zhang H, Wang JX, Zheng P, et al. Cisplatin-induced non-apoptotic death of pancreatic cancer cells requires mitochondrial cyclophilin-D-p53 signaling. Biochem Biophys Res Commun. 2013;437:526–531. doi: 10.1016/j.bbrc.2013.06.103. [DOI] [PubMed] [Google Scholar]
- 115.Zhao LP, Ji C, Lu PH, Li C, Xu B, et al. Oxygen glucose deprivation (OGD)/re-oxygenation-induced in vitro neuronal cell death involves mitochondrial cyclophilin-D/P53 signaling axis. Neurochem Res. 2013;38:705–713. doi: 10.1007/s11064-013-0968-5. [DOI] [PubMed] [Google Scholar]
- 116.Zhen YF, Wang GD, Zhu LQ, Tan SP, Zhang FY, et al. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. J Cell Physiol. 2014;229:1475–1483. doi: 10.1002/jcp.24589. [DOI] [PubMed] [Google Scholar]
- 117.Bergeaud M, Mathieu L, Guillaume A, Moll UM, Mignotte B, et al. Mitochondrial p53 mediates a transcription-independent regulation of cell respiration and interacts with the mitochondrial F(1)F0-ATP synthase. Cell Cycle. 2013;12:2781–2793. doi: 10.4161/cc.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hernandez JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med. 2014;18:709–720. doi: 10.1111/jcmm.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karch J, Molkentin JD. Is p53 the long-sought molecular trigger for cyclophilin D-regulated mitochondrial permeability transition pore formation and necrosis? Circ Res. 2012;111:1258–1260. doi: 10.1161/CIRCRESAHA.112.280990. [DOI] [PubMed] [Google Scholar]
- 120.Starkov AA. The molecular identity of the mitochondrial Ca2 + sequestration system. Febs J. 2010;277:3652–3663. doi: 10.1111/j.1742-4658.2010.07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGee AM, Baines CP. Complement 1q-binding protein inhibits the mitochondrial permeability transition pore and protects against oxidative stress-induced death. Biochem J. 2011;433:119–125. doi: 10.1042/BJ20101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barreto-Torres G, Hernandez JS, Jang S, Rodriguez-Munoz AR, Torres-Ramos CA, et al. The beneficial effects of AMP kinase activation against oxidative stress are associated with prevention of PPARalpha-cyclophilin D interaction in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2015;308:H749–H758. doi: 10.1152/ajpheart.00414.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barreto-Torres G, Javadov S (2016) Possible role of interaction between PPARalpha and Cyclophilin D in cardioprotection of AMPK against in vivo ischemia-reperfusion in rats. PPAR Res 2016:9282087 [DOI] [PMC free article] [PubMed] [Retracted]
- 124.Li Y, Johnson N, Capano M, Edwards M, Crompton M. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochem J. 2004;383:101–109. doi: 10.1042/BJ20040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schubert A, Grimm S. Cyclophilin D, a component of the permeability transition-pore, is an apoptosis repressor. Cancer Res. 2004;64:85–93. doi: 10.1158/0008-5472.CAN-03-0476. [DOI] [PubMed] [Google Scholar]
- 126.Javadov S, Hunter JC, Barreto-Torres G, Parodi-Rullan R. Targeting the mitochondrial permeability transition: cardiac ischemia-reperfusion versus carcinogenesis. Cell Physiol Biochem. 2011;27:179–190. doi: 10.1159/000327943. [DOI] [PubMed] [Google Scholar]
- 127.Machida K, Ohta Y, Osada H. Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J Biol Chem. 2006;281:14314–14320. doi: 10.1074/jbc.M513297200. [DOI] [PubMed] [Google Scholar]
- 128.Ghosh JC, Siegelin MD, Dohi T, Altieri DC. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res. 2010;70:8988–8993. doi: 10.1158/0008-5472.CAN-10-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, et al. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 130.Schusdziarra C, Blamowska M, Azem A, Hell K. Methylation-controlled J-protein MCJ acts in the import of proteins into human mitochondria. Hum Mol Genet. 2013;22:1348–1357. doi: 10.1093/hmg/dds541. [DOI] [PubMed] [Google Scholar]
- 131.Sinha D, D’Silva P. Chaperoning mitochondrial permeability transition: regulation of transition pore complex by a J-protein, DnaJC15. Cell Death Dis. 2014;5:e1101. doi: 10.1038/cddis.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen SH, Li DL, Yang F, Wu Z, Zhao YY, et al. Gemcitabine-induced pancreatic cancer cell death is associated with MST1/cyclophilin D mitochondrial complexation. Biochimie. 2014;103:71–79. doi: 10.1016/j.biochi.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 133.Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, et al. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem. 2009;284:9692–9699. doi: 10.1074/jbc.M808750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 135.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 136.Calne RY, White DJ, Thiru S, Evans DB, McMaster P, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. The Lancet. 1978;2:1323–1327. doi: 10.1016/S0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 137.Calne RY, Rolles K, White DJ, Thiru S, Evans DB, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. The Lancet. 1979;2:1033–1036. doi: 10.1016/S0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 138.Thiel G, Brunner FP, Hermle M, Stahl RA, Mihatsch MJ. Effect of cyclosporine A on ischemic renal failure in the rat. Clin Nephrol. 1986;25(Suppl 1):S155–S161. [PubMed] [Google Scholar]
- 139.Jablonski P, Harrison C, Howden B, Rae D, Tavanlis G, et al. Cyclosporine and the ischemic rat kidney. Transplantation. 1986;41:147–151. doi: 10.1097/00007890-198602000-00002. [DOI] [PubMed] [Google Scholar]
- 140.Hayashi T, Nagasue N, Kohno H, Chang YC, Nakamura T. Beneficial effect of cyclosporine pretreatment in preventing ischemic damage to the liver in dogs. Transplantation. 1988;46:923–924. [PubMed] [Google Scholar]
- 141.Kawano K, Kim YI, Kaketani K, Kobayashi M. The beneficial effect of cyclosporine on liver ischemia in rats. Transplantation. 1989;48:759–764. doi: 10.1097/00007890-198911000-00007. [DOI] [PubMed] [Google Scholar]
- 142.Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem J. 1991;274(2):611–614. doi: 10.1042/bj2740611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-K. [DOI] [PubMed] [Google Scholar]
- 144.Gomez L, Chavanis N, Argaud L, Chalabreysse L, Gateau-Roesch O, et al. Fas-independent mitochondrial damage triggers cardiomyocyte death after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2005;289:H2153–H2158. doi: 10.1152/ajpheart.00165.2005. [DOI] [PubMed] [Google Scholar]
- 145.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/S0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 147.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, et al. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weinbrenner C, Liu GS, Downey JM, Cohen MV. Cyclosporine A limits myocardial infarct size even when administered after onset of ischemia. Cardiovasc Res. 1998;38:676–684. doi: 10.1016/S0008-6363(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 149.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 150.Leshnower BG, Kanemoto S, Matsubara M, Sakamoto H, Hinmon R, et al. Cyclosporine preserves mitochondrial morphology after myocardial ischemia/reperfusion independent of calcineurin inhibition. Ann Thorac Surg. 2008;86:1286–1292. doi: 10.1016/j.athoracsur.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther. 2010;24:85–87. doi: 10.1007/s10557-010-6219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zalewski J, Claus P, Bogaert J, Driessche NV, Driesen RB, et al. Cyclosporine A reduces microvascular obstruction and preserves left ventricular function deterioration following myocardial ischemia and reperfusion. Basic Res Cardiol. 2015;110:18. doi: 10.1007/s00395-015-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kay JE, Moore AL, Doe SE, Benzie CR, Schonbrunner R, et al. The mechanism of action of FK 506. Transplant Proc. 1990;22:96–99. [PubMed] [Google Scholar]
- 154.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, et al. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol. 2012;52:1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 155.Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, et al. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- 156.Lim WY, Messow CM, Berry C. Cyclosporin variably and inconsistently reduces infarct size in experimental models of reperfused myocardial infarction: a systematic review and meta-analysis. Br J Pharmacol. 2012;165:2034–2043. doi: 10.1111/j.1476-5381.2011.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karlsson LO, Zhou AX, Larsson E, Astrom-Olsson K, Mansson C, et al. Cyclosporine does not reduce myocardial infarct size in a porcine ischemia-reperfusion model. J Cardiovasc Pharmacol Ther. 2010;15:182–189. doi: 10.1177/1074248410362074. [DOI] [PubMed] [Google Scholar]
- 158.Lie RH, Stoettrup N, Sloth E, Hasenkam JM, Kroyer R, et al. Post-conditioning with cyclosporine A fails to reduce the infarct size in an in vivo porcine model. Acta Anaesthesiol Scand. 2010;54:804–813. doi: 10.1111/j.1399-6576.2010.02241.x. [DOI] [PubMed] [Google Scholar]
- 159.Kuznetsov AV, Margreiter R. Heterogeneity of mitochondria and mitochondrial function within cells as another level of mitochondrial complexity. Int J Mol Sci. 2009;10:1911–1929. doi: 10.3390/ijms10041911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. Am J Physiol Heart Circ Physiol. 2014;307:H1–H14. doi: 10.1152/ajpheart.00747.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Palmer JW, Tandler B, Hoppel CL. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am J Physiol. 1986;250:H741–H748. doi: 10.1152/ajpheart.1986.250.5.H741. [DOI] [PubMed] [Google Scholar]
- 162.Kuznetsov AV, Troppmair J, Sucher R, Hermann M, Saks V, et al. (2006) Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: possible physiological role? Biochim Biophys Acta 1757: 686–691 [DOI] [PubMed]
- 163.Arieli Y, Gursahani H, Eaton MM, Hernandez LA, Schaefer S. Gender modulation of Ca(2+) uptake in cardiac mitochondria. J Mol Cell Cardiol. 2004;37:507–513. doi: 10.1016/j.yjmcc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 164.Milerova M, Drahota Z, Chytilova A, Tauchmannova K, Houstek J, et al. Sex difference in the sensitivity of cardiac mitochondrial permeability transition pore to calcium load. Mol Cell Biochem. 2016;412:147–154. doi: 10.1007/s11010-015-2619-4. [DOI] [PubMed] [Google Scholar]
- 165.Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74:456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 166.Vijay V, Han T, Moland CL, Kwekel JC, Fuscoe JC, et al. Sexual dimorphism in the expression of mitochondria-related genes in rat heart at different ages. PLoS One. 2015;10:e0117047. doi: 10.1371/journal.pone.0117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Milerova M, Charvatova Z, Skarka L, Ostadalova I, Drahota Z, et al. Neonatal cardiac mitochondria and ischemia/reperfusion injury. Mol Cell Biochem. 2010;335:147–153. doi: 10.1007/s11010-009-0251-x. [DOI] [PubMed] [Google Scholar]
- 168.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 169.Roy S, Sileikyte J, Neuenswander B, Hedrick MP, Chung TD, et al. N-Phenylbenzamides as Potent Inhibitors of the Mitochondrial Permeability Transition Pore. ChemMedChem. 2016;11:283–288. doi: 10.1002/cmdc.201500545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fancelli D, Abate A, Amici R, Bernardi P, Ballarini M, et al. Cinnamic anilides as new mitochondrial permeability transition pore inhibitors endowed with ischemia-reperfusion injury protective effect in vivo. J Med Chem. 2014;57:5333–5347. doi: 10.1021/jm500547c. [DOI] [PubMed] [Google Scholar]
- 171.Martin LJ, Fancelli D, Wong M, Niedzwiecki M, Ballarini M, et al. (2014) GNX-4728, a novel small molecule drug inhibitor of mitochondrial permeability transition, is therapeutic in a mouse model of amyotrophic lateral sclerosis. Front Cell Neurosci 8: 433 [DOI] [PMC free article] [PubMed]
- 172.Richardson AP, Halestrap AP. Quantification of active mitochondrial permeability transition pores using GNX-4975 inhibitor titrations provides insights into molecular identity. Biochem J. 2016;473:1129–1140. doi: 10.1042/BCJ20160070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Roy S, Sileikyte J, Schiavone M, Neuenswander B, Argenton F, et al. Discovery, Synthesis, and Optimization of Diarylisoxazole-3-carboxamides as Potent Inhibitors of the Mitochondrial Permeability Transition Pore. ChemMedChem. 2015;10:1655–1671. doi: 10.1002/cmdc.201500284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 175.Bell RM, Botker HE, Carr RD, Davidson SM, Downey JM, et al. 9th Hatter Biannual Meeting: position document on ischaemia/reperfusion injury, conditioning and the ten commandments of cardioprotection. Basic Res Cardiol. 2016;111:41. doi: 10.1007/s00395-016-0558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–513. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 177.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.CJ-09-0338. [DOI] [PubMed] [Google Scholar]
- 178.Magid DJ, Wang Y, Herrin J, McNamara RL, Bradley EH, et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction. JAMA. 2005;294:803–812. doi: 10.1001/jama.294.7.803. [DOI] [PubMed] [Google Scholar]
- 179.Schneider A, Ad N, Izhar U, Khaliulin I, Borman JB, et al. Protection of myocardium by cyclosporin A and insulin: in vitro simulated ischemia study in human myocardium. Ann Thorac Surg. 2003;76:1240–1245. doi: 10.1016/S0003-4975(03)00830-0. [DOI] [PubMed] [Google Scholar]
- 180.Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005;289:H237–H242. doi: 10.1152/ajpheart.01192.2004. [DOI] [PubMed] [Google Scholar]
- 181.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 182.Hausenloy D, Kunst G, Boston-Griffiths E, Kolvekar S, Chaubey S, et al. The effect of cyclosporin-A on peri-operative myocardial injury in adult patients undergoing coronary artery bypass graft surgery: a randomised controlled clinical trial. Heart. 2014;100:544–549. doi: 10.1136/heartjnl-2013-304845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chiari P, Angoulvant D, Mewton N, Desebbe O, Obadia JF, et al. Cyclosporine protects the heart during aortic valve surgery. Anesthesiology. 2014;121:232–238. doi: 10.1097/ALN.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 184.Ghaffari S, Kazemi B, Toluey M, Sepehrvand N. The effect of prethrombolytic cyclosporine-A injection on clinical outcome of acute anterior ST-elevation myocardial infarction. Cardiovasc Ther. 2013;31:e34–e39. doi: 10.1111/1755-5922.12010. [DOI] [PubMed] [Google Scholar]
- 185.Mewton N, Cung TT, Morel O, Cayla G, Bonnefoy-Cudraz E, et al. Rationale and design of the Cyclosporine to ImpRove Clinical oUtcome in ST-elevation myocardial infarction patients (the CIRCUS trial) Am Heart J. 2015;169(758–766):e756. doi: 10.1016/j.ahj.2015.02.020. [DOI] [PubMed] [Google Scholar]