Graphical abstract
Keywords: Semecarpus anacardium, Catechol derivatives, Toxicity, Hematology
Highlights
-
•
Evaluation of acute and subacute toxicity of catechol derivatives.
-
•
In acute study, no behavioral adverse effects and mortality up to 800 mg/kg.
-
•
LD50 values of catechol derivatives I–III and IV (1600 and 1250 mg/kg b.wt).
-
•
In subacute study, (300 mg/kg b.wt daily, 30 days) shows no mortality, catechol derivative 1 and IV boost up immune system and HDL.
-
•
Results prove that catechol derivatives are potentially toxic but therapeutically effective.
Abstract
The present study was aimed at evaluating the acute and subacute toxicity of catechol derivatives (I–IV, isolated from Semecarpus anacardium nuts) in Wistar Albino rats. In acute study (14 days), catechol derivatives I–IV 800 mg/kg caused no behavioral adverse effects and mortality. Fifty percent (LD50) of mortality was observed in catechol derivatives I–III (1600 mg/kg b.wt) and catechol derivative IV (1250 mg/kg b.wt). In subacute study, daily oral administration of catechol derivatives I–IV (300 mg/kg b.wt) for 30 days did not result in death or significant changes in the body weight and organ weight, In hematological and some biochemical analysis showed few beneficial effects particularly in catechol derivatives I and IV treated rats that is transient rise in WBC count and HDL cholesterol and decrease in LDL, plasma and tissue lipid profile. These results indicate the impact of catechol derivatives in boosting the immune system and reducing cardiovascular risk factors and thereby they possess cardio protective and immunopotentiating effect. Further, histopathological examination of liver and kidney showed normal architecture that suggests no morphological disturbances. Based on the results obtained, it may be concluded that the catechol derivatives are potentially toxic but therapeutically effective.
1. Introduction
Conventional use of plant basis medications for treatment of various ailments is widely experienced in both developed and developing countries. It has been estimated that around 60% of the world’s population relies on plants for medications. This quantity elevate to more than 80% due to the increase of populations in developing world, easy access and increasing drug expenses [[1], [2]]. Thus, plants remain the chief provider of active drugs from natural sources [3]. Flora contain pharmacologically active components which are quite safe and often considered to be less toxic and free from side effects than synthetic ones [4]. Plants derived components play a vital role in world health and have long been known to have biological activity [5]. Thirty percent of all recent drugs are derived from plants [6]. According to the World Health Organization about 80% of the world's population living in developing countries relies basically on plants for primary health care [7]
Most repeatedly, these herbal medication methods are used in most disease conditions over a long period of time without proper dosage monitoring and consequently toxic effects maybe produced from such prolonged traditional practice. The danger associated with the potential toxicity of such herbal therapies used over a long period of time imposes in keeping abreast reported occurrences of renal and hepatic toxicities, consequential from intake of these medicinal herbs [8]. Thus, in modern medicine, animal toxicity studies are also required to establish the potential adverse effect of newly plant derived drugs [9]. Therefore, the present study is intended to investigate the basic toxic evaluation of four catechol derivatives (isolated from Semecarpus anacardium seeds) for establishing the safety of each drug.
2. Materials and methods
2.1. Plant material
Semecarpus anacardium seeds were purchased from K.R. Vasan Traditional & Herbal Medicine shop, Parris, Chennai, Tamil Nadu, India. The identity of the plant was confirmed by Prof. Raman, plant taxonomist, Centre for Advanced Studies in Botany, University of Madras, Guindy Campus, Chennai-600025. A voucher specimen (MUCASB- H105) was preserved in the Department herbarium for future reference.
2.2. Extraction and isolation
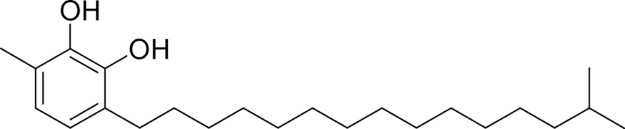
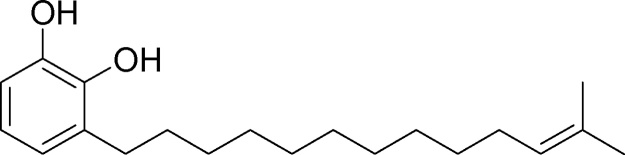
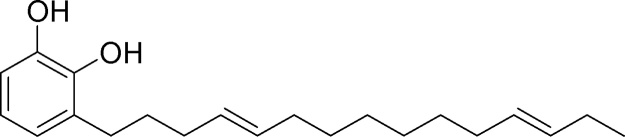
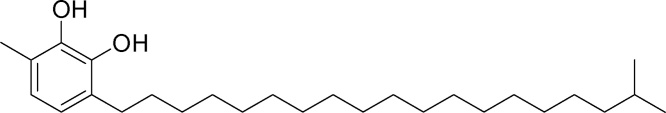
In our previous study, we have explained the isolation and characterization of catechol derivatives I, II, III and IV from Semecarpus anacardium seeds and their antibacterial property against gram positive and gram negative bacteria [10]. The chemical structure of catechol derivatives are given in Fig. 1, Fig. 2, Fig. 3, Fig. 4.
Fig. 1.
Isolation and characterization of catechol derivatives from Semecarpus anacardium seeds. Catechol derivative 1.
Fig. 2.
Isolation and characterization of catechol derivatives from Semecarpus anacardium seeds. Catechol derivative 2.
Fig. 3.
Isolation and characterization of catechol derivatives from Semecarpus anacardium seeds. Catechol derivative 3.
Fig. 4.
Isolation and characterization of catechol derivatives from Semecarpus anacardium seeds. Catechol derivative 4.
3. Acute toxicity study
3.1. Animals
Wistar albino rats of either sex weighing 100 ± 20 g were secured from Central Animal House, Institute of Basic Medical Sciences, University of Madras, Taramani Campus, Chennai, India. The rats were housed in clean, sterile and polypropylene cages under standard conditions 12 h light/12 h dark cycle and constant temperature (25 ± 2 °C) with free access to standard commercial rat chow
and water. The study has got the approval from the Institutional Animal Ethical Committee (IAEC No.01/03/08) of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
3.2. Experimental study design for acute toxicity study
The single dose of acute oral toxicity study was evaluated following the recommendations by OECD/OCDE [11]. The animals were randomly divided into twenty nine groups of four animals in each group. After overnight fasting, four catechol derivatives were separately dissolved in 1 ml of olive oil and administered only once/per day orally to experimental groups through gastric intubation at doses of 50, 100, 200, 400, 800, 1600, and 2000 mg/kg b.wt, and control group received 1 ml of olive oil (vehicle) alone. Group II–VIII received catechol I at above mentioned doses individually. Likewise, Group IX–XV received catechol II, Group XVI–XXII received catechol III and Group XXIII–XXIX received catechol IV, respectively. Feeding was restarted 4 h after dosing All animals were observed for clinical signs including mortality and morbidity, immediately after dosing at 1, 2, 4, 8 and 12 h, then twice daily until 14 days. Abnormal findings were observed with the time of onset and disappearance. Body weights and food consumption were measured on day 0, 1, 3, 5, 7, 10 and 14. On the 14th day, all animals were sacrificed and all organs and tissues were observed macroscopically. The abnormal organs were placed in 10% formalin and observed by pathological examination.
3.3. Experimental design
| Groups | Treatment |
|---|---|
| Group I | Served as vehicle treated normal control |
| Group II | Animals received single dose of catechol I (50 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group III | Animals received single dose of catechol I (100 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group IV | Animals received single dose of catechol I (200 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group V | Animals received single dose of catechol I (400 mg/kg b.w) dissolved in 1 ml of olive oil for only one day |
| Group VI | Animals received single dose of catechol I (800 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group VII | Animals received single dose of catechol I (1600 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group VIII | Animals received single dose of catechol I (2000 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group IX | Animals received single dose of catechol II (50 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group X | Animals received single dose of catechol II (100 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XI | Animals received single dose of catechol II (200 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XII | Animals received single dose of catechol II (400 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XIII | Animals received single dose of catechol II (800 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XIV | Animals received single dose of catechol II (1600 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XV | Animals received single dose of catechol II (2000 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XVI | Animals received single dose of catechol III (50 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XVII | Animals received single dose of catechol III (100 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XVIII | Animals received single dose of catechol III (200 mg/kg b.w orally) dissolved in 1 ml of olive for only one day |
| Group XIX | Animals received single dose of catechol III (400 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XX | Animals received single dose of catechol III (800 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXI | Animals received single dose of catechol III (1600 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXII | Animals received single dose of catechol III (2000 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXIII | Animals received single dose of catechol IV (50 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXIV | Animals received single dose of catechol IV (100 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXV | Animals received single dose of catechol IV (200 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXVI | Animals received single dose of catechol IV (400 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXVII | Animals received single dose of catechol IV (800 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXIVIII | Animals received single dose of catechol IV (1600 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
| Group XXIX | Animals received single dose of catechol IV (2000 mg/kg b.w orally) dissolved in 1 ml of olive oil for only one day |
3.4. Sub-acute toxicity study
Wistar albino rats of either sex weighing 180 ± 20 g were divided into five groups of six animals in each group and were housed under the same conditions as described earlier. Catechol derivatives I–III was individually administered for 30 days at doses of 300 mg/kg/b.wt. Whereas catechol IV was administered 30 days at doses of 250 mg/kg/b.wt. The control animals received 0.5 ml of the vehicle alone. Toxic manifestations and mortality were monitored daily and body weight changes were recorded every seven days till the end of the experimental study.
3.4.1. Experimental design
Group I: Control animals [Normal healthy animals received (0.5 ml) of olive oil orally by gastric intubation for 30 days].
Group II: Catechol derivative I treated [Drug (300 mg/kg body weight dissolved in 0.5 ml of olive oil was administered orally by gastric intubation for 30 days].
Group III: Catechol derivative II treated [Drug (300 mg/kg body weight dissolved in 0.5 ml of olive oil was administered orally by gastric intubation for 30 days].
Group IV: Catechol derivative III treated [Drug (300 mg/kg body weight dissolved in 0.5 ml of olive oil was administered orally by gastric intubation for 30 days].
Group V: Catechol derivative IV treated [Drug (250 mg/kg body weight dissolved in 0.5 ml of olive oil was administered orally by gastric intubation for 30 days].
On 28th day, 24 h urine samples were collected by introducing the animals in the metabolic cage to avoid contamination of urine with animal excreta. Animals had free access to tap water but no feed was given. A 50 ml of beaker maintained at 0 °C in an ice bath was used for collection of urine sample. Toluene was used as a preservative while collecting the sample. The sediments present in the urine were removed by centrifugation and the collected urine was used for biochemical estimation.
On 30th day, animals were fasted for 12 h, then anesthetized with ether and blood was collected from retro orbital sinus in two tubes: one with EDTA for immediate analysis of hematological parameters and to separate plasma for biochemical estimations, the other without any additives and was centrifuged at 4000 rpm at 4 °C for l0 min to obtain the serum. Both plasma and serum were stored at −20 °C until analyzed for biochemical parameters. Animals were then sacrificed by ether anesthesia and liver, kidney, lung, heart, spleen and adrenals were dissected out, washed and transferred to an ice-cold saline solution. The organs were weighed and portions of organs were fixed in 10% formalin for histopathological examinations. Ten percent tissue homogenates of liver and kidney were prepared by homogenizing a weighed amount of tissues in 0.01 M Tris HC1 buffer, pH 7.4. The homogenates were analyzed for biochemical parameters.
3.4.2. Hematological indices
Haemoglobin content in blood was determined by the method of Drabkin and Austin [12]. Red and white blood cells were estimated according to the method of Chesbrough and McArthur [13]. PCV was estimated by the Wintrobe macro method [14].
3.4.3. Biochemical parameters
Protein content of the tissue sample was estimated by the method of Lowry et al. [15]. Alanine aspartate aminotransferases and alkaline phosphatase were assayed by the method of King [16]. Blood urea nitrogen was estimated by the method of Natelson et al. [17]. Plasma uric acid was estimated by the method of Caraway [18]. Creatinine was estimated by the method of Owen et al. [19]. Glucose was estimated by the method of Trinder [20] using reagent kit. The plasma total cholesterol, triglycerides and HDL cholesterol were measured using diagnostic kits (Ensure Biotech, Hyderabad, India). For the determination of very low density lipoprotein (VLDL) and low density lipoprotein (LDL) cholesterol Friedewald’s formula was used which states: VLDL cholesterol = Triglyceride/5 and LDL cholesterol = Total cholesterol − (VLDL + HDL cholesterol). Total lipids were extracted from liver and kidney tissues according to the method of Folch et al. [21] using chloroform: methanol mixture (2:1, v/v). Cholesterol content was estimated by the method of Parekh and Jung [22]. Triglyceride in tissue was estimated by the method of Rice [23] based on the method of Van Handel ([24]. Free fatty acids were estimated by the method of Itaya [25]. Phospholipids were estimated by the method of Rouser et al. [26].
3.4.4. Urine analysis
In 24 h urine sample, protein, uric acid and creatinine were estimated by the method of Lowry et al. [15], Caraway [18] and Owen et al. [19] respectively.
3.4.5. Statistical analysis
The results are expressed as mean ± standard deviation (S.D.). Differences between groups were assessed by ANOVA using the SPSS software package for Windows. Post hoc testing was performed for inter-group comparisons using the least significance difference (L.S.D.) test; p-values < .05 were considered as significantly altered.
4. Results
4.1. Acute toxicity study
In the acute toxicity study (Table 1a, Table 1b), 100% of death was observed in all the animals that received 2000 mg/kg bw, of all catechol derivatives and even 1600 mg/kg/b.w. of catechol derivatives IV received animals. But, 50% death of was observed in the case of catechol derivatives I–III received animals at a dose of 1600 mg/kg/b.w. (To find out the lethal dose of catechol derivatives IV, the animals further divided into three groups and those received 1000, 1250 and 1500 mg/kg bw, respectively.) In this connection, 100% of death was observed in the animals that received 1500 mg/kg and 50% of death was noticed in the animals that received 1250 mg/kg and no death occurred in the animals that received a dose of 1000 mg/kg). No death was occurred during 14 days the animals that received 50, 100, 200, 400 and 800 mg/kg and no significant differences in body weights and food consumption was observed between the catechol derivatives administrated group and the controls (data not shown). A short time of sluggish appeared in all catechol derivatives treated rats after oral gavage in the first hour (when the dosage level goes beyond 800–1000 mg/kg), but the rats return to life later. Otherwise, there were no abnormal findings at other clinical signs and autopsy in the experimental or control group. But, when dosage goes above 1000 mg/kg, the animals show clinical signs of toxicity which include, loose motion, difficulty in breathing and lethargic and eventually all the animals died at another 48 h. Based on these, the median acute toxicity (LD50) of the catechol derivatives I–III were determined to be 1600 and 1250 mg/kg of catechol derivatives IV.
Table 1a.
Acute toxicity study Determination of LD50 values for catechol derivatives I–IV isolated from Semecarpus anacardium nut extract.
| Groups | Compound | Dose (mg/kg/b.w.) | No of animals | No of mortality (24 h) | Survival rate |
|---|---|---|---|---|---|
| Group I Control | – | – | 4 | – | 4 |
| Group II | Catechol derivative I | 50 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group III | Catechol derivative I | 100 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group IV | Catechol derivative I | 200 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group V | Catechol derivative I | 400 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group VI | Catechol derivative I | 800 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group VII | Catechol derivative I | 1600 (mg/kg/b.w.) | 4 | 2 | 2 |
| Group VIII | Catechol derivative I | 2000 (mg/kg/b.w.) | 4 | 4 | Nil |
| Group IX | Catechol derivative II | 50 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group X | Catechol derivative II | 100 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XI | Catechol derivative II | 200 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XII | Catechol derivative II | 400 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XIII | Catechol derivative II | 800 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XIV | Catechol derivative II | 1600 (mg/kg/b.w.) | 4 | 2 | 2 |
| Group XV | Catechol derivative II | 2000 (mg/kg/b.w.) | 4 | 4 | Nil |
| Group XVI | Catechol derivative III | 50 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XVII | Catechol derivative III | 100 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XVIII | Catechol derivative III | 200 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XIX | Catechol derivative III | 400 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XX | Catechol derivative III | 800 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XXI | Catechol derivative III | 1600 (mg/kg/b.w.) | 4 | 2 | 2 |
| Group XXII | Catechol derivative III | 2000 (mg/kg/b.w.) | 4 | 4 | Nil |
| Group XXIII | Catechol derivative IV | 50 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XXIV | Catechol derivative IV | 100 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XXV | Catechol derivative IV | 200 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XXVI | Catechol derivative IV | 400 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XXVII | Catechol derivative IV | 800 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group XXVIII | Catechol derivative IV | 1600 (mg/kg/b.w.) | 4 | 4 | Nil |
| Group XXIX | Catechol derivative IV | 2000 (mg/kg/b.w.) | 4 | 4 | Nil |
Table 1b.
Acute toxicity study- Determination of LD50 values for catechol derivatives IV isolated from Semecarpus anacardium nut extract.
| Groups | Compound | Dose (mg/kg/b.w.) | No of animals | No of mortality (24 h) | Survival rate |
|---|---|---|---|---|---|
| Group I | Catechol derivative IV | 1000 (mg/kg/b.w.) | 4 | Nil | 4 |
| Group II | Catechol derivative IV | 1250 (mg/kg/b.w.) | 4 | 2 | 2 |
| Group III | Catechol derivative IV | 1500 (mg/kg/b.w.) | 4 | 4 | Nil |
| Group IV | Catechol derivative IV | 2000 (mg/kg/b.w.) | 4 | 4 | Nil |
4.2. Sub acute toxicity study
4.2.1. Effect of catechol derivatives on body and organ weights
No lethality and any abnormal behavioral changes were recorded in the animals that received catechol derivatives I–III up to the dose of 300 mg/kg and catechol derivatives IV up to the dose of 250 mg/kg body weight during the 30 days period of treatment (Table 1c). All the animals were active as control animals. Moreover, there were no significant differences in the body weight and organ weight gain between the control and all catechol derivatives treated groups (Table 2).
Table 1c.
Sub acute toxicological studies of Catechol derivatives I–IV for the period of 30 days.
| Groups | Compound | Dose (mg/kg/b.w.) | No of Animals | No of Mortality | Survival rate |
|---|---|---|---|---|---|
| Group I Control | – | – | 6 | Nil | 6 |
| Group II Treated | Catechol derivative I | 300 (mg/kg/b.w.) | 6 | Nil | 6 |
| Group III Treated | Catechol derivative II | 300 (mg/kg/b.w.) | 6 | Nil | 6 |
| Group IV Treated | Catechol derivative III | 300 (mg/kg/b.w.) | 6 | Nil | 6 |
| Group V Treated | Catechol derivative IV | 250 (mg/kg/b.w.) | 6 | Nil | 6 |
Table 2.
Effect of Catechol derivatives I–IV on body and organ weight changes in control and experimental rats during subacute toxicity studies.
| Parameters | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Control | Catechol derivative I | Catechol derivative II | Catechol derivative III | Catechol derivative IV | |
| Body weight (g) | |||||
| Initial | 162 ± 11.82 | 158.8 ± 10.25 | 161.6 ± 21.37 | 166.5 ± 10.64 | 169.1 ± 10.97 |
| Final | 201.1 ± 9.35 | 196.8 ± 17.84 | 195.3 ± 79.55 | 199.6 ± 12.13 | 207 ± 16.83 |
| Body weight gain | 39.16 ± 10.34 | 38 ± 7.58 | 33.66 ± 58.18 | 33.16 ± 1.48 | 37.8 ± 5.85 |
| Organ weight | |||||
| Liver (g) | 6.90 ± 0.64 | 7.03 ± 0.60 | 7.29 ± 0.67 | 7.40 ± 0.49 | 7.53 ± 0.55 |
| Kidney (g) | 2.24 ± 0.25 | 2.19 ± 0.22 | 2.10 ± 0.19 | 2.03 ± 0.17 | 2.09 ± 0.12 |
| Heart (g) | 0.76 ± 0.07 | 0.80 ± 0.09 | 0.79 ± 0.08 | 0.82 ± 0.08 | 0.80 ± 0.09 |
| Lungs (g) | 1.18 ± 0.12 | 1.21 ± 0.15 | 1.25 ± 0.14 | 1.23 ± 0.15 | 1.22 ± 0.17 |
| Spleen (g) | 0.85 ± 0.09 | 0.82 ± 0.08 | 0.80 ± 0.06 | 0.83 ± 0.09 | 0.81 ± 0.07 |
Values are expressed as mean ± SD for 6 animals. Comparisons are made between: a- Group I Vs Groups II, III, IV and V. The symbol * also represent the statistically significance at p < .05.
4.2.2. Effect of catechol derivatives on the hematological and biochemical parameters
The effect of catechol derivatives on the hematological parameters is presented in Table 3. The catechol derivatives treatment did neither improve nor produced any deleterious effects on the hematological parameters (Hb, RBC, WBC and PCV). As depicted in Table 3, there was no significant change observed in the levels of plasma protein, uric acid and creatinine in all catechol derivative treated groups. But, in the case of catechol derivative I and IV treated rats, the levels of plasma glucose were significantly decreased when compared to control and catechol derivatives II and III treated rats.
Table 3.
Effect of catechol derivatives I–IV on hematological and blood biochemical parameters in control and experimental rats during subacute toxicity studies.
| Parameters | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Control | Catechol derivative I | Catechol derivative II | Catechol derivative III | Catechol derivative IV | |
| Protein (g/dl) | 6.92 ± 0.62 | 7.11 ± 0.92 | 6.69 ± 0.64 | 7.35 ± 0.75 | 7.19 ± 0.63 |
| Urea (mg/dl) | 27.94 ± 2.56 | 29.07 ± 2.81 | 25.63 ± 2.30 | 28.60 ± 3.29 | 25.74 ± 2.49 |
| Uric acid (mg/dl) | 1.78 ± 0.11 | 1.70 ± 0.09 | 1.75 ± 0.08 | 1.80 ± 0.09 | 1.71 ± 0.08 |
| Creatinine (mg/dl) | 0.72 ± 0.09 | 0.80 ± 0.10 | 0.76 ± 0.10 | 0.84 ± 0.07 | 0.79 ± 0.10 |
| Glucose (mg/dl) | 87.23 ± 8.89 | 76.53 ± 5.74* | 81.44 ± 7.62 | 78.3 ± 6.43 | 74.90 ± 6.94* |
| Hb (g/dl) | 13.11 ± 1.24 | 12.63 ± 1.11 | 13.38 ± 1.06 | 13.65 ± 1.07 | 14.1 ± 1.07 |
| RBC (Millions/mm2) | 4.37 ± 0.41 | 4.21 ± 0.37 | 4.46 ± 0.35 | 4.55 ± 0.35 | 4.7 ± 0.35 |
| WBC(thousands/mm3) | 7.38 ± 0.81 | 9.46 ± 0.75* | 7.95 ± 0.76 | 8.13 ± 0.67 | 10.01 ± 0.92* |
| PCV (%) | 39.35 ± 3.74 | 37.9 ± 3.35 | 40.15 ± 3.20 | 40.95 ± 3.21 | 42.3 ± 3.22 |
Values are expressed as mean ± SD for 6 animals. Comparisons are made between:- Group I with Groups II, III, IV and V. The symbol * also represent the statistically significance at p < .05.
Table 4 represents the marker enzymes (GOT, GPT and ALP) in serum, liver and kidney. No significant changes were observed in GOT, GPT and ALP levels in serum, liver and kidney in all the groups tested.
Table 4.
Effect of catechol derivatives I–IV on marker enzymes in serum, liver and kidney in control and experimental rats during subacute toxicity studies.
| Parameters | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Control | Catechol derivative I | Catechol derivative II | Catechol derivative III | Catechol derivative IV | |
| SERUM | |||||
| Aspartate amino transferase | 33.64 ± 2.50 | 32.51 ± 3.32 | 29.60 ± 2.97 | 32.14 ± 2.97 | 30.69 ± 3.61 |
| Alanine amino transferase | 25.05 ± 2.11 | 23.83 ± 2.76 | 27.33 ± 2.80 | 24.76 ± 2.78 | 26.56 ± 2.89 |
| Alkaline phosphatase | 1.77 ± 0.18 | 1.84 ± 0.20 | 1.71 ± 0.17 | 1.79 ± 0.16 | 1.82 ± 0.18 |
| LIVER | |||||
| Aspartate amino transferase | 26.11 ± 2.51 | 28.31 ± 2.39 | 27.10 ± 2.51 | 28.84 ± 2.47 | 24.91 ± 2.35 |
| Alanine amino transferase | 16.21 ± 1.73 | 15.06 ± 1.89 | 15.55 ± 1.44 | 14.65 ± 2.12 | 18.07 ± 1.70 |
| Alkaline phosphatase | 1.17 ± 0.01 | 1.22 ± 0.13 | 1.13 ± 0.01 | 1.19 ± 0.01 | 1.15 ± 0.01 |
| KIDNEY | |||||
| Aspartate amino transferase | 23.84 ± 2.21 | 24.06 ± 3.0 | 22.63 ± 2.57 | 24.27 ± 2.35 | 26.08 ± 2.79 |
| Alanine amino transferase | 12.14 ± 1.28 | 13.86 ± 1.30 | 13.65 ± 1.79 | 11.93 ± 1.03 | 12.83 ± 1.97 |
| Alkaline phosphatase | 0.74 ± 0.09 | 0.80 ± 0.10 | 0.69 ± 0.05 | 0.72 ± 0.06 | 0.83 ± 0.07 |
Values are expressed as mean ± SD for 6 animals. Aspartate amino transferase (AST) & Alanine amino transferase (ALT): μmoles × 10−2 of pyruvate liberated/min/mg/protein; Alkaline phosphatase (ALP): μmoles × 10−2 of phenol liberated/min/mg/protein. Comparisons are made between:- Group I with Groups II, III, IV and V. The symbol * also represent the statistically significance at p < .05.
Table 5 shows the plasma, liver and kidney lipid profiles (TC, TG, PL and FFA) and lipoprotein (LDL and HDL) levels in control and experimental animals. The levels of TC, TG, PL and LDL were significantly decreased in plasma, liver and kidney of catechol derivative I and catechol derivative IV whereas, the levels of HDL were significantly increased when compared to control group and catechol derivative II, catechol derivative III.
Table 5.
Effect of catechol derivatives I–IV on lipid profile in serum, liver and kidney in control and experimental rats during subacute toxicity studies.
| Parameters | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Control | Catechol derivative I | Catechol derivative II | Catechol derivative III | Catechol derivative IV | |
| SERUM (mg/dl) | |||||
| TC | 86.98 ± 6.46 | 77.00 ± 6.14* | 82.96 ± 6.73 | 79.85 ± 6.14 | 70.01 ± 6.26* |
| TG | 115.25 ± 10.09 | 85.35 ± 8.65* | 110.33 ± 9.05 | 107.7 ± 9.75 | 82.87 ± 7.09* |
| PHOSPHOLIPIDS | 38.67 ± 4.02 | 28.22 ± 2.98* | 35.76 ± 2.86 | 36.77 ± 3.18 | 25.84 ± 3.12* |
| FFA | 27.69 ± 2.81 | 18.31 ± 1.97 | 25.75 ± 2.43 | 25.11 ± 2.45 | 16.99 ± 2.37 |
| HDL | 25.96 ± 3.15 | 29.07 ± 3.48* | 26.57 ± 2.35 | 27.38 ± 2.28 | 31.83 ± 2.94* |
| LDL | 37.94 ± 1.29 | 30.86 ± 0.93* | 36.93 ± 2.76 | 33.75 ± 2.08 | 21.60 ± 1.89* |
| VLDL | 23.05 ± 2.01 | 17.07 ± 1.73 | 19.45 ± 1.61 | 18.70 ± 1.77 | 16.57 ± 1.41 |
| LIVER (mg/gm) | |||||
| TC | 8.98 ± 0.55 | 6.28 ± 0.98* | 8.38 ± 0.48 | 8.26 ± 0.48 | 5.13 ± 0.71* |
| TG | 13.28 ± 1.67 | 8.44 ± 0.79* | 12.33 ± 1.27 | 12.59 ± 1.26 | 6.54 ± 0.59* |
| PHOSPHOLIPIDS | 20.76 ± 2.36 | 16.88 ± 1.71* | 19.21 ± 2.10 | 18.99 ± 2.31 | 14.02 ± 1.32* |
| FFA | 6.06 ± 0.52 | 4.21 ± 0.58 | 5.51 ± 0.49 | 5.41 ± 0.45 | 3.04 ± 0.53 |
| KIDNEY (mg/gm) | |||||
| TC | 5.34 ± 0.57 | 3.70 ± 0.46* | 4.84 ± 0.71 | 4.70 ± 0.63 | 3.07 ± 0.46* |
| TG | 9.15 ± 0.51 | 5.86 ± 0.79* | 8.41 ± 0.66 | 8.50 ± 0.65 | 4.43 ± 0.67* |
| PHOSPHOLIPIDS | 15.42 ± 1.65 | 13.24 ± 1.72* | 14.47 ± 1.85 | 13.64 ± 1.69 | 12.15 ± 1.36* |
| FFA | 4.84 ± 0.55 | 3.63 ± 0.44 | 4.31 ± 0.59 | 4.24 ± 0.54 | 3.05 ± 0.23 |
Values are expressed as mean ± SD for 6 animals. Comparisons are made between:- Group I with Groups II, III, IV and V. The symbol * also represent the statistically significance at p < .05.
Table 6 portrays the urine levels of protein, urea, uric acid and creatinine. The catechol derivatives I–III treated rats (300 mg/kg b.wt) as well as the catechol derivatives- IV treated rats (250 mg/kg b.wt) showed no significant changes in the levels of protein, urea, uric acid and creatinine during the 30 days period of treatment.
Table 6.
Effect of catechol derivatives I–IV on urinary parameters in control and experimental rats during subacute toxicity studies.
| Parameters | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Control | Catechol derivative I | Catechol derivative II | Catechol derivative III | Catechol derivative IV | |
| Protein (g/dl) | 6.650 ± 0.634 | 6.295 ± 0.500 | 6.940 ± 0.842 | 7.416 ± 1.086 | 7.625 ± 0.745 |
| Urea (mg/dl) | 1.863 ± 0.192 | 1.846 ± 0.167 | 1.784 ± 0.175 | 1.825 ± 0.125 | 1.871 ± 0.141 |
| Ureic acid (mg/dl) | 0.800 ± 0.01 | 0.082 ± 0.011 | 0.085 ± 0.008 | 0.078 ± 0.006 | 0.078 ± 0.006 |
| Creatinine (mg/dl) | 0.736 ± 0.654 | 0.795 ± 0.075 | 0.755 ± 0.072 | 0.781 ± 0.096 | 0.821 ± 0.093 |
Values are expressed as mean ± SD for 6 animals. Comparisons are made between:- Group I with Groups II, III, IV and V. The symbol * also represent the statistically significance at p < .05.
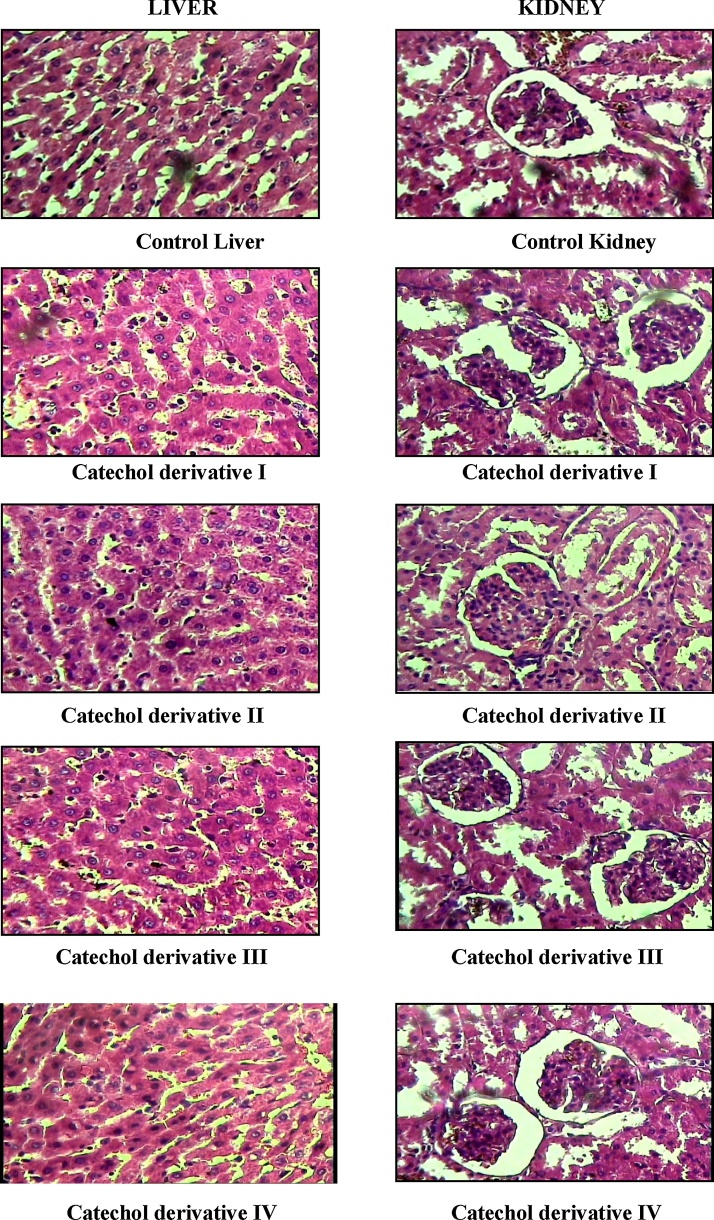
4.3. Histology of liver and kidney
Fig. 5 Liver and kidney samples were obtained and stained with haematoxylin and eosin and observed under light microscope. Histopathological sections of liver and kidney of all catechol derivatives treated groups revealed the normal architecture of hepatocytes and glomerulus with functional integrity on comparison with control rats.
Fig. 5.
Effect of catechol derivative (I–IV) on histopathological changes in liver and kidney of control and experimental animals (Subacute study, 30 days treatment).
Control liver: Section shows liver tissue with normal architecture of hepaocytes. The central vein appeared normal. Few of the focal tracts exhibit mild infiltration by lymphocytes.
Catechol derivative I treated liver: Section shows liver tissue with normal histology.
Catechol derivative II treated liver: Section shows liver tissue with normal architecture of histology.
Catechol derivative III treated liver: Section shows liver tissue with normal architecture of histology.
Catechol derivative IV treated liver: Section shows liver tissue with normal arrangement hepatocytes. The focal tract and central vein appeared normal.
Control kidney: Section shows renal tissue with normal glomeruli and tubules.
Catechol derivative I treated kidney: Section shows renal tissue with normal cortex, glomeruli and tubules appeared normal.
Catechol derivative II treated kidney: Section shows kidney tissue with normal histology.
Catechol derivative III treated kidney: Section shows kidney tissue with normal histology.
Catechol derivative IV treated kidney: Section shows renal tissue with normal cortex, glomeruli and tubules appeared normal.
5. Discussion
Plant-based medications had served from the beginning as therapeutic weapon available to man to fight against various diseases. Man cannot survive on this earth for long time without plant kingdom because the plant products and their active constituents played an important role. Herbs have always been the principal form of medicine in India and currently they are becoming popular throughout the world, as they are healthier and more harmless or safer than synthetic ones [27]. Hence, herbal drugs have received greater attention as an alternative to clinical therapy and the demand for these remedies has currently increased [28]. The exclusive use of herbal drugs prepared and distributed by irrationally trained herbalists, for the treatment of diseases over a long period of time without proper dosage monitoring which produces renal and hepatic toxicities [8]. To overcome this problem, experimental screening method to be established to find out the safety and efficacy of the herbal products as well their active components [3]. On this basis, structurally similar of four catechol derivatives have been isolated from the well renowned medicinal plant Semecarpus anacardium seeds and tested its lethal dose and effective dose in Wistar albino rats mainly for safe usage proposed as a future medicine in humans.
Acute toxicity test gives clues on the range of doses that could be toxic to the animal; it could also be used to estimate the therapeutic index (LD50/ED50) of drugs and xenobiotics [29]. In acute toxicity study, all the animals died within 48 h as soon as received the catechol derivatives I–IV dosages from 1600 to 2000 mg/kg bw due to loose motion and labored breathing. One fifth concentration was taken for sub acute toxic study from the LD50 values of each catechol derivative.
In the subacute toxicity study, the catechol derivatives I–III (300 mg/kg) treated groups and catechol derivatives IV (250 mg/kg) treated group showed no mortality and changes in animal behaviour, body and organ weights gain during the long term treatment. The changes in body weight have been used as an indicator of adverse effects of drugs and chemicals [30]. Therefore, the present results suggest that oral administration of catechol derivatives I–III at a dose of 300 mg/kg and catechol derivative IV at a dose 250 mg/kg are non toxic for 30 days of treatment.
There were no significant changes observed in various hematological parameters such as Hb, RBC and PCV in catechol derivatives I–IV treated groups compared to control group which indicates that catechol derivatives may not be toxic and does not affect the circulating red blood cells. But the levels of WBC count were significantly augmented in catechol derivatives IV treated group compared to control and other catechol derivatives I–III treated groups. This raise in the WBC level may indicate the impact of catechol derivatives in boosting the immune system of treated groups. This may be due to antioxidant potential of catechol derivatives. Because, two OH group present in the aromatic ring which plays a vital in scavenging free radical during diseased state. On the other hand, lipid oxidation is also initiated by free radicals. Moreover, free radicals can also interfere with immune system and reduces immune supremacy. Therefore, antioxidant is essential for protecting human being against oxidative damage caused by free radicals. In this connection, elevated levels of WBC count in catechol derivatives IV treated animals are obscure. But, there are slight structural variations among the catechol derivatives. Catechol derivatives I and IV are structurally similar, like that catechol derivatives II and III are structurally similar and their activities are also varied from one another. Catechol derivative IV possesses methyl group (CH3) in the aromatic ring and absence of double bond in the aliphatic side chain which was not present in the catechol derivatives II and III. These structural variations of catechol IV may have increased the WBC count.
The levels of glucose were significantly decreased in catechol derivatives I and IV treated groups when compared to control and other catechol derivatives II and III treated groups. This observation gives confidence to the use of the catechol derivatives as a hypoglycemic agent.
An increased level of protein, urea and creatinine in both plasma and urine are considered as significant markers of renal dysfunction [31]. In the present study, there were no significant changes observed in the levels of protein, urea and creatinine in both plasma and urine of all catechol derivatives treated rats when compared to control. These findings thereby illustrate the nontoxic effect of catechol derivatives on renal function/tissue in treated rats when compared to control groups.
The measurement of enzyme activities in tissue or body fluid plays a significant role in investigation and diagnosis of diseases [32]. Tissue enzyme analyze can indicate cellular damage long before structural damage of tissues can be picked up by conventional histological techniques. Such measurement can also give an insight into the site of cellular tissue damage as a result of attack by sub-acute or chronic use of plant derived drugs. Transaminases (GPT and GOT) and ALPs are good indices of liver and kidney damage, respectively [33]. There were no deleterious alternations found in the level of transaminases and ALPs in serum, liver and kidney of treated rats when compared with the control rats. Hence, from the above outcomes, it can be delineated that catechol derivatives did not aggravate any detrimental effects on liver and kidney tissues in treated groups. The organ protective efficiency of catechol derivatives is further confirmed by the histological assessments which are shown in Fig. 5.
The levels of plasma lipid, tissue lipid profile were significantly decreased in catechol derivatives I and IV treated groups when compared to catechol derivatives II and III drug, whereas, the levels of LDL cholesterol were found to be decreased whereas the levels of HDL cholesterol were significantly elevated in the catechol derivatives I and IV treated rats. This showed that the catechol derivatives had some beneficial effects by reducing cardiovascular risk factors and thereby it acts as a protective factor against coronary heart disease.
Histopathological examination of, liver and kidney from both treated and control animals showed normal architecture, suggesting no detrimental changes and morphological turbulence were caused on the administration of catechol derivatives for 30 days. This study reveals that the compound at low and moderate doses does not provoke the toxic effects in the animals’ tissues.
6. Conclusion
Based on the results obtained from the acute and sub acute toxic study, it may be suggested that single dose of (800 mg/kg) each catechol derivatives is practically non- toxic and safe. But, each catechol derivative goes beyond the 1000 mg/kg bw, produces adverse effect. Moreover, Low and moderate dose (300 mg/kg bw) of all catechol derivatives show some beneficial effects but predominant beneficial effects was observed particularly in catechol derivatives I and IV treated groups. This may be attributed to the presence of methyl group (CH3) in the aromatic region and absence of double bond in alkenes region. Both acute and sub acute studies clearly indicate that the catechol derivatives are potentially toxic but therapeutically effective.
Conflict of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
First author acknowledges the financial assistance provided by University Grant Commission, New Delhi, in the form of Research Fellowship in Sciences to Meritorious Students (RFSMS).
References
- 1.Fransworth N.R., Soejarto D.D. Potencial consequence of plant extinction in the United States on the current and future availability of prescription drugs. Econ. Bot. 1994;39:231–240. [Google Scholar]
- 2.Cordell G.A. Biodiversity and drug discovery–a symbiotic relationship. Phytochemistry. 2002;55:463–480. doi: 10.1016/s0031-9422(00)00230-2. [DOI] [PubMed] [Google Scholar]
- 3.Ogbonnia S., Adekunle A.A., Bosa M.K., Enwuru V.N. Evaluation of acute and subacute toxicity of Alstonia congensis Engler Apocynaceae) bark and Xylopia aethiopica (Dunal) A. Rich (Annonaceae) fruits mixtures used in the treatment of diabetes. Afr. J. Biotechnol. 2008;7:701–705. [Google Scholar]
- 4.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Pribitkin E.A. Herbal medicine and surgery. Semin. Integr. Med. 2005;3:17–23. [Google Scholar]
- 6.Burns M.M. Alternative medicine: herbal preparation. Clin. Pediatr. Emerg. Med. 2000;1:186–190. [Google Scholar]
- 7.McKay D.L., Blumberg J.B. A review of the bioactivity of South African herbal teas: roobos (Aspalathus linearis) and Honey comb (Cyclopia Intermedia) Phytother. Res. 2007;21:1–16. doi: 10.1002/ptr.1992. [DOI] [PubMed] [Google Scholar]
- 8.Tedong L., Dzeufiet P.D.D., Dimo T., Asongalem E.A., Sokeng S.N., Flejou J.F., Callard P., Kamtchouing P. Acute and Subchronic toxicity of Anacardium occidentale Linn (Anacardiaceae) leaves hexane extract in mice. Afr. J. Tradit. Altern. Med. 2007;4:140–147. doi: 10.4314/ajtcam.v4i2.31194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Fragoso L., Reyes-Esparza J., Burchiel S.W., Herrera-Ruiz D., Torres E. Risks and benefits of commonly used herbal medicines in Mexico. Toxicol. Appl. Pharmacol. 2008;227:125–135. doi: 10.1016/j.taap.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundaram R., Muthu K., Nagaraj S., Shanthi P., Sachdanandam P. Isolation and characterization of catechol derivatives from Semecarpus anacardium seeds and their antibacterial potential in in vitro. Biomed Prev Nutr. 2014;4:177–180. [Google Scholar]
- 11.OECD . 2001. Guideline for Testing of Chemicals. 423 Acute Oral Toxicit–Acute Toxic Class Method. [Google Scholar]
- 12.Drabkin D.L.R., Austin J.N. Spectrophotometric studies spectrophotometric constants for common hemoglobin derivatives in human, dog and rabbit blood. J. Biol. Chem. 1932;98:719–733. [Google Scholar]
- 13.Chesbrough M., McArthur J. The English language book society and Churchill Livingstone; 1972. A Laboratory Manual for Rural Tropical Hospital; p. 145. [Google Scholar]
- 14.Wintrobe M.M. Macroscopic examination of the blood in retrospect. Am. J. Med. Sci. 1976;271:103–105. doi: 10.1097/00000441-197601000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O.H., Rosenbrough N.J., Farn A.L., Randall R.J. Protein measurement with Folin's phenol reagent. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.King J. The phosphohydrolases-acid and alkaline phosphatases. In: Van D., editor. Practical Clinical Enzymology. Nostrand Company Limited; London: 1956. pp. 191–208. [Google Scholar]
- 17.Natelson S., Scott M.L., Beffa C. A rapid method for the estimation of urea in biological fluid by means of the reaction between diacetely and urea. Am. J. Clin. Pathacol. 1951;21:275–281. doi: 10.1093/ajcp/21.3_ts.275. [DOI] [PubMed] [Google Scholar]
- 18.Caraway W.T. Uric acid. In: Seligson D., editor. Standard Methods of Clinical Chemistry. Academic Press; New York: 1956. pp. 191–208. [Google Scholar]
- 19.Owen J.A., Iggo T.B., Scandrett F.J., Stemart I.P. Determination of creatinine in plasma (or) serum and in urine. Biochem. J. 1954;58:426–437. doi: 10.1042/bj0580426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969;6:24–27. [Google Scholar]
- 21.Folch J., Less M., Stanley G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Parekh A.C., Jung D.H. Cholesterol determination with ferric chloride-uranyl acetate and sulphuric acid-ferrous sulphate reagent. Anal. Chem. 1970;42:1423–1427. [Google Scholar]
- 23.Rice E.W. Triglyceride in serum. In: Roderick P., MacDonald C.H., editors. Standards Methods of Clinical Chemistry. 6th ed. Academic Press; New York: 1970. pp. 215–222. [Google Scholar]
- 24.Van Handel E. Suggested modifications of the micro determination of triglycerides. Clin. Chem. 1961;7:249–251. [PubMed] [Google Scholar]
- 25.Itaya K. A more sensitive and stable colorimetric determination of free fatty acid in plasma. J. Lipid Res. 1977;18:653–665. [PubMed] [Google Scholar]
- 26.Rouser G.R., Fleisher S., Yamanoro A. Two dimensional thin layer chromatographic separation of lipids and determinations of phospholipids by phosphorous analysis of spot. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 27.Parvathi S., Brindha R. vol. XXII. 2003. Ethnobotanical medicines of Anaimalai union. (Ancient Science of Life). [PMC free article] [PubMed] [Google Scholar]
- 28.Mythilypriya R., Shanthi P., Sachdanandam P. Oral acute and subacute toxicity studies with Kalpaamruthaa, a modified indigenous preparation on rats. J. Health Sci. 2007;53:351–358. [Google Scholar]
- 29.Rang H.P., Dale M., Ritter J. In: Pharmacology. 4th ed. USA, editor. Churchill Livingstone; New York: 2001. [Google Scholar]
- 30.Teo S., Stirling D., Thomas S., Hoberman A., Kiorpes A., Khetani V. A 90-day oral gavage toxicity study of D-methylphenidate and D: L-methylphenidate in Sprague-Dawley rats. Toxicology. 2002;179:183–196. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim M.Y., Abdul1 A.B., Ibrahim T.A.T., Abdelwahab S.I., Elhassan M.M., Syam M.M. Evaluation of acute toxicity and the effect of single injected doses of zerumbone on the kidney and liver functions in Sprague Dawley rats. African J. Biotec. 2010;28:4442–4450. [Google Scholar]
- 32.Malomo S.O. Toxicological implication of ceftriaxone administration in rats. Niger. J. Biochem. Mol. Biol. 2000;15:33–38. [Google Scholar]
- 33.Martin D.W., Mayes A., Rodwel Y.W. Harper's Review of Biochemistry. 18th ed. Lange Medical; CA: 1981. Transaminases; p. 61. [Google Scholar]