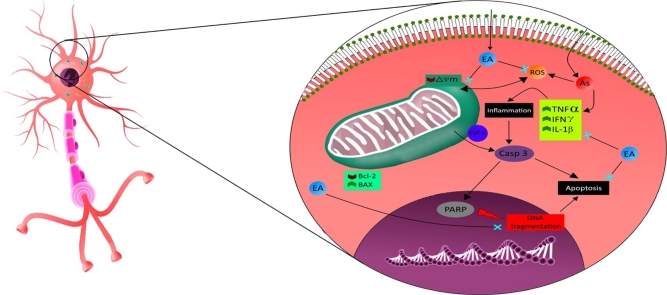
Graphical abstract
Keywords: Arsenic, Neurotoxicity, Ellagic acid, ROS generation, Inflammation, Mitochondrial membrane potential
Highlights
-
•
Ellagic acid mitigates arsenic mediated genotoxicity in rat brain hippocampi.
-
•
Ellagic acid ameliorates arsenic induced exacerbation in levels of ROS and pro-inflammatory cytokines in rat brain hippocampi.
-
•
Ellagic acid has the propensity to modulate mRNA expression of BAX, Bcl2 and caspase3, suggestive of its neuroprotective efficacy.
Abstract
Arsenic, being a global pollutant needs a potential remedy which could fight against its associated toxicities. Ellagic acid (EA) is a known agent for its anti-inflammatory, antioxidant and antiapoptotic effects, and it is commonly found in fruits. The present study is designed to determine protective efficacy of EA against arsenic induced toxicity with special mention to inflammation and mitochondrial dysfunction in hippocampi of wistar rats. Rats were pre-treated with EA (20 and 40 mg/kg b.wt; p.o. for 11 days) along with arsenic (10 mg/kg; p.o. for 8 days). Total reactive oxygen species level and mitochondrial membrane potential were analyzed using flow cytometry. Protein and mRNA expression of apoptotic and inflammatory markers were also evaluated in rat hippocampus. Our results show that arsenic exposure increased total ROS generation and DNA fragmentation, decreased mitochondrial membrane potential alongwith an increase in expression of pro-apoptotic and inflammatory markers. suggesting that EA complementation downregulated total ROS generation dose dependently. Apoptotic markers, BAX and Bcl2 as well as inflammatory markers, IL-1β, TNFα, INFγ got altered significantly on its administration. Moreover, it also attenuated effects on mitochondrial membrane potential. Based on our findings, EA might substantiate to be a budding therapeutic candidate against arsenic induced neurotoxicity.
1. Introduction
Arsenic, a toxic metalloid broadly distributed in soil and water bodies due to its natural existence and anthropogenic sources [1,2]. It is suggested that inorganic forms of arsenic are more toxic than organic forms. Numerous experimental studies have been carried out to investigate morphological, physiological, pharmacological and neurochemical effects following its exposure. Arsenic effortlessly crosses blood brain barrier and its concentration builds up in brain parts to exert its neurotoxic effects [[3], [4], [5]].
Arsenic has been reported to modify levels of biogenic amines and affects the behavioral and neurochemical functions in developing and adult rat brains [6,7]. Experimental studies on rodents have revealed learning and memory insufficiency with alteration in motor behavior [8,9]. Exposure to sodium arsenate/arsenite through drinking water decreased AChE activity in brain of rats and also inhibited synthesis and release of acetylcholine in brain slices [6,10]. Interestingly, decrease in AChE activity following arsenic and gallium arsenite treatment was correlated with impairment in learning and memory in rats [11]. Sub-chronic exposure of sodium arsenite augmented dopamine and 5-hydroxyindoleacetic acid (5-HIAA) levels in mid brain and cortex [4].
Oxidative stress is suggested to be a likely mechanism involved in arsenic neurotoxicity [1,12,13]. Arsenic enhances generation of free radicals including hydroxyl radicals, superoxide anions, dimethyl arsenic peroxy radical, dimethyl arsenic radical, nitric oxide. It also impairs the antioxidant system in brain and other biological tissues thereby increasing the oxidative stress [[14], [15], [16]]. Arsenic has high affinity towards GSH which boosts its oxidative stress vulnerability by creating an imbalance in the cellular redox status [[17], [18], [19]].
Arsenic is a major health concern according to WHO which has recommended chelation therapy alongwith antioxidants supplementation to treat related intoxications [20]. Thus, it is extremely necessary that new compounds are screened for management of arsenic neurotoxicity and other related dysfunctions.
Ellagic acid, EA (2,3,7,8-tetrahydroxybenzopyrano [5,4,3-cde] benzopyran-5-10-dione) is a phenolic compound and an excretion product of many plant species. It is generally present in fruits and nuts including blueberries, blackberries, raspberries, strawberries and walnuts [[21], [22], [23]]. It has a variety of beneficial effects including anti-oxidant, anti-inflammatory, anti-fibrotic and anti-cancer properties [[24], [25], [26]]. EA’s protective effect has been attributed to its superoxide and hydroxide scavenging action. Further, EA has also been reported to inhibit lipid peroxidation and 8-OhdG formation both in vitro and in vivo [[27], [28], [29], [30]].
In view of its potential therapeutic value, EA may have the propensity for the management of arsenicosis and related neurotoxicological implications, though the molecular mechanism pertaining to its anti-oxidative efficacy is largely unknown. It is in this context that we carried out the present work to study the protective role of EA in arsenic-induced neurotoxicity, genotoxicity, inflammation and mitochondrial dysfunction with special reference to ROS generation.
2. Materials and methods
2.1. Chemicals and reagents
DCFDA, Rhodamine 123, TRI® reagent, corn oil and sodium arsenate were purchased from Sigma–Aldrich Chemicals Pvt. Ltd (MO, USA). EA was purchased from TCI, Japan. M-MLV Reverse Transcriptase (AM2043), Hibernate®, B27®, Glutamax-I, 2X Mastermix, RNase inhibitor, dNTP mix and Random primers were purchased from Thermo Fisher (CA, USA). CBA kits for TNFα (Cat no. 558309) and INF-γ (Cat no. 558305) were purchased from BD Biosciences, USA. Ac-DEVD-pNA substrate was bought from Santa Cruz, USA.
2.2. Animals
Wistar rats (200 ± 25 g) were used for the current study. Animals were procured from Central Animal House facility of Jawaharlal Nehru Medical College, Aligarh Muslim University and housed individually in cages maintained under suitable conditions with temperature (25 ± 1 °C), humidity (60 ± 10%) in a well ventilated room with 12-h light and dark cycle. Rats were provided ad-libitum diet and water. All animal experimental procedures were approved by the Institutional Animal Ethics Committee and carried out as per CPCSEA guidelines and the doses and schedules of EA and arsenic were based on the pilot studies and previously published reports [1,[31], [32], [33]].
2.3. Experimental design
Rats were randomly divided into four groups containing 12 animals each.
Group 1: Control, Vehicle only, [C]
Group 2: Arsenic as sodium arsenate (10 mg/kg; p.o in drinking water) for 8 days [As]
Group 3: EA1 (20 mg/kg, p.o) pre-treatment for 3 consecutive days followed by administration with Arsenic up to 11th day [As + EA1]
Group 4: EA2 (40 mg/kg; p.o) pre-treatment for 3 consecutive days followed by administration with Arsenic up to 11th day [As + EA2]
2.4. Tissue preparation for biochemical analysis
At the end of the experimental period, animals were anaesthetized using 400 mg/kg chloral hydrate and sacrificed by cervical dislocation. Brains were dissected on ice cold saline to remove blood and for harvesting hippocampus. Hippocampi were immediately homogenized in ice cold phosphate buffer (0.1 mM, pH 7.4). The homogenate was centrifuged at 10,000g for 20 min at 4 °C to get tissue supernatant, which was used for estimation of caspase 3 activity and bead based assay for pro-inflammatory cytokines.
2.5. Preparation of single cell suspension for flow cytometry and comet assay
Freshly isolated hippocampi were kept in ice cold Hibernate A® medium (supplemented by B27® serum free supplement and Glutamax-I). Single cell suspension was obtained by mechanical dissociation and trituration using a pipette and was passed through 70 μm cell strainer followed by centrifugation at 450g for 10 min at 4 °C. The supernatant was discarded and the pellet was resuspended in Hibernate A® and kept at 37 °C for incubations with respective fluorescent probes.
2.5.1. Assessment of total reactive oxygen species (ROS) generation
2′,7′-Dichlorofluorescin diacetate (DCFDA) is a cell-permeable non-fluorescent probe. DCFDA measures hydroxyl, peroxyl and other reactive oxygen species (ROS) levels within the cell. The cells were incubated with 20 μM DCFDA for 40 min at 37 °C in the dark. The incubation was terminated and cytometric acquisition was done using BD FACS ARIA II flow Cytometer and results were analyzed using FACS DIVA® analysis software.
2.5.2. Assessment of change in mitochondrial membrane potential (ΔYm)
Rhodamine 123 is a cationic fluorescent dye that gets distributed according to the negative membrane potential across the mitochondrial inner membrane in respiring mitochondria. Loss of potential results in loss of the dye and, therefore, the fluorescence intensity. It has been used to monitor mitochondrial function in living cells. The cells were incubated with 5 μM Rhodamine 123 for 30 min at 37 °C in the dark. The incubation was terminated and cytometric acquisition was done using BD FACS ARIA II flow Cytometer and results were analyzed using FACS DIVA® analysis software.
2.5.3. Assessment of DNA fragmentation by comet assay
DNA damage was assessed using the alkaline comet assay as described by Singh et al. (1988) with slight modifications [34]. Cell suspensions with 0.5% low melting point agarose (LMPA) were overlaid on slides precoated with a fine layer of 1% normal melting agarose (NMA). A third layer of 1% LMPA was poured and slides were immersed overnight at 4 °C in lysing solution containing (2.5 M NaCl, 100 mM EDTA, 10 mM Tris base, 0.2 mM NaOH, 0.1% Triton X-100 and DMSO; pH 10) followed by an alkali unwinding solution for 20 min. Electrophoresis was performed at 25 V in 1X TBE buffer for 45 min. Slides were dried and stained immediately with propidium iodide (1X) solution. Photographs were obtained at 40X using Nikon Eclipse Ci-L fluorescence microscope.
2.6. RNA isolation, cDNA synthesis and reverse transcriptase PCR
RNA was isolated from hippocampi of rats of each group using TRI® Reagent (Sigma-Aldrich, MO, USA) as mentioned in the manufacturer guidelines. High quality 2 μg RNA was reverse transcribed into cDNA as per manufacturer guidelines. Reaction mixture containing denatured RNA, random primers, 10X reaction buffers, dNTP mix (10 mM each), RNase inhibitor and M-MLV RT enzyme was incubated at 42 °C. The resulting cDNA was used as a template for semi-quantitative PCR using TCS Biocycler-1000. Amplification was done using specific primers (1 μM). Primer sequences used were β-actin (Forward CAACCTTCTTGCAGCTCCTC; Reverse TTCTGACCCATACCCACCAT), BAX (Forward GCCTCCTTTCCTACTTCGGG; Reverse CTTTCCCCGTTCCCCATTCA), Bcl2 (Forward CGACTTTGCAGAGATGTCCA; Reverse CATCCACAGAGCGATGTTGT), IL-1β (Forward TCAAGCAGAGCACAGACCTG; Reverse ACTGCCCATTCTCGACAAGG), TNFα (Forward GAATTGTGGCTCTGGGTCCA; Reverse TCCAGTGAGTTCCGAAAGCC), IFNγ (Forward TGTCATCGAATCGCACCTGA; Reverse TCAGCACCGACTCCTTTTCC). PCR was programmed for 35 cycles; denaturation at 95 °C, annealing at 58 °C and renaturation at 72 °C. The amplicons were run on 1.7% agarose gel. β-actin was used as an internal control. Band intensity analysis was done using Image J software (version 1.50, NIH, USA).
2.7. Bead based immunoassay for TNFα and INFγ
Pro-inflammatory cytokines, TNFα and IFNγ in hippocampi of rats were analyzed and quantitated according to instructions provided with the CBA kit (BD Biosciences, U.S.A.).The data was acquired on LSR-II Flow Cytometer at BD FACS Academy, Jamia Hamdard, New Delhi, India and analyzed using FCAP ARRAY software version 3.0 (BD Biosciences, U.S.A.).
2.8. Assay for caspase 3 activity
Caspase 3 activity was estimated by using Ac-DEVD-pNA substrate (Santa Cruz Biotech, CA, USA). Tissue supernatant was first diluted in assay buffer (1X) containing 20 mM HEPES (pH 7.4), 0.1% CHAPS, 5 mM DTT and then DEVD-pNA substrate (2 mM) was added. The reaction plate was incubated at 37 °C overnight in a humidified incubator. Subsequently, contents of the plate were read at 405 nm in a BioRad Elisa plate reader.
2.9. Evaluation of neuronal damage by cresyl violet
The animals were perfused transcardially with ice cold PBS (0.1 M, pH 7.4) followed by cold 4% formalin in PBS (0.1 M; pH 7.4). Brains were removed quickly, post fixed in 4% Neutral buffered formalin solution for 48 h, dehydrated in graded alcohol and embedded in wax. Coronal sections containing hippocampus and 6 μm thickness were dewaxed and sections were stained using 1% cresyl violet solution for 5–8 min. The sections were then rinsed in distilled water and differentiated in alcohol followed by clearing in xylene and mounting in DPX. Images were acquired using light microscope (Nikon ƐCIL, Japan).
2.10. Statistical analysis
Results were expressed as mean ± standard deviation (SD). Data was analyzed using analysis of variance (ANOVA) followed by Tukey’s as a post hoc test. Values of p ≤ 0.05 were considered as significant. Significant differences were marked with (*) when compared with control and with (#) when compared with any other group except control. All statistical analyses were performed using Graph pad prism 7 software (Graph Pad Software Inc., San Diego, CA).
3. Results
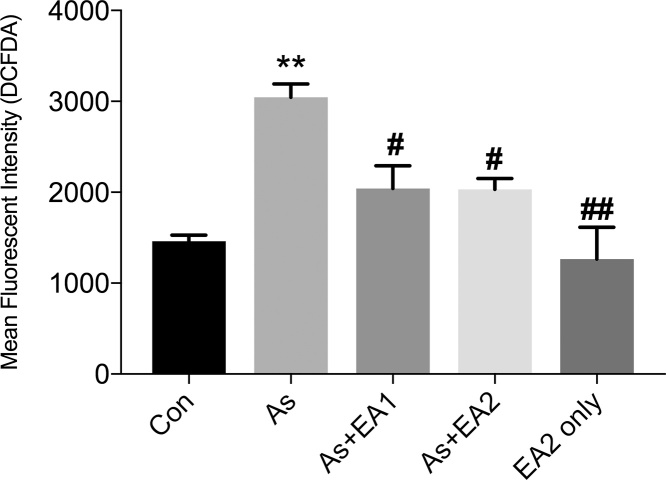
3.1. Effect of EA on arsenic mediated ROS
To establish oxidative stress, we estimated total ROS content through DCFDA flow cytometry. Arsenic ingestion to rats has significantly increased (*p ≤ 0.05) ROS generation in hippocampus region (Fig. 1). Pretreatment with EA to arsenic treated animals restrained (#p ≤ 0.05) ROS generation in a dose dependent manner (Fig. 1 c, d).
Fig. 1.
Effect of ellagic acid on arsenic induced ROS generation by flow cytometric analysis using DCFDA. Cells were treated with 20 μM DCFDA for 40 min at 37 °C. Data was expressed as Mean ± SEM. Significant differences were expressed as (**p ≤ 0.01) when comparison was done in control and arsenic and (#p ≤ 0.05) when compared with arsenic ellagic acid pretreatment groups, As + EA1 (20 mg/kg) and As + EA2 (40 mg/kg) respectively. No significant difference was observed between control and EA2 only group.
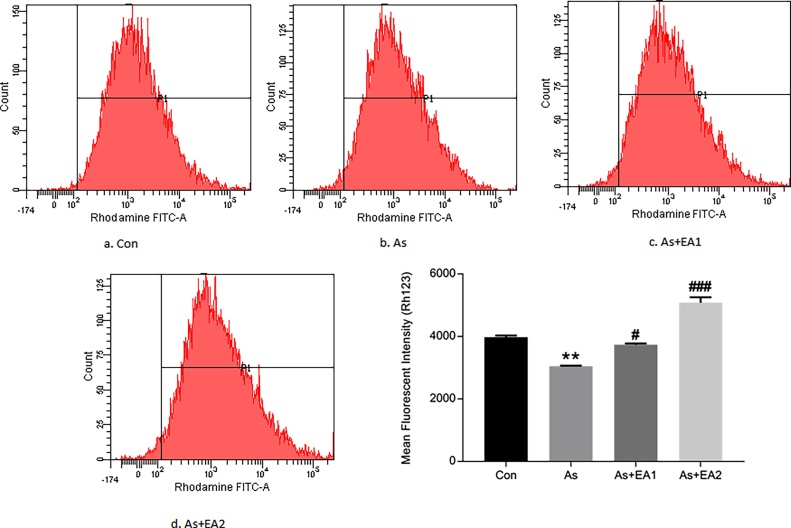
3.2. Effect of EA on arsenic mediated disruption of MMP (ΔΨm)
MMP (ΔΨm) is an important hallmark of mitochondrial health as its disruption is an early and unavoidable event preceding mitochondrial dysfunction and is generally related with predisposition of cells towards apoptosis. Arsenic caused a remarkable decrease (**p ≤ 0.01) in MMP, while EA supplementation significantly restored MMP (ΔΨm) dose dependently viz., #p ≤ 0.05 at 20 mg/kg and ###p ≤ 0.001 at 40 mg/kg (Fig. 2).
Fig. 2.
Effect of ellagic acid on the arsenic related loss of Mitochondrial Membrane Potential (Δψm) by flow cytometric analysis using Rh 123. Cells were treated with 5 μM Rh 123 for 30 min at 37 °C. Data was represented as Mean ± SEM. Significant differences were expressed as (**p ≤ 0.01) when comparison was done between arsenic and control groups and (#p ≤ 0.05; ##p ≤ 0.01) when compared with arsenic and ellagic acid pretreatment groups As + EA1 (20 mg/kg) and As + EA2 (40 mg/kg) respectively.
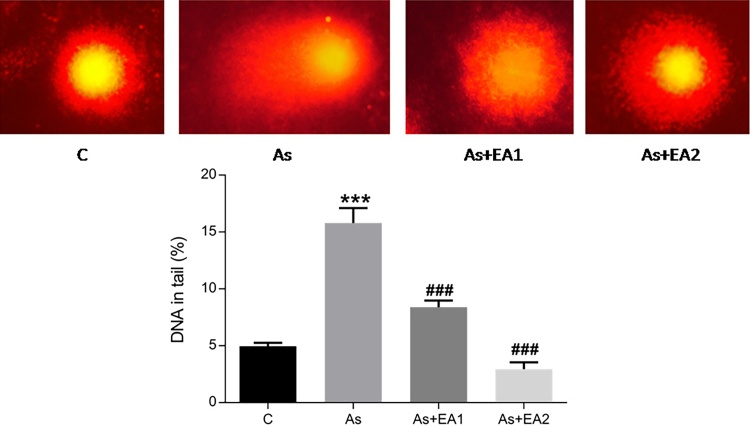
3.3. Effect of EA against arsenic induced DNA damage
Comet assay was done for the assessment of arsenic induced genotoxicity. In terms of percent DNA in tail, arsenic exposure group showed a remarkable amount of DNA fragmentation (***p ≤ 0.001) in the hippocampal neurons as evident from Fig. 3(b), while EA pretreatment mitigated the DNA damage associated with it as shown in Fig. 3 c and d (###p ≤ 0.001).
Fig. 3.
Comet assay analysis of the effect of ellagic acid on arsenic induced DNA fragmentation. Comet tail length and tail intensities were quantified using Cometscore v1.5 software. The data was expressed as Mean ± SEM. Significant differences in the tail length were expressed as (***p ≤ 0.001) when comparison was done in between arsenic and control groups and (###p ≤ 0.001) when compared with arsenic and ellagic acid pretreatment groups As + EA1 (20 mg/kg) and As + EA2 (40 mg/kg) respectively.
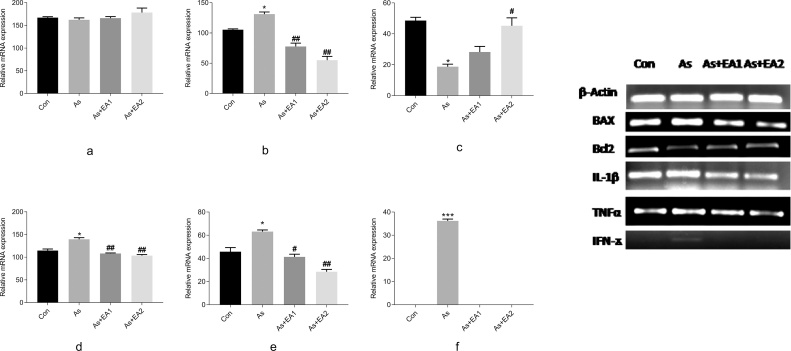
3.4. Effect of arsenic and EA on mRNA expression of apoptotic and inflammatory markers
Our results show that arsenic exposure has significantly increased apoptosis as evidenced by increased expression of proapoptotic BAX and decreased expression of Bcl2 markers respectively (Fig. 4). Likewise, increased expression of inflammatory markers IL-1β, TNFα, and IFNϫ was observed in the arsenic exposed group. Interestingly, EA pretreatment significantly protected the mRNA levels of all these markers with the higher concentration becoming more effective (Fig. 4).
Fig. 4.
Relative mRNA expression of β-actin, BAX, Bcl2, IL-1β, TNFα and INFγ shown in Fig. 4 (a,b,c,d,e,f) respectively. Band intensities are calculated using Image J (version 1.50, NIH, USA). Significant differences in intensities were expressed as (* ≤ 0.05, ***p ≤ 0.001) when comparison was done between arsenic and control groups. Fig. 4 (a,b,c,d,e), Fig. 4(f); (##p ≤ 0.01, ##p ≤ 0.05) when compared with arsenic and ellagic acid pretreatment groups As + EA1 (20 mg/kg) and As + EA2 (40 mg/kg) Fig. 4 (b,d,e) and 4 (c) respectively.
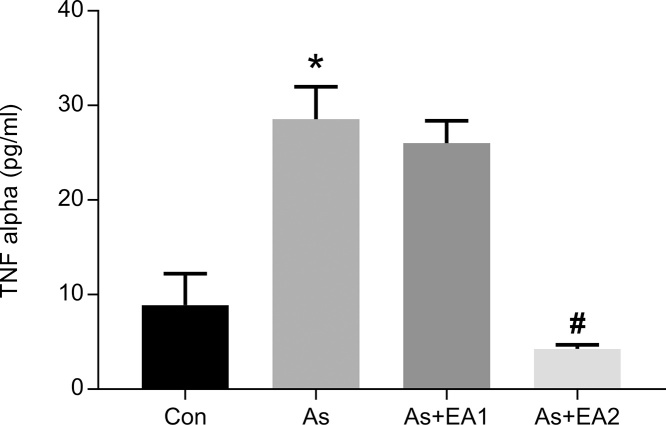
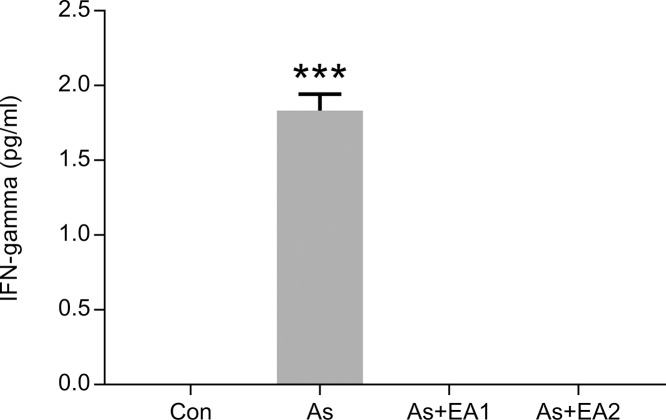
3.5. Effect of arsenic and EA on bead-based assay of pro-inflammatory cytokines-TNFα and IFNγ
Pro-inflammatory cytokine level, TNF-α in tissue supernatant of rat hippocampi was significantly higher ***p ≤ 0.001 in the arsenic exposed group compared with the untreated control, while it declined dose dependently with a significant decrease of #p ≤ 0.05 at 40 mg/kg in the EA treated animals (Fig. 5). Moreover, IFN-γ only showed its expression in the arsenic treated group (see Fig. 6) with a remarkable increase (***p ≤ 0.001), which is a clear cut indicator of inflammation in arsenic neuropathies. Other treatment groups as well as negative control demonstrated undetectable expression of IFN-γ as shown in Fig. 6.
Fig. 5.
Effect of ellagic acid and arsenic on hippocampal TNFα levels. TNFα protein level in hippocampal lysate of rat brain were assessed using CBA kit (BD Biosciences). Results are expressed as protein levels of TNFα in pg/ml. Significant differences were expressed as (*p ≤ 0.05) when comparison was done in between arsenic and control groups and (#p ≤ 0.05) when compared with arsenic and ellagic acid pretreatment groups As + EA2 (40 mg/kg) respectively.
Fig. 6.
Effect of ellagic acid and arsenic on hippocampal INFγ levels. INFγ in hippocampal lysate of rat brain were assessed using CBA kit (BD Biosciences). Results are expressed as protein levels of INFγ in pg/ml. Significant differences were expressed as (***p ≤ 0.001) when comparison was done between arsenic and control groups.
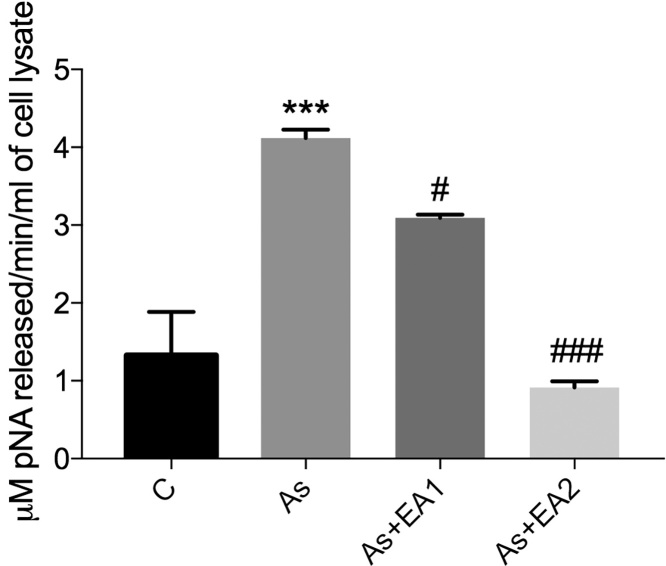
3.6. Effect of EA on arsenic induced caspase 3 activity
Caspase 3 activity is a crucial marker of apoptosis. It was found elevated in the arsenic exposed group (***p ≤ 0.001), while it got drastically reduced in arsenic treated animals pretreated with different doses of EA (##p ≤ 0.01 at 20 mg/kg and ###p ≤ 0.001 at 40 mg/kg respectively) as shown in Fig. 7.
Fig. 7.
Effect of ellagic acid on the arsenic induced exacerbation in Caspase 3 activity using Ac-DEVD-pNA a selective probe for caspase 3 having maximum absorbance at 405 nm. Activity has been measured in terms of μM of pNA released/min/ml of cell lysate. Data was represented as Mean ± SEM. Significant differences were expressed as (***p ≤ 0.001) when comparison was done between arsenic and control groups and (#p ≤ 0.05, ###p ≤ 0.001) when compared with arsenic and ellagic acid pretreatment groups As + EA1 (20 mg/kg) and As + EA2 (40 mg/kg) respectively.
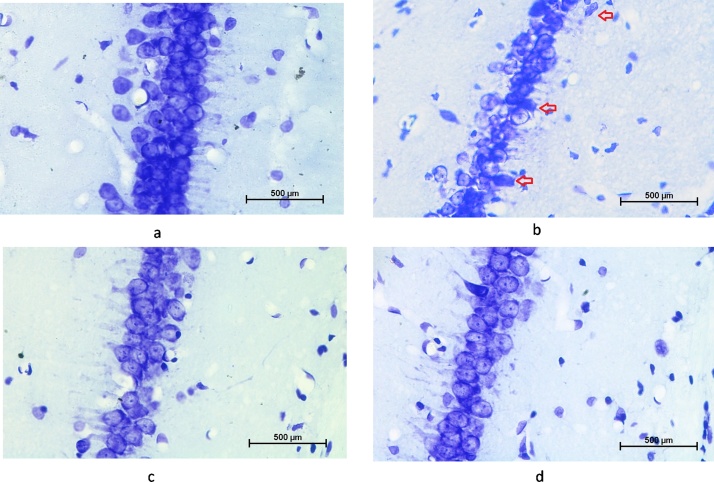
3.7. Assessment of hippocampal regions using cresyl violet (CV) staining
The CV stained sections of hippocampus (CA1) from control have shown a distinct layering pattern as compared to the As administered group. Sequentially arranged pyramidal cells with preserved structural integrity and packaging were observed in control (Fig. 8a). While, As-exposure group has shown disintegrated pyramidal cell layer with greater number of pyknotic cells, ectopic and misaligned cells with improper axonal processes as compared to control animals. EA pre-treatment significantly reversed the As-induced alterations via maintaining the structural integrity of hippocampal neurons (Fig. 8c, d).
Fig. 8.
Photomicrograph of CV stained coronal section of hippocampus showing pyramidal cells in pyramidal cell layer of CA1 region of the hippocampus. Comparison between Fig. a (control) and Fig. b, Arsenic exposure showed loss in the granule cell layer thickness, cell shrinkage, misaligned pyramidal neuron and pycnotic cells (arrow head). EA pre-treatment showed significant restoration in cell morphology and thickness of granule cell layer (Fig c,d).
4. Discussion
In the present study, we have investigated the mitigatory competence of EA in arsenic exposure associated mitochondrial dysfunction, ROS generation, DNA fragmentation and apoptosis in hippocampus of arsenic administered rats.
Our results show that pre and co-administration of ellagic acid at 20 mg/kg and 40 mg/kg brought about a marked reduction in the total ROS generation in a dose dependent manner which is consistent with the previous studies [[35], [36], [37]]. The arsenic exposed group was found to have increased levels of ROS. It has been reported that higher levels of ROS within the cells cause mitochondrial membrane depolarization or decrease in mitochondrial membrane potential, and excessive ROS generated under certain pathological conditions act as an intermediary for apoptotic signaling pathway activation [[38], [39], [40]]. Moreover, mitochondrial membrane depolarization or MMP has been known to be one of the earliest intracellular events of apoptosis and often considered as the “point of no return” in the cascade of events terminating into cell death [41,42]. Thus, our results demonstrated that disproportionate ROS production (Fig. 1) concomitant with decreased MMP (Fig. 2) occurs in arsenic exposure. Furthermore, EA mediated decrease in the levels of ROS might have contributed to amelioration of mitochondrial dysfunction (ΔΨm).
Multiple studies have reported a decrease in ΔΨm following arsenic exposure; however, the underlying mechanism still remains obscure. ΔΨm is peculiarly important in physiological cell functioning as its maintenance is essential for ATP synthesis [43]. Moreover, ΔΨm also provides energy for Ca2+ uptake into mitochondria as it functions as cellular Ca2+ buffer [44]. However, under certain conditions, most notably increased ROS generation the concomitant oxidative stress can trigger opening of mitochondrial permeability transition pore (mPTP) causing collapse of ΔΨm [45,46]. In the present study, EA restored ΔΨm in a dose dependent manner in arsenic treated rats which may be attributed to its antioxidative properties, presumably by a direct effect on the redox status of mitochondrion (Fig. 2). Finally, our results suggest that EA can act as a neuroprotective candidate by directly and selectively targeting mitochondria.
Arsenic exposure seems to induce cell death through ROS mediated mitochondrial pathway which would finally sets in BAX and Caspase 3 activation (Fig. 4). Moreover, a significant decrease in mRNA expression of another apoptotic marker, namely Bcl2 was also observed. These apoptotic markers got altered accordingly in EA treated rats. Furthermore, increased ROS control expression of pro-inflammatory cytokines through activation of protein kinase C and mitogen-activated protein kinase as evidenced by elevated mRNA levels of pro-inflammatory markers, IL-1β, IFNϫ and TNFα (Fig. 4) and protein synthesis of IFNϫ and TNFα (Fig. 5, Fig. 6) respectively in arsenic administered rats. Inflammation plays a key role in many neurodegenerative conditions and neuronal damage [47,48]. One of the chief features of inflammation is that IFNϫ, TNFα, and IL-1β are responsible for microglial activation and ROS generation [49,50].
Comet assay conducted on hippocampal cells confirmed apoptosis specifically in terms of DNA fragmentation in arsenic exposed rats exhibiting elongated tails of DNA comets. It is believed that increased caspase 3 enzyme activity too, can induce DNA fragmentation through PARP activation [51]. To further confirm damage caused by As, considerable loss of pyramidal neuronal layers in Cornu ammonis 1 (CA1) following Nissl staining of hippocampal region was observed. This loss suggests an alteration of the neuronal morphology in rat’s hippocampus in As-administered rats. This damage was attenuated dose-dependently by EA, which clearly show cytoprotection and cellular integrity (Fig. 8). Our results are consistent with the previous studies of Uzar et al. (2012) [52].
Interestingly, in our study EA pre- and co-administration dose-dependently downregulated DNA fragmentation, caspase 3 activity and mRNA expression of IL-1β, IFNϫ, TNF α and BAX along with upregulation of Bcl2 mRNA expression in arsenic exposed rats (Fig. 4).
5. Conclusion
In view of the above results, we propose that EA could serve as a strong candidate for diet supplementation alongwith chelation therapy in arsenic related neurotoxicities as it shows all propensities to modulate hippocampal associated mitochondrial dysfunction, inflammation and apoptosis associated with it.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors acknowledge Department of Science and Technology, Government of India for supporting F.F. through its INSPIRE Fellowship Programme (IF-120626). We deeply acknowledge BD-FACS Academy, Jamia Hamdard for conducting flow cytometric analysis of our samples and also to Dr. Pradeep Kumar Rai for his expert help in analyzing results.
References
- 1.Das A.K., Bag S., Sahu R., Dua T.K., Sinha M.K., Gangopadhya M. Protective effect of Corchorus olitorius leaves on sodium arsenite-induced toxicity in experimental rats. Food Chem. Toxicol. 2010;48:326–335. doi: 10.1016/j.fct.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez V.M., Jiménez-Capdeville M.E., Giordano M. The effects of arsenic exposure on the nervous system. Toxicol. Lett. 2003;145:1–18. doi: 10.1016/s0378-4274(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 3.Jin Y., Xi S., Li X., Lu C., Li G., Xu Y., Qu C., Niu Y., Sun G. Arsenic speciation transported through the placenta from mother mice to their newborn pups. Environ. Res. 2006;101:349–355. doi: 10.1016/j.envres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez V.M., Carrizales L., Jimenez-Capdeville M.E., Dufour L., Giordano M. The effect of sodium arsenite exposure on behavioral parameters in the rat. Brain Res. Bull. 2001;55:301–308. doi: 10.1016/s0361-9230(01)00477-4. [DOI] [PubMed] [Google Scholar]
- 5.Rosado J.L., Ronquillo D., Kordas K., Rojas O., Alatorre J., Lopez P. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ. Health Perspect. 2007;115:1371–1375. doi: 10.1289/ehp.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannan G.M., Tripathi N., Dube S.N., Gupta M., Flora S.J. Toxic effects of arsenic (III) on some hematopoietic and central nervous system variables in rats and guinea pigs. J. Toxicol. Clin. Toxicol. 2001;39:675–682. doi: 10.1081/clt-100108508. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez V.M., Dufour L., Carrizales L., Diaz-Barriga F., Jimenez-Capdeville M.E. Effects of oral exposure to mining waste on in vivo dopamine release from rat striatum. Environ. Health Perspect. 1998;106:487–491. doi: 10.1289/ehp.106-1533203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J.H., Qiu Z.Q., Shu W.Q., Zhang Y.Y., Zhang L., Chen J.A. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 2009;184:121–125. doi: 10.1016/j.toxlet.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Li S., Piao F., Hong Y., Liu P., Zhao Y. Arsenic down-regulates the expression of Camk4, an important gene related to cerebellar LTD in mice. Neurotoxicol. Teratol. 2009;31:318–322. doi: 10.1016/j.ntt.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 10.Tripathi N., Kannan G.M., Pant B.P., Jaiswal D.K., Malhotra P.R., Flora J.S. Arsenic-induced changes in certain neurotransmitter levels and their recoveries following chelation in rat whole brain. Toxicol. Lett. 1997;92:201–208. doi: 10.1016/s0378-4274(97)00058-1. [DOI] [PubMed] [Google Scholar]
- 11.Flora S.J., Bhatt K., Mehta A. Arsenic moiety in gallium arsenide is responsible for neuronal apoptosis and behavioral alterations in rats. Toxicol. Appl. Pharmacol. 2009;240:236–244. doi: 10.1016/j.taap.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Flora S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Sinha M., Manna P., Sil P.C. Protective effect of arjunolic acid against arsenic-induced oxidative stress in mouse brain. J. Biochem. Mol. Toxicol. 2008;22:15–26. doi: 10.1002/jbt.20209. [DOI] [PubMed] [Google Scholar]
- 14.Barchowsky A., Dudek E.J., Treadwell M.D., Wetterharn K.E. Arsenic induces oxidant stress and NF-κB activation in cultured aortic endothelial cells. Free Radic. Biol. Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 15.Gurr J.R., Yih L.H., Samikkannu T., Bau D.T., Lin S.Y., Jan K.Y. Nitric oxide production by arsenite. Mutat. Res. 2003;533:173–182. doi: 10.1016/j.mrfmmm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Lynn S., Gurr J.R., Lai H.T., Jan K.Y. NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ. Res. 2000;86:514–519. doi: 10.1161/01.res.86.5.514. [DOI] [PubMed] [Google Scholar]
- 17.Aposhian H.V., Aposhian M.M. Arsenic toxicology: five questions. Chem. Res. Toxicol. 2006;19:1–15. doi: 10.1021/tx050106d. [DOI] [PubMed] [Google Scholar]
- 18.Shila S., Kokilavani V., Subathra M., Panneerselvam C. Brain regional response in antioxidant system to a-lipoic acid in arsenic intoxicated rat. Toxicology. 2005;210:25–36. doi: 10.1016/j.tox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Shila S., Ramanathan K., Tamilselvan J., Panneerselvam C. Protein oxidative damage in arsenic induced rat brain: influence of DL-αlipoic acid. Toxicol. Lett. 2005;155:27–34. doi: 10.1016/j.toxlet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 20.WHO . WHO Press; Geneva: 2010. Exposure to Arsenic: A Major Public Health Concern. [Google Scholar]
- 21.Anderson K.J., Teuber S.S., Gobeille A., Cremin P., Waterhouse A.L., Steinberg F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001;131:2837–2842. doi: 10.1093/jn/131.11.2837. [DOI] [PubMed] [Google Scholar]
- 22.Sellappan S., Akoh C.C., Krewer G. Phenolic compounds and antioxidant capacity of Georgia grown blueberries and blackberries. J. Agric. Food Chem. 2002;50:2432–2438. doi: 10.1021/jf011097r. [DOI] [PubMed] [Google Scholar]
- 23.Wada L., Ou B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002;50:3495–3500. doi: 10.1021/jf011405l. [DOI] [PubMed] [Google Scholar]
- 24.Iino T., Tashima K., Umeda M., Ogawa Y., Takeeda M., Takata K., Takeuchi K. Effect of ellagic acid on gastric damage induced in ischemic rat stomachs following ammonia or reperfusion. Life Sci. 2002;70:1139–1150. doi: 10.1016/s0024-3205(01)01493-x. [DOI] [PubMed] [Google Scholar]
- 25.Lei F., Xing D.M., Xiang L. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2003;796:189–194. doi: 10.1016/s1570-0232(03)00610-x. [DOI] [PubMed] [Google Scholar]
- 26.Priyadarsini K.I., Khopde S.M., Kumar S.S., Mohan H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002;50:2200–2206. doi: 10.1021/jf011275g. [DOI] [PubMed] [Google Scholar]
- 27.Cozzi R., Ricordy R., Bartolini F., Ramadori L., Perticone P., De Salvia R. Taurine and ellagic acid: two differently-acting natural antioxidants. Environ. Mol. Mutagen. 1995;26:248–254. doi: 10.1002/em.2850260310. [DOI] [PubMed] [Google Scholar]
- 28.Iino T., Nakahara K., Miki W., Kiso Y., Ogawa Y., Kato S., Takeuchi K. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol: role of ellagic acid the nonalcoholic component. Digestion. 2001;64:214–221. doi: 10.1159/000048864. [DOI] [PubMed] [Google Scholar]
- 29.Laranjinha J., Vierira O., Almeida L., Madeira V. Inhibition of metmyoglobin/H2O2-dependent low density lipoprotein lipid peroxidation by naturally occurring phenolic acids. Biochem. Pharmacol. 1996;51:395–402. doi: 10.1016/0006-2952(95)02171-x. [DOI] [PubMed] [Google Scholar]
- 30.Takagi A., Sai K., Umemura T., Hasegawa R., Kurokawa Y. Inhibitory effects of vitamin E and ellagic acid on 8-hydroxydeoxyguanosine formation in liver nuclear DNA of rats treated with 2-nitropropane. Cancer Lett. 1995;91:139–144. doi: 10.1016/0304-3835(95)03734-e. [DOI] [PubMed] [Google Scholar]
- 31.Ayhanci A., Cengiz M., Mehtap Kutlu H., Vejselova D. Protective effects of ellagic acid in d-galactosamine-induced kidney damage in rats. Cytotechnol. 2016;68:1763–1770. doi: 10.1007/s10616-015-9928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharya S., Haldar P.K. Ameliorative effect Trichosanthes dioica root against experimentally induced arsenic toxicity in male albino rats. Environ. Toxicol. Pharmacol. 2012;33:394–402. doi: 10.1016/j.etap.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Gu L., Deng W.S., Liu Y., Jiang C.H., Sun L.C., Sun X.F., Xu Q., Zhou H. Ellagic acid protects lipopolysachharide/d-galactosamine-induced acute hepatic injury in mice. Int. Immunopharmacol. 2014;22:341–345. doi: 10.1016/j.intimp.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 35.Baluchnejadmojarad T., Rabiee N., Zabihnejad S., Roghani M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson's disease: possible involvement of ERβ/Nrf2/HO-1 signaling. Brain Res. 2017;1662:23–30. doi: 10.1016/j.brainres.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Hwang J.M., Cho J.S., Kim T.H., Lee Y.I. Ellagic acid protects hepatocytes from damage by inhibiting mitochondrial production of reactive oxygen species. Biomed. Pharmacother. 2010;64:264–270. doi: 10.1016/j.biopha.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Kiasalari Z., Heydarifard R., Khalili M., Afshin-Majd S., Baluchnejadmojarad T., Zahedi E., Sanaierad A., Roghani M. Ellagic acid ameliorates learning and memory deficits in a rat model of Alzheimer's disease: an exploration of underlying mechanisms. Psychopharmacology (Berl) 2017;234:1841–1852. doi: 10.1007/s00213-017-4589-6. [DOI] [PubMed] [Google Scholar]
- 38.Deng W., Garrett C., Dombert B., Soura V., Banks G., Fisher E.M., van der Brug M.P., Hafezparast M. Neurodegenerative mutation in cytoplasmic dynein alters its organization and dynein-dynactin and dynein-kinesin interactions. J. Biol. Chem. 2010;285:39922–39934. doi: 10.1074/jbc.M110.178087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufmann S.H., Hengartner M.O. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 40.Mc Donald E.R., El-Deiry W.S. Cell cycle control as a basis for cancer drug development. Int. J. Oncol. 2000;16:871–886. [PubMed] [Google Scholar]
- 41.Desagher S., Martinou J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 42.Green D.R., Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 43.Kühlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13:89. doi: 10.1186/s12915-015-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmers S., McCarron J.G. The mitochondrial membrane potential and Ca2 + oscillations in smooth muscle. J. Cell Sci. 2008;121:75–85. doi: 10.1242/jcs.014522. [DOI] [PubMed] [Google Scholar]
- 45.Marchi S., Giorgi C., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Missiroli S., Patergnani S., Poletti F., Rimessi A., Duszynski J., Wieckowski M.R., Pinton P. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchenko N.D., Moll U.M. Mitochondrial death functions of p53. Mol. Cell Oncol. 2014;1 doi: 10.1080/23723548.2014.955995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amor S., Puentes F., Baker D., Van der Valk P. Inflammation in neurodegenerative diseases. Immunol. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glass C.K., Saijo K., Beate W., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carniglia L., Ramírez D., Durand D., Saba J., Turati J., Caruso C., Scimonelli T.N., Lasaga M. Neuropeptides and microglial activation in inflammation, painand neurodegenerative diseases. Mediators Inflamm. 2017:5048616. doi: 10.1155/2017/5048616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraft A.D., Harry G.J. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health. 2011;8:2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Los M M., Mozoluk M., Ferrari D., Stepczynska A., Stroh C., Renz A., Herceg Z., Wang Z.Q., Schulze-Osthoff K. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblastnecrosis and apoptosis in death receptor signaling. Mol. Biol. Cell. 2002;13:978–988. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uzar E., Alp H., Cevik M.U., Fırat U., Evliyaoglu O., Tufek A., Altun Y. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol. Sci. 2012;33:567–574. doi: 10.1007/s10072-011-0775-1. [DOI] [PubMed] [Google Scholar]