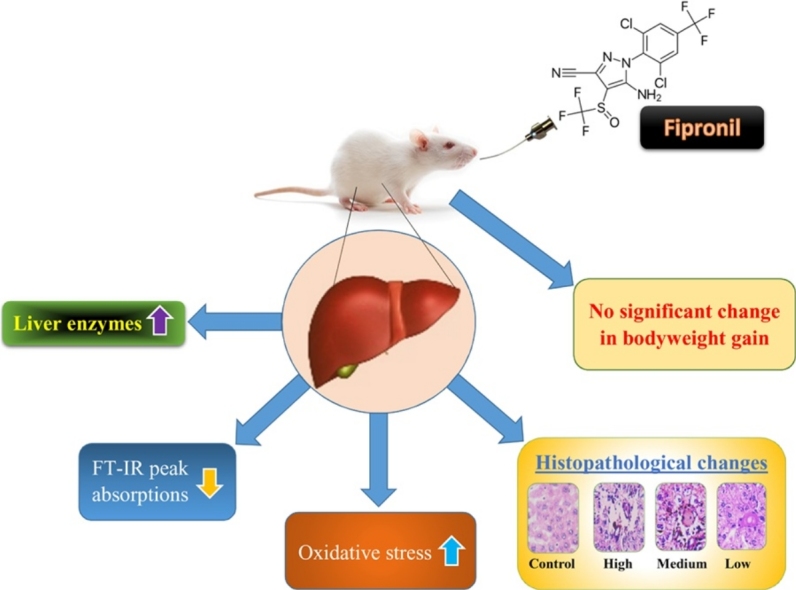
Graphical abstract
Keywords: Antioxidants, Fipronil, Hepatotoxicity, Necrosis, Pesticide
Highlights
-
•
Fipronil was found to induce oxidative stress.
-
•
Exposure to fipronil resulted in histopathology of hepatic tissue.
-
•
Peak absorption changes in FT-IR was evident in liver of rats on Fipronil exposure.
-
•
Fipronil modulated the enzymatic threshold of hepatic enzymes.
Abstract
Extensive pesticide application has contributed to environmental contamination globally, imposing adverse health effects on non-target organisms. Need for an understanding of cellular response following pesticide exposure is, therefore, paradigmatic for elucidating perturbations occurring within biological systems. The present investigation was aimed to examine safe and toxic dose level of a persistent, synthetic, phenylpyrazole based insecticide, Fipronil (FPN) on rat liver. Experimental animals were divided into four groups and gavaged with 0.0 (control), 32.33 (high), 12.12 (medium) and 6.46 mg/kg body weight/day (low dose) of FPN for 90 days. While results for liver catalase and glutathione S-transferase indicated significant changes in high and medium dose groups, the superoxide dismutase and glutathione peroxidase activity suggested significant changes in all exposed groups as compared to control. Elevated levels of liver malondialdehyde reflected oxidative damage potential under the exposed groups but remained insignificant for low dose. Histologically, structural irregularities with findings like impaired portal vein and hypertrophy of hepatocytes were prominent under all the exposed groups. The FT-IR based spectral investigation further revealed changes in absorption patterns and peak intensities in rats exposed to FPN. Significant elevation was also noticed in liver enzymes; alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase in rat serum suggesting the toxicity in dose -dependent pattern. Based on the outcome, it could be ascertained that the toxicity of FPN is certain at high and medium dose levels but remains ambiguous at a low dose of 6.46 mg/kg body weight/day. The current upshots serve as a preliminary report thereby advising the farming community against the usage of FPN insecticide.
1. Introduction
Large -scale anthropogenic activities under chemical scale have predominantly contributed to environmental contamination globally [1]. Increased chemical persistence, mainly pesticides, in soil and water have found their direct correlation to deteriorating health conditions of non-target species ranging from amphibians to mammals [[2], [3]]. Pesticides belong to the only group of chemical toxicants that are intentionally applied to the environment with an objective of enhancing food production through countering insect pests and controlling disease vectors additionally [4]. Even though the consequences of pesticide toxicity are known to be many, establishment of oxidative stress is high paradigm due to the occurrence of reactive oxygen species (ROS) in all aerobic individuals [5]. Upshots in the imbalance of oxidant and antioxidant magnitudes, basically due to elevated proportions of ROS in vivo is therefore considered as an imperative indicator of chemical stress [6]. ROS, as known, is an inevitable and obligatory composite of aerobic mechanism, often produced paradoxically in hepatocytic mitochondria and endoplasmic reticulum, channelized through cytochrome P450 cluster [7]. Although its role in signaling pathways is highly critical, its existence in surplus amounts could impart catastrophic damage to cellular proteins, with the processes often being irreversible [8].
Besides, the mammalian liver is of exceptional concern for studying pesticide -induced toxicity in various experimental models [9]. Its ability to carry out detoxification contributes to homeostasis [10]. Hepatocytic proteins, lipids, and DNA on other hand are among the cellular structures that are primarily affected by ROS that basically arises through exposure to toxic chemicals [11]. The process has been found to result in structural and functional discrepancies of hepatic tissue, ultimately resulting in the impaired health. Attrition of liver as a consequence of incessant detoxification is often reflected by an upsurge in liver enzymes [12]. Basic tools for determining the damage to the liver include the assay of liver enzymes; alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels from blood serum. These components and the end products of the metabolic pathways are highly sensitive for determining the incurred abnormality and have been considered as biochemical markers of liver dysfunction [13].
Fipronil (5- amino- 1- (2, 6-dichloro- α, α, α- trifluoro- p- tolyl)- 4-trifluoromethylsulfinylpyrazole-3- carbonitrile) a broad spectrum, first N- phenylpyrazole insecticide was introduced with an objective of controlling insect pest [14]. It has been found to be effective even against those pests which have gained resistance to the conventional insecticides [15]. The mechanism of FPN toxicity is presumed to be due to the inhibition of a neurotransmitter, a GABAA-gated chloride channel, rendering the organism to suffer from insufficient neurological threshold finally resulting in death [14]. Earlier reports have suggested the involvement of FPN in impeding the metabolic enzymatic systems that are often known to contain sulfhydryl groups and uncoupling of oxidative phosphorylation in the mitochondrial complex [16]. The process is often thought to result in increased lipid peroxidation, DNA damage, depletion of sulfhydryls, altered calcium homeostasis, hepatic congestion, ischemia, hypoxia and death [17].
FT-IR (Fourier Transformed Infra-Red) based spectroscopy analysis has been considered as one of the important tools under vibrational spectroscopic technique, widely used for detection and quantification of macromolecules in biological samples [[18], [19], [20]]. Increase in the levels of serum AST, ALT and ALP reflects the loss of biochemical and structural integrity of the liver and is the most preferred diagnostic tool to evaluate hepatocellular injury [12]. Even though FPN is known to generate oxidative stress and cause the histopathological alterations in hepatic tissue [21], its lipophilic nature and the mechanism underlining its metabolic fate and disposition through mechanisms involved in mammal liver have been poorly understood. Therefore, an attempt has been made in the current study to investigate the FPN induced hepatotoxicity by evaluating biochemical, histopathological and biophysical means following subchronic exposure tenures.
2. Materials and methods
2.1. Animal procurement
Male Wistar albino rats (8 weeks) were obtained from animal house facility, Department of PG studies and Research in Zoology, Karnatak University, Dharwad, Karnataka, India. Rats were housed in the polypropylene cages with ad libitum access to standard pellet feed and drinking water. The room was maintained under a 12/12 h light– dark cycle, an ambient temperature of 23–30 °C with a relative humidity of 45.0 (±15)%.
2.2. Experimental design and test doses
For a 90 day study of oral toxicity, the 6–7 week old Wistar rats were randomly assigned into four groups of six males each. While first group of rats served as the control, the other three group received 32.33 (high), 12.12 (medium) and 6.46 mg/kg body weight/day (low dose) of FPN dose respectively in drinking water. On completion of the exposure duration, the rats were euthanized by carbon dioxide inhalation followed by cervical dislocation. The necessary tissue/organs were isolated.
2.3. Antioxidant enzyme assay
2.3.1. Catalase activity
The Catalase (CAT; EC 1.11.1.6) activity was determined by measuring the decrease of hydrogen peroxide concentration at 240 nm according to Luck [22]. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0) and 10 mM H2O2 freshly added. The enzyme activity was expressed as U/mg protein.
2.3.2. Superoxide dismutase activity
Superoxide dismutase (SOD; EC 1.15.1.1) activity was measured by methodology as described by Kakkar et al., [23]. The reaction mixture consisted of 0.052 M sodium pyrophosphate buffer (pH 8.4), 186 μM phenazine methosulphate (PMS), 30 μM nitroblue tetrazolium chloride (NBT) and 780 μM NADH. Activity is reported in units of SOD per milligram of protein. The activity was calculated wherein, one unit of the enzyme concentration required to inhibit 50% of the optical density of chromagen formed in one unit at 560 nm under the assay condition. The values were expressed in terms of U/mg protein.
2.3.3. Glutathione peroxidase activity
Glutathione peroxidase (GPx; EC 1.11.1.9) activity was measured by the method of Paglia and Valentine [24]. The (GSSG) generated by GPx was reduced by GR, and NADPH oxidation was monitored at 340 nm using Spectrophotometer. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7), 1 mM EDTA, 0.1 E.U/mL oxidised glutathione reductase (GSSG), 1 mM sodium azide, 1 mM reduced glutathione (GSG), 0.2 mM NADPH, and 25 mM H2O2. The enzyme activity was expressed as mU/mg protein. Wherein one mU is the amount that catalyses 1 nmoles of NADPH per minute.
2.3.4. Glutathione S-transferase activity
Glutathione S-transferase (GST; EC 2.5.1.18) activity was assayed by the method of Habig et al. [25]. The increase in absorbance was noted at 340 nm using 1-chloro-2,4-dinitrobenzene (CDNB). The reaction mixture 0.3 mM phosphate buffer (pH 6.5), 30 mM 1-chloro-2, 4-dinitrobenzene (CDNB) and double distilled water. After pre incubating the reaction mixture at 37 °C for 5 min, the reaction was started by the addition of homogenate and glutathione as substrate. The absorbance was followed for 5 min at 340 nm. The enzyme activity was calculated as U/mg protein.
2.4. Lipid peroxidation
Lipid peroxidation (LPO) level was performed according to the method of Buege and Aust [26] and estimated by thiobarbituric acid reactive substance (TBARS) assay performed by an optically measured malondialdehyde (MDA) reaction with TBA. To the liver homogenate, 10% trichloroacetic and 0.67% thiobarbituric acid were added to adjust to a final volume of 1.0 ml. The reaction mixture was placed in a microcentrifuge tube and incubated for 15 min at 95 °C. After cooling, it was centrifuged at 5000×g for 15 min, and optical density was measured by a spectrophotometer at 530 nm. TBARS levels were expressed as nmol MDA/mg protein.
2.5. Histopathology
For the histopathological examination, the method was followed as described by Humason [27]. The liver was isolated and immediately fixed in Bouin’s fluid for 24–48 h. The tissue was processed in a series of graded alcohol and embedded in paraffin which was being filtered thrice earlier. The organs embedded in paraffin were sectioned into 5 μm-thick ribbons by using semi-automated microtome (LeicaRM 2255), and sections were stained primarily with haematoxylin and counterstained with eosin (H&E) for light microscopic examination [28]. The sections were observed under ×200 magnification. The microscopic view was photographed by using an Olympus phase-contrast microscope (Olympus BX51, Tokyo, Japan) with attached photography machinery (ProgResC3, Jenoptic- Germany). The photographed images were further observed for differences, and the findings were recorded. The photomicrograph was processed with Adobe Photoshop 7.0 for slight management in brightness and contrast. The captured images were processed with ImageJ (V. 1.50i) software for enhanced understanding of structural changes.
2.6. FT-IR spectroscopy
The liver was gently washed with phosphate buffered saline, blot dried and immediately immersed in liquid nitrogen and crushed in a mortar and pestle to obtain homogenate. This was further lyophilized prior to infrared analysis. For the infrared measurement, KBr/sample disks were prepared (0.1998 g of powdered KBr with 0.0002 g sample) to reach a total of 0.2 g of KBr/sample mixture and were measured IR spectrophotometrically according to Paul and Robert [29]. The IR for each sample was measured in triplicate using a Shimadzu FTIR–8400 s spectrophotometer with a continuous nitrogen purge. The IR spectra recorded from different KBr disks represented only one spectrum for each experimental group after being co-added. Twenty scans were signal-averaged for a single spectrum with 4 cm−1 spectral resolution in the spectral range of 3600–500 cm−1, obtained at room temperature. IR spectra recorded from each group were co-added, and the entire spectra were normalized and baseline corrected, then subjected to the Kubelka-Munk algorithm [30] by the means of IR solution software [31].
2.7. Serum biochemistry
The blood samples were obtained from the experimental rats by cardiac puncture and were transferred to anticoagulant/heparin free tubes. The serum was separated by centrifugation. The separated serum was subjected to the method of Reitman and Frankel [32] to determine the levels of serum ALT and AST. The method of MacComb and Bowers [33] was carried out to determine the level of serum ALP.
2.8. Statistical analysis
The antioxidant activities are reported as the mean ± standard error of the mean (SEM) obtained from triplicates. The data were subjected to one-way analysis of variance and further subjected to Tukey’s test for post hoc analysis by defining the significance level at *p < 0.05, **p < 0.01.
2.9. Ethics statement
The present study was carried out at the Department of PG Studies and Research in Zoology, Karnatak University, Dharwad (Karnataka, India) as per the ethics committee regulations. The test animals used were maintained and subjected to experimentation process as well as disposed upon completion of the experiment as per the guidelines issued by CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) (Animal House Registration No. 639/02/a/CPCSEA) guidelines were followed for maintenance, use and disposal of the experimental animals. All the experiments were conducted in accordance with IAEC (Institutional Animal Ethics Committee).
3. Results and discussion
3.1. Toxicity signs
No mortality was recorded in rats under control or exposed groups. Additionally, no severe clinical signs or symptoms were noticed in rats exposed to sublethal doses of FPN or under control.
3.2. Body weight gain
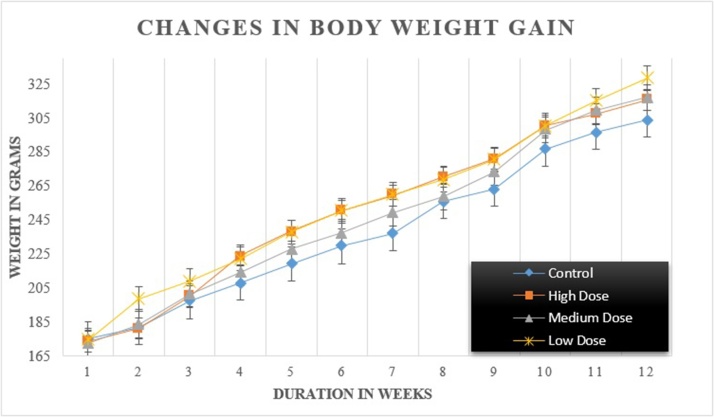
The changes in body weight gain under control rats and those exposed to different sublethal doses of FPN are given in Fig. 1.
Fig. 1.
Changes in body weight gain of male rats under control and exposed group.
Significant variation in enzymatic activity of antioxidant enzymes was noticed in the liver of rats exposed to high and medium dose FPN unlike in rats under low dose group when compared to control (Table 1). Percent decline of −54.37% was noticed for CAT in rats administered with a high dose of FPN, this was followed by −21.19% in rats intoxicated with medium dose. A mild elevation (6.25%) in CAT was noticed in the liver of rats dosed with low FPN which, however, remained insignificant with respect to control group. The SOD activity under FPN stress also reduced significantly with percent decline of −54.49 and −48.43% under high and medium dose respectively. However, the rats under low dose demonstrated a significant elevation in SOD which was recorded to be 24.89%. The existence of SOD–CAT combined system is known to act as the first line of defence against ROS induced damage, thus these activities are used as biomarkers to indicate the exorbitant production of ROS [34]. The current outcome is consistent with studies of Sayeed et al. [35]; Crestani et al., [36] and Kartheek and David [37] who reported a decrease in CAT and SOD magnitudes in the liver following exposure to deltamethrin, clomazone, and sodium cyanide respectively. The present condition characterized by the metabolism of FPN on regular intervals could have led to the production of free radicals that might have chemically modified essential biomolecules specifically proteins and lipids resulting in the condition of LPO [38].
Table 1.
Changes in antioxidant status of wistar rats on exposure to selected doses of fipronil.
| Sl. No. | Parameter | Groups |
|||
|---|---|---|---|---|---|
| Control | Exposed |
||||
| High | Medium | Low | |||
| 1. | Catalase (U/mg protein) | 13.26 ± 0.19 | 6.05 ± 0.43** | 10.45 ± 0.87* | 14.09 ± 0.52 |
| 2. | Superoxide dismutase (U/mg protein) | 48.37 ± 0.27 | 22.01 ± 0.8** | 24.94 ± 0.66** | 60.41 ± 0.72* |
| 3. | Glutathione peroxidase (U/mg protein) | 190.07 ± 1.14 | 51.77 ± 0.93** | 124.94 ± 0.44* | 156.41 ± 0.91* |
| 4. | Glutathione S-transferase (U/mg protein) | 45.11 ± 0.71 | 19.77 ± 0.29** | 24.51 ± 0.53* | 42.64 ± 0.66 |
| 5. | Malondialdehyde (nmol of MDA formed/mg protein) | 2.01 ± 0.08 | 7.33 ± 0.44** | 4.15 ± 0.13* | 2.59 ± 0.22* |
Values represented in groups are means ± SE; significantly different from control.
p < 0.05.
p < 0.01.
Evaluation of GPx in the liver of exposed rats suggested a contrasting trend in its activity wherein the decline of −51.71, −34.26 and −17.70% was recorded for high, medium and low dose respectively. The possibilities for observing this trend may be due to the fact that GPx is oxidised completely if hydroperoxide and GSH concentrations are similar. The significant loss of GST activity in a similar pattern was witnessed, while the maximum decline (−56.17%) was recorded against high dose, a negligible change of −5.47% was noticed recorded for low dose group. The same, when recorded for medium dose revealed decline with a percent change of −45.66%. This decline could be as a consequence of depleted levels of cellular thiol which has been recognized as a crucial component of GST. A number of toxicants have been known to deplete the thiol content [[39], [40]]. Nonetheless, the mechanism by which the decline occurs remains unknown.
MDA is a major end product of LPO and is often thought to propagate and amplify oxidative injury [41]. Its evaluation helps in validating the intensity of damage to biological membranes [37]. In the present study, a significant elevation in LPO level was observed in exposure groups as compared with control group. While rats exposed to high dose of FPN indicated the highest MDA level which was found to be 284.57% as compared to control. While medium dose imparted a percent change of 106.46%, the low dose administration resulted in MDA elevation by 28.85%. A likely explanation for this could be that a much stronger oxidant than superoxide anion-radical could initiate the chain oxidation of polyunsaturated phospholipids, thus leading to impairment of membrane function [42]. The observed findings are in agreement with previously reported studies by Mossa et al., [21] wherein significant decline in antioxidant enzymes and elevation in MDA levels was observed in rats administered with sublethal FPN dose following 45 days of exposure.
3.3. Histological analysis
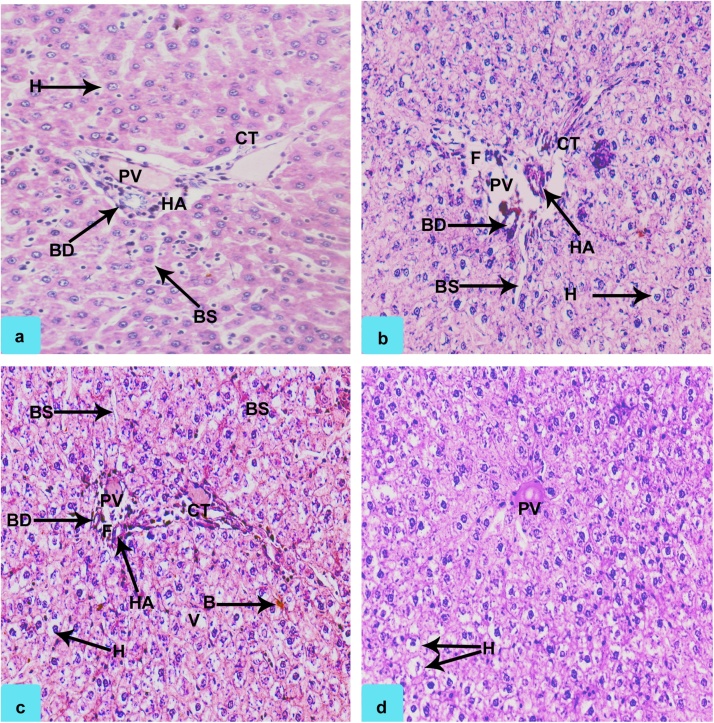
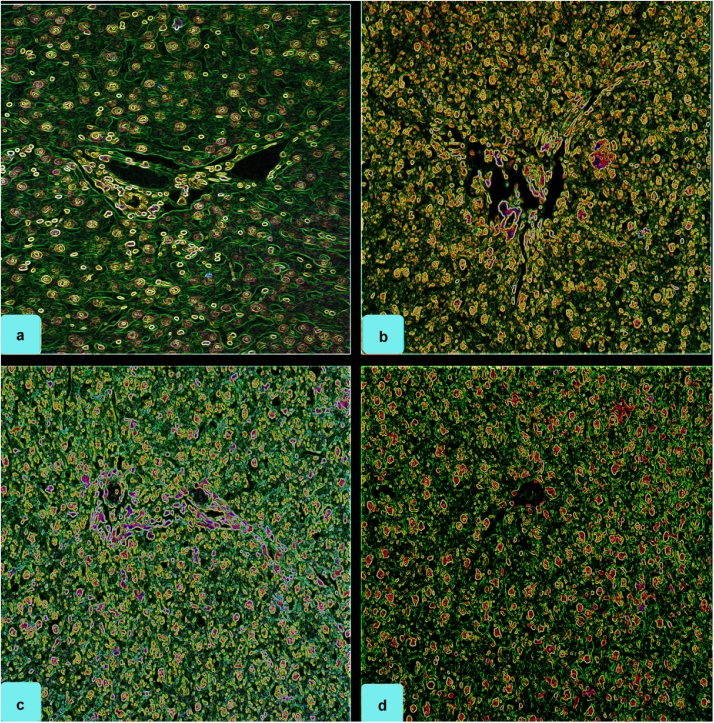
The histopathological findings in the liver of rats under control and exposed group is given in Table 2; Fig. 2, Fig. 3. Changes in histoarchitecture of liver was evidenced with findings like dilation of blood sinusoids (BS), ruptured/partial clogging of bile duct (BD) and portal vein (PV), damaged hepatic artery (HA) and connective tissue (CT), hypertrophy of hepatocytes (H), stagnation of bile (B), mild vacuolation (V) and focal necrosis (F). Nevertheless, no changes were recorded for the control group. Hepatic portal was found to exist intact with hepatic sinusoids indicating normal liver histoarchitecture. Similar reports have been presented previously in different animal models by various authors [11]. The previous reports indicate that defenestration of the sinusoidal endothelium occurs early in the pathogenesis of cirrhosis, both in humans exposed to hepatotoxins and in animal models of cirrhosis [43]. Correlating the outcome from current investigation and previous reports it could be ascertained that the further exposure to FPN dosage could probably incur the cirrhosis like condition in the exposed animal. In another study, authors have suggested the propensity of FPN and its metabolites in promoting adipogenesis [44]. Since FPN is found to be a lipophilic agent, its ability to accumulate in lipids cannot be overlooked [[45], [46]] since its effect in similar approach on certain organisms like earthworms are also quite evident [47] which has been evidenced by increase in body weight gain of the exposed animal. The rats under high dose group showed maximum damage with findings like necrosis, degeneration of bile duct and hepatocytes, impaired sinusoids and damaged portal vein were clearly visible. Changes in rats of medium dose group were found to be comparatively lesser. However, the intensity of damage was found to be more than that of rats under low dose group. The rats under medium dose group were characterized with changes like vacuolization, degenerated portal vein, pyknotic nucleus, dilated sinusoid, degeneration of hepatocytes and cytoplasmic degeneration. Stagnation of bile was noticed for high and medium doses. Our findings are in agreement with Kerem et al., [48], who as well reported that fenitrothion treated rats demonstrated similar histopathological alterations in liver which led to severe effects. Correlating the histoarchitectural findings with antioxidant outcome, it is ascertained that decline in enzymatic threshold of ROS scavenging enzymes might have triggered the oxidation reactions thereby causing the catastrophic damage to the cells and tissues [49].
Table 2.
Histopathological changes in liver of male Wistar rats following oral exposure to different doses of FPN.
| Findings | Groups |
|||
|---|---|---|---|---|
| Control | Exposed |
|||
| High | Medium | Low | ||
| Vacuolation (V) | ND | * * | * * | * |
| Dilated sinusoid (DS) | ND | * * | * * | ND |
| Hypertrophy of hepatocytes (H) | ND | * * * | * * | * |
| Stagnation of bile (B) | ND | * | ** | ND |
| Damaged hepatic artery (HA) | ND | * | * * | ND |
| Degeneration/Clogging of bile duct (BD) | ND | * * * * | * * * | ND |
| Focal necrosis (F) | ND | * | ND | ND |
| Degenerated/Thrombosis/Clogged portal vein (PV) | ND | ND | ND | * * * * |
Histopathological findings in the liver of male Wistar albino rats with different groups indicated, (ND) as Not detected, (*) as low, (* *) as medium, (* * *) as high and (* * * *) as severe levels of histoarchitectural damage.
Fig. 2.
Photomicrograph showing sections of rat liver of Control (0.0 mg/kg BW/day) a: Hepatocytes (H), Portal Vein (PV), Blood sinusoids (BS), Hepatic Artery (HA), Bile Duct (BD), interlobular Connective Tissue; rats exposed to High dose (32.33 mg/kg BW/day) b: Damaged Bile Duct (BD), Ruptured Portal Vein (PV), dilated blood sinusoids (BS), Damaged Hepatic Artery (HA), Hypertrophy of Hepatocytes (H), Disrupted Connective Tissue (CT), Focal Necrosis (F); rats exposed to medium dose (12.12 mg/kg BW/day) c: Mild Dilation of Blood Sinusoids (BS), Partial clogging of Bile Duct (BD) and Portal Vein (PV), Damaged Hepatic Artery (HA) and Connective Tissue (CT), Hypertrophy of Hepatocytes (H), Stagnation of Bile (B), mild Vacuolation (V), Focal Necrosis (F); rats exposed to with low dose (6.46 mg/kg BW/day) d: Hypertrophy of Hepatocytes (H), Clogged Portal Vein (PV), (H and E staining, 200 X).
Fig. 3.
Photomicrograph showing control and FPN exposed sections of rat liver; notice intact hepatocytes, blood sinusoids, portal vein, and bile duct in (a): Control (0.0 mg/kg BW/day); Degenerated hepatocytes, damaged portal vein, bile duct and necrotized area around portal vein (b): High dose- 32.33 mg/kg BW/day, c: Medium dose- 12.12 mg/kg BW/day and (d): Low dose- 6.46 mg/kg BW/day (H and E staining, processed with ImageJ, 200 X).
3.4. FT-IR analysis
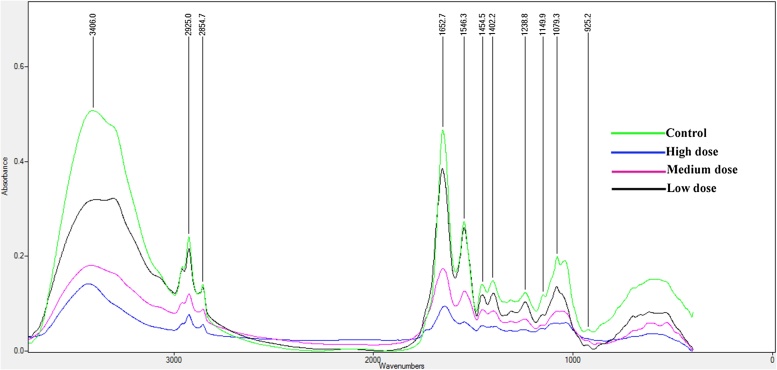
The four mean normalized spectra corresponding to wavenumber 4000–500 cm−1 have been presented as control (curve 1), high (curve 2), medium (curve 3) and low dose (curve 4) groups of rat liver (Table 3; Fig. 4). The bands at 1654 and 1534 cm−1 correspond to amide I and II vibrations of structural proteins respectively, with the first band associated with protein amide C—O stretching vibration and second band corresponding to amide N—H bending vibration coupled to C—N stretching vibration of polypeptide and protein backbone [50]. The current outcome points to the possibilities of the antagonistic approach of FPN against membrane-bound lipids which are the primarily affected structures due to pesticide toxicity [51]. The observation supports the hypothesis that polyunsaturated fatty acids (PUFA) are particularly susceptible to hydrogen removal resulting in the initiation of a radical chain reaction [52]. An increase in the intensity of the carbonyl-stretching mode (1732 cm−1) may indicate an increase in the accumulation of lipids [53] which suggests the synthesis of lipids in excess due to FPN.
Table 3.
General band assignments for FTIR spectra of the liver of rats under control and groups exposed to sublethal doses of FPN.
| Peak | Groups |
Assignments of peaks | |||
|---|---|---|---|---|---|
| Control | Exposed |
||||
| High | Medium | Low | |||
| 1 | 3406 | 3428 | 3413 | 3303 | Amine N—H asymmetric stretching |
| 2 | 2925 | 2924 | 2924 | 2924 | CH3 asymmetric stretching: mainly lipids |
| 3 | 2854 | 2855 | 2857 | 2854 | CH2 asymmetric stretching: mainly lipids |
| 4 | 1652 | 1642 | 1652 | 1654 | Amide I: Protein C O stretching |
| 5 | 1546 | 1550 | 1543 | 1545 | Amide II: Protein N—H bending, C—N stretching |
| 6 | 1454 | 1456 | 1452 | 1454 | C—H bending (scissoring) (in CH3groups)/aromatic —C C stretching vibrations |
| 7 | 1402 | 1383 | 1400 | 1399 | –OH bending vibrations, —C—O—H in-plane bending vibrations,—CH3 out-of-plane bending vibrations, —CH2— wagging and twisting vibrations |
| 8 | 1238 | 1268 | 1315 | 1315 | C(O)—O stretching vibrations and —OH in plane vibrations/amide III (e.g. in aromatic ethers) |
| 9 | 1149 | 1162 | 1241 | 1239 | C—O stretching vibrations (e.g. in C—O—C glycosidic linkages of oligosaccharides or in triacylglycerols) |
| 10 | 1079 | 1036 | 1080 | 1081 | PO2− symmetric stretching: Phospholipids and nucleic acids |
| 11 | 925 | 806 | 870 | 926 | γzOH of carboxylic group |
Fig. 4.
Co-added and overlaid infrared spectra (4000–500 cm−1 spectral region) of liver tissue for the control (0.0) and groups treated with high (32.33), medium (12.12) and low (6.46 mg/kg BW) dose of FPN.
The stretch of 3500–2500 cm−1 with bands centred at 3300, 2990, 2925, and 2854 cm−1, corresponding to the stretching mode of OH and/or NH, H—C C—H, asymmetrical CH3, and both asymmetrical and symmetrical CH2 vibrations, respectively and were found to be affected significantly following the exposure to FPN under subchronic duration. The region corresponding to fatty acid has been highlighted in the current study as the FPN is found to be an adipogenic chemical as reported by Sun et al. [44]. A slight increase in the intensity of the band absorption pattern at 3406 cm−1 and a highly significant reduction (Table 4; Fig. 4) in the ratio of the absorption at 2924 cm−1 (CH3 asymmetric stretching vibration) to that at 2925 cm−1 (CH2 asymmetric and weak CH3 symmetric stretching vibrations) were observed. Moreover, it was noticed that a significant decrease from the control group to the treated one in the ratio of the peak area at 2854 cm−1 (CH2 asymmetric vibration) to that at 2855 and 2857 cm−1 (CH2 symmetric vibration) were witnessed in high and medium dose groups with rats under low dose remaining undisturbed. It is known that the symmetric CH2 stretching band at 2854 cm−1 is particularly valuable as a monitor for probing the “state of order” of biological membranes because its frequency and bandwidth parameters respond sensitively to order–disorder transitions and to changes in mobility of the fatty acid chains [54]. According to Liu et al. [55], “the main amide I absorption in infarcted tissue was shifted from 1650 to 1628 cm−1, a shift indicative of either a structural rearrangement of the existing tissue proteins or the expression of a new set of proteins with different structural characteristics”. This kind of outcome has been witnessed in the peak number 4 where in the variation was from 1652 to 1642, 1652 and 1654 cm−1 for high, medium and low doses respectively.
Table 4.
Changes in the absorption of IR peaks in the liver of male Wistar rats following oral administration to different doses of FPN.
| Range | Groups |
||||
|---|---|---|---|---|---|
| Peak | Control | Exposed |
|||
| High | Medium | Low | |||
| 1 | 3303–3428 | 144.39 | 29.83 | 36.139 | 76.708 |
| 2 | 2924–2925 | 2.189 | 0.602 | 0.722 | 1.584 |
| 3 | 2854–2857 | 0.678 | 0.159 | 0.366 | 0.647 |
| 4 | 1642–1654 | 18.283 | 2.927 | 6.038 | 15.124 |
| 5 | 1543–1550 | 6.45 | 0.241 | 2.34 | 7.157 |
| 6 | 1452–1456 | 1.031 | 0.145 | 0.452 | 1.017 |
| 7 | 1383–1402 | 0.929 | 0.019 | 0.373 | 0.947 |
| 8 | 1268–1328 | 1.154 | 0.03 | 0.381 | 1.166 |
| 9 | 1156–1241 | 0.181 | 0.05 | 0.055 | 0.143 |
| 10 | 1036–1081 | 0.534 | 0.041 | 0.065 | 0.483 |
| 11 | 806–926 | 0.605 | 0.137 | 0.046 | 0.401 |
The bands observed at ∼1454, 1402, 1328, 1156, 1079 and 925 cm−1 are mainly due to C—H bending —OH bending vibrations C(O)–O stretching vibrations C—O stretching vibrations PO2− symmetric stretching and γzOH of the carboxylic group respectively were also found to be largely affected. The medium intensity band observed at ∼1235 cm−1 is that of the PO2-asymmetric stretching modes of the phosphodiester indication of phospholipids and the amide III/CH2 wagging vibration from the glycine backbone and protein side chain. Liver being the site of detoxification, is also known to maintain stable equilibrium through synthesis and degradation its glycogen [56]. Considering the involvement of polysaccharides, the band at 1081 cm−1 was more appropriate for study and is assigned as mainly to the C—O stretching vibrations of polysaccharides inclusive of glycogen component [57]. Cakmak et al. [58] had reported that the band observed at 1237 and 1235 cm−1 corresponds to PO2− asymmetric stretching of nucleic acids with little contribution from phospholipids and 1081 and 1082 cm−1 are mainly due to symmetric stretching of PO2− group in nucleic acids and phospholipids. All the band absorptions were found to decline and minimal absorption was observed for rats under low dose group which also suggested the decline in the concentration of membrane-bound lipids and damage to DNA as well.
3.5. Liver function test
In the current study, FPN caused a significant elevation in the levels of AST, ALT and ALP in the serum of rats (Table 5). The percent change noticed for AST was 184.79, 35.46 and 28.73% for high, medium and low dose respectively. ALT a pyridoxal phosphate-dependent enzyme of hepatic origin also showed an increase with percent change of 304.02, 82.46 and 46.39% for high, medium and low dose exposed groups. A Similar trend was noticed for ALP levels suggesting significantly elevated levels with percent change of 165.08% (high dose) followed by 124.42% (medium dose) and 10.64% (low dose) of FPN. The changes in levels of liver maker enzymes are often utilized as a crucial indicator of cellular damage among hepatocytes with a further indication of altered plasma membrane permeability [59]. Several reports have suggested similar findings wherein the increase in liver marker enzymes has been attributed to the histopathological incidence. Our results are in agreement with Celik et al. [60] who reported similar conditions of elevated ALT in the serum of rats following dichlorvos exposure.
Table 5.
Changes in serum biochemical levels of wistar rats on exposure to selected doses of FPN.
| Sl. No. | Parameter | Groups |
|||
|---|---|---|---|---|---|
| Control | Exposed |
||||
| High | Medium | Low | |||
| 6. | Aspartate aminotransferase | 85.01 ± 2.64 | 242.19 ± 2.22** | 115.16 ± 1.47* | 109.44 ± 1.44 * |
| 7. | Alanine aminotransferase | 55.37 ± 1.97 | 223.71 ± 2.64** | 101.03 ± 2.46* | 81.06 ± 0.91* |
| 8. | Alkaline phosphatase | 133.88 ± 2.42 | 354.91 ± 3.46** | 300.46 ± 3.32** | 148.13 ± 2.31* |
Values represented in groups are means ± SE; significantly different from control.
p < 0.05.
p < 0.01.
The type of liver injury is associated with the relative rise of ALT and ALP is documented by Hussaini and Farrington [61]. A review made by Pandit et al. [62] suggests the elevated level of liver enzymes following administration of diclofenac. The ratio among the maker enzymes is also crucial for determining the type of injury. In ischemic or toxic liver injury, AST levels are found to peak and condition is known to progress even before those of ALT. The background of this process being the enzyme's peculiar intralobular distribution [63]. An additional investigation has suggested that the Zone 3 of the acinus is more vulnerable to both hypoxic (hepatocytes are exposed to an already oxygen-poor milieu) and toxic (hepatocytes are richer in microsomal enzymes) damage. Giannini et al. [12] opined similar changes in serum AST and emphasized that the hepatic damage could be either due ischemic or toxic nature of the compound. The present condition which was characterized by the elevation in AST levels of serum was a substantial evidence of damage to zone 3 of hepatic acinus and has been confirmed by histopathological analysis under current investigation.
4. Conclusion
Based on the overall findings from the present investigation, it may be inferred that FPN is toxic to liver following its ingestion at the selected doses for 90 days, as it is known to alter the biochemical levels with additionally conferring structural impairments as well. However, owing to the limitations of the current investigation, more studies are recommended to confirm the toxicity of low dose chosen under present study which showed ambiguous results for parameters considered. In addition to this, the endpoints obtained from the current investigation could be critically supportive in evaluating the changes under molecular levels. The study could be concluded by further cautioning the farming community about the hepatic damage upon intake of FPN.
Acknowledgements
The authors are thankful to the UGC for providing financial assistance through UGC SAP scheme [No. F.4-18/2015/DSA-I (SAP-II)] and through UGC-UPE/FAR I [(37-245/2009 (SR)]. The authors are also thankful to Department of Science and Technology for providing financial support through DST PURSE Phase II Program.
Contributor Information
R.M. Kartheek, Email: karthikraobiotech@gmail.com.
M. David, Email: mdavid.kud@gmail.com.
References
- 1.Rhind S.M., Evans N.P., Bellingham M., Sharpe R.M., Cotinot C., Mandon-Pepin B., Loup B., Sinclair K.D., Lea R.G., Pocar P., Fischer B., van der Zalm E., Hart K., Schmidt J.S., Amezaga M.R., Fowle P.A. Effects of environmental pollutants on the reproduction and welfare of ruminants. Animal. 2010;4:1227–1239. doi: 10.1017/S1751731110000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasconcelos A.M., Daam M.A., dos Santos L.R., Sanches A.L., Araújo C.V., Espíndola E.L. Acute and chronic sensitivity, avoidance behavior and sensitive life stages of bullfrog tadpoles exposed to the biopesticide abamectin. Ecotoxicology. 2016;25:500–509. doi: 10.1007/s10646-015-1608-4. [DOI] [PubMed] [Google Scholar]
- 3.Kartheek R.M., David M. Assessment of renal toxicity in rats exposed to commercial formulations of fipronil. Int. J. Pharm. Chem. Biol. Sci. 2017;7:296–303. [Google Scholar]
- 4.Aktar M.W., Sengupta D., Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2009;2:1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuter K., Nowak P., Gołembiowska K., Ossowska K. Increased reactive oxygen species production in the brain after repeated low-dose pesticide paraquat exposure in rats. A comparison with peripheral tissues. Neurochem. Res. 2010;35:1121–1130. doi: 10.1007/s11064-010-0163-x. [DOI] [PubMed] [Google Scholar]
- 6.David M., Kartheek R.M. In vivo studies on hepato-renal impairments in freshwater fish Cyprinus carpio following exposure to sublethal concentrations of sodium cyanide. Environ. Sci. Pollut. Res. Int. 2016;23:722–733. doi: 10.1007/s11356-015-5286-9. [DOI] [PubMed] [Google Scholar]
- 7.Poljsak B., Šuput D., Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/956792. (11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol.: CB. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn B.E., Baker T.A. Oxidization without substrate unfolding triggers proteolysis of the peroxide-sensor, PerR. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E23–E31. doi: 10.1073/pnas.1522687112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trapani L., Segatto M., Pallottini V. Regulation and deregulation of cholesterol homeostasis: the liver as a metabolic power station. World J. Hepatol. 2012;27:184–190. doi: 10.4254/wjh.v4.i6.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mello T., Zanieri F., Ceni E., Galli A. Oxidative stress in the healthy and wounded hepatocyte: a cellular organelles perspective. Oxid. Med. Cell. Longev. 2016;15 doi: 10.1155/2016/8327410. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ: Can. Med. Assoc. J. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schomaker S., Warner R., Bock J., Johnson K., Potter D., Van Winkle J., Aubrecht J. Assessment of emerging biomarkers of liver injury in human subjects. Toxicol. Sci. 2013;132:276–283. doi: 10.1093/toxsci/kft009. [DOI] [PubMed] [Google Scholar]
- 14.Simon-Delso N., Amaral-Rogers V., Belzunces L.P., Bonmatin J.M., Chagnon M., Downs C., Furlan L., Gibbons D.W., Giorio C., Girolami V., Goulson D., Kreutzweiser D.P., Krupke C.H., Liess M., Long E., McField M., Mineau P., Mitchell E.A., Morrissey C.A., Noome D.A., Pisa L., Settele J., Stark J.D., Tapparo A., Van Dyck H., Van Praagh J., Van der Sluijs J.P., Whitehorn P.R., Wiemers M. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015;22:5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda T., Zhao X., Nagata K., Kono Y., Shono T., Yeh J.Z., Narahashi T. Fipronil modulation of gamma-aminobutyric acid (A) receptors in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2001;296:914–921. [PubMed] [Google Scholar]
- 16.Vidau C., González-Polo R.A., Niso-Santano M., Gómez-Sánchez R., Bravo-San Pedro J.M., Pizarro-Estrella E., Blasco R., Brunet J.L., Belzunces L.P., Fuentes J.M. Fipronil is a powerful uncoupler of oxidative phosphorylation that triggers apoptosis in human neuronal cell line SHSY5Y. Neurotoxicology. 2011;32:935–943. doi: 10.1016/j.neuro.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Bharavi K., Gopala Reddy A., Rao G.S., Ravikumar P., Rajasekhar Reddy A., Rama Rao S.V. Reversal of cadmium induced oxidative stress and its bioaccumulation by culinary herbs Murraya koenigii and Allium sativum. Res. J. Pharmacol. 2010;4:60–65. [Google Scholar]
- 18.Lu X., Webb M., Talbott M.J., Van Eenennaam J.P., Doroshov S.I., Rasco B.A. A study of biochemical parameters associated with ovarian atresia and quality of caviar in farmed white sturgeon (Acipenser transmontanus) by Fourier Transform Infrared (FT–IR) Spectroscopy. Aquaculture. 2011;315:298–305. [Google Scholar]
- 19.Turker S., Ilbay G., Severcan M., Severcan F. Investigation of compositional, structural, and dynamical changes of pentylenetetrazol induced seizures on a rat brain by FT-IR spectroscopy. Anal. Chem. 2014;86:1395–1403. doi: 10.1021/ac402992j. [DOI] [PubMed] [Google Scholar]
- 20.Krishnakumar N., Prabu S.M., Sulfikkarali N.K. Quercetin protects against cadmium-induced biochemical and structural changes in rat liver revealed by FT-IR spectroscopy. Biomed. Prev. Nutr. 2012;2:179–185. [Google Scholar]
- 21.Mossa A.-T.H., Swelam E.S., Mohafrash S.M.M. Sub-chronic exposure to fipronil induced oxidative stress: biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015;2:775–784. doi: 10.1016/j.toxrep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luck H. 2nd edn. Academic Press; New York: 1974. Methods in Enzymatic Analysis. [Google Scholar]
- 23.Kakkar P., Das B., Viswanathan P. A modified method for assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:131–132. [PubMed] [Google Scholar]
- 24.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 25.Habig W.J., Pabst M.J., Jacoby W.B. Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 26.Buege J.A., Aust S.D. Microsomal lipid peroxidation methods. Enzymology. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 27.Humason G.L. 3rd edn. Freeman; San Francisco: 1972. Animal Tissue Techniques. [Google Scholar]
- 28.Lille R.D. 8th edn. Williams and Wilkins; Baltimore: 1969. Biological Stains. [Google Scholar]
- 29.Paul G.L., Robert D.S. Cancer grading by Fourier transform infrared spectroscopy. Biospectroscopy. 1998;4:37–46. doi: 10.1002/(sici)1520-6343(1998)4:1<37::aid-bspy4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza L., Devi P., Shridhar M.P., Naik G. Use of fourier transform infrared (FTIR) spectroscopy to study cadmium-Induced changes in Padina tetrastromatica. Anal. Chem. Insights. 2008;3:135–143. [PMC free article] [PubMed] [Google Scholar]
- 31.Ke Y., Wang S.W., Lu Q.Y., Huang P., Xing B., Wang Z.Y. The correlation between postmortem interval and Fourier transform infrared spectra in rat’s brain. Guang Pu Xue Yu Guang Pu Fen Xi. 2008;28:2545–2549. [PubMed] [Google Scholar]
- 32.Reitman S., Frankel S. Glutamic – pyruvate transaminase assay by colorimetric method. Am. J. Clin. Pathol. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 33.MacComb R.B., Bowers G.N., Jr. Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin. Chem. 1972;18:97–104. [PubMed] [Google Scholar]
- 34.Monteiro D.A., de Almeida J.A., Rantin F.T., Kalinin A.L. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion) Comp. Biochem. Physiol.: C Toxicol. Pharmacol. 2006;143:141–149. doi: 10.1016/j.cbpc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Sayeed L., Parvez S., Pandey S., Bin-Hafeez B., Haque R., Raisuddin S. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus. Bloch. Ecotoxicol. Environ. Saf. 2003;56:295–301. doi: 10.1016/s0147-6513(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 36.Crestani M., Menezes C., Glusczak L., dos Santos Miron D., Spanevello R., Silveira A., Gonçalves F.F., Zanella R., Loro V.L. Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere. 2007;67:2305–2311. doi: 10.1016/j.chemosphere.2006.09.070. [DOI] [PubMed] [Google Scholar]
- 37.Kartheek R.M., David M. Fipronil induced modulations in biochemical and histopathological aspects of male Wistar albino rats: a subchronic study. J. Environ. Biosci. 2016;5:26–32. [Google Scholar]
- 38.Young S., Woodside J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elsaid F.G., Elkomy M.M. Aqueous garlic extract and sodium thiosulphate as antidotes for cyanide intoxication in Albino rats. Res. J. Med. Med. Sci. 2006;1:50–56. [Google Scholar]
- 40.David M., Kartheek R.M. In vivo studies on hepato-renal impairments in freshwater fish Cyprinus carpio following exposure to sublethal concentrations of sodium cyanide. Environ. Sci. Pollut. Res. Int. 2016;23:722–733. doi: 10.1007/s11356-015-5286-9. (2016) [DOI] [PubMed] [Google Scholar]
- 41.Pizzimenti S., Ciamporcero E., Daga M., Pettazzoni P., Arcaro A., Cetrangolo G., Minelli R., Dianzani C., Lepore A., Gentile F., Barrer G. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider B.P., Wang M., Radovich M., Sledge G.W., Badve S., Thor A., Flockhart D.A., Hancock B., Davidson N., Gralow J., Dickler E.A., Cobleigh M., Shenkier T., Edgerton S., Miller K.D. ECOG 2100. association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced Breast cancer: ECOG 2100. J. Clin. Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braet F., Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Q., Qi W., Yang J.J., Yoon K.S., Clark J.M., Park Y. Fipronil promotes adipogenesis via AMPKα-mediated pathway in 3T3-L1 adipocytes. Food Chem. Toxicol. 2016;92:217–223. doi: 10.1016/j.fct.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Bonmatin J.M., Giorio C., Girolami V., Goulson D., Kreutzweiser D.P., Krupke C., Liess M., Long E., Marzaro M., Mitchell E.A.D., Noome D.A., Simon-Delso N., Tapparo A. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 2015;22:35–67. doi: 10.1007/s11356-014-3332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Case K.M., Vega N.M., Gupta R.C., Lasher M.A., Canerdy T.D. Safety evaluation of Parastar® plus in dogs and assessment of transferable residue of fipronil and cyphenothrin from dogs to humans. Front. Vet. Sci. 2016;3:89. doi: 10.3389/fvets.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horwood M.A. Rapid degradation of termiticides under field conditions. Aust. J. Entomol. 2007;46:75–78. [Google Scholar]
- 48.Kerem M., Bedirli N., Gurbus N., Ekinci O., Bedirli A., Akkaya T., et al. Effects of acute fenthion toxicity on liver and kidney function and histology in rats. Turk. J. Med. Sci. 2007;37:281–288. [Google Scholar]
- 49.Perni M., Galvagnion C., Maltsev A., Meisl G., Müller M.B.D., Challa P.K., Kirkegaard J.B., Flagmeier P., Cohen S.I.A., Cascella R., Chen S.W., Limbocker R., Sormanni P., Heller G.T., Aprile F.A., Cremades N., Cecchi C., Chiti F., Nollen E.A.A., Knowles T.P.J., Vendruscolo M., Bax A., Zasloff M., Dobso C.M. A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity. Proc. Natl. Acad. Sci. U. S. A. 2017;114(6):1009–1017. doi: 10.1073/pnas.1610586114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akkas S.B., Severcan M., Yilmaz O., Severcan F. Effects of lipoic acid supplementation on rat brain tissue, an FTIR spectroscopic and neural network study. Food Chem. 2007;105:1281–1288. [Google Scholar]
- 51.Dianzani M., Barrera G. In: Free Radical Pathophysiology. Álvarez S., Evelson P., editors. Transworld Research Network; Kerala, India: 2008. Pathology and physiology of lipid peroxidation and its carbonyl products; pp. 19–38. [Google Scholar]
- 52.Nimse S.B., Pal D. Free radicals natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. [Google Scholar]
- 53.Takahashi H., French S.W., Wong P.T.T. Alterations in hepatic lipids and proteins by chronic ethanol intake: a high pressure fourier transform infrared spectroscopic study on alcoholic liver disease in the rat. Alcohol. Clin. Exp. Res. 1991;15:219–223. doi: 10.1111/j.1530-0277.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 54.Casal H.L., Cameron D.G., Jarell H.D., Smith I.C.P., Mantsch H.H. Lipid phase transitions in fatty acid-homogeneous membranes of Acholeplasma laidlawii B. Chem. Phys. Lipids. 1982;30:17–26. [Google Scholar]
- 55.Liu K.Z., Jackson M., Sowa M.G., Ju H., Dixon I.M.C., Mantsch H.H. Modification of the extracellular matrix following myocardial infarction monitored by FTIR spectroscopy. Biochem. Biophys. Acta. 1996;1315:73–77. doi: 10.1016/0925-4439(95)00118-2. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Novell J.M., Lopez-Iglesias C., Ferrer J.C., Guinovart J.J. Zonal distribution of glycogen synthesis in isolated rat hepatocytes. FEBS Lett. 2002;531:222–228. doi: 10.1016/s0014-5793(02)03506-8. [DOI] [PubMed] [Google Scholar]
- 57.Toyran N., Lasch P., Naumann D., Turan B., Severcan F. Early alterations in myocardia and vessels of the diabetic rat heart: an FTIR microspectroscopic study. Biochem. J. 2006;397:427–436. doi: 10.1042/BJ20060171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cakmak G., Togan I., Severcan F. 17β-Estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: a comparative study with nonylphenol. Aquatic Toxicol. 2006;77:53–63. doi: 10.1016/j.aquatox.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 59.Dhouib El-Bini, Annabi A., Montassar M.L., Gharbi N., El-Fazaa S. Anti-inflammatory effects of N-acetylcysteine against carbosulfan-induced hepatic impairment in male rats. Recent Adv. Biolo. Med. 2015;1:29–40. [Google Scholar]
- 60.Celik I., Yilmaz Z., Turkoglu V. Hematotoxic and hepatotoxic effects of dichlorvos at sublethal dosages in rats. Environ. Toxicol. 2009;24:128–132. doi: 10.1002/tox.20390. [DOI] [PubMed] [Google Scholar]
- 61.Hussaini S.H., Farrington E.A. Idiosyncratic drug-induced liver injury: an overview. Expert Opin. Drug Saf. 2007;6:673–684. doi: 10.1517/14740338.6.6.673. [DOI] [PubMed] [Google Scholar]
- 62.Pandit A., Sachdeva T., Bafna P. Drug-induced hepatotoxicity: a review. J. Appl. Pharm. Sci. 2012;02:233–243. [Google Scholar]
- 63.Seeto R.K., Fenn B., Rockey D.C. Ischemic hepatitis: clinical presentation and pathogenesis. Am. J. Med. 2000;109:109–113. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]