Highlights
-
•
Carboplatin and thalidomide induced neuro-nephrotoxicity.
-
•
Carboplatin and thalidomide caused inflammation.
-
•
Carboplatin and thalidomide upregulate tumor suppressor protein p53.
-
•
Carboplatin and thalidomide disturbed cytokine production.
-
•
Neuro-and nephroprotective effect of grape seed proanthocyanidin.
Keywords: Thalidomide, Carboplatin, Grape seed proanthocyanidin extract, Neurotoxicity, Nephrotoxicity
Abstract
The combination of thalidomide and carboplatin is one of the most potent chemotherapeutic strategies for the treatment of cancer. However, limited studies have been conducted on the neurotoxicity and nephrotoxicity of both chemotherapeutic agents. The aim of our study was to assess the toxicity of thalidomide and carboplatin combination on brain and kidney and investigate the protective effect of grape seed proanthocyanidin extract (GSPE). Thalidomide and carboplatin induced up-regulation of the expression of p53, tumor necrosis factor-α and interleukin-6 in brain and kidney. Acetylcholinesterase, dopamine and serotonin were decreased and norepinephrine was increased. Thiobarbituric acid reactive substances, nitric oxide, lipid profile, bilirubin and creatinine were elevated, while antioxidants enzymes (GST, GPX, CAT and SOD), total antioxidant capacity and the levels of glutathione were decreased. A microscopic examination showed shrinkage of capillaries, degeneration with pyknotic nuclei, loss of normal structure and neuronal degeneration. GSPE co-treatment with thalidomide and carboplatin reduced their brain and renal damage, oxidative stress, diminished cytokines, p53, neurotransmitters and biochemical parameters, and inhibited brain and renal cell apoptosis. It can be concluded that, the protective effects of GSPE against thalidomide and carboplatin induced-brain and renal damage was associated with the minimization of oxidative stress.
1. Introduction
Chemotherapy drugs have many mechanisms of actions and may belong to more than one group. One group of chemotherapeutic drugs is alkylating agents that directly damage DNA. Alkylating agents include the platinum drugs (cisplatin, carboplatin and oxalaplatin) [1]. High dose of carboplatin induces ototoxicity in cancer patients and oxidative injury in rats via the generation of free radicals and the depletion of antioxidants [2].
Immunomodulating drugs such as thalidomide, lenalidomide and revlimid is another group of chemotherapeutic agents. Thalidomide was used as a sedative drug to treat morning sickness in pregnant women in the 1950s, but was subsequently withdrawn from the market in 1961 because of severe teratogenicity and neurotoxicity [3]. Interestingly, subsequent studies on the mechanisms of thalidomide teratogenicity revealed that the compound was an effective anticancer and anti-inflammatory agent. The US Food and Drug Administration approved thalidomide for the treatment of lepromatous leprosy and multiple myeloma in 1998 and 2006, respectively [4]. Thalidomide is used experimentally to treat various cancers, dermatological, neurological and inflammatory diseases. Thalidomide and its immunomodulatory analogues have numerous effects on the body's immune system, including potential anti-cancer and anti-inflammatory activities [5].
Antineoplastic agents induce oxidative stress in biological systems. During cancer chemotherapy, oxidative stress induced lipid peroxidation generates numerous electrophilic aldehydes that can attack many cellular targets. These products of oxidative stress can slow cell cycle progression of cancer cells and cause cell cycle checkpoint arrest, effects that may interfere with the ability of anticancer drugs to kill cancer cells. The aldehydes may also inhibit drug-induced apoptosis (programmed cell death) by inactivating death receptors and inhibiting caspase activity. These effects would also diminish the efficacy of the treatment. The use of antioxidants during chemotherapy may enhance therapy by reducing the generation of oxidative stress-induced aldehydes [6].
Oxidative damage represents a major mechanism of cytotoxicity to normal cells by most chemotherapeutic agents since the drugs of many classes of antineoplastic agents are known to generate a high level of oxidative stress in biological systems. A good deal of evidence suggests that reactive oxygen species (ROS) have an important role in certain chemotherapy-induced side effects. Examples include doxorubicin-induced cardiotoxicity, bleomycin-induced pulmonary fibrosis, and cisplatin-induced nephrotoxicity, ototoxicity and neurotoxicity [6].
Antioxidants are potent scavengers of free radicals and serve as inhibitors of neoplastic processes. A large number of synthetic and natural antioxidants have been demonstrated to induce beneficial effects on human health and disease prevention [7]. A broad spectrum of pharmacological and therapeutic benefits of grape seed proanthocyanidin extract (GSPE) against oxidative stress and degenerative diseases, including cardiovascular dysfunctions, acute and chronic stress, gastrointestinal distress, neurological disorders, pancreatitis, various stages of neoplastic processes and carcinogenesis including detoxification of carcinogenic metabolites, have been reported [8].
GSPE is a superior scavenger as compared to vitamins C, E and carotene and prevents hepatic and brain lipid peroxidation and DNA damage in animals. Besides antioxidant activity, GSPE has been described as anti- microbial, anti-cancer, anti-inflammatory and anti-fatigue agent [9]. GSPE protects against structurally diverse drug and chemical-induced multi-organ toxicity, induces selective cytotoxicity toward human breast, lung, gastric and pancreatic cancer cells while maintaining growth and viability of normal cells [8]. Our previous studies showed that GSPE is capable of alleviating cisplatin-induced damage in kidney genomic DNA, nephrotoxicity and oxidative stress in male rats [10,11]. The aim of the present study was to investigate the chemo-protective effect of GSPE against neurotoxicity and nephrotoxicity induced by thalidomide and carboplatin via reactive oxygen species, nitric oxide, total antioxidant capacity, antioxidant enzymes, neurotransmitters, cytokines (tumor necrosis factor and interleukin-6), tumor suppressor gene P53, biochemical parameters and histopathological changes in male rats.
2. Materials and methods
2.1. Tested compounds and doses
Thalidomide (C13H10N2O4) was purchased from Sigma Chemical Company (St. Louis, MO, USA). Carboplatin (C6H14N2O4Pt) obtained from Vitafor additives and pharmaceuticals, Germany (www.vitafor.com). A dried, powdered grape seed proanthocyanidin extract (GSPE) was obtained from Pharco Pharmaceuticals Company (Alexandria, Egypt). The dose of thalidomide was 60 mg/kg according to the study of Kaczmarczyk-Sedlak et al. [12]. The dose of carboplatin was 196 mg/kg according to Husain et al. [13]. The dose of grape seed proanthocyanidin extract (GSPE) was 200 mg/kg BW according to Yousef et al. [11].
2.2. Animals and experimental groups
Forty Wistar male rats weighing 160–180 g (6–8 weeks old) were used. Animals were obtained from Faculty of Medicine, Alexandria University, Alexandria, Egypt. The local committee approved the design of the experiments, and the protocol conforms to the guidelines of the National Institutes of Health (NIH). Animals were housed in a stainless steel wire cages (five animals were housed in each cage) and kept on basal diet and given feed and water ad libitum. Animals were maintained in a controlled atmosphere at temperature of 25 ± 5 °C and 50–70% humidity. After two weeks of acclimation, animals were divided into four equal groups (10 animals each) as follows: the first group was used as control, the second group was treated orally for 28 consecutive days with GSPE (200 mg/kg BW in 5 ml), the third group was treated intraperitoneally (i.p.) daily with thalidomide (60 mg/kg BW in 0.1 ml/100 g BW) for 14 consecutive days then followed by oral administration with carboplatin (196 mg/kg BW in 1 ml/kg BW) for another 14 days and the fourth group was treated with the combination of GSPE (200 mg/kg BW) and thalidomide (60 mg/kg BW) for 14-day and then followed by GSPE (200 mg/kg BW) and carboplatin (196 mg/kg BW) for other 14-day.
2.3. Blood samples collection and tissue preparations
At the end of the 28th day of the experimental period, all animals of each group were anaesthetized with diethyl ether and sacrificed. Blood samples were collected from anaesthetized rats in test tubes containing heparin as an anticoagulant and placed immediately on ice. The collected blood was centrifuged at 860×g for 20 min for the separation of plasma. The plasma was kept at −80 °C until analyses of the tested parameters. Brain and kidney were immediately excised, washed using chilled saline solution and the adhering fat and connective tissues were removed. Brain and kidney were divided into two parts; one part was immersed immediately in formalin for histological analysis, the other part was minced and homogenized (10%, w/v), separately, in ice-cold sucrose buffer (0.25 M) in a Potter–Elvehjem type homogenizer, the homogenates were centrifuged at 10,000×g for 20 min at 4 °C, to pellet the cell debris, and the supernatant was collected and stored at −80 °C.
2.4. ELISA measurements
Tumor suppressor gene p53, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were assayed, using Enzyme-linked Immunosorbent Assay (ELISA) kits, in brain and kidney tissue homogenates according to the methods of Yang et al. [14], Hedayati et al. [15] and Ferguson-Smith et al. [16], respectively. Dopamine and serotonin levels and norepinephrine hormone were determined by using a competitive inhibition enzyme immunoassay technique kits for the in vitro quantitative measurement from Cloud-Clone Corp. Houston, USA.
2.5. Markers of oxidative stress
Tissue supernatant thiobarbituric acid-reactive substances (TBARS) were measured at 532 nm by using 2-thiobarbituric acid (2,6-dihydroxypyrimidine-2-thiol; TBA). Total antioxidant capacity (TAC) and the level of nitric oxide (NO) were assayed in brain and kidney homogenates. Superoxide dismutase (SOD) was determined and the assay procedure involves the inhibition of epinephrine auto-oxidation in an alkaline medium (pH 10.2) to adrenochrome, which is markedly inhibited by the presence of SOD. Epinephrine was added to the assay mixture, containing tissue supernatant and the change in extinction coefficient was followed at 480 nm in a spectrophotometer. Glutathione peroxidase (GPX) activity was determined in brain and kidney homogenates. Glutathione S-transferase (GST) catalyzes the conjugation reaction with glutathione in the first step of mercapturic acid synthesis. The activity of GST was measured in tissue homogenates and P-nitrobenzylchloride was used as substrate. The absorbance was measured spectrophotometrically at 310 nm using UV-Double Beam Spectrophotometer. The catalase enzyme (CAT) converts H2O2 into water. The CAT activity in tissue homogenates was measured spectrophotometrically at 240 nm by calculating the rate of degradation of H2O2, the substrate of the enzyme. Reduced glutathione content was determined. The method utilized metaphosphoric acid for protein precipitation and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) for color development, and density was measured at 412 nm. All the above assays were determined according to the manual instructions of Biodiagnostic Kit, Egypt.
2.6. Biochemical parameters
Acetylcholinesterase (AChE) activity was estimated in plasma and brain according to the method of Ellman et al. [17]. Plasma creatinine, total bilirubin, total lipids, cholesterol and triacylglycerol (TAG) were measured with kits from Biosystems S.A (Biosystems S.A. Costa Brava 30, Barcelona, Spain).
2.7. Histological section preparation of brain and kidney
Brain and kidney specimens were obtained from rats, and immediately fixed in 10% formalin, and then treated with conventional grade of alcohol and xylol, embedded in paraffin and sectioned at 4–6 μm thickness. The sections were stained with Haematoxylin and Eosin (H&E) stain for studying the histopathological changes [18].
2.8. Statistical analysis
Results are reported as means ± SE. Statistical analysis for all studied parameters were performed using the general linear model (GLM) produced by Statistical Analysis Systems Institute [19]. Duncan's New Multiple Range Test was used to test the significance of the differences between means [20]. Values of p > 0.05 were considered statistically significant.
3. Results
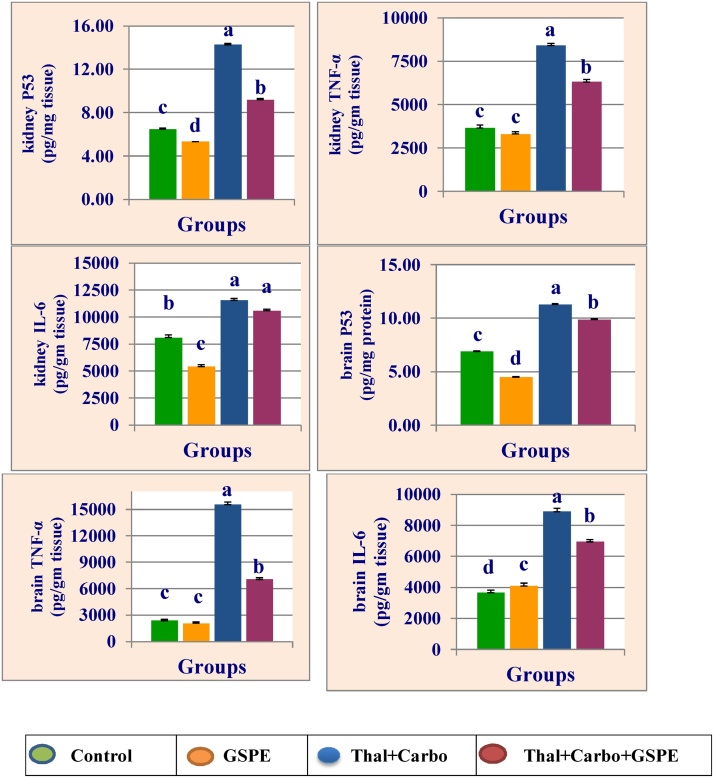
Fig. 1 represent the data of kidney and brain tumor suppressor P53 (P53), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) of rats treated with GSPE, thalidomide for 14 days followed by carboplatin for 14 days and their combination with GSPE. Treatment with thalidomide for 14 days followed by carboplatin for 14 significantly (P > 0.05) increased the levels of P53, IL-6 and TNF-α in kidney and brain compared to control group. The combination group of GSPE with thalidomide and carboplatin reduced the elevation of P53, IL-6 and TNF-α compared to group treated with thalidomide and carboplatin.
Fig. 1.
Mean values ± SE of kidney and brain levels of tumor suppressor P53 (P53), tumor necrosis factor-α (TNF-α) interleukin 6 (IL-6) of male rats treated with grape seed proanthocyanidin extract (GSPE), thalidomide (Thal.), carboplatin (Carbo.) and their combination.
Mean values not sharing a common superscript letters (a–d) were significantly different, p < 0.05.
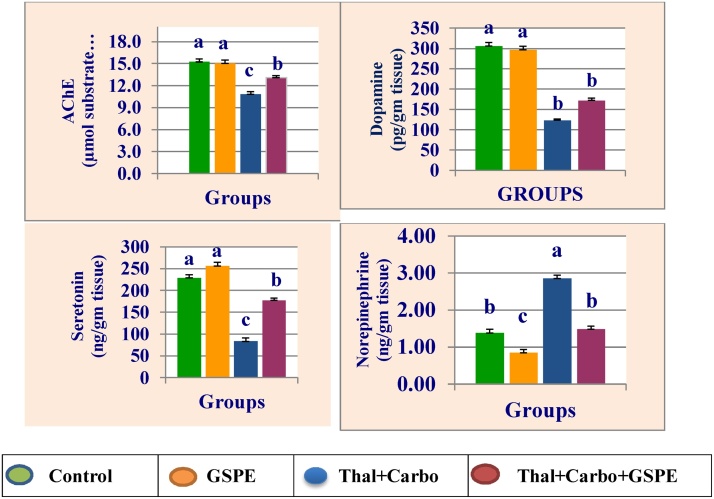
The mean values of brain acetylcholine esterase (AChE), dopamine, serotonin and norepinephrine of rats treated with grape seed proanthocyanidin extract (GSPE), thalidomide, carboplatin and their combination are presented in Fig. 2. Treatment with thalidomide for 14 days followed by carboplatin for 14 significantly (P > 0.05) significantly decreased brain AChE, dopamine and serotonin, while significantly increased norepinephrine compared to control group. The presence of GSPE with thalidomide and carboplatin in the combination group reduced their neurotoxicity compared to the group treated with thalidomide and carboplatin.
Fig. 2.
Mean values ± SE of brain acetylcholine esterase (AChE), dopamine serotonin, and norepinephrine of male rats treated with grape seed proanthocyanidin extract (GSPE), thalidomide (Thal.), carboplatin (Carbo.) and their combination.
Mean values not sharing a common superscript letters (a–d) were significantly different, p < 0.05.
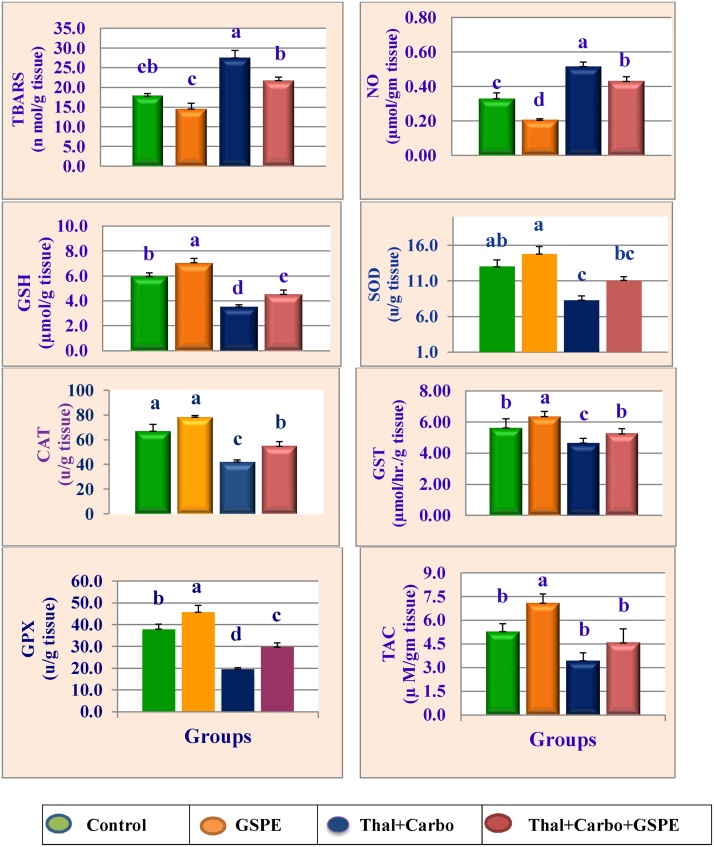
Kidney and brain thiobarbituric acid reactive substances (TBARS), nitric oxide (NO), reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione peroxidase (GPx) and total antioxidant capacity (TAC) of rats treated with GSPE, thalidomide, carboplatin and their combination are expressed in Fig. 3, Fig. 4. Data showed that treatment with thalidomide for 14 days followed by carboplatin for 14 significantly increased the levels of TBARS and NO, while significantly (P > 0.05) decreased the activities of antioxidant enzymes (SOD, GST, GPx, CAT and TAC) and the level of GSH compared to control group. Treatment with the antioxidant GSPE alone significantly (P > 0.05) reduced the levels of TBARS and NO, while significantly (P > 0.05) increased the antioxidant enzymes and GSH in kidney and brain compared to control group. The presence of GSPE with thalidomide and carboplatin overcame their oxidative effect and reduced the elevation in TBARS and NO, and increased the reduction in TAC, GSH and antioxidant enzymes.
Fig. 3.
Mean values of ±SE kidney thiobarbituric acid-reactive substances (TBARS), nitric oxide (NO), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione peroxidase (GPX) and total antioxidant capacity (TAC) activities levels of male rats treated with grape seed proanthocyanidin extract (GSPE), thalidomide (Thal.), carboplatin (Carbo.) and their combination.
Mean values not sharing a common superscript letters (a–d) were significantly different, p < 0.05.
Fig. 4.
Mean values of ±SE brain thiobarbituric acid-reactive substances (TBARS), nitric oxide (NO), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione peroxidase (GPX) and total antioxidant capacity (TAC) activities levels of male rats treated with grape seed proanthocyanidin extract (GSPE), thalidomide (Thal.), carboplatin (Carbo.) and their combination.
Mean values not sharing a common superscript letters (a–d) were significantly different, p < 0.05.
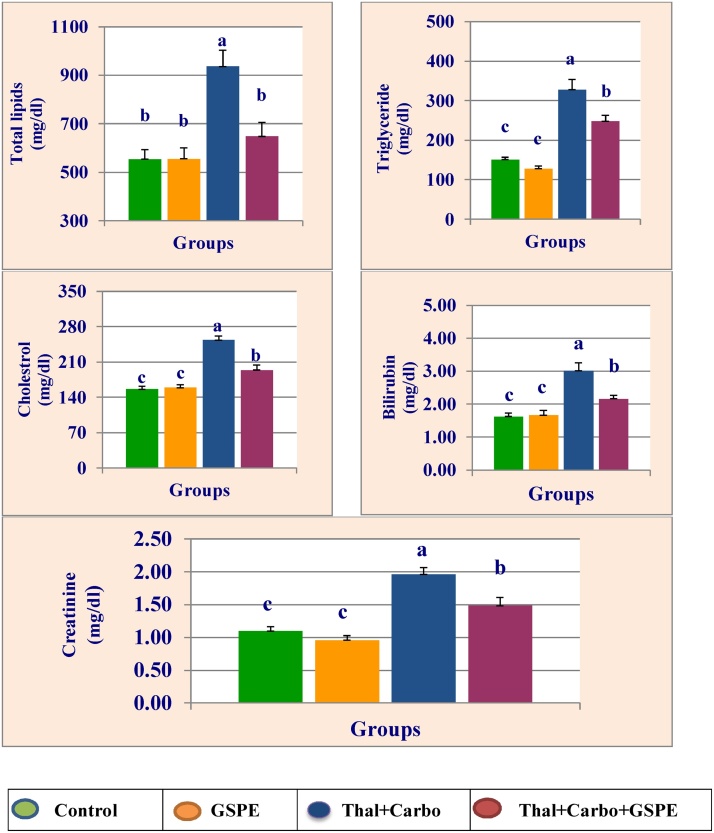
Total lipids, triglyceride, cholesterol, bilirubin and creatinine of plasma are presented in Fig. 5. Treatment with thalidomide for 14 days followed by carboplatin for 14 days significantly (P > 0.05) increased the levels of lipid profile, bilirubin and creatinine compared to control group. The combination of GSPE with thalidomide and carboplatin alleviated their effects on above parameters compared to thalidomide and carboplatin group.
Fig. 5.
Mean values ± SE of plasma total lipid, triglyceride, cholesterol, bilirubin and creatinine of male rats treated with grape seed proanthocyanidin extract (GSPE), thalidomide (Thal.), carboplatin (Carbo.), and their combination.
Mean values not sharing a common superscript letters (a, b, c, d) were significantly different, p < 0.05.
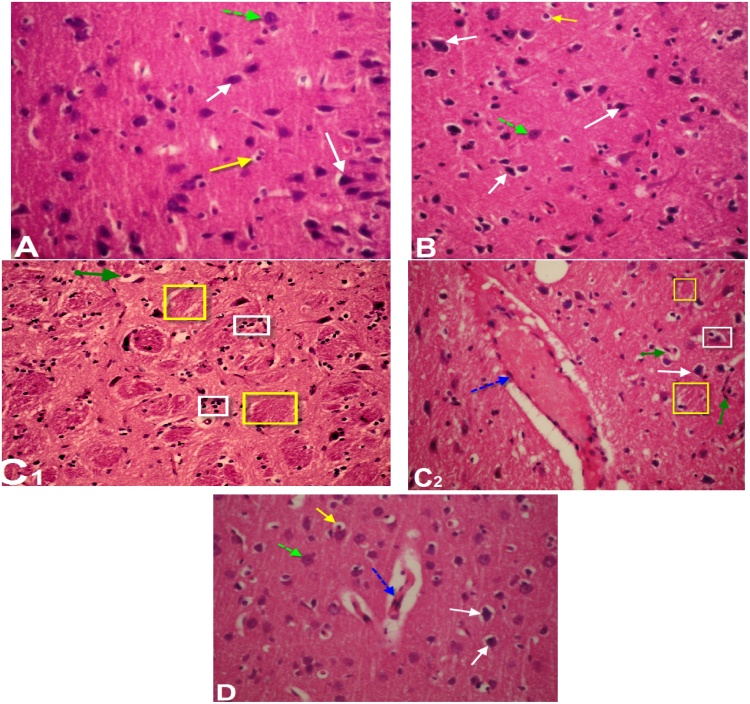
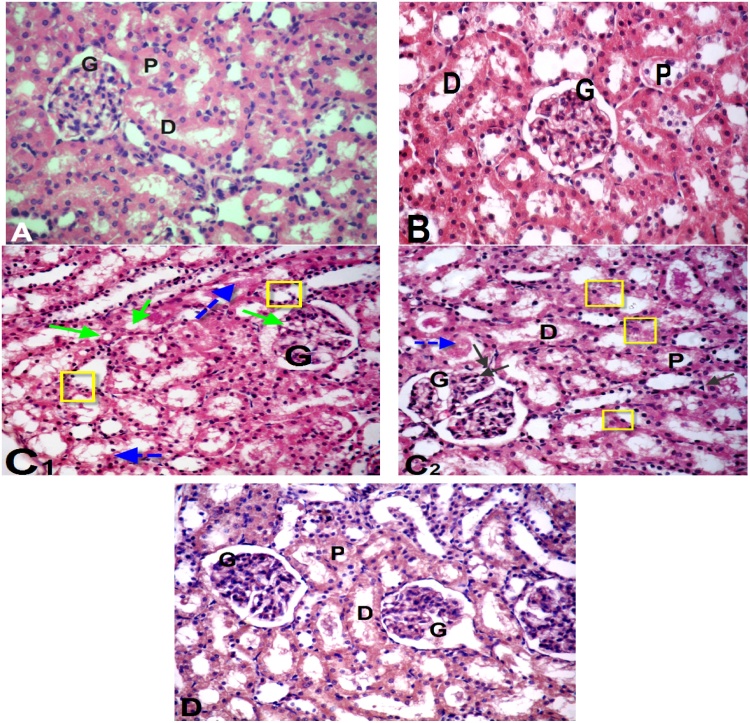
Histological study of brain sections of control and GSPE (Fig. 6A & B) revealed normal histoarchitecture of the pyramidal and polymorphic cells in the cerebral cortex. Sections in cerebral cortex of thalidomide and carboplatin-treated group showed loss of normal structure, neuronal degeneration with pyknotic nuclei, dilatation of blood capillary with hemorrhage (Fig. 6C1 & C2) compared to control group. However, sections of brain of rats treated with thalidomide, carboplatin and GSPE exhibited slightly restored neuronal cells more or less near to normal structure with few glail cells and residual dilatation and hemorrhage of blood vessels (Fig. 6D). The histological examination of kidney of the control and GSPE groups showed normal kidney structure which contains normal glomeruli, proximal and distal convoluted tubules (Fig. 7A & B). On the other hand, thalidomide and carboplatin treatment induced histopathological alterations in the kidney such as shrinkage of capillaries in the glomerulus with vacuolization and capsular space, slightly degeneration with pyknotic nuclei of both proximal and distal tubules compared to the control group (Fig. 7C1 & C2). Supplementation of GSPE in combination with thalidomide and carboplatin showed partial improvement of histopathological alterations compared to thalidomide and carboplatin treated group (Fig. 7D).
Fig. 6.
Light micrograph of brain of male rats: (A) Control: normal histo-architecture. (B) Section of the cerebral cortex of rats treated with grape seed proanthocyanidin extract (GSPE), showing normal histology of the pyramidal cells layer and the polymorphic layer (white and green dotted arrows) and few glail cells (yellow arrow). (C1 & C2): Thalidomide and carboplatin-treated rats showing diffused gliosis (white square), dilatation of blood capillary with hemorrhage and pyknotic nuclei (blue dotted arrow), neuronal degeneration (green arrow) and encephalomelacia (yellow square) (D). Section of brain of rat treated with thalidomide, carboplatin and GSPE showing slightly restores of the neuronal cells more or less near to normal structure (black arrow) with few glail cells (yellow arrow) and residual dilatation & hemorrhage of blood vessels (blue dotted dotted arrow). (H & E, 400 X).
Fig. 7.
Photomicrograph of kidney sections: (A) Control group and (B) Group of grape seed proanthocyanidin extract (GSPE) treated rats showing normal cortical architecture with normal glomerulus (G), proximal convoluted tubules (P), distal convoluted tubules (D). (C1 & C2) Sections in kidney tissue of thalidomide and carboplatin -treated group showing shrunken glomeruli (G) with the capsular space, degenerative changes in the epithelial cells lining the renal tubules with pyknotic nuclei (yellow square & black arrows), vacuolization (green arrows) and tubular lumen with cellular debris & congestion (blue dotted arrows). Histological alterations induced after thalidomide and carboplatin treatment were markedly reduced in the combination group (D) thalidomide, carboplatin + GSPE treated rats showing moderate improvement in kidney tissue. (H. & E. 400 X).
4. Discussion
4.1. Carboplatin and thalidomide
Our work aimed to investigate the neurotoxicity and nephrotoxicity induced by carboplatin and thalidomide through changes in cytokines, tumor suppressor protein p53, neurotransmitters, oxidative stress and histopathology. The obtained data revealed that treatment with thalidomide and carboplatin enhanced the expression of kidney and brain tumor suppressor P53 (P53), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (Fig. 1). Tumor necrosis factor-alpha (TNF-α) is a cytokine that plays a central role in the regulation of host immune and inflammatory response to infection. In the central nervous system, TNF-α is involved in induction of a fever response and triggers the release of other cytokines, and may also influence transport of compounds into the brain, leading to cerebrospinal fluid leukocytosis, increased protein influx, and lactate accumulation [21]. Cytokines regulate function of many cells in an additive, synergistic or antagonistic manner. Interleukin-6 (IL-6) is mainly secreted by fibroblasts, macrophages and lymphocytes. It promotes tumor growth by up-regulating antiapoptotic and angiogenic proteins in tumor cells [22,23]. Activation of the p53 pathway has been known as one of the central mechanisms for DNA-damaging agents to induce cell apoptosis and tumor inhibition [24]. Cisplatin and its second generation drug carboplatin act similarly, i.e. both drugs cause a concomitant decrease in p53 mRNA and an increase in p53 protein level [25]. The present study revealed that carboplatin and thalidomide induced oxidative stress through increasing the levels of free radicals and nitric oxide, and the depletion of glutathione levels and inhibition of antioxidant enzymes. The induction of the cytokines and P53 might be due to the oxidative stress. Also, Ramesh and Reeves [26] stated that hydroxyl radicals, either directly or indirectly, activate p38 MAPK (mitogen activated protein kinase) which plays an important role in mediating cisplatin-induced acute renal injury and inflammation, through the production of TNF-α. The p53 tumor suppressor pathway is a key mediator of the stress response that protects the organism from accumulating genetically altered and potentially cancerous cells by inducing growth arrest or apoptosis in damaged cells [27]. Activation of microglia in hippocampus results in releasing many neuro-inflammatory mediators such as TNF-α, IL-1β and NF-κB [28]. Moreover, microglial activation can induce an inflammatory-oxidative cascade leading to cognitive deficit [29]. In addtion, TNF-α was capable of inducing the death of cultured dopaminergic neurons [30].
Neurotoxicity, severe cumulative myelosuppression, renal toxicity and ototoxicity are commonly caused by platinum-based chemotherapy [31]. The dorsal root ganglia (DRG) are the main target of platinum drug-induced peripheral neuropathies induced by chemotherapy (CIPN) [32]. The body of the experimental evidence points toward 2 different putative mechanisms, not necessarily mutually exclusive, because both can eventually produce DRG neuron apoptosis: (i) the formation of platinum intra-strand adducts and inter-strand crosslinks, which influence the tertiary structure of the nuclear DNA [33], alternating cell-cycle kinetics [34] and (ii) the interaction with mitochondrial DNA, leading to oxidative stress [35] and possibly to p53 increased activity and mitochondrial release of cytochrome-c pathway [36].
Carboplatin causes dose-limiting and cumulative myelosuppression, characterized by frequent and severe thrombocytopenia, granulocytopenia and anaemia. Likewise, cisplatin is associated with several cumulative and irreversible toxicities, including dose-dependent renal tubule toxicity and neurotoxicity [37]. Cisplatin induces decrease in immunoreactivity for most of the selected neurotransmitter markers [38]. Our results reveal that treatment with thalidomide and carboplatin causes neurotoxicity via decreased AChE, dopamine and serotonin and increased norepinephrine in brain (Fig. 2). Administration of chemicals disturbs the spontaneous activity of the cells and influences neurotransmitter turnover. Thus the level of neurotransmitters in brain and plasma of rats has been evaluated. Neurotransmitters are known to play a key role in memory, awareness, thought, and consciousness and allow the organism to become alert and guards against the intensification of reflex reactions and other behavior. Our results suggest that treatment with the carboplatin and thalidomide resulted in altered synthesis and release of certain neurotransmitters and receptors in nerve cells, leading to neural damage. In addition, ROS could attack the various kinds of neuronal connections and decrease the memory in experimental animals [39]. The resulting oxidative stress and brain injury in the present study might be due to a cascade of reactions triggered by chemotherapeutic agents, such as lipid peroxidation, decreased activity of antioxidant enzymes, release of nitric oxide, reduction of glutamic acid, and changeable levels of AChE, dopamine, serotonin and norepinephrine. Astrocytes are involved in several homeostatic functions such as regulating neurotransmitter concentrations (i.e., glutamate, GABA), secreting neurotrophic factors and maintaining BBB integrity [40]. As well as, cytokines and chemokines, enabling the regulation of immune signalling in both normal and diseased brain. Cisplatin induced decrease in immunoreactivity for most of the selected neurotransmitter markers, thereby altering the postnatal development of circuits in the hippocampal formation. Cisplatin also brought out clear evidence for an interaction between excitatory and inhibitory neurotransmitter markers during the postnatal maturation of cells and fiber projections containing GluR2/3 and GAD65, despite the fact that glutamatergic neurons and GABAergic interneurons are divergent in their source of genesis and in their mode of migration [38]. Mechanistically, both autonomic and neuroendocrine function may promote stress-induced inflammation. Norepinephrine enhances proinflammatory cytokines by inducing nuclear factor kB (NF-kB) transcription, an intracellular signaling molecule that regulates proinflammatory cytokine gene expression [41,42]. Furthermore, higher levels of parasympathetic activity can reduce inflammation via the cholinergic anti-inflammatory pathway that induces acetylcholine release [43].
Antioxidant enzymes are the first line of defense against free radicals/reactive oxygen species (ROS)-induced oxidative renal injury [13]. Thiobarbituric acid reactive substances (TBARS) are produced by lipid peroxidation and are considered as indicators of oxidative stress [44]. Anticancer agents usually demolish the physiological homoeostasis in various organs during treatment of cancer. Physiological side effects can occur that are induced in non-tumor cells mostly by radical formation and oxidant injury [45,46]. The present results indicate that treatment with thalidomide and carboplatin induces oxidative stress via increased TBARS and NO, and decreased antioxidant enzymes, GSH and TAC in brain and kidney. This is agreement with Yousef and Hussien [47] who found that cisplatin increased the levels of kidney TBARS, xanthine oxidase and nitric oxide, and decreased the activities of antioxidant enzymes (GST, GPX, CAT and SOD), and the levels of GSH. Moreover, our previous studies showed that treatment with cisplatin induced lipid peroxidation and inhibited the activities of the antioxidant enzymes in kidney [10,11].
Kidneys represent the major control system maintaining body homeostasis. Plasma concentration of creatinine determines renal function and is thus considered as biomarker of kidney disease [48]. The obtained results indicate that thalidomide and carboplatin induce nephrotoxicity via increased creatinine and bilirubin. The kidney is considered to be the primary target organ for carboplatin toxicity. Also, cisplatin significantly increased the levels of plasma total lipid, cholesterol, urea and creatinine, and the relative weight of kidney [11]. Carboplatin-induced nephrotoxicity may be due to impaired antioxidant enzyme activities and suppression of antioxidant enzymes protein expression in the kidney [44], and this is in agreement with the obtained data that shows increase in TBARS, NO, and decrease in the antioxidant enzymes activity and GSH levels in kidney of rats treated with carboplatin and thalidomide.
Histological alterations in kidney showed that thalidomide and carboplatin administration induces morphological changes in the kidney tissues compared to the control group. They caused atrophy in glomerulus and degeneration of epithelial of proximal and distal tubules. These results are in the same line with Kim et al. [49] who reported that injection of cisplatin caused renal morphological changes including tubular necrosis desquamation and degeneration in the proximal and distal tubules. Carboplatin nephrotoxicity has suggested that higher cumulative dose is associated with greater tubular toxicity [50]. Increased TNF-α production in response to cisplatin treatment has been demonstrated in renal epithelial cells in vitro, raising the possibility that renal parenchymal cells may be a major source of TNF-α production [26]. In the same manner kidney cytokines (IL-6 and TNF-α) increased due to treatment with carboplatin and thalidomide. We hypothesize that the induction of oxidative stress could cause kidney damage and increase the levels of cytokines.
Histological study of brain sections of thalidomide and carboplatin-treated group showed loss of normal structure, neuronal degeneration with pyknotic nuclei, and dilation of blood capillary with hemorrhage. Similarly, cisplatin has negative effects on cerebellar cortex neurons [51]. Gulec et al. [52] suggested that sufficient levels of cisplatin have been shown to cause toxicity in the brain. Our result indicate that cytokines induced due to exposure to carboplatin and thalidomide in brain which caused neurotoxicity and this may be due to the upregulation of free radicals, downregulation in the antioxidant enzymes, and the depletion in the levels of glutathione.
4.2. Grape seed proanthocyanidin exract
The present results indicate the ability of GSPE to ameliorate carboplatin and thalidomide induced neurotoxicity and nephrotoxicity. Similarly, our previous studies tested the protective effect of GSPE against cisplatin in kidney showed that GSPE treatment significantly protected genomic DNA, lipid peroxidation, inhibition of antioxidant enzymes and alterations of biochemical parameters in plasma and kidney of rats [10,11]. It seems likely that GSPE inhibits the inflammatory mediators by several mechanisms. Macrophages sense the presence of pathogens through Toll-like receptors (TLRs) and other receptors. Among TLRs, TLR4 has a dominant role in various inflammatory diseases [53]. Stimulation of TLR4 by lipopolysaccharide (LPS) triggers the recruitment of the cytoplasmic adaptor protein MyD88 and the activation of TAK1, which subsequently activates downstream signaling pathways such as the MAPKs and nuclear factor-kappa B (NF-κB). Also, Chu et al. [54] reported that GSPE has been found to suppress the mRNA expression of pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and inflammatory molecule of cyclooxygenase-2 (COX-2), while mRNA level of IL-10 was greatly promoted. Furthermore, GSPE inhibited the phosphorylation of extracellular signal-regulated protein kinase (ERK), c-Jun terminal kinase (JNK) and P38, IKK α/β and nuclear factor-kappa B (NF-κB) p65 subunit. GSPE may exert its therapeutic effects on the collagen-induced arthritis (CIA) animal model through its anti-inflammatory effects as well as its antioxidant activity [54,55]. Our results showed that GSPE showed its anti-inflammatory by suppressing the induction of cytokines (IL-6 and TNF-α) and P53. Ramesh and Reeves [26] stated that hydroxyl radicals, either directly or indirectly, activate p38 MAPK (mitogen activated protein kinase) which plays an important role in mediating cisplatin-induced acute renal injury and inflammation, through the production of TNF-α. Our previous in vivo studies demonstrated that GSPE has specificity for the hydroxyl radical and decrease the over generation of free radicals [10,11].
Lipid peroxidation is ascribed to a free radical–mediated chain reaction that damages cell membranes and inhibition of this process by GSPE is mainly attributed to its high ability of scavenging free radicals. In the present study, combined treatment of carboplatin and thalidomide and GSPE resulted in significant decrease in TBARS, significant elevation in GSH content of brain and renal tissue indicating the protection offered by GSPE against carboplatin and thalidomide damage. GSPE also maintained the activities of CAT, SOD, GPx, GST and TAC and GSH-dependent antioxidant enzymes when compared to control group which indicates the protective nature of GSPE against carboplatin and thalidomide induced oxidative stress. The obtained results are in agreement with Saad et al. [10] and Yousef et al. [11] who reported the beneficial effects of GSPE in nephroprotection against cisplatin-induced kidney injury, in which oxidative stress was long known to contribute to the pathogenesis. The chemical properties of proanthocyanidins in term of the availability of the phenolic hydrogens as hydrogen donating radical scavengers and singlet oxygen quenchers predict their antioxidant activity [56]. This antioxidant activity has been proven to be significantly more potent than that of vitamin C, E or beta carotene [57].
Moskaug et al. [58] reported that dietary plant polyphenols, namely the flavonoids, modulate expression of an important enzyme (gamma-glutamylcysteine synthetase) in both cellular antioxidant defenses and detoxification of xenobiotics. This enzyme is rate limiting in the synthesis of the most important endogenous antioxidant in cells, glutathione. Since, proanthocyanidins are naturally occurring polyphenolic compounds [56], the protective effect of GSPE against cisplatin-induced toxicity may be attributed to GSPE potential to enhance glutathione synthesis. Also, GSPE exhibits free radical scavenging ability, inhibition of DNA topoisomerase II activity, inhibition of protein kinase C, anti-endonucleolytic activity and cytochrome P450 2E1 inhibitory effect. It was also reported to show anti-carcinogenic activities, modulatory effects on oxidative and apoptotic regulatory genes such as Bcl2, c-myc and p53, which may be responsible for the novel chemo-preventive properties exhibited by GSPE [56]. In vivo studies have shown that GSPE is a better free radical scavenger and inhibitor of oxidative tissue damage, DNA fragmentation, and subsequent apoptosis than all the antioxidant vitamins. In fact, it is twenty times more potent than Vitamin C, and 50 times stronger than Vitamin E [59].
The present results showed that GSPE alone or in combination with carboplatin and thalidomide significantly lowered the lipid profile. Oral administration of proanthocyanidins from grape seed produced a hypocholesterolemic effect in a high-cholesterol animal feed model; specifically, it prevented an increase in total and LDL plasma cholesterol and a decrease in HDL [60]. Natella et al. [61] stated that oligomeric proanthocyanidins supplementation resulted in decreased lipid peroxidation, increased plasma antioxidant levels, and improved resistance of LDL to oxidation in volunteers consuming a lipid-rich test meal. The significant decrease in the elevated levels of both parameters in rats receiving GSPE prior to cisplatin indicated the ability of GSPE to counteract cisplatin-induced toxicity.
Histological examination of kidney showed that the co-administration of GSPE in combination with thalidomide and carboplatin revealed attenuation in the kidney tissues (Fig. 7). Also, the results of Ray et al. [62] suggested that GSPE is bioavailable, and its significant potential to prevent cisplatin-induced acute renal failure may be attributed to the attenuation of renal tubular damage and enhancement of the regenerative response of the damaged tubular cells. GSPE supplementation might ameliorate cisplatin [56], anti-inflammatory effects [63] and this is in agreement with the obtained data that shows decrease of cytokines and tumor suppressor P53 expression in kidney (Fig. 1). From the view point of pathophysiology, the major cytotoxicity of cisplatin is inhibition of DNA synthesis and the production of reactive oxygen species; consequently, the mechanism underlying proanthocyanidins nephroprotection may be due to their marked radical scavenging ability [64].
5. Conclusion
Chemotherapy diminishes the normal homeostasis of the body, a fact which is particularity applicable for carboplatin and thalidomide treatment. Despite their effectiveness, the doses of carboplatin and thalidomide that can be administered are limited by their neurotoxicity and nephrotoxicity. In the present study, it is clear that carboplatin and thalidomide exposure resulted in the induction of inflammatory cytokines, P53, lipid peroxidation, and reduction in neurotransmitters and antioxidant enzymes, as well as alterations of biochemical parameters and histological examinations in brain and kidney tissues of rats. Carboplatin and thalidomide-induced oxidative stress has been proven to be responsible for the induced neurotoxicity and nephrotoxicity of the drugs. Antioxidants have proven to be effective in ameliorating chemotherapeutic drugs-induced toxicity in many preclinical and few clinical interventions. Concomitant treatment of GSPE with the combination of carboplatin and thalidomide provided near complete protection in terms of brain and kidney cytokines, P53, neurotransmitters, oxidative stress, antioxidant enzymes activity, biochemical and histological changes. Therefore, we suggest that GSPE may be a potential preventive agent against neurotoxicity and nephrotoxicity induced by carboplatin and thalidomide.
Declaration of interest
The authors report no conflict of interest.
Acknowledgement
We thank Dr. Lamiaa El-Shennawy, Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University for language editing.
References
- 1.Cheung-Ong K., Giaever G., Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem. Biol. 2013;20(5):648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Husain K., Scott R.B., Whitworth C., Somani S.M., Rybak L.P. Dose response of carboplatin-induced hearing loss in rats: antioxidant defense system. Hear. Res. 2001;151(1):71–78. doi: 10.1016/s0300-2977(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 3.Franks M.E., Macpherson G.R., Figg W.D. Thalidomide. Lancet. 2004;363(9423):1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.H., Scialli A.R. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol. Sci. 2011;122(1):1–6. doi: 10.1093/toxsci/kfr088. Epub 2011 Apr 19. [DOI] [PubMed] [Google Scholar]
- 5.Teo S.K., Stirling D.I., Zeldis J.B. Thalidomide as a novel therapeutic agent: new uses for an old product. Drug Discov. Today. 2005;10(2):107–114. doi: 10.1016/S1359-6446(04)03307-0. [DOI] [PubMed] [Google Scholar]
- 6.Conklin K.A. Cancer chemotherapy and antioxidants. J. Nutr. 2004;134:3201S–3204S. doi: 10.1093/jn/134.11.3201S. https://www.ncbi.nlm.nih.gov/pubmed/15514307 [DOI] [PubMed] [Google Scholar]
- 7.Bagchi D., Bagchi M., Stohs S.J., Das D.K., Ray S.D., Kuszynski C.A., Joshi S.S., Pruess H.G. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148(2-3):187–197. doi: 10.1016/s0300-483x(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 8.Bagchi D., Swaroop A., Preuss H.G., Bagchi M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: an overview. Mutat. Res. 2014;768:69–73. doi: 10.1016/j.mrfmmm.2014.04.004. Epub 2014 Apr 19. [DOI] [PubMed] [Google Scholar]
- 9.Shan Y., Ye X.H., Xin H. Effect of the grape seed proanthocyanidin extract on the free radical and energy metabolism indicators during the movement. Sci. Res. Essays. 2010;5(2):148–153. [Google Scholar]
- 10.Saad A.A., Youssef M.I., El-Shennawy L.K. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: the protective effect of grape seed proanthocyanidin extract. Food Chem. Toxicol. 2009;47(7):1499–1506. doi: 10.1016/j.fct.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Yousef M.I., Saad A.A., El-Shennawy L.K. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem. Toxicol. 2009;47(6):1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Kaczmarczyk-Sedlak I., Zych M., Rotko K., Sedlak L. Effects of thalidomide on the development of bone damage caused by prednisolone in rats. Pharmacol. Rep. 2012;64:386–395. doi: 10.1016/s1734-1140(12)70779-x. http://www.if-pan.krakow.pl/pjp/pdf/2012/2_386.pdf [DOI] [PubMed] [Google Scholar]
- 13.Husain K., Jagannathan R., Hasan Z., Trammell G.L., Rybak L.P., Hazelrigg S.R., Somani S.M. Dose response of carboplatin-induced nephrotoxicity in rats. Pharmacol. Toxicol. (Oxf. U. K.) 2002;91(2):83–89. doi: 10.1034/j.1600-0773.2002.910207.x. https://www.ncbi.nlm.nih.gov/pubmed/12420797 [DOI] [PubMed] [Google Scholar]
- 14.Yang A., Kaghad M., Caput D., McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. TRENDS Genet. 2002;18(2):90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 15.Hedayati M., Yazdanparast R., Azizi F. Determination of human tumor necrosis factor α by a highly sensitive enzyme immunoassay. Biochem. Biophys. Res. Commun. 2001;289(1):295–298. doi: 10.1006/bbrc.2001.5886. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson-Smith A.C., Chen Y.F., Newman M.S., May L.T., Sehgal P.B., Ruddle F.H. Regional localization of the interferon-β2B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21. Genomics. 1988;2(3):203–208. doi: 10.1016/0888-7543(88)90003-1. [DOI] [PubMed] [Google Scholar]
- 17.Ellman G.L., Courtney K.D., Anders V.J.R., Featherstone R.M. A new rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 18.Drury R.A., Wallington E.A., Carleton S. 5th ed. Oxford University Press; London, New York, Toronto: 1980. Histological Techniques; pp. 241–242. [Google Scholar]
- 19.SAS, Statistical Analysis System . SAS Institute Inc.; Cary, Nc, U.S.A: 1998. SAS Procedure Guide. Release 6.03 Edition. [Google Scholar]
- 20.Duncan D.B. Multiple range and multiple F tests. Biometrics. 1955;11(1):1–42. [Google Scholar]
- 21.Pasa S., Altintas A., Cil T., Ustun C., Bayan K., Danis R., Urakci Z., Tuzun Y., Ayyildiz O. Two cases of bacterial meningitis accompanied by thalidomide therapy in patients with multiple myeloma: is thalidomide associated with bacterial meningitis? Int. J. Infect. Dis. 2009;13(1):e19–e22. doi: 10.1016/j.ijid.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Purohit A., Newman S.P., Reed M.J. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4(2):65–69. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer Z.T., Brugge J.S. IL-6 involvement in epithelial cancers. J. Clin. Invest. 2007;117(12):3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnstone R.W., Ruefli A.A., Lowe S.W. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 25.Siemer S., Ørnskov D., Guerra B., Boldyreff B., Issinger O.G. Determination of mRNA, and protein levels of p53, MDM2 and protein kinase CK2 subunits in F9 cells after treatment with the apoptosis-inducing drugs cisplatin and carboplatin. Int. J. Biochem. Cell Biol. 1999;31(6):661–670. doi: 10.1016/s1357-2725(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 26.Ramesh G., Reeves W.B. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol. Ren. Physiol. 2005;289(1):F166–74. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 27.Reinhardt H.C., Schumacher B. The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 2012;28(3):128–136. doi: 10.1016/j.tig.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva B., Sousa L., Miranda A., Vasconcelos A., Reis H., Barcelos L., Arantes R., Teixeira A., Rachid M.A. Memory deficit associated with increased brain proinflammatory cytokine levels and neurodegeneration in acute ischemic stroke. Arquivos de neuro-psiquiatria. 2015;73(8):655–659. doi: 10.1590/0004-282X20150083. [DOI] [PubMed] [Google Scholar]
- 29.Khalil R.M., Khedr N.F. Curcumin protects against monosodium glutamate neurotoxicity and decreasing NMDA2B and mGluR5 expression in rat hippocampus. Neurosignals. 2016;24(1):81–87. doi: 10.1159/000442614. Epub 2016 Aug 17. [DOI] [PubMed] [Google Scholar]
- 30.Chen S.C., Chen W.C. Vascular leakage induced by exposure to arsenic via increased production of NO, hydroxyl radical and peroxynitrite. Microvasc. Res. 2008;75(3):373–380. doi: 10.1016/j.mvr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Windebank A.J., Grisold W. Chemotherapy‐induced neuropathy. J. Peripher. Nerv. Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 32.Cavaletti G., Tredici G., Marmiroli P., Petruccioli M.G., Barajon I., Fabbrica D. Morphometric study of the sensory neuron and peripheral nerve changes induced by chronic cisplatin (DDP) administration in rats. Acta Neuropathol. 1992;84(4):364–371. doi: 10.1007/BF00227662. https://link.springer.com/article/10.1007/BF00227662 [DOI] [PubMed] [Google Scholar]
- 33.Ta L.E., Espeset L., Podratz J., Windebank A.J. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum–DNA binding. Neurotoxicology. 2006;27(6):992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Gill J.S., Windebank A.J. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J. Clin. Invest. 1998;101(12):2842–2850. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H., Mizumachi T., Carcel-Trullols J., Li L., Naito A., Spencer H.J., Spring P.M., Smoller B.R., Watson A.J., Margison G.P., Higuchi M. Targeting human 8-oxoguanine DNA glycosylase (hOGG1) to mitochondria enhances cisplatin cytotoxicity in hepatoma cells. Carcinogenesis. 2007;28(8):1629–1637. doi: 10.1093/carcin/bgm072. [DOI] [PubMed] [Google Scholar]
- 36.McDonald E.S., Windebank A.J. Cisplatin-Induced apoptosis of DRG neurons involves Bax redistribution and cytochrome cRelease but not fas receptor signaling. Neurobiol. Dis. 2002;9(2):220–233. doi: 10.1006/nbdi.2001.0468. [DOI] [PubMed] [Google Scholar]
- 37.Pignata S., De Placido S., Biamonte R., Scambia G., Di Vagno G., Colucci G., Febbraro A., Marinaccio M., Lombardi A.V., Manzione L., Cartenì G. Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the multicenter Italian trial in ovarian cancer (MITO-4) retrospective study. BMC Cancer. 2006;6(1):5. doi: 10.1186/1471-2407-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccolinia V.M., Cerri S., Romanelli E., Bernocchi G. Interactions of neurotransmitter systems during postnatal development of the rat hippocampal formation: effects of cisplatin. Exp. Neurol. 2012;234(1):239–252. doi: 10.1016/j.expneurol.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 39.Ma L., Liu J., Li N., Wang J., Duan Y., Yan J., Liu H., Wang H., Hong F. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO 2 delivered to the abdominal cavity. Biomaterials. 2010;31(1):99–105. doi: 10.1016/j.biomaterials.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Speth C., Dierich M.P., Sopper S. HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol. Immunol. 2005;42(2):213–228. doi: 10.1016/j.molimm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Bierhaus A., Wolf J., Andrassy M., Rohleder N., Humpert P.M., Petrov D., von Eynatten R.FerstlM., Wendt T., Rudofsky G., Joswig M., Morcos M., Schwaninger M., McEwen B., Kirschbaum C., Nawroth P.P. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straub R., Härle P. Sympathetic neurotransmitters in joint inflammation. Rheum. Dis. Clin. North Am. 2005;31(1):43–59. doi: 10.1016/j.rdc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Tracey K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karthikeyan K., Bai B.S., Devaraj S.N. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int. J. Cardiol. 2007;115(3):326–333. doi: 10.1016/j.ijcard.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Yagmurca M., Bas O., Mollaoglu H., Sahin O., Nacar A., Karaman O., Songur A. Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch. Med. Res. 2007;38(4):380–385. doi: 10.1016/j.arcmed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Yagmurca M., Fadillioglu E., Erdogan H., Ucar M., Sogut S., Irmak M.K. Erdosteine prevents doxorubicin-induced cardiotoxicity in rats. Pharmacol. Res. 2003;48(4):377–382. doi: 10.1016/s1043-6618(03)00185-3. https://www.ncbi.nlm.nih.gov/pubmed/12902208 [DOI] [PubMed] [Google Scholar]
- 47.Yousef M.I., Hussien H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin-6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem. Toxicol. 2015;78:17–25. doi: 10.1016/j.fct.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Levey S.A., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann. Intern. Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. https://www.ncbi.nlm.nih.gov/pubmed/10075613 [DOI] [PubMed] [Google Scholar]
- 49.Kim Y.J., Lee M.Y., Son H.Y., Park B.K., Ryu S.Y., Jung J.Y. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014;80(08/09):645–654. doi: 10.1055/s-0034-1368571. [DOI] [PubMed] [Google Scholar]
- 50.English M.W., Skinner R., Pearson A.D.J., Price L., Wyllie R., Craft A.W. Dose-related nephrotoxicity of carboplatin in children. Br. J. Cancer. 1999;81(2):336–341. doi: 10.1038/sj.bjc.6690697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owoeye O., Onwuka S.K. Tomato pomace powder ameliorated cisplatin-induced microanatomical alterations in brain of Wistar rats. Int. J. Biol. Chem. Sci. 2015;9(1):1–11. [Google Scholar]
- 52.Gulec M., Oral E., Dursun O.B., Yucel A., Hacimuftuoglu A., Akcay F., Suleyman H. Mirtazapine protects against cisplatin‐induced oxidative stress and DNA damage in the rat brain. PCN. 2013;67(1):50–58. doi: 10.1111/j.1440-1819.2012.02395.x. [DOI] [PubMed] [Google Scholar]
- 53.Zuany-Amorim C., Hastewell J., Walker C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat. Rev. Drug Discov. 2002;1(10):797–807. doi: 10.1038/nrd914. [DOI] [PubMed] [Google Scholar]
- 54.Chu H., Tang Q., Huang H., Hao W., Wei X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264. 7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2016;41:159–166. doi: 10.1016/j.etap.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Tang Q., Zou P., Jin H., Fu J., Yang J., Shang L., Wei X. Grape-seed proanthocyanidins ameliorate contact hypersensitivity induced by 2, 4-dinitrofluorobenzene (DNFB) and inhibit T cell proliferation in vitro. Toxicol. Lett. 2012;210(April (1)):1–8. doi: 10.1016/j.toxlet.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Bagchi D., Bagchi M., Stohs S.J., Ray S.D., Sen C.K., Preuss H.G. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002;957(1):260–270. doi: 10.1111/j.1749-6632.2002.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 57.Joshi S.S., Kuszynski C.A., Bagchi D. The cellular and molecular basis of health benefits of grape seed proanthocyanidin extract. Curr. Pharm. Biotechnol. 2001;2(2):187–200. doi: 10.2174/1389201013378725. [DOI] [PubMed] [Google Scholar]
- 58.Moskaug J.Ø., Carlsen H., Myhrstad M.C., Blomhoff R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005;81(Suppl. (1):277S–283S. doi: 10.1093/ajcn/81.1.277S. https://www.ncbi.nlm.nih.gov/pubmed/15640491 [DOI] [PubMed] [Google Scholar]
- 59.Bagchi D., Garg A., Krohn R.L., Bagchi M., Tran M.X., Stohs S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997;95(2):179–189. https://www.ncbi.nlm.nih.gov/pubmed/9090754 [PubMed] [Google Scholar]
- 60.Fine A.M. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Altern. Med. Rev.: J. Clin. Ther. 2000;5(2):144–151. http://europepmc.org/abstract/med/10767669 [PubMed] [Google Scholar]
- 61.Natella F., Belelli F., Gentili V., Ursini F., Scaccini C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J. Agric. Food Chem. 2002;50(26):7720–7725. doi: 10.1021/jf020346o. [DOI] [PubMed] [Google Scholar]
- 62.Ray S.D., Patel D., Wong V., Bagchi D. In vivo protection of DNA damage associated apoptotic and necrotic cell deaths during acetaminophen-induced nephrotoxicity, amiodarone-induced lung toxicity and doxorubicin-induced cardiotoxicity by a novel IH636 grape seed proanthocyanidin extract. Res. Commun. Mol. Pathol. Pharmacol. 2000;107(1–2):137–166. https://www.ncbi.nlm.nih.gov/pubmed/11334364 [PubMed] [Google Scholar]
- 63.Bayeta E., Lau B.H. Pycnogenol inhibits generation of inflammatory mediators in macrophages. Nutr. Res. 2000;20(2):249–259. [Google Scholar]
- 64.Sato S., Hori Y., Yamate J., Saito T., Kurasaki M., Hatai A. Protective effect of dietary azuki bean (Vigna angularis) seed coats against renal interstitial fibrosis of rats induced by cisplatin. Nutrition. 2005;21(4):504–511. doi: 10.1016/j.nut.2004.07.019. [DOI] [PubMed] [Google Scholar]