Abstract
Objectives
To explore whether complex glandular patterns (CGPs) have a potential role in the clinical management of patients with lung adenocarcinoma.
Methods
We included 356 patients with lung adenocarcinoma with available clinicopathologic information, gene mutations, and clinical outcomes for analysis.
Results
We identified 54 (15.2%) CGP-predominant cases. The CGPs were associated with ALK rearrangement and HER2 mutation. Survival analysis showed that the clinical outcome of CGP-predominant patients was worse than that for acinar-predominant patients (overall survival [OS], 66.4 vs 90.3 months, P < .01; recurrence-free survival [RFS], 50.1 vs 73.1 months, P = .022) but was comparable with solid-predominant subtype tumors (OS, 66.4 vs 67.8 months, P = .558; RFS, 50.1 vs 41.3 months, P = .258). In particular, the coexistence of the cribriform and fused gland pattern was associated with the poorest survival, with a death risk increased by 2.25-fold (hazard ratio, 3.25; 95% confidence interval, 1.35-7.86, P = .009).
Conclusions
Our results provide new insight into the potential role of CGPs in clinical management and will be beneficial for treatment decision making in patients with lung adenocarcinoma.
Keywords: Complex glandular patterns, Cribriform, Fused gland, Lung adenocarcinoma
Lung cancer is one of the most common cancer types and also the leading cause of cancer-related deaths worldwide.1,2 In 2011, the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) proposed a new classification scheme for lung adenocarcinoma.2 Four years later, the World Health Organization (WHO) proposed the classification of tumors of the lung, with only minor changes compared with the lung adenocarcinoma classification (2011) published by the IASLC/ATS/ERS, which classified lung cancer into five main pathologic subtypes, including acinar, solid, micropapillary, papillary, and lepidic.3 Both the 2011 IASLC/ATS/ERS and 2015 WHO classifications were widely adopted in clinical and pathologic practice, and their predictive roles for patient clinical outcomes had been confirmed by several studies.4-7 Interestingly, during the clinical practice of the 2015 WHO classification, we noted that some complex glandular patterns (CGPs) (eg, cribriform and fused gland) presented distinct pathologic features apart from the five main subtypes of adenocarcinoma (acinar, solid, micropapillary, papillary, and lepidic). In a white population-based study, CGPs were mainly found in high-grade lung adenocarcinoma.8 Before the 2015 WHO classification was published, whether CGPs, especially the cribriform pattern, should be classified as a new type of lung adenocarcinoma remained controversial.8-10
With aims to explore whether CGPs have a potential role in the clinical management of patients with lung adenocarcinoma, we included 356 patients with lung adenocarcinoma who had available clinical information and survival data. This cohort represented a relatively homogeneous and well-defined eastern Asian population. Furthermore, we investigated the associations of CGPs with some related gene mutations and rearrangement to reveal whether genetic alternation may be induced by CGPs. In particular, the coexistence of the cribriform and fused gland (CCFG) pattern was also taken into investigation as a distinct CGP subtype for its association with survival and clinical characteristics of these patients.
Materials and Methods
Source of Clinical Data
In the present study, we included 356 patients with lung adenocarcinoma (stages I-III) who had undergone surgical resection at the Fudan University Shanghai Cancer Center from 2006 to 2013. Patients were restaged according to the eighth edition of the TNM classification.11 The last follow-up date was October 9, 2016. All procedures performed in the present study involving human participants were in accordance with the ethical standards of the Committee for Ethical Review of Research of Fudan University Shanghai Cancer Center and also conformed to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Tumor tissues were obtained with signed consent for the purpose of scientific research from the included patients.
Histologic Analysis and Evaluation
All resected specimens were formalin fixed immediately after resection and stained with H&E. The slides were measured independently by two pathologists (X.S. and Y.L.) who were blinded to the clinical data. The evaluation criterion was according to the WHO and IASLC/ATS/ERS classification of adenocarcinoma.2,3 The criterion of the cribriform pattern was in accordance with Moreira et al,8 who defined the cribriform pattern as nests of tumor cells with a sieve-like perforation and the fused gland pattern as fused glands with irregular borders, back-to-back glands without intervening stroma, or ribbon-like formations. In the present study, CGPs were subdivided into the single cribriform pattern, single fused gland pattern, and CCFG pattern. The single cribriform pattern (SCP) is judged according to the following criteria: SCP ≥10% and single fused gland (SFG) ≤9%. The SFG is judged according to the following criteria: SFG ≥10% and SCP ≤9%. CCFG pattern was defined as coexistence of cribriform (≥10%) and fused glands (≥10%).
Statistical Analysis
The χ2 test was applied to compare the association between CGPs (including three subtypes, respectively) and clinical features as well as several gene mutations (the Fisher exact test was used when the number of patients in one compared group was fewer than five). Overall survival (OS) was defined as the time from resection to death from any cause. Recurrence-free survival (RFS) was defined as the time from resection to the first time of recurrence. The Kaplan-Meier method was used to assess the association of clinicopathologic factors (eg, the CGPs and five main pathologic subtypes) with OS and RFS. Univariate and multivariable analyses for the related association with death or recurrence risk of the patients were performed using the Cox regression hazards model. A two-tailed P value less than .05 was considered statistically significant for interpretation of the results. All statistical analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL).
Results
Distribution of CGPs in the Predominant Type of Adenocarcinoma
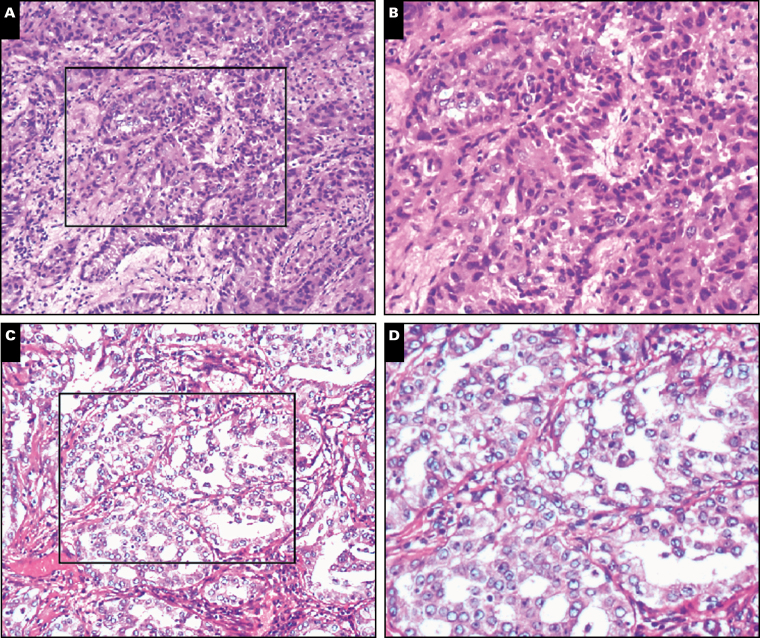
The stained results for nonstandard, cribriform, and fused gland biopsy specimens are shown in Image 1. Of all 356 cases, 172 (48.3%) were diagnosed as acinar predominant, 40 (11.2%) as solid predominant, 61 (17.1%) as micropapillary predominant, 47 (13.2%) as papillary predominant, and 36 (10.1%) as lepidic predominant according to the 2015 WHO classification criteria (Supplementary Table 1; all supplemental materials can be found at American Journal of Clinical Pathology online). A total of 156 (43.82%) patients with CGPs were also identified, and 54 (15.2%) were CGP predominant. The single cribriform pattern, single fused gland pattern, and CCFG pattern were observed in 50 (14.0%), 89 (25.0%), and 17 (4.8%) tumors, respectively. CGPs had a significant tendency to coexist with acinar subtypes (P < .01, Supplementary Figure 1A). The single fused gland pattern was more likely to coexist with the acinar subtype (P < .01, Supplementary Figure 1B), while the single cribriform pattern was most likely to coexist with the solid subtype (P < .01, Supplementary Figure 1C).
Image 1.
Corresponding paraffin-embedded complex glandular pattern tissues were subjected to H&E staining. A, B, Fused gland (×20). C, D, Cribriform (×20).
Association Between Clinicopathologic Characteristics and CGPs
CGPs had strong associations with lymph/vascular invasion (P = .001) and higher TNM stage (P = .035) Table 1. Further analysis revealed that the single fused gland pattern was correlated with a higher TNM stage (P = .041) and a tumor size of 20 mm or less (P = .020), and the single cribriform pattern was associated with lymph/vascular invasion (P < .001), a tumor size greater than 20 mm (P = .021), and a higher TNM stage (P < .001), while no clinicopathologic features were significantly associated with the CCFG pattern.
Table 1.
Associations of CGPs With Clinical Characteristics in Patients With Lung Cancer
| Characteristic | No. of Cases | Predominant Pattern, No. (%) | χ2 | P Value | |
|---|---|---|---|---|---|
| Patterns With CGPs | Patterns Without CGPs | ||||
| Age, y | |||||
| ≤59 | 186 | 80 (43.0) | 106 (57.0) | 0.104 | .747 |
| >59 | 170 | 76 (44.7) | 94 (55.3) | ||
| Sex | |||||
| Male | 140 | 60 (42.9) | 80 (57.1) | 0.087 | .768 |
| Female | 216 | 96 (44.4) | 120 (55.6) | ||
| pT | |||||
| 1 | 205 | 90 (43.9) | 115 (56.1) | 0.296 | .961 |
| 2 | 138 | 60 (43.5) | 78 (56.5) | ||
| 3 | 10 | 5 (50.0) | 5 (50.0) | ||
| 4 | 3 | 1 (33.3) | 2 (66.7) | ||
| pN | |||||
| 0 | 259 | 103 (39.8) | 156 (60.2) | 6.342 | .042 |
| 1 | 24 | 13 (54.2) | 11 (45.8) | ||
| 2 | 73 | 40 (54.8) | 33 (45.2) | ||
| 3 | — | — | — | — | — |
| Stage | |||||
| 1 | 251 | 99 (39.4) | 152 (60.6) | 6.713 | .035 |
| 2 | 27 | 14 (51.9) | 13 (48.1) | ||
| 3 | 78 | 43 (55.1) | 35 (44.9) | ||
| Tumor size | |||||
| Small (≤20 mm) | 182 | 80 (3.8) | 102 (56.0) | 0.003 | .958 |
| Large (>20 mm) | 174 | 76 (5.7) | 98 (56.3) | ||
| Lymph/vascular invasion | |||||
| No | 266 | 103 (38.7) | 163 (61.3) | 11.110 | .001 |
| Yes | 90 | 53 (58.9) | 37 (41.1) | ||
| Smoking | |||||
| Nonsmoker | 258 | 111 (43.0) | 147 (57.0) | 0.242 | .623 |
| Current/ex-smoker | 98 | 45 (45.9) | 53 (54.1) | ||
CGP, complex glandular pattern.
Gene Mutations Analysis in CGPs
With a further interest to find out CGP-related gene mutations, we evaluated the EGFR, KRAS, AKT1, HER2, BRAF, ALK, ROS1, and P110 mutational profile in these 356 adenocarcinomas. The CGPs were associated with ALK rearrangement (P = .006) and HER2 mutation (P = .047) Table 2. The single cribriform pattern was associated with ALK rearrangement (P < .001), EGFR mutation (P = .003), and AKT1 mutation (P = .013). However, we did not find a significant association of the single fused gland group and CCFG group with all molecular alterations and gene mutations. To compare with previous studies, we also analyzed whether these potential genetic associations were exhibited in the cribriform pattern and fused gland pattern. As indicated by our results, the cribriform pattern was associated with EGFR mutation (P = .016), AKT1 mutation (P = .038), and ALK rearrangement (P < .001), while the fused gland pattern was associated with EGFR mutation (P = .049).
Table 2.
Correlations Between CGPs and Related Gene Mutations
| Gene Mutation | No. of Cases | Predominant Pattern, No. (%) | χ2 | P Value | |
|---|---|---|---|---|---|
| Patterns With CGPs | Patterns Without CGPs | ||||
| EGFR | |||||
| No | 120 | 54 (45.0) | 66 (55.0) | 0.102 | .749 |
| Yes | 236 | 102 (43.2) | 134 (56.8) | ||
| ALK | |||||
| No | 332 | 139 (41.9) | 193 (58.1) | 7.628 | .006 |
| Yes | 24 | 17 (70.8) | 7 (29.2) | ||
| KRAS | |||||
| No | 335 | 146 (43.6) | 189 (56.4) | 0.131 | .718 |
| Yes | 21 | 10 (47.6) | 11 (52.4) | ||
| AKT1 | |||||
| No | 355 | 155 (43.7) | 200 (56.3) | 1.286 | .257 |
| Yes | 1 | 1 (100) | 0 (0) | ||
| HER2 | |||||
| No | 351 | 156 (44.4) | 195 (55.6) | 3.956 | .047 |
| Yes | 5 | 0 (0) | 5 (100) | ||
| BRAF | |||||
| No | 355 | 155 (43.7) | 200 (56.3) | 1.286 | .257 |
| Yes | 1 | 1 (100) | 0 (0) | ||
| ROS1 | |||||
| No | 355 | 156 (43.9) | 199 (56.1) | 0.782 | .376 |
| Yes | 1 | 0 (0) | 1 (100) | ||
| P110 | |||||
| No | 346 | 150 (43.4) | 196 (56.6) | 0.531 | .466 |
| Yes | 9 | 5 (55.6) | 4 (44.4) | ||
CGP, complex glandular pattern.
Clinical Outcome in Cases With CGPs
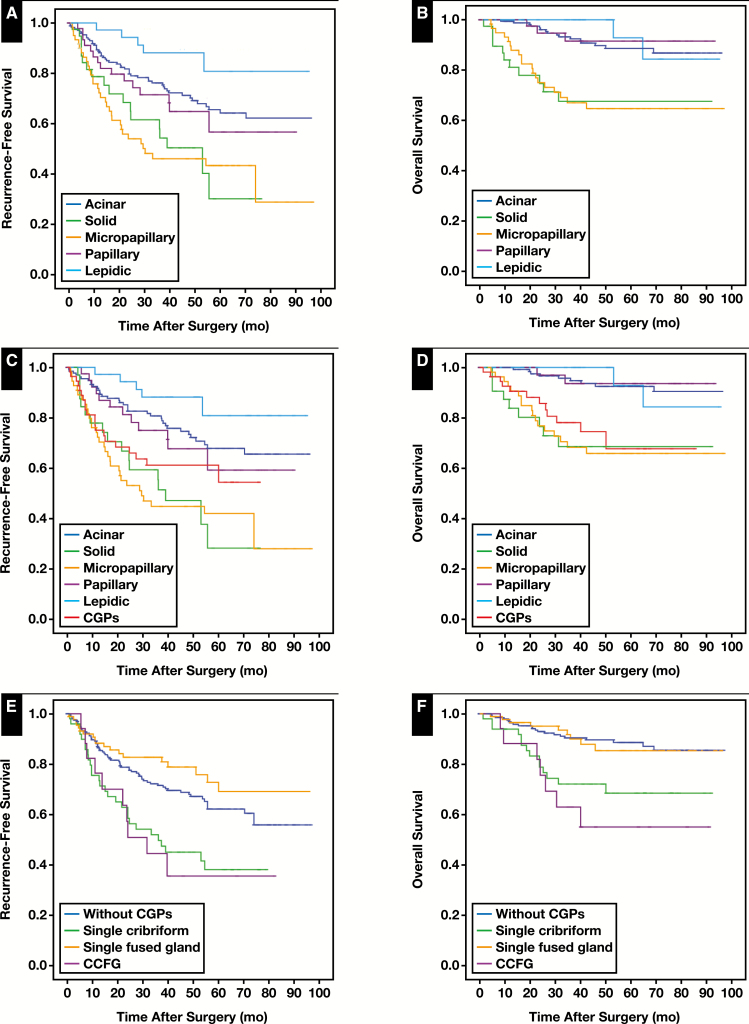
Survival analysis was performed for the clinicopathologic factors listed in Table 3. In this cohort, we set up several sets of histologic classification systems: one was consistent with the current WHO classification system Figure 1A and Figure 1B, and the second made some changes by defining CGPs as a new independent type of invasive lung adenocarcinoma Figure 1C and Figure 1D. CGP-predominant patients had a worse clinical outcome (mean OS, 66.4 months; mean RFS, 50.1 months) compared with acinar-predominant patients, whose survival was closer to that of patients with solid and micropapillary predominant tumors. Last, we compared the clinical outcomes among patients with the single cribriform pattern, single fused gland pattern, CCFG pattern, and those without CGPs Figure 1E and Figure 1F. Patients with CCFG pattern tumors had the worst clinical outcome, with a mean OS of 60.8 months and mean RFS of 42.0 months. Multivariable analysis for the association with death risk was carried out using the Cox regression model Table 4. Our results showed that pT (P = .005), pN (P < .001), and CCFG pattern (P = .009) were independent prognostic factors. To restrict the survival analysis to a more homogeneous group of patients, we carried out a multivariate Cox regression analysis in stage I patients treated with lobectomy/segmentectomy (Supplementary Table 2). This subgroup contained 228 patients; lymph/vascular invasion (hazard ratio [HR], 2.18; 95% confidence interval [CI], 1.03-4.61; P = .042) was able to independently and adversely affect the RFS, and the CCFG pattern (HR, 6.87; 95% CI, 1.34-35.34; P = .021) was an independent and negative predictor for OS after adjustment for age, sex, smoking status, pathologic pattern, and lymph/vascular invasive status, which was consistent with the results calculated from the overall population. Although the impact on RFS by CCFG reached only a borderline significance (P = .086), we observed a similar trend toward unfavorable RFS in the presence of CCFG (HR, 2.52), further indicating the clear role of CCFG in the poor prognosis of patients with lung cancer.
Table 3.
Survival Analysis for Clinical Characteristics of the Patients
| Parameter | No. of Patients | OS, mo | RFS, mo | ||
|---|---|---|---|---|---|
| Mean (SD) | P Value | Mean (SD) | P Value | ||
| Age, y | |||||
| ≤59 | 186 | 83.5 (2.3) | .771 | 69.0 (3.0) | .151 |
| >59 | 170 | 82.8 (2.3) | 60.6 (3.2) | ||
| Sex | |||||
| Male | 140 | 77.9 (2.9) | .021 | 57.6 (3.5) | .009 |
| Female | 216 | 86.9 (1.8) | 70.2 (2.7) | ||
| pT | |||||
| 1 | 205 | 90.1 (1.6) | <.001 | 74.6 (2.6) | <.001 |
| 2 | 138 | 76.9 (3.0) | 55.3 (3.6) | ||
| 3 | 10 | 31.3 (6.0) | 17.2 (5.1) | ||
| 4 | 3 | 38.5 (9.0) | 14.5 (5.0) | ||
| pN | |||||
| 0 | 259 | 90.9 (1.3) | <.001 | 77.3 (2.2) | <.001 |
| 1 | 24 | 62.4 (5.3) | 36.6 (6.3) | ||
| 2 | 73 | 60.0 (4.6) | 28.8 (3.3) | ||
| 3 | — | — | — | ||
| Stages | |||||
| 1 | 251 | 91.7 (1.3) | <.001 | 79.2 (2.2) | <.001 |
| 2 | 27 | 64.1 (5.2) | 34.3 (6.0) | ||
| 3 | 78 | 59.0 (4.5) | 28.0 (3.0) | ||
| Location | |||||
| Left lobes | 145 | 86.7 (2.3) | .147 | 69.0 (3.4) | .274 |
| Right lobes | 211 | 81.2 (2.2) | 62.8 (2.9) | ||
| Tumor size | |||||
| Small (≤20 mm) | 182 | 90.8 (1.6) | <.001 | 77.1 (2.6) | <.001 |
| Large (>20 mm) | 174 | 74.0 (2.7) | 51.8 (3.2) | ||
| Smoking | |||||
| Nonsmoker | 258 | 86.1 (1.8) | .028 | 69.3 (2.5) | .009 |
| Current/ex-smoker | 98 | 74.3 (3.4) | 53.8 (4.0) | ||
| Operation | |||||
| Partial resection | 24 | 73.3 (2.9) | .469 | 73.1 (3.0) | .028 |
| Segmentectomy | 7 | 54.0 (6.4) | 38.2 (9.6) | ||
| Lobectomy or more | 325 | 83.4 (1.7) | 64.3 (2.3) | ||
| Lymph/vascular invasion | |||||
| No | 266 | 89.3 (1.5) | <.001 | 75.2 (2.3) | <.001 |
| Yes | 90 | 64.5 (4.0) | 35.5 (3.8) | ||
OS, overall survival; RFS, recurrence-free survival.
Figure 1.
Survival analysis stratified by pathologic type of patients with lung cancer. A, B, Recurrence-free survival (RFS) and overall survival (OS) curve for current World Health Organization–based five main subtypes of lung adenocarcinoma. C, D, RFS and OS curve for all predominant growth pathologic patterns, and CGPs were also included for analysis as an additional subtype. E, F, RFS and OS curve for patients with three subtypes of CGPs and those without CGPs, respectively. P < .001.
Table 4.
Multivariable Analysis for Assessing Death Risk Using Cox Regression Hazards Model
| Characteristic | HR (95% CI) | P Value |
|---|---|---|
| Age | 0.80 (0.45-1.41) | .443 |
| Sex | 0.57 (0.28-1.18) | .129 |
| pT | 1.84 (1.20-2.82) | .005 |
| pN | 2.05 (1.42-2.95) | <.001 |
| Smoking | 0.92 (0.44-1.94) | .821 |
| With single cribriform pattern | 1.11 (0.54-2.30) | .772 |
| With single fused gland pattern | 1.40 (0.63-3.13) | .409 |
| With CCFG pattern | 3.25 (1.35-7.86) | .009 |
| Lymph/vascular invasion | 1.79 (0.93-3.43) | .081 |
CCFG, cribriform coexisting with fused gland; CI, confidence interval; HR, hazard ratio.
Discussion
CGPs, especially the cribriform pattern, have been regarded as a subtype of adenocarcinoma or a distinct prognostic factor in many types of tumors.12-14 Moreira et al8 suggested that cribriform and fused glands should be considered patterns of high-grade pulmonary adenocarcinoma in a population-based homogeneous white cohort. In 2015, the WHO proposed a new classification of lung adenocarcinoma3 based on the 2011 IASLC/ATS/ERS classification.2 However, CGPs were not classified as one of the main subtypes of lung adenocarcinoma according to that classification strategy. In this study, we described the distribution of CGPs in lung adenocarcinoma and further investigated the clinical relevance of CGPs on common clinicopathologic characteristics, gene mutations, and patients’ prognosis. Once they are validated by larger studies, our results will provide some new evidence to support taking the CGPs into clinical practice to make more reasonable treatment decisions for patients with lung cancer.
In the present study, we found that CGPs were associated with lymph/vascular invasion and a higher TNM stage, which is in accordance with previous studies.15,16 However, results in the present cohort do not support a significant correlation between CGPs and smoking history, which is inconsistent with a previous study.9 Furthermore, the cribriform and fused gland patterns had different clinicopathologic correlations, as indicated by our results: the single cribriform pattern was associated with lymph/vascular invasion, a tumor size greater than 20 mm, and a higher TNM stage, while the single fused gland pattern was associated with a higher TNM stage and a tumor size of 20 mm or less. Interestingly, as a coexistence pattern of SCP and SFG, CCFG showed a worse prognosis, but there was no significant relationship between CCFG and clinicopathologic factors in this cohort.
The EGFR mutation is more prevalent in Asian people.17 In our study, the most frequent gene mutation in CGP adenocarcinoma was EGFR (more than 50% of CGP cases). In the present study, HER2 and ALK mutations had significant correlations with CGPs, but results became insignificant for the association between KRAS rearrangement and CGPs, which is inconsistent with a previous study, likely due to racial discrepancy.10 By further analysis, we found that EGFR and AKT1 mutations and ALK rearrangement were associated with the single cribriform pattern, which is, however, in accordance with previous studies based on Japanese cohorts.18,19 In addition to ALK rearrangements, alterations in KRAS and ROS1 were detected in tumors with the cribriform pattern in a previous study,20 while we did not find any significant correlation of KRAS and ROS1 with the cribriform pattern in the present study. No related gene mutation presented significant associations with the single fused gland pattern and CCFG pattern in the present study. However, when CGPs were divided into two groups (cribriform and fused gland), the fused gland pattern was associated with EGFR mutations. These results might provide some implications to estimate which patients should benefit a great deal from a genetic target, such as anti-EGFR therapy.
Individualized therapy has reached great success in the past decades in light of novel molecular factors identified to be responsible for the variability in clinical outcome for patients with lung cancer. Our results were mostly in line with previous studies that suggested that the lepidic subtype was associated with prolonged survival,21-24 while solid and micropapillary subtypes were associated with worse clinical outcomes.25,26 Maeshima et al27 reported first that the CGPs were associated with poor prognosis. Our study confirmed that the recurrence risk for patients with CGP lung adenocarcinoma was significantly higher than that for patients with acinar- or papillary-predominant adenocarcinoma but was close to that for patients with micropapillary- or solid-predominant adenocarcinoma. By further research on CGPs, we found that the CCFG pattern was associated with worst OS and RFS compared with the single cribriform pattern and single fused gland pattern, so we conducted a multivariate Cox analysis in which a much higher death risk was observed in the presence of the CCFG pattern, indicating a need for close survival surveillance of patients with this pathologic subtype of lung cancer.
The present study has some potential limitations that should be considered when interpreting the results. Like other single institution–based, retrospective, observational cohort studies, there was potential for referral and selection bias. In addition, given the relatively small number of patients included in the present study, further larger studies with sufficient statistical power are warranted to validate the association of CGPs with clinicopathologic characteristics, gene mutations, and clinical outcome of patients with lung cancer.
Conclusions
Although CGPs were not included in the new WHO pathologic classification criteria, it is notable that patients with cribriform and fused gland patterns tumors have been taken into account for predicting metastasis and survival of patients with multiple types of cancer.28-30 Regarding CGPs as a new category of lung adenocarcinoma was also suggested based on recent studies.5,10,31 In conclusion, based on the retrospective analysis in the present study, it is reasonable to classify CGPs as a new type of lung adenocarcinoma for advancing the clinical management of patients in terms of either survival surveillance or treatment decision.
Supplementary Material
Acknowledgments
We thank Zhenhua Zhou, MD, for valuable suggestions in revising the manuscript.
This work was supported by the National Natural Science Foundation of China (No. 81422029, No. 81572264, and No. 81372525), Chinese Minister of Science and Technology grant (No. 2016YFA0501800).
References
- 1. Sakurai H, Asamura H, Miyaoka E, et al. ; Japanese Joint Committee of Lung Cancer Registry Differences in the prognosis of resected lung adenocarcinoma according to the histological subtype: a retrospective analysis of Japanese lung cancer registry data. Eur J Cardiothorac Surg. 2014;45:100-107. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Noguchi M, et al. . International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Burke AP, et al. . WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC; 2015. [DOI] [PubMed] [Google Scholar]
- 4. Travis WD, Brambilla E, Noguchi M, et al. . The new IASLC/ATS/ERS international multidisciplinary lung adenocarcinoma classification. J Thoracic Oncol. 2011;6:244-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petersen I, Warth A. Lung cancer: developments, concepts, and specific aspects of the new WHO classification. J Cancer Res Clin Oncol. 2016;142:895-904. [DOI] [PubMed] [Google Scholar]
- 6. Okada M. Subtyping lung adenocarcinoma according to the novel 2011 IASLC/ATS/ERS classification: correlation with patient prognosis. Thorac Surg Clin. 2013;23:179-186. [DOI] [PubMed] [Google Scholar]
- 7. Behera M, Owonikoko TK, Gal AA, et al. . Lung adenocarcinoma staging using the 2011 IASLC/ATS/ERS classification: a pooled analysis of adenocarcinoma in situ and minimally invasive adenocarcinoma. Clin Lung Cancer. 2016;17:e57-e64. [DOI] [PubMed] [Google Scholar]
- 8. Moreira AL, Joubert P, Downey RJ, et al. . Cribriform and fused glands are patterns of high-grade pulmonary adenocarcinoma. Hum Pathol. 2014;45:213-220. [DOI] [PubMed] [Google Scholar]
- 9. Kadota K, Yeh YC, Sima CS, et al. . The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2014;27:690-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warth A, Muley T, Kossakowski C, et al. . Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol. 2015;10:638-644. [DOI] [PubMed] [Google Scholar]
- 11. Detterbeck FC, Nicholson AG, Franklin WA, et al. . The IASLC lung cancer staging project: summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11:639-650. [DOI] [PubMed] [Google Scholar]
- 12. Pradhan D, Sharma A, Mohanty SK. Cribriform-morular variant of papillary thyroid carcinoma. Pathol Res Pract. 2015;211:712-716. [DOI] [PubMed] [Google Scholar]
- 13. Kir G, Sarbay BC, Gumus E, et al. . The association of the cribriform pattern with outcome for prostatic adenocarcinomas. Pathol Res Pract. 2014;210:640-644. [DOI] [PubMed] [Google Scholar]
- 14. Lino-Silva LS, Salcedo-Hernández RA, Herrera-Gómez A, et al. . Colonic cribriform carcinoma, a morphologic pattern associated with low survival. Int J Surg Pathol. 2015;23:13-19. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Durra HY, Huang Y, et al. . Interobserver reproducibility study of the histological patterns of primary lung adenocarcinoma with emphasis on a more complex glandular pattern distinct from the typical acinar pattern. Int J Surg Pathol. 2014;22:149-155. [DOI] [PubMed] [Google Scholar]
- 16. Xu L, Tavora F, Burke A. Histologic features associated with metastatic potential in invasive adenocarcinomas of the lung. Am J Surg Pathol. 2013;37:1100-1108. [DOI] [PubMed] [Google Scholar]
- 17. Sun Y, Ren Y, Fang Z, et al. . Lung adenocarcinoma from east Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010; 28:4616-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida A, Tsuta K, Nakamura H, et al. . Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011;35:1226-1234. [DOI] [PubMed] [Google Scholar]
- 19. Jokoji R, Yamasaki T, Minami S, et al. . Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol. 2010;63:1066-1070. [DOI] [PubMed] [Google Scholar]
- 20. Mackinnon AC Jr, Luevano A, de Araujo LC, et al. . Cribriform adenocarcinoma of the lung: clinicopathologic, immunohistochemical, and molecular analysis of 15 cases of a distinctive morphologic subtype of lung adenocarcinoma. Mod Pathol. 2014;27:1063-1072. [DOI] [PubMed] [Google Scholar]
- 21. Kadota K, Villena-Vargas J, Yoshizawa A, et al. . Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol. 2014;38:448-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mäkinen JM, Laitakari K, Johnson S, et al. . Nonpredominant lepidic pattern correlates with better outcome in invasive lung adenocarcinoma. Lung Cancer. 2015;90:568-574. [DOI] [PubMed] [Google Scholar]
- 23. Strand TE, Rostad H, Strøm EH, et al. . The percentage of lepidic growth is an independent prognostic factor in invasive adenocarcinoma of the lung. Diagn Pathol. 2015; 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Araki K, Kidokoro Y, Hosoya K, et al. . Excellent prognosis of lepidic-predominant lung adenocarcinoma: low incidence of lymphatic vessel invasion as a key factor. Anticancer Res. 2014;34:3153-3156. [PubMed] [Google Scholar]
- 25. Zhang Y, Wang R, Cai D, et al. . A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J Thorac Oncol. 2014;9:1772-1778. [DOI] [PubMed] [Google Scholar]
- 26. Urer HN, Kocaturk CI, Gunluoglu MZ, et al. . Relationship between lung adenocarcinoma histological subtype and patient prognosis. Ann Thorac Cardiovasc Surg. 2014;20:12-18. [DOI] [PubMed] [Google Scholar]
- 27. Maeshima AM, Tochigi N, Yoshida A, et al. . Histological scoring for small lung adenocarcinomas 2 cm or less in diameter: a reliable prognostic indicator. J Thorac Oncol. 2010;5:333-339. [DOI] [PubMed] [Google Scholar]
- 28. Kweldam CF, Wildhagen MF, Steyerberg EW, et al. . Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol. 2015;28:457-464. [DOI] [PubMed] [Google Scholar]
- 29. Iczkowski KA, Torkko KC, Kotnis GR, et al. . Digital quantification of five high-grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am J Clin Pathol. 2011;136:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Epstein JI, Egevad L, Amin MB, et al. . The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244-252. [DOI] [PubMed] [Google Scholar]
- 31. Travis WD. The 2015 WHO classification of lung tumors. Der Pathologe. 2014;35(suppl 2):188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.