Abstract
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor is the cornerstone of pharmacologic management of patients with acute coronary syndrome (ACS) and/or those receiving coronary stents. Long-term (>1 year) DAPT may further reduce the risk of stent thrombosis after a percutaneous coronary intervention (PCI) and may decrease the occurrence of non-stent-related ischaemic events in patients with ACS. Nevertheless, compared with aspirin alone, extended use of aspirin plus a P2Y12 receptor inhibitor may increase the risk of bleeding events that have been strongly linked to adverse outcomes including recurrent ischaemia, repeat hospitalisation and death. In the past years, multiple randomised trials have been published comparing the duration of DAPT after PCI and in ACS patients, investigating either a shorter or prolonged DAPT regimen. Although the current European Society of Cardiology guidelines provide a backup to individualised treatment, it appears to be difficult to identify the ideal patient profile which could safely reduce or prolong the DAPT duration in daily clinical practice. The aim of this consensus document is to review contemporary literature on optimal DAPT duration, and to guide clinicians in tailoring antiplatelet strategies in patients undergoing PCI or presenting with ACS.
Keywords: Coronary artery disease, Dual antiplatelet therapy, Long-term dual antiplatelet therapy, Prior myocardial infarction
Table of contents
1. Epidemiology of coronary artery disease in Italy
2. Acute coronary syndrome and stable angina, two different entities
3. Rationale for long-term antiplatelet therapy
4. Antiplatelet therapies available in Italy
4.1 Aspirin
4.2 Clopidogrel
4.3 Ticagrelor
4.4 Prasugrel
5. Evidence to support the reduced duration of dual antiplatelet therapy after percutaneous coronary intervention: from the risk of late thrombosis to the latest generation of stents
6. Duration of dual antiplatelet therapy: recommendations from international guidelines
7. Ischaemic and haemorrhagic risk in secondary prevention: is it time to change perspective?
7.1 The risk of further cardiovascular events in patients with coronary artery disease
7.2 The risk of haemorrhagic events in patients with coronary artery disease treated with dual antiplatelet therapy
7.3 Achieving a balance
8. New paradigms on the duration of dual antiplatelet therapy: profiling patients eligible for prolonged antiplatelet treatment
8.1 Vorapaxar and the TRA 2P-TIMI 50 study
8.2 Ticagrelor and the PEGASUS-TIMI 54 study
8.3 Who should be given long-term antiplatelet therapy?
9. Dual long-term antiplatelet therapy: adherence and managing the switch
9.1 The scale of the problem
9.2 Poor treatment adherence: definitions, causes and possible solutions
9.3 Switch management
9.4 Data on the pharmacodynamics of switching
Management and therapeutic pathway in patients undergoing dual antiplatelet therapy. Treatment schedules
10. Antiplatelet therapy in patients with chronic ischaemic heart disease undergoing a percutaneous coronary intervention
11. Dual antiplatelet therapy in patients with myocardial infarction with or without ST segment elevation undergoing a percutaneous coronary intervention
12. Dual antiplatelet therapy in patients with acute coronary syndrome undergoing surgical revascularisation
13. Dual antiplatelet therapy in patients with acute coronary syndrome not treated with revascularisation
14. Dual antiplatelet therapy in elderly and frail patients
14.1 Dual antiplatelet therapy in the elderly person
14.1.1 Antiplatelet drugs
14.1.2 P2Y12 receptor antagonists
14.1.3 Dual antiplatelet therapy in elderly patients on oral anticoagulant therapy
14.1.4 Duration of dual antiplatelet therapy
14.2 Dual long-term antiplatelet therapy in the frail patient
14.3 Conclusions
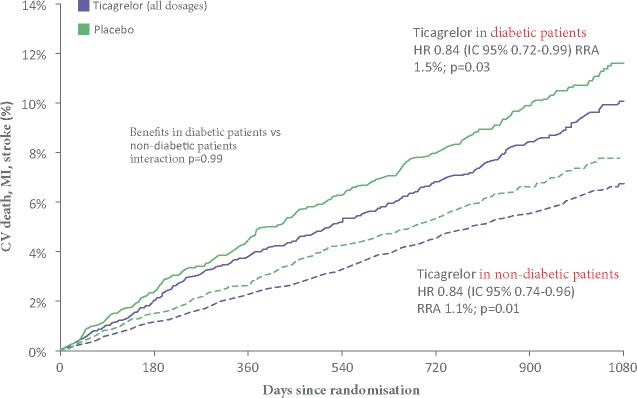
15. Dual antiplatelet therapy in diabetic patients and/or those with renal dysfunction
15.1 Atherothrombotic risk in diabetic patients
15.1.1 Which anti-thrombotic therapy in diabetic patients?
15.1.2 Duration of dual antiplatelet therapy in diabetic patients
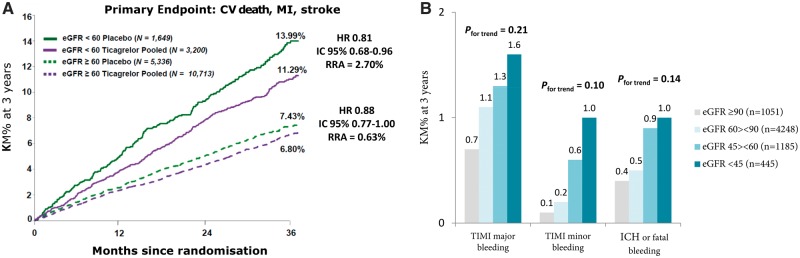
15.2 Atherothrombotic risk in patients with renal insufficiency
15.2.1 Anti-thrombotic therapy in patients with acute coronary syndrome and chronic renal failure
15.2.2 Dual antiplatelet therapy prolonged beyond 12 months after acute coronary syndrome in patients with chronic renal failure
16. Dual antiplatelet therapy in patients with peripheral obliterative arteriopathy
16.1 Epidemiological background
16.2 Dual antiplatelet therapy in patients with peripheral obliterative arteriopathy
16.3 Indications in the guidelines
16.4 Conclusions
17. Duration of dual antiplatelet therapy in patients with recurrent ischaemic events
17.1 Recurrence events in the first year following acute coronary syndrome
17.2 Recurrence events beyond the first year following acute coronary syndrome
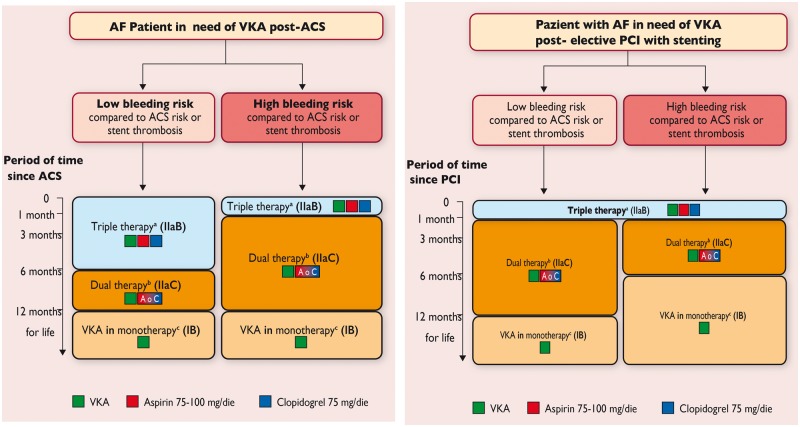
18. Dual antiplatelet therapy in patients receiving oral anticoagulation
19. Dual antiplatelet therapy in patients at high bleeding risk
19.1 How to reduce the risk of bleeding in high-risk patients
20. Dual antiplatelet therapy in patients undergoing a preoperative cardiovascular evaluation for non-cardiac surgery
21. Management of patients who develop haemorrhages during dual antiplatelet therapy
21.1 Bleeding during dual antiplatelet therapy
21.2 Indication for dual antiplatelet therapy
21.3 Haemorrhage management
21.4 Grades of dual antiplatelet therapy
21.5 Resumption of dual antiplatelet therapy
21.6 Conclusions
Summary
Bibliography
ABBREVIATIONS AND ACRONYMS
- ACC
American College of Cardiology
- ACS
Acute Coronary Syndrome
- NSTE-ACS
Acute Coronary Syndrome without ST Segment Elevation
- ACTION
Acute Coronary Treatment and Intervention Outcomes Network
- ADP
Adenosine Diphosphate
- AF
Atrial Fibrillation
- AHA
American Heart Association
- AMI
Acute Myocardial Infarction
- ANCE
Italian Association of Territorial Cardiology
- ANMCO
Italian Association of Hospital Cardiologists
- ANNEXA-4
Andexanet Alfa, a Novel Antidote for the Anti coagulation Effects of FXA Inhibitors
- ARCA
Regional Ambulatory Cardiologists Association
- ARCTIC
Double Randomisation of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation, and Interruption Versus Continuation of Double Antiplatelet Therapy
- ASA
Aspirin
- ATP
Adenosine Triphosphate
- ATVB
Working Group on Atherosclerosis, Thrombosis and Vascular Biology
- BARC
Bleeding Academic Research Consortium
- BMS
Bare Metal Stent
- BRIDGE
Maintenance of Platelet Inhibition with Cangrelor After Discontinuation of Thienopyridines in Patients Undergoing Surgery
- BRS
Bioresorbable Stent
- BVS
Bioresorbable Vascular Scaffold
- CABG
Coronary Artery Bypass Grafting
- cAMP
Cyclic Adenosine Monophosphate
- CAPRIE
Clopidogrel vs Aspirin in Patients at Risk of Ischaemic Events
- CCS
Canadian Cardiovascular Society
- CHARISMA
Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilisation, Management and Avoidance
- CI
Confidence Interval
- CLARITY-TIMI 28
Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myocardial Infarction 28
- CLI
Critical Limb Ischaemia
- COMMIT
Clopidogrel and Metoprolol in Myocardial Infarction Trial
- COX
Cyclooxygenase
- CREDO
Clopidogrel for the Reduction of Events During Observation
- CRF
Chronic Renal Failure
- CSC
Italian Council of Cardiovascular Societies
- CSHA
Canadian Study on Health and Aging
- CURE
Clopidogrel in Unstable Angina to Prevent Recurrent Events
- CURRENT-OASIS 7
Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events-Seventh Organisation to Assess Strategies in Ischaemic Syndromes
- DAPT
Dual Antiplatelet Therapy
- DES
Drug-Eluting Stent
- DES-LATE
Optimal Duration of Clopidogrel Therapy with DES to Reduce Late Coronary Arterial Thrombotic Event
- DISPERSE
Dose Confirmation Study Assessing the Antiplatelet Effects of AZD6140 vs Clopidogrel in Non-ST-Segment Elevation Myocardial Infarction
- EASD
European Association for the Study of Diabetes
- EES
Everolimus Releasing Stent
- eGFR
Estimated Glomerular Filtration Rate
- EMA
European Medicines Agency
- EPICOR
Long-term Follow-up of Antithrombotic Management Patterns in Acute Coronary Syndrome Patients
- ESC
European Society of Cardiology
- EXCELLENT
Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting
- EYESHOT
Antithrombotic Therapies Used in Patients with Acute Coronary Syndromes Hospitalised in Italian Cardiac Care Units
- FDA
Food and Drug Administration
- GENERATIONS
Comparison of Prasugrel and Clopidogrel in Very Elderly and Non-Elderly Patients with Stable Coronary Artery Disease
- GICR-IACPR
Italian Group of Rehabilitation and Preventive Cardiology
- GIEC
Italian Group of Emergency Cardiology
- GISE
Italian Society of Interventional Cardiology
- GP
Glycoprotein
- GRAPE
Greek Antiplatelet Registry
- HR
Hazard Ratio
- HSICOA
Società Italiana Cardiologia Ospedalità Accreditata [Italian Society of Accredited Hospitals]
- ITAHFA
Italian Heart Failure Association
- MACE
Major Adverse Cardiovascular Events
- MCVA
Atherosclerotic Cardiovascular Disease
- NNH
Number Needed to Harm
- NNT
Number Needed to Treat
- NORSTENT
Norwegian Coronary Stent Trial
- NSTEMI
Non-ST Segment Elevation Myocardial Infarction
- OAT
Oral Anticoagulant Therapy
- OEC/HES
Cardiovascular Epidemiological Observatory/Health Examination Survey
- OR
Odds Ratio
- OPTIDUAL
Optimal Dual Antiplatelet Therapy
- PAD
Peripheral Artery Disease
- PCI
Percutaneous Coronary Intervention
- PEGASUS-TIMI 54
Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54
- PES
Paclitaxel Eluting Stent
- PIONEER AF-PCI
Open-Label, Randomised, Controlled, Multicentre Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Interventions
- PLATO
Platelet Inhibition and Patient Outcomes
- PNE
Programma Nazionale Esiti [National Outcomes Programme]
- PPI
Proton Pump Inhibitors
- PRODIGY
Prolonging Dual Antiplatelet Treatment after Grading Stent-Induced Intimal Hyperplasia
- PROSPECT
Providing Regional Observations to Study Predictors of Events in the Coronary Tree
- REACH
Reduction of Atherothrombosis for Continued Health
- RECLOSE 2-ACS
Responsiveness to Clopidogrel and Stent Thrombosis 2-Acute Coronary Syndrome
- RE-DUAL PCI
Randomised Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Interventions
- RESET
Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-Eluting Stent Implantation
- RE-VERSE AD
Reversal Effects of Idarucizumab on Active Dabigatran
- RR
Relative Risk
- SHIFT-OVER
Platelet Aggregation During the Shift from Clopidogrel to Ticagrelor
- SICP
Italian Society of Pediatric Cardiology
- SICOA
Italian Society of Accredited Cardiology Hospital Care
- SIT
Italian Society of Digital Medicine & Telemedicine
- STEMI
ST-elevation Myocardial Infarction
- SWAP
Switching Antiplatelet
- SWEDEHEART
Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies
- TASC
Trans-Atlantic Inter-Society Consensus
- THINKPAD
Atherosclerosis of the Lower Extremities as a Linked Comorbidity in Patients Admitted for Cardiac Rehabilitation
- TIA
Transient Ischaemic Attack
- TRANSLATE-ACS
Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome
- TRA 2P-TIMI 50
Thrombin Receptor Antagonist for the Secondary Prevention of Atherothrombotic Ischaemic Events-Thrombolysis in Myocardial Infarction 50
- TRIPLET
Transferring from Clopidogrel Loading Dose to Prasugrel Loading Dose in Acute Coronary Syndrome Patients
- TRITON-TIMI 38
Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38
- WOEST
What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting
- ZES
Zotarolimus Releasing Stent
1. EPIDEMIOLOGY OF CORONARY ARTERY DISEASE IN ITALY
Cardiovascular diseases are still the leading cause of death in Italy, and are responsible for 44% of all deaths. In particular, Coronary Artery Disease is the leading cause of death in Italy, accounting for 28% of all deaths, while cerebrovascular accidents are third with 13%, just after malignancies. Those who survive a heart attack become chronically ill, while the disease changes the quality of life and entails a significant economic cost for society. In Italy, the prevalence of significant cardiovascular disability is 4.4 per thousand (Istat data). Overall, 23.5% of Italian pharmaceutical spending expenditure (1.34 of the gross domestic product) is for cardiovascular drugs. (Report on the country's state of health, 2000)1.
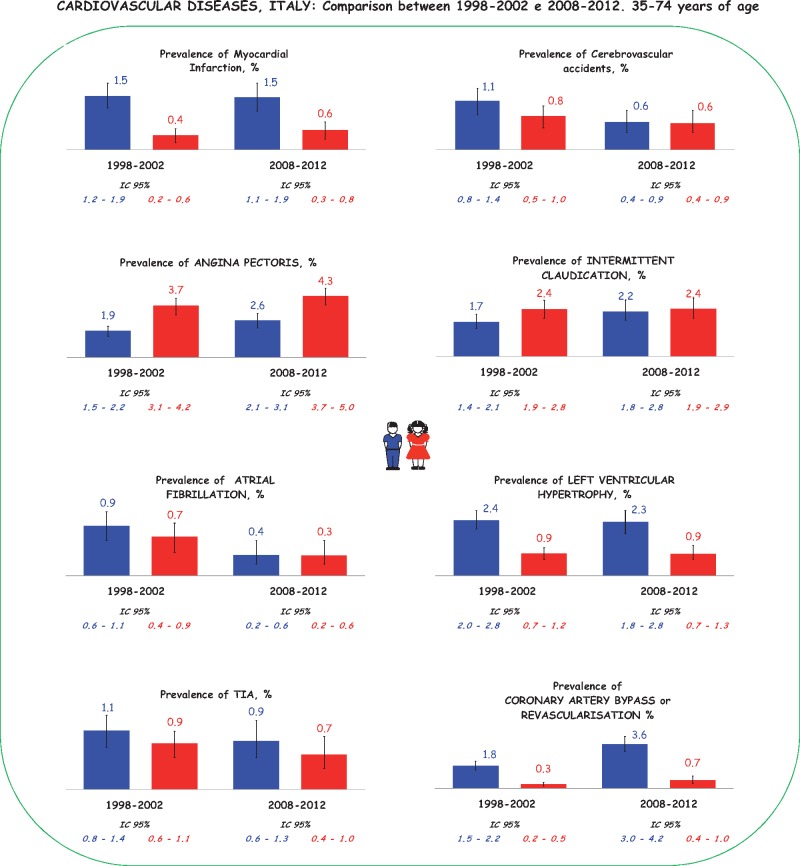
The Cardiovascular Epidemiological Observatory/Health Examination Survey (OEC/HES) of the Italian Superior Health Institute has has provided epidemiological data on both prevalence and incidence of cardiovascular diseases in Italy (acute myocardial infarction [AMI], cerebrovascular accident, angina pectoris, intermittent claudication, atrial fibrillation [AF], left ventricular hypertrophy, transient ischaemic attack [TIA]). Such data were collected in two surveys, made possible by the cooperation between the Italian Superior Health Institute and the Italian Association of Hospital Cardiologists, and formed part of Progetto CUORE, a first survey was conducted between 1998 and 2002 and the second one between 2008 and 20122,3.
Figure 1 shows a comparison between the prevalence of cardiovascular diseases in both samples of the general population (aged 34-75 years) examined in 1998-1902 (n = 9612) and in 2008-2012 (n = 8141). The presence of previous AMI or coronary revascularisation has been derived anamnestically, while those who were positive for the Rose questionnaire were considered as effected by angina pectoris. All data were adjusted by age using direct methods, considering the European population of 2013 as a standard, and are presented by gender. The prevalence of AMI in Italy remained stable in the two surveys: 1.5% in men and 0.4% in women in 1998-2002 vs. 1.5% in men and 0.4% in women in 2008-2012. On the contrary, the prevalence of stable angina is increasing, rising from 1.9% in men and 3.7% in women in the 1998-2002 survey to 2.6% in men and 4.2% in women in 2008-2012 (Figure 1)4.
Figure 1.
Prevalence of cardiovascular diseases in Italy. Data from the Cardiovascular Epidemiological Observatory/Health Examination Survey.
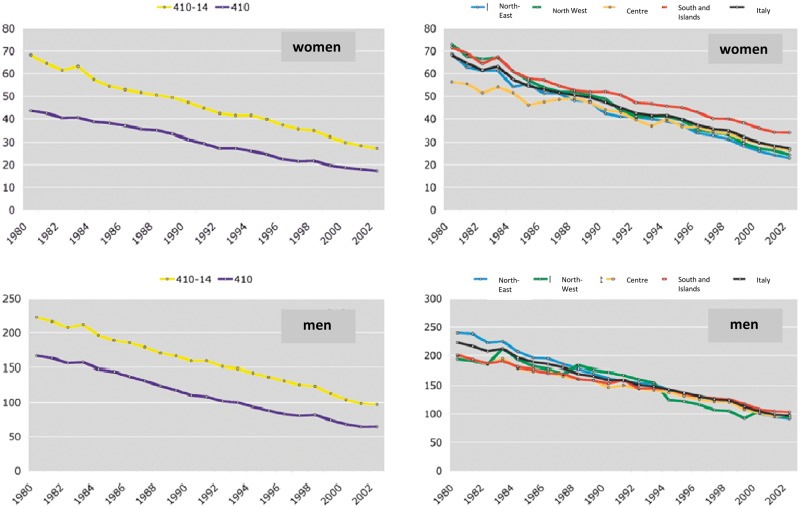
According to population data, mortality for ischaemic heart diseases among the adult population (35-74 years) is equal to 12% of all deaths, of which 8% for AMI. Figure 2 illustrates the trend in mortality rates for ischaemic heart disease and for AMI from 1980 to 2002 in Italy, standardised for the 35-74-year age range using the European population as a base reference. As shown in the graph, mortality from ischaemic heart disease (codes ICD-9 410-14) and mortality from AMI (code ICD-9 410) are constantly decreasing through the different geographical areas of the country.
Figure 2.
Mortality from ischaemic heart disease (codes ICD-9 410-14) and mortality from acute myocardial infarction (ICD-9 410). Data from the Cardiovascular Epidemiological Observatory/Health Examination Survey.
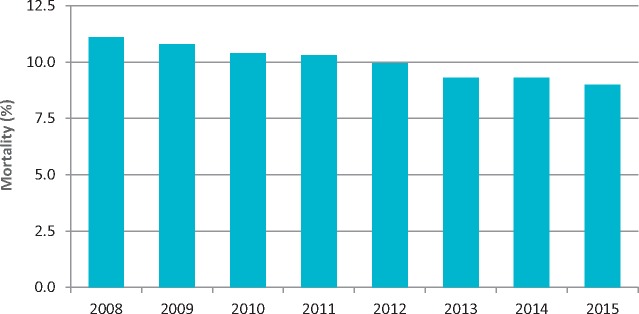
The most recent data on the epidemiology of AMI, however, derives from the National Outcomes Programme (PNE), which includes data from Regional Health Authorities not yet available at national level. In the 2016 report, AMI cases in Italy were estimated to be at 132,896/year. The 30-day mortality for AMI continues to decline, from 10.4% in 2010 to 9.0% in 2015 (Figure 3). There is low inter-regional variability and reasonable intra-regional variability, with values for hospital structures ranging from a minimum of 1.3% to a maximum of 25% (such data should be regarded with caution, as specified below). The 30-day mortality is inversely proportional to the hospital's volume of activity: 92% survival at 30 days in hospitals (n = 391) that treat at least 100 cases a year compared to 83% at 30 days in hospitals (n = 252) that treat less than 100 cases a year.
Figure 3.
Acute myocardial infarction: 30-day mortality, Italy 2008-2015. Data from the National Outcomes Project.
The results may have been partially altered by the incorrect identification of AMIs, for example unstable angina could have been coded as AMI or limited secondary myocardial damage coded as infarction without ST segment elevation NSTEMI. The overall trend shows a progressive reduction of mortality in the acute phase, while there is a reduction of 834 AMI cases each year.
Other Italian National databases, such as the Mattoni Project, provide information about the progressive increase of angioplasty procedures in Italy (141,830 angioplasties were performed in 2014, 32,557 of which were primary), while isolated coronary artery bypass surgeries (CABG) performed within Italian cardiac surgeries are decreasing over time5.
Although in Italy there has been a progressive reduction of in-hospital mortality for decades, which has also been confirmed by the aforementioned recent PNE data the post-discharge mortality trend appears as surprisingly stable or even increasing in several studies6. The epidemiology of acute coronary syndromes (ACS) has changed radically over recent years, both in Italy and worldwide, and these changes have affected both the post-acute and chronic phase7,8.
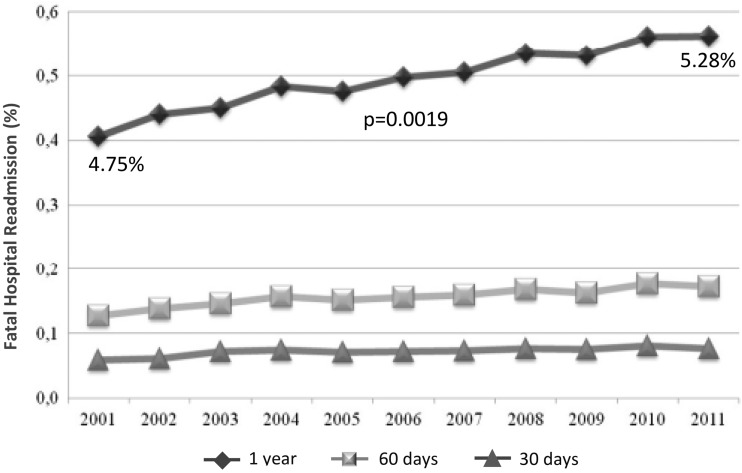
Other administrative sources, such as the Italian Hospital Discharge Record Database, provide further information or the changing scenario of ACS in Italy. In fact, a recent retrospective analysis of more than one million hospital discharge records, collected between 2001 and 2011, allowed the evaluation of 30-day, 60-day and one-year mortality and readmission rates after AMI. As amatter of fact, the index event mortality rate and total in-hospital mortality were reduced from 11.34% to 8.99% and from 16.46% to 14.68% respectively in the years 2001 to 2011 (both p <0.0001)9. On the other hand, one-year fatal readmissions increased from 4.75% to 5.28% (p = 0.0019) (Figure 4). This trend was even more evident in patients who had heart failure during the index hospitalisation, who showed a reduction of in-hospital mortality from 26.5% to 23.2%, but an increase of fatal re-hospitalisations at one year, with a mortality between discharge and the first year of 10% in 20119 .
Figure 4.
Trends in fatal hospital admission (F-RR) rates at one year, 30 days and 60 days in the Italian population with acute myocardial infarction. Data derived from hospital discharge records 2001-2011.
The risk of fatal readmission at one year therefore shows a slow, though progressive increase over time, despite the considerable improvements in the treatment of the acute phase of AMI. Accordingly, more effective risk stratification and early secondary prevention strategies should be implemented in AMI patients.
Based on such clear epidemiological data, the 2014 ANMCO/GICR-IACPR/GISE Consensus Document7 has already clarified how the risk of both ischemic recurrences and heart failure should be addressed by practicing clincicians in patients with ACS.
Patients with ejection fraction <40% patients with an ejection fraction between 40% and 45% and evidence of left ventricular remodelling, with as well as patients with a major, elevation of natriuretic peptide (BNP or NT-prpBNP) should be considered at high risk of developing heart failure after an ACS. On the other hand, the presence of diabetes mellitus, chronic renal failure, peripheral arterial disease, history of angina or of a previous AMI, multi-vessel coronary disease, incomplete revascularisation may identify patients at high risk of recurrence after an ACS.
Precisely based on the evidence of an increase in residual risk in the post-acute and chronic phase of coronary syndromes, and in particular in relation to thrombotic risk, it can be hypothesised that more intensive treatments in subgroups of selected patients may offer a prognostic benefit10,11. This observation confirms not only the importance of the period immediately following the ACS but opens the prospect to the need for long-term secondary prevention programmes in this type of patient and also independently of the cardiac failure variable, which remains the major predictor of mortality and re-hospitalisation.
A document from the World Heart Federation, which takes stock of secondary prevention in the world and in Italy12, indicating the goals to be achieved for the health of the planet until 2025, highlights the lack of a national plan for the secondary prevention of cardiovascular diseases in Italy. Secondary prevention is often integrated into routine care, performed at the discretion of the general practitioner, and only a minority of post myocardial infarction and post cardiac re-vascularisation patients access rehabilitation programmes. There are no diagnostic-therapeutic assistance plans integrated between the acute and post-acute phase which are synergistic between hospital and territory and above all harmonic and standardised in the regions and in the country. While there are no significant recovery problems for rehabilitation after an acute or chronic cardiac event, there is a disparity between the potential number of participating patients and the availability of rehabilitative cardiology units. A survey by the Italian Group of Rehabilitative Cardiology/Italian Association of Cardiovascular Prevention and Rehabilitation (GICR-IACPR) has described that access to rehabilitation cardiology units is able to determine an increase in adherence13, in addition to demonstrating a net reduction in cardiovascular events and re-infarction in subjects started on cardiological rehabilitation programmes after myocardial infarction14.
In conclusion, despite the considerable improvements currently achieved in the prognosis after an ACS, the risk of fatal one-year readmission is steadily increasing. It is necessary to identify the subgroups at risk and intervene by optimising and harmonising the available resources. Sustainable solutions are those able to determine a resource gradient proportional to the complexity and appropriateness of the prescriptions.
2. ACUTE CORONARY SYNDROME AND STABLE ANGINA, TWO DIFFERENT ENTITIES
ACS and stable angina are clinical profiles belonging to the spectrum of ischaemic heart disease, mainly due to atherosclerosis of the coronary arteries or to different causes such as, for example, congenital anomalies of coronary arteries, coronary arteries, myocardial bridges, etc.
Coronary atherosclerosis resulting in a varying degree of obstruction of the vessel lumen can lead to the development of myocardial ischaemia when the supply of oxygen to the myocardium is not adequate with respect to the requirement.
Although in some manifestations of coronary artery disease chest pain may be absent or non-predominant (silent ischaemia, arrhythmias, sudden death, heart failure, diabetic subjects), the most typical clinical manifestation of myocardial ischaemia is however angina, generally described as severe oppression or chest tightness and/or difficulty breathing, often radiated to the neck or arm.
Chest pain characterises both stable angina and ACS frameworks, but clinical profiles differ in the disorder’s duration and progression.
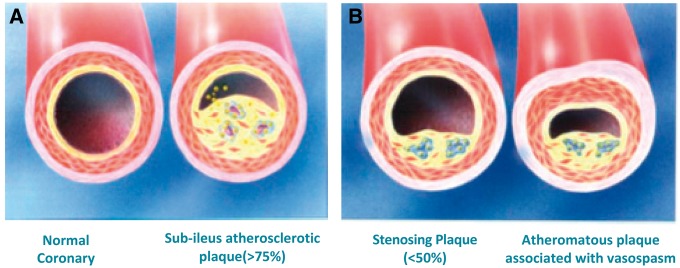
The definition of stable angina is, in fact, a negative definition: we speak of stable angina when the characteristics of unstable angina are missing, i.e. onset at rest, appearance from less than one-two months or progression in crescendo15. Stable angina is therefore a manifestation of reversible myocardial ischaemia, typically caused by physical or emotional and reproducible stress, lasting 5-10 min; the symptom is traditionally attributed to the presence of stenosis ≥50% in the common trunk or ≥70% in one or more coronary arteries; however, episodes of angina may also occur at rest due to the overlap of vasospasm on a coronary plate16 (Figure 5).
Figure 5.
Normal coronary artery and different types of Atheromatous plaque with or without spasm.
The latest guidelines from the European Society of Cardiology (ESC)17 on known or suspected stable coronary artery disease include several groups of subjects:
with stable angina or other symptoms (dyspnoea) reasonably attributed to coronary artery disease;
with obstructive or non-obstructive coronary artery disease already known and becoming asymptomatic in therapy (therefore also in phases of successive stability, after one year, at ACS);
that report the angina symptom for the first time but are believed to already be in a phase of stability (e.g. symptoms already present for months).
The same guidelines identify different clinical presentations of stable coronary artery disease, associated with different ischaemic mechanisms:
stenosis related to an atherosclerotic plaque in epicardial coronary arteries;
focal or diffuse spasm of normal coronary arteries or with atherosclerotic plaques;
microvascular dysfunction or spasm;
left ventricular dysfunction caused by previous necrosis or myocardial hibernation (ischaemic cardiomyopathy).
These mechanisms can act in isolation or in combination and thus justify the different clinical angina pectoris frameworks:15
exercise-induced: due to insufficient coronary artery obstructions to induce myocardial ischaemia at rest but which cause it in case of increased demand;
variant: transitory alteration of the blood supply by coronary vasospasm or platelet aggregation in the presence or absence of atherosclerotic plaques. Highlighted at rest, while it should be framed in unstable forms, but its long-term prognosis is good. Among these forms we must also include that of Prinzmetal, rarely, due to a vasospasm of a large coronary artery causing transmural ischaemia with elevation of the ST segment at rest or with exercise;
syndrome X: typical exercise-induced angina positive to provocation tests but with anatomically normal coronary arteries, due to reduced vasodilating capacity of the microcirculation, with a good long-term prognosis.
Furthermore, other mechanisms may alter the oxygen supply/demand balance, causing myocardial ischaemia (e.g. aortic valvulopathies, hypertrophic and dilated cardiomyopathy, uncontrolled hypertension), as well as some comorbidities that may cause functional type angina (e.g. oxygen demand for hyperthyroidism, hyperthermia and cocaine use, reduction of oxygen supply for anaemia, pneumopathies hypoxaemia, microemboli, blood hyperviscosity).
What we are interested in highlighting in this discussion in order to emphasise the role of antiplatelet therapy in the various clinical scenarios is the diversity of the histopathological substratum that characterises the atherosclerotic lesions of stable angina compared to those of ACS. Indeed, in stable clinical pictures, unlike when found in ACS, the plaques rarely show surface erosion or ruptures - they are typically fibrotic, with poor cellularity, often with a fibrotic cap and free of superimposed thrombi16.
It is therefore the reduced calibre of the vessel that plays the main role in the genesis of ischaemia.
Clinically, the severity of stable angina and its impact on the quality of life are estimated according to several classifications, the most frequently used is that of the Canadian Cardiovascular Society (CCS), which goes from Class 1 (angina only with prolonged intense activities without compromising normal activity) to Class 4 (angina at rest and inability to perform any physical activity)4.
As already mentioned, in some cases of angina at rest, theoretically attributable to the unstable angina chapter, the cause of ischaemia may be a spasm occurring on a plaque, in a context therefore of stable angina. In the same way, an angina at the beginning but which occurs after an intense effort, can fall into the definition of stable angina rather than unstable angina. This gives an idea of how difficult it sometimes is to classify the two entities (stable and unstable angina), which can in some cases be different phases of a continuum: this is also confirmed by the finding, in some cases of stable angina, of minimum elevations in troponin at high specificity that, even though well below the threshold for causing the infarction, have nevertheless been shown to have a certain prognostic impact19,20.
However, in general the two pictures are easily distinguishable and unstable angina is classically defined as pain that occurs: 1) at rest, with a duration of >20 min, 2) of new onset (CCS II or III), or 3) worsening (reduction of the threshold of a stable angina with rapid increase in number and intensity of episodes over a period of four weeks or less).
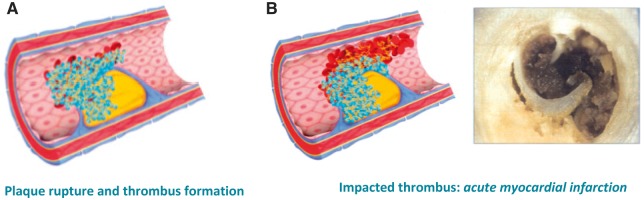
The unstable angina falls, on the pathophysiological level, in the chapter of ACS, whose substratum is represented by the rupture of a coronary atherosclerotic plaque, with formation of a thrombus on its surface that partially or completely obstructs the lumen of the vessel. The plaque of the culprit lesion in these cases, unlike what is seen in stable angina, presents rupture, erosion or fixation of the fibrous cap, is thin, with exposure of the necrotic core containing prothrombotic material; the contact of this material with the blood flow leads to the formation of the platelet thrombus21. Based on whether the plaque thrombosis is occlusive or not, the clinical picture will be that of an infarction with (STEMI) or persistent ST-segment elevation (NSTEMI) evidenced by the ECG (Figure 6).
Figure 6.
Plaque rupture and thrombosis formation leading to acute myocardial infarction.
As for stable angina, other mechanisms may be involved in the development of the acute clinical picture in the case of ACS as well: progressive dynamic obstruction, favoured by a spasm of an epicardial coronary, or constriction of small intramural arteries or endothelial dysfunction or local vasoconstrictors (thromboxane A2 released from the platelets); severe narrowing of the coronary lumen by progression of atherosclerosis from restenosis during percutaneous intervention; inflammation; severe myocardial ischaemia secondary to oxygen demand/supply imbalance (tachycardia, fever, hypotension, anaemia, etc.)22.
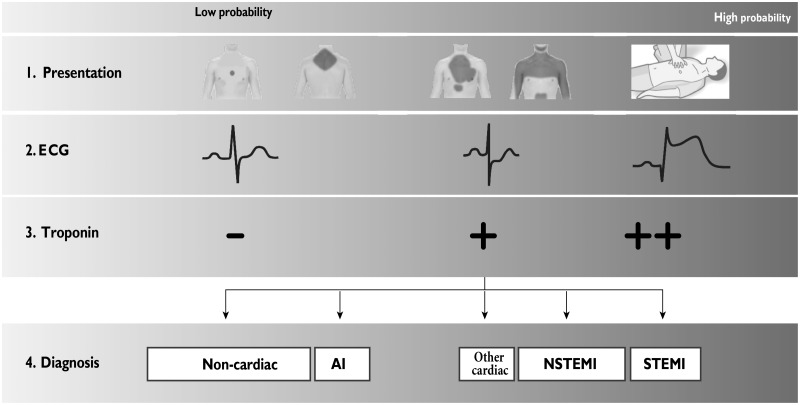
The introduction of high sensitivity troponins in the evaluation of patients with acute chest pain that seek emergency medical attention has reduced the number of unstable angina diagnoses in favour of those of NSTEMI over time (Figure 7)23. The diagnosis of unstable angina, in fact, presupposes the absence of troponin modifications, and therefore implies the absence of myocardial necrosis, which brings with it a lower risk of death compared to NSTEMI, as well as less benefit deriving from intense antiplatelet therapies or from early invasive strategies24–27 .
Figure 7.
Initial assessment of patients with acute coronary syndrome.
UA, unstable angina; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Used with permission from Roffi et al.23
The diagnosis of AMI, on the other hand, presupposes the presence of myocardial necrosis in a clinical context compatible with acute myocardial ischaemia (there are indeed other clinical conditions in which there is evidence of an increase in myocardial-troponin damage indexes but in completely different contexts where the clinical meaning is therefore completely different). A combination of elements for the diagnosis of infarction is therefore required, i.e. the presence of a characteristic necrosis marker curve (troponin, in crescendo-decrescendo) and with at least one value above the 99th percentile of the upper reference limit, associated with at least one of the other factors: ischaemia symptoms, new significant ST-T alterations to the ECG or new onset left branch block, development of pathological Q waves in the ECG, evidence of new segmental kinesis abnormalities or loss of vital myocardium for imaging tests, intra-coronary thrombosis with angiography or autopsy.
The type of infarction referred to is type one according to the universal classification of the infarction24, whose physio-pathological substrate, as already described, is that of acute thrombosis on plaque rupture. The other types of infarction predicted by the classification are not included here, considering the context of the treatment that deals with the use of antiplatelet therapy in coronary heart disease.
Despite having the same pathophysiological mechanism at its base, STEMI and NSTEMI differ from a clinical point of view for the prompt intervention required to treat them. The thrombosis that completely occludes the lumen of the culprit vessel in STEMI implies the need to reduce as much as possible the time of diagnosis and intervention to save as much as possible of the myocardial tissue put at risk by the occlusion itself. In these cases, anti-thrombotic therapy (antiplatelets and anticoagulants) should be intense and start as early as possible.
In the case of NSTEMI, clinical presentations include a broader spectrum, ranging from pauci/asymptomatic to patient presentation to cardiac arrest at the onset, passing through intermediate pictures of haemodynamic and electrical instability. According to the stratification of the risk of submitting the individual patient to the presentation, the most suitable strategy to follow among those indicated also in the guidelines will be established23.
In all cases, however, anti-thrombotic therapy is a cornerstone of treatment, given the physio-pathological substrate at the base of the clinical event.
In conclusion, this brief summary of the definition, classification and description of the clinical forms of ischaemic heart disease aims to underline the different role that the formation of the platelet thrombus has in ACS, where it is generally the protagonist, and in stable angina, where it is definitely in the background. This supports the different use of antiplatelet agents in the two clinical conditions: need for intensive and early therapy in the case of ACS to reduce serious events already in the acute phase, and long-term therapy useful to improve the outcome in secondary prevention in the stable angina case.
3. RATIONALE FOR LONG-TERM ANTIPLATELET THERAPY
In the last 30 years, we have witnessed a progressive reduction of deaths caused by the main cardiovascular diseases in Italy. However, AMI and strokes remain very frequent pathologies and are among the main causes of permanent disability in the Italian population. These acute events represent the main clinical manifestations of atherosclerotic cardiovascular disease (MCVA), whose pathogenetic basis is in the progressive atherosclerotic damage of the arterial vessels28.
Atherosclerosis is characterised in its early stages as a systemic inflammatory process, featuring an initial and circumscribed lipid accumulation (lipid strie) located at the level of the intima of the arterial vessels. The following periods in the development of vascular lesions are dominated by an inflammatory reaction, mediated by the cellular component (macrophages and monocytes)29. Finally, the combination of lipid overload and inflammatory reaction leads to the formation of the arterial lesions that characterise the disease: atherosclerotic plaques. The site (affected arterial district, size of the injured vessel) and the characteristics of the individual vascular lesions (extension, erosion, breakage or relative stability of the plaques) will condition the subsequent clinical development of the disease and the severity of any acute manifestations. In the context of disease progression to its acute clinical manifestations, the role of platelets dominates the pathophysiological and clinical picture.
In the more advanced phases of MCVA, the integrity of the endothelium covering the atherosclerotic plaque is potentially compromised and the arterial pathology is transformed, complicating itself, from atherosclerotic into atherothrombotic29. In fact, the acute clinical manifestations of the disease, AMI and ischaemic stroke, recognise the most important pathogenetic component in the thrombotic phenomena, mediated by the aggregation of the platelets28. In the continuum of MCVA, the platelets are placed, therefore, in a central position conditioning the acute clinical expression. The adhesion and aggregation of the platelets on the exposed surface of the eroded or lacerated atherosclerotic plaque represent, in fact, the initial moment of acute thrombosis and the consequent subsequent tissue ischaemia.
The synthetic pathophysiological scheme illustrated is the basic rationale for the use of antiplatelet agents in the prevention and treatment of acute manifestations of MCVA30. These drugs have the capacity of platelets to adhere to the damaged endothelium and aggregate, preventing thrombotic phenomena superimposed on complicated atherosclerotic lesions. The particular positioning of the platelets in the development of the MCVA explains why antiplatelet drugs have proved to be effective above all in the treatment of the acute phase of AMI and stroke and in secondary prevention.
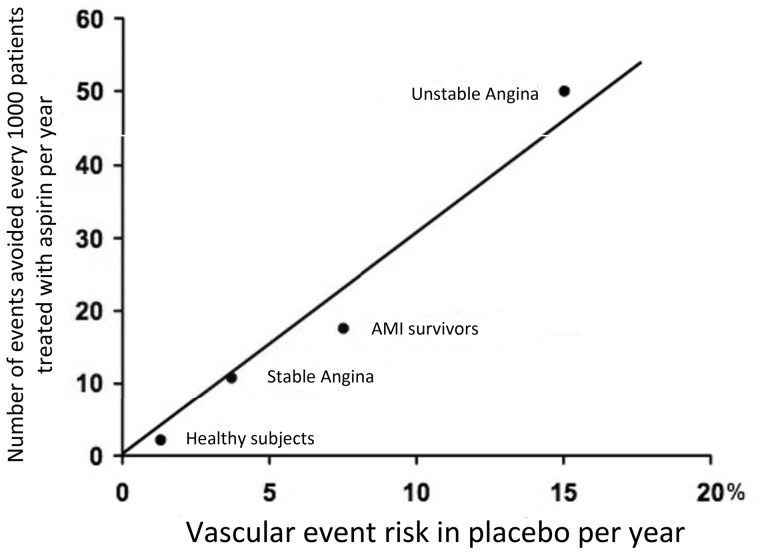
Wanting to disregard the vast range of data related to the treatment of acute manifestations of MCVA, the long-term benefits of antiplatelet therapy with aspirin (ASA) in secondary cardiovascular prevention have been conclusively demonstrated by the methylation of the Antithrombotic Trialists' Collaboration30. This study included data from over 135,000 patients with previous atherosclerotic cardiovascular events from 195 randomised controlled trials. The meta-analysis showed that ASA therapy is able to reduce the relative risk (RR) of ischaemic recurrences by 22% (Figure 8). In absolute terms, for example, antiplatelet therapy with ASA would avoid 36 major ischaemic events for every 1000 patients with previous AMI treated for at least 27 months.
Figure 8.
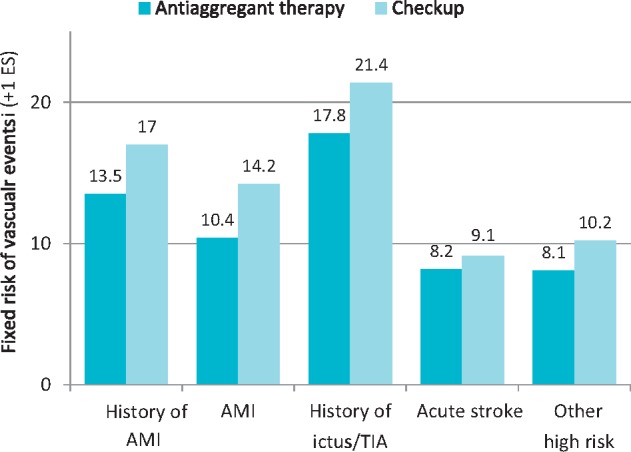
Effect of antiplatelet therapy on the risk of vascular events (myocardial infarction, stroke or vascular death) in five categories of high-risk patients.
SE, standard error; AMI, acute myocardial infarction; TIA, transient ischaemic attack.
Modified by Antithrombotic Trialists’ Collaboration30
Some studies have compared long-term treatment with thienopyridine, P2Y12 platelet receptor inhibitor drugs of first and second generation (ticlopidine or clopidogrel) compared to ASA. In particular, the CAPRIE study (Clopidogrel vs Aspirin in Patients at Risk of Ischaemic Events) conducted on about 20,000 patients with MCVA (previous AMI, previous stroke or peripheral arterial disease) showed a modest, albeit significant, effect in favour of clopidogrel compared to ASA31. Over a time-range of about two years, in fact, the incidence of adverse cardiovascular events was 5.3% per year in patients treated with clopidogrel and 5.8% in patients treated with ASA. Similar results have also been obtained with ticlopidine, which however showed a less favourable safety profile than clopidogrel32. Overall, the metanalytic data indicate that therapy with thienopyridine (clopidogrel or ticlopidine) would be able to prevent an additional 10 major cardiovascular events for every 1000 patients treated for two years compared to ASA therapy. Furthermore, therapy with thienopyridine was associated with a lower risk of gastrointestinal haemorrhagic events32.
Ultimately, the information deriving from the large-intervention clinical studies gives us a substantially unequivocal picture. Long-term antiplatelet therapy reduces the risk of further ischaemic events in patients with clinical evidence of MCVA and/or previous major atherothrombotic ischaemic events. The ASA is the recommended choice in international guidelines33 for treatments of indefinite duration in secondary prevention, even if the first and second generation thienopyridines seem to have a slightly higher safety and efficacy profile.
A new question related to the secondary prevention of MCVA now arises.
DAPT, which involves the association of a second antiplatelet (P2Y12 receptor inhibitor) with ASA, has been shown to be particularly effective in reducing ischaemic recurrences in patients with ACS and in clinically stable patients undergoing percutaneous revascularisation interventions33. The two clinical situations mentioned above are characterised by a high instability of atherosclerotic vascular lesions and/or by the presence of intravascular stents28,29. In these conditions, characterised by high vascular reactivity, the role of the platelets is crucial in favouring further possible thrombotic events. Therefore, a more incisive antiplatelet intervention can certainly be beneficial34. But is this paradigm also applicable in secondary prevention? When and in which clinically stable patients with clinical evidence of MCVA should an antiplatelet treatment of higher intensity be considered that includes the association of an additional P2Y12 receptor inhibitor with ASA?
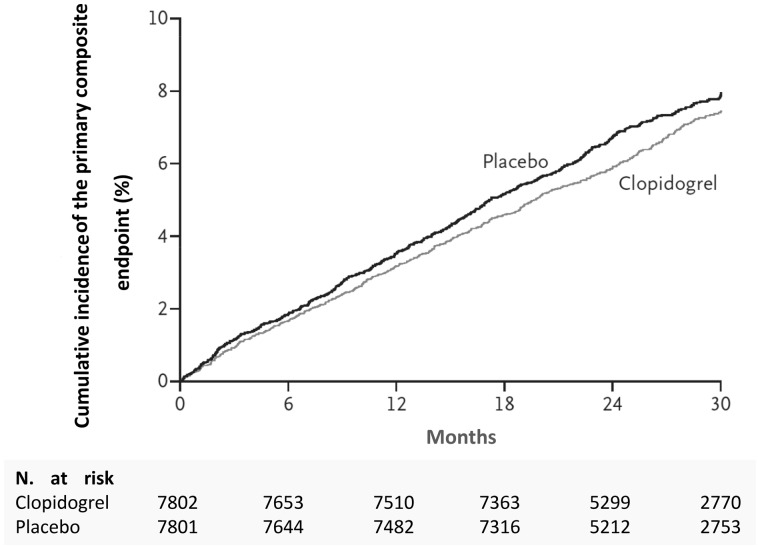
In the CHARISMA study (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilisation, Management and Avoidance), 15,600 patients with a high atherothrombotic risk profile (ischaemic heart disease, previous stroke, peripheral arteriopathy, asymptomatic with multiple risk factors) were randomised to treatment with DAPT (clopidogrel and ASA) against ASA alone35. At a follow-up of over two years, the incidence of adverse cardiovascular events was 6.8% in patients treated with DAPT and 7.3% in patients treated with ASA (p = 0.22) (Figure 9). Overall, the data from this study seems to indicate that DAPT is not a viable therapeutic strategy in all patients with either direct or indirect evidence of clinically stable MCVA. In fact, the selection of patients to start this treatment must be particularly accurate, as suggested by the sub-analyses of the same CHARISMA study. These ex post facto assessments indicate a possible benefit of DAPT in patients with previous AMI, previous stroke or peripheral arterial disease (Figure 10)36.
Figure 9.
Cumulative 30-month incidence of primary endpoint (myocardial infarction, stroke or cardiovascular death) in the CHARISMA study.
Modified from Bhatt et al.35
Figure 10.
Cumulative incidence of primary endpoint (myocardial infarction, stroke or cardiovascular death) in patients with previous myocardial infarction enrolled in the CHARISMA study.
ASA, aspirin; HR, hazard ratio; CI, confidence interval.
Modified from Bhatt et al.36
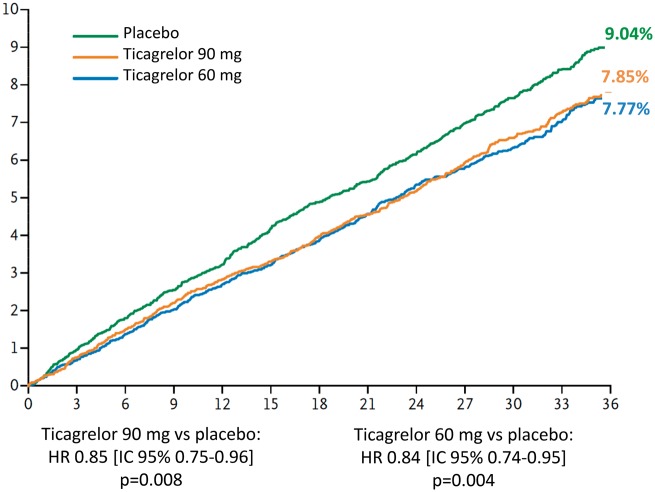
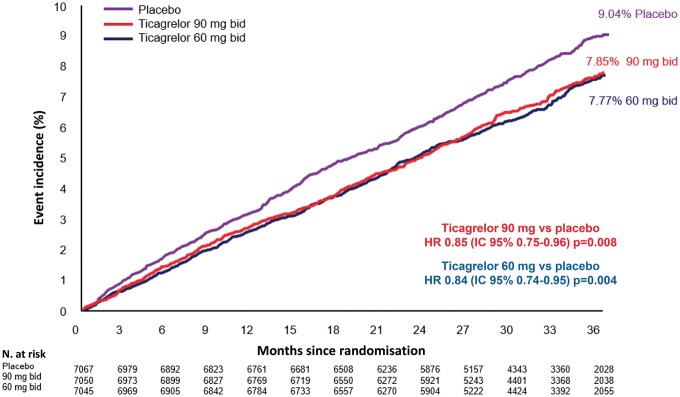
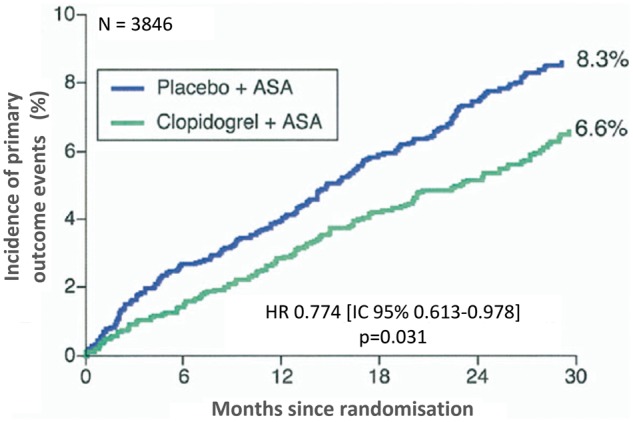
Recently, the hypothesis that DAPT can reduce ischaemic recurrences in patients with a history of AMI was demonstrated in the PEGASUS-TIMI 5411 study (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54). A cohort of more than 21,000 patients with a history of previous AMI was randomised to receive ticagrelor, a reversible P2Y12 platelet receptor inhibitor, at a dose of 90 mg bid or 60 mg bid or placebo. At the time of enrolment, all patients were on ASA therapy. In a median follow-up of 33 months, both ticagrelor doses significantly reduced the incidence of major cardiovascular events compared to placebo (incidence of three-year events of 7.8% in the ticagrelor 90 mg group, 7.7% in the ticagrelor 60 mg group and 9.04% in the placebo group) (Figure 11).
Figure 11.
Three-year cumulative incidence of cardiovascular death, myocardial infarction and stroke in the PEGASUS-TIMI 54 study.
ASA, aspirin; HR, hazard ratio; CI, confidence interval.
Modified by Bonaca et al.11
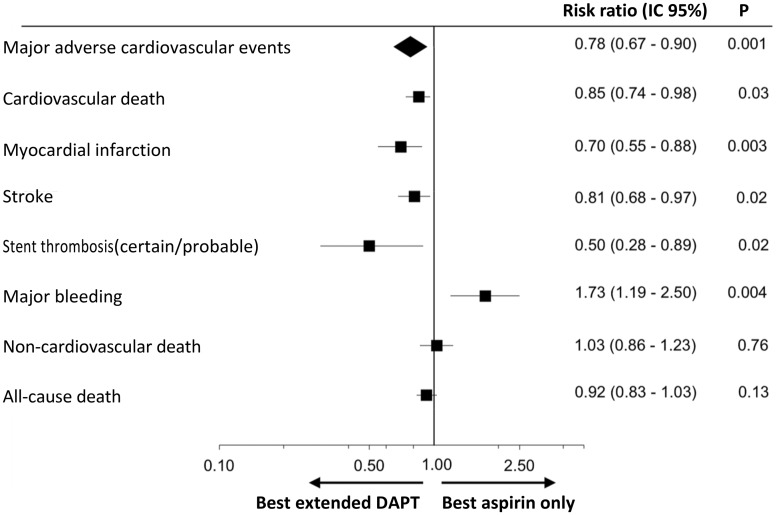
The positive potential of DAPT in patients with a history of previous AMI is also confirmed by a recent meta-analysis which included data from over 33,000 patients included in six randomised clinical trials37. In this study, DAPT demonstrated the ability to reduce the RR of major medium-term cardiovascular events by 32% in patients with a history of previous AMI (Figure 12).
Figure 12.
Risk of cardiovascular and haemorrhage events in patients treated with dual antiplatelet therapy (DAPT) vs aspirin alone.
CI, confidence interval.
Adapted from Udell et al.37
Therefore, long-term DAPT is a particularly interesting therapeutic option, especially in patients with a history of previous AMI. The individual risk profile must, however, be assessed with particular attention, also because of the not inconsiderable risk of bleeding that this treatment entails.
4. ANTIPLATELET THERAPIES AVAILABLE IN ITALY
Coronary atherosclerosis is a pathological process that affects the wall of the coronary arteries, characterised by the development of atheromatous plaques that may undergo ulceration, rupture or bleeding, with subsequent activation of the haemostatic system and thrombus formations that constitute the main complication of the atherosclerotic process within the ACS.
The correction of cardiovascular risk factors plays a fundamental role in the development and progression of coronary plaque, but at the same time the modulation of platelet activation and aggregation plays an important role both in the formation of plaque and in the pathogenesis of acute coronary artery disease in the context of ACS.
In fact, it is known that the breaking of the coronary artery's intima in the context of an atheromatous plaque directly exposes the structures of the damaged wall to the blood flow, in particular the von Willebrand factor and collagen, and the platelet membrane surface receptors interact with these structures, causing the platelets to adhere to the damaged wall and the formation of intercellular bridges between the platelets, a process that involves coagulation factors and glycoprotein receptors (GP) IIb/IIIa and which leads to the formation of the platelet thrombus39. Platelet activation causes the release of substances such as adenosine diphosphate (ADP), arachidonic acid and platelet activation factor. ADP plays a fundamental role in platelet activation because it interacts with P2X1 and P2Y1 receptors and favours the first reversible phase of activation; interacting with P2Y12 receptors, it prolongs the platelet aggregation in an irreversible phase of the same40. Arachidonic acid is metabolised by the cyclo-oxygenases (COX), in particular COX-1, a thromboxane A2, a powerful platelet agonist41. Moreover, platelets can also favour the formation of plaque as well as intervene at the time it ruptures. They can adhere to the endothelium by attracting monocytes that penetrate the same, transforming into macrophages, the same endothelial progenitor cells recruited by activated platelets can turn into foam cells, favouring atherogenesis42.
From a clinical point of view, antiplatelet therapy with drugs that inhibit platelet activation or aggregation should be able to interfere with plaque formation and thrombosis following its breakdown. Therefore, antiplatelet agents should be able to exert a favourable action in terms of both primary and secondary prevention. The results of clinical trials in this regard seem to confirm a very positive role of antiplatelet therapy in secondary prevention of cerebro- and cardiovascular events in patients judged to be at high-risk for a previous cardiovascular event, with a reduction of the RR of 25%30, while in terms of primary prevention, the risk/benefit balance of antiplatelet therapy does not appear as certain as shown by the meta-analysis of primary prevention trials conducted by the Antithrombotic Trialists' Collaboration in 200943, even in populations such as those of diabetics where the benefit seemed obvious enough to indicate a use not corroborated by the evidence in the literature in the international guidelines. Two trials in progress, ASCEND44 and ACCEPT-D45, will assess the net clinical benefit of ASA (and omega-3 and simvastatin respectively) in primary prevention on a large population of diabetics with a very long follow-up.
4.1 Aspirin
Discovered as a good antiplatelet agent in the 1960s, its antiplatelet action is performed by means of a specific irreversible COX-1 inhibition which results in a reduced production of thromboxane A2, a strong platelet agonist. As this inhibition is irreversible, platelet aggregation can be restored only with the synthesis of new platelets. A daily dose between 75 and 160 mg/day has been shown to significantly reduce cardiovascular events in secondary prevention. There is a lack of reliable data on the net clinical benefit in primary prevention, also as aforesaid in high-risk patient populations similar to those who have already had an event, for example diabetics (Figure 13).
Figure 13.
Prevention of cardiovascular events, aspirin vs placebo.
AMI, acute myocardial infarction.
Modified by Antithrombotic Trialists’ Collaboration30
4.2 Clopidogrel
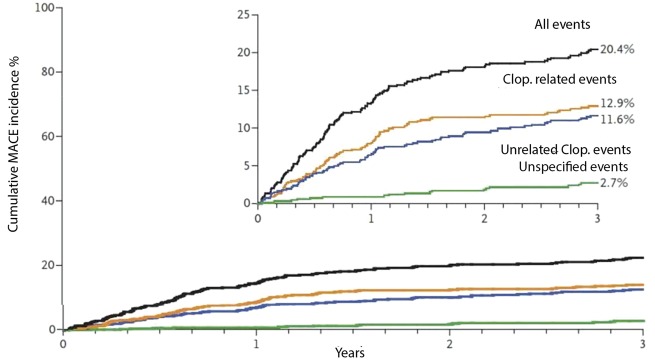
Clopidogrel was released onto the market in 1997 in the United States, and in 1998 in Europe, and is a second-generation thienopyridine that exerts its anti-aggregating action by irreversibly inhibiting the bond between ADP and the P2Y12 surface purinergic receptors. This binding activates the inhibitory G protein, which results in a reduction of intra-platelet concentration of cyclic adenosine monophosphate (cAMP), which promotes the expression of GPIIb/IIIa aggregation receptors on the platelet surface. Thus, ADP is the P2Y12 receptor agonist, while adenosine triphosphate (ATP) is the receptor antagonist that increases the production of cAMP and therefore reduces platelet aggregation. It is a pro-drug whose intestinal absorption is linked to the intervention of esterases which causes inactivation of 85% of the molecules; the remaining 15% are oxidised at a hepatic level, in particular by the CYP2C19 isoform of cytochrome P450, obtaining the active metabolite46. The P2Y12 receptor binding causes an irreversible modification in the chemical conformation of the P2Y12 receptor, which prevents bonding with ADP, then causes an increase in AMP and a consequent reduction in the expression of the GPIIb/IIIa aggregation receptor complex and the platelet aggregating ability47. It is now known that the response, in terms of effective antiplatelet aggregation by clopidogrel, is subject to a wide inter-individual variability that can lead to a percentage of so-called "poor responders" between 5% and 40% depending on the cut-offs used to assess the residual platelet reactivity of treated patients48. Some genetic polymorphisms of cytochrome P450 isoforms that metabolise the drug are among those responsible for this phenomenon.
The drug has been tested in numerous clinical trials; in the field of ischaemic heart disease, the founder was certainly the CURE study (Clopidogrel in Unstable Angina to Prevent Recurrent Events) in patients with ACS without ST segment elevation (NSTE-ACS)49. Compared to ASA monotherapy, the ASA + clopidogrel association reduces the frequency of ischaemic recurrences in patients with NSTE-ACS by 20%49. It also reduces the composite frequency of acute cardiac events by 25-30%, such as AMI, urgent revascularisation and 30-day cardiac mortality and 12 months after placement of a coronary stent as shown in the CREDO study (Clopidogrel for the Reduction of Events During Observation) and PCI-CURE study50,51. It also reduces the composite risk of death, reinfection and recurrent myocardial ischaemia in patients with AMI undergoing thrombolysis by 20%52. Nevertheless, many patients, also in dual therapy with ASA + clopidogrel, develop major thrombotic complications (early recurrence of myocardial ischaemia, coronary restenosis after angioplasty, stent thrombosis, etc.)53, and in many of these patients the in vitro tests of platelet aggregation show a sub-optimal response to therapy with both ASA54 and with clopidogrel, then manifest a form of pharmacological resistance to antiplatelet agents55.
Clopidogrel is now used according to international guidelines in the treatment of patients with stable coronary heart disease undergoing a percutaneous coronary intervention (PCI) in association with ASA for a duration of three-six months when treated with a drug-eluting stent (DES) and one month if treated with a metallic stent (BMS)56. It can also be used as an alternative to ASA in patients with chronic allergic diseases or those intolerant to ASA. In ACS its use is limited to cases in which therapy with ticagrelor or prasugrel is not feasible23,57. Regarding the much-discussed interaction between proton pump inhibitors (PPI) and clopidogrel, combined with the inhibitory action that PPIs as a class would have on the CYP2C19 isoform, the same involved in the hepatic bio transformation of clopidogrel, the American and European Regulatory Agencies (Food and Drug Administration [FDA] and European Medicines Agency [EMA]/Italian Drug Agency), based on numerous contrasting studies (Table 1)58–65, first advised against combining these two classes of drugs, then restricted this caution to esoprazole and omeprazole alone, thus denying an overall class effect and rather corroborating the concept of different degrees of inhibition of CYP2C19 isoform by drugs of the same class66–69.
Table 1.
Characteristics of the studies on the interaction between clopidogrel and proton pump inhibitors
| Author | Year | Design | Population | Exposure | Duration | Results |
|---|---|---|---|---|---|---|
| Ho et al.58 | 2009 | Retrospective cohort study | 8,205 | Clopidogrel + PPI (omeprazole, lansoprazole, rabeprazole) | 521 days (average) | The concomitant use of clopidogrel and PPI increases the risk of death or ACS (OR 1.27, 95% CI 1.10-1.46) |
| Juurlink et al.59 | 2009 | Case-control study | 13,636 | Clopidogrel + PPI (omeprazole, lansoprazole, rabeprazole) | 90 days | Increased risk of reinfarction in co-therapy patients with the exception of pantoprazole (OR 1.27, 95% CI 1.03-1.57) |
| Sibbing et al.60 | 2009 | Transversal observational study | 1,000 | Clopidogrel + PPI (omeprazole, pantoprazole, esomeprazole) | NA | Reduction of the platelet response to clopidogrel in subjects receiving omeprazole (p = 0.001). No effect with esomeprazole and pantoprazole |
| Siller-Matula et al.61 | 2009 | Prospective study | 300 | DAPT + PPI (pantoprazole, esomeprazole) | 3 months | Esomeprazole and pantoprazole do not alter the response to clopidogrel |
| O'Donoghue et al.62 | 2009 | Post-hoc analysis on two RCTs |
|
Prasugrel or clopidogrel at high dosages + PPI |
|
|
| Bhatt et al.63 | 2010 | RCT | 3,761 | DAPT + omeprazole | 106 days | No CV interaction between clopidogrel and omeprazole (HR 0.99, 95% CI 0.68-1.44) |
| Douglas et al.64 | 2012 |
|
24,741 | DAPT + PPI | 303 days (average) |
|
CV, cardiovascular; DAPT, dual antiplatelet therapy; HR, hazard ratio; CI, confidence interval; NA, not available; OR, odds ratio; PPI, proton pump inhibitors; RCT, randomised controlled trial; ACS, acute coronary syndrome.
Adapted from Casula et al.65
A recent meta-analysis of non-randomised and uncontrolled studies on the subject concluded that if only randomised trials are considered, there is no negative interference between PPI and clopidogrel antiplatelets70.
4.3 Ticagrelor
Ticagrelor belongs to the cyclopentyl-triazolopyrimidine class, it differs from thienopyridines as it is a direct and reversible inhibitor of the P2Y12 receptor for the ADP71. Ticagrelor is not a pro-drug, requires no biotransformation, is not subject to action latency or drug-resistance phenomena. It binds to the P2Y12 receptor reversibly, and causes a temporary modification of its conformation so as to prevent, in a non-competitive way, binding to ADP72. Ticagrelor is a structural analogue of ATP, which in turn is a natural inhibitor with a short half-life of P2Y12 receptor but with respect to which, due to a modification of the chemical structure, has a greater affinity for the receptor72. Pharmacokinetic studies show that the latency of the anti-aggregating effect of ticagrelor depended only on the rate of intestinal absorption of the drug, so that the "resistance" to the antiplatelet effect in continuous treatment rarely arises73,74. All this means that, for example in patients treated with clopidogrel, a single administration of ticagrelor of 180 or 270 mg, produces a further decrease in the platelet aggregation rate in the subsequent 12 hours: a reduction of the average aggregation values from 28-38% (clopidogrel) to about 5% (after ticagrelor). Furthermore, the maximum platelet inhibition rate (identified by residual aggregation <10%) goes from 20% after clopidogrel to over 90% after ticagrelor, which would show how ticagrelor is also able to recruit and prevent the aggregation of that residual amount of clopidogrel-resistant platelets in patients more responsive tothienopyridine74. In this sense, the phase II trial DISPERSE-1 (Dose Confirmation Study Assessing Antiplatelet Effects of AZD6140 vs Clopidogrel in Non-ST-Segment Elevation Myocardial Infarction) was to provide pharmacokinetic and pharmacodynamic information as well as safety and tolerability of the ASA + ticagrelor association at increasing doses (50, 100 or 200 mg bid or 400 mg/day) vs ASA + clopidogrel in standard fixed combination (ASA 75/100 mg + clopidogrel 75 mg/day) in 200 patients with known atherosclerotic disease by excluding patients with ACS or who had undergone PCI in the previous four months. The study showed that a 100 mg bid dose of ticagrelor is already able to allow more rapid and boosted platelet inhibition than clopidogrel (inhibition of platelet activity, respectively >90% ticagrelor and about 60% clopidogrel), with a safety and tolerability profile similar to that of clopidogrel, with only one episode of major bleeding but in a patient with a ticagrelor 400 mg/day intake regimen. A 10-20% incidence of dyspnoea was already evident among the adverse effects, but only one patient was forced to stop treatment.73,74 The Phase II DISPERSE-2 trial was instead designed to test the safety and efficacy of two different ticagrelor dosages in comparison with standard clopidogrel dosage but on a population of 990 patients with NSTE-ACS also undergoing surgical or percutaneous treatment, randomised to receive, in addition to ASA, a dose of ticagrelor 90 or 180 mg bid or clopidogrel 75 mg/day for 12 weeks75. The primary endpoints were the incidence of bleeding (major and minor) in the first four weeks of treatment, incidence of myocardial infarction, cardiovascular death, stroke and recurrent ischaemia and the determination of platelet inhibition. There were no significant differences in bleeding between the two groups; patients undergoing CABG were bleeding even less in the ticagrelor group. There was no statistically significant difference between ticagrelor and clopidogrel in the four-week incidence of myocardial infarction, stroke or recurrent ischaemia, but there was a trend favourable to ticagrelor for the incidence of myocardial infarction at 12 weeks75.
The efficacy and safety of ticagrelor have been evaluated in important phase III clinical trials. The most important is PLATO (Platelet Inhibition and Patient Outcomes), a randomised, double-blind study comparing ticagrelor (loading dose of 180 mg followed by 90 mg bid) with clopidogrel (loading dose of 300-600 mg followed by 75 mg/day), both added to ASA treatment, in 18,624 patients with ACS (STEMI and NSTE-ACS) enrolled within 24 hours from the onset of the symptoms that could go against an invasive or conservative therapeutic strategy76. The primary composite endpoint was death due to cardiovascular causes, myocardial infarction and stroke; the main safety endpoint was major bleeding (including those associated with any CABG interventions). At 12 months the primary combined endpoint occurred in 9.8% of patients treated with ticagrelor versus 11.7% of patients treated with clopidogrel (hazard ratio [HR] 0.84, confidence interval [CI] 95% 0.77-0.92; p<0.001); the curves had already diverged after the first 30 days of therapy and were maintained throughout the study observation period. The individual components of the composite endpoint showed the following incidences (ticagrelor vs clopidogrel): myocardial infarction 5.8 vs 6.9% (p = 0.005); death due to cardiovascular causes 4.0 vs 5.1% (p = 0.001); total mortality 4.5 vs 5.9% (p <0.001). Only the incidence of stroke was not significantly different in the two groups (1.5 vs 1.3%; p = 0.22) with a similar number of ischaemic events, but with an increase in haemorrhagic strokes in the ticagrelor group, although not statistically significant (0.2 vs 0.1%; p = 0.10). With regard to safety endpoints, there were no statistically significant differences in major bleeding between the two treatments (11.6 vs 11.2%; p = 0.43), whereas the combination of major and minor haemorrhage was in favour of clopidogrel (16.1 vs 14.6%; p = 008). The PLATO study confirmed the presence in the ticagrelor group of known side effects with respect to clopidogrel, as an increased incidence of dyspnoea (13.8 vs 7.8%) that rarely causes treatment withdrawal. However, PLATO shows a statistically significant reduction in cardiovascular mortality and total mortality in the arm treated with ticagrelor, both with conservative strategy and invasive strategy.
Ticagrelor is used on the basis of the indications provided by the European guidelines in the treatment of patients with NSTE-ACS or STEMI for one year regardless of the type of treatment, whether conservative or interventional (Class I recommendation, level of evidence B)23,57,77. The PEGASUS-TIMI 54 multicentre clinical study11 was designed to evaluate the efficacy of dual antiplatelet treatment with ticagrelor in reducing recurrent ischaemia over a period of more than one year from the index event in high-risk patients. The double-blind study recruited 21,162 patients with previous myocardial infarction over a period of about 20 months. Patients were randomised at least one year from the acute event into one of the three arms: treatment with ticagrelor 90 mg bid, treatment with ticagrelor 60 mg bid, or placebo. The recruited population had a medium-high ischaemic risk profile, the inclusion criterion being at least one additional risk characteristic between: age ≥65 years, diabetes mellitus, chronic renal failure (CRF), multivessel coronary disease and recurrent myocardial infarction. Patients with a history of or predisposition to bleeding, previous stroke/TIA or the need for anticoagulant therapy were excluded. The primary efficacy endpoint was cardiovascular mortality, myocardial infarction and stroke; the primary safety endpoint was major TIMI bleeding. The primary analysis was performed by comparing each dose of ticagrelor with placebo. Administered at two different doses, ticagrelor demonstrated a significant reduction in favour of prolonged treatment of the primary efficacy endpoint (ticagrelor 90 mg: HR 0.85, 95% CI 0.75-0.96; p = 0.008; ticagrelor 60 mg: HR 0.84, 95% CI 0.74-0.95; p = 0.004). Both doses showed a significant reduction in terms of myocardial infarction, whereas only at the 60 mg dose, ticagrelor showed a significant difference in the incidence of ischaemic stroke. No difference emerged between ticagrelor (at both doses) and placebo in terms of death from any cause, unstable angina, urgent revascularisation procedures and TIA. Taking into consideration the safety endpoints, a significant difference emerged to the detriment of both ticagrelor doses compared to placebo in terms of major and minor TIMI bleeding, bleeding requiring transfusion or determining discontinuation of treatment, but with a lower incidence of haemorrhagic events at 60 mg bid. The incidence of dyspnoea was greater (ticagrelor 90 mg: HR 3.55, 95% CI 3.16- 3.98, p < 0.001; ticagrelor 60 mg: HR 2.81, 95% CI 2.50-3.17; p < 0.001) in discontinuation of therapy due to dyspnoea (ticagrelor 90 mg: HR 8.89, 95% CI 6.65-11.88, p < 0.001; ticagrelor 60 mg: HR 6.06, 95% CI 4.50-8.15; p < 0.001) and gout attacks compared to the placebo arm; no difference emerged in terms of bradycardia. Treatment with ticagrelor at 60 mg bid showed a more favourable risk/benefit balance than the 90 mg bid regimen, which would make it plausible to reduce platelet aggregation even over one year from an infarction event in patients at high risk of ischaemia recurrences11.
4.4 Prasugrel
In order to overcome the limits of clopidogrel in terms of rapidity of action, antiplatelet potency and individual variability, the third-generation thienopyridine prasugrel was formulated. It is an irreversible indirect inhibitor of the P2Y12 receptor which shows rapid initiation of the antiplatelet effect (the drug is biologically active after 15 min from a loading dose of 60 mg) and a greater antiaggregating capacity compared to clopidogrel, whose biotransformation from pro-drug to active metabolite is not influenced by cytochrome P45078–80. Given, however, the numerous phase II and III studies that showed the greater rapidity of antiplatelet action initiation and the superior antiaggregant potency of prasugrel compared to clopidogrel81–85.
The largest phase III trial comparing prasugrel with clopidogrel is TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38)86–92. In a population of 13,600 patients with ACS undergoing PCI, the trial tested the efficacy of ASA + prasugrel vs ASA + clopidogrel, showing: a) a statistically significant reduction of approximately 21% in terms of main composite endpoint RR of cardiovascular death, myocardial infarction, and stroke, in favour of the combination ASA + prasugrel vs ASA + clopidogrel (9.9 vs 12.1; p = 0.003; number needed to treat [NNT]=46); b) a statistically significant reduction of around 42% of the RR of both late and early stent thrombosis is independent of the type of stent (DES or BMS) in favour of ASA + prasugrel (RR 1.1 vs 2.4; p = 0.0001; NNT = 77); c) net clinical benefit in favour of ASA + prasugrel if the relative outcome of reduction of cardiovascular events vs major bleeding is assessed (p = 0.004), except in three subgroups of patients: those with prior stroke/TIA, those older than 75 years and those weighing < 60 kg. In these three subgroups, the impact of haemorrhagic events negatively influenced the avoided cardiovascular events 86. However, some variables were identified that increase the risk of haemorrhage of the prasugrel group, such as age, female gender, presence of CRF, use of GPIIb/IIIa inhibitors, femoral access and duration of the procedure. Bleeding was associated with a higher rate of mortality only if it occurred in the first 40 days87. The clear clinical benefit was favourable, especially in the STEMI population and in that of diabetic patients88,89. In patients undergoing CABG there was an increase in bleeding, the need for transfusions and exploratory reopening, with a mortality rate lower than the clopidogrel group90. As regards the possible interference related to the concomitant use of PPIs, no differences in efficacy were found with respect to the combination PPI + prasugrel91. Furthermore, prasugrel compared to clopidogrel seems to reduce not only the incidence of a first event subsequent to the index one, but also of subsequent recurrent events92. The efficacy of prasugrel in comparison with clopidogrel was also tested in the TRILOGY ACS trial in the context of patients with unstable angina or NSTEMI treated conservatively, i.e. those who did not undergo PCI for a period of 30 months, reducing the dosage of the drug from 10 to 5 mg in the group of patients over 75 years of age, weight under 60 kg or previous stroke/TIA without obtaining a significant benefit in terms of reduction of cardiovascular death, reinfarction or stroke, compared to clopidogrel93. The drug is now also indicated on the basis of the European guidelines in treatment with a dose of 10 m/day after a loading dose of 60 mg for patients with STEMI and those with NSTE-ACS, provided that the coronary anatomy is known and therefore who are candidates for a percutaneous revascularisation procedure (class I, level of evidence B)56. It is not indicated in patients aged over 75 years or weighing below 60 kg; it is certainly contraindicated in patients with a previous stroke/TIA56,57.
5. EVIDENCE TO SUPPORT THE REDUCED DURATION OF DUAL ANTIPLATELET THERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION: FROM THE RISK OF LATE THROMBOSIS TO THE LATEST GENERATION OF STENTS
The introduction of DES has been revolutionary in coronary intervention, mainly due to the need to limit what turned out to be one of the problems of the first BMS devices, namely restenosis, even before stent thrombosis. In fact, in the age of BMS, the risk of stent thrombosis was higher in the immediate post-revascularisation period than the incidence in the medium and long term.
DESs, starting from the metal structures of the BMS devices, were thus coated with physical agents capable of carrying pharmacological agents which can inhibit intimal neoproliferation, the main mechanism underlying restenosis.
In the first studies, where DAPT was recommended for three months after the implantation of sirolimus-eluting stents and six months after paclitaxel-eluting stents (PES), the first-generation DESs showed, in comparison with BMSs, excellent results in terms of complete inhibition of coronary intimal hyperplasia and incidence of restenosis, without additional problems of thrombotic risk in the short and medium term (30 days and one year of follow-up)94–99.
However, even in the early days of these new findings, the link between DAPT and the safety of DES was evident, although the optimal duration of pharmacological antiplatelet therapy to minimise the risk of acute thrombotic occlusion of implanted stents was not completely clear.
Subsequently, further scientific evidence showed that the first-generation DES devices, when compared to BMSs, were associated with a higher risk of very late stent thrombosis (after one year from implantation), underlining the increased related thrombogenicity, in the first instance, to the delayed re-endothelisation of the stent mesh, in turn a direct effect of the antiproliferative drug released by the device100,101.
The "the longer the better" concept was introduced for the duration of DAPT in patients treated with DES, while underlining how, in addition to antiplatelet therapy, stent thrombosis is a multifactorial mechanism in which the different variables interact with each other: the "patient" variable (e.g. stenting indication – AMI stable coronary artery disease, cardiovascular risk factors, comorbidity), type of procedure performed (direct stenting, balloon catheter predilation, bifurcation stenting, chronic coronary occlusion stenting, multiple stenting, length of the vascular segment treated with angioplasty and stent implantation), vessel response to the stent implantation, characteristics of the implanted stent102–112.
Scientific research has led to the introduction of more technologically sophisticated DES devices with ever less cumbersome profiles, polymers with greater safety features as regards the possible inflammatory stress on the vessel wall and antiproliferative drugs with more stable and largely predictable release kinetics.
Consequently, on the basis of improved safety in relation to stent thrombosis and myocardial infarction on the target vessel, first-generation DESs have been progressively replaced by new generation devices113–115.
In this context, given the need to validate the duration of the DAPT more accurately, the new generation DESs should be compared above all with devices that already require short DAPT periods, i.e. BMS. A broad analysis of 4,896 patients with ACS showed a statistically significant reduction in the incidence of angiographically defined stent thrombosis (RR 0.42, 95% CI 0.22-0.78; p = 0.006), myocardial infarction (RR 0.71, 95% CI 0.55-0.92; p = 0.01) and cardiac-related mortality (RR 0.67, 95% CI 0.49-0.91; p = 0.01) in patients in whom a second-generation everolimus-chromium-cobalt stent was implanted (EES), compared to patients treated with BMS. The effect of this treatment was independent of the duration of the DAPT116.
In the PRODIGY (Prolonging Dual Antiplatelet Treatment after Grading Stent-Induced Intimate Hyperplasia) randomised trial, which compared EES, zotarolimus-eluting stents (ZES) or PES and BMS, both composite major cardiovascular event endpoints (EES: 19.2%; ZES 27.8%; PES: 26.2%; BMS: 32.1%; p = 0.00029) and certain/probable stent thrombosis (EES: 1.0%; ZES: 1.4%; PES: 4.6%; BMS: 3.6%; p = 0.0001) in the two-year follow-up were significantly higher in BMS patients than in new-generation DESs117–119.
Recently in the NORSTENT study (Norwegian Coronary Stent Trial), 9,013 patients with stable or acute coronary artery disease were randomised with DES (95% of which were new generation) or BMS. At six years of follow-up, the primary endpoint incidence (composite of all-cause death and non-fatal myocardial infarction) did not significantly differ between the two groups (16.6 vs 17.1%; p = 0.66). However, the use of DES was associated with better results with regard to the need for new revascularisation (16.5 vs 19.8%; p <0.001) and the incidence of stent thrombosis (0.8 vs 1.2%; p = 0.0498)120.
Also in the DAPT trial, a secondary analysis showed an advantage of DES over BMS in terms of stent thrombosis at a follow-up of 33 months. But the most significant results were those on the duration of DAPT, where a prolonged 30-month DAPT regimen, compared to 12 months post-PCI, was associated with a significant reduction in stent thrombosis and major adverse cardiovascular events (MACE), although at the cost of a greater risk of bleeding121.
In recent years, the so-called resorbable stents (BRS) have entered the scene, of which there is limited long-term follow-up data and the real performance compared with the new generation DES in unselected patients is not known. The first BRS prototypes introduced onto the market with the EC mark and FDA approval were Absorb, bioresorbable vascular scaffolds (BVS). The available meta-analyses show that patients with BVS have an increased risk of myocardial infarction (4.3 vs 2.3%; odds ratio [OR] 1.63, 95% CI 1.18-2.25; p <0.01) and certain or probable stent thrombosis (1.3 vs 0.6%; OR 2.10, 95% CI 1.13-3.87; p = 0.02) during the first year of follow-up122,123. The high thickness of the struts has been indicated as a probable explanation of the increased rate of stent thrombosis and new BRS technologies are currently being developed with lower strut thicknesses. There are currently no studies focused on the optimal duration of DAPT after implantation of BRS but the preliminary available data would seem to indicate the need for a prolonged DAPT.
In conclusion, the evidence available in the literature quite clearly highlights the best safety profile of most second-generation DESs when compared to BMSs with similar DAPT duration. Moreover, thanks to the advanced technology of the new devices that allow even very short DAPT cycles, the choice of a BMS does not seem to be justified in cases where there is a clinical picture in which the prolonged intake of antiplatelet drugs is inadvisable.
Recently, the new ESC guidelines focusing on DAPT in patients with coronary artery disease have clearly recommended that the decision on duration is independent of the type of stent implanted57.
6. DURATION OF DUAL ANTIPLATELET THERAPY: RECOMMENDATIONS FROM INTERNATIONAL GUIDELINES
In patients with NSTE-ACS (Table 2), the ESC guidelines23 recommend the administration of a P2Y12 receptor inhibitor in addition to ASA for the 12 months following the index event, unless there are contraindications such as excessive bleeding risk. In particular, prasugrel (loading dose 60 mg followed by 10 mg/day) is recommended only in patients who are candidates for PCI and where the coronary anatomy is known (class I, level of evidence B). Ticagrelor (loading dose 180 mg followed by 90 mg bid) is recommended in all patients at moderate to high risk of ischaemic events (e.g., elevated troponin), regardless of the initial treatment strategy, including patients pre-treated with clopidogrel (which should be suspended before administering ticagrelor) (class I, level of evidence B). Clopidogrel (loading dose 600 mg followed by 75 mg/day) should only be given when prasugrel or ticagrelor are not available or are contraindicated (class I, level of evidence B) or when patients should receive an oral anticoagulant. The guidelines specify that prasugrel pre-treatment is not recommended in patients with unknown coronary anatomy (class III, level of evidence B). In patients with NSTE-ACS who are DES carriers and at high risk of bleeding, the administration of a P2Y12 receptor inhibitor can be shortened to three or six months (class IIB, level of evidence B).
Table 2.
Oral antiplatelet therapy in patients with acute coronary syndrome without ST segment elevation
| Classa | Levelb | |
|---|---|---|
| In the absence of contraindications, ASA therapy (loading dose 150-300 mg followed by 75-100 mg/day) is recommended in all patients. | I | A |
| The administration of P2Y12 receptor inhibitors in addition to ASA is recommended for 12 months unless there are contraindications such as an excessive haemorrhage risk. | I | A |
|
I | B |
|
I | B |
|
I | B |
| Administration of P2Y12 receptor inhibitors may be considered for a short period of 3-6 months after DES implantation for patients considered at high risk of bleeding. | IIb | A |
ASA, aspirin; DES, drug-eluting stent; PCI, percutaneous coronary intervention.
aRecommendation class.
bLevel of evidence.
Adapted from Roffi et al.23
In patients with STEMI (Table 3) undergoing PCI, the latest ESC guidelines77 recommend treatment for 12 months with a potent P2Y12 receptor inhibitor (ticagrelor or prasugrel) in combination with ASA to be administered before or at least at the time of the procedure, unless there are contraindications such as a high risk of bleeding. Clopidogrel should only be used when prasugrel or ticagrelor are not available or are contraindicated (class I, level of evidence A)77.
Table 3.
Oral antiplatelet therapy in patients with ST segment elevation myocardial infarction
| Classa | Levelb | |
|---|---|---|
| The administration of a potent P2Y12 receptor inhibitor (prasugrel or ticagrelor), or clopidogrel if the former are contraindicated or not available, is recommended before (or at most during) PCI and should be continued for 12 months, unless there are contraindications such as an excessive risk of bleeding. | I | A |
PCI, percutaneous coronary intervention.
aRecommendation class.
bLevel of evidence.
Modified from Ibanez et al.77
Alongside the new ESC guidelines on STEMI, guidelines focused on DAPT were published at the same time57. These guidelines have tried to harmonise the previous ones, inserting some recommendations regarding the use of antiplatelet agents:
Pre-treatment with a P2Y12 receptor inhibitor is generally recommended in patients whose coronary anatomy is known and the decision to proceed with PCI has been taken, as well as in patients with STEMI (class I, level of evidence A);
In patients with ACS undergoing stent implantation, DAPT with a P2Y12 receptor inhibitor co-administered with ASA is recommended for 12 months unless there are contraindications such as an excessive risk of bleeding (PRECISE-DAPT score ≥25) (class I, level of evidence A);
In patients with ACS who have been previously treated with clopidogrel, the switch from clopidogrel to ticagrelor is recommended as soon as possible after hospitalisation with a dose of 180 mg, regardless of the loading dose of clopidogrel and timing of administration, unless ticagrelor is contraindicated (class I, level of evidence B).
Regarding patients with stable coronary artery disease undergoing PCI17,124, the above ESC guidelines recommend the administration of DAPT for a period of six months (class I, level of evidence A), regardless of the type of implanted stent, setting the risk of bleeding as a restriction on a shorter DAPT (3 months, class IIa, level of evidence B) (Figure 14).
Figure 14.
Recommendations on the use and duration of dual antiplatelet therapy (DAPT) in patients undergoing percutaneous coronary procedures.
BMS, metallic stent; BRS, reabsorbable stent; DCB, medicated balloon; DES, medicated stent; MI, myocardial infarction.
1After PCI treatment with DCB, DAPT must be considered for six months (class IIa B).
2Patients with stable coronary artery disease or acute coronary syndrome are not eligible for treatment with prasugrel or ticagrelor.
3Applicable to patients who are not eligible for treatment with prasugrel or ticagrelor.
4Applicable to patients who are not eligible for treatment with ticagrelor.
Reproduced with permission from Valgimigli et al.57
Regarding the extension of the duration of DAPT, in the updated guidelines57 the ESC states that DAPT beyond the 12 months currently recommended may be continued in those patients who did not have bleeding complications during that period (class II, level of evidence A) and that ticagrelor should be preferred to clopidogrel and prasugrel (class II, level of evidence B) (Table 4). These recommendations are based on evidence derived from the DAPT study10 and the PEGASUS-TIMI 54 study11.
Table 4.
Oral antiplatelet therapy over 12 months
| Classa | Levelb | |
|---|---|---|
| In patients with ACS who did not develop bleeding complications during DAPT, an extension of DAPT beyond 12 months is recommended. | IIb | A |
| In patients with acute myocardial infarction and high ischaemic risk who did not develop bleeding complications during DAPT, a 60 mg bid dose of ticagrelor in combination with ASA for more than 12 months may be preferable to clopidogrel or prasugrel. | IIb | B |
ASA, aspirin; ACS, acute coronary syndrome.
aRecommendation class.
bLevel of evidence.
cDefined by age ≥ 50 years and one or more of the following high-risk characteristics: age ≥ 65 years, undergoing treatment for diabetes mellitus, previous spontaneous myocardial infarction, multivessel coronary disease, chronic renal failure defined by a creatinine clearance < 60 ml/min.
Modified from Ibanez et al.57
In the DAPT study10, patients treated with DAPT that did not manifest any ischaemic or haemorrhagic adverse event in the 12 months after the PCI were randomised to receive an additional 18 months of thienopyridines (clopidogrel or prasugrel) or placebo. The extension of DAPT, compared with the 12-month treatment, showed a significant reduction in the risk of major adverse cardio- and cerebrovascular events (4.3 vs 5.9%, HR 0.71, 95% CI 0.59-0.85, p <0.001), stent thrombosis (0.4 vs 1.4%; HR 0.29; 95% CI 0.17-0.48; p < 0.001) and myocardial infarction (2.1 vs 4.1%; HR 0.47; p <0.001). The rate of all-cause mortality was higher in patients who continued treatment with DAPT compared to placebo (HR 1.36; 95% CI 1.00-1.85; p = 0.05). The study also showed an increase in the rate of moderate or severe bleeding in patients who prolonged treatment with DAPT compared to placebo (2.5 vs 1.6%; HR 1.61; 95% CI 1.21-2.16; p = 0.001).
The PEGASUS-TIMI 54 study11 randomised 21,162 patients with a history of heart attack one to three years before and high ischaemic risk to treatment with ticagrelor 90 mg bid, ticagrelor 60 mg bid or placebo. At a median follow-up of 33 months, the study showed a reduction in the risk of cardiovascular death, myocardial infarction or stroke (HR 0.85, 95% CI 0.75-0.96; p = 0.008; and HR 0.84; 95% CI 0.74-0.95; p = 0.004 for 90 and 60 mg of ticagrelor vs placebo, respectively) but an increase in major bleeding events (2.60% with ticagrelor 90 mg, 2.30% with ticagrelor 60 mg and 1.06% with placebo, p <0.001).
In 2016, the American College of Cardiology (ACC) and the American Heart Association (AHA)125 also published an update of the guidelines focused on DAPT duration. The update was based on recent evidence that emerged as part of the extension of the treatment duration (primarily the DAPT study10 and the PEGASUS-TIMI 54 study11), which show that there is a favourable risk/benefit ratio in prolonging treatment with DAPT over 12 months in patients with a history of myocardial infarction.
In patients treated with DAPT, ASA should be used at a low dose (80 or 100 mg/day).
Although the optimal duration of treatment with DAPT over 12 months has not yet been established with certainty through specific clinical studies, the extension of DAPT beyond 12 months is considered reasonable in the recommendations made by the guidelines in patients with ACS managed conservatively or invasively, who have tolerated DAPT in the first 12 months of treatment without bleeding complications and who are not at high risk of bleeding (class IIb, level of evidence A).
More recently, the National Institute for Health and Care Excellence (NICE)126, the British organisation that processes the main national guidelines in the area of health on the basis of cost effectiveness, published the ultimate guide to using the drug ticagrelor 60 mg, drafted by a group of experts on the basis of the evidence available to date on the medication. The evaluation committee established that ticagrelor 60 mg, in association with ASA, is recommended as a treatment option for the prevention of atherothrombotic events in patients with a history of myocardial infarction and who are at high risk of developing a new atherothrombotic event, and that treatment should be discontinued when clinically indicated or after a maximum of three years.
7.ISCHAEMIC AND HAEMORRHAGIC RISK IN SECONDARY PREVENTION: IS IT TIME TO CHANGE PERSPECTIVE?
7.1 The risk of further cardiovascular events in patients with coronary artery disease
Several clinical and instrumental features are particularly useful in defining the risk of clinical recurrence in patients with evidence of coronary heart disease15 (Table 5).
Table 5.
High risk criteria for ischaemic ulcer recurrence in patients with coronary heart disease
| Clinical assessment |
|
| Level of control of risk factors |
|
| Diagnostic tests |
|
| Angiographic variables |
|
AV, atrioventricular; eGFR, estimated glomerular filtration rate; IV, intraventricular; LDL, low density lipoprotein; AP, arterial pressure.
In general, clinical features such as old age, comorbidity including diabetes mellitus, chronic kidney disease, previous ischaemic stroke, peripheral or carotid artery disease and history of angina, are associated with a significant increase in the risk of recurrent clinical events15,17,127. These elements must be associated with the assessment of the level of effective control of traditional risk factors. Smokers with high blood pressure and hypercholesterolaemia that are not controlled with treatment have significantly higher risk levels15,17,128.
The presence of left ventricular dysfunction (ejection fraction <40%), i.e. the evidence of inducible ischaemia during provocative tests (ergometric test, myocardial scintigraphy, echocardiography with pharmacological stress) contribute to define the individual risk profile15,17,128.
For patients undergoing coronary angiography, in addition to the variables mentioned above, some simple parameters exist, such as the number of coronary vessels affected by significant stenosis, the number and type of stents implanted or an incomplete revascularisation, which must be considered when estimating the level of individual risk of clinical relapse. There are also more complex risk scores based mainly on angiographic parameters, including for example the SYNTAX score based on nine anatomical criteria, including the number, location and complexity of coronary lesions, or rather, on angiographic parameters integrated with clinical ones (Clinical SYNTAX score and SYNTAX score II, ACUITY-PCI risk score)15,17.
7.2 The risk of haemorrhagic events in patients with coronary artery disease treated with dual antiplatelet therapy
Bleeding complications of variable entity are a common possibility during DAPT129,130. Observational studies seem to indicate that the medium-long term haemorrhagic risk during DAPT is estimated in the range of 4-7% per year129,131. The assessment of the bleeding risk in the individual patient does not, however, appear to be a simple matter, also in view of the complex and multifactorial genesis of haemorrhage events. However, some clinical features are associated with a significant increase in bleeding risk during DAPT: old age, female gender, history or evidence of heart failure, history of peptic ulcer, reduction of renal function, anaemia and history of cerebrovascular disease131,132. It is evident that some factors affecting the risk of ischaemic recurrences also represent predisposing factors to haemorrhagic events. This paradox has been known for some time and has already been widely tested for oral anticoagulant therapy (OAT) in patients with AF133. The haemorrhagic risk stratification, therefore, appears to be difficult to define in the individual patient.
7.3 Achieving a balance
Long-term DAPT reduces the risk of ischaemic relapse in patients with coronary heart disease. The benefit is particularly significant especially in patients with low risk of bleeding, where the incidence of adverse events is similar to that recorded during therapy with ASA alone134. Therefore, when considering long-term DAPT in the patient with coronary heart disease it is appropriate:
to consider whether the patient has already followed DAPT in the past and how he or she has tolerated this treatment. Patients who have tolerated DAPT well for 12 months without bleeding events have a low probability of facing significant complications in case of continuation of therapy;
not to consider DAPT in patients with evident bleeding diathesis. Very elderly patients with documented cerebrovascular disease, unstable haemodynamic conditions, severe renal failure or persistent anaemia should not be started on this therapy;
to only refer patients with a high ischaemic risk profile for DAPT (Table 5), such as diabetic patients with multivessel disease or peripheral arterial disease. In these subjects, long-term DAPT can give the best clinical results.
8. NEW PARADIGMS ON THE DURATION OF DUAL ANTIPLATELET THERAPY: PROFILING PATIENTS ELIGIBLE FOR PROLONGED ANTIPLATELET TREATMENT
Patients with previous myocardial infarction have a high incidence of recurrent ischaemic events (death, myocardial infarction and stroke), despite current pharmacological and non-pharmacological secondary prevention therapies135–137. Recent data from international multicentre registries suggests that between one and five years after the index event, about 20% of patients with myocardial infarction present a new ischaemic event138. The PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) study included patients with myocardial infarction who underwent PCI and intracoronary imaging of both the culprit vessel and the remaining vessels, and were subsequently treated with maximal pharmacological therapy139. This natural history study also revealed a cumulative rate of events equal to 21% at three years (on average 7%/year), of which 70% occurred in the first year, and then decreased exponentially. Half of these events occurred on a previously treated lesion and the other half on an initially non-culprit lesion139. This means that the rationale of DAPT is not only in the prevention of stent thrombosis (an increasingly unusual event with the latest generation of DESs) but also in the prevention of recurrent events, also unrelated to the vessel that caused the heart attack, potentially correlated to instability of silent coronary plaques at the time of the index event. The only randomised trial that in the past suggested a benefit for DAPT extension beyond one year was CHARISMA, which recruited over 15,000 patients with a prior atherothrombotic event or at high-risk for the development of such events35. In this study, the addition of clopidogrel to ASA for a median time of 28 months did not demonstrate any benefit in terms of cardiovascular death, myocardial infarction, and stroke, if not precisely in patients with prior heart attack, stroke or symptomatic peripheral vascular disease, with a 27% reduction in ischaemic events, without a significant increase in fatal or severe bleeding35. Furthermore, haemorrhagic events were concentrated in the first year of DAPT (recommended in all patients diagnosed with ACS), beyond which no difference was recorded between DAPT and ASA alone140.
Recently, further studies have tested the hypothesis that the prolongation of DAPT or the addition of a third agent (antiplatelet or anticoagulant) to traditional DAPT can bring advantages in terms of prognosis and reduce the residual risk in infarcted patients (Figure 15). In this chapter we will cover the only antiplatelet therapy and the only drugs that have currently been approved by the FDA for long term post-infarction treatment - vorapaxar, studied in addition to ASA and/or clopidogrel, in the TRA 2P-TIMI 50 trial (Thrombin Receptor Antagonist for the Secondary Prevention of Atherothrombotic Ischaemic Events-Thrombolysis in Myocardial Infarction 50), and ticagrelor, assessed in addition to ASA in the PEGASUS-TIMI 54 trial.
Figure 15.
Antithrombotic strategies tested and/or approved in the long-term treatment of myocardial infarction.
ASA, aspirin; Clopi, clopidogrel; FDA, Food and Drug Administration.
8.1 Vorapaxar and the TRA 2P-TIMI 50 study
Vorapaxar is the first of a new class of antiplatelet drugs, PAR-1 (protease-activated receptor 1) antagonists, the major platelet receptor for thrombin. Platelet aggregation induced by thrombin is not influenced by ASA or P2Y12 receptor inhibitors and could contribute to atherothrombotic events occurring in high-risk patients despite standard antiplatelet treatments. Furthermore, preclinical pharmacology and phase II clinical studies of vorapaxar had generated the hypothesis that the antithrombotic effect of this drug could be separate from an increase in bleeding complications, based on a presumed late participation of thrombin in the primary haemostasis process141. The TRA 2P-TIMI 50 trial evaluated the efficacy and safety in secondary prevention in 26,449 patients with prior myocardial infarction or ischaemic stroke in the previous 2-52 weeks or peripheral arterial vascular disease142. After the completion of the recruitment process and about 24 months of follow-up, the study’s Data and Safety Monitoring Board found an excess of intracranial bleeding in patients with a history of stroke treated with vorapaxar and recommended discontinuing experimental treatment in all patients with a history of stroke and the continuation of the trial for the other patients. At three years the primary endpoint, a composite of cardiovascular death, myocardial infarction or stroke, occurred in 9.3% of patients treated with vorapaxar and in 10.5% of patients treated with placebo (added to either single or double standard antiplatelet therapy) (HR 0.87, 95% CI 0.80-0.94; p<0.001) (Figure 16). The safety endpoint of primary interest, moderate or severe bleeding according to the GUSTO criteria, occurred in 4.2% of patients treated with vorapaxar and 2.5% of patients receiving placebo (HR 1.66, 95% CI 1.43-1.93; p < 0.001), with a statistically significant increase also in solely intracranial bleeding142. However, it is important to point out that in 17,779 patients with prior myocardial infarction, vorapaxar reduced the risk of primary endpoint events more convincingly than in other categories of patients (8.1 vs 9.7%; HR 0.80, 95% CI 0.72-0.89; p<0.0001; NNT = 62), with a moderately reduced absolute excess of bleeding complications (3.4 vs 2.1%; HR 1.61; 95% CI 1.31-1.97; p < 0.0001; number needed to harm [NNH] = 77)143. Furthermore, among patients with previous myocardial infarction and diabetes mellitus (n=3,623), vorapaxar significantly reduced the primary endpoint compared to placebo (11.4 vs 14.3%; HR 0.73; 95% CI 0.60-0.89; p = 0.002; NNT = 29)144. It remains to be noted that the benefit demonstrated by vorapaxar was observed in addition to ASA and/or clopidogrel (less than 1% received prasugrel during the study) and the efficacy and safety of vorapaxar in patients treated with ASA and modern P2Y12 receptor inhibitors remains to be verified.
Figure 16.
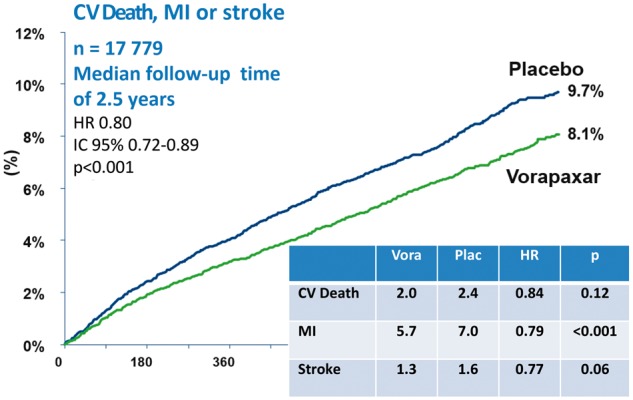
Primary endpoint in the population with previous myocardial infarction (MI) in the TRA 2P-TIMI 50 trial.
CV, cardiovascular; HR, hazard ratio; CI, confidence interval.
8.2 Ticagrelor and the PEGASUS-TIMI 54 study
The multicentre PEGASUS-TIMI 54 study recruited more than 21,000 patients with history of myocardial infarction between one and three years and additional risk criteria such as age >65 years, multivessel disease, diabetes mellitus, kidney failure and recurrent heart attack. Patients with previous stroke/TIA (also in light of the analysis of the TRA 2P-TIMI 50 study), predisposition or history of bleeding, or need for anticoagulant therapy145 were excluded. Patients were randomised at least one year from the acute event into one of the three arms: treatment with ticagrelor 90 mg bid, treatment with ticagrelor 60 mg bid, or placebo, in addition to low-dose ASA. The primary efficacy endpoint was composite cardiovascular mortality, myocardial infarction and stroke; the primary safety endpoint was major bleeding according to the TIMI classification145. Ticagrelor, administered at the two different doses, demonstrated a significant reduction in the primary efficacy endpoint (ticagrelor 90 mg: HR 0.85, 95% CI 0.75-0.96; p = 0.008; ticagrelor 60 mg: HR 0.84, 95% CI. 0.74 -0.95; p = 0.004) (Figure 17); these results also remained consistent in the analysis by subgroups11. Regarding the exploratory analysis of the other endpoints, both doses showed a significant reduction in terms of myocardial infarction, whereas exclusively at the 60 mg dose, ticagrelor showed a significant reduction in the incidence of ischaemic stroke compared to placebo and a favourable trend regarding cardiovascular death. No difference emerged between ticagrelor (at both doses) and placebo in terms of cardiovascular death, death from any cause, unstable angina, urgent revascularisation procedures and TIA. As expected, considering the safety endpoints with the understanding that the PEGASUS-TIMI 54 is the first trial in which a new P2Y12 receptor inhibitor was compared with placebo, there was a significant difference to the disadvantage of both ticagrelor doses compared to placebo in terms of major and minor TIMI bleedings, but without any difference between ticagrelor (both doses) and placebo in terms of fatal or intracerebral bleeding11. Moreover, in both ticagrelor arms, the incidence of dyspnoea was greater (ticagrelor 90 mg: HR 3.55, 95% CI 3.16-3.98; p < 0.001; ticagrelor 60 mg: HR 2.81, 95% CI 2.50-3.17; p < 0.001), while no differences emerged in terms of bradycardia. Overall, treatment with ticagrelor at 60 mg bid showed a better risk/benefit profile than the 90 mg bid regimen, compatible with a reasonable need to modulate platelet inhibition over time after an infarction event. If we aggregate the ischaemic and haemorrhagic data into a view of irreversible damage (death, stroke, myocardial infarction, fatal and intracranial haemorrhages), ticagrelor 60 mg bid led to a relative reduction in risk of 14% compared to placebo (p = 0.0160)11. For this reason, it was submitted for approval to the FDA and only the dosage of 60 mg bid of ticagrelor was subsequently approved for chronic use post-infarction.
Figure 17.
Primary endpoint of the PEGASUS-TIMI 54 study.
ASA, aspirin; HR, hazard ratio; CI, confidence interval.
The benefits of DAPT extension in patients with previous myocardial infarction have also recently been confirmed in a meta-analysis of five trials where only patients with previous myocardial infarction (37% of the total population) were selected37. The extension of DAPT reduced the risk of MACE compared to ASA (6.4% vs 7.5; RR 0.78, 95% CI 0.67-0.90; p = 0.001) and reduced cardiovascular mortality (2.3 vs 2.6%; RR 0.85, 95% CI 0.74-0.98; p = 0.03), without any increase in non-cardiac mortality (RR 1.03, 95% CI 0.86-1.23; p = 0.76). Prolonged DAPT also led to a significant reduction in the incidence of myocardial infarction (RR 0.70, 95% CI 0.55-0.88; p = 0.003), stroke (RR 0.81, 95% CI 0.68-0.97; p = 0.02) and stent thrombosis (RR 0.50, 95% CI 0.28-0.89; p = 0.02). On the other hand, there was an increase in major bleeding (1.85 vs 1.09%, RR 1.73, 95% CI 1.19-2.50; p = 0.004) but not in fatal bleeding (0.14 vs 0.17%; RR 0.91, 95% CI 0.53-1.58; p = 0.75)37.
There are many sub-analyses of the PEGASUS-TIMI 54 study, some of which have been published and some which will be published in the coming months and will help us to better understand the population that can benefit most from long-term DAPT. The most interesting sub-study among those submitted to date is that on the timing of the suspension of DAPT146. Patients enrolled in PEGASUS-TIMI 54 were categorised into three groups based on the time frame from the last intake of a P2Y12 receptor inhibitor: ≤30, >30-360 and >360 days. The benefits of ticagrelor were closely related to the intervening time from the last dose of P2Y12 receptor inhibitor, with HR (95% CI) for ticagrelor (pooled doses) vs placebo of 0.73 (0.61-0.87), 0.86 (0.71-1.04) and 1.01 (0.80-1.27), respectively (p for interaction <0.001)146. The benefit for patients who discontinued treatment with P2Y12 inhibitors ≤30 days in favour of ticagrelor was independent of the time since myocardial infarction (<2 years, HR 0.73 95% CI 0.89, 0.60-0.89 vs ≥2 years, HR 0.71 95% CI 0.50-1.00). In essence, the benefits of ticagrelor were greater in patients who continued antiplatelet therapy or re-started it within 30 days of stopping compared with patients who had by then permanently stopped DAPT for longer. The increase in the frequency of bleeding in the ticagrelor groups vs placebo was independent of the time of therapy withdrawal146.
A recent analysis on tolerability showed that at three years, premature discontinuation of the drug was found to be more frequent in the ticagrelor arm (32% for 90 mg bid, 29% for 60 mg bid) versus placebo (21%) due in most cases to adverse events147. Although the majority of discontinuations of treatment in the ticagrelor arms were induced by side effects such as dyspnoea and bleeding, most events were non-severe (>95%) and bleeding was minimal or required medical treatment in the vast majority of cases. However, in clinical practice PEGASUS-TIMI 54 should result in a continuation of ticagrelor in patients who have tolerated therapy for at least one year after stroke, rather than in a resumption of therapy from scratch (as in fact happened in the vast majority of patients enrolled in PEGASUS-TIMI 54). For this reason, the frequency of discontinuation in general, and that due to adverse events, should be higher in the early stages from the resumption of therapy and lower in patients who have already tolerated the drug for one year. For this reason, a one-year "land-mark" analysis vs 2-3 years of therapy was performed in PEGASUS-TIMI 54 and the frequency of discontinuation and the reasons for interruption of therapy in the three treatment arms were compared. After the first year of therapy, discontinuation due to an adverse event was low (∼3% per year) and among the patients who did not stop therapy or stopped it for a short time (≤30 days), the benefit of ticagrelor in terms of reduction of ischaemic events was highly significant (ticagrelor 60 mg bid vs placebo: HR 0.79, 95% CI 0.68-0.91; p < 0.001)147.
A landmark analysis of the PEGASUS-TIMI 54 study was recently published, showing how the effect of ticagrelor 60 mg is consistent in all three years of treatment. At the same time, major TIMI bleedings increased with ticagrelor 60 mg at each landmark analysis, although with a higher risk in the first year of therapy (1-year HR: 3.22; 2-year HR: 2.07; 3-year HR: 1.65)148.
Finally, it seems appropriate to add the analysis performed by applying an indication approved by the EMA (high-risk post-myocardial infarction patients with previous myocardial infarction less than two years before and discontinuation of ADP inhibitor therapy less than one year before), although this was not prespecified and is therefore to be considered carefully149. Post-hoc analysis performed on this specific population showed a greater benefit of prolonged DAPT with ticagrelor 60 mg with a significant reduction of the primary endpoint (composite of infarction, stroke and cardiovascular death) as well as of the individual components of cardiovascular death and infarction (primary endpoint: HR 0.80, 95% CI 0.70-0.91; p = 0.001; cardiovascular death: HR 0.71; 95% CI 0.56-0.90; p = 0.0041; myocardial infarction: HR 0.83; 95% CI 0.70-0.99; p = 0.041) (Table 6)149.
Table 6.
The subpopulation primary endpoint in the PEGASUS-TIMI 54 study with the indication approved by the European Medicines Agency (previous myocardial infarction <2 years, suspension of ADP's inhibitor <1 year)
| Outcomes | Ticagrelor 60 mg bid (n = 5388) |
Placebo (n = 5391) |
HR (95% CI) | p | ||
|---|---|---|---|---|---|---|
| No. | KM% 3-year survival | No. | KM% 3-year survival | |||
| Composite CV death, MI or stroke outcome | 373 | 7.9 | 463 | 9.6 | 0.80 (0.70-0.91) | 0.001 |
| CV death | 119 | 2.6 | 167 | 3.6 | 0.71 (0.56-0.90) | 0.0041 |
| MI | 230 | 4.8 | 274 | 5.6 | 0.83 (0.70-0.99) | 0.041 |
| Stroke | 71 | 1.5 | 95 | 2.0 | 0.74 (0.55-1.01) | 0.058 |
| Mortality for each cause | 206 | 4.4 | 256 | 5.4 | 0.80 (0.67-0.96) | 0.018 |
CV, cardiovascular; HR, hazard ratio; KM, Kaplan-Meier; CI, confidence interval; MI, myocardial infarction.
8.3 Who should be given long-term antiplatelet therapy?
Based on data available to date, we can say that it appears reasonable to prolong DAPT rather than re-start it in patients with prior myocardial infarction and additional risk factors. But does this apply to all patients with a previous heart attack? To date, the prespecified sub-studies TRA 2P-TIMI 50 and PEGASUS-TIMI 54 do not allow us to clearly identify the subgroup of patients who benefit most from long-term DAPT. We will need other sub-studies that will be presented in the coming months as well as post-hoc analyses with the complementary assessment of additional risk factors or probably validated risk scores specifically for the post-infarction population.
Meanwhile, we can suggest the patients who should not receive prolonged DAPT:
patients with a history of stroke/TIA: as previous analyses have suggested, there is an increase in fatal bleeding in this patient population when DAPT is prolonged;
patients who experience major bleeding events in the first year of DAPT;
patients requiring oral anticoagulants, with bleeding diathesis or requiring major surgery;
frail patients for whom clinical reasoning does not suggest prolonging DAPT.
In this sense it is evident that the magnitude of the ischaemic and haemorrhagic risks, as well as their modifications during follow-up, conditions the risk/benefit ratio of prolonged DAPT. It is therefore reasonable to think that for long-term therapy, we must consider a dynamic instead of static equilibrium, with seriated assessment of patients who can benefit from DAPT by carefully assessing the risk of bleeding events over time. This is essentially what we have been doing for years with DAPT based on ASA and clopidogrel. In fact, data from observational studies, including the international EPICOR registry (Long-term Follow-Up of Antithrombotic Management Patterns in Acute Coronary Syndrome Patients), which between September 2010 and March 2011 recruited over 10,500 patients diagnosed with ACS in 555 hospitals in 20 countries around the world, suggests that DAPT is prolonged beyond the year recommended in more than 60% of cases and more than 55% in Italy150. This demonstrates that we know how to identify those at risk where the prolongation of DAPT is reasonable and beneficial, and recent trials have only scientifically confirmed the reasonable choices we make in clinical practice.
To date ticagrelor 60 mg is the only oral antiplatelet approved for secondary prevention of high-risk post-infarction patients beyond 12 months. However, not all patients are eligible for continuation and it is for this reason that a careful re-evaluation performed at 12 months is recommended. Figure 18 is a follow-up model for post-infarction patients, which would make it possible to tailor their examinations according to their level of risk. Moreover, if the patient is considered a candidate for continuation, a simple diagram can allow those who could benefit from a continuation over 12 months with ticagrelor to be selected (Figure 19).
Figure 18.
Clinical pathway for the post-acute phase in myocardial infarction patients with and without ST segment elevation1.
DAPT, dual antiplatelet therapy; DAPT-LT, long-term dual antiplatelet therapy; ARF, additional risk factors (age ≥65 years, mellitus, renal insufficiency [creatinine clearance 60 ml/min], multivessel coronary disease, recurrent ischaemic events); EF, ejection fraction; HF, heart failure (signs and/or symptoms).
1In stable asymptomatic patients, excluding those with known moderate-severe valve lesions as well as those with EF ≤ 0% and defibrillator indication and after appropriate assessment of the organisational context.
2For patients with ARFs (Additional Risk Factors).
3No smoking; regular physical activity (30 min, 5/7 days); waist circumference <89 cm in women and <102 cm in men; blood pressure 140/70 mmHg; LDL cholesterol 70 mg/dl; non-HDL cholesterol <100 mg/dl; glycated haemoglobin ≤7%; optimisation of bradycardial, antihypertensive and antianginal therapies; consider the influenza vaccine and cognitive state + continuation of DAPT and/or therapy optimisation with renin-angiotensin-antialdosterone system inhibitors, where indicated.
Figure 19.
Decisional flow-chart.
ClCr, creatinine clearance; CV, cardiovascular; DAPT, dual antiplatelet therapy; ACS, acute coronary syndrome; TIA, transient ischaemic attack.
9. LONG-TERM DUAL ANTIPLATELET THERAPY: ADHERENCE AND MANAGING THE SWITCH
9.1 The scale of the problem
Cardiovascular diseases today are the leading cause of death and it is predicted that by 2030, they will be responsible for more than 23 million deaths per year. This is despite the impressive development of effective medicines, especially in the primary and secondary prevention of ischaemic heart disease. Lack of adherence influence the incidence of AMI, stroke and death from all causes (Figure 20)151, causing almost 200,000 deaths per year and an increase of 125 billion euros/year in costs in Europe alone.
Figure 20.
Heart attack, stroke and total mortality in patients with different levels of adherence to statin therapy.
AMI, acute myocardial infarction.
Reproduced with permission from Volpe et al.151
9.2 Poor treatment adherence: definitions, causes and possible solutions
The term "adherence" refers to a behaviour according to which patients comply with all instructions and take the drugs in the manner prescribed by the physician152. The term "perseverance" has a different meaning that considers the duration of taking the drug, even intermittently, before stopping it prematurely and permanently.
In general, good adherence is achieved in the presence of a medication possession ratio (MPR) ≥80%, although in the cardiovascular field, owing to the potential consequences of poor adherence to some drugs such as antiplatelets and anticoagulants, the target should be 100%. In several observational studies on cardiovascular prevention as well as in an authoritative estimate of the World Health Organisation on patients under chronic treatment, inadequate adherence was present in 40-60% of patients153,154. There are many causes of non-adherence and they are attributable not only to the patient but also to the healthcare service, physicians or sociocultural and environmental conditions155–160. Recognising and correcting these factors can lead to considerable benefits, not only in clinical but also economic terms.
Recent registry studies and research on unselected populations have reported interesting facts about therapeutic adherence with DAPT161–165. Adherence with DAPT and thienopyridines is high in the first month, then begins to decline at six months, decreasing significantly at 12 months (Figure 21)165. ASA adherence, on the other hand, remains higher161,164,165. The main factors of non-adherence were age, multimorbidity, polytherapy, a low level of education and a lack of social support, inadequate continuity of care and incomplete information on DAPT, previous haemorrhaging and the use of anticoagulants. In contrast, a previous myocardial infarction, CABG and/or diabetes seem to increase adherence161,163,164.
Figure 21.
Persistence of antiplatelet treatment at six and 12 months after discharge from hospitalisation for acute coronary syndrome.
Adapted from Maggioni et al.165
The premature interruption of antiplatelet therapy after an AMI in patients undergoing PCI with DES strongly increases the risk of stent thrombosis, myocardial infarction, death and rehospitalisation166,167.
Finally, as may be expected, there is a negative impact on healthcare costs. In an Italian study on the ARNO database of 7,082 patients hospitalised for AMI, not following DAPT according to the indications of the guidelines was associated with an increase in rehospitalisations, which were only due to a new acute episode in a third of cases (Figure 22)165.
Figure 22.
Frequency and causes of rehospitalisation during a one-year follow-up in patients admitted for acute coronary syndrome (ACS).
CV, cardiovascular.
Adapted from Maggioni et al.165
It is important to focus on reducing bleeding risk with a more careful selection of candidates for prolonged DAPT and improved communication between patient and physician or healthcare staff, in particular with the risk of premature discontinuation of treatment. In one of the centres studied, patients were contacted by telephone after seven days and thereafter at three, six and nine months from DES implantation, achieving almost perfect adherence with treatment162.
There are many means of resolving this situation, but the physician-patient relationship remains of fundamental importance and in particular:
comprehensive information about the disease and the need to take medication for the prescribed period;
the patient should inform the physician of any medication side effects before changing/discontinuing treatment;
the treatment plan must be agreed upon and shared by the physician and patient, also on the basis of the patient's needs;
the psychological state of the patient must be taken into consideration in choosing treatment;
the number of treatment changes and the introduction of unknown drugs to the patient must be limited;
it is useful to involve the general practitioner, other professional figures (e.g. nurse) and the patient's caregiver in the therapeutic process;
the use of reminders by physicians, pharmacists or patients themselves is useful;
the treatment plan should be simplified as much as possible.
9.3 Switch management
Little is known about the effects of the transition from a P2Y12 receptor inhibitor drug to another and particularly from clopidogrel to the new antiplatelet therapies, prasugrel or ticagrelor, or vice versa. In this context the ESC guidelines restrict the use of clopidogrel to patients who cannot take prasugrel or ticagrelor56,168. ACC/AHA guidelines instead leave the choice up to the clinician based on the ischaemic and haemorrhagic risk169,170.
In clinical practice, switching is not uncommon and can be expected in cases of171:
the need for a more potent antiplatelet drug (upgrading) in patients initially treated with clopidogrel (usually pre-treated prior to coronarography/PCI), who are subsequently reclassified at higher risk (switch clopidogrel-prasugrel or clopidogrel-ticagrelor);
downgrading (switching back) of antiplatelet therapy in case of side effects (intolerance, allergic reactions to prasugrel or ticagrelor);
patients treated with prasugrel or ticagrelor (change) who develop an indication for OAT for intercurrent AF.
The most relevant data on switching originates from registries and from small studies. In the GRAPE (Greek Antiplatelet Registry) study172, 638 (35.5%) of the 1,794 ACS patients undergoing PCI had a documented switch of therapy: 575 (90.4%) from clopidogrel to prasugrel (40.1%) or ticagrelor (50.3%), 34 (5.3%) from prasugrel or ticagrelor to clopidogrel and 27 (4.3%) between prasugrel and ticagrelor. Reverse switching (from prasugrel/ticagrelor to clopidogrel) occurred in 34 patients (6.5%). In the subgroup initially treated with clopidogrel, the independent predictors of switching (upgrading) in the multivariate analysis were presenting to hospital with no haemodynamics and the use of bivalirudin, while the group aged ≥75 and regional differences were in favour of the continuation of clopidogrel.
The Italian EYESHOT registry (Employed Antithrombotic Therapies in Patients with Acute Coronary Syndromes Hospitalised in Italian Cardiac Care Units)173 assessed the antithrombotic strategies in 2,585 patients diagnosed with ACS (41.2% with STEMI and 58.8% with NSTEMI). Among the patients treated with PCI (n=1,755), switching from one P2Y12 platelet receptor inhibitor to another occurred in 3.6% of cases in the haemodynamics laboratory and in 14.2% of cases before discharge. Among patients who did not undergo PCI (n=790), switching occurred in 5.7% of cases. On discharge, a new antiplatelet (prasugrel or ticagrelor) in association with the ASA was prescribed in 59.5% of patients with STEMI and 33.9% of those with NSTEMI. The main independent predictor of switching (upgrade) was PCI, while age ≥75 years was the major predictor of clopidogrel use. Other conditions strongly associated with the prescription of clopidogrel were neoplasms, a high risk of bleeding, previous stroke or TIA, kidney failure and peripheral arterial disease (Figure 23)173.
Figure 23.
Independent predictors of prescription of a novel P2Y12 platelet receptor inhibitor (prasugrel/ticagrelor) in association with aspirin at discharge after acute coronary syndrome.
CAD, coronary heart disease; CI, confidence interval; GP, glycoprotein; OR, odds ratio; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; STEMI, ST elevation myocardial infarction; TIA, transient ischaemic attack.
Adapted from De Luca et al.173
Therefore, although current European guidelines recommend the preferential use of prasugrel or ticagrelor over clopidogrel, the latter is still the most widely-used drug in association with ASA in DAPT in Italian intensive cardiac care units. This data confirms that of the GRAPE registry.
Data from European registries on STEMI174 shows how in-hospital switching, particularly from clopidogrel to prasugrel, is very common in some studies. A smaller percentage of switching is reported by data from European registries on NSTEMI. The most frequent switching was still from clopidogrel to prasugrel, although it is less common175.
Among the few trials on the issue, the TRANSLATE-ACS study (Adenosine Diphosphate Receptor Inhibitors Treatment with: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome)176 analysed the outcome at 6 months of 11,999 AMI patients treated with PCI. Of the 8,715 patients initially treated with clopidogrel, 994 (11.4%) were switched to prasugrel or ticagrelor. Of the 3,284 patients initially treated with prasugrel or ticagrelor, 448 (13.6%) switched to clopidogrel. Compared to maintenance of clopidogrel therapy, switching to ticagrelor or prasugrel was not associated with an increase of bleeding or MACE. In a later study on the same cohort, among patients on DAPT at discharge, 663 (7.6%) performed the switch during the first year of follow-up (median of 50 days after discharge), most frequently in patients discharged with ticagrelor (64/226; 28.3%), followed by prasugrel (383/2,489; 15.4%) and clopidogrel (216/5,957; 3.6%). In the first two cases, the main reason for switching was the cost, while the switch from clopidogrel to a new drug was motivated by a clinical decision (previous MACE and/or stent thrombosis).
9.4 Data on the pharmacodynamics of switching
Pharmacodynamic studies have shown that the switch from clopidogrel to ticagrelor determines a reduction in platelet reactivity that reaches the values observed in patients treated with ticagrelor76,177. The pharmacodynamic impact of a loading dose of ticagrelor in patients with ACS was investigated in the SHIFT-OVER study (Platelet Aggregation During the Shift from Clopidogrel to Ticagrelor)178, without showing significant differences in residual platelet aggregation. This result suggests that switching from clopidogrel to ticagrelor without a loading dose does not cause a delay in platelet inhibition in patients with ACS (Figure 24)57.
Figure 24.
Switching mode between the P2Y12 platelet receptor inhibitors, acute and chronic phase.
LD, loading dose; MD, maintenance dose.
Reproduced with permission from Valgimigli et al.57
Similarly, some small-scale studies have demonstrated the feasibility of the switch between clopidogrel and prasugrel83,179,180. Because the metabolites of clopidogrel irreversibly bind P2Y12 receptors, although by less than prasugrel, administration of prasugrel in a patient already undergoing loading with clopidogrel should complete the binding with the remaining receptors being similar to that of prasugrel alone. Two randomised trials investigated the pharmacodynamic effects of the switch from chronic therapy with clopidogrel to prasugrel with or without loading83,180. In the SWAP (Switching Anti Platelet)83 study, switching from clopidogrel to prasugrel 10 mg resulted in increased platelet aggregation at seven days and within 2h (with loading only), without increased major bleeding events. The TRIPLET study180 (Transferring from Clopidogrel Loading Dose to Prasugrel Loading Dose in Acute Coronary Syndrome Patients) analysed the effect of 60 mg prasugrel loading in patients with or without 600 mg clopidogrel loading precoronarography, without showing significant differences in platelet inhibition or bleeding events.
Downgrading, i.e. switching from a more potent drug to clopidogrel may be necessary in case of relevant adverse events (usually bleeding) or if there is a need for concomitant oral anticoagulant therapy. Downgrading from ticagrelor to clopidogrel was associated with an increase in platelet reactivity even after 600 mg clopidogrel loading177. A pharmacological interaction is possible since the sites of action of the drugs are different as well as the affinity of the active metabolites of the two drugs at the P2Y12 receptor level – the occupation of P2Y12 receptors by ticagrelor could prevent clopidogrel binding during the early phase of switching. The SWAP-4 study, which is still underway, should clarify this point. The switch from prasugrel to clopidogrel after ACS was investigated in a small-scale study181 demonstrating a ten-fold increase in platelet reactivity in one third of patients at 15 days from switching to clopidogrel. Therapeutic efficacy (low platelet reactivity) was reduced in 10% of subjects while about 60% of patients were in the therapeutic window of platelet inhibition. Since the thresholds defining the low platelet reactivity are often arbitrary, the therapeutic consequences of switching from prasugrel to clopidogrel are not very predictable, especially in the context of ACS and revascularisation procedures182.
The switch from ticagrelor to prasugrel or vice versa is a relatively rare possibility and is mainly correlated to side effects of ticagrelor (dyspnoea or hypokinetic arrhythmias). In these cases, the switch should be performed on prasugrel rather than the downgrading to clopidogrel, especially when closer to the acute event and weighing up the thrombotic and haemorrhagic risk. The SWAP-2 study183 hypothesised a pharmacological interaction (elevated platelet reactivity in the first seven days) in switching from ticagrelor to prasugrel only partially reduced by the use of a loading dose in patients with stable chronic ischaemic heart disease. The active metabolite of prasugrel would in fact not be able to bind to the receptor until the ticagrelor was dissociated, perhaps also due to transient allometric changes determined by the ticagrelor itself184. Little research has been done on the clinical consequences. However, some studies report an increased incidence of adverse events including sudden death, reinfarction and stent thrombosis185. In the switch between ticagrelor and prasugrel, it is therefore advisable to use the loading dose of the latter even if the exact mechanisms are not perfectly clear. The current SWAP-3 study should clarify this aspect. The methods currently proposed for the different types of switching, particularly in the post-acute phase, are summarised in Figure 24.
Management and Therapeutic Pathway in Patients Undergoing Dual Antiplatelet Therapy. Treatment Schedules
10. ANTIPLATELET THERAPY IN PATIENTS WITH CHRONIC ISCHAEMIC HEART DISEASE UNDERGOING A PERCUTANEOUS CORONARY INTERVENTION
In patients with chronic ischaemic heart disease who undergo percutaneous revascularisation, there is evidence for DAPT with ASA and clopidogrel to reduce the risk of periprocedural thrombotic complications and cancel the risk of long-term stent thrombosis. In patients not taking ASA, in the case of PCI, a loading dose of 150-300 mg orally or 80-150 mg intravenously (i.v.) preceding the procedure, with 75-100 mg/day maintenance dose186 is advisable. There is no conclusive evidence either for or against the administration of clopidogrel prior to coronary angiography. In clinical practice, however, pre-treatment is widespread, based on the pathophysiological rationale of having an effective dual antiplatelet therapy at the time of a PCI with a stent implantation, at least in patients with a lower risk of bleeding187. If pre-treatment is given, a loading dose of 300 mg should be administered at least six hours before the procedure or 600 mg at least two hours before the procedure, followed by 75 mg/day50. This optional approach to unknown coronary anatomy is instead recommended in patients in whom coronary anatomy is already known and a percutaneous elective procedure is indicated124. In patients with chronic ischaemic heart disease, the duration of DAPT is recommended for one month after replace with bare metal stent implantation and for six months after DES implantation. Given the availability of new generation DES for which the risk of late stent thrombosis is significantly lower than the first-generation DES used in the past, it is possible to foresee a reduction in the duration of the DAPT even under six months in case of significant clinical risk of haemorrhagic events (or if clinically relevant bleeding occurs), as in non-cardiac surgery188. However, it is considered reasonable not to replace with withdraw the DAPT sooner than three months even in the case of a high risk of bleeding (e.g. for patients receiving concomitant anticoagulant therapy), except in case of severe bleeding189,190. On the basis of recently accumulated evidence, it is reasonable to continue the DAPT for a period of more than six months, up to 12 months or even longer, in cancel patients with a high thrombotic risk based on both angiographic features (e.g. in the case of multiple stents, of a stent on the anterior proximal descending artery or common trunk) and clinical features (presence of diabetes, previous myocardial infarction, incomplete revascularisation, non-coronary atherosclerosis) and at a low risk of bleeding 10. DAPT duration after implantation of a BVS is recommended for a period of at least 12 months and even longer in the presence of complex procedures (“overlapping”, bifurcations, tortuosity, fibrocalcific lesions, suboptimal procedural outcomes)191. In patients who have not had moderate or severe haemorrhagic events in the first 12 months, DAPT prolonged for up to 30 months reduces stent thrombosis and AMI (both correlated and uncorrelated with stent thrombosis), but with an increase in moderate bleeding.192 It should therefore be emphasised that this should be considered only for a selected group of patients, with a low risk of bleeding but high ischaemic risk. A score is available to help identify patients with the greatest potential benefit from this strategy (http://www.daptstudy.org). In case of allergy or intolerance to ASA and the need for DAPT, empirical use of another COX inhibitor, indobufen at a dose of 200 mg bid in association combined with clopidogrel, is suggested. The use of new P2Y12 receptor inhibitors, prasugrel or ticagrelor, in the context of chronic ischaemic heart disease, has so far been little researched, most likely in relation to the low level of platelet activation in stable patients and therefore to an unfavourable balance between the prevention of recurrences and bleeding risk193. The only study currently available is the PEGASUS-TIMI 54 trial11, in which 21,162 patients who had had an AMI from one to three years before and who presented with some additional risk elements (age ≥65 years, diabetes mellitus under treatment, second previous myocardial infarction, multivessel coronary disease or CRI) were randomised to double antiaggregation with ASA and ticagrelor at 60 mg bid or 90 mg bid vs placebo, up to 36 months of follow-up. Both doses of ticagrelor reduced the primary composite endpoint of cardiovascular death, AMI and stroke (ticagrelor 90 mg vs placebo: HR 0.85, CI 95% 0.75-0.96; p=0.008; ticagrelor 60 mg vs placebo: HR 0.84, CI 95% 0.74-0.95; p=0.004). However, major TIMI bleeding was more frequent with ticagrelor (2.60% with 90 mg and 2.30% with 60 mg) than with placebo (1.06%, p <0.001 for both doses versus placebo). In the light of these results it is evident that for long-term treatment, the lower dosage has equal advantages and better tolerance. Data from a sub-analysis of PEGASUS-TIMI 54 has shown that the advantage of prolongation of DAPT with ASA and ticagrelor is greater the closer the scheduled date of withdrawal, indicated by the class with a suspension >1 year at 6.9%, in 30 days-≤1 year to 8.7% and in ≤30 days to 9.9%. In particular, the advantage of ticagrelor treatment was significant in the group with interruption ≤30 days and not significant or absent in the other groups146. It therefore seems appropriate in patients at high risk of thrombosis to continue treatment with ticagrelor beyond the first year or in any case to resume it within 30 days of withdrawal, while its reinstatement after more than one year increases bleeding but does not produce benefits. The efficacy and safety of ticagrelor in women was also analysed compared to men with previous AMI194. In 24% of female patients enrolled (n=5,060) there was a reduction of the primary endpoint similar to that seen in males (P for interaction = 0.84), with a more marked effect of stroke reduction in women. An additional sub-analysis evaluated the level of platelet inhibition obtained with the 60 mg bid dose, lower than the standard dose.195 In a sample of 180 patients in the study, who were treated for more than four weeks, ticagrelor plasma levels were approximately one third lower with 60 mg compared to 90 mg (post dose: 448 vs 717 ng/ml, p <0.001) but both doses achieved high levels of platelet inhibition before and after administration, with slight variability using 60 mg. High platelet reactivity, measured with VerifyNow, appeared to be rare with a dose of 60 mg pre-dose (3.5%) and none post-dose. Pre- and post-dose platelet reactivity measured by light-transmission aggregometry was numerically but not significantly lower with the 90 mg dose than 60 mg. This explains the good clinical results obtained with the lower dose.
Prasugrel was compared with clopidogrel at a loading dose of 300 mg in the TRITON-TIMI 38 study, with the beginning of the administration of both drugs in the haemodynamics laboratory after diagnostic angiography, showing favourable effects on the combined outcome of thromboembolic and ischaemic events85. A significant reduction in recurrences of cardiovascular events was observed in patients treated with prasugrel, but an increase in major bleeding complications, especially in patients with a history of stroke and TIA, in elderly patients (≥75 years) and in underweight patients (<60 kg). The rate of bleeding was highest in patients treated with prasugrel started early on CABG. Excluding patients with a higher risk of bleeding, prasugrel offers a significant benefit compared to clopidogrel with respect to cardiovascular events without causing an increase in serious bleeding. In diabetic patients with ACS, prasugrel gives a significant advantage over clopidogrel without any increase in bleeding88. Prasugrel should be administered to patients who have had stent thrombosis concomitantly with clopidogrel.
11. DUAL ANTIPLATELET THERAPY IN PATIENTS WITH MYOCARDIAL INFARCTION WITH OR WITHOUT ST SEGMENT ELEVATION UNDERGOING A PERCUTANEOUS CORONARY INTERVENTION
Treatment with DAPT after PCI was introduced to prevent acute and subacute stent thrombosis in the first 30 days from implantation as an alternative to anticoagulant therapy196–198. The attention of research in antiplatelet therapy has therefore focused on the type of clinical presentation of the patient undergoing PCI, whether elective or with ACS, and on the duration of therapy in the medium and then in the long-term199. The effectiveness of DAPT with ASA and clopidogrel 12 months after NSTE-ACS, regardless of the invasive strategy, was initially demonstrated by the CURE study49. A previously specified population of this trial consisting of 2,658 patients to undergo PCI was randomised to pre-treatment with clopidogrel or placebo, with a median of ten days before performing PCI51. Including events both before and after PCI, there was a reduction in cardiovascular mortality or myocardial infarction of 30% at one year in the clopidogrel group. The CREDO study50 then randomised 2,116 patients anticipating PCI, of which around 65% for NSTEMI, to receive clopidogrel 75 mg/day in addition to standard therapy. Therapy was continued for one year with a 27% reduction in the risk of death, myocardial infarction and stroke. The introduction of clopidogrel over the medium to long-term after STEMI is due to the result of the CLARITY-TIMI 28 (Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myocardial Infarction 28)200 and COMMIT (Clopidogreland Metoprolol in Myocardial Infarction Trial)52 studies, which streamlined treatment with ASA and clopidogrel for one year in all forms of ACS, demonstrating the benefit in patients undergoing mainly thrombolysis as a riperfusive strategy and subsequent to PCI.
However, clopidogrel showed some important limitations, such as slow onset of action and marked variability of response, with a mean of 30% of "non-responders". The CURRENT-OASIS 7 (Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events-Seventh Organisation to Assess Strategies in Ischaemic Syndromes) study201 assessed whether it was possible to overcome these limitations by increasing the dose, but in the general population of the study, which included 17,263 patients undergoing PCI, there were no significant differences in cardiovascular death, heart attack or stroke at 30 days between the administration of high doses (75 mg bid) or standard doses of clopidogrel. The reduced efficacy and variable action of clopidogrel was overcome by the introduction of a second generation of P2Y12 receptor inhibitors with a more predictable, powerful and rapid action: prasugrel and ticagrelor202. The TRITON-TIMI 38 study85 enrolled 13,608 patients with ACS (74% NSTEMI and 26% STEMI) at medium/high risk, who all underwent PCI with a follow-up of 14.5 months. In the prasugrel arm, an absolute reduction of 2.2% and relative reduction of 19% was recorded in the incidence of the primary endpoint of cardiovascular death, myocardial infarction or stroke (12.1 vs 9.9%, p<0.001) and of 54% in stent thrombosis (2.4 vs. 1.1%, p <0.001). The reduction in the level of ischaemic events with prasugrel was however accompanied by an increase in bleeding complications (1.8 versus 2.4%; p=0.03), which limited the net clinical benefit in the overall population of the study. Excluding patients in whom bleeding complications were concentrated (those with previous stroke, which became a contraindication, and those weighing <60 kg or aged >75 years), prasugrel showed a clear net clinical benefit (HR 0.80, 95% CI 0.71-0.89, p <0.001).
Ticagrelor was tested in the PLATO trial76, which evaluated 624 patients with ACS for 18 months, of which 37% had STEMI and 43% had NSTEMI at medium/high risk (20% with TIMI score >5), with or without pre-treatment, randomised to clopidogrel (300-600 mg followed by 75 mg/day) or to ticagrelor (180 mg followed by 90 mg bid). Not only was there a 16% reduction in the composite endpoint, consisting of cardiovascular death, infarction or stroke (9.8 vs. 11.7%, p <0.001), but a reduction in overall mortality (4.5 vs. 5.9%; HR 0.78) CI 95% 0.69-0.89; p<0.001) was also demonstrated in the ticagrelor arm. In the ticagrelor arm there was a significant increase in major TIMI bleeding not correlated to the CABG procedure, but not fatal or life-threatening bleeding. The incidence of the primary endpoint was reduced by ticagrelor in the subgroup of 13 408 patients undergoing invasive strategy, 77% revascularised by PCI (10.7% vs 9.0; HR 0.84, CI 95% 0.75-0.94; p=0.0025)203.
The 2016 ACC/AHA guidelines specifically dedicated to the duration of DAPT125 had the superiority of ticagrelor in NSTEMI patients undergoing PCI in all patients in priority class IIa and level of evidence BR (randomised), and that of prasugrel only in those who did not present contraindications of previous stroke or TIA.
European guidelines on DAPT from 201757 put both the use of ticagrelor in all patients with NSTEMI undergoing PCI (with or without pre-treatment) and the use of prasugrel into class I with level of evidence B, but only in patients not responding to other P2Y12 receptor inhibitors, after the coronary anatomy is known (pre-treatment is contraindicated) and in the absence of history of stroke or TIA. For STEMI, pre-treatment with either drug is instead permitted, whether the patient is referred to primary PCI or to an early invasive strategy. When ticagrelor or prasugrel is available, the use of clopidogrel should be reserved only for patients who have contraindications (such as a previous intracranial haemorrhage, or history of cerebral ischaemia for prasugrel alone) or who require a concomitant OAT.
In all types of ACS undergoing PCI, the guidelines agree on a class I indication for the continuation of DAPT for 12 months with type A (ESC)57 or BR (ACC/AHA)125 evidence. However, it is clear from the "landmark" analyses of the studies with clopidogrel, prasugrel and ticagrelor, that the benefit of DAPT in the reduction of ischaemic events is greater in the first months and gradually decreases, without disappearing even in the long-term204.
In cases of high bleeding risk, the P2Y12 receptor inhibitor can be stopped after six months (ESC: class IIa, level of evidence B, ACC/AHA: class IIb, level of evidence CLD); conversely, in patients who tolerated DAPT for one year without complications it appears to indicate continuing the P2Y12 receptor inhibitor beyond the 12 months (ESC: class IIb, level of evidence A; ACC/AHA: class IIb, level of evidence ASR) after evaluation of the ischaemic and residual haemorrhagic risk. Upon prolongation of DAPT specifically with ticagrelor, the ESC guidelines ascribe a class IIb recommendation with level of evidence B.
Some studies that have predominantly recruited patients undergoing PCI, most of whom for ACS, have predicted a DAPT duration of less than 12 months (PRODIGY205, RESET [Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-Eluting Stent Implantation]206, EXCELLENT [Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting]207) without showing an increase in ischaemic complications but with a reduction in haemorrhagic complications, although these data are not definitive due to the low number of events204. Other trials have instead evaluated the duration of long-term DAPT. CHARISMA35 studied patients, including a 14% subgroup with a previous infarction (STEMI/NSTEMI), and demonstrated a significant reduction in the risk of death, heart attack or stroke (HR 0.774, CI 95% 0.613-0.978) in the arm of active treatment with clopidogrel over 12 months. The DAPT study10 evaluated the prolongation of therapy beyond the 12 months in 9,961 patients undergoing PCI (10% STEMI, 15% NSTEMI) who had tolerated the first year of therapy without bleeding complications, represented in two thirds of patients by clopidogrel and in one third by prasugrel. Overall, long-term therapy resulted in a reduction in primary stent thrombosis endpoints (0.4 vs 1.4%, HR 0.29, CI 95% 0.17-0.48, p<0.001) and death, heart attack or stroke (4.3 vs 5.9%; HR 0.71, CI 95% 0.59-0.85; p<0.001). The reduction in infarction-affected events on lesions with stents and events on lesions at other points of the coronary tree equally, suggesting a prevention effect conferred by DAPT independent of being stent carriers. Moreover, in the subgroup of 43% of patients undergoing stent implantation for ACS, the benefits were even greater than the general population in the reduction of death, heart attack or stroke (3.9 vs. 6.8%, HR 0.56, CI 95% 0.42-0.76, p=0.001), with a tendency to reduce cardiac death (HR 0.67, CI 95% 0.31-1.44) and without the excess of non-cardiovascular mortality recorded on the totality of patients 208. The analysis of the DAPT study allowed a score209 to be calculated that was able to identify the type of patients that benefit the most from continuing the DAPT after 12 months on the basis of the following characteristics: age <75 years, smoking, diabetes, small vessel, myocardial infarction at presentation, previous PCI or previous infarction, ejection fraction <30%, stent implanted in the saphenous vein.
In the TRA 2P-TIMI 50 trial142, vorapaxar (platelet thrombin receptor inhibitor) was effective in reducing the composite endpoint of mortality, heart attack or stroke in addition to ASA and clopidogrel in long-term secondary prevention in the group of patients with previous myocardial infarction (67% of the global population, of which 65% with previous revascularisation). Enhanced antiplatelet therapy reduced events in the first year as well as after the first year up to 36 months, with the two event curves continuing to diverge. An independent trial, OPTIDUAL (Optimal Dual Antiplatelet Therapy)210, enrolled about 1,300 patients at 12 months following PCI (performed in 36% for ACS, 17% of which were myocardial infarction) randomising them to continue with ASA alone or with the addition of clopidogrel for another 36 months. The trial was discontinued early due to low recruitment rates with a borderline benefit trend (p = 0.06) in reducing the composite endpoint of death, infarction or stroke without increased bleeding, but the results are limited by the small sample size. Another two independent post-PCI studies (DES-LATE [Optimal Duration of Clopidogrel Therapy with DES to Reduce Late Coronary Arterial Thrombotic Event]211, ARCTIC [Double Randomisation of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation, and Interruption Versus Continuation of Double Antiplatelet Therapy]-Interruption212), reached analogous results, and did not achieve statistical significance.
The largest study to analyse prolonged DAPT more than 12 months after myocardial infarction (53% NSTEMI, 41% STEMI) was the PEGASUS-TIMI 5411, within which the subgroup of patients with previous PCI with stent was analysed (n=16,891, 80% of the total), which with the dosage of 60 mg presented a significant reduction in the primary endpoint in line with that of the entire population of the trial (HR 0.84, 95% CI 0.73-0.97; p=0.016) not only for minor stent thrombosis, but also to reduce ischaemic events on other coronary segments. This figure is highly significant for patients who do not suspend the DAPT with ticagrelor (24% reduction in RR) or at least resume it within 30 days after the traditional 12 months.
Recently, attention has been focused on the complexity of coronary anatomy and PCI performed to identify the type of patients that could benefit from the prolongation of DAPT beyond 12 months. The meta-analysis by Giustino et al.213 included six randomised trials with 9,577 patients (50% of whom with ACS) treated with complex or simpler PCI and with short (three-six months) or long (>12 months) DAPT duration. The complexity of PCI has been defined based on the presence of one of the following characteristics: three vessels or three stents or three lesions or length >60 mm or bifurcation with two stents or total chronic occlusion. Brief DAPT compared with the extension beyond 12 months led to a significant reduction in MACE (death, heart attack or stent thrombosis: 0.56 HR 95% CI 0.35-0.89). The magnitude of the benefit was greater with prolonged DAPT as the complexity of the PCI increased. The result of this study suggests that in addition to the well-known and coded clinical risk criteria (PEGASUS-TIMI 5411 and DAPT score209criteria), anatomical complexity and PCI treatment type, criteria can be introduced as influential elements in the decision on the extension of DAPT long-term. In the PRODIGY study205 2,013 patients undergoing PCI (22% NSTEMI, 33% STEMI) were randomised to receive four DES models and brief (six months) or long (24 months) DAPT with ASA and clopidogrel, and an analysis of the anatomical complexity of the lesions compared to the utility of prolonging the DAPT214 was performed. The subgroup with lesions located on the left main or proximal add left anterior descending (953 patients) showed a reduction in the incidence of each type of stent thrombosis if treated for 24 months compared to six months (2.8 vs 5.6%; p=0.02), but with an increase in Bleeding Academic Research Consortium (BARC) bleeding 2, 3 and 5 (8.7 vs. 3.5%; p = 0.003)214. These treatment sites may therefore be associated with the criteria of anatomical complexity and other clinical features derived from the PEGASUS-TIMI 54 and DAPT studies11,209.
The possibility of assessing the patient's bleeding risk at the time of admission for STEMI and NSTEMI and therefore establishing DAPT duration (<12 or >12 months) is provided by the PRECISE-DAPT score215, derived from the analysis of 14,963 patients included in eight randomised studies on the duration of DAPT and validated on the PLATO population and the Berna Rotterdam registry. It is based on five simple clinical variables detected on admission: age, creatinine, leukocyte count, haemoglobin level and bleeding history. These parameters allow the identification of patients at high risk of bleeding (score ≥25) for which it is preferable to set a duration of DAPT <12 months. Patients with a score <25 can instead take DAPT for a standard (12 months) or prolonged (>12 months) duration without being exposed to a significant increase in bleeding risk.
In any case, it is important to evaluate the patient's clinical case on a case-by-case basis and not only consider the score attributed to the patient at the end of DAPT prescription, as the international guidelines also never consider the scores separate from the clinical context to be exclusive and mandatory (Table 7)10,11,35,142,205,209,213,214.
Table 7.
Clinical and anatomic criteria for thrombotic risk
| Clinical criteria | Anatomical criteria |
|---|---|
DES, drug-eluting stent; EF, ejection fraction; AIA, anterior interventricular artery; NSTEMI, non-ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
In conclusion, patients undergoing PCI who have a high risk of bleeding and must undergo surgery or require anticoagulant treatment, DAPT can be reduced from 12 to six months and in some cases even three months after DES implantation, or even at one month after DES implantation without polymer216 or in the case of triple therapy.
In patients with a low risk of bleeding, however, who have tolerated the first 12 months of DAPT without bleeding complications, especially those with clinical presentation with ACS, with recurrent events, or diabetics who have undergone complex PCI, it may be reasonable to continue the DAPT beyond the 12 months, in some cases even indefinitely204 and ticagrelor 60 mg bid should be preferred to prasugrel and clopidogrel. In any case, any kind of DAPT duration strategy should be individualised on an individual patient, based on the relationship between ischaemic and haemorrhagic risk that must be constantly reassessed in the follow-up.
12. DUAL ANTIPLATELET THERAPY IN PATIENTS WITH ACUTE CORONARY SYNDROME UNDERGOING SURGICAL REVASCULARISATION
ASA therapy after CABG improves venous graft patency, especially during the first postoperative year, reducing the incidence of MACE217–220.
The CURE study randomised 12,562 patients within 24 hours of onset of symptoms of an ACS to receive clopidogrel or placebo over the next 3-12 months in addition to ASA49. The study showed a reduction in the primary endpoint (cardiovascular death, non-fatal myocardial infarction or stroke) in the group treated with DAPT (from 11.4% to 9.3%). In the study, 2,072 (16.5%) patients had undergone CABG after randomisation, 2,658 (21.2%) to PCI and the remaining to medical therapy49. The following incidences of the primary endpoint were observed in the three groups: (a) percutaneous revascularisation: 9.6% in the clopidogrel group, 13.2% in the placebo group (RR 0.72); (b) CABG: 14.5% in the clopidogrel group, 16.2% in the placebo group (RR 0.89); (c) medical therapy: 8.1% in the clopidogrel group, 10.0% in the placebo group (RR 0.80)222. Although the CABG group as a whole showed a statistically insignificant difference between the two groups, the endpoint trend in the actuarial curves led the authors to consider the benefit induced by consistent and homogeneous DAPT between the three groups, thus independent of the treatment received222.
Among patients undergoing CABG the benefit lay predominantly in the phase prior to surgery: 6.7% of patients randomised to placebo developed an event vs 5.6% in the group randomised to clopidogrel (RR 0.82)49. After CABG a number of similar events between the two groups (112 vs 103; RR 0.97) was observed. However, it should be noted that the median duration of treatment was ten days and that the treatment was only resumed in 75% of patients222.
A Danish registry study of 3,545 patients with a first myocardial infarction and CABG-treated patients within 180 days of admission showed that only 27% of the study population were treated with clopidogrel after CABG223. At a mean follow-up of 466 days, 4.1% of patients treated with clopidogrel developed an endpoint event (death, myocardial infarction) vs 7.8% of patients not taking clopidogrel (p=0.0003), 86.1% of whom had been treated with ASA. In the study, a propensity score analysis was performed and of the 945 matched patients who underwent or did not undergo treatment with clopidogrel, the endpoint occurred in 4% of patients taking clopidogrel vs 6% of patients not taking clopidogrel (HR 0.67; p=0.05), demonstrating that the percentage of patients treated with clopidogrel after CABG is modest and, on the assumption that the presence of clopidogrel is usually an expression of DAPT, this conditions an improvement in prognosis even at an average >one-year follow-up223.
Some studies, including post-hoc analyses, assessed the impact of clopidogrel on graft occlusion after on-pump CABG224–227, reporting the absence of significant differences between monotherapy patients and those treated with DAPT for graft patency at a time interval ranging from one month to one year post-CABG.
The only randomised controlled trial was conducted on 249 patients – 124 randomised to receive DAPT (ASA and clopidogrel) and 125 to receive ASA alone228. Antiplatelet therapy was instituted when the loss in postoperative thoracic drainage was ≤30 ml/h for 2 hours. The patency of the grafts was evaluated after three months by angiotomography. Of the participants, 224 (90%) underwent angiotomography; venous graft patency was 91.6% in patients on DAPT vs 85.7% in patients on monotherapy (p=0.043). In multivariate analysis, DAPT was an independent predictor of graft patency228.
Two meta-analyses and one systematic review assessed the potential benefits of DAPT after CABG, providing results that were partly contradictory229–231. In the largest meta-analysis of five randomised controlled trials and six observational studies, DAPT was associated with a reduced frequency of venous graft occlusion and reduced 30-day mortality compared to ASA monotherapy229. A meta-analysis of only five randomised controlled trials confirmed a significantly lower venous graft occlusion incidence during DAPT without an improvement in arterial graft patency230. Major bleeding was significantly more common in subjects treated with DAPT229–231.
Some monocentric observational studies and a randomised controlled clinical trial also showed a higher incidence of graft patency in DAPT, as well as an improvement in clinical outcome232–234.
The only available data on new P2Y12 platelet inhibitors are from post-hoc analyses. In a post-hoc analysis of the TRITON-TIMI 38 study85, 338 patients with ACS were evaluated who were randomised to receive ASA and clopidogrel or prasugrel and then underwent CABG89. A significantly higher drainage loss was observed in patients receiving prasugrel without a significant difference in the need for transfusion. Regarding the clinical outcome, a significantly lower 30-day mortality was found in the group randomised to receive prasugrel compared to that randomised to clopidogrel (2.31 vs 8.67%; p = 0.025)89.
With regard to a post-hoc analysis of the PLATO study76, data are available for 1,899 patients with ACS who underwent CABG after randomisation to ASA with clopidogrel or ticagrelor235. The analysis refers to the 1,261 patients who received treatment <7 days before CABG. The incidence of the primary endpoint (time from CABG to the first adverse event – cardiovascular death, myocardial infarction, stroke) was similar in the two groups, while mortality from any cause (4.7 vs 9.7%; p<0.01), cardiovascular mortality (4.1 vs 7.9%; p<0.01) and non-cardiovascular mortality (9.7 vs 2.0%; p=0.07) were lower in the ticagrelor group compared with clopidogrel.
Based on the data above, the American guidelines125 recommend resuming DAPT in patients who, after placement of a coronary stent or after ACS (STEMI or NSTEMI), undergo CABG. DAPT duration is correlated to the characteristics of the stent in the first case and has a duration of 12 months in the case of ACS. The recommended ASA dose is 75-100 mg. The above recommendations are all class I. The recommendation for administering DAPT (with clopidogrel started early post-operatively) in the 12 months following the intervention in patients with stable ischaemic heart disease in order to increase venous graft patency is class IIb.
The European guidelines on management of patients with NSTE-ACS23 recommend the administration of a P2Y12 platelet receptor inhibitor in addition to ASA in the 12 months following surgery in all patients who do not present an excessive risk of bleeding. This risk is to be assessed individually along with the Heart Team's ischaemic risk assessment. ASA is recommended in the 6-24h post-CABG in the absence of haemorrhagic events. The above recommendations are all class I.
With a class IIa recommendation, the same guidelines recommend, for patients already in DAPT and sent for CABG, to suspend ticagrelor and clopidogrel five days prior to surgery; unlike the recommendation for prasugrel, which is seven days. After CABG, the resumption of DAPT should be considered as soon as possible.
13. DUAL ANTIPLATELET THERAPY IN PATIENTS WITH ACUTE CORONARY SYNDROME NOT TREATED WITH REVASCULARISATION
In recent years, thanks to increased lifespan and the simultaneous increase in co-morbidities, attention has increasingly focused on conservative treatment in patients not treated with myocardial revascularisation.
Although there is mutual agreement on the importance of revascularisation in patients with NSTE-ACS, a large proportion of these patients do not benefit from this treatment. In fact, in the Italian EYESHOT registry 42.7% of patients with NSTE-ACS were started on conservative therapy, of which 17.2% did not even receive coronary angiographic examination, while 25.5% were not revascularised following coronary angiography236.
Populations who did not undergo myocardial revascularisation may be divided into:
patients who did not undergo clinical examination were studied for high risk as a result of major comorbidities and/or old age;
patients who underwent coronarography but who were not revascularised for the detection of subcritical coronary artery disease or for significant stenosis of a secondary coronary vessel;
patients who underwent coronarography not treated with revascularisation due to a widespread and severe coronary artery disease that was not susceptible to revascularisation;
patients with a type 2 myocardial infarction, i.e. a pathological condition other than ischaemic heart disease (coronary endothelial dysfunction, coronary spasm, coronary embolisation, arrhythmias, anaemia, respiratory failure, hypotension, hypertension) leads to an imbalance between oxygen supply and demand such as to cause myocardial damage24.
These groups appear to be very heterogeneous and are often initiated on different pharmacological treatment with a consequent different prognosis237. Patients not undergoing coronarographic examination show a higher percentage of risk factors and comorbidities with an increase of about three times the incidence of mortality during hospitalisation. Often these are frail patients of advanced age with significant comorbidities, such as renal insufficiency, where the risk of undergoing coronary angiography and/or myocardial revascularisation is not offset by the benefit that would result.
The EYESHOT registry analysis also shows that 58.8% of the patients treated with a conservative strategy were discharged with a dual antithrombotic therapy that combined ASA with a P2Y12 platelet inhibitor (DAPT)236, while 29.2% received ASA alone. This percentage clashes with the latest European guidelines23 recommending the same antithrombotic strategy in patients with NSTE-ACS undergoing myocardial revascularisation and initiated onto conservative medical therapy. In fact, the use of DAPT with ASA associated with ticagrelor or clopidogrel is suggested in case the bleeding risk is too high. Ticagrelor is also preferred to clopidogrel, if not contraindicated. This data is also supported by the PLATO study238, which included 48.8% of non-revascularised patients, in which there was a 17% reduction in endpoint for cardiovascular death, heart attack or stroke in patients treated with ticagrelor compared to those treated with clopidogrel.
Additional data to support the use of DAPT arise from the CURE222 study, where there was a reduction of about 20% of the composite endpoint for cardiovascular death, myocardial infarction and ischaemic stroke in patients treated with DAPT (ASA + Clopidogrel) 12 months after the acute event.
For the duration of the DAPT, according to the most recent guidelines57, as already indicated in the NSTEMI European guidelines23, a timescale of not less than 12 months is recommended (class I, level of evidence A), extendable beyond the 12 months, based on data from the PEGASUS-TIMI 54 study11, in cases of high thrombotic risk and/or previous myocardial infarction (class IIb, level of evidence B) (Figure 25).
Figure 25.
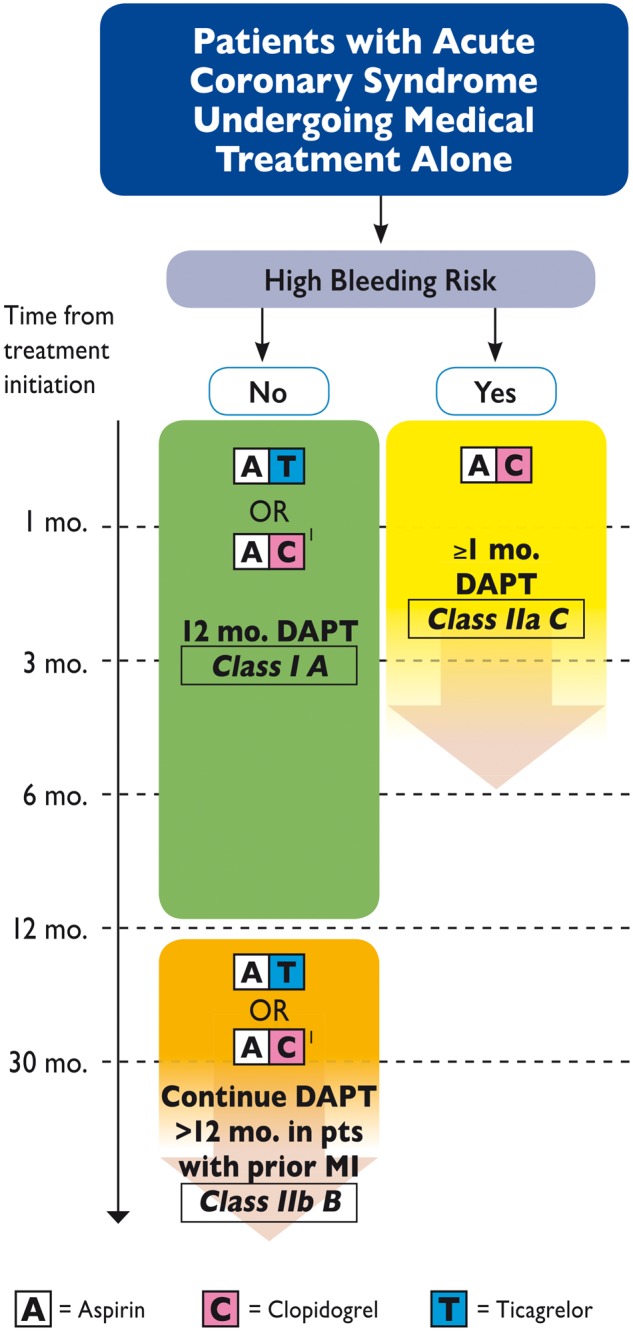
Algorithm for dual antiplatelet therapy (DAPT) in patients with acute coronary syndrome not subjected to revascularisation.
1If the patient is not eligible for treatment with ticagrelor.
Reproduced with permission from Valgimigli et al.57
Therefore, despite the CURRENT widespread presence of catheterization laboratories, many patients with NSTE-ACS are treated conservatively. This population is of increasing clinical and scientific interest, though deserving of more attention and further research239.
As seen in such patients an optimisation of drug therapy involving DAPT is desirable, possibly with a new-generation P2Y12 for at least 12 months, with a clear clinical benefit even in frail patients.
14. DUAL ANTIPLATELET THERAPY IN ELDERLY AND FRAIL PATIENTS
The elderly are a growing population in Western countries as a result of increased life expectancy and improved socioeconomic conditions240. The definition of "elderly patient", however, varies according to the studies; on the basis of the greater evidence we defined as elderly all patients aged ≥75 and <85 years, and as senior patients those aged >85 years. Very often then, the elderly patient is confused with the frail patient, two different nosographic conditions that may or may not be concomitant in the same patient.
As age increases, the risk of chronic diseases increases, so it is almost obvious that there is a significant increase in the number of elderly people affected by a progressive pathology such as cardiovascular disease. The data from the BLITZ 4 study shows that 41% of patients with NSTEMI and 29% of those with STEMI who are admitted to the Italian cardiological intensive care units are more than 75 years old241.
However, the profound heterogeneity of elderly subjects, some in good health, others with numerous comorbidities or signs of frailty, must be underlined. Therefore, age does not in itself constitute a sufficient element for a correct clinical classification, but is necessary to evaluate the biological age and the frailty of the patient. A correct personalised assessment of the elderly patient is therefore fundamental to define a correct prognostic and therapeutic classification.
14.1 Dual antiplatelet therapy in the elderly person
DAPT with ASA and a P2Y12 inhibitor is currently recommended by international guidelines in all patients with ACS and in patients undergoing PCI irrespective of age, although the duration of treatment varies depending on the type of clinical presentation (elective vs acute) and type of implanted stents (BMS vs DES)124. There are some important considerations for DAPT in the elderly; the evaluation of ischaemic and haemorrhagic risk is essential, as is the presence of renal insufficiency, history of stroke, diabetes mellitus, previous PCI or heart failure.
Current guidelines were developed based on data from randomised clinical trials in which the elderly population is often poorly represented. The results relate to younger populations and were extended to elderly patients only secondarily. Furthermore, different pharmacological aspects influence the management of antiplatelet therapy in this subgroup of patients, relating to the reduced capacity of intestinal absorption, changes in the volume of body distribution for a reduction of lean mass and increase of fat mass, the alterations of renal function that cause a reduction of clearance resulting in drug build-up, lower efficiency of hepatic metabolism (Table 8)242,243.
Table 8.
Age-related changes in organs that influence drug pharmacokinetics
| Physiological changes | Pharmacological consequences |
|---|---|
| Gastrointestinal tract | |
|
|
| Body composition and drug distribution | |
|
|
| Liver | |
|
|
| Kidney | |
|
|
Vd, volume of distribution
Adapted from Andreotti et al.243
14.1.1. Antiplatelet drugs
Aspirin. The European guidelines recommend the use of ASA both after elective PCI and in patients with ACS, with a loading dose of 150-300 mg orally or 80-150 mg via IV followed by 75-100 mg/day as a maintenance dose, without changing the dosage based on age43,124. This is the highest dose that can be administered with a lower risk of toxicity and gastrointestinal bleeding. Use of PPIs is recommended in patients with a history of upper gastrointestinal bleeding and in patients requiring DAPT with multiple risk factors for superior digestive bleeding244.
14.1.2. P2Y12 receptor antagonists
Clopidogrel. In stable patients undergoing PCI, clopidogrel is the only P2Y12 inhibitor indicated for DAPT. In patients with ACS undergoing PCI, however, clopidogrel is considered a second choice compared to the new antiplatelet agents. The recommended loading dose for faster platelet inhibition after PCI is 600 mg, followed by a maintenance dose of 75 mg/day. In the CURE study, the combination of clopidogrel and ASA was associated with a relative 20% reduction of the combined ischaemic endpoint (cardiovascular death, non-fatal myocardial infarction or stroke) and a relative 38% increase in greater bleeding at one year in the general population, compared to ASA alone49. Compared to younger patients, those aged >65 years showed a similar absolute reduction (2.0 vs. 2.2%) and a smaller relative reduction (13.1 vs 28.9%) of the endpoint combined with the addition of clopidogrel. Clopidogrel, however, was shown to be significantly more effective than the placebo in both groups. In particular, some features are associated with a greater benefit of clopidogrel, such as a high TIMI risk score or previous revascularisation. Age-related bleeding was not reported.
Pharmacodynamic studies showed increased platelet reactivity in elderly patients with clopidogrel 75 mg compared with younger patients245. At present there is conflicting evidence about whether an individualised antiplatelet strategy can improve the clinical outcome.
In addition, clopidogrel is indicated in patients with concomitant AF who must then take an OAT, previous ischaemic or haemorrhagic stroke, moderate-severe liver disease (Child-Pugh B and C), recent fibrinolytic therapy (in this case the loading dose should not be administered), a high bleeding risk defined by the presence of two or more characteristics reported in Table 9246–250.
Table 9.
Rapid clinical evaluation of increased bleeding risk (defined by the presence of ≥2 of the listed characteristics)
|
|
|
|
|
Prasugrel. In the TRITON-TIMI 38 study, prasugrel showed a faster and more potent antiplatelet effect when compared to clopidogrel in patients with ACS treated with PCI, reducing the rate of ischaemic events85. However, in the subgroup of patients ≥75 years, prasugrel was associated with an increase in major and fatal bleeding so that there was no significant difference between the two treatment groups in terms of mortality; the reduction of ischaemic events was offset by an increase in bleeding events. In the TRILOGY ACS study251, patients with ACS undergoing conservative treatment were compared. No significant differences were observed in the incidence of ischaemic and haemorrhagic events in those treated with prasugrel 5 mg vs clopidogrel 75 mg, and an increase in age-related bleeding risk was observed. Furthermore, pharmacodynamic studies such as the GENERATIONS (Comparison of Prasugrel and Clopidogrel in Very Elderly and Non-Elderly Patients With Stable Coronary Artery Disease) study 252 have shown that the administration of 5 mg of prasugrel in patients ≥75 years induced platelet inhibition that was not lower than the antiplatelet effect obtained with 10 mg.
Because of these findings, prasugrel is not recommended in elderly patients, with a reduced dosage of 5 mg indicated in selected cases; the loading dose of 60 mg remains unchanged.
Ticagrelor. In the PLATO study, 18,624 patients with ACS were randomised to ticagrelor or clopidogrel; of these, 2,878 patients (around 15%) were ≥75 years old. A pre-specified sub-analysis of this subgroup253 showed that the significant clinical benefit and overall safety of ticagrelor compared to clopidogrel are not age dependent. The absolute reduction in all-cause mortality was numerically greater (2.6%) in the elderly than younger patients (1.2%) and the absolute reduction in the primary endpoint was 1.1% in the elderly and 1.8% in younger patients.
Based on these data, ticagrelor appears to be the P2Y12inhibitor to be used in elderly patients with ACS who should not take concomitant OAT, do not have a history of previous haemorrhagic stroke, CRI undergoing dialysis, severe hepatopathy (Child-Pugh C) or have not received recent fibrinolytic therapy and do not have a high haemorrhagic risk defined by the presence of two or more characteristics shown in Table 9. Moderate hepatopathy (Child-Pugh B) and previous ischaemic stroke are not absolute contraindications to the use of ticagrelor in the elderly patient, but they are important conditions in the overall assessment of ischaemic-haemorrhagic risk in the frail patient. In addition, ticagrelor can be administered with caution in patients without pacemakers presenting advanced sinoatrial disease and in patients with history of asthma and/or chronic obstructive pulmonary disease.
The use of ticagrelor is recommended with a loading dose of 180 mg followed by a maintenance dose of 90 mg bid124.
14.1.3. Dual antiplatelet therapy in elderly patients on oral anticoagulant therapy
Approximately 5% to 8% of the population of patients undergoing PCI require OAT for the presence of AF, mechanical valve prosthesis or venous thromboembolism. In these patients triple antithrombotic therapy (OAT with vitamin K antagonists or new oral anticoagulants in association with DAPT) can be considered. The only indicated P2Y12 receptor inhibitor is clopidogrel, while ticagrelor and prasugrel are not recommended. Risk stratification occurs through CHA2DS2-VASc and HAS-BLED scores, remembering that age is a factor that influences both thrombotic and haemorrhagic risk. In the recent AF guidelines (Figure 26)133, for patients at high risk of bleeding (defined by a HAS-BLED ≥3), administration with OAT of ASA and clopidogrel for one month is recommended; thereafter, OAT with ASA or clopidogrel up to six or 12 months depending on the clinical context presented. If there is a low risk of stent thrombosis but a high risk of bleeding, OAT may be considered with only one antiplatelet agent for 12 months.
Figure 26.
Antithrombotic therapy after percutaneous coronary intervention (PCI) (see left) and post-acute coronary syndrome (ACS) (see right) in patients with atrial fibrillation (AF) requiring oral anticoagulant therapy (OAT).
Reproduced with permission from Kirchhof et al.133
14.1.4. Duration of dual antiplatelet therapy
Current guidelines recommend therapy with ASA and a P2Y12 receptor inhibitor for 12 months in ACS124. In patients undergoing elective PCI with DES, DAPT is indicated for six months and for one month in case of BMS. In patients with bleeding or at high risk of bleeding, DAPT can be reduced to three months for the latest generation DES. Results of recent trials and meta-analyses10,11,192 have demonstrated how a DAPT >12 months may be associated with a lower incidence of ischaemic events in patients with high risk of thrombosis, although an increase in bleeding has been observed. The extension of this benefit to the elderly population is currently debated; the benefit of prolongation of DAPT is evaluated only in patients with a high ischaemic risk but with a low risk of bleeding254 and the choice of the second antiplatelet to be associated with ASA, ticagrelor or clopidogrel.
For example, the results of the DAPT study10, one of the most recent studies designed along with the FDA to evaluate the optimal duration of DAPT, showed a benefit of protracted therapy in terms of reduction of ischaemic events, especially in patients with a history of stroke, against an increase in bleeding, although the data cannot be easily extrapolated to an elderly population, as the average age of the approximately 10,000 patients enrolled was only 61 years. However, as is confirmed, in the subgroup of elderly patients, the benefit is equal to that of younger subjects, while worsening the haemorrhagic risk profile. Based on this data, the authors also extrapolated a risk/benefit assessment score to consider before starting long-term DAPT. In the high-score group the probability of ischaemic events, AMI or stent thrombosis was 2.9% in patients who continued DAPT compared to 4.7% in the placebo group, compared to 1.7 vs 2.2% in patients with low scores. On the other hand, the probability of bleeding was 1.8 vs 1.2% in high-risk patients compared to 3% in patients with low scores who continued the DAPT and 1.4% in the placebo group. Age >75 years in itself determines a score of -2, which effectively reduces the ability to achieve high scores, for which the choice of a prolonged DAPT is cost-effective. Therefore, based on this data, the age factor limits the choice to those categories with very high thrombotic risk, such as a previous heart attack, widespread coronary artery disease, incomplete revascularisation, diabetes, dilated cardiomyopathy with low ejection fraction, venous grafts.
14.2 Dual long-term antiplatelet therapy in the frail patient
Frailty is a biological syndrome that reflects a state of reduced reserve and an increased multiple organ vulnerability to sources of stress, and is linked to an increased risk of cognitive and functional decline, hospitalisation and disability, representing an independent risk factor for cardiovascular disease morbidity and mortality255. There are more than 20 tools to assess the frailty of a patient256; we found the Rockwood scale the most suitable and applicable in the context of an outpatient cardiology evaluation257. This notwithstanding the biomedical paradigms that define fragility as "a physiological syndrome characterised by the decline in functional reserves and the decreased resistance to ‘stressors’, resulting from the cumulative decline of multiple physiological systems that cause vulnerability and adverse consequences"258 and on the other biopsychosocial paradigms that instead recognise "a dynamic state that affects an individual who experiences losses in one or more functional domains (physical, mental, social), caused by the influence of several variables that increase the risk of adverse health results"124.
The prolongation of DAPT over the 12 months classically prescribed for ACS necessarily implies recognising in this population, on the one hand, those who can easily manifest reduced adherence with therapy, with secondary unfavourable coronary events, and on the other, those who while complying with the prescriptions, may undergo adverse events linked to the antiplatelet therapy itself. In this regard, the 70 items of the CSHA study (Canadian Study on Health and Aging)257 or the 35 items of Kamaruzzaman et al.259 can be useful when divided into seven subgroups.
The TRILOGY ACS260 study designed specifically to address the topic showed that those aged >65 years who meet the above Fried criteria present a more severe prognosis as the number of items increases. The above applies to both the general population of the study and to the subdivision of patients by type of antiplatelet treatment carried out (clopidogrel 75 mg/day or prasugrel 10 mg/day; 5 mg if >75 years or <60 kg protracted up to 30 months), although there was no correlation between frailty and the risk of bleeding.
Patients who had "pre-frailty features" (1-2 items) and "full frailty" (≥ 3 items) randomised to prasugrel, however, showed a higher incidence of adverse cardiac events than those classified as not frail. However, also in this case without an increased risk for bleeding, despite the low body weight and reduced muscle mass. The reason is likely to be found in inactivity, due to the reduced risk of trauma.
But regardless of age, haematological, neoplastic, kidney dialysis patients and non-haematopoietic patients with potentially bleeding gastrointestinal diseases, immunological pathologies and anything else that could provide "frailty" features were for obvious reasons "the major absentees" from the trials that primarily studied the antiplatelet agents in use today, such as TRILOGY ACS, where these populations were not represented.
ESC guidelines on NSTEMI23 in this respect are not robust, equating the word "frail" with advanced age. In the ANMCO/SICI-GISE paper on antithrombotic therapy in elderly patients with ACS261, on the other hand, a precise definition of the frail patient is adopted, which aims to be as objective as possible by using specific assessment scales. This document identifies several possible scenarios:
post-STEMI (whether treated with primary PCI, with fibrinolysis, or not given reperfusion); the DAPT duration should be at least 12 months; in cases of higher risk of bleeding, DAPT duration is preferably 6-12 months;
post-NSTEMI (whether undergoing coronary revascularisation or not, and in the absence of frailty conditions or high risk of bleeding): the recommended duration of DAPT is at least 12 months.
ESC 2017 DAPT guidelines do not adopt a clear definition of frail patient, and dedicate a paragraph specifically to the elderly patient. The only reference to the concept of frailty is the section devoted to measures to minimise the risk of bleeding during dual antiplatelet therapy. For the purposes of choosing a P2Y12 inhibitor to combine with ASA, it is stated that past intracranial haemorrhage or current bleeding contraindicate the use of prasugrel and ticagrelor. Furthermore, a history of stroke is identified as a marker of the patient's frailty, which increases the risk of haemorrhagic stroke in particular in the subsequent 12 months57.
The PEGASUS-TIMI 54 study results11 demonstrate that the addition of ticagrelor 60 mg bid to standard therapy with ASA in stable patients with a history of IMA reduces the risk of death, myocardial infarction and stroke in all major subgroups analysed - therefore, even in the elderly. The downside is a moderate increase in major TIMI bleeding, but not in fatal bleeding. The primary endpoint reduction of risk of death, heart attack and stroke was 23%, particularly in the subgroup of patients aged >75, with an NNT of 40 vs 90 in the placebo group11, an even more favourable outcome than in the general population of the study.
Dyspnoea is a potential factor that negatively affects patient adherence to long-term therapy with ticagrelor and is found for both 90 mg and 60 mg doses assessed in the PEGASUS-TIMI 54 study, although to a lesser extent for 60 mg (6.5 vs 4.6%). It is now well known, however, that it is an event that manifests itself in particular in the first phase of drug intake and is often self-limiting. In patients with chronic obstructive pulmonary disease and asthma and in the elderly, the appearance of dyspnoea is more frequent and often not limited to the first seven days of therapy, which is why in these subgroups of patients the quality of life and consequently adherence to therapy can be reduced.
These data suggest that it is not only the risk of bleeding that drives the choice of duration and type of DAPT in the frail patient, as the appearance of other potential adverse effects, such as dyspnoea and bradycardia, should be adequately considered, as they are potentially able to alter patient adherence.
14.3 Conclusions
Advanced age and frailty are two common conditions for which the risk-benefit balance of long-term DAPT should be carefully considered. If in fact the benefit of the prolongation of DAPT also determines the advantages in terms of reduction of ischaemic events in these categories of patients, the greater risk of haemorrhagic events that this therapeutic choice may cause in older subjects, especially in the very elderly, and in those affected by frailty, should be carefully considered.
The interpretation of the data in these patients is affected on the one hand by the heterogeneity of presentation, whereby age cannot be considered a uniform criterion in all patients; on the other hand, it is affected by the lack of data in literature on these categories, which is often excluded from large trials.
It is before an elderly or fragile subject, therefore, that the concept of a tailored treatment is best applied, in which the decision to prolong the DAPT must be decided on the basis of the patient before us, with his or her own characteristics and expectations, and not on the basis of a generic classification.
15. DUAL ANTIPLATELET THERAPY IN DIABETIC PATIENTS AND/OR THOSE WITH RENAL DYSFUNCTION
The safety of antithrombotic therapy is strongly influenced by a delicate balance between drug characteristics and effects, patient ischaemic/haemorrhagic risk and quality of clinical management (Table 10). In particular, comorbidities (old age, diabetes mellitus, kidney failure, anaemia, etc.) greatly increase the risk of bleeding, but the same happens for thrombotic-ischaemic disease (Table 11). This makes it very difficult in clinical practice to choose antithrombotic therapy that can balance the individual thrombotic and haemorrhagic risk and above all maintain this balance in the long term261–265. Fortunately, in the coronary heart patient the ischaemic and haemorrhagic risk are expressed at different times; the first one, in ACS (initial phase), while the risk of major bleeding is constant over time.
Table 10.
Risk and benefit of antithrombotic drugs: interaction between factors related to the patient, medication and the quality of care
| Drugs | Patient | Quality of care |
|---|---|---|
|
|
|
PCI, percutaneous coronary intervention.
Table 11.
Clinical factors related to thrombotic/haemorrhage risk
| Parameter | Thrombotic risk | Haemorrhage risk |
|---|---|---|
| Age | +++ | ++ |
| Female gender | – | ++ |
| Kidney failure | +++ | +++ |
| Anaemia | ++ | +++ |
| Anterior wall myocardial infarction | ++ | – |
| Heart rate | ++ | ++ |
| Arterial blood pressure | ++ | ++ |
| Killip Class >1 | +++ | ++ |
| Stroke | ++ | ++ |
| Diabetes mellitus | ++ | + |
| Decreased body weight | + | ++ |
| Previous major bleeding | – | ++ |
15.1 Atherothrombotic risk in diabetic patients
Without going into the characteristics of coronary artery disease (multivessel and pluri-segmental, progressive and accelerated, characterised by complex or vulnerable plaques or dissection, with high incidence of restenosis after PCI and saphenous graft occlusion) in patients with diabetes mellitus, especially type 2, insulin-resistance and hyperglycaemia contribute to the pathogenesis of a prothrombotic state (Table 12).
Table 12.
Factors that influence atherothrombotic risk in diabetes mellitus
| ↑ PAI-1 |
| ↑ Factor VII, XII, fibrinogenous |
| ↓ tPA |
| ↑Platelet reactivity (adhesion, activation, aggregation) |
| Upregulation of glycoproteins IIb/IIIa and P-selectin |
| Increased P2Y12 platelet receptor signalling |
| Accelerated platelet turnover |
| Resistance to certain antiplatelet agents |
PAI-1, type 1 plasminogen activator inhibitor; tPA, tissue plasminogen activator.
In diabetic patients there is therefore an intense activation and platelet aggregation associated with resistance to some antiplatelets (clopidogrel). In addition, patients with diabetes are older, more often women, have more co-morbidities such as hypertension and renal failure, and are more prone to developing complications, especially heart failure and bleeding. All these result in an adverse prognosis right after and long past the acute coronary event. However, intervention records against this worsen prognosis, have consistently shown that diabetics are treated as sub-optimal compared to non-diabetics; European registries record all forms of revascularisation, treatment with GPIIb/IIIa receptor inhibitor thienopyridines, and are prescribed less frequently in this population.
15.1.1. Which anti-thrombotic therapy in diabetic patients?
DAPT with ASA, an inhibitor of the PY212 receptor, is one of the pillars of the treatment of ACS: the introduction of DAPT in clinical practice began with ASA and clopidogrel after the CURE222 study results, which showed the association's superiority compared to ASA alone; however, diabetic patients had only a modest benefit from the treatment (combined endpoint of death, heart attack or stroke: 14.2 vs 16.7%), and the net benefit among diabetic patients treated with PCI was slightly less than that of non-diabetics.
In the CREDO study, which showed a significant long-term (one year) benefit of clopidogrel in patients undergoing PCI (combined risk of death, heart attack or stroke 8.5% with clopidogrel vs 11.5% with placebo), the magnitude of the benefit observed was lower in patients with diabetes mellitus50. Platelet hyperactivity combined with reduced susceptibility to antiplatelet agents may explain the increased atherothrombotic risk in these patients.
It remains to be seen whether the optimisation of DAPT using the new antithrombotic drugs can help improve the outcome of these patients.
In the TRITON-TIMI 38 study, the PY212 platelet receptor inhibitor prasugrel in patients with ACS, after initial angiography, has been shown to be superior to clopidogrel in reducing the composite endpoint of cardiovascular death, heart attack and stroke, without an excess of major bleeding in diabetic patients (12.2 vs 17%), both insulin-dependent and not insulin-dependent88.
In the PLATO study, ticagrelor compared with clopidogrel reduced the percentage of ischaemic events and mortality from any cause in patients with ACS, regardless of the diabetic situation and glycaemic control, without an increase in major bleeding266. Unlike the TRITON-TIMI 38 study, in which patients were treated only after coronary angiography, in PLATO, diabetic patients with ACS could not undergo any invasive or conservative therapeutic strategy.
15.1.2. Duration of dual antiplatelet therapy in diabetic patients
The joint ESC/EASD guidelines (European Association for the Study of Diabetes)267 published in 2013 recommend the use of a P2Y12 platelet receptor inhibitor in all patients with diabetes mellitus and ACS for one year. In patients with ACS undergoing PCI, the use of prasugrel or ticagrelor is recommended with a class I criterion, level of evidence A, further endorsing the use of the best antiplatelet therapy in diabetic patients considered to be at particularly high risk (prasugrel or ticagrelor compared to clopidogrel).
In 2017, new ESC guidelines for the management of DAPT57 were published. The latter recommend treating diabetic patients as non-diabetics for either type or duration of the DAPT (recommendation class IIa, level of evidence B) (table 13).
Table 13.
Indications for dual antiplatelet therapy (DAPT) in diabetic patients
| Recommendations | Classa | Levelb |
|---|---|---|
| The same DAPT typology and duration is recommended for both sexes. | I | A |
| In patients who are potentially at risk of bleeding complications, it is recommended that type, dosage and duration of DAPT be periodically reassessed. | I | C |
| The same DAPT typology and duration is recommended for both diabetic and non-diabetics patients. | IIa | B |
aRecommendation class.
bLevel of evidence.
Several studies have addressed the problem of defining the right duration of DAPT in patients with diabetes mellitus.
In the PEGASUS-TIMI 54 study, the sub-analysis of patients with diabetes (n = 6,806) and history of myocardial infarction one to three years in the past, plus additional risk factors, randomised for ticagrelor (90-60 mg bid) or placebo followed for a median of 33 months showed a reduction in absolute risk (1.5% vs. 1.1%) of cardiovascular mortality by 22% and coronary mortality by 34%. Major TIMI bleedings were significantly increased in diabetic patients (2.56% vs. 0.98%), similarly to what was observed in non-diabetic patients (2-3 vs. 1.09%) without differences in fatal or intracranial bleeding rates (<1% over 3 years). However, the study protocol envisaged the exclusion of patients with recent bleeding, previous stroke or who needed OAT.
Important data on the benefits/risks and duration of DAPT in diabetic patients is also identified in the DAPT study, which included 11,648 patients undergoing percutaneous revascularisation, both stable and post-infarction (62%), comparing DAPT suspension after 12 months vs its continuation for another 18 months with a thienopyridine (65% clopidogrel, 35% prasugrel) and ASA. The strategy of extended DAPT (30 months overall) resulted in a significant reduction of co-primary ischaemic events (real or suspected thrombosis intrastent, heart attack, stroke), especially in patients treated with first-generation DES, those with previous AMI or PCI, diabetic patients and patients with heart failure and/or <30% ejection fraction.
A risk score was defined (Table 14) when identifying any patients who had benefited from long-term DAPT. Patients with a DAPT score >2 that stayed on thienopyridines versus placebo exhibited a reduction of ischaemic events by 2.7% vs 5.7%; in those with DAPT scores <2, the reduction of ischaemic events was only 1.7% vs 2.3% in the placebo group. There was a small increase in bleeding in the higher score group. In clinical practice, DAPT scores could steer the definition of the proper DAPT duration; its value remains uncertain, however, because it has not been tested prospectively in randomised clinical trials. Present data emphasises the need to individualise the duration of DAPT for each patient. Patients with increased risk of ischaemic events, e.g., diabetic patients, especially those with lower risk of bleeding, should be strongly considered for extending DAPT beyond the year currently recommended by the guidelines.
Table 14.
Factors used to calculate the DAPT score
| Variable | Score |
|---|---|
| Age ≥ 75 years | −2 |
| Age 65 - < 75 years | -1 |
| Age < 65 years | 0 |
| Smoking | 1 |
| Diabetes mellitus | 1 |
| MI on admission | 1 |
| Previous PCI or MI | 1 |
| Stent diameter < 3 mm | 1 |
| Stent with elution of paclitaxel | 1 |
| CHF or LVEF < 30% | 2 |
| PCI with saphenous vein graft | 2 |
LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; CHF, chronic heart failure.
The ACC/AHA 2016 guidelines on the duration of DAPT in patients with coronary artery disease125 emerge from a review of 11 studies of patients undergoing stent implantation (predominance of DES), treated with shorter or longer duration DAPT, and from the results of a randomised trial of patients with previous AMI (1-3 years) treated with DAPT vs ASA monotherapy. In comparison, an extended DAPT determined a greater reduction of the ischaemic risk, but at the expense of an increased risk of bleeding, especially by using the newer and stronger P2Y12 receptor inhibitors. In conclusion, these guidelines recommend:
DAPT must be administered for a minimum period of 6-12 months (class I recommendation);
DAPT may be considered beyond this period in patients with higher thrombotic risk and lower risk of bleeding (e.g. patients with diabetes mellitus) (class IIb recommendation);
shorter DAPT may be considered in patients with lower ischaemic risk and higher risk of bleeding (class IIb recommendation).
The DAPT score derived from the DAPT study may be useful for deciding whether to continue DAPT in patients treated for one year without significant bleeding or ischaemic events (DAPT score >2). On the contrary, in patients with a DAPT score <2, the risk/benefit ratio of a DAPT extension is not favourable. It should, however, be noted that this score is applicable selectively to the DAPT study population, which included both stable patients and post-stroke patients, and thus its systematic application on a large scale seems unlikely.
In conclusion, the combination of a history of diabetes and coronary artery disease leads to a very high risk for future cardiovascular events; therapy with more powerful anti-thrombotic drugs significantly reduces ischaemic events and cardiovascular mortality with an increased risk of bleeding, although not of an intracranial or fatal nature. In diabetic patients with no history of bleeding, this approach of intense and prolonged platelet inhibition seems to counter the excess of ischaemic risk observed in this population.
15.2 Atherothrombotic risk in patients with renal insufficiency
In ACS, the coexistence of CRF is a powerful, independent and unfavourable condition in prognostic terms11,268. In fact, the CRF poses an additional and independent risk of thrombotic events, but also a significant bleeding risk that determines an especially unfavourable mix. This combination causes significant difficulties in the choice of the initial strategy, but also in identifying the most appropriate antiplatelet therapy to be used, introducing other potentially unfavourable prognostic indicators.
Already in 2002, analysing the short-and long-term prognosis of patients with CRF and STEMI, Wright et al.269 had documented a correlation between worsening of renal function and risk of death, including patients with mild impairment. A subsequent study on over 130,000 patients with STEMI and ≥65 years of age confirmed the same relationship, and a 30-day mortality in patients with moderate renal impairment (serum creatinine between 2.5 and 3.9 md/dl) equal to 44%270. More recent studies such as the ACTION registry (Acute Coronary Treatment and Intervention Outcomes Network), which evaluated over 19,000 patients, confirmed the relationship between CRF and mortality in patients with STEMI271.
Even in patients with NSTE-ACS, short- and long-term mortality is worse for CRF. Studies such as that of Gibson et al.272 showed a negative correlation between deterioration of estimated glomerular filtration rate (eGFR) and cardiovascular ischaemic cerebrovascular events (Figure 27) over a 30-day period, with a concomitant increase in risk for bleeding.
Figure 27.
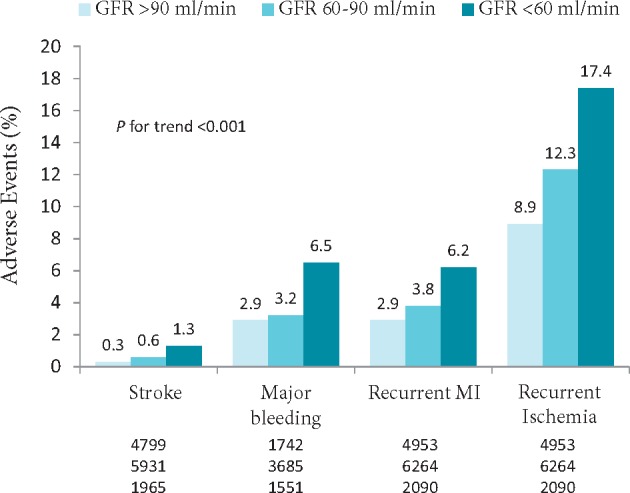
Association between atherothrombotic and haemorrhage events in patients with acute coronary syndrome and chronic renal failure.
GFR, glomerular filtration rate.
Adapted from Gibson et al.272
Two recent studies (ACTION and SWEDEHEART [Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies]) have documented, also for NSTE-ACS patients, a close correlation between CRF and an increase of in-hospital mortality and over a one-year period271,273.
15.2.1. Anti-thrombotic therapy in patients with acute coronary syndrome and chronic renal failure
Almost one third of patients with STEMI and over 40% of those with NSTEMI have renal dysfunction. Antithrombotic therapy in patients with CRF and ACS is still one of the greatest unmet therapeutic needs. On the one hand, the CRF is associated with prolonged bleeding time and alterations of platelet aggregation and adhesion; on the other hand, a state of hypercoagulability is documented. This combination of alterations places patients simultaneously at risk of thrombosis and bleeding. Current guidelines still recommend DAPT extended for 12 months, also in patients with CRF, giving preference to the so-called new P2Y12 receptor inhibitors (prasugrel and ticagrelor)23,56,57. Indeed, in the PLATO study, the efficacy of ticagrelor vs clopidogrel in patients with ACS and renal dysfunction (eGFR <60 ml/min) was similar - if not greater - than that of patients with conserved renal function, with a reduction of absolute risk without adjustments of dosage274.
15.2.2. Dual antiplatelet therapy prolonged beyond 12 months after acute coronary syndrome in patients with chronic renal failure
Thus, the presence of CRF poses a long-term atherothrombotic risk that can be controlled, for example, by extending DAPT beyond the year. However, it should be noted that the extended DAPT produces a benefit in terms of thrombotic events but may increase the risk of bleeding. Because the risk of bleeding in patients with CRF itself is increased, the choice to extend the DAPT in these patients should be evaluated carefully.
The hypothesis that long-term DAPT may reduce the risk of major cardiovascular events compared to the use of a single antiplatelet (ASA) was explored by the CHARISMA study35, in which patients with high atherothrombotic risk were eligible with a documented cardio-cerebrovascular disease and/or cardiovascular risk factors, including diabetic nephropathy (43.2% of the entire population treated with DAPT), but not CRF. Over a 30-month follow-up, DAPT with clopidogrel 75 mg/day + ASA 75-162 mg/day proved to be superior to mere ASA 75-162 mg. The study did not conduct an analysis of interaction regarding the sub-population with diabetic nephropathy, hence no hypothesis can be generated in this subgroup. The study, however, in a later post-hoc analysis36, presented certain benefits of DAPT in patients with full-blown cardio-cerebrovascular disease, particularly in patients with myocardial infarction. In this analysis, the percentage of patients with diabetic nephropathy represented only 4.1%, and again, no analysis of interaction was performed for this subgroup.
Recently, the PEGASUS-TIMI study 5411 analysed the effect of ticagrelor 90 and 60 mg bid in patients infarcted one to three years before who were previously treated with ASA and considered to be at high cardiovascular risk due to the coexistence of at least one of the five risk conditions (age ≥65 years, one or more infarctions beyond the qualifying one, multi-vessel coronary disease, non-terminal renal failure and diabetes). In the study, patients with CRF (eGFR <60 ml/min) accounted for about 22% of the entire population. The pre-specified analysis275 of this subgroup showed that the reduction of RR due to ticagrelor (data analysis of 60 and 90 mg) of the primary endpoint was similar between patients with and without CRF (HR 0.81, 95% CI 0.68-0.96 vs HR 0.88, CI 95% 0.77-1.00; p for interaction = 0.44). However, given the increased incidence of cardiovascular events in patients with CRF, the reduction of absolute three-year risk in the latter population was extremely important, about four times greater than that of patients without CRF (2.70%, 95% CI 0.49-4.93 vs. 0.63%, 95% CI 0.32-1.57) (Figure 28A).
Figure 28.
Risk of thrombotic events (A) and haemorrhage risk (B) in patients participating in the PEGASUS-TIMI 54 study in relation to renal function.
CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence interval; ICH, intracranial haemorrhage; MI, myocardial infarction; KM, Kaplan-Meier; RAR, reduction of absolute risk; TIMI, Thrombolysis in Myocardial Infarction.
Adapted from Magnani et al.275
The RR of major TIMI bleeding (primary safety endpoint), minor TIMI and fatal bleeding/intracranial haemorrhages, as was to be expected, increased overall in patients under study (Figure 28B). However, the RR of major TIMI bleeding was similar between patients with and without CRF (ticagrelor 90 and 60 mg vs placebo; eGFR < 60 ml/min: HR 95% CI 1.13-1.98, 3.46; eGFR ≥ 60 ml/min: HR 95% CI 1.87-2.65, 3.76; p for interaction = 0.38). It is noteworthy that the combination of fatal bleeding/intracranial haemorrhages was not significantly increased with ticagrelor, regardless of renal function. Finally, the analysis did not show an increased risk of adverse renal events with ticagrelor.
Overall, these results have important implications for the treatment of post-infarcted patients with a renal function impairment, which exposes them to a greater risk of future events. Whilst increased haemorrhagic risk is observed in patients with CRF who continue DAPT beyond the year, available data from the PEGASUS-TIMI 54 study indicate that the net clinical benefit is still favourable, without serious bleeding complications. Overall, therefore, as the population of patients with CRF who have suffered an ACS is very broad, the choice of extending DAPT should consider the greater relative benefit, and the individual bleeding risk that depends on the severity of renal dysfunction, but also on other factors such as age, sex, the presence or absence of anaemia and/or diabetes.
16. DUAL ANTIPLATELET THERAPY IN PATIENTS WITH PERIPHERAL OBLITERATIVE ARTERIOPATHY
16.1 Epidemiological background
Peripheral obliterative arteriopathy, which represents, after coronary heart disease, the most frequent complication of atherosclerosis, offers a broad phenotype of clinical presentations ranging from asymptomatic atherosclerosis to intermittent claudication, pain at rest, tissue lesions up to critical limb ischaemia (CLI), classified by Rutherford (Table 15). Symptoms may be typical in 10-30% or atypical in 30-40% of cases (easy fatigue, coldness, sexual impotence), but the disease may be asymptomatic in 10-30% of cases, where diagnosis is possible with the measurement of the systolic ankle-arm pressure index276,277. It follows that epidemiological data, depending on the diagnostic criteria used, can vary widely. In an Italian study278, the prevalence of the disease was equal to 1.6% of the general population (2.4% in men and 1.0% in women), thus lower relative to the populations of Northern Europe and North America, with a progressive increase with increasing age; in industrialised countries, the PAD is found in 4-12% of the subjects between 55 and 70 years, and in 20% of the subjects over the age of 70279,280. The prevalence of PAD, and in particular of CLI, is increasing dramatically. From 2005 to 2009, admissions in Germany due to PAD increased by 20.7%, and interventional procedures by 46%281. The PAD shares the same risk factors as other atherosclerotic manifestations, but the predominant ones are smoking, for the most proximal localisations (aorto-iliac-femoral), and diabetes mellitus, for the more peripheral ones (poplitee)282–285.
Table 15.
The Rutherford peripheral obliterative arteriopathy classification
| Grade | Category | Clinical assessment |
|---|---|---|
| 0 | 0 | Asymptomatic |
| I | 1 | Mild claudication |
| I | 2 | Moderate claudication |
| I | 3 | Severe claudication |
| II | 4 | Ischaemic pain at rest |
| III | 5 | Slight loss of tissue |
| III | 6 | Significant loss of tissue |
Regarding the prognosis, PAD is considered a powerful marker of cardiovascular risk286. In the face of a modest probability of local complications (five years after diagnosis, only 5% have CLI and 1-4% undergoes amputation), the risk of cardiovascular events increases from two to six times287 and total mortality, mainly cardiovascular, exceeds 50% in five years279,280. Over 10 years, at least 60% of persons with symptomatic claudication dies due to ischaemic heart disease, of which 12% due to stroke279,288,289. The prognosis is related to co-morbidities, especially to diabetes290 and the severity of the disease. Based on Rutherford class 1 to 6, four-year mortality risk grows from 18.9% to 63.5%, and that of amputation from 4.6% to 67.3%291. CLI patients exhibit up to 90% incidence of coronary events and one-year mortality of 25-50%292.
PAD is a frequent co-morbidity in coronary arteries; more generally, given the substantial coexistence of risk factors and etiopathogenesis, atherosclerotic manifestations often coexist in the same patient. In the REACH Register (Reduction of Atherothrombosis for Continued Health)286, about three-fifths of patients with PAD suffered an atherothrombotic disease in other areas. In particular, an isolated PAD was present in 4.7% of subjects, and a similar proportion had either PAD or coronary heart disease, whilst another 1.2% had either PAD or a cerebrovascular disease. The diagnosis of coronary artery disease depends on the sensitivity of the method used. Clinical history and ECG identify a prevalence of ischaemic heart disease in 40% of patients with PAD; two thirds have positive functional tests, and more than 70% have at least one vessel with coronary angiography287. The extension of the coronary artery disease, both to the coronarography and the angio-tomography, also correlates with the ABI287. At least a quarter of patients with PAD treated with peripheral angiography are carriers of advanced coronary artery disease. In patients with suspected coronary artery disease who are candidates for coronarography, the presence of PAD makes it more likely to detect an anterior interventricular artery disease (18 vs <1%) of a multivessel disease (63 vs 11%), and any form of obstructive coronary artery disease (98 vs 81%)293. In an English prospective study (Oxfordshire)294 on patients with acute PAD manifestations, 77.2% had a previous cardiovascular disease. Conversely, patients with documented coronary artery disease have a higher probability (10% to 30%) of having PAD287. Regardless of the perspective applied (stable patient, treated with angiographic procedure, revascularisation or post-acute event), even in the most specifically represented rehabilitative and preventive contexts (as specifically demonstrated by the THINKPAD registry [Atherosclerosis of the Lower Extremities as a Linked Comorbidity in Patients Admitted for Cardiac Rehabilitation])295, the coronary artery disease-PAD association denotes a more complex patient in terms of risk profile and co-morbidity (chronic obstructive pulmonary disease, CRF, prior revascularisation). PAD associated with coronary artery disease is more serious and has a poorer prognosis296–299. In a retrospective series of patients undergoing PCI in the National Heart, Lung, and Blood Institute Dynamic Registry300, those with PAD were at high risk of cardiovascular adverse events; over time, the cumulative mortality rate decreased from 13.7% to 9.8%, that of myocardial infarction remained stable (from 9.8% to 10%), whilst that of a new revascularisation decreased from 26.8% to 17.2%. The negative prognostic impact of PAD is also evidenced by the PLATO study in post-ACS subset analyses301.
16.2 Dual antiplatelet therapy in patients with peripheral obliterative arteriopathy
Therapeutic interventions in patients with PAD are focused on cardio protection rather than on the prevention of events related to arterial vascularisation of the limbs. The benefit of antiplatelet therapy in all groups of patients with PAD has long been known302, with a reduction of about 23% of the composite endpoint of cardiovascular death, infarction and stroke, but with a cost in terms of major extracranial haemorrhagic events (RR 1.6, 95% CI 1.4-1.8)30. The most used drugs are ASA and clopidogrel; ticagrelor was as effective as a clopidogrel monotherapy303. In a Danish administrative registry304, two groups of patients with PAD were identified with and without coronary artery disease in the "real world". The use of antiplatelet agents increased from 29% to 59% from 2000 to 2007 for patients with isolated PAD, but use within 18 months from diagnosis was modest compared to patients with isolated coronary artery disease (53% vs 66%).
There is a lack of consensus on the advantages and disadvantages of DAPT in PAD. After uncovering the benefits of DAPT in coronary artery patients, it has been hypothesised that these could extend to larger populations with stable atherosclerosis. In an observational study305 of 629 patients with claudication or CLI on a 3-year follow-up, the use of DAPT was associated with a significant reduction in the incidence of adverse cardiovascular events (HR 0.65) and total mortality (HR 0.55), and non-significant in the risk of major amputations (HR 0.69). In the CHARISMA study, however, which randomised 15,603 patients with stable atherosclerotic disease or multiple risk factors to monotherapy with ASA in low doses (75-162 mg) or clopidogrel + ASA association, the latter did not fare better in reducing major cardiovascular adverse events; in the subgroup of 3,096 patients with PAD under DAPT, however, a lower frequency of myocardial infarction and hospitalisations for ischaemic events was observed (even if not of the primary composite endpoint), and an increase in minor bleeding, albeit not in major bleeding.306 In another post-hoc analysis of the PLATO study301, as regards the post-ACS subgroup of patients with PAD, ticagrelor entailed a reduction in MACE (18 vs 20.6%; HR 0.85, CI 95% 0.64-1.11) and mortality (HR 0.74, CI 95% 0.50-1.08) compared with clopidogrel, without an increase in haemorrhagic events (HR 0.81, CI 95% 0.59-1.1). A subset analysis of the PRODIGY study307 evaluated the efficacy and safety of extended DAPT for two years, compared to a short (<6 months) one in patients undergoing PCI. Extended DAPT was associated with a reduced risk of efficacy endpoints in patients with PAD (HR 0.54, 95% CI 0.31-0.95; p = 0.03) but not in those without it (HR 1.28, CI 95% 0.92-1.77; p = 0:15); the risk of stent thrombosis was also significantly lower, with no apparent increase in bleeding risk.
However, there are no modern trials defining the optimal antiplatelet therapy after peripheral revascularisation308. In current practice, long-term ASA therapy is considered sufficient after an angioplasty. In more recent trials with DES, a more protracted therapy with ASA and clopidogrel was used; in the "real-world" European registries, however, the use of clopidogrel for one to three months associated with long-term ASA was recorded. The duration and intensity of antiplatelet therapy should consider the bleeding risk and complexity of the lesion. Patients with more complex anatomy are treated with DAPT longer, but there is no evidence of the risks and benefits of this procedure308. The incremental safety/efficacy of the new P2Y12 receptor inhibitors is being studied in non-coronary interventions. Even in a recent meta-analysis of antiplatelet therapy, DAPT in a patient with PAD (49 trials out of 34,518 patients treated with ASA and with ticlopidine, clopidogrel, ticagrelor, cilostazol, picotamide and vorapaxar monotherapy or in combination with ASA)309 seems to be valid in post-revascularisation, but it is difficult to say in what kind of revascularisation; there is a lack of evidence, above all, on the coronaropathy-PAD association. A significant reduction in MACE was observed with the use of ticagrelor associated with ASA (RR 0.67, CI 95% 0.46-0.96, NNT = 66), clopidogrel (RR 0.72, CI 95% 0.58-0.91, NNT = 80) and with the clopidogrel + ASA association (RR 0.78, CI 95% 0.61-0.99, NNT = 98), which compared to ASA also reduced major amputations after revascularisation (RR 0.68, CI 95% 0.46-0.99, NNT = 94) at the expense of an increase in major bleeding (RR 1.48, CI 95% 1.05-2.1, NNH = 215). The review of Hanna310 confirms the absence of indications for DAPT in stable PAD; however, it opens some subgroups to the extended DAPT >3 months such as the superficial femoral stent, which is exposed to considerable external forces, including compression, torsion and elongation, with a consequent high degree of restenosis. Regarding duration, one to three months after angioplasty of the superficial femoral artery seem reasonable in a context of low risk and one month after iliac stenting. It is suggested that the decision to initiate DAPT should be individualised by evaluating technical parameters (type, length and risk of fracture of the stent or quality of the downstream run-off), as well as clinical ones (presence of critical ischaemia and spread of the disease)310.
In a recent analysis of the PEGASUS-TIMI 54 study311, high-risk patients with PAD, in the presence of a reduction of the RR similar to that of the global population, benefited from a greater reduction in absolute risk (4.1%, NNT = 25), with an absolute increase in TIMI bleeding above 0.12% (NNH = 834). Moreover, the 60 mg dose produced particularly favourable effects on overall and cardiovascular mortality, and the absolute risk reduction was 5.2% at three years. Overall treatment with ticagrelor reduced the risk of major adverse events in the limbs that are defined as acute ischaemia of the limb or peripheral revascularisation due to ischaemia (HR 0.65, CI 95% 0.44-0.95; p = 0.026).
16.3 Indications in the guidelines
According to the TASC II (Trans-Atlantic Inter-Society Consensus)312, all symptomatic patients with and without a history of other cardiovascular disease should receive an antiplatelet to reduce the risk of mortality and cardiovascular events (class A), but no indications for DAPT are provided. In the guidelines of the American College of Chest Physicians of 2012313, DAPT is not recommended in symptomatic stable PAD (2B). Even for patients undergoing endovascular revascularisation with angioplasty, with or without stent implantation, the recommendation is for 75-100 ASA or clopidogrel (grade 1A), with a single rather than dual therapy (grade 2C). Similarly, after peripheral arterial bypass surgery, ASA 75-100 mg or clopidogrel (grade 1A) and the use of a single antiplatelet (grade 2B) is recommended. A niche use for dual antiplatelet treatments (ASA 75-100 mg + clopidogrel) is granted for one year in popliteal-distal prosthetic grafts (grade 2C). The perspective of the ACC/AHA guidelines of 2013 is interesting314, as they seem to open up somewhat to a wider use of DAPT after adequate assessment of the thrombotic and haemorrhagic balance. The ASA + clopidogrel combination may be considered to reduce cardiovascular risk in patients with symptomatic PAD, including those with claudication or CLI, prior revascularisation or amputations with high cardiovascular risk and low risk of bleeding (class IIb). The ACC/AHA guidelines for 2016276 confirm that the efficacy of DAPT with ASA and clopidogrel in reducing the risk of ischaemic events in patients with symptomatic PAD is not well established (class IIb, level of evidence B), but combination therapy may be reasonable to reduce the risk of local events in patients with symptomatic PAD after revascularisation (class IIb, level of evidence C).
Finally, recent ESC guidelines for 2017315 indicate a more modern management of antiplatelet treatment in PAD. Whilst recognising the absence of specific trials covering the full spectrum of clinical presentations of PAD, the guidelines highlight the absence of evidence on the use of antiplatelet agents in asymptomatic patients, and on the superiority of new antiplatelet agents such as ticagrelor303, for which clopidogrel is indicated as a first-choice drug in monotherapy. Specifically, the latest European guidelines, compared to those of 2013, are largely open to DAPT, placing it in class IIa with level of evidence C in the first month after angioplasty (regardless of the type of stent used and the peripheral revascularised site); what is more, DAPT up to one year may be considered in place of monotherapy in the presence of a recent ACS and/or PCI in patients with symptomatic PAD, outcomes of angioplasty or peripheral surgical revascularisation. A particular category of patients potentially eligible for this "extended DAPT" consists of diabetics with incomplete revascularisation. In patients with an anticoagulation indication for AF, initiating a triple antithrombotic DAPT/anticoagulant therapy is generally discouraged, with the exception of cases with popliteal-distal peripheral stents or complex lesions at high risk of thrombosis.
Finally, it should be noted that the recent ESC 2017 guidelines on the subject of DAPT in the coronary patient57, regarding the patient with PAD following ACS and/or PCI, based on the PRODIGY307 and PEGASUS-TIMI 54311 trials, suggest considering a more long-term DAPT, also achievable with the use of the new antiplatelet agents with reduced dosages (e.g. ticagrelor 60 mg bid), potentially and favourably impacting not only mortality and coronary events, but also vascular events in the lower limbs affected by PAD.
16.4 Conclusions
Despite the lack of specific evidence, it seems reasonable to say that where a patient with coronary artery disease requires extended DAPT, the presence of PAD is not a limitation, as indeed is the presence of coronary artery disease in a patient with PAD where an extended DAPT is necessary. Being at high ischaemic risk, patients with coronary artery disease and PAD seem to particularly benefit from extended DAPT.
17. DURATION OF DUAL ANTIPLATELET THERAPY IN PATIENTS WITH RECURRENT ISCHAEMIC EVENTS
Patients with recurrent ischaemic events have an extra risk of recurrent ischaemic events (following the initial event) that is independent of the optimal therapeutic treatment, including myocardial revascularisation and DAPT, both during the first 12 months and following the first year.
By analysing the natural history of coronary artery disease in patients with ACS, the PROSPECT study provides some useful information to support this claim. This is a prospective study that included patients with NSTEMI-ACS or STEMI undergoing percutaneous revascularisation of the culprit lesion, integrated with intravascular ultrasound and virtual histology, for over 24 hours. After the procedure, patients were treated with hypolipidemic and antiplatelet drug therapy (clopidogrel) and clinical follow-up for about three years until the appearance of a new ischaemic event, which entailed an angiographic revaluation using the same methodology139. The follow-up analysis shows an incidence of recurrent ischaemic events equal to 20.4% at three years (on average 7%/year) with maximum expression (about 70%) in the first year and an exponential decrease thereafter. In half of the cases, the recurrent event was due to instability of the previously treated culprit lesion, and in the other half, to non-culprit coronary lesions which, although initially silent, presented a complex morphology under intra-coronary ultrasound (Figure 29).
Figure 29.
Recurrent ischaemic events in the PROSPECT study.
MACE, major adverse cardiovascular events.
Modified from Stone et al.139
There is therefore a real risk that patients with ACS develop a recurrent ischaemic event during the first year and in subsequent years, although with progressively decreasing incidence, which is not just attributable to stent pathology316, but also to the progression of coronary atherosclerotic disease. Patient populations exposed to increased risk include diabetics, those with CRF and females. In the context of ACS, diabetes is a strong predictor of recurrent short-term and long-term ischaemic events, both in patients treated with medical therapy and with PCI317. Hence the importance of the duration of DAPT for 12 months after ACS, which is enshrined in the European and American guidelines, and the search for more powerful antiplatelet drugs in the hypothesis that "poor responders" are more predisposed to such relapses.
The most recent European guidelines recommend at least 12 months of DAPT after the initial event in patients with NSTEMI, regardless of PCI execution57 and the type of stent implanted.
17.1 Recurrence events in the first year following acute coronary syndrome
The analysis methodology of large trial results on DAPT envisages the exclusion of patients from any further analysis after the first occurrence of any component of the primary endpoint. This limits any type of information on subsequent events and leaves questions unanswered regarding the efficacy or risk of continuing therapy in patients with the initial event during the study318.
Recently, many publications have shown the utility of analysing the incidence of recurring events, i.e. not only the initial occurrence of a component of the primary endpoint, but also of subsequent events in patients who survived the initial event. It should be noted that in the TRITON-TIMI 38 study, prasugrel not only reduced the composite endpoint of cardiovascular death, non-fatal myocardial infarction or non-fatal stroke, but also the incidence of recurring events among patients who survived the initial event91.
The same type of analysis on PLATO results shows that ticagrelor, compared to clopidogrel, can not only prevent the first but also subsequent cardiovascular events (Figures 30-32)319. In the PLATO study, in patients randomised for treatment with ticagrelor, there were a total of 1,057 primary endpoints vs. 1,225 in patients randomised for treatment with clopidogrel (OR 0.86, CI 95% 0.79-0.93, p = 0.003). The number of additional recurrent events was numerically lower in the ticagrelor group (189 vs 205; p = 0.40), resulting in a reduction in the risk of recurrent events and/or death of 0.80 (CI 95% 0.70-0.90; p = 0.001), and generating an NNT of 54 for the composite endpoint of cardiovascular death/myocardial infarction, without a significant increase in major bleeding and a similar benefit in the reduction of stent thrombosis320. A similar NNT value was demonstrated with a high-dose statin therapy (NNT = 59) for the composite endpoint of coronary death and myocardial infarction (atorvastatin 80 mg vs pravastatin 40 mg)321.
Figure 30.
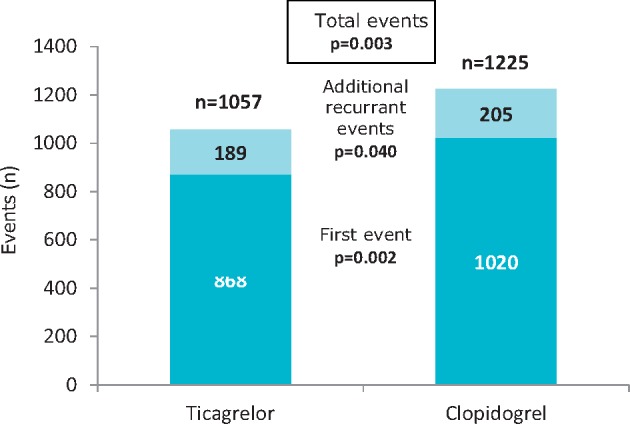
Reduction of the first event and additional recurring events at one year in the PLATO study.
Modified from Kholi et al.319
Figure 31.
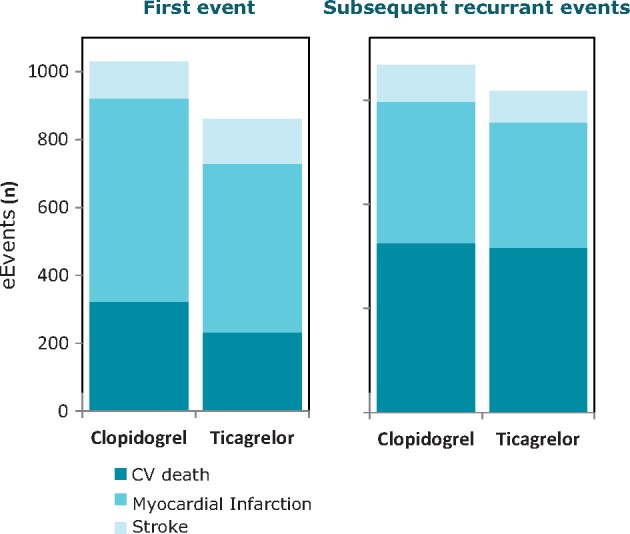
Reduction of the composite endpoint broken down according to the first event and additional one-year recurring events in the PLATO study.
CV, cardiovascular.
Modified from Kholi et al.319
Figure 32.
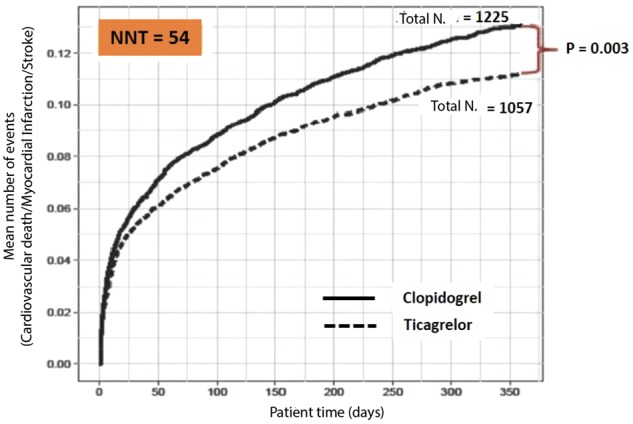
"Number needed to treat" (NNT) to reduce a recurrent event in the first year after acute coronary syndrome in the PLATO study.
CV, cardiovascular.
Modified from Kholi et al.319
Treatment with ticagrelor compared to clopidogrel results in more effective prevention with less haemorrhagic risk. The study found that two-thirds of patients who continued ticagrelor treatment after the first episode of bleeding did not experience any increase in major bleeding events (according to TIMI criteria). Finally, considering all the events, the benefit of treatment with ticagrelor in patients with ACS is even bigger than previously reported by analyses based on protocols that exclusively considered the first event319. This data has important clinical implications in guiding physicians to continue ticagrelor therapy, even in patients experiencing haemorrhagic events during treatment.
The TRITON-TIMI 38 study demonstrates how the combination of prasugrel with ASA can reduce both recurrent ischaemic events and stent thrombosis, being far superior to the clopidogrel + ASA association91,322 in that respect. However, it should be remembered that the PLATO study yields this result even for patients treated conservatively, showing a favourable efficacy and safety profile for ticagrelor when administered "upstream" (without knowledge of coronary anatomy) in patients with NSTE-ACS, even to those not undergoing percutaneous revascularisation.
17.2 Recurrence events beyond the first year following acute coronary syndrome
In recent years, several trials have been carried out to evaluate the potential benefit of extending DAPT over 12 months considering the risk of recurrent coronary and cerebrovascular ischaemic events that, as described by the post-hoc analysis of the RECLOSE 2-ACS study (Responsiveness to Clopidogrel and Stent Thrombosis 2-Acute Coronary Syndrome), is closely related to the presence of additional risk factors such as diabetes mellitus, renal failure and multivessel coronary disease323. On the other hand, the fact that haemorrhagic risk tends to increase - albeit not so evidently - precisely in the presence of the same risk factors, cannot be overlooked. Therefore, the goal would be to strike a dynamic balance between the need for extending DAPT to reduce the risk of recurrent ischaemic events without excessively increasing the bleeding risk, so that the patient enjoys a favourable net clinical benefit.
There is still no conclusive data that universally supports the continuation of DAPT beyond 12 months after an ACS in high-risk patients. In the CHARISMA study conducted on over 15,000 patients with a prior acute thrombotic event or at high risk of ischaemic events, the addition of clopidogrel versus ASA alone over a period of about 2.5 years has shown an advantage only in patients with a previous event, reporting a reduction in ischaemic events of 27% without a significant increase in fatal or severe bleeding but only in moderate ones35.
The DAPT study envisages the involvement of patients undergoing PCI with DES (of which less than 50% in a clinical setting of ACS) and randomised for extended DAPT until 30 months compared with placebo. Among the limitations of the study, we find the exclusion of randomisation for patients with high ischaemic and haemorrhagic risk (patients with new ischaemic events within the first 12 months after the initial event or a bleeding episode of at least moderate nature according to GUSTO criteria). This study does not even allow a comparison between the two thienopyridines used (clopidogrel and prasugrel), as the treatment assignment was not performed randomly10.
The multi-centre clinical study PEGASUS-TIMI 54 deserves special attention. In particular, this study involved 21,162 patients with a prior heart attack suffered at least one year after the acute event (median 1.7 years) exhibiting at least one additional risk criterion (age ≥65 years, diabetes mellitus, CRF, multivessel coronary disease, second myocardial infarction). It is important to underline that inclusion criteria included a medium to high ischaemic risk profile, whilst patients with a predisposition or bleeding history, or need for an anticoagulant therapy, were excluded. In this study, patients were randomised into three groups: ticagrelor 90 mg bid, ticagrelor 60 mg bid and placebo. Both ticagrelor doses demonstrated a significant reduction in the primary efficacy endpoint: cardiovascular death, myocardial infarction and stroke (ticagrelor 90 mg: HR 0.85, 95% CI 0.75-0.96; p = 0.008; ticagrelor 60 mg: HR 0.84, CI 95% 0.74-0.95; p = 0.004)11. It should be emphasised that periprocedural infarctions were excluded, as lacking prognostic significance, and considered only spontaneous ones.
Although a composite endpoint of net clinical benefit was not recorded, it should be noted that treatment with ticagrelor at 60 mg bid showed a better risk-benefit profile than the 90 mg bid regimen, which is compatible with a reasonable need to modulate platelet inhibition over time after an infarction event. This consideration seems to be supported by the fact that:
at the 60 mg bid dose, ticagrelor showed a reduction of cardiovascular death and stroke compared to placebo;
the authors estimated that when treating 10,000 patients with ticagrelor 60 mg bid, one may prevent 42 ischaemic events by causing 31 haemorrhagic events, while the 90 mg bid would prevent 40 ischaemic events whilst causing 41 haemorrhagic events, with a net result favourable to the lower dose by -11 events.
Both ticagrelor doses demonstrate a significant reduction in the primary efficacy endpoint (cardiovascular death, myocardial infarction, stroke), with increased risk of bleeding, but without statistically significant differences in intracranial and fatal bleeding. Ultimately, ticagrelor 60 mg bid is not worse than 90 mg bid in reducing ischaemic events and has a better safety profile. As further confirmation of the need to identify the best risk/benefit ratio in extended patient treatment, the sub-analysis of the diabetic population with previous myocardial infarction showed a significant benefit from long-term ticagrelor therapy compared to placebo, when combined with ASA (Figure 33)324. As regards haemorrhagic risk, there was an increase in major bleeding according to TIMI criteria in the ticagrelor group, albeit without significant differences in the incidence of intracranial and/or fatal bleeding. The reduction of recurrent ischaemic events included the reduction of RR of cardiovascular mortality by 22% (Figure 34), and of coronary mortality by 34% (Figure 35); this hypothesis, however, needs further confirmation by future studies.
Figure 33.
Incidence of major adverse cardiovascular events in patients with and without diabetes in the PEGASUS-TIMI 54 study.
CV, cardiovascular; HR, hazard ratio; CI, confidence interval; MI, myocardial infarction; RAR, reduction of absolute risk.
Modified from Bhatt et al.324
Figure 34.
Incidence of cardiovascular death in patients with and without diabetes in the PEGASUS-TIMI 54 study.
HR, hazard ratio; CI, confidence interval; RAR, reduction of absolute risk; RRR, reduction of relative risk.
Modified by Bonaca et al.146
Figure 35.
Incidence of coronary death in patients with and without diabetes in the PEGASUS-TIMI 54 study.
HR, hazard ratio; CI, confidence interval; RAR, reduction of absolute risk; RRR, reduction of relative risk.
Modified by Bonaca et al.146
It is noted that the earlier the interruption of a P2Y12 receptor inhibitor is identified after ACS, the higher the risk of a new recurring event. This concept would seem to be validated even after a year by an ACS event. In the context of the PEGASUS-TIMI 54 study, the benefit seems more evident in patients who do not stop therapy with ticagrelor and those who resume treatment after a brief interruption, relative to patients who discontinued therapy for over one year, even if stable from the initial event (Figure 36)146. The increase in haemorrhagic events with ticagrelor was similar regardless of the period that had elapsed since the interruption. This figure, albeit with due caution, seems to suggest a greater benefit in continuing DAPT without interruption after myocardial infarction. Further investigation is needed to clarify the risk profile of patients who, after myocardial infarction, would benefit from continued DAPT, and those where bleeding may increase with no reduction of recurrent ischaemic events.
Figure 36.
Cardiovascular death, myocardial infarction (MI) or stroke in randomised placebo patients at 90 days and at three years from the moment of interruption of the P2Y12 receptor inhibitor.
CV, cardiovascular; HR, hazard ratio; CI, confidence interval; AMI, acute myocardial infarction.
Modified by Bonaca et al.146
These results confirm, once again, the need for a secondary prevention therapy of recurrent ischaemic events, which must be conducted with a personalised approach based on the features of each patient, the risk of bleeding and the type of event in a context characterised by the availability of multiple molecules and re-perfusive strategies.
18. DUAL ANTIPLATELET THERAPY IN PATIENTS RECEIVING ORAL ANTICOAGULATION
Approximately 6-8% of patients treated with PCI have indications to take an oral anticoagulant due to the presence of AF, mechanical valve prostheses or venous thromboembolism325.
The pharmacological management of the patient undergoing coronary stent implantation that also requires OAT still presents some decisional difficulties, and exposes the patient to an increased risk of bleeding. This increase is determined by the need, at least in the first months, to maintain, where possible, a triple antithrombotic therapy, represented by two antiplatelet agents associated with the OAT. This association is in many respects indispensable because on the one hand, antiplatelet therapy cannot significantly reduce the cardioembolic risk linked to AF, and on the other the anticoagulant therapy is not sufficient to prevent the risk of stent thrombosis in the first months after stent implantation326,327.
The difficulty of balancing the risk-benefit of this triple therapy is made even more complicated by the lack of ad hoc randomised studies on the subject. With the currently available evidence, the use of prasugrel and ticagrelor in association with OAT is contraindicated; therefore, clopidogrel is the P2Y12 receptor inhibitor of choice in these patients.
There is some published data on the use of prasugrel and ticagrelor with ASA and sodium warfarin after PCI. In particular, the study of the Kastrati group showed how the association of ASA, warfarin sodium and prasugrel increases the risk of bleeding four-fold328. Similarly, the Ottawa group reported an increased risk of bleeding ranging from 8% to 11% in the ticagrelor group329. Such data could be linked to a selection bias toward patients with more complex coronary anatomy, who may have been treated with a more effective antiplatelet. Given that the risk factors that predispose a patient to widespread coronary disease are similar to those that increase the risk of bleeding (diabetes mellitus, extremely low body mass index, renal failure), which could explain the high incidences of haemorrhage; nevertheless, lacking a randomised trial on the subject, they pose a serious warning signal in this context.
As for patients with atrial fibrillation, the ESC guidelines' recommended treatment for patients with NSTE-ACS is a therapy based on bleeding risk (assessed with the HAS-BLED score) of each patient: for low risk patients (HAS-BLED 0-2), triple therapy is recommended for the first six months, continuing with OAT for six months and a single antiplatelet for an extra six months; in case of higher bleeding risk (HAS-BLED ≥3), the triple therapy is only recommended for the first four weeks, then one of the two antiplatelets for a year133. As an alternative, in patients at high risk of bleeding, dual therapy with OAT + clopidogrel is proposed immediately, based on the results of the WOEST study (What is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting) where patients treated with PCI saw significant reductions in the risk of bleeding without increasing thrombotic events330, relative to the triple therapy. At 12 months, the suspension of any antiplatelet therapy is encouraged, with the exception of patients with a high risk of coronary events; this suggestion, however, is based on the recommendations of a consensus document, and not on the results of a clinical trial23,325.
When using a direct oral anticoagulant, European guidelines recommend using the lowest effective dose in preventing strokes133. Further reductions in dosage below that approved by phase III clinical trials are not currently recommended and should be tested in ongoing studies.
Unfortunately, there is little available data on the safety and efficacy of triple therapy with new oral anticoagulants in patients with AF. In fact, the need for DAPT represented a criterion for exclusion in trials on new oral anticoagulants, except for the RE-LY trial. A post-hoc analysis of this trial showed that the triple therapy (administered in 800 patients) resulted in an increase in the bleeding RR relative to patients taking only one antiplatelet drug, and that the absolute risk of bleeding in the group treated with dabigatran 110 was lower331. It should be noted, however, that this analysis did not indicate the duration of the triple therapy, which was identified as the assumption "in any period of the study" of DAPT associated with the anticoagulant. Furthermore, the number of patients who underwent coronary angiography and/or coronary revascularisation by PCI or CABG during the study was extremely limited332,333.
Data on the association of new oral anticoagulants and antiplatelet therapy has recently been published, while other studies are still ongoing (Table 16). It should be emphasised that the primary endpoint is represented exclusively by haemorrhagic events in all studies. Therefore, we cannot have conclusive data regarding the efficacy in terms of ischaemic events, such as stent thrombosis, especially in subgroups treated since the early days with an antiplatelet and the new anticoagulant exclusively334–337.
Table 16.
Studies on the binding of new oral anticoagulants and antiplatelet therapy after percutaneous coronary intervention.
| Trial | No.of patients | Endpoint | Exclusion of patients with a previous stroke | Treatment arms |
|---|---|---|---|---|
| PIONEER AF-PCI334 | 2,124 |
|
Previous stroke/TIA |
|
| RE-DUAL PCI335 | 2,725 |
|
Stroke in the previous month |
|
| AUGUSTUS336 | 4,600 |
|
History of intracranial haemorrhage |
|
| ENTRUST-AF-PCI337 | 1,500 |
|
Ischaemic stroke in the previous 2 weeks |
|
ASA, acetylsalicylic acid; CV, cardiovascular; MI, myocardial infarction; ISTH, International Society on Thrombosis and Haemostasis; TIA, transient ischaemic attack; TIMI, Thrombolysis in Myocardial Infarction.
The recently published PIONEER AF-PCI trial (Open-Label, Randomised, Controlled, Multicentre Study Exploring Two Treatment Strategies of Rivaroxaban and a recently published Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention), involved 2,100 patients with non-valvular AF and undergoing PCI 334. The study showed that the combination of rivaroxaban with a dose of 15 mg/day and a P2Y12 receptor inhibitor for 12 months, or the combination of rivaroxaban 2.5 mg bid and DAPT for 1, 6 or 12 months, resulted in a significant reduction in bleeding events compared to standard warfarin and DAPT therapy for 1, 6 or 12 months. On the contrary, there were no significant differences in terms of ischaemic events amongst the three groups. The trial excluded patients with a previous stroke, was undersized to highlight any differences amongst the groups compared to ischaemic events, and has not provided data to date on the complexity of PCI and coronary anatomy. Despite these limitations, it is important to note that PIONEER AF-PCI represents the first randomised trial designed to evaluate the best association of antithrombotic therapy in the patient with AF, undergoing PCI, and that it demonstrated the safety of the association between new oral anticoagulants and antiplatelet therapy in this context.
The RE-DUAL PCI study (Randomised Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention)335 randomised 2,725 patients with AF undergoing PCI for warfarin treatment with a P2Y12 receptor inhibitor and ASA (1 to 3 months) (triple therapy group), or dual therapy with dabigatran (110 mg or 150 mg bid) with a P2Y12 receptor inhibitor without ASA (dual therapy groups with 110 mg or 150 mg). The study showed that the risk of bleeding was significantly higher in the triple therapy group compared to the dual therapy group. In particular, the lowest bleeding values were observed in the randomised double-therapy group treated with dabigatran 110 mg.
In view of the increased risk of bleeding in patients taking both antiplatelet therapy and anticoagulant therapy, it should be noted that some direct oral anticoagulants are available that could be used in cases of major bleeding. Idarucizumab, an antibody which specifically inhibits dabigatran, has recently been approved for cases of major bleeding complications. Idarucizumab has been shown to completely neutralise the anticoagulant effect of dabigatran within a few minutes in a cohort of patients with major bleeding or urgent surgery candidates in the RE-VERSE AD study (Reversal Effects of Idarucizumab on Active Dabigatran)338. Andexanet alfa is capable of antagonising factor Xa inhibition. In the ANNEXA-4 study (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors)339, andexanet reduced anti-factor Xa inhibition activity in patients with acute major bleeding in therapy with factor Xa inhibitors, resulting in effective haemostasis in the majority of treated patients.
19. DUAL ANTIPLATELET THERAPY IN PATIENTS AT HIGH BLEEDING RISK
As has already been pointed out, the implementation of DAPT must always consider the relationship between the risk of thrombotic events and that of major bleeding that may cause permanent damage correlating with mortality57. In the CURE study49, the first to demonstrate the superiority of DAPT compared to ASA alone in patients with ACS, the addition of clopidogrel increased bleeding by more than 38% despite the absence of a significant difference between fatal bleeding and cerebral haemorrhage. Compared to the association of ASA + clopidogrel, in the PLATO study76 ASA and ticagrelor increased major bleeding by more than 19%, while in the TRITON-TIMI 38 study85 ASA and prasugrel increased it by 31%.
Currently, there are no validated criteria for defining patients at high risk of bleeding. The 2016 ACC/AHA guidelines on DAPT125 included in this class elderly subjects, those who must take the triple antithrombotic therapy with DAPT and OAT, those with lesions or neoplasias at risk of major bleeding, those with iron deficiency anaemia and/or positive occult blood test results, thrombocytopenia, but also women and diabetics (Table 17).
Table 17.
Clinical conditions associated with a high risk of bleeding in patients with ischaemic heart disease
|
|
|
|
|
|
|
|
|
|
|
NSAIDs, nonsteroidal anti-inflammatory drugs; eGFR, estimated glomerular filtration rate.
Recently, the ESC guidelines on DAPT57 indicated that a PRECISE-DAPT score ≥25215 based on five readily available variables - haemoglobin, white blood cells, age, creatinine clearance, history of major bleeding - is the preferred manner for defining patients with a high risk of bleeding, even if the degree of recommendation is of type IIb. Therefore, as already expressed in this consensus document, it is reasonable to always associate the clinical assessment with the simple rating derived from the score.
19.1 How to reduce the risk of bleeding in high-risk patients
In general, the ESC guidelines on DAPT do not describe cases in which such therapy is contraindicated, i.e. those in which there is bleeding in progress or where the risk of major bleeding is very high. Obviously, in such cases, the implantation of a coronary stent is also contraindicated.
In order to minimise the specific risk of bleeding in DAPT candidate patients at high risk of bleeding, the recent ESC guidelines express a preference for the combination of ASA with clopidogrel, instead of ticagrelor or prasugrel, and list the following recommendations57:
use the radial vascular access instead of the femoral one (class I, level of evidence A)340,341;
associate a PPI to reduce the risk of gastric bleeding (class I, level of evidence B)63,342,343;
use ASA with ≤ 100 mg dosage (class I, level of evidence A)344,345.
Regarding DAPT duration, a minimum of one month is considered sufficiently "safe" in cases of stable patients with revascularisation (class IIb, level of evidence C). Three months applies for cases in class IIa, level of evidence B, while at least six months is required for ACS cases, whether the revascularisation took place with stent implantation (class IIa, level of evidence B) or if it was carried out surgically (class IIa, level of evidence C). Duration can also be limited to one month in patients treated with medical therapy (class IIa, level of evidence C).
Despite extensive literature on the subject, there is currently no indication on the use of platelet or genetic functional tests to activate a DAPT (class III, level of evidence A).
It is interesting to note that, compared to the 2016 ACC/AHA guidelines125, those of the 2017 ESC57 no longer consider the type of implanted stent as a useful parameter for defining the duration of a DAPT.
Patients taking triple antithrombotic therapy (DAPT + OAT) present a particular situation characterised by high haemorrhage risk. In these cases, the ESC guidelines on reducing the risk of bleeding list the following recommendations:
use ASA at the dosage of < 100 mg;
prefer clopidogrel to ticagrelor and prasugrel;
prefer an oral anticoagulant directed towards a vitamin K antagonist;
continue the therapy for the shortest possible time;
maintain the international normalised ratio (INR) at the level of the lower limits of the therapeutic range;
associate PPIs.
20. DUAL ANTIPLATELET THERAPY IN PATIENTS UNDERGOING A PREOPERATIVE CARDIOVASCULAR EVALUATION FOR NON-CARDIAC SURGERY
The number of patients with coronary stent requiring non-cardiac surgery in the first year after the revascularisation procedure has been estimated to vary between 4% and 8% and is constantly increasing346. The suspension of one or both antiplatelet agents involves, especially in the first months after the procedure, a significant risk of stent thrombosis, a potentially fatal event347–351. On the other hand, antiplatelet therapy greatly increases the risk of bleeding during surgical or endoscopic procedures352.
Antiplatelet therapy in the perioperative phase is often managed individually and not necessarily jointly between cardiologists and surgeons. Furthermore, the current guidelines on patient management for non-cardiac surgery do not provide clear operational protocols in relation to the patient's thrombotic risk and the different types of surgical interventions and refer, for the most part, to the clinical evaluation of individual cases353,354.
Recent European guidelines on the management of antiplatelet therapy in patients with ischaemic heart disease recommend a DAPT with ASA and P2Y12 receptor inhibitor over a variable period of one to 12 months based on the patient's ischaemic and haemorrhagic risk profile57. In fact, the premature suspension of this therapy has proved to be an important risk factor for stent thrombosis355 and the first cause of DAPT suspension was the need to subject the patient to surgery356.
Perioperative management of the patient with ischaemic heart disease represents a relevant problem, not only in epidemiological terms but also in prognostic terms. It is known that a perioperative AMI is associated with an in-hospital mortality of 15-25%357,358.
ASA can significantly reduce the risk of cardio-cerebrovascular events in secondary prevention359. One of the problems associated with discontinuing ASA therapy is the risk of a rebound effect30,360,361. On the other hand, surgery itself induces a hypercoagulable state362,363.
Previous studies have shown that the perioperative suspension of ASA therapy, independently of any previous coronary stent placement, is associated with a significant increase in MACE347–351.
Data on the effect of clopidogrel in association with ASA in non-stent patients is very poor and derives, mostly, from post-hoc analyses of randomised clinical trials and a number of records364.
Perioperative risk is certainly high in the cases of patients with coronary stents, especially in the first days following surgery365. One of the hypotheses that can explain the significant increase of perioperative phase MACE in patients implanted with stents could be the premature suspension of antiplatelet therapy. The risk of cardiac ischaemic events tends to decrease with increasing time between PCI and surgery366,367. It is important to highlight that patients with a stent will always carry an increased risk, both for possible complications of the stent itself, and because such patients suffer from ischaemic heart disease.
In some studies, an increase in overall MACE was observed, rather than an increase in stent thrombosis349. Other studies have reported similar rates of combined cardiac events368,369. In this regard, it is important to highlight that coronary heart disease is often multifocal370,371.
Based on data from an extensive retrospective database on a cohort of 8,116 patients (with an average age of 40 years) undergoing major non-cardiac surgery in Ontario (Canada) between 2003 and 2009 and carriers of coronary stents implanted in the preceding 10 years, the optimal minimum time to discontinue therapy before subjecting a patient to non-cardiac elective surgery was estimated to be 46-180 days after BMS implantation and over 180 days after DES implantation372.
The incremental risk of non-cardiac surgery on adverse cardiac events after stent implantation is higher in the first six months and stabilises at around 1% after six months. The greatest benefit in delaying at least six months applies to inpatients undergoing surgery, high-risk patients and patients with a DES357.
A recent analysis examined the association between perioperative cardiovascular events and the previous implantation of strata stents for the time between the procedure and non-cardiac surgery368. A sub-analysis conducted according to the type of stent (BMS or DES) indicated that the perioperative risk beyond six months could be at least partly attributable to patients with previous BMS implantation, probably related to a higher incidence of restenosis372. This latest data is in line with other recent studies and contradicts the common belief that perioperative risk is high only in the first month after implantation of a BMS. Therefore, the use of BMS does not reduce the incidence of adverse events in this time frame in patients undergoing surgery, compared to DES.
It is known that DAPT is associated with an increased risk of bleeding371,372. Unfortunately, studies on intraoperative haemorrhage risk linked to antiplatelet therapy, although numerous, often lack sufficient statistical soundness and there are few prospective randomised trials. The latter were mostly conducted in orthopaedics and cardiac surgery368.
An inter-company consent document has recently been published with the aim of standardising haemorrhagic and thrombotic risk in patients who are carriers of coronary stents and candidates for cardiac and non-cardiac surgery188,373. This document was jointly compiled by the national scientific societies of the main surgical and anaesthesiological disciplines who, together with the Italian cardiological societies (GISE and ANMCO), prepared a series of tables containing practical information on the type of antiplatelet regimen envisaged in the most important surgical procedures. The specific haemorrhage risk for each type of intervention related to the various surgical branches was defined by the specialists who participated in the drafting of the document. The definition of high, medium and low haemorrhage risk, therefore, depends on evaluations expressed by interviewed surgeons based on their experience as well as on data published in the literature, where present.
Thrombotic risk was defined on the basis of four factors:
the type of stent implanted (BMS vs DES);
the time from PCI to surgery;
the angiographic features of the lesions treated;
the clinical factors.
The recommendations provided are general indications and do not take account of particular clinical situations. Therefore, an assessment of the individual case with regard to individual ischaemic and haemorrhage risk is always recommended. However, the recent ESC guidelines on DAPT57 recommend that this therapy should not be discontinued before one month if the patient is expected to undergo non-cardiac elective surgery (class III, level of evidence B) (Figure 37). In patients who must be operated on within a few days, the suspension of clopidogrel for five days and prasugrel for seven days before surgery is recommended, whereas the interruption of ticagrelor may be limited to three days (Figure 38). The timing of the resumption of antiplatelet therapy in the postoperative period may also be deferred in cases of clinically relevant bleeding complications. Moreover, in the case of therapy with prasugrel and ticagrelor suspended in the preoperative phase, the possibility of switching to clopidogrel therapy in the postoperative period, with relative loading dose in relation to the estimated bleeding risk, should be assessed.
Figure 37.
Criteria for the interruption of dual antiplatelet therapy (DAPT) in patients undergoing percutaneous coronary intervention (PCI) and candidates for non-cardiac surgery.
ACS - Acute coronary syndrome.
124-hour site cath lab is recommended if major surgery is undertaken within six months post-PCI.
2Prevention of stent thrombosis during adequate antiplatelet therapy; stenting of the last patent coronary artery; extensive multivessel coronary disease, particularly in diabetic patients; chronic renal failure (creatinine clearance < 60 ml/min); at least 3 implanted stents; at least 3 lesions treated; bifurcation with 2 implanted stents; total length of the stent >60 mm; treatment of chronic total occlusions.
Reproduced with permission from Valgimigli et al.57
Figure 38.
Minimal interruption of the P2Y12 receptor inhibitors and postoperative recovery.
OAC, oral anticoagulation.
Reproduced with permission from Valgimigli et al.57
The results of the BRIDGE trial have recently been published (Maintenance of Platelet Inhibition with Cangrelor after Discontinuation of Thienopyridines in Patients Undergoing Surgery)374. The latter prescribed the intravenous administration of cangrelor (a new potent antiplatelet, which competitively inhibits the P2Y12 platelet receptor) as a "bridging" therapy in CABG intervention candidate patients taking thienopyridine. The trial, conducted on 210 patients, showed that subjects treated with cangrelor had significantly lower platelet reactivity values compared to the reference group treated with placebo. No increase in CABG-related bleeding was observed in the cangrelor group compared to the placebo group. On the basis of this data, although derived from an albeit limited population, we may hypothesise the use of this drug as a "bridging" therapy also in patients implanted with coronary stents and non-cardiac surgery candidates. However, ad hoc clinical studies must be undertaken to support this hypothesis.
As an alternative to cangrelor, a "bridging" therapy could be applied by using GPIIb/IIIa antagonists with a short half-life in the perioperative phase preceding interventions with high thrombotic and haemorrhage risk as a reasonable substitute for oral antiplatelet therapy. The protocol for a "bridging" therapy with cangrelor or with intravenous inhibitors of GPIIb/IIIa platelet receptors is reserved for patients at high risk of stent thrombosis, for whom the surgeon suggests the interruption of therapy with P2Y12 receptor inhibitors due to an unacceptable haemorrhage risk. The protocol prescribes the suspension of clopidogrel and ticagrelor five days before surgery (seven days of suspension of prasugrel are recommended) and replacement by an intravenous infusion of cangrelor or tirofiban or eptifibatide, starting from the third day before surgery. The infusion should be discontinued at least one hour before surgery for patients taking cangrelor and at least four hours before surgery (eight hours in patients with creatinine clearance <30 ml/min). In the postoperative period, the resumption of P2Y12 receptor inhibitor therapy is recommended on the first day, and in any case as soon as possible, with the applicable loading dosages (300 mg for clopidogrel, 60 mg for prasugrel and 180 mg for ticagrelor). Continuation of oral therapy with ASA is strongly recommended when possible.
Since the most critical period for the development of ischaemic complications is the postoperative phase, careful clinical and electrocardiographic surveillance of the patient is recommended, using serial ECGs (2-3 times per day), or better still, with continuous ECG monitoring. Indeed, post-operative antalgic therapy could alleviate anginal symptoms in the case of ACS, thus making a timely diagnosis more difficult.
21. MANAGEMENT OF PATIENTS WHO DEVELOP HAEMORRHAGES DURING DUAL ANTIPLATELET THERAPY
Bleeding during DAPT is associated with a significant risk of complications and death, particularly if it occurs during ACS, to the extent that haemorrhage events are now routinely included among the components of the main endpoint of the studies on the topic. The goal is now the "net clinical benefit", consisting of ischaemic events (usually death, reinfarction, stroke and recurrence of ischaemia) and major bleeding.
Managing haemorrhages in the course of DAPT is extremely complicated due to the conflicting requirements which arise: on the one hand, the insurgence of haemorrhaging with its risk variability as a function of extent and location may require temporary or definitive suspension or the reduction of the DAPT; on the other hand, the possible complications from withdrawal of therapy, such as stent thrombosis, could be catastrophic. When making decisions, we must then consider the "weight" of the clinical indication for DAPT in the specific context in which the case occurs. Once bleeding is resolved, it remains very difficult to decide if, how and when to return to administering DAPT.
In the absence of consistent data derived from trials on this topic, the ESC has developed a consensus paper in an attempt to define behaviour strategies375 based on the BARC classification of haemorrhages, a stratification of both ischaemic and thrombotic risk at five levels (Table 18), and suggestions on some possible strategies for managing specific frameworks.
Table 18.
Graduation of thrombotic and haemorrhagic risk
| Risk category | Thrombotic risk | Haemorrhage risk |
|---|---|---|
| Very high |
|
Non-treatable intracranial bleeding; severe extracranial bleeding not detected or not effectively treatable |
| High |
|
Major extracranial bleeding with identified origin but not effectively treatable |
| Moderate | ACS or PCI with DES 1-12 months | Effectively treated intracranial bleeding; effectively treated major extracranial bleeding with identified origin |
| Mild-moderate | SCAD (ACS > 12 months or PCI with DES) but with complex conditions (CT, bifurcations, recurring ACS) | Minor extracranial bleeding |
| Mild | SCAD (ACS > 12 months or PCI with DES) without complex conditions | Minor extracranial haemorrhage |
BVS, bioabsorbable vascular scaffold; DES, drug-eluting stent; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome; SCAD, stable coronary artery disease; CT, common trunk.
21.1 Bleeding during dual antiplatelet therapy
The scientific community has shown a heightened perception of the clinical relevance of bleeding in recent years. The incidence of bleeding in the major trials on antithrombotic drugs during the past decades stands at 1-8% at 30 days85,201,376–378, but we must bear in mind that these studies excluded patients at greatest risk of bleeding and older patients in particular, which instead make up a significant proportion of the actual population. Indeed, data from treatment records indicates that major bleeding can occur even more frequently than in trials, and that it is associated with a substantial increase in mortality risk379–384.
The impact of haemorrhage, especially if it occurs in the context of a hospitalisation for ACS, can increase the risk of death, stroke and heart attack respectively up to seven, five and three times, and can weigh on the prognosis even more than a reinfarction385.
The mechanisms through which bleeding can manifest its effects are varied and it is not clear whether it is bleeding itself that determines the increase in mortality or whether this occurs due to worsening of renal function, predisposition to ischaemia and/or as the result of platelet activation. The increase in reinfarction and stroke is partly related to the fact that bleeding and anaemia often induce a reduction or suspension of DAPT, predisposing the patient to severe ischaemic recurrences, and that transfusion, in the absence of haemodynamic compromise, increases mortality386.
It is improper to classify the risk of bleeding from an exclusively quantitative point of view, since a haemorrhage which is small in extent can have great clinical relevance in relation to its location and the possibility of recurrence (a typical example being a cerebral haemorrhage), and, vice versa, haemorrhages with a significant drop in haemoglobin can be easily controlled (e.g. by cutting wounds).
21.2 Indication for dual antiplatelet therapy
An ideal scheme for managing the intensity of DAPT and the risk of bleeding would apply a simple inverse relationship: as the risk of haemorrhage increases, antiplatelet therapy should be reduced/discontinued. However, the clinical indication for DAPT must be carefully considered, given its relative importance under various conditions. The current recommendations on the duration of the therapy are shown in Table 19.
Table 19.
Recommended duration of dual antiplatelet therapy according to indications
| Indication | Stent | Duration |
|---|---|---|
| Long-term prevention | Y/N | From 12 to 36 months |
| ACS | N | 12 months |
| ACS | Y | 12 months |
| BVS | Y | 12 months |
| Stable angina | Y | 6 months (new generation DES) |
BVS, bioresorbable vascular scaffold; DES, drug-eluting stent; ACS, acute coronary syndrome.
Prolonged treatment with DAPT for more than 12 months after a heart attack due to a high ischaemic risk profile is probably the most suitable condition for suspending a DAPT, given the related incidence of ischaemic complications. Nevertheless, this incidence is lower than other conditions, resulting in a lower risk of suspension, also for haemorrhages considered less dangerous. Thus, we may have a condition of low-risk bleeding (e.g. recurrent epistaxes) but at more than one year from the ACS or stent implantation, and in such cases suspension of the DAPT would be appropriate. Conversely, if the same epistaxes were to occur within a few days after a primary PCI, then the DAPT should be continued without hesitation.
Should DAPT be prescribed following ACS, the decision to reduce or suspend the treatment even before the standard 12-month duration will be more straightforward, provided a stent has not been implanted, compared to the case of a patient at a few weeks from a multiple stent implantation.
Finally, in elective conditions, the availability of the latest generation stents, with their very low incidence of stent thrombosis, makes it possible to reduce the duration of DAPT to three months, which is not advisable in case of a BVS implantation, given the known incidence of thrombosis387.
21.3 Haemorrhage management
Since no dedicated studies are available, we propose a DAPT management approach in the presence of bleeding which is applicable to the most common clinical situations (see Table 20). It is obviously not possible to contemplate all the possible clinical scenarios, but the intention is to establish a number of principles which will assist in the formulation of specific decisions.
Table 20.
Management scheme for dual antiplatelet therapy in the event of the most common forms of haemorrhage
| Bleeding severity | Minimal (does not require changes in therapy or medical intervention) | Mild (requires medical consultation but no intervention or hospitalisation) | Moderate (significant anaemisation without haemodynamic instability) | Severe (drop in Hb >5 g/dl without haemodynamic instability) | Life-threatening (any bleeding is potentially lethal) |
| Type of bleeding | Mild epistaxis, modest conjunctival bleeding, mild bruising | Major or recurrent epistaxis, moderate conjunctival bleeding, GI bleeding, GU or mild haemoptysis without significant anaemia | GU, GI bleeding with significant anaemia and/or need for transfusion | Bleeding of any kind with anaemia >5 g/dl, or uncontrollable GU bleeding (prostate, bladder) | Bleeding of any kind with serious anaemisation and/or haemodynamic and/or severe site instability (intracranial) |
| DAPT management | Continue DAPT | Continue the DAPT | Consider administering SAPT, preferably with P2Y12 inhibitor | Consider administering SAPT, preferably with P2Y12 inhibitor or suspension of the DAPT | Suspension of DAPT |
| Measures | Reassure the patient, explain the importance of DAPT, prevent recurrences | Treat the causes of bleeding, asses the possibility of reducing DAPT duration or reducing inhibition | Treat the cause of bleeding (interventional endoscopy), iv PPI in case of GI bleeding, assess the possibility of reducing DAPT duration or reducing inhibition | Transfuse RBCs for Hb <7-8 g/dl; surgical/endoscopic haemorrhage treatment; consider the transfusion of platelets; once bleeding has ceased, reassess the possibility of restarting DAPT/SAPT, possibly with clopidogrel as an alternative to prasugrel/ticagrelor and reassess relevant duration | RBC Transfusion, volume expansion, surgical/endoscopic/endovascular treatment, consider indefinite suspension of DAPT, consider transfer to hospital with 24-hour cath lab |
DAPT, dual antiplatelet therapy; GI, gastrointestinal; RBCs, red blood cells; GU, genitourinary; Hb, haemoglobin; PPI, proton pump inhibitors; SAPT, single antiplatelet therapy.
Society legend: ANMCO -Italian Association of Hospital Cardiologists; ANCE -Italian Association of Territorial Cardiology; ARCA-Regional Ambulatory Cardiologists Association; ATVB- Working Group on Atherosclerosis, Thrombosis and Vascular Biology; GICR-IACPR-Italian Group of Rehabilitation and Preventive Cardiology; GIEC- Italian Group of Emergency Cardiology; GISE -Italian Society of Interventional Cardiology; ITAHFA – Italian Heart Failure Association; SICP- Italian Society of Pediatric Cardiology; SICOA - Italian Society of Accredited Cardiology Hospital Care (SICOA); SIT - Italian Society of Digital Medicine & Telemedicine
In cases of trivial bleeding, DAPT should not be suspended, whatever the indication. For example, in cases of moderate bruising, mild epistaxis or mild conjunctival bleeding, treatment should continue unchanged, but patients should be reassured by stressing the importance of DAPT in relation to their specific indication. In cases of more significant haemorrhages, such as appreciable digestive or genital-urinary bleeding, in-depth causal diagnosis and treatment is essential, since several haemorrhage classes can be controlled with endoscopic or surgical interventions.
For moderate to severe bleeding, reducing or suspending the DAPT should be considered; whereas in the presence of appreciable mortality risk, it must be suspended and priority must be given to treating the haemorrhage itself.
21.4 Grades of dual antiplatelet therapy
In order to modulate the DAPT as a function of diverse requirements, we devised an intensity scale as shown in Figure 39. The maximum intensity is represented by the association of ASA with prasugrel and ticagrelor, or with clopidogrel. Downgrading from prasugrel and ticagrelor to clopidogrel is a reasonable approach given the lower incidence of bleeding in trials which compared the drugs in question76,85, even though this effect could be due more to variability in its metabolic response rather than an intrinsic lower efficacy. We could thus be faced with a case of a good "responder" to clopidogrel, for whom the degree of platelet inhibition is comparable to that of prasugrel and ticagrelor. Conversely, in the case of a "non-responder", particularly if we were to opt for a single antiplatelet strategy using clopidogrel, we would risk exposing the patient to insufficient platelet inhibition and therefore significant risk of thrombosis.
Figure 39.
Modulation of dual antiplatelet therapy (DAPT).
APT, antiplatelet therapy; ASA, aspirin; SAPT, single antiplatelet therapy.
Possible alternatives could include the combined administration of prasugrel 5 mg with ASA (in a patient whose indication would be 10 mg) or of ticagrelor at 60 mg bid; or yet again, we may consider a single antiplatelet approach with prasugrel and ticagrelor at standard dosages. It should be noted that we lack data on the safety and effectiveness of these strategies.
21.5 Resumption of dual antiplatelet therapy
At times, it may be necessary to resume DAPT after a previous suspension or reduction, albeit with possibly minor intensity or shorter duration. Factors to be reconsidered include the weight of the indication to proceed with the DAPT, the clinical relevance of the type of bleeding and its risk of relapse.
21.6 Conclusions
In the absence of randomised trials, the management of bleeding during DAPT can only be addressed on the basis of individual cases. In conditions of combined high ischaemic and haemorrhage risk, maintaining a balanced treatment regimen is a truly complex undertaking and requires careful consideration of all the factors that can help to ensure that the DAPT is managed optimally.
The proposed approach remains inevitably incomplete due to the complexity of the possible scenarios; nevertheless, it can serve as a reference for achieving the best possible therapeutic compromise.
22. SUMMARY
Dual antiplatelet therapy (DAPT) based on aspirin and a P2Y12 platelet receptor inhibitor is the cornerstone of the pharmacological treatment of patients with acute coronary syndrome (ACS) and those who have had coronary artery stenting. Long-term (>1 year) DAPT can further reduce the risk of stent thrombosis following percutaneous coronary intervention (PCI) and may limit the incidence of ischaemic events unrelated to the stent in patients with ACS. However, compared to aspirin alone, the prolonged use of aspirin and a P2Y12 receptor inhibitor is associated with an increased risk of haemorrhaging that is closely associated with adverse events such as recurrent ischaemia, repeated hospitalisations and mortality. The results of several randomised clinical trials have been published in recent years; these compared the duration of DAPT in ACS patients and post-PCI cases by evaluating the outcomes of both short-term and prolonged DAPT regimens.
Although current European guidelines provide initial framework for the individualisation of antiplatelet treatment, it is becoming increasingly complex in clinical practice to identify the ideal patient for whom treatment with DAPT can be reduced or prolonged in a safe manner. The purpose of this consensus document is to summarise the evidence related to the optimal duration of DAPT and to provide guidance for the clinician engaged in defining the antiplatelet strategy for patients undergoing PCI or for those suffering from ACS.
Keywords Dual antiplatelet therapy; Dual long-term antiplatelet therapy; Coronary heart disease; Prior myocardial infarction.
Contributor Information
ESC Scientific Document Group:
Alberto Maria Cappelletti, Gavino Casu, Giuseppe Di Pasquale, Giuseppe Di Tano, Stefano Domenicucci, Giuseppina Maura Francese, Claudio Fresco, Gian Franco Gensini, Maria Teresa La Rovere, Fabiana Lucà, Ciro Mauro, Adriano Murrone, Andrea Rubboli, Maria Giovanna Russo, Maurizio Santomauro, Corrado Tamburino, Giuseppe Tarantini, Ugo Vairo, and Guerrino Zuin
Faculty for approval of the Consensus Document:
Maurizio Giuseppe Abrignani, Marco Ambrosetti, Antonio Francesco Amico, Nadia Aspromonte, Vincenzo Aulitto, Gabriella Barile, Giacinto Calculli, Pasquale Caldarola, Roberto Caporale, Alberto Maria Cappelletti, Alessandro Carbonaro, Giancarlo Casolo, Gavino Casu, Claudio Cavallini, Emilia Chiuini, Furio Colivicchi, Leonardo De Luca, Andrea Di Lenarda, Giuseppe Di Pasquale, Giuseppe Di Tano, Stefano Domenicucci, Pompilio Faggiano, Giuseppina Maura Francese, Claudio Fresco, Domenico Gabrielli, Gian Franco Gensini, Giovanna Geraci, Loreto Gesualdo, Simona Giubilato, Michele Massimo Gulizia, Alessio Gaetano La Manna, Maria Teresa La Rovere, Fabiana Lucà, Aldo Pietro Maggioni, Alfredo Marchese, Ferdinando Maria Massari, Ciro Mauro, Alberto Menozzi, Gian Francesco Mureddu, Adriano Murrone, Giuseppe Musumeci, Federico Nardi, Patrizia Noussan, Antonio Vittorio Panno, Guido Parodi, Roberto Franco Enrico Pedretti, Gian Piero Perna, Massimo Piredda, Enrico Pusineri, Carmine Riccio, Roberta Rossini, Andrea Rubboli, Maria Giovanna Russo, F Saia, Maurizio Santomauro, Marino Scherillo, Giampaolo Scorcu, Fortunato Scotto di Uccio, Corrado Tamburino, Giuseppe Tarantini, Stefano Urbinati, Ugo Vairo, Ferdinando Varbella, Giovanni Battista Zito, and Guerrino Zuin
BIBLIOGRAPHY
- 1. EpiCentro. Il portale dell’epidemiologia per la sanità pubblica a cura del Centro Nazionale per la Prevenzione delle Malattie e la Promozione della Salute dell’Istituto Superiore di Sanità. http://www.epicentro.iss.it/focus/cardiovascolare/cardiovascolari.asp [ultimo accesso 26 marzo 2018].
- 2.Il Progetto CUORE: epidemiologia e prevenzione delle malattie cerebro e cardiovascolari. http://www.cuore.iss.it [ultimo accesso 26 marzo 2018].
- 3. Giampaoli S, Palmieri L, Donfrancesco C, Lo Noce C, Pilotto L, Vanuzzo D; Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey Research Group. Cardiovascular health in Italy. Ten-year surveillance of cardiovascular diseases and risk factors: Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey 1998-2012. Eur J Prev Cardiol 2015;22(2 Suppl):9–37. [DOI] [PubMed] [Google Scholar]
- 4. Giampaoli S, Vanuzzo D; Gruppo del Progetto Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey. La salute cardiovascolare degli italiani. Terzo Atlante Italiano delle Malattie Cardiovascolari - Edizione 2014. G Ital Cardiol 2014;15(4 Suppl 1):7S–31S. [Google Scholar]
- 5. Seccareccia F, D’Errigo P, Maraschini A, Casali G, Rosato S, Badoni G; Gruppo di Lavoro dello Studio Mattone Outcome-BYPASS. Lo studio Mattone Outcome-BYPASS: sopravvivenza a breve termine in pazienti sottoposti a intervento di bypass aortocoronarico nelle cardiochirurgie italiane. Risultati finali. G Ital Cardiol 2011;12:439–49. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT.. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012;344:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greco C, Bovenzi FM, Berti S, et al. Documento ANMCO/GICR-IACPR/GISE: L’organizzazione dell’assistenza nella fase post-acuta delle sindromi coronariche. G Ital Cardiol 2014;15(1 Suppl 1):3S–27S. [Google Scholar]
- 8. Kostis WJ, Deng Y, Pantazopoulos JS, Moreyra AE, Kostis JB; Myocardial Infarction Data Acquisition System (MIDAS14) Study Group. Trends in mortality of acute myocardial infarction after discharge from the hospital. Circ Cardiovasc Qual Outcomes 2010;3:581–9. [DOI] [PubMed] [Google Scholar]
- 9. Greco C, Rosato S, D’Errigo P, Mureddu GF, Lacorte E, Seccareccia F.. Trends in mortality and heart failure after acute myocardial infarction in Italy from 2001 to 2011. Int J Cardiol 2015;184:115–21. [DOI] [PubMed] [Google Scholar]
- 10. Mauri L, Kereiakes DJ, Yeh RW, et al. ; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonaca MP, Bhatt DL, Cohen M, et al. ; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800. [DOI] [PubMed] [Google Scholar]
- 12. World Heart Federation. Secondary cardiovascular disease prevention and control: focus on Italy. A World Heart Federation report. August 2014. www.worldheart.org
- 13. Griffo R, Ambrosetti M, Tramarin R, et al. ; ICAROS Investigators. Effective secondary prevention through cardiac rehabilitation after coronary revascularization and predictors of poor adherence to lifestyle modification and medication. Results of the ICAROS Survey. Int J Cardiol 2013;167:1390–5. [DOI] [PubMed] [Google Scholar]
- 14. Giannuzzi P, Temporelli PL, Marchioli R, et al. ; GOSPEL Investigators. Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Arch Intern Med 2008;168:2194–204. [DOI] [PubMed] [Google Scholar]
- 15. Riccio C, Gulizia MM, Colivicchi F, et al. Documento di consenso ANMCO/GICR-IACPR/SICI-GISE: La gestione clinica del paziente con cardiopatia ischemica cronica. G Ital Cardiol 2016;17:529–69. [DOI] [PubMed] [Google Scholar]
- 16. Depré C, Wijns W, Robert AM, Renkin JP, Havaux X.. Pathology of unstable plaque: correlation with the clinical severity of acute coronary syndromes. J Am Coll Cardiol 1997;30:694–702. [DOI] [PubMed] [Google Scholar]
- 17. Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 18. Kaul P, Naylor CD, Armstrong PW, Mark DB, Theroux P, Dagenais GR.. Assessment of activity status and survival according to the Canadian Cardiovascular Society angina classification. Can J Cardiol 2009;25:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ndrepepa G, Braun S, Mehilli J, et al. Prognostic value of sensitive troponin T in patients with stable and unstable angina and undetectable conventional troponin. Am Heart J 2011;161:68–75. [DOI] [PubMed] [Google Scholar]
- 20. Omland T, de Lemos JA, Sabatine MS, et al. ; Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 2009;361:2538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crea F, Andreotti F.. The unstable plaque: a broken balance. Eur Heart J 2009;30:1821–3. [DOI] [PubMed] [Google Scholar]
- 22. Cannon CP, Braunwald E.. Angina instabile e infarto miocardico senza sopraslivellamento del tratto ST In: Braunwald’s Heart Disease: A Texbook of Cardiovascular Medicine. IX ed. Traduzione italiana. Milano: Elsevier; 2012:1222–3. [Google Scholar]
- 23. Roffi M, Patrono C, Collet JP, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 24. Thygesen K, Alpert JS, Jaffe AS, et al. ; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur Herat J 2012;33:2551–67. [DOI] [PubMed] [Google Scholar]
- 25. Braunwald E, Morrow DA.. Unstable angina: is it time for a requiem? Circulation 2013;127:2452–7. [DOI] [PubMed] [Google Scholar]
- 26. Mueller C. Biomarkers and acute coronary syndromes: an update. Eur Heart J 2014;35:552–6. [DOI] [PubMed] [Google Scholar]
- 27. Reichlin T, Twerenbold R, Reiter M, et al. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med 2012;125:1205–13. [DOI] [PubMed] [Google Scholar]
- 28. Jackson SP. Arterial thrombosis – insidious, unpredictable and deadly. Nat Med 2011;17:1423–36. [DOI] [PubMed] [Google Scholar]
- 29. Rondina MT, Weyrich AS, Zimmerman GA.. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res 2013;112:1506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–39. [DOI] [PubMed] [Google Scholar]
- 32. Sudlow CL, Mason G, Maurice JB, Wedderburn CJ, Hankey GJ.. Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database Syst Rev 2009;(4):CD001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 society and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016;23:NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 34. Cattaneo M. New P2Y12 inhibitors. Circulation 2010;121:171–9. [DOI] [PubMed] [Google Scholar]
- 35. Bhatt DL, Fox KA, Hacke W, et al. ; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. [DOI] [PubMed] [Google Scholar]
- 36. Bhatt DL, Flather MD, Hacke W, et al. ; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007;49:1982–8. [DOI] [PubMed] [Google Scholar]
- 37. Udell JA, Bonaca MP, Collet JP, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J 2016;37:390–9. [DOI] [PubMed] [Google Scholar]
- 38. Davì G, Patrono C.. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–94. [DOI] [PubMed] [Google Scholar]
- 39. Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost 2009;102:248–57. [DOI] [PubMed] [Google Scholar]
- 40. Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost 2008;99:466–72. [DOI] [PubMed] [Google Scholar]
- 41. Yuhky K, Kashiwagi H, Kojima F, Kawabe J, Ushikubi F.. Roles of prostanoids in the pathogenesis on cardiovascular diseases. Int Angiol 2010;29:19–27. [PubMed] [Google Scholar]
- 42. Lindermann S, Kramer B, Daub K, Stellos K, Gawaz M, Molecular pathways used by platelets to initiate and accelerate atherogenesis. Curr Opin Lipidol 2007;18:566–73. [DOI] [PubMed] [Google Scholar]
- 43. Baigent C, Blackwell L, Collins R, et al. ; Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bowman L, Mafham M, Stevens W, et al. ; ASCEND Study Collaborative Group. ASCEND: A Study of Cardiovascular Events in Diabetes: characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am Heart J 2018;198:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Berardis G, Sacco M, Evangelista V, et al. ; ACCEPT-D Study Group. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomised study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gurbel PA, Tantry US.. Combination antithrombotic therapies. Circulation 2010;121:569–83. [DOI] [PubMed] [Google Scholar]
- 47. Lins R, Broekhuysen J, Necciari J, Derubaix X.. Pharmacokinetic profile of 14C-labeled clopidogrel. Semin Thromb Hemost 1999;25 Suppl 2:29–33. [PubMed] [Google Scholar]
- 48. Cattaneo M. Resistance to anti-platelet agents. Thromb Res 2011;127 Suppl 3:S61–3. [DOI] [PubMed] [Google Scholar]
- 49. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 50. Steinhubl SR, Berger PB, Mann JT 3rd, et al. ; CREDO Investigators (Clopidogrel for the Reduction of Events During Observation). Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288:2411–20. [DOI] [PubMed] [Google Scholar]
- 51. Mehta SR, Yusuf S, Peters RJ, et al. ; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527–33. [DOI] [PubMed] [Google Scholar]
- 52. Chen ZM, Jiang LX, Chen YP, et al. ; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607–21. [DOI] [PubMed] [Google Scholar]
- 53. Kasotakis G, Pipinos II, Lynch TG.. Current evidence and clinical implications of aspirin resistance. J Vasc Surg 2009;50:1500–10. [DOI] [PubMed] [Google Scholar]
- 54. Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM.. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908–13. [DOI] [PubMed] [Google Scholar]
- 55. Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005;46:1827–32. [DOI] [PubMed] [Google Scholar]
- 56. Steg PG, James SK, Atar D, et al. ; Task Force on the Management of ST-segment Elevation Acute Myocardial Infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. [DOI] [PubMed] [Google Scholar]
- 57. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60. [DOI] [PubMed] [Google Scholar]
- 58. Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009;301:937–44. [DOI] [PubMed] [Google Scholar]
- 59. Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009;180:713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009;101:714–9. [PubMed] [Google Scholar]
- 61. Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B.. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009;157:148.e1–5. [DOI] [PubMed] [Google Scholar]
- 62. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: analysis of two randomized trials. Lancet 2009;374:989–97. [DOI] [PubMed] [Google Scholar]
- 63. Bhatt DL, Cryer BL, Contant CF, et al. ; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–17. [DOI] [PubMed] [Google Scholar]
- 64. Douglas IJ, Evans SJ, Hingorani AD, et al. Clopidogrel and interaction with proton pump inhibitors: comparison between cohort and within person study designs. BMJ 2012;345:e4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Casula M, Cantoni A, Tragni E.. Interazione tra clopidogrel e inibitori di pompa protonica. Giornale Italiano di Farmacoeconomia e Farmacoutilizzazione 2013;5:18–30. [Google Scholar]
- 66. Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole Clopidogrel Aspirin) study. J Am Coll Cardiol 2008;51:256–60. [DOI] [PubMed] [Google Scholar]
- 67. Yano H, Tsukahara K, Morita S, et al. Influence of omeprazole and famotidine on the antiplatelet effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes: a prospective, randomized, multicenter study. Circ J 2012;76:2673–80. [DOI] [PubMed] [Google Scholar]
- 68. Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 2006;108:2244–7. [DOI] [PubMed] [Google Scholar]
- 69. Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001;409:202–7. [DOI] [PubMed] [Google Scholar]
- 70. Cardoso RN, Benjo AM, DiNicolantonio JJ, et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis. Open Heart 2015;2:e000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van Giezen JJ, Humphries RG.. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin Thromb Hemost 2005;31:195–204. [DOI] [PubMed] [Google Scholar]
- 72. Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G.. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J 2006;27:1038–47. [DOI] [PubMed] [Google Scholar]
- 73. Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007;50:1852–6. [DOI] [PubMed] [Google Scholar]
- 74. Farid NA, Payne CD, Small DS, et al. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther 2007;81:735–41. [DOI] [PubMed] [Google Scholar]
- 75. Cannon CP, Husted S, Harrington RA, et al. ; DISPERSE-2 Investigators. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007;50:1844–51. [DOI] [PubMed] [Google Scholar]
- 76. Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- 77. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- 78. Michelson AD, Frelinger AL 3rd, Braunwald E, et al. ; TRITON-TIMI 38 Investigators. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J 2009;30:1753–63. [DOI] [PubMed] [Google Scholar]
- 79. Wiviott SD, Antman EM, Winters KJ, et al. ; JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation 2005;111:3366–73. [DOI] [PubMed] [Google Scholar]
- 80. Wiviott SD, Trenk D, Frelinger AL, et al. ; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007;116:2923–32. [DOI] [PubMed] [Google Scholar]
- 81. Jakubowski JA, Winters KJ, Naganuma H, Wallentin L.. Prasugrel: a novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev 2007;25:357–74. [DOI] [PubMed] [Google Scholar]
- 82. Montalescot G, Sideris G, Cohen R, et al. Prasugrel compared with high-dose clopidogrel in acute coronary syndrome. The randomized, double-blind ACAPULCO study. Thromb Haemost 2010;103:213–23. [DOI] [PubMed] [Google Scholar]
- 83. Angiolillo DJ, Saucedo JF, DeRaad D, et al. ; SWAP Investigators. Increased platelet inhibition after switching from maintenance clopidogrel to prasugrel in patients with acute coronary syndromes: results of the SWAP (SWitching Anti Platelet) study. J Am Coll Cardiol 2010;56:1017–23. [DOI] [PubMed] [Google Scholar]
- 84. Alexopoulos D, Dimitropoulos G, Davlouros P, et al. Prasugrel overcomes high on-clopidogrel platelet reactivity post-stenting more effectively than high-dose (150-mg) clopidogrel: the importance of CYP2C19*2 genotyping. JACC Cardiovasc Interv 2011;4:403–10. [DOI] [PubMed] [Google Scholar]
- 85. Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. [DOI] [PubMed] [Google Scholar]
- 86. Hochholzer W, Wiviott SD, Antman EM, et al. Predictors of bleeding and time dependence of association of bleeding with mortality: Insights from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38 (TRITON-TIMI 38). Circulation 2011;123:2681–9. [DOI] [PubMed] [Google Scholar]
- 87. Montalescot G, Wiviott SD, Braunwald E, et al. ; TRITON-TIMI 38 Investigators. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet 2009;373:723–31. [DOI] [PubMed] [Google Scholar]
- 88. Wiviott SD, Braunwald E, Angiolillo DJ, et al. ; TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation 2008;118:1626–36. [DOI] [PubMed] [Google Scholar]
- 89. Smith PK, Goodnough LT, Levy JH, et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol 2012;60:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. O’Donoghue M, Antman EM, Braunwald E, et al. The efficacy and safety of prasugrel with and without a glycoprotein IIb/IIIa inhibitor in patients with acute coronary syndromes undergoing percutaneous intervention: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) analysis. J Am Coll Cardiol 2009;54:678–85. [DOI] [PubMed] [Google Scholar]
- 91. Murphy SA, Antman EM, Wiviott SD, et al. ; TRITON-TIMI 38 Investigators. Reduction in recurrent cardiovascular events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON-TIMI 38 trial. Eur Heart J 2008;29:2473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pride YB, Wiviott SD, Buros JL, et al. ; TIMI Study Group. Effect of prasugrel versus clopidogrel on outcomes among patients with acute coronary syndrome undergoing percutaneous coronary intervention without stent implantation: a TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet inhibitioN with prasugrel (TRITON)-Thrombolysis in Myocardial Infarction (TIMI) 38 substudy. Am Heart J 2009;158:e21–6. [DOI] [PubMed] [Google Scholar]
- 93. Roe MT, Armstrong PW, Fox KA, et al. ; TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–309. [DOI] [PubMed] [Google Scholar]
- 94. Morice MC, Serruys PW, Sousa JE, et al. ; RAVEL Study Group. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773–80. [DOI] [PubMed] [Google Scholar]
- 95. Moses JW, Leon MB, Popma JJ, et al. ; SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315–23. [DOI] [PubMed] [Google Scholar]
- 96. Schofer J, Schluter M, Gershlick AH, et al. ; E-SIRIUS Investigators. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet 2003;362:1093–9. [DOI] [PubMed] [Google Scholar]
- 97. Schampaert E, Cohen EA, Schluter M, et al. ; C-SIRIUS Investigators. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol 2004;43:1110–5. [DOI] [PubMed] [Google Scholar]
- 98. Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 2003;107:38–42. [DOI] [PubMed] [Google Scholar]
- 99. Stone GW, Ellis SG, Cox DA, et al. ; TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221–31. [DOI] [PubMed] [Google Scholar]
- 100. Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 2012;125:2873–91. [DOI] [PubMed] [Google Scholar]
- 101. Kalesan B, Pilgrim T, Heinimann K, et al. Comparison of drug-eluting stents with bare metal stents in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012;33:977–87. [DOI] [PubMed] [Google Scholar]
- 102. Urban P, Gershlick AH, Guagliumi G, et al. ; e-Cypher Investigators. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation 2006;113:1434–41. [DOI] [PubMed] [Google Scholar]
- 103. Valgimigli M, Percoco G, Malagutti P, et al. ; STRATEGY Investigators. Tirofiban and sirolimus-eluting stent vs abciximab and bare-metal stent for acute myocardial infarction: a randomized trial. JAMA 2005;293:2109–17. [DOI] [PubMed] [Google Scholar]
- 104. Kuchulakanti PK, Chu WW, Torguson R, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation 2006;113:1108–13. [DOI] [PubMed] [Google Scholar]
- 105. Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol 2005;45:995–8. [DOI] [PubMed] [Google Scholar]
- 106. Iakovou I, Ge L, Colombo A.. Contemporary stent treatment of coronary bifurcations. J Am Coll Cardiol 2005;46:1446–55. [DOI] [PubMed] [Google Scholar]
- 107. Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 2007;297:159–68. [DOI] [PubMed] [Google Scholar]
- 108. Benezet-Mazuecos J, Ibanez B, Badimon JJ.. Dual antiplatelet therapy and drug eluting stents: a marriage of convenience. Thromb J 2007;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202. [DOI] [PubMed] [Google Scholar]
- 110. Matter CM, Rozenberg I, Jaschko A, et al. Effects of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol 2006;48:286–92. [DOI] [PubMed] [Google Scholar]
- 111. Parry TJ, Brosius R, Thyagarajan R, et al. Drug-eluting stents: sirolimus and paclitaxel differentially affect cultured cells and injured arteries. Eur J Pharmacol 2005;524:19–29. [DOI] [PubMed] [Google Scholar]
- 112. Farb A, Burke AP, Kolodgie FD, Virmani R.. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 2003;108:1701–6. [DOI] [PubMed] [Google Scholar]
- 113. Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv 2013;6:1263–6. [DOI] [PubMed] [Google Scholar]
- 114. Serruys PW, Farooq V, Kalesan B, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv 2013;6:777–89. [DOI] [PubMed] [Google Scholar]
- 115. Wijns W, Steg PG, Mauri L, et al. ; PROTECT Steering Committee and Investigators. Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared with cypher sirolimus-eluting stent: 4-year results of the PROTECT randomized trial. Eur Heart J 2014;35:2812–20. [DOI] [PubMed] [Google Scholar]
- 116. Valgimigli M, Sabate M, Kaiser C, et al. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. BMJ 2014;349:g6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Valgimigli M, Borghesi M, Tebaldi M, Vranckx P, Parrinello G, Ferrari R; PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY Investigators. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY). Eur Heart J 2013;34:909–19. [DOI] [PubMed] [Google Scholar]
- 118. Valgimigli M, Tebaldi M, Borghesi M, et al. ; PRODIGY Investigators. Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (PROlonging Dual Antiplatelet Treatment After Grading stent-induced Intimal hyperplasia studY). JACC Cardiovasc Interv 2014;7:20–8. [DOI] [PubMed] [Google Scholar]
- 119. Valgimigli M, Campo G, Percoco G, et al. Randomized comparison of 6- versus 24-month clopidogrel therapy after balancing anti-intimal hyperplasia stent potency in all-comer patients undergoing percutaneous coronary intervention Design and rationale for the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia study (PRODIGY). Am Heart J 2010;160:804–11. [DOI] [PubMed] [Google Scholar]
- 120. Bonaa KH, Mannsverk J, Wiseth R, et al. ; NORSTENT Investigators. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med 2016;375:1242–52. [DOI] [PubMed] [Google Scholar]
- 121. Kereiakes DJ, Yeh RW, Massaro JM, et al. ; DAPT Study Investigators. Stent thrombosis in drug-eluting or bare-metal stents in patients receiving dual antiplatelet therapy. JACC Cardiovasc Interv 2015;8:1552–62. [DOI] [PubMed] [Google Scholar]
- 122. Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta-analysis. JACC Cardiovasc Interv 2016;9:12–24. [DOI] [PubMed] [Google Scholar]
- 123. Mukete BN, van der Heijden LC, Tandjung K, et al. Safety and efficacy of everolimus-eluting bioresorbable vascular scaffolds versus durable polymer everolimus-eluting metallic stents assessed at 1-year follow-up: a systematic review and meta-analysis of studies. Int J Cardiol 2016;221:1087–94. [DOI] [PubMed] [Google Scholar]
- 124. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619. [DOI] [PubMed] [Google Scholar]
- 125. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082–115. [DOI] [PubMed] [Google Scholar]
- 126. National Institute for Health and Care Experience (NICE). Ticagrelor for preventing atherothrombotic events after myocardial infarction. Technology appraisal guidance [TA420]. 14 December 2016. https://www.nice.org.uk/guidance/ta420 [accessed March 28, 2018].
- 127. Wilson PW, D’Agostino R Sr, Bhatt BL, et al. ; REACH Registry. An international model to predict recurrent cardiovascular disease. Am J Med 2012;125:695–703. [DOI] [PubMed] [Google Scholar]
- 128. Hjemdahl P, Eriksson SV, Held C, Forslund L, Nasman P, Rehnqvist N.. Favourable long term prognosis in stable angina pectoris: an extended follow up of the Angina Prognosis Study in Stockholm (APSIS). Heart 2006; 92:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sorensen R, Hansen ML, Abildstrom SZ, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet 2009;374:1967–74. [DOI] [PubMed] [Google Scholar]
- 130. Abraham NS, Hartman C, Richardson P, Castillo D, Street RL Jr, Naik AD.. Risk of lower and upper gastrointestinal bleeding, transfusions, and hospitalizations with complex antithrombotic therapy in elderly patients. Circulation 2013;128:1869–77. [DOI] [PubMed] [Google Scholar]
- 131. Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 2012;126:1185–93. [DOI] [PubMed] [Google Scholar]
- 132. Mrdovic I, Savic L, Krljanac G, et al. Simple risk algorithm to predict serious bleeding in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: RISK-PCI bleeding score. Circ J 2013;77:1719–27. [DOI] [PubMed] [Google Scholar]
- 133. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 134. Bonaca MP, Sabatine MS.. Antiplatelet therapy for long-term secondary prevention after myocardial infarction. JAMA Cardiol 2016;1:627–8. [DOI] [PubMed] [Google Scholar]
- 135. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 136. Bhatt DL, Eagle KA, Ohman EM, et al. ; REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–7. [DOI] [PubMed] [Google Scholar]
- 137. Fox KA, Carruthers KF, Dunbar DR, et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J 2010;31:2755–64. [DOI] [PubMed] [Google Scholar]
- 138. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M.. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163–70. [DOI] [PubMed] [Google Scholar]
- 139. Stone GW, Maehara A, Lansky AJ, et al. ; PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–35. [DOI] [PubMed] [Google Scholar]
- 140. Berger PB, Bhatt DL, Fuster V, et al. ; CHARISMA Investigators. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010;121:2575–83. [DOI] [PubMed] [Google Scholar]
- 141. Krantz MJ, Kaul S.. Secondary prevention of cardiovascular disease with vorapaxar: a new era of 3-drug antiplatelet therapy? JAMA Intern Med 2015;175:9–10. [DOI] [PubMed] [Google Scholar]
- 142. Morrow DA, Braunwald E, Bonaca MP, et al. ; TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404–13. [DOI] [PubMed] [Google Scholar]
- 143. Scirica BM, Bonaca MP, Braunwald E, et al. ; TRA 2°P-TIMI 50 Steering Committee Investigators. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet 2012;380:1317–24. [DOI] [PubMed] [Google Scholar]
- 144. Cavender MA, Scirica BM, Bonaca MP, et al. Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: findings from the thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events-TIMI 50 trial. Circulation 2015;131:1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bonaca MP, Bhatt DL, Braunwald E, et al. Design and rationale for the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. Am Heart J 2014;167:437–444.e5. [DOI] [PubMed] [Google Scholar]
- 146. Bonaca MP, Bhatt DL, Steg PG, et al. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients with prior myocardial infarction: insights from PEGASUS-TIMI 54. Eur Heart J 2016;37:1133–42. [DOI] [PubMed] [Google Scholar]
- 147. Bonaca MP, Bhatt DL, Oude Ophuis T, et al. Long-term tolerability of ticagrelor for the secondary prevention of major adverse cardiovascular events: a secondary analysis of the PEGASUS-TIMI 54 trial. JAMA Cardiol 2016;1:425–32. [DOI] [PubMed] [Google Scholar]
- 148. Bonaca MP, Storey RF, Theroux P, et al. Efficacy and safety of ticagrelor over time in patients with prior MI in PEGASUS-TIMI 54. J Am Coll Cardiol 2017;70:1368–75. [DOI] [PubMed] [Google Scholar]
- 149. Dellborg M, Bonaca MP, Storey RF, et al. Efficacy and safety with ticagrelor in patients with prior myocardial infarction in the approved European label: insights from PEGASUS-TIMI 54 [abstract]. Eur Heart J 2017;38(Suppl):794–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bueno H, Pocock S, Danchin N, et al. International patterns of dual antiplatelet therapy duration after acute coronary syndromes. Heart 2017;103:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Volpe M, Degli Esposti L, Romeo F, et al. Il ruolo dell’aderenza al trattamento farmacologico nella terapia cronica delle malattie cardiovascolari: documento intersocietario di consenso. G Ital Cardiol 2014;15(Suppl 1):3S–10S. [DOI] [PubMed] [Google Scholar]
- 152. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–7. [DOI] [PubMed] [Google Scholar]
- 153. Naderi SH, Bestwick JP, Wald DS.. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med 2012;125:882–7.e1. [DOI] [PubMed] [Google Scholar]
- 154. Sabaté E. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization, 2003. [Google Scholar]
- 155. Mohammed S, Arabi A, El-Menyar A, et al. Impact of polypharmacy on adherence to evidence-based medication in patients who underwent percutaneous coronary intervention. Curr Vasc Pharmacol. 2016;14:388–93. [DOI] [PubMed] [Google Scholar]
- 156. Yu J, Baber U, Mastoris I, et al. Sex-based differences in cessation of dual-antiplatelet therapy following percutaneous coronary intervention with stents. JACC Cardiovasc Interv 2016;9:1461–9. [DOI] [PubMed] [Google Scholar]
- 157. Pinnarelli L, Mayer F, Bauleo L, et al. Adherence to antiplatelet therapy after percutaneous coronary intervention: a population study in a region of Italy. J Cardiovasc Med 2015;16: 230–7. [DOI] [PubMed] [Google Scholar]
- 158. De Servi S, Roncella A, Reimers B.. Causes and clinical implications of premature discontinuation of dual antiplatelet therapy. Curr Opin Cardiol 2011;26(Suppl 1):S15–21. [DOI] [PubMed] [Google Scholar]
- 159. Kovacic JC, Lee P, Karajgikar R, et al. Safety of temporary and permanent suspension of antiplatelet therapy after drug eluting stent implantation in contemporary “real-world” practice. J Interv Cardiol 2012;25:482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Fennessy MM, Devon HA, Ryan C, Lopez JJ, Zerwic JJ.. Changing illness perceptions and adherence to dual antiplatelet therapy in patients with stable coronary disease. J Cardiovasc Nurs 2013;28:573–83. [DOI] [PubMed] [Google Scholar]
- 161. Czarny MJ, Nathan AS, Yeh RW, Mauri L.. Adherence to dual antiplatelet therapy after coronary stenting: a systematic review. Clin Cardiol 2014;37:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Rinfret S, Rodes-Cabau J, Baguer R. et al. Telephone contact to improve adherence to dual antiplatelet therapy after drug-eluting stent implantation. Heart 2013;99:562–9. [DOI] [PubMed] [Google Scholar]
- 163. Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013;382:1714–22. [DOI] [PubMed] [Google Scholar]
- 164. Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842–7. [DOI] [PubMed] [Google Scholar]
- 165. Maggioni AP, Rossi E, Cinconze E, et al. ; ARNO Cardiovascular Observatory. Outcomes, health costs and use of antiplatelet agents in 7082 patients admitted for an acute coronary syndrome occurring in a large community setting. Cardiovasc Drugs Ther 2013;27:333–40. [DOI] [PubMed] [Google Scholar]
- 166. Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation 2006;113:2803–9. [DOI] [PubMed] [Google Scholar]
- 167. Roy P, Bonello L, Torguson R, et al. Temporal relation between clopidogrel cessation and stent thrombosis after drug-eluting stent implantation. Am J Cardiol 2009;103:801–5. [DOI] [PubMed] [Google Scholar]
- 168. Hamm CW, Bassand JP, Agewall A, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 169. Kneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:645–81. [DOI] [PubMed] [Google Scholar]
- 170. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:485–510. [DOI] [PubMed] [Google Scholar]
- 171. De Luca L, Capranzano P, Patti G, Parodi G.. Switching of platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: review of the literature and practical considerations. Am Heart J 2016;176:44–52. [DOI] [PubMed] [Google Scholar]
- 172. Alexopoulos D, Xanthopoulou I, Deftereos S, et al. In-hospital switching of oral P2Y12 inhibitor treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention: prevalence, predictors and short-term outcome. Am Heart J 2014;167:68–76. [DOI] [PubMed] [Google Scholar]
- 173. De Luca L, Leonardi S, Cavallini C. et al. ; EYESHOT Investigators. Contemporary antithrombotic strategies in patients with acute coronary syndrome admitted to cardiac care units in Italy: The EYESHOT Study. Eur Heart J Acute Cardiovasc Care 2015;4:441–52. [DOI] [PubMed] [Google Scholar]
- 174. Danchin N, Lettino M, Zeymer U, et al. ; PIRAEUS Group. Use, patient selection and outcomes of P2Y12 receptor inhibitor treatment in patients with STEMI based on contemporary European registries. Eur Heart J Cardiovasc Pharmacother 2016;2:152–67. [DOI] [PubMed] [Google Scholar]
- 175. Zeymer U, Widimsky P, Danchin N, et al. ; PIRAEUS Group. P2Y12 receptor inhibitors in patients with non-ST-elevation acute coronary syndrome in the real world: use, patient selection, and outcomes from contemporary European registries. Eur Heart J Cardiovasc Pharmacother 2016;2:229–43. [DOI] [PubMed] [Google Scholar]
- 176. Zettler ME, Peterson ED, McCoy LA, et al. ; TRANSLATE-ACS Investigators. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: insights from the Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) observational study. Am Heart J 2017;183:62–8. [DOI] [PubMed] [Google Scholar]
- 177. Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel non-responders and responders and effect of switching therapies. The RESPOND study. Circulation 2010;121:1188–99. [DOI] [PubMed] [Google Scholar]
- 178. Caiazzo G, De Rosa S, Torella D, et al. Administration of a loading dose has no additive effect on platelet aggregation during the switch from ongoing clopidogrel treatment to ticagrelor in patients with acute coronary syndrome. Circ Cardiovasc Interv 2014;7:104–12. [DOI] [PubMed] [Google Scholar]
- 179. Rollini F, Franchi F, Angiolillo DJ.. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat Rev Cardiol 2016;13:11–27. [DOI] [PubMed] [Google Scholar]
- 180. Diodati JG, Saucedo JF, French JK, et al. Effect on platelet reactivity from a prasugrel loading dose after a clopidogrel loading dose compared with a prasugrel loading dose alone: Transferring From Clopidogrel Loading Dose to Prasugrel Loading Dose in Acute Coronary Syndrome Patients (TRIPLET): a randomized controlled trial. Circ Cardiovasc Interv 2013;6:567–74. [DOI] [PubMed] [Google Scholar]
- 181. Kerneis M, Silvain J, Abtain J, et al. Switching acute coronary syndrome patients from prasugrel to clopidogrel. JACC Cardiovasc Interv 2013;6:158–65. [DOI] [PubMed] [Google Scholar]
- 182. Angiolillo DJ, Rollini F.. Switching from prasugrel to clopidogrel: navigating in unknown waters. JACC Cardiovasc Interv 2013;6:166–8. [DOI] [PubMed] [Google Scholar]
- 183. Angiolillo DJ, Curzen N, Gurbel P, et al. Pharmacodynamic evaluation of switching from ticagrelor to prasugrel in subjects with stable coronary artery disease: results of the SWAP-2 study (Switching Anti Platelet-2). J Am Coll Cardiol 2014;63:1500–9. [DOI] [PubMed] [Google Scholar]
- 184. Parodi G, Bellandi B, Antoniucci D.. Switching from ticagrelor to prasugrel: a warning. Int J Cardiol 2014;176:1089–90. [DOI] [PubMed] [Google Scholar]
- 185. Wu H, Wang Q, Zhou J, et al. First report of stent thrombosis after a switch therapy resulting from ticagrelor-related dyspnea. Int J Cardiol 2014;176:e127–8. [DOI] [PubMed] [Google Scholar]
- 186. Patrono C, Andreotti F, Arnesen H, et al. Antiplatelet agents for the treatment and prevention of atherothrombosis. Eur Heart J 2011;32:2922–32. [DOI] [PubMed] [Google Scholar]
- 187. Caporale R, Geraci G, Gulizia MM, et al. Documento di consenso ANMCO/SIC/SICI-GISE/SICCH: Approccio clinico al pretrattamento farmacologico in pazienti candidati a procedure di rivascolarizzazione miocardica. G Ital Cardiol 2016;17:462–91. [DOI] [PubMed] [Google Scholar]
- 188. Rossini R, Musumeci G, Oltrona Visconti L, et al. ; Italian Society of Invasive Cardiology (SICI-GISE); Italian Association of Hospital Cardiologists (ANMCO); Italian Society for Cardiac Surgery (SICCH); Italian Society of Vascular and Endovascular Surgery (SICVE); Italian Association of Hospital Surgeons (ACOI); Italian Society of Surgery (SIC); Italian Society of Anaesthesia and Intensive Care Medicine (SIAARTI); Lombard Society of Surgery (SLC); Italian Society of Maxillofacial Surgery (SICMF); Italian Society of Reconstructive Plastic Surgery and Aesthetics (SICPRE); Italian Society of Thoracic Surgeons (SICT); Italian Society of Urology (SIU); Italian Society of Orthopaedics and Traumatology (SIOT); Italian Society of Periodontology (SIdP); Italian Federation of Scientific Societies of Digestive System Diseases Lombardia (FISMAD); Association of Obstetricians Gynaecologists Italian Hospital Lombardia (AOGOI); Society of Ophthalmology Lombardia (SOL). Perioperative management of antiplatelet therapy in patients with coronary stents undergoing cardiac and non-cardiac surgery: a consensus document from Italian cardiological, surgical and anaesthesiological societies. EuroIntervention 2014;10:38–46. [DOI] [PubMed] [Google Scholar]
- 189. Palmerini T, Sangiorgi D, Valgimigli M, et al. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol 2015;65:1092–102. [DOI] [PubMed] [Google Scholar]
- 190. Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014;64:2086–97. [DOI] [PubMed] [Google Scholar]
- 191. Tarantini G, Saia F, Capranzano P, et al. Documento di posizione SICI-GISE: Utilizzo di Absorb BVS nella pratica clinica. G Ital Cardiol 2016;17(10 Suppl 1):28S–44S. [DOI] [PubMed] [Google Scholar]
- 192. Montalescot G, Brieger D, Dalby AJ, Park SJ, Mehran R.. Duration of dual antiplatelet therapy after coronary stenting a review of the evidence. J Am Coll Cardiol 2015;66:832–47. [DOI] [PubMed] [Google Scholar]
- 193. Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol 2012;59:2159–64. [DOI] [PubMed] [Google Scholar]
- 194. O’Donogue ML, Bonaca MP, Magnani G, et al. The efficacy and safety of ticagrelor in women versus men with a prior myocardial infarction: insights from the PEGASUS-TIMI 54 trial [abstract]. Eur Heart J 2015;36(Suppl 1):549. [Google Scholar]
- 195. Storey RF, Angiolillo DJ, Bonaca MP, et al. Ticagrelor 60 mg twice-daily provides effective platelet inhibition in patients with prior myocardial infarction: the PEGASUS-TIMI 54 platelet function substudy [abstract]. Eur Heart J 2015;36 (Suppl1):549. [Google Scholar]
- 196. Colombo A, Hall P, Nakamura S, et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation 1995;91:1676–88. [DOI] [PubMed] [Google Scholar]
- 197. Schömig A, Neumann FJ, Kastrati A. et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996;334:1084–9. [DOI] [PubMed] [Google Scholar]
- 198. Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998;339:1665–71. [DOI] [PubMed] [Google Scholar]
- 199. Varbella F. Introduzione In: Varbella F, Musumeci G, Marchese A, Tarantini G, eds. Ottimizzazione della terapia antiaggregante nelle sindromi coronariche acute. Torino: Edizioni Minerva Medica; 2016. [Google Scholar]
- 200. Sabatine MS, Cannon CP, Gibson CM, et al. ; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179–89. [DOI] [PubMed] [Google Scholar]
- 201. Mehta SR, Bassand JP, Chrolavicius S, et al. ; CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010;363:930–42. [DOI] [PubMed] [Google Scholar]
- 202. Wiviott SD, Steg PG.. Clinical evidence for oral antiplatelet therapy in acute coronary syndromes. Lancet 2015;386:292–302. [DOI] [PubMed] [Google Scholar]
- 203. Cannon CP, Harrington RA, James S, et al. ; PLATelet inhibition and patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 2010;375:283–93. [DOI] [PubMed] [Google Scholar]
- 204. Montalescot G, Sabatine MS.. Oral dual antiplatelet therapy: what have we learnt from recent trials? Eur Heart J 2016;37:344–52. [DOI] [PubMed] [Google Scholar]
- 205. Valgimigli M, Campo G, Monti M, et al. ; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125:2015–26. [DOI] [PubMed] [Google Scholar]
- 206. Kim BK, Hong MK, Shin DH, et al. ; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012:1340–8. [DOI] [PubMed] [Google Scholar]
- 207. Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012;125:505–13. [DOI] [PubMed] [Google Scholar]
- 208. Yeh RW, Kereiakes DJ, Steg PG, et al. ; DAPT Study Investigators. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol 2015;65:2211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Yeh RW, Secemsky EA, Kereiakes DJ, et al. ; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Helft G, Steg PG, Feuvre CL, et al. ; OPTImal DUAL Antiplatelet Therapy Trial Investigators. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 2016;37:365–74. [DOI] [PubMed] [Google Scholar]
- 211. Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014;129:304–12. [DOI] [PubMed] [Google Scholar]
- 212. Collet JP, Silvain J, Barthélémy O, et al. ; ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014;384:1577–85. [DOI] [PubMed] [Google Scholar]
- 213. Giustino G, Chieffo A, Palmerini T, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol 2016;68:1851–64. [DOI] [PubMed] [Google Scholar]
- 214. Costa F, Adamo M, Ariotti S, et al. Left main or proximal left anterior descending coronary artery disease location identifies high-risk patients deriving potentially greater benefit from prolonged dual antiplatelet therapy duration. EuroIntervention 2016;11:e1222–30. [DOI] [PubMed] [Google Scholar]
- 215. Costa F, van Klaveren D, James S, et al. , PRECISE-DAPT Study Investigators Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025–34. [DOI] [PubMed] [Google Scholar]
- 216. Urban P, Meredith IT, Abizaid A, et al. ; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–47. [DOI] [PubMed] [Google Scholar]
- 217. Farooq V, Serruys PW, Bourantas C, et al. Incidence and multivariable correlates of long-term mortality in patients treated with surgical or percutaneous revascularization in the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial. Eur Heart J 2012;33:3105–13. [DOI] [PubMed] [Google Scholar]
- 218. Johnson WD, Kayser KL, Hartz AJ, et al. Aspirin use and survival after coronary bypass surgery. Am Heart J 1992;123:603–8. [DOI] [PubMed] [Google Scholar]
- 219. Chesebro JH, Clements IP, Fuster V, et al. A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med 1982;307:73–8. [DOI] [PubMed] [Google Scholar]
- 220. Chesebro JH, Fuster V, Elveback LR, et al. Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations. N Engl J Med 1984;310:209–14. [DOI] [PubMed] [Google Scholar]
- 221. Goldman S, Copeland J, Moritz T, et al. Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a Veterans Administration Cooperative Study. Circulation 1988;77:1324–32. [DOI] [PubMed] [Google Scholar]
- 222. Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004;110:1202–8. [DOI] [PubMed] [Google Scholar]
- 223. Sørensen R, Abildstrøm SZ, Hansen PR, et al. Efficacy of post-operative clopidogrel treatment in patients revascularized with coronary artery bypass grafting after myocardial infarction. J Am Coll Cardiol 2011;57:1202–9. [DOI] [PubMed] [Google Scholar]
- 224. Ebrahimi R, Bakaeen FG, Uberoi A, et al. Effect of clopidogrel use post coronary artery bypass surgery on graft patency. Ann Thorac Surg 2014;97:15–21. [DOI] [PubMed] [Google Scholar]
- 225. Kulik A, Le May MR, Voisine P, et al. Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the Clopidogrel After Surgery for Coronary Artery Disease (CASCADE) Trial. Circulation 2010;122:2680–7. [DOI] [PubMed] [Google Scholar]
- 226. Gao C, Ren C, Li D, et al. Clopidogrel and aspirin versus clopidogrel alone on graft patency after coronary artery bypass grafting. Ann Thorac Surg 2009;88:59–62. [DOI] [PubMed] [Google Scholar]
- 227. Sun JC, Teoh KH, Lamy A, et al. Randomized trial of aspirin and clopidogrel versus aspirin alone for the prevention of coronary artery bypass graft occlusion: the Preoperative Aspirin and Postoperative Antiplatelets in Coronary Artery Bypass Grafting study. Am Heart J 2010;160:1178–84. [DOI] [PubMed] [Google Scholar]
- 228. Gao G, Zheng Z, Pi Y, et al. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery: a single-center, randomized, controlled trial. J Am Coll Cardiol 2010;56:1639–43. [DOI] [PubMed] [Google Scholar]
- 229. Deo SV, Dunlay SM, Shah IK, et al. Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J Card Surg 2013;28:109–16. [DOI] [PubMed] [Google Scholar]
- 230. Nocerino AG, Achenbach S, Taylor AJ.. Meta-analysis of effect of single versus dual antiplatelet therapy on early patency of bypass conduits after coronary artery bypass grafting. Am J Cardiol 2013;112:1576–9. [DOI] [PubMed] [Google Scholar]
- 231. de Leon N, Jackevicius CA.. Use of aspirin and clopidogrel after coronary artery bypass graft surgery. Ann Pharmacother 2012;46:678–87. [DOI] [PubMed] [Google Scholar]
- 232. Ibrahim K, Tjomsland O, Halvorsen D, et al. Effect of clopidogrel on midterm graft patency following off-pump coronary revascularization surgery. Heart Surg Forum 2006;9:E581–856. [DOI] [PubMed] [Google Scholar]
- 233. Mannacio VA, Di Tommaso L, Antignan A, De Amicis V, Vosa C.. Aspirin plus clopidogrel for optimal platelet inhibition following off-pump coronary artery bypass surgery: results from the CRYSSA (prevention of Coronary arteRY bypaSS occlusion After off-pump procedures) randomised study. Heart 2012;98:1710–5. [DOI] [PubMed] [Google Scholar]
- 234. Gurbuz AT, Zia AA, Vuran AC, Cui H, Aytac A.. Postoperative clopidogrel improves mid-term outcome after off-pump coronary artery bypass graft surgery: a prospective study. Eur J Cardiothorac Surg 2006;29:190–5. [DOI] [PubMed] [Google Scholar]
- 235. Held C, Asenblad N, Bassand JP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol 2011;57:672–84. [DOI] [PubMed] [Google Scholar]
- 236. De Luca L, Leonardi S, Smecca IM, et al. ; EYESHOT Investigators. Contemporary antithrombotic strategies in patients with acute coronary syndromes managed without revascularization: insights from the EYESHOT study. Eur Heart J Cardiovasc Pharmacother 2015;1:168–78. [DOI] [PubMed] [Google Scholar]
- 237. Boden WE, Lansky A, Angiolillo DJ.. Refining the role of antiplatelet therapy in medically managed patients with acute coronary syndrome. Am J Cardiol 2013;111:439–44. [DOI] [PubMed] [Google Scholar]
- 238. James SK, Roe MT, Cannon CP, et al. ; PLATO Study Group. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ 2011;342:d3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Menozzi A, De Luca L, Olivari Z, et al. I pazienti con sindrome coronarica acuta senza sopraslivellamento persistente del tratto ST non sottoposti a rivascolarizzazione coronarica: una popolazione sottotrattata G Ital Cardiol 2016;17:816–26. [DOI] [PubMed] [Google Scholar]
- 240. Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Olivari Z, Steffenino G, Savonitto S, et al. The management of acute myocardial infarction in the cardiological intensive care units in Italy: the ‘BLITZ 4 Qualità’ campaign for performance measurement and quality improvement. Eur Heart J Acute Cardiovasc Care 2012;1:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Capodanno D, Angiolillo DJ.. Antithrombotic therapy in the elderly. J Am Coll Cardiol 2010;56:1683–92. [DOI] [PubMed] [Google Scholar]
- 243. Andreotti F, Rocca B, Husted S, et al. ; ESC Thrombosis Working Group. Antithrombotic therapy in the elderly: expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2015;36:3238–49. [DOI] [PubMed] [Google Scholar]
- 244. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 2010;56:2051–66. [DOI] [PubMed] [Google Scholar]
- 245. Silvain J, Cayla G, Hulot JS, et al. High on-thienopyridine platelet reactivity in elderly coronary patients: the SENIOR-PLATELET study. Eur Heart J 2012;33:1241–9. [DOI] [PubMed] [Google Scholar]
- 246. Tarantini G, Berti S, De Luca L, et al. Documento di posizione della Società Italiana di Cardiologia Interventistica (SICI-GISE): terapia antitrombotica nel paziente anziano con sindrome coronarica acuta. G Ital Cardiol 2016;17:64–79. [DOI] [PubMed] [Google Scholar]
- 247. Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003;24:1815–23. [DOI] [PubMed] [Google Scholar]
- 248. Spencer FA, Moscucci M, Granger CB, et al. ; GRACE Investigators. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation 2007;116:2793–801. [DOI] [PubMed] [Google Scholar]
- 249. Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009;119:1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010;55:2556–66. [DOI] [PubMed] [Google Scholar]
- 251. Gurbel PA, Erlinge D, Ohman EM, et al. ; TRILOGY ACS Platelet Function Substudy Investigators. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA 2012;308:1785–94. [DOI] [PubMed] [Google Scholar]
- 252. Erlinge D, Gurbel PA, James S, et al. Prasugrel 5 mg in the very elderly attenuates platelet inhibition but maintains noninferiority to prasugrel 10 mg in nonelderly patients: the GENERATIONS trial, a pharmacodynamic and pharmacokinetic study in stable coronary artery disease patients. J Am Coll Cardiol 2013;62:577–83. [DOI] [PubMed] [Google Scholar]
- 253. Husted S, James S, Becker RC, et al. ; PLATO Study Group. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the Prospective Randomized PLATelet Inhibition and Patient Outcomes (PLATO) Trial. Circ Cardiovasc Qual Outcomes 2012;5:680–8. [DOI] [PubMed] [Google Scholar]
- 254. Capodanno D, Capranzano P, Buccheri S, Tamburino C.. Risk stratification for secondary prevention with ticagrelor and aspirin: a closer look to patient subsets from the PEGASUS-TIMI 54 trial. Int J Cardiol 2015;201:276–8. [DOI] [PubMed] [Google Scholar]
- 255. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW.. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011;10:104–14. [DOI] [PubMed] [Google Scholar]
- 257. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Gobbens RJ1, Luijkx KG, Wijnen-Sponselee MT, Schols JM.. In search of an integral conceptual definition of frailty: opinions of experts. J Am Med Dir Assoc 2010;11:338–43. [DOI] [PubMed] [Google Scholar]
- 259. Kamaruzzaman S, Ploubidis GB, Fletcher A, Ebrahim S.. A reliable measure of frailty for a community dwelling older population. Health Qual Life Outcomes 2010;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. White HD, Westerhout CM, Alexander KP, et al. ; TRILOGY ACS Investigators. Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care 2016;5:231–42. [DOI] [PubMed] [Google Scholar]
- 261. De Luca L, Bolognese L, Valgimigli M, et al. ; Gruppo di Lavoro dell’Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO); Società Italiana di Cardiologia Invasiva (SICI-GISE). Documento ANMCO/SICI-GISE sulla terapia antiaggregante nelle sindromi coronariche acute. G Ital Cardiol 2013;14:839–66. [DOI] [PubMed] [Google Scholar]
- 262. Alexander KP, Peterson ED.. Minimizing the risks of anticoagulants and platelet inhibitors. Circulation 2010;121:1960–70. [DOI] [PubMed] [Google Scholar]
- 263. Steg PG, Huber K, Andreotti F, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J 2011;32:1854–64. [DOI] [PubMed] [Google Scholar]
- 264. Mureddu GF,, Rizzello V,, Boccanelli A.. La terapia alla dimissione tra standardizzazione e personalizzazione. G Ital Cardiol 2008;9(7 Suppl 1):31S–42S. [Google Scholar]
- 265. Rossini R, Cimino A, De Servi S, et al. ; Sezione Lombarda dell’Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO), della Società Italiana di Cardiologia Invasiva (GISE), dell’Associazione Medici Diabetologi (AMD). Gestione multidisciplinare del paziente con sindrome coronarica acuta e diabete mellito: dalla terapia antitrombotica al trattamento dell’iperglicemia. G Ital Cardiol 2014;15:378–92. [DOI] [PubMed] [Google Scholar]
- 266. James S, Angiolillo DJ, Cornel JH, et al. ; PLATO Study Group. Ticagrelor vs clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2010;31:3006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035–87. [DOI] [PubMed] [Google Scholar]
- 268. Marenzi G, Cabiati A, Assanelli E.. Chronic kidney disease in acute coronary syndromes. World J Nephrol 2012;1:134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med 2002;137:563–70. [DOI] [PubMed] [Google Scholar]
- 270. Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB.. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 2002;137:555–62. [DOI] [PubMed] [Google Scholar]
- 271. Fox CS, Muntner P, Chen AY, et al. ; Acute Coronary Treatment and Intervention Outcomes Network Registry. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 2010;121:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Gibson CM, Dumaine RL, Gelfand EV, et al. ; TIMI Study Group. Association of glomerular filtration rate on presentation with subsequent mortality in non-ST-segment elevation acute coronary syndrome; observations in 13,307 patients in five TIMI trials. Eur Heart J 2004;25:1998–2005. [DOI] [PubMed] [Google Scholar]
- 273. Szummer K, Lundman P, Jacobson SH, et al. ; SWEDEHEART. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation 2009;120:851–8. [DOI] [PubMed] [Google Scholar]
- 274. James S, Budaj A, Aylward P, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2010;122:1056–67. [DOI] [PubMed] [Google Scholar]
- 275. Magnani G, Storey RF, Steg G, et al. Efficacy and safety of ticagrelor for long-term secondary prevention of atherothrombotic events in relation to renal function: insights from the PEGASUS-TIMI 54 trial. Eur Heart J 2016;37:400–8. [DOI] [PubMed] [Google Scholar]
- 276. Gerhard-Herman MD, Gornik HL, Barrett C,. et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;69:e71–126. [DOI] [PubMed] [Google Scholar]
- 277. Kullo IJ, Rooke TW.. Peripheral artery disease. N Engl J Med 2016;374:861–71. [DOI] [PubMed] [Google Scholar]
- 278. Brevetti G, Oliva G, Silvestro A, Scopacasa F, Chiariello M; Peripheral Arteriopathy and Cardiovascular Events (PACE) Study Group. Prevalence, risk factors and cardio-vascular comorbidity of symptomatic peripheral arterial disease in Italy. Atherosclerosis 2004;175:131–8. [DOI] [PubMed] [Google Scholar]
- 279. Peach G, Griffin M, Jones KG, Thompson MM, Hinchliffe RJ.. Diagnosis and management of peripheral arterial disease. BMJ 2012;345:e5208. [DOI] [PubMed] [Google Scholar]
- 280. Pande RL, Perlstein TS, Beckman JA, Creager MA.. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Malyar N, Furstenberg T, Wellmann J, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J 2013;34:2706–14. [DOI] [PubMed] [Google Scholar]
- 282. Novo S, Avellone G, Di Garbo V, et al. prevalence of risk factors in patients with peripheral arterial disease: a clinical and epidemiological evaluation. Int Angiol 1992;11:218–29. [PubMed] [Google Scholar]
- 283. Peinetti F, Bellandi G, Cappelli A, et al. Linee guida SICVE. Patologia ostruttiva cronica aorto-iliaca e delle arterie degli arti inferiori. Ital J Vasc Endovasc Surg 2015;22(3 Suppl 2):25–68. [Google Scholar]
- 284. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 285. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26:3333–41. [DOI] [PubMed] [Google Scholar]
- 286. Bhatt DL, Steg PG, Ohman EM, et al. ; REACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–9. [DOI] [PubMed] [Google Scholar]
- 287. Gallino A, Aboyans V, Diehm C, et al. ; European Society of Cardiology Working Group on Peripheral Circulation. Non-coronary atherosclerosis. Eur Heart J 2014;35:1112–9. [DOI] [PubMed] [Google Scholar]
- 288. Steg PG, Bhatt DL, Wilson PW, et al. ; REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197–206. [DOI] [PubMed] [Google Scholar]
- 289. Meves SH, Diehm C, Berger K, et al. ; getABI Study Group. Peripheral arterial disease as an independent predictor for excess stroke morbidity and mortality in primary-care patients: 5-year results of the getABI study. Cerebrovasc Dis 2010;29:546–54. [DOI] [PubMed] [Google Scholar]
- 290. Mueller T, Hinterreiter F, Poelz W, Haltmayer M, Dieplinger B.. Mortality rates at 10 years are higher in diabetic than in non-diabetic patients with chronic lower extremity peripheral arterial disease. Vasc Med 2016;21:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Reinecke H, Unrath M, Freisinger E, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J 2015;36:932–8. [DOI] [PubMed] [Google Scholar]
- 292. Teraa M, Conte MS, Moll FC, Verhaar MC.. Critical limb ischemia: current trends and future directions. J Am Coll Cardiol 2016;5:e002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Sukhija R, Yalamanchili K, Aronow WS.. Prevalence of left main coronary artery disease, of three- or four-vessel coronary artery disease, and of obstructive coronary artery disease in patients with and without peripheral arterial disease undergoing coronary angiography for suspected coronary artery disease. Am J Cardiol 2003;92:304–5. [DOI] [PubMed] [Google Scholar]
- 294. Howard DPJ, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell DM; Oxford Vascular Study. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events. Implications for prevention. Circulation 2015;132:1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Ambrosetti M, Temporelli PL, Faggiano P, et al. ; THINKPAD Investigators. Lower extremities peripheral arterial disease among patients admitted to cardiac rehabilitation: the THINKPAD registry. Int J Cardiol 2014;171:192–8. [DOI] [PubMed] [Google Scholar]
- 296. Hussein AA, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol 2011;57:1220–5. [DOI] [PubMed] [Google Scholar]
- 297. Inglis SC, Bebchuk J, Al-Suhaim SA, et al. Peripheral artery disease and outcomes after myocardial infarction: an individual-patient meta-analysis of 28,771 patients in CAPRICORN, EPHESUS, OPTIMAAL and VALIANT. Int J Cardiol 2013;168:1094–101. [DOI] [PubMed] [Google Scholar]
- 298. Welten GM, Schouten O, Hoeks SE, et al. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol 2008;51:1588–96. [DOI] [PubMed] [Google Scholar]
- 299. Paraskevas KI, Mukherjee D, Whayne TF.. Peripheral arterial disease: implications beyond the peripheral circulation. Angiology 2013;64:569–71. [DOI] [PubMed] [Google Scholar]
- 300. Parikh SV, Saya S, Divanji P, et al. Risk of death and myocardial infarction in patients with peripheral arterial disease undergoing percutaneous coronary intervention (from the National Heart, Lung and Blood Institute Dynamic Registry). Am J Cardiol 2011;107:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Patel MR, Becker RC, Wojdyla DM, et al. Cardiovascular events in acute coronary syndrome patients with peripheral arterial disease treated with ticagrelor compared with clopidogrel: data from the PLATO Trial. Eur J Prev Cardiol 2015;22:734–42. [DOI] [PubMed] [Google Scholar]
- 302. Bonaca MP, Creager MA.. Pharmacological treatment and current management of peripheral artery disease. Circ Res 2015;116:1579–98. [DOI] [PubMed] [Google Scholar]
- 303. Hiatt WR, Fowkes FG, Heizer G, et al. ; EUCLID Trial Steering Committee and Investigators. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376:32–40 [DOI] [PubMed] [Google Scholar]
- 304. Subherwal S, Patel MR, Kober L, et al. Missed opportunities. Despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation 2012;126:1345–54. [DOI] [PubMed] [Google Scholar]
- 305. Armstrong EJ, Anderson DR, Yeo KK, et al. Association of dual-antiplatelet therapy with reduced major adverse cardiovascular events in patients with symptomatic peripheral arterial disease. J Vasc Surg 2015;62:157–65. [DOI] [PubMed] [Google Scholar]
- 306. Cacoub PP, Bhatt DL, Steg PG, Topol EJ, Creager MA; CHARISMA Investigators. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J 2009;30:192–201. [DOI] [PubMed] [Google Scholar]
- 307. Franzone A, Piccolo R, Gargiulo G, et al. Prolonged vs short duration of dual antiplatelet therapy after percutaneous coronary intervention in patients with or without peripheral arterial disease: a subgroup analysis of the PRODIGY randomized clinical trial. JAMA Cardiol 2016;1:795–803. [DOI] [PubMed] [Google Scholar]
- 308. Sobieszczyk p, Eisenhauer A.. Management of patients after endovascular interventions for peripheral artery disease. Circulation 2013;128:749–57. [DOI] [PubMed] [Google Scholar]
- 309. Katsanos K, Spiliopoulos S, Saha P, et al. Comparative efficacy and safety of different antiplatelet agents for prevention of major cardiovascular events and leg amputations in patients with peripheral arterial disease: a systematic review and network meta-Analysis. PLoS One 2015;10:e0135692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Hanna EB. Dual antiplatelet therapy in peripheral arterial disease and after peripheral percutaneous revascularization. J Invasive Cardiol 2012;24:679–84. [PubMed] [Google Scholar]
- 311. Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol 2016;67:2719–28. [DOI] [PubMed] [Google Scholar]
- 312. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45 SupplS:S5–67. [DOI] [PubMed] [Google Scholar]
- 313. Alonso-Coello P, Bellmunt S, McGorrian C, et al. Antithrombotic therapy in peripheral artery disease. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl):e669S–e690S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Anderson JL, Halperin JL, Albert NM, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:1425–43. [DOI] [PubMed] [Google Scholar]
- 315. Aboyans V, Ricco JB, Bartelink. et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO). The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 316. Tada T, Byrne RA, Simunovic I, et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18 334 patients. JACC Cardiovasc Interv 2013;6:1267–74. [DOI] [PubMed] [Google Scholar]
- 317. Cavender MA, Steg PG, Smith SC Jr, et al. ; REACH Registry Investigators. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015;132:923–31. [DOI] [PubMed] [Google Scholar]
- 318. Luscher TF. The search for optimal dual antiplatelet therapy after PCI: fine-tuning of initiation and duration. Eur Heart J 2016;37:319–21. [DOI] [PubMed] [Google Scholar]
- 319. Kohli P, Wallentin L, Reyes E, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation 2013;127:673–80. [DOI] [PubMed] [Google Scholar]
- 320. Steg PG, Harrington RA, Emanuelsson H, et al. ; PLATO Study Group. Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective randomized PLATO trial. Circulation 2013;128:1055–65. [DOI] [PubMed] [Google Scholar]
- 321. Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. [DOI] [PubMed] [Google Scholar]
- 322. Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008;371:1353–63. [DOI] [PubMed] [Google Scholar]
- 323. Valenti R, Marcucci R, Capodanno D, et al. Residual platelet reactivity to predict long-term clinical outcomes after clopidogrel loading in patients with acute coronary syndromes: comparison of different cutoff values by light transmission aggregometry from the responsiveness to clopidogrel and stent thrombosis 2-acute coronary syndrome (RECLOSE 2-ACS) study. J Thromb Thrombolysis 2015;40:76–82. [DOI] [PubMed] [Google Scholar]
- 324. Bhatt DL, Bonaca MP, Bansilal S, et al. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol 2016;67:2732–40. [DOI] [PubMed] [Google Scholar]
- 325. Lip GY, Windecker S, Huber K, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J 2014;35:3155–79. [DOI] [PubMed] [Google Scholar]
- 326. Connolly S, Pogue J, Hart R, et al. ; ACTIVE Writing Group of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12. [DOI] [PubMed] [Google Scholar]
- 327. Schömig A, Neumann FJ, Walter H, et al. Coronary stent placement in patients with acute myocardial infarction: comparison of clinical and angiographic outcome after randomization to antiplatelet or anticoagulant therapy. J Am Coll Cardiol 1997;29:28–34. [DOI] [PubMed] [Google Scholar]
- 328. Sarafoff N, Martischnig A, Wealer J, et al. Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug-eluting stent implantation and an indication for oral anticoagulation. J Am Coll Cardiol 2013;61:2060–6. [DOI] [PubMed] [Google Scholar]
- 329. Fu A, Singh K, Abunassar J, et al. ; CAPITAL Investigators. Ticagrelor in triple antithrombotic therapy: predictors of ischemic and bleeding complications. Clin Cardiol 2016;39:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Dewilde WJ, Oirbans T, Verheugt FW, et al. ; WOEST Study Investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107–15. [DOI] [PubMed] [Google Scholar]
- 331. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013;127:634–40. [DOI] [PubMed] [Google Scholar]
- 332. Healey JS, Eikelboom J, Douketis J, et al. ; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012;126:343–8. [DOI] [PubMed] [Google Scholar]
- 333. Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 2012;125:669–76. [DOI] [PubMed] [Google Scholar]
- 334. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–34. [DOI] [PubMed] [Google Scholar]
- 335. Cannon CP, Bhatt DL, Oldgren J, et al. ; RE-DUAL PCI Steering Committee and Investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–24. [DOI] [PubMed] [Google Scholar]
- 336.A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis (Blood Clots) Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart. ClinicalTrials.gov Identifier: NCT02415400. https://clinicaltrials.gov/ct2/show/NCT02415400 [accessed April 4, 2018].
- 337.Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI). ClinicalTrials.gov Identifier: NCT02866175. https://clinicaltrials.gov/ct2/show/NCT02866175 [accessed April 4, 2018].
- 338. Pollack CV, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511–20. [DOI] [PubMed] [Google Scholar]
- 339. Connolly SJ, Milling TJ Jr, Eikelboom JW, et al. ; ANNEXA-4 Investigators. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med 2016;375:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Valgimigli M, Gagnor A, Calabrò P, et al. ; MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015;385:2465–76. [DOI] [PubMed] [Google Scholar]
- 341. Ferrante G, Rao SV, Juni P, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv 2016;9:1419–34. [DOI] [PubMed] [Google Scholar]
- 342. Agewall S, Cattaneo M, Collet JP, et al. ; ESC Working Group on Cardiovascular Pharmacology and Drug Therapy and ESC Working Group on Thrombosis. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J 2013;34:1708–13. [DOI] [PubMed] [Google Scholar]
- 343. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J 2016;174:95–102. [DOI] [PubMed] [Google Scholar]
- 344. Steinhubl SR, Bhatt DL, Brennan DM, et al. ; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med 2009;150:379–86. [DOI] [PubMed] [Google Scholar]
- 345. Mehta SR, Tanguay JF, Eikelboom JW, et al. ; CURRENT-OASIS 7 Trial Investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 2010;376:1233–43. [DOI] [PubMed] [Google Scholar]
- 346. Savonitto S, Caracciolo M, Cattaneo M, De Servi S.. Management of patients with recently implanted coronary stents on dual antiplatelet therapy who need to undergo major surgery. J Thromb Haemost 2011;9:2133–42. [DOI] [PubMed] [Google Scholar]
- 347. Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011;97:1566–72. [DOI] [PubMed] [Google Scholar]
- 348. Schouten O, van Domburg RT, Bax JJ, et al. Noncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac events. J Am Coll Cardiol 2007;49:122–4. [DOI] [PubMed] [Google Scholar]
- 349. Anwaruddin S, Askari AT, Saudye H, et al. Characterization of post-operative risk associated with prior drug-eluting stent use. JACC Cardiovasc Interv 2009;2:542–9. [DOI] [PubMed] [Google Scholar]
- 350. Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM.. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013;310:1462–72. [DOI] [PubMed] [Google Scholar]
- 351. Rossini R, Musumeci G, Capodanno D, et al. Perioperative management of oral antiplatelet therapy and clinical outcomes in coronary stent patients undergoing surgery. Results of a multicentre registry. Thromb Haemost. 2015;113:272–82. [DOI] [PubMed] [Google Scholar]
- 352. Devereaux PJ, Mrkobrada M, Sessler DI, et al. ; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014;370:1494–503. [DOI] [PubMed] [Google Scholar]
- 353. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383–431. [DOI] [PubMed] [Google Scholar]
- 354. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:e77–137. [DOI] [PubMed] [Google Scholar]
- 355. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126–30. [DOI] [PubMed] [Google Scholar]
- 356. Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011;107:186–94. [DOI] [PubMed] [Google Scholar]
- 357. Holcomb CN, Graham LA, Richman JS, et al. The incremental risk of noncardiac surgery on adverse cardiac events following coronary stenting. J Am Coll Cardiol 2014;64:2730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Kumar R, McKinney WP, Raj G, et al. Adverse cardiac events after surgery: assessing risk in a veteran population. J Gen Intern Med 2001;16:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW.. Myocardial infarction after noncardiac surgery. Anesthesiology 1998;88:572–8. [DOI] [PubMed] [Google Scholar]
- 360. Beving H, Zhao C, Albage A, Ivert T.. Abnormally high platelet activity after discontinuation of acetylsalicylic acid treatment. Blood Coagul Fibrinolysis 1996;7:80–4. [DOI] [PubMed] [Google Scholar]
- 361. Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and metaanalysis on the hazards of discontinuing or not adhering to aspirin among 50 279 patients at risk for coronary artery disease. Eur Heart J 2006;27:2667–74. [DOI] [PubMed] [Google Scholar]
- 362. Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004;93:9–20. [DOI] [PubMed] [Google Scholar]
- 363. Dahl OE. Mechanisms of hypercoagulability. Thromb Haemost 1999;82:902–6. [PubMed] [Google Scholar]
- 364. Levy JH, Tanaka KA.. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 2003;75:S715–20. [DOI] [PubMed] [Google Scholar]
- 365. Firanescu CE, Martens EJ, Schonberger JP, Soliman Hamad MA, van Straten AH.. Postoperative blood loss in patients undergoing coronary artery bypass surgery after preoperative treatment with clopidogrel. A prospective randomised controlled study. Eur J Cardiothorac Surg 2009;36:856–62. [DOI] [PubMed] [Google Scholar]
- 366. Berger PB, Kleiman NS, Pencina MJ, et al. ; EVENT Investigators. Frequency of major noncardiac surgery and subsequent adverse events in the year after drug-eluting stent placement: results from the EVENT (Evaluation of Drug-Eluting Stents and Ischemic Events) Registry. JACC Cardiovasc Interv 2010;3:920–7. [DOI] [PubMed] [Google Scholar]
- 367. Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016;68:2622–32. [DOI] [PubMed] [Google Scholar]
- 368. Mahmoud KM, Sanon S, Habermann EB, et al. Perioperative cardiovascular risk of prior coronary stent implantation among patients undergoing noncardiac surgery. J Am Coll Cardiol 2016;67:1038–49. [DOI] [PubMed] [Google Scholar]
- 369. van Kuijk JP, Flu WJ, Schouten O, et al. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. Am J Cardiol 2009;104:1229–34. [DOI] [PubMed] [Google Scholar]
- 370. Rabbitts JA, Nuttall GA, Brown MJ, et al. Cardiac risk of noncardiac surgery after percutaneous coronary intervention with drug-eluting stents. Anesthesiology 2008;109:596–604. [DOI] [PubMed] [Google Scholar]
- 371. Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW.. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med 2000;343:915–22. [DOI] [PubMed] [Google Scholar]
- 372. Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012;126:1355–62. [DOI] [PubMed] [Google Scholar]
- 373. Rossini R, Angiolillo DJ, Musumeci G, et al. Antiplatelet therapy and outcome in patients undergoing surgery following coronary stenting: results of the surgery after stenting registry. Catheter Cardiovasc Interv 2017;89:E13–E25. [DOI] [PubMed] [Google Scholar]
- 374. Angiolillo DJ, Firstenberg MS, Price MJ, et al. ; BRIDGE Investigators. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA 2012;307:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Halvorsen S, Storey RF, Rocca B, et al. ; ESC Working Group on Thrombosis. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2017;38:1455–62. [DOI] [PubMed] [Google Scholar]
- 376. Budaj A, Eikelboom JW, Mehta SR, et al. ; OASIS 5 Investigators. Improving clinical out-comes by reducing bleeding in patients with non-ST-elevation acute coronary syndromes. Eur Heart J 2009;30:655–61. [DOI] [PubMed] [Google Scholar]
- 377. Stone GW, McLaurin BT, Cox DA, et al. ; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006;355:2203–16. [DOI] [PubMed] [Google Scholar]
- 378. Shahzad A, Kemp I, Mars C, et al. ; HEAT-PPCI Trial Investigators. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;384:1849–58. [DOI] [PubMed] [Google Scholar]
- 379. Lopes RD, Subherwal S, Holmes DN, et al. The association of in-hospital major bleeding with short-, intermediate-, and long-term mortality among older patients with non-ST-segment elevation myocardial infarction. Eur Heart J 2012;33:2044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S.. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006;114:774–82. [DOI] [PubMed] [Google Scholar]
- 381. Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention: results from a patient-level pooled analysis of the REPLACE-2, ACUITY, and HORIZONS-AMI trials. JACC Cardiovasc Interv 2011;4:654–64. [DOI] [PubMed] [Google Scholar]
- 382. Ndrepepa G, Neumann FJ, Schulz S, et al. Incidence and prognostic value of bleeding after percutaneous coronary intervention in patients older than 75 years of age. Catheter Cardiovasc Interv 2014;83:182–9. [DOI] [PubMed] [Google Scholar]
- 383. Kikkert WJ, Delewi R, Ouweneel DM, et al. Prognostic value of access site and nonaccess site bleeding after percutaneous coronary intervention: a cohort study in ST-segment elevation myocardial infarction and comprehensive meta-analysis. JACC Cardiovasc Interv 2014;7:622–30. [DOI] [PubMed] [Google Scholar]
- 384. Ducrocq G, Schulte PJ, Becker RC, et al. Association of spontaneous and procedure-related bleeds with short- and long- term mortality after acute coronary syndromes: an analysis from the PLATO trial. EuroIntervention 2015;11:737–45. [DOI] [PubMed] [Google Scholar]
- 385. Hamon M, Filippi-Codaccioni E, Riddell JW, Lepage O.. Prognostic impact of major bleeding in patients with acute coronary syndromes. A systematic review and meta-analysis. EuroIntervention 2007;3:400–8. [DOI] [PubMed] [Google Scholar]
- 386. Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007;49:1362–8. [DOI] [PubMed] [Google Scholar]
- 387. Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2016;387:537–44. [DOI] [PubMed] [Google Scholar]