Abstract
Background
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have been widely used in the treatment of non-small cell lung cancer (NSCLC) patients with sensitive EGFR mutations. However, the survival of patients with EGFR-TKI administration is limited by the inevitable development of acquired drug resistance. Recently, multi-targeted drugs combination has been shown to be a promising strategy to improve the efficacy of EGFR-TKI treatment and enable the reduction of drug resistance in NSCLC.
Material/Methods
Humanized NSCLC cell lines PC9 and A549 were co-cultured with thalidomide and/or icotinib to test for anti-tumor efficiency. Cell proliferation was measured by MTT assay, cell apoptosis by flow cytometry and cell migration by wound healing assay. Western blot was performed to determine the expression of caspase-3, -8, -9, Bax, EGFR, VEGF-R, AKT, ERK, MMP2, MMP9, and NF-κB. The xenograft mouse model was used to explore the effects of thalidomide and icotinib in vivo. Immunohistochemical testing was used to determine the expression of Ki-67 and TUNEL staining in tumor tissues.
Results
Treatments of thalidomide and/or icotinib reduced cell viability, induced apoptosis, and suppressed migration. Attenuation of pEGFR and pVEGF-R resulted in deactivation of ERK and AKT pathways, which eventually increased the anti-proliferative response. In PC9 xenograft model, combined administration of thalidomide and icotinib restrained tumor growth with remarkable reduced Ki-67 index and increased TUNEL positive cells.
Conclusions
Thalidomide sensitizes icotinib to increase apoptosis and prevent migration, and it may be a potentially promising anti-tumor drug in lung cancer multi-modality therapy.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Drug Therapy, Combination; Receptor, Epidermal Growth Factor; Thalidomide
Background
Lung cancer is still the most common cancer and the leading cause of cancer-related mortality in the world [1]. Non-small cell lung cancer (NSCLC) accounts for more than 80% of lung cancers [2] with 40%–80% of NSCLC patients over-expressing epidermal growth factor receptor (EGFR), which leads to poor prognosis [3]. Small-molecule tyrosine kinase inhibitors targeting EGFR (EGFR-TKIs) such as gefitinib and icotinib which are widely used in NSCLC treatment, often result in dramatically improvement in postponing disease progression in NSCLC patients [4]. Although TKI treatment has increased, to some degree, the response rate of NSCLCs, a study showed that it had limited prolongation effect a for overall survival time compared with placebo intervention in patients harboring sensitive EGFR mutations [5]. The long-term benefits of NSCLC patients receiving EGFR-TKI mono-treatment were far from the hoped-for expectation. Moreover, almost all patients with initial sensitivity to the treatment, after about 1-year of continuous application of TKI, inevitably developed acquired drug resistance that results in disease progression and subsequently therapeutic failure [6]. The combination of traditional chemotherapeutic drugs and TKIs has been used in various studies to achieve an increase in efficacy or to reduce the incidence of TKI resistance. However, it has been reported that chemotherapeutic drugs, such as gemcitabine, cisplatin, paclitaxel, and carboplatin, when combined with gefitinib had neither survival extension nor response rate elevation for enrolled NSCLC patients and the combined drug regimen only increased toxicity reactions [7,8].
Thalidomide, which had originally been used for alleviating morning sickness, was withdrawn from the market following reports of it causing birth defects and neuropathy [9,10]. As part of the potential anti-tumor effects, thalidomide has been shown to exhibit a strong inhibitory effect on angiogenesis by blocking vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) signaling pathway in pre-clinical studies [11,12]. Angiogenesis is considered an indispensable process to mediate the formation and distant metastasis of tumors, and thalidomide may be able to inhibit the growth of tumor tissues by preventing neovascularization. In addition, studies have demonstrated other biological functions of thalidomide such as inhibiting tumor necrosis factor-α (TNF-α) synthetization [13], promoting proliferation and lymphokine production of T cells by generating a co-stimulation signal in the presence of additional stimulus [14], and blocking the NF-κB activation by dynamic regulating of the reactivity of NF-κB transcription, IKK phosphorylation and IκBα clearance [15]. Thalidomide became one of the “hotspots” in treatment of multiple myeloma and showed an amazing effect: 80% of patients derived clinical benefits from thalidomide mono-treatment [16]. Based on the data in multiple myeloma studies, thalidomide has been recommended for use in numerous clinical trials for treating solid tumors, including lung cancer, esophageal cancer, hepatocellular carcinoma, and renal cell carcinoma [17–20], of the utilization has suggested certain therapeutic efficacy.
In our present study, we investigated the anti-cancer effects of thalidomide combined with EGFR-TKI icotinib in NSCLC cells. Several methods were used to clarify the anti-cancer effects of the combined therapy, including MTT(3-(4,5-dimethyl-thiazoyl-2-yl)-2,5-diphenyltetrazolium bromide), flow cytometry, western blotting, and wound healing assay. A NSCLC cell-based xenograft model was established to explore the anti-tumor effects of the 2 drugs and combination treatment. Furthermore, the effects of thalidomide and/or icotinib on ERK, AKT, and NF-κB signaling were also assessed to illustrate the mechanisms.
Material and Methods
Cell lines and cell culture
The humanized NSCLC cell lines PC9 and A549 harboring sensitive EGFR mutations and wild-type EGFR gene, respectively, was provided by Shanghai Cell Resource Center of Chinese Academy of Sciences. The cells were maintained in DMEM medium (Gibco Life Technologies, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco Life Technologies, USA), 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in an atmosphere of 5% CO2.
MTT assay
MTT assay was performed to determine cell survival. Cells were seeded in 96well plates at a density of 10 000 cells/well. After attachment, cells were treated with various concentrations of icotinib and/or thalidomide; altogether 40 groups for 24 hours. We added MTT (Sigma, USA) 10 μL to each well, and incubate for 4 hours at 37°C. The supernatant was then removed, and the formed precipitates were dissolved using 100 μL DMSO (dimethyl sulfoxide); the absorbance was read at 570 nm. All experiments were repeated 3 times.
Flow cytometry
Cell apoptosis were employed by flow cytometry. PC9 cells were seeded in 6-well plates at a density of 3×105 cells/well, and treated with either 0.1 μM icotinib, 200 μg/mL thalidomide, or a combination. After 24-hour culture, cells were collected and stained with Annexin V-FITC and propidium iodide (Apoptosis Kit, BestBio Biotechnology). Data were acquired on a FACSCalibur HG flow cytometer (BD, Franklin Lakes, NJ, USA) and analyzed using Cell-Quest software (BD Bioscience). For each experiment, 10 000 events per sample were recorded. FlowJo 7.6 software was used for flow cytometry analysis. All experiments were repeated 3 times.
Western blot
Cells lysates were prepared with radioimmunoprecipitation assay buffer (RIPA) lysis buffer supplement with protease inhibitor cocktail (Calbiochem) and phosphatase inhibitor (Roche). The proteins are electrophoresed on 6–15% SDS-polyacrylamide gel (Beyotime Biotechnology) and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked in the TBS solution (100 mM Tris, 150 mM NaCl, pH 7.6) containing 5% (w/v) skim milk, and 0.5% (v/v) Tween-20 for 1 hour at room temperature. The membranes were incubated with primary antibodies overnight at 4°C followed by incubation with secondary antibodies for 1 hour. Primary antibodies used in this study were antibodies against caspase-3, -8, -9, Bax, pEGFR, pVEGF-R2, pAKT, AKT, and NK-κB, metal matrix protease (MMP) 2, MMP9, β-actin (Cell Signaling Technology, USA), pERK, and ERK (Abcam, UK). Goat polyclonal anti-mouse IgG-horseradish peroxidase (HRP) or goat polyclonal anti-rabbit IgG-HRP were used as secondary antibodies (Abcam, UK). Bands were visualized by chemiluminescence detection kit (Millipore, USA). All experiments were repeated 3 times.
Wound healing assay
Cell migration effects were assessed by wound healing assay. PC9 cells were seeded in 6-well plates at a density of 6×105 cells/well, cultured for 12 hours with complete DMEM medium. Straight scratch was made using a 200 μL pipette tip at an angle of around 30 degrees. The medium was removed, and we washed off the cell debris with phosphate buffered saline (PBS) 3 times. FBS-free DMEM was used as the control, and DMEM containing 0.1 μM icotinib, DMEM containing 200 μg/mL thalidomide, and these 2 drugs combined were added to wells, respectively. The plates were placed in an incubator and observed every 4 hours for 24 hours. All experiments were repeated 3 times.
Tumor xenografts in nude mice
We purchased male BALB/c nude mice (5- to 6-weeks-old) from Experimental Animal Center of Anhui Province. The research protocol was approved, and mice were maintained in accordance with the institutional guidelines of Ethics Committee of Anhui Medical University. Mice were challenged by subcutaneous injection with 5×106 PC9 cells and were then randomized into 4 groups: the 5% hydroxy propyl methyl cellulose-treated group (control group), the thalidomide-treated group, the icotinib-treated group, and the combination group. After 10 days for tumor xenograft growth, mice had daily administration by gavage of thalidomide 200 mg/kg, icotinib 100 mg/kg or a combination. Mice were treated for 18 days and sacrificed on day 29. The removed tumor tissues were fixed with 10% formalin for subsequent testing. Tumor sizes were measured using a caliper, and tumor volumes (mm3) were calculated using the following formula: tumor volume (mm3)=(A×B2)/2, where A is the larger diameter and B is the smaller diameter of the tumor. Then the fixed tumor tissues were embedded in a paraffin block, sectioned transversely and stained with rabbit monoclonal antibody to Ki-67 (Abcam Co., UK) or TUNEL Kit (Abcam Co., UK) followed by hematoxylin.
Statistical analysis
GraphPad Prism 6.0 (La Jolla, CA, USA) was used for statistical significance evaluation and Biosoft CalcuSyn software 2.0 (Biosoft, Cambridge, UK) for Combination Index (CI) calculation. Data are presented as the mean ± standard deviation (SD) for 3 independent experiments. The 1-way analysis of variance (ANOVA) and 2-way ANOVA were used for comparison between 2 groups or more than 3 groups. A value of P<0.05 was considered significant.
Result
Effects of thalidomide and icotinib combined treatment on the proliferation of NSCLC cells
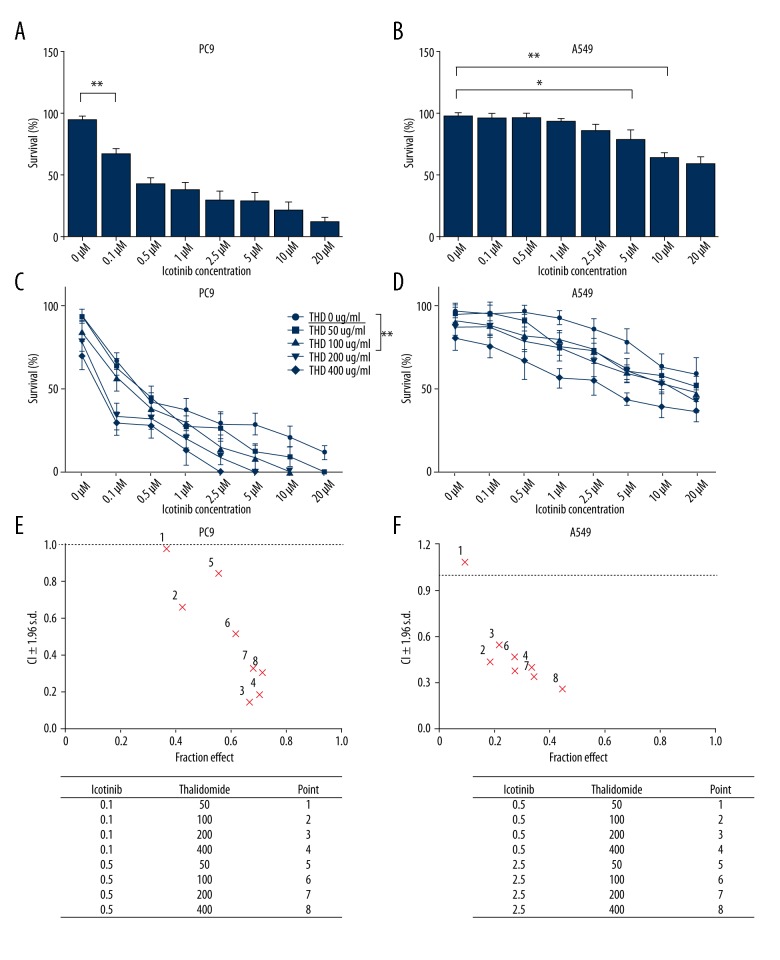
To examine whether thalidomide potentiate the anti-tumor activity of icotinib in vitro, we observed the effects of thalidomide and icotinib on proliferation of NSCLC cells PC9 and A549 using the MTT colorimetric assay. Growth of both PC9 and A549 cells was inhibited by icotinib: with the increased concentration of icotinib, the proliferation activity of PC9 was significantly inhibited at only 0.1 μM (Figure 1A, P<0.01). Comparatively, no apparent effects were observed in A549 cells until the concentration reached 5 μM (Figure 1B, P<0.05). PC9 cells carrying EGFR mutations showed higher sensitivity to icotinib than wild-type A549 cells. Both PC9 and A549 cell proliferation was inhibited when icotinib was combined with more than 50 μg/mL thalidomide (Figure 1C, 1D). The CI index of inhibitory effects was obviously <1 when thalidomide was at 100 μg/mL combined with icotinib at 0.1 μM in PC9 cells or 0.5 μM in A549 cells respectively (Figure 1E, 1F), which indicated synergistic anti-proliferation effects on both NSCLC cell lines with more effective growth inhibition towards PC9 cells observed.
Figure 1.
Effects of thalidomide and icotinib on proliferation of PC9 and A549 cells. (A, B) Icotinib inhibited proliferation of PC9 and A549 cells increasingly with the elevation of drug concentration. PC9 and A549 cells were treated with various doses of icotinib (0, 0.1, 0.5, 1, 2.5, 5, 10, and 20 μM) for 24 hours. MTT assay was used to determine cell viability. (C, D) Combination treatment with thalidomide (50, 100, 200, and 400 μg/mL) and icotinib inhibited cell proliferation in PC9 and A549 cells. Data are represented as the means ±SD, n=3, * P<0.05, ** P<0.01. (E, F) Combination index and fractional effect values of thalidomide and icotinib combination treatment in PC9 and A549 cells.
Proliferation inhibitory effects of thalidomide and icotinib highly correlated with apoptosis
In comparison with NSCLC patients harboring wild-type EGFR, over-expressing mutant EGFR were predictive of response to EGFR-TKI therapy. Oncologists considered that TKIs would be optional in patients with wild-type EGFR as a second-line treatment [21]. However, TKIs in treating EGFR wild-type NSCLCs has not been adopted by NCCN Guidelines.
The proliferation assay confirmed that icotinib combined with thalidomide exerted higher proliferation inhibiting effect in the PC9 cell line. Thus, we picked PC9 as the target cell line for further experiments. Icotinib (0.1 μM) plus thalidomide (200 μg/mL) showed the best synergistic effect in PC9 cells according to CI index analysis (Figure 1E, 1F).
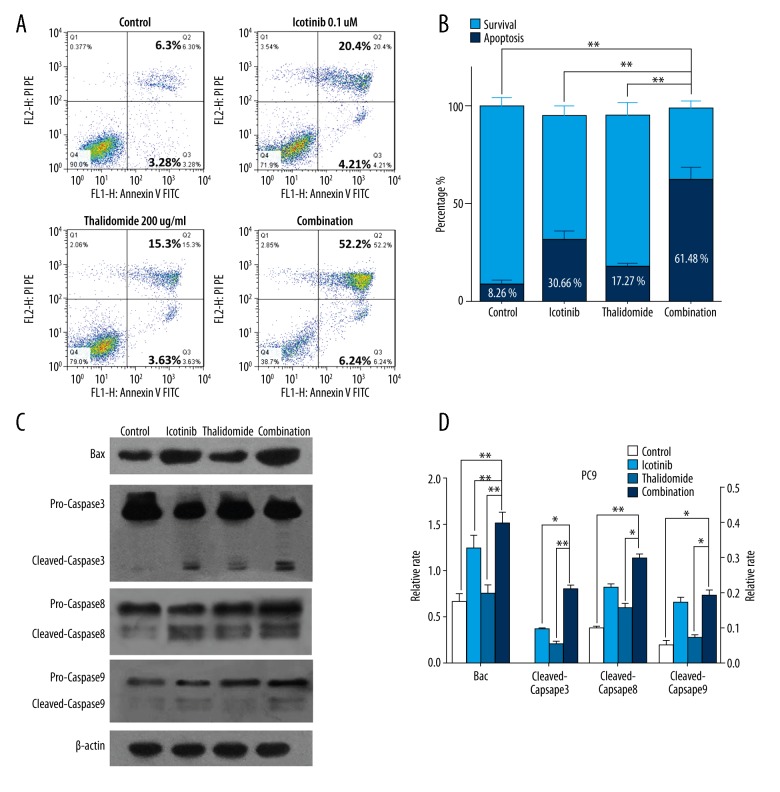
Two main pathways may be involved in apoptosis activation, which are the intrinsic pathway mediated by mitochondria and the exogenous pathway initiated by the death receptor [22]. The cell apoptosis evaluated by Annexin V/propidium iodide staining showed dramatically improvement with the combination treatment (61.48%) compared with single treatments of thalidomide (17.27%) or icotinib (30.66%) in PC9 cells (Figure 2A, 2B, P<0.01). Therefore, we considered that the growth inhibitory effects may be correlated with apoptosis induction.
Figure 2.
Thalidomide and icotinib induced apoptosis in PC9 cells. (A, B) Flow cytometry analysis showed the percentage of apoptotic cells in the control group, thalidomide group, icotinib group, and combination group. (C, D) Western blot analysis showed the expression of caspase-3, -8, -9, and Bax in the control group, thalidomide group, icotinib group, and combination group. Data are represented as the means ±SD, n=3. * P<0.05, ** P<0.01. Relative rate of Bax was put on the left Y axis and caspase-3, -8, -9 on the right Y axis.
To further clarify the molecules essential in apoptosis, we evaluated the proteolytic activity of the apoptotic “executioners” caspase-3, -8, -9 as well as the alteration of Bax, a regulator of mitochondrial function. Compared with the control and mono-therapy groups, increased cleaved forms of caspase-3 were detected following the combined treatment (P<0.05), cleavage of caspase-8 and caspase-9 levels remained unchanged between the combination and icotinib treatment (P>0.05). The expression of Bax was upregulated by the combination treatment compared with both single treatments (Figure 2C, 2D, P<0.01), which indicated translocation of Bax to the mitochondria membrane may contribute to apoptosis.
Cell migration was involved in enhanced anti-tumor activity of thalidomide combined with icotinib
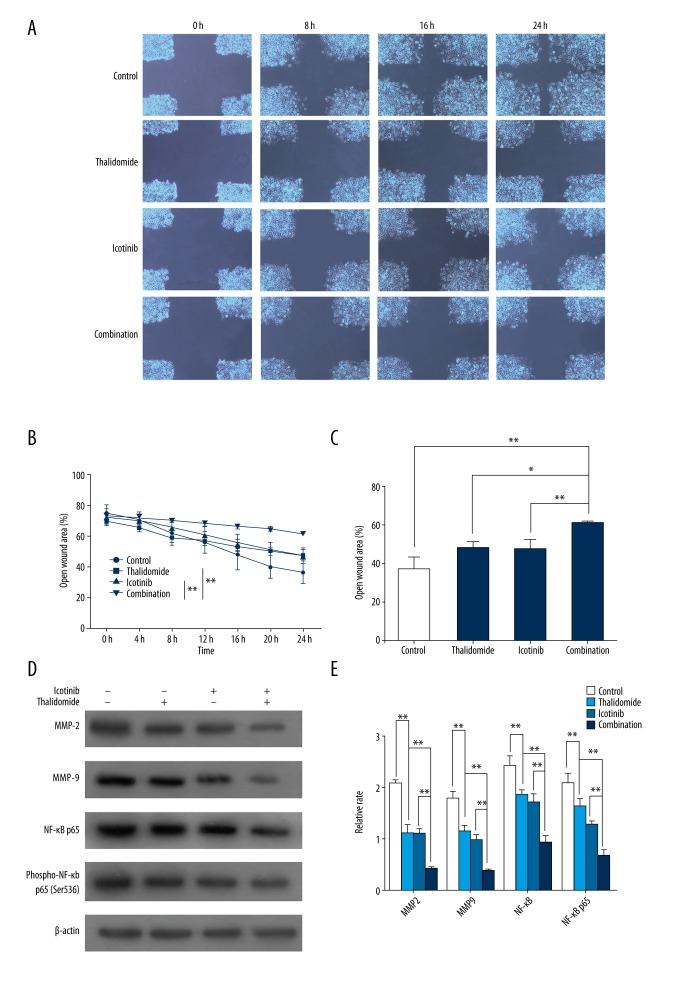
Cell migration is closely associated with tumor invasion and metastasis, which is regulated by various invasive factors, including MMP2 and MMP9 [23]. MMPs overexpressed tumor cells are recognized as an initiation factor for metastasis; NF-κB, a transcription factor whose activation is related to p65 nuclear translocation, exhibits a pro-metastatic factor through upregulating the expression of MMP-9 [24,25]. The effect of thalidomide and icotinib on cell migration in our study was assessed by wound healing assay which showed that PC9 cells receiving thalidomide or icotinib treatment exhibited significantly depressed migration (Figure 3A, 3B, P<0.05). Furthermore, the combination-treatment exhibited the most potent inhibitory effect on the migration of PC9 cells (Figure 3A, 3B, P<0.05). Migration related proteins including MMP2, MMP9, NF-κB p65, and Ser-536 p65 phosphorylation were also downregulated by the combination-treatment (Figure 3C, 3D, P<0.05).
Figure 3.
Thalidomide and icotinib inhibited migration of PC9 cells. (A) Images of wounded PC9 cells (200×). (B) Quantitative analysis of the percentage of open wound area during and after the 24 hours incubation. Data are represented as the mean ±SD, n=4, * P<0.05, ** P<0.01. (C, D) Western blot analysis showed the expression of MMP2, MMP9, NF-κB p65, and phospho-NF-κB p65 in the control group, thalidomide group, icotinib group, and combination group. β-actin was used as a loading control. Data are represented as the means ±SD, n=3, * P<0.05, ** P<0.01.
EGFR and VEGF as the key modulators regulating tumor cells migration
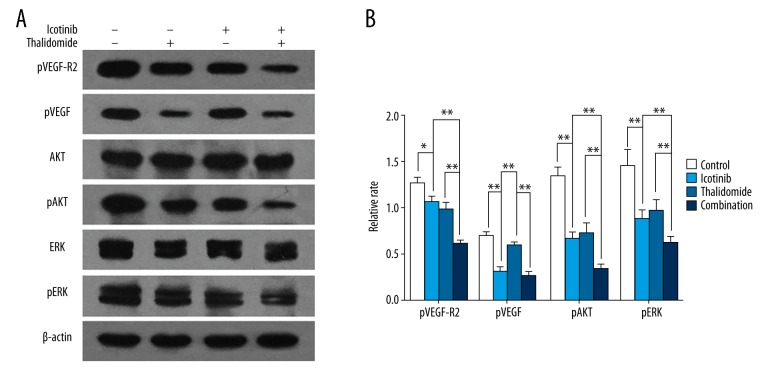
The cross-talk of EGFR and VEGF pathways in neoplastic cells highlighted the promising strategy of inhibiting both proliferation and angiogenesis [26]. To further clarify the mechanism of combined treatment in proliferation inhibition and pro-apoptosis, we investigated the phosphorylation of EGFR, VEGF-R2, and downstream signaling pathways. Western blotting assay revealed that phosphorylated EGFR was suppressed in both the icotinib group and the combination group and not the thalidomide group, whereas VEGF-R2 phosphorylation was suppressed in all 3 treatment groups compared with the control group. Activation of 2 pivotal molecules shared by EGFR and VEGF-R, the AKT and ERK, and also their phosphorylation dependent activation, were monitored. Treatment with thalidomide or icotinib significantly reduced efficiency of AKT and ERK phosphorylation, furthermore, the combined-treatment resulted in enhanced inhibition (Figure 4A, 4B, P<0.05).
Figure 4.
Thalidomide and icotinib inhibited the EGFR and VEGF-R2 pathways in PC9 cells. Western blot analysis showed the expression of pEGFR, pVEGF-R2, AKT, pAKT, ERK, and pERK in PC9 cells in the control group, thalidomide group, icotinib group, and combination group. β-actin was used as a loading control. Data are represented as the means ±SD, n=3, * P<0.05, ** P<0.01.
Combined treatment of thalidomide and icotinib exerted synergistic anti-tumor effects in vivo
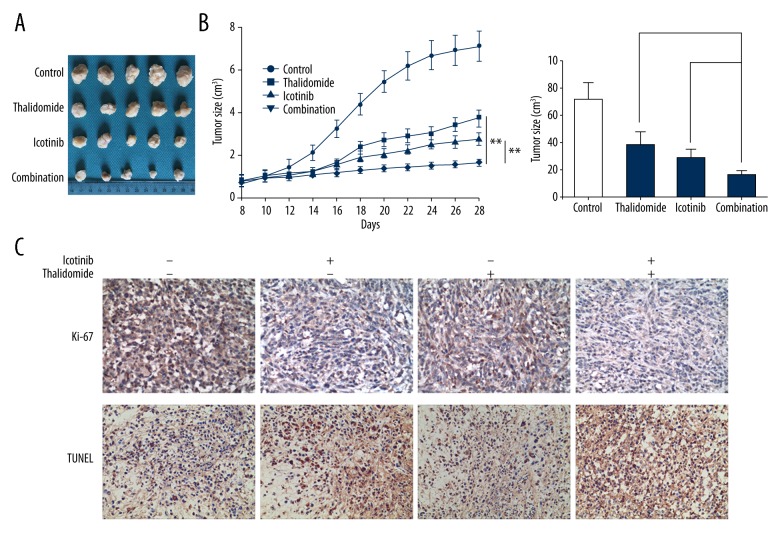
We further studied the effects of thalidomide and icotinib on tumor growth in nude mice subcutaneously transplanted with PC9 cells. The tumor growth was dramatically suppressed by the intervention with thalidomide and/or icotinib compared with the control group (Figure 5A, 5B, P<0.01). Combination treatment showed the greatest attenuation of tumor size compared with both mono-treatment groups (Figure 5A, 5B, P<0.05). Cell proliferative index (Ki-67 immunohistochemistry) and apoptosis index (TUNEL staining) was conducted on tumoral tissue sections. The pathological staining showed apparently downregulated expression of Ki-67 and upregulated TUNEL levels in the combination group compared with that in either control or mono-treatment groups (Figure 5C).
Figure 5.
Thalidomide and icotinib inhibits tumor growth and promote tumor death in vivo. (A, B) PC9 tumor cells were injected subcutaneously. Mice were divided into groups as follow: control, thalidomide, icotinib, and combination group. Data represent the mean tumor volume of 5 mice. The tumor size in combination group is significantly smaller than that in individual icotinib group and individual thalidomide group. Data are represented as the means ±SD, n=5, * P<0.05, ** P<0.01. (C) Immunohistochemical reaction to Ki-67 and TUNEL staining in control, thalidomide, icotinib and combination group (200×).
Discussion
Thalidomide has been reported to be successfully used alone or with other chemotherapeutics in treatment of multiple myeloma [16,27]. In addition, thalidomide and its analogs can also be used in the treatment of immune disorders such as graft-versus-host disease and autoimmune diseases [28,29]. However, there are little or no effective benefits obtained from mono-therapies with thalidomide or its analogs in the treatment of solid tumors. Recently, thalidomide combined with typical chemotherapeutic drugs or immunomodulatory drugs showed curative effects in pre-clinical studies of tumor and leukemia [30,31]. Our presented study developed a combination of thalidomide with icotinib which showed distinctly synergic cytostatic and pro-apoptotic effects in lung cancer cells. Co-administration of thalidomide and icotinib exhibited cooperative effects on proliferation inhibition in PC9 and A549 cells, as confirmed by calculating the CI index. Combined treatment of these 2 drugs accelerated the process of apoptosis in PC9 cells evaluated by AnnexinV-FITC/propidium iodide staining and reconfirmed by apoptotic related proteins analysis. Cleavage of caspase-8, -3, and -9 was found in the activation of caspase cascade for apoptosis induced by the combination treatment. Furthermore, the elevation of Bax expressions suggested the inducement of mitochondrial membrane permeabilization and dysfunction in the process of apoptosis.
It was observed that thalidomide and icotinib concertedly blocked the migration process of PC9 cells by wound healing assay. Migration-related factors, including NF-κB, MMP2, and MMP9 were detected as well. The activated transcription factor NF-κB plays a critical role in the development of tumorigenesis by promoting neoplastic cell proliferation and migration. The canonical p65/p50 heterodimer is a prototypical form of NF-κB, whose optimal activity requires the phosphorylation at p65 subunit [32]. Activation of NF-κB was reported to be induced by VEGF and EGF binding to their receptors (VEGF-R and EGFR) respectively [33,34]. Activated NF-κB was also considered a promoter of angiogenic molecules such as VEGF, CXCL1, IL-8, and COX-2 [35], and a positive regulator of the EGFR pathway [34]. Therefore, positive feedback loops were established, in which NF-κB activation may be a crucial factor. We found that both NF-κB and its phosphorylation in PC9 cells were strikingly downregulated by thalidomide combined with icotinib, which suggested that combined treatment exerts its effects in PC9 cells through blockade of the NF-κB pathway. MMPs belong to the neutral proteinases, critical to the process of embryo development, organs remodeling, and inflammation, and are the key enzymes in extracellular matrix degradation and metastasis during tumorigenesis. Previous research has established that activated NF-κB induced the synthesis and secretion of MMPs remarkably for boosting tumor cells migration via degradation of extracellular matrix and destruction of basement membrane [36]. Our data have clearly shown that MMP2 and MMP9, the main members of the MMP family involved in tumor metastasis and invasion, were also suppressed by combined thalidomide/icotinib therapy in PC9 cells. Downregulations of NF-κB activation induced MMP2 and MMP9 expressions and may be responsible for the decrease of cell migration ability by the combined-treatment.
Angiogenesis, with great relevance to activation of VEGF receptor (VEGF-R) including VEGF-R1, -R2, and -R3, was deemed indispensably necessary for malignant tumor formation. Among these receptors, phosphorylation of VEGF-R2 plays a major role in neo-vascularization correlating the aggressive biological behavior and metastasis-forming capacity in tumors [37]. According to our current study, icotinib showed significantly inhibitory effect on EGFR phosphorylation that was indiscernible by treatment of thalidomide. Furthermore, the combined-treatment with icotinib and thalidomide showed no significant difference compared with that of icotinib. In contrast, the combined treatment showed the greatest inhibitory effect compared with both single treatment groups on VEGF-R2 phosphorylation-mediated activation in PC9 cells. Phosphorylation, but not expression level of AKT and ERK (the common downstream proteins involved in EGFR and VEGF signal transduction), declined by both treatment with thalidomide and with icotinib. The main biological influences of activated AKT and ERK on tumor cell growth could be roughly divide into 2 categories: cell proliferation-promoting and protection for cell survival. In terms of tumor angiogenesis, AKT was known as an essential molecule by phosphorylating Girdin, an indispensable protein in VEGF-mediated cell transplantation and vascular tube formation [38]. ERK was reported to stimulate MMP2 and MMP9 activity for increasing motility and invasiveness of tumor cells [39]. Our data have shown that both thalidomide and icotinib can inhibit the phosphorylation of AKT and ERK in lung cancer cells and by means of these cross-talks, thalidomide and icotinib exerted synergistically cytostatic, pro-apoptotic, and migration inhibitory activities. Similarly, Noman et al. confirmed that thalidomide manifested suppression of EGF induced tumor cell growth without downregulation of EGFR in monocytic leukemia cells [40]. In addition, phosphorylated ERK was reported to be a positive regulator of VEGF expression [41]. In this report, VEGF-R2 was found to be concertedly downregulated by icotinib and thalidomide. A possible explanation for this was that both drugs cooperatively reduced phosphorylation level of ERK and subsequently attenuated the expression of VEGF. Existence of other undiscovered feed-back loops between VEGF and EGFR pathways remained a possibility and thereby suggest the potential of targeting dual pathways.
In the in vivo experiment, we further confirmed that the combination therapy of thalidomide plus icotinib remarkably suppressed tumor growth of subcutaneously xenografted PC9 cells in nude mice. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) is a technique reacting with DNA strand breaks that permits the detection of apoptotic programmed cell death. Reduced Ki-67 expression and increased TUNEL positive cells also demonstrated that combination treatment can motivate apoptosis and inhibit proliferation of xenografted cancer cells. As one of the cell proliferation markers, over-expression of Ki-67 was associated with tumor proliferation, invasion, and metastasis, which served as an indicator of poor prognosis in NSCLCs [42]. In comparison to our data, in vitro combination of both drugs exerted tumor suppressive effects but no measurable toxic or adverse effects in a xenograft model, given that no conspicuous difference was detected in body weight of nude mice between the control group and treatment groups, within the therapeutic range. Such treatment in vivo exhibited satisfactory biosafety and tolerability that can provide reference data for compatibility of the drugs in clinical treatment of NSCLCs.
Our experiments had satisfied results and conveyed the idea that compatibility of thalidomide and icotinib show a synergistic effect and might be a potential therapeutic method for lung cancer treatment. In addition, both thalidomide and icotinib are frequently used medicines, thalidomide in multiple myeloma [16,27] and icotinib in lung cancer [43], which means utilization of the 2 drugs has been recognized as safe and well-tolerated for their respective indications. Hence, both drugs will be soon be ubiquitous once proven to have therapeutic actions in lung cancer therapy. Furthermore, thalidomide displays obvious superiority on potency ratio and is covered by medical insurance such that patients can benefit from the anti-angiogenic therapy provided by thalidomide and its analogues administration.
Conclusions
This study has shown the improved curative effects of both thalidomide and icotinib on PC9 cells and a xenograft model. Using combined treatment, biological functions of several tumor growth or metastasis associated proteins, including EGFR, VEGF-R2, AKT, ERK, NF-κB, MMP2, and MMP9 were all suppressed considerably. In contrast, the executive molecules of apoptosis: cleaved caspase-8, -3, and -9 were upregulated by the combined-treatment, accompanied by an increase in the mitochondrial apoptotic protein Bax. According to these data, the underlying mechanisms of thalidomide sensitizing icotinib in lung cancer cells were revealed and this study demonstrated the direction for study of the drug combination in treatment of lung cancer. The utilization of thalidomide combined with icotinib requires further conduction of large-scale, randomized, prospective clinical trials for NSCLC patients.
Footnotes
Source of support: Departmental sources
Reference
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs. 2008;12(4):587–96. doi: 10.1188/08.CJON.587-596. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch FR, Varellagarcia M, Cappuzzo F. Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene. 2009;28(1):32–37. doi: 10.1038/onc.2009.199. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–88. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med. 2005;353(353):133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 6.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–47. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial – INTACT 1. J Clin Oncol. 2004;22(5):777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial – INTACT 2. J Clin Oncol. 2004;22(5):785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 9.Mcbride WG. Thalidomide and congenital abnormalities. Lancet. 1961;278(7216):912–13. [Google Scholar]
- 10.Fullerton PM, Kremer M. Neuropathy after intake of thalidomide (Distaval) Br Med J. 1961;2(5256):855–58. doi: 10.1136/bmj.2.5256.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon BM, Browne F, D’Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64(6):971–78. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 12.D’Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91(9):4082–85. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng L, Ding W, Granstein RD. Thalidomide inhibits tumor necrosis factor-alpha production and antigen presentation by Langerhans cells. J Invest Dermatol. 2003;121(5):1060–65. doi: 10.1046/j.1523-1747.2003.12565.x. [DOI] [PubMed] [Google Scholar]
- 14.Haslett PA, Corral LG, Albert M, et al. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187(11):1885–92. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keifer JA, Guttridge DC, Ashburner BP, et al. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276(25):22382–87. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 16.Desikan R. Antitumor activity of thalidomide in refractory multiple myeloma. New Engl J Med. 1999;341(21):1565–71. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 17.Jazieh AR, Komrokji R, Gupta A, et al. Phase II trial of thalidomide, irinotecan and gemcitabine in chemonaive patients with advanced non-small cell lung cancer. Cancer Invest. 2009;27(9):932–36. doi: 10.3109/07357900801944856. [DOI] [PubMed] [Google Scholar]
- 18.Wilkes EA, Selby AL, Cole AT, et al. Poor tolerability of thalidomide in end-stage oesophageal cancer. Eur J Cancer Care. 2011;20(5):593–600. doi: 10.1111/j.1365-2354.2011.01255.x. [DOI] [PubMed] [Google Scholar]
- 19.Yau T, Chan P, Wong H, et al. Efficacy and tolerability of low-dose thalidomide as first-line systemic treatment of patients with advanced hepatocellular carcinoma. Oncology. 2007;72(Suppl 1):67–71. doi: 10.1159/000111709. [DOI] [PubMed] [Google Scholar]
- 20.Margulis V, Matin SF, Tannir N, et al. Randomized trial of adjuvant thalidomide versus observation in patients with completely resected high-risk renal cell carcinoma. Urology. 2009;73(2):337–41. doi: 10.1016/j.urology.2008.08.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemoth Pharm. 2012;69(5):1241–46. doi: 10.1007/s00280-012-1831-0. [DOI] [PubMed] [Google Scholar]
- 22.Hassen S, Ali N, Chowdhury P. Molecular signaling mechanisms of apoptosis in hereditary non-polyposis colorectal cancer. World J Gastrointest Pathophysiol. 2012;3(3):71–79. doi: 10.4291/wjgp.v3.i3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee SU, Ahn KS, Sung MH, et al. Indacaterol inhibits tumor cell invasiveness and MMP-9 expression by suppressing IKK/NF-κB activation. Mol Cells. 2014;37(8):585–91. doi: 10.14348/molcells.2014.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H, Du T, Xu G, et al. Matrine suppresses invasion of castration-resistant prostate cancer cells by downregulating MMP-2/9 via NF-κB signaling pathway. Int J Oncol. 2016;50(2):640–48. doi: 10.3892/ijo.2016.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen AK, Ouaret D, El OK, et al. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Therap. 2011;131(1):80–90. doi: 10.1016/j.pharmthera.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Barlogie B, Desikan R, Eddlemon P, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: Identification of prognostic factors in a phase II study of 169 patients. Blood. 2001;98(2):492–94. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 28.Koc S, Leisenring W, Flowers ME, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96(12):3995–96. [PubMed] [Google Scholar]
- 29.Karim MY, Ruiz-Irastorza G, Khamashta MA, et al. Update on therapy – thalidomide in the treatment of lupus. Lupus. 2001;10(3):188–92. doi: 10.1191/096120301677213822. [DOI] [PubMed] [Google Scholar]
- 30.Qiao Z, Yuan J, Shen J, et al. Effect of thalidomide in combination with gemcitabine on human pancreatic carcinoma SW-1990 cell lines in vitro and in vivo. Oncol Lett. 2015;9(5):2353–60. doi: 10.3892/ol.2015.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Mi R, Fan R, et al. Effects of thalidomide combined with interferon on inhibiting Kasumi-1 cell proliferation. Adv Clin Exp Med. 2016;25(3):403–8. doi: 10.17219/acem/41048. [DOI] [PubMed] [Google Scholar]
- 32.Chaturvedi MM, Sung B, Yadav VR, et al. NF-κB addiction and its role in cancer: ‘One size does not fit all’. Oncogene. 2011;30(14):1615–30. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dell’Eva R, Ambrosini C, Minghelli S, et al. The Akt inhibitor deguelin, is an angiopreventive agent also acting on the NF-κB pathway. Carcinogenesis. 2007;28(2):404–13. doi: 10.1093/carcin/bgl162. [DOI] [PubMed] [Google Scholar]
- 34.Shostak K, Chariot A. EGFR and NF-κB: Partners in cancer. Trends Mol Med. 2015;21:385–93. doi: 10.1016/j.molmed.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Basseres D, Baldwin A. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 36.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: A tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 37.Nishida N, Yano H, Komai K, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer. 2004;101(6):1364–74. doi: 10.1002/cncr.20449. [DOI] [PubMed] [Google Scholar]
- 38.Fukushima H, Nakao A, Okamoto F, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10(3):329–37. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 39.Yue P, Gao ZH, Xue X, et al. Des-γ-carboxyl prothrombin induces matrix metalloproteinase activity in hepatocellular carcinoma cells by involving the ERK1/2 MAPK signalling pathway. Eur J Cancer. 2011;47(7):1115–24. doi: 10.1016/j.ejca.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Noman ASM, Koide N, Khuda IE, et al. Thalidomide inhibits epidermal growth factor-induced cell growth in mouse and human monocytic leukemia cells via Ras inactivation. Biochem Bioph Res Commun. 2008;374(4):683–8. doi: 10.1016/j.bbrc.2008.07.090. [DOI] [PubMed] [Google Scholar]
- 41.Wang FS, Wang CJ, Chen YJ, et al. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1α and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem. 2004;279(11):10331–37. doi: 10.1074/jbc.M308013200. [DOI] [PubMed] [Google Scholar]
- 42.He LY, Zhang H, Wang ZK, et al. Diagnostic and prognostic significance of E-cadherin and Ki-67 expression in non-small cell lung cancer patients. Eur Rev Med Pharmacol Sci. 2016;20(18):3812–17. [PubMed] [Google Scholar]
- 43.Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): A randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14(10):953–61. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]