Abstract
Ag/BiVO4/Mn1−xZnxFe2O4 was synthesized with a dip-calcination in situ synthesis method. This work was hoped to provide a simple method to synthesis three-phase composite. The phase structure, optical properties and magnetic feature were confirmed by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), ultraviolet-visible diffuse reflectance spectrophotometer (UV-vis DRS), and vibrating sample magnetometer (VSM). The photocatalytic activity was investigated by Rhodamine B (RhB) photo-degradation under visible light irradiation. The photo-degradation rate of RhB was 94.0~96.0% after only 60 min photocatalytic reaction under visible light irradiation, revealing that it had an excellent visible-light-induced photocatalytic activity. In the fifth recycle, the degradation rate of Ag/BiVO4/Mn1−xZnxFe2O4 still reached to 94.0%. Free radical tunnel experiments confirmed the dominant role of •O2− in the photocatalytic process for Ag/BiVO4/Mn1−xZnxFe2O4. Most importantly, the mechanism that multifunction Ag could enhance photocatalytic activity was explained in detail.
Keywords: Ag/BiVO4/Mn1−xZnxFe2O4, photocatalytic activity, magnetic property, wastewater treatment, reaction mechanism
1. Introduction
Semiconductor photocatalysts have been paid more attention in the application of research of clean energy exploration and environmental pollutants removal. These photocatalysts possess superior physicochemical and magneto-optical properties [1,2,3,4]. Non-toxic bismuth vanadate (BiVO4), with good light absorption and high ionic conductivity [5,6,7,8], has attracted strong interest from scientists. Although the visible-light sensitivity and photocatalytic activity of monoclinic scheelite BiVO4 (m-BiVO4) are the largest among three crystals including additionally tetragonal zircon and tetragonal scheelite [9,10,11,12], the light absorption and the catalytic property of m-BiVO4 can be further improved by various strategies. The photocatalytic activity can be greatly improved when the photo-generated electrons and holes are efficiently separated. BiVO4-based composites with a high separation efficiency of photo-generated electrons and holes have been developed to enlarge the quantum efficiency of BiVO4 and the photocatalytic activity of BiVO4. Meanwhile, doping noble metal in photocatalysts is an effective way of promoting the efficient separation of photo-generated electrons and holes. Researchers have reported that the electron–hole separation in doping compounds was strengthened by the charge transfer between semiconductor and noble metal [13,14,15,16,17]. Ag is the most promising noble metal because of the low cost and strong electron trapping ability. Ag-doped catalyst could induce surface plasmon resonance, involving in a better absorption of visible light [18].
For the convenience of recovery and separation of photocatalysts after reaction, magnetic photocatalysts have been fabricated in recent years [19,20,21,22]. Magnetic photocatalysts could be recovered with an external magnetic field, and a high recovery ratio would be conducive to promote their industrial application. In our previous research, both the soft-magnetic Mn1−xZnxFe2O4/Bi2O3 [21] and hard-magnetic SrFe12O19/BiVO4 [22] with photocatalytic properties were prepared with dip-calcination method. Further exploration is necessary to synthesize m-BiVO4-based composite with high photocatalytic activity as well as large recovery ratio. In this work, Ag was doped in BiVO4/Mn1−xZnxFe2O4 with in situ synthesis method. The photocatalytic activity of Ag/BiVO4/Mn1−xZnxFe2O4 was investigated under sunlight irradiation. Further insights are focused on characteristic structure, magnetic property, and photocatalytic mechanism of Ag/BiVO4/Mn1−xZnxFe2O4.
In fact, fabrication of Ag/BiVO4/Mn1−xZnxFe2O4 was a continuation of our research about the syntheses and application of BiVO4/Mn1−xZnxFe2O4 [19]. The RhB degradation reaction using BiVO4/Mn1−xZnxFe2O4 as photocatalyst was very slow (take 3 h). The incorporation of Ag could enhance the photocatalytic activity of BiVO4/Mn1−xZnxFe2O4.
2. Experimental
2.1. Synthesis of Ag/BiVO4/Mn1−xZnxFe2O4
Mn1−xZnxFe2O4 was prepared according to the literature [19,21].
The precursor of BiVO4 was produced by the chemical co-precipitation way [22].
486 mg Mn1−xZnxFe2O4 was dispersed into the precursor of BiVO4 and dried at 80 °C for 12 h. BiVO4/Mn1−xZnxFe2O4 (15.0 wt %) (marked BiVO4/Mn1−xZnxFe2O4) was gained after roasting at 450 °C for 3 h. After dip-roasting, 600 mg BiVO4/Mn1−xZnxFe2O4 was put into 50 mL AgNO3 solution (10 mmol/L) under stirring conditions at room temperature for 2 h to form dispersion solution A. 1.0 g polyvinylpyrrolidone (PVP) was added into 50 mL ethanol to obtain the solution B. The dispersion solution A mixed with the solution B. Then the mix solution was heated in water-bath at 70 °C for 4 h. The as-formed mixture was filtered, and washed with water and ethanol, respectively. 12.0 wt % Ag/BiVO4/ 15.0 wt % Mn1−xZnxFe2O4 (marked Ag/BiVO4/Mn1−xZnxFe2O4) was obtained after the filtration residues was dried at 50 °C for 12 h.
2.2. Material Characterization
The structure of samples was determined by X-ray diffractometer (Shimadzu, XRD-6000, Kyoto, Japan), Fourier transform infrared spectroscopy (FTIR, Perkin-Elmersystem 2000, Perkin Elmer, Waltham, MA, USA). The ultraviolet-visible diffuse reflectance spectrophotometer (UV-vis DRS, TU1901, Beijing Purkinje, Beijing, China) was employed to examine the light absorption performance of the as-obtained composites. Their morphologies were observed by transmission electron microscopy (TEM, FEI, Tecnai G2 F20, Hillsboro, OR, USA). The element content was analyzed by X-ray photoelectron spectrometer (XPS-XSAM800, Kratos, Manchester, UK) with a base pressure 2 × 10−7 Pa and X-ray gun180 W (12 kV, 15 mA). The magnetic properties were investigated using a vibrating sample magnetometer (VSM, Lakeshore 7410, LakeShore, Carson, CA, USA).
2.3. Measurement of the Photocatalytic Performance
The photocatalytic activity of the as-prepared composites was investigated by the photodegradation of simulated dye wastewater (Rhodamine B, RhB) under visible light irradiation. 100 mg photocatalyst was put into 100 mL RhB solution of 5 mg/L. Then the suspension liquid was placed in dark for 0.5 h with stirring to reach the adsorption–desorption equilibrium. A 500 W Xe lamp, equipping with UV cut-off filter, was used as the visible light source (λ ≥ 420 nm). At the given irradiation time intervals, a series of the reaction solution was sampled and measured the absorption with the UV-vis spectrophotometer (TU-1901). The photocatalytic mechanism of Ag/BiVO4/Mn1−xZnxFe2O4 was explored by holes-radical trapping experiments with p-benzoquinone (BZQ, •O2− radical scavenger), EDTA-Na2 (hole scavenger), and tert-butanol (t-BuOH, •OH radical scavenger).
The repeatability of the photocatalyst was detected by cycling tests. After each cycle, the catalyst was separated by an external magnetic field, then washed and dried for the next cycle.
3. Results and Discussion
3.1. Synthesis Condition and Structure Identification
The appropriate mass ratio of Mn1−xZnxFe2O4 and BiVO4 was essential for BiVO4/Mn1−xZnxFe2O4. Thus, the composite possessed not only a good magnetization but also a high photocatalytic activity. By the comparison experiments, it is found that the composite held the largest magnetic property without the reduction of photocatalytic activity when 15.0 wt % Mn1−xZnxFe2O4 was loaded in BiVO4 by the dip-calcination approach. The doping quantity of Ag was not only closely related to the photocatalytic activity but also affected the cost of Ag/BiVO4/Mn1−xZnxFe2O4. PVP was confirmed as an efficient stabilizer and reductant in the synthesis process of Ag/BiVO4/Mn1−xZnxFe2O4. Its suitable dosage was 1.0 g in 50 mL ethanol solution. With a series of tests, the optimized doping dosage of Ag in the magnetic composite was determined to be 12.0 wt %.
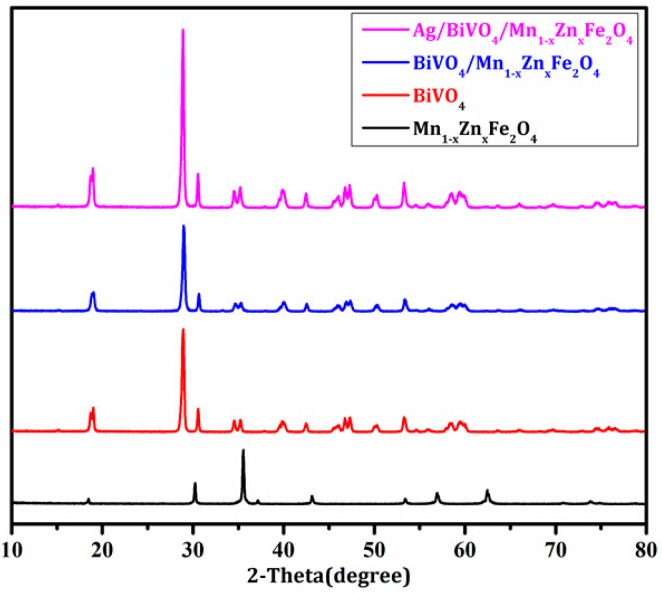
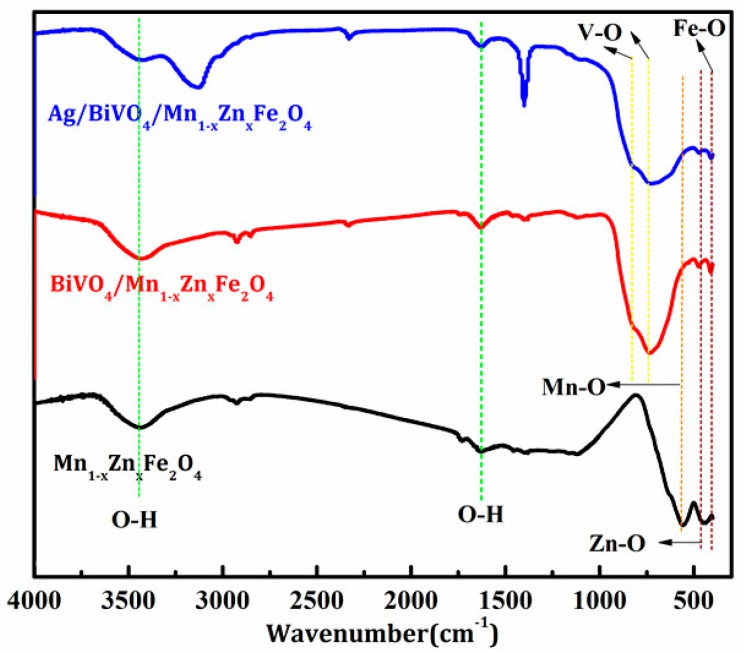
XRD patterns of Mn1−xZnxFe2O4, BiVO4, BiVO4/Mn1−xZnxFe2O4, and Ag/BiVO4/Mn1−xZnxFe2O4 were illustrated in Figure 1. It was noticed that each diffraction peak of Mn1−xZnxFe2O4 was indexed to the franklinite (cubic spinel) phase [23] which belonged to the Fd-3m (227) space group with a lattice size of 0.8474 nm. Three peaks at 28.9°, 35.2°, and 46.0° were clearly attributed to the iconic twin peaks of monoclinic scheelite BiVO4 (JCPDS 14-0688) [9]. The lattice parameters of the prepared BiVO4 was a = 5.1175 nm, b = 11.6697 nm, and c = 5.1084 nm. The peak at 28.9° (121) was used to calculate the average crystallite size that was 27.2 nm, while the average size of BiVO4/Mn1−xZnxFe2O4 and Ag/BiVO4/Mn1−xZnxFe2O4 was 29.0 nm and 32.8 nm, respectively. To gain further insight into the structure of Ag/BiVO4/Mn1−xZnxFe2O4, we carried out the measurement of bonds vibration absorption with Fourier transform infrared spectroscopy. Figure 2 showed the FTIR spectra of the composites. The vibration peaks of Mn-O, Zn-O, and Fe-O bands of Mn1−xZnxFe2O4 were severally at 560.1 cm−1, 473.7 cm−1, and 412.4 cm−1, while the V-O vibration absorption peaks of BiVO4 was at 734.3 cm−1 and 823.4 cm−1. This result confirmed the coexistence of Mn1−xZnxFe2O4 and BiVO4 in the composites. The absorption peaks at 2341.7 cm−1 and 3433.6 cm−1 were ascribed to CO2 and the surface adsorption H2O. There were not observable characteristic peaks of Ag in Figure 1 and Figure 2 due to its low content [24].
Figure 1.
XRD patterns of Mn1−xZnxFe2O4, BiVO4, BiVO4/Mn1−xZnxFe2O4, and Ag/BiVO4/Mn1−xZnxFe2O4.
Figure 2.
FTIR spectra of Mn1−xZnxFe2O4, BiVO4/Mn1−xZnxFe2O4, and Ag/BiVO4/Mn1−xZnxFe2O4.
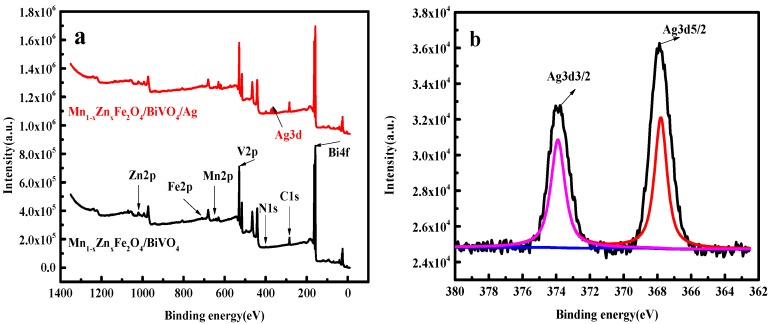
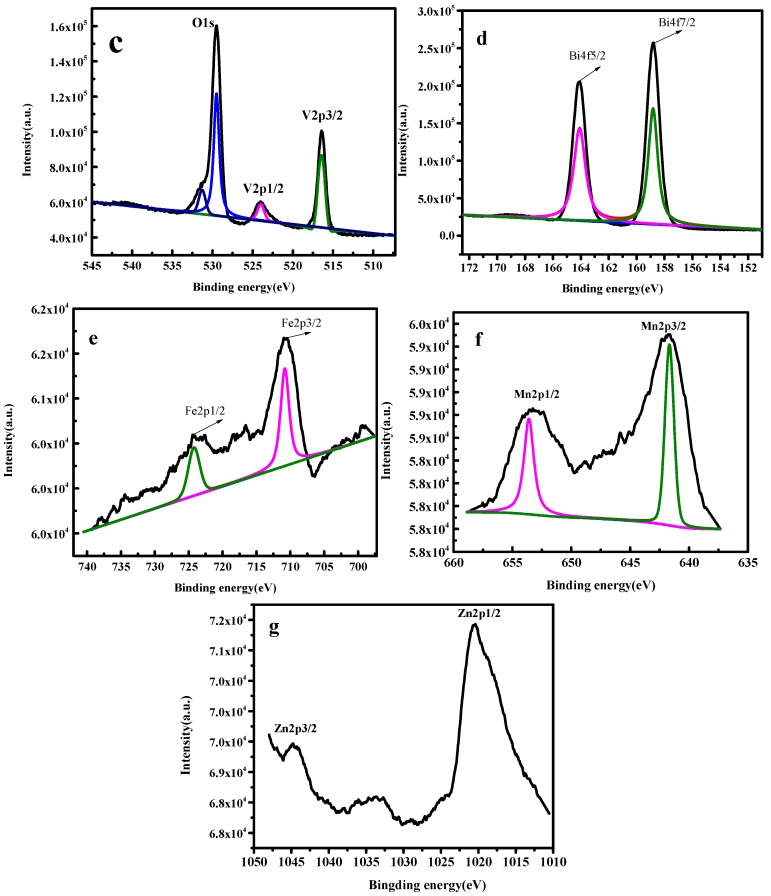
To discern the element contents in Ag/BiVO4/Mn1−xZnxFe2O4 and determine their valence states, XPS study was carried out. The binding energy peaks of Ag, O, V, Bi, Fe, Zn, and Mn were recorded in Figure 3. The peaks of O, V, Bi, Fe, Mn, and Zn elements were clearly observed in Figure 3a. Further comparing the fully scanning XPS spectra, it can be seen that the characteristic profile of Ag3d was obvious in Ag/BiVO4/Mn1−xZnxFe2O4 while Ag peak in BiVO4/Mn1−xZnxFe2O4 was not observed. Thus, it was deduced that the doping Ag in BiVO4/Mn1−xZnxFe2O4 was successful [25]. The peaks at the binding energy of 373.9 eV and 367.9 eV in Figure 3b was severally ascribed to Ag 3d3/2 and 3d5/2 [19], revealing the existence of Ag+. In Figure 3c, the peaks of O1s, V2p3/2, and V2p1/2 were located at 530.5 eV, 516.5 eV, and 523.4 eV, which were assigned to O2− and V-O bands. There were peaks of Bi4f5/2 and 4f7/2 at 164.1 eV and 158.3 eV in Figure 3d, indicating the presence of bismuth species of Bi3+ in BiVO4. Figure 3f displayed peaks at the binding energy of 641.5 eV (Mn2p3/2) and 653.1 eV (Mn2p1/2). The high resolution spectra of Fe2p as well as Zn2p were shown in Figure 3e,g. These peaks verified the presence of Mn1−xZnxFe2O4, which was consistent with the results of XRD and FTIR detection [26]. So, Ag/BiVO4/Mn1−xZnxFe2O4 was successfully assembled by in situ wet-chemistry synthesis method. This synthesis approach was simple, low cost, and environmentally friendly.
Figure 3.
XPS spectra of the magnetic composite (a) fully scanned spectra of BiVO4/Mn1−xZnxFe2O4 and Ag/BiVO4/Mn1−xZnxFe2O4; (b–g) narrow scan spectrum of Ag3d, O1s and V2p, Bi4f, Fe2p, Mn2p, and Zn2p of Ag/BiVO4/Mn1−xZnxFe2O4.
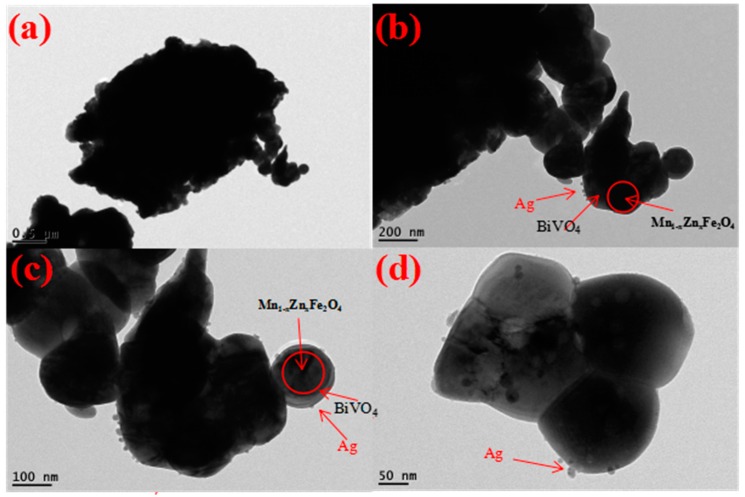
The morphological analysis of Ag/BiVO4/Mn1−xZnxFe2O4 was studied with transmission electron microscopy (TEM), and the results were displayed in Figure 4. By comparative experiments, it was demonstrated that the surface property of Ag/BiVO4/Mn1−xZnxFe2O4 was significantly improved when the appropriate dosage of polyvinylpyrrolidone (PVP) was used in the fabrication process of the composite. The improvement in properties resulted from the surface activity of PVP and the full uniform dispersion of Ag ions in the reaction solution. In addition, ethanol (solvent) further promoted the complete interface reaction of the ions with BiVO4/Mn1−xZnxFe2O4 particles. Namely, PVP could prompt the formation of nano-structural particles through in situ wet-chemistry method.
Figure 4.
TEM images of Ag/BiVO4/Mn1−xZnxFe2O4.
It was noted in Figure 4a,b that the bright surface sphere of BiVO4 attached the dark particles of Mn1−xZnxFe2O4. A small amount of Ag granular particles uniformly dispersed in the spherical surface in Figure 4c,d. As estimated from the images of BiVO4/Mn1−xZnxFe2O4, the average size of Ag granular particles was about 30 nm. The granular nanostructure particle of Ag favored production of rich active sites in the photocatalyst.
3.2. Light Absorption Property and Magnetic Property
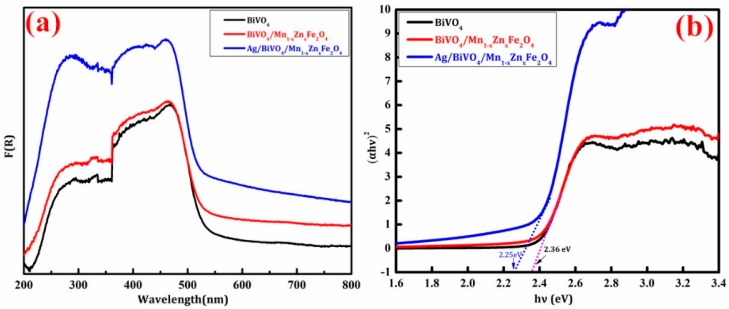
UV-vis diffuse reflectance spectrophotometry was a suitable and important technique to determine the light absorption for semiconductor photocatalysts [27]. Figure 5 showed UV-vis diffuse reflectance spectra (UV-vis DRS) of BiVO4, BiVO4/Mn1−xZnxFe2O4, and Ag/BiVO4/Mn1−xZnxFe2O4. It can be discovered from Figure 5a that the maximum absorption edge of Ag/BiVO4/Mn1−xZnxFe2O4 shifted to red light region, leading to the main absorption edge around 400 nm. First, the red shift was directly related to the electrons and Ag+ transformation between the conduction band and the valence band of BiVO4 [17]. Second, Ag particles had darkened color to enhance absorption of the visible-light for BiVO4/Mn1−xZnxFe2O4. Third, Ag particles could produce a strong surface plasmon resonance absorption. The band-gap energy (Eg) for Ag/BiVO4/Mn1−xZnxFe2O4 was about 2.25 eV. Eg values of BiVO4 and BiVO4/Mn1−xZnxFe2O4 in Figure 4b were about 2.36 eV. The incorporation of Mn1−xZnxFe2O4 did not change the optical properties of BiVO4 [18]. The relatively low Eg of Ag/BiVO4/Mn1−xZnxFe2O4 appeared to strengthen the absorption and sensitivity response for visible light. The significant enhancement of optics properties would be conducive to bringing high photocatalytic activity.
Figure 5.
(a) UV-vis diffuse reflection spectra of products and (b) plot of (Ahυ)2 versus photon energy (hυ) according to the UV-vis DRS.
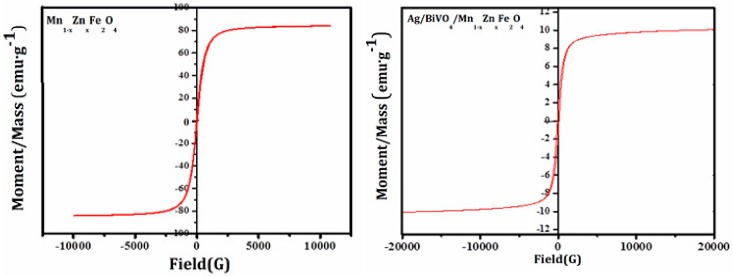
Hysteresis loops are the key way to characterize magnetization for magnetic materials. Figure 6 recorded the hysteresis loops of Ag/BiVO4/Mn1−xZnxFe2O4 and pure Mn1−xZnxFe2O4. The saturation magnetization (Ms) of Ag/BiVO4/Mn1−xZnxFe2O4 in Figure 6 was 10.04 emu/g. It was noted that large Ms was conducive towards the separation and recovery with an external magnet. Compared with Mn1−xZnxFe2O4, the Ms of Ag/BiVO4/Mn1−xZnxFe2O4 was declined by 87.5% due to the decrease of magnetic content per unit mass. More importantly, Ms of Ag/BiVO4/Mn1−xZnxFe2O4 was larger than that (7.01 emu/g) of Mn1−xZnxFe2O4/Bi2O3 [21]. The magnetic property was conducive to the stable activity of Ag/BiVO4/Mn1−xZnxFe2O4. The result revealed that the as-prepared magnetic photocatalyst was easily recovered by an external magnet. Therefore, it was concluded that Ag/BiVO4/Mn1−xZnxFe2O4 with good magnetic property possessed a high recovery rate.
Figure 6.
Hysteresis loops of BiVO4/Mn1−xZnxFe2O4 and Ag/BiVO4/Mn1−xZnxFe2O4.
3.3. Photocatalytic Activity
It was well-known that the photocatalytic ability was vital to photocatalytic materials, which was the base property for their industrial application. Generally, the photocatalytic activity was assessed with the degradation reaction of dyes.
3.3.1. Visible-Light-Driven Photocatalytic Activity
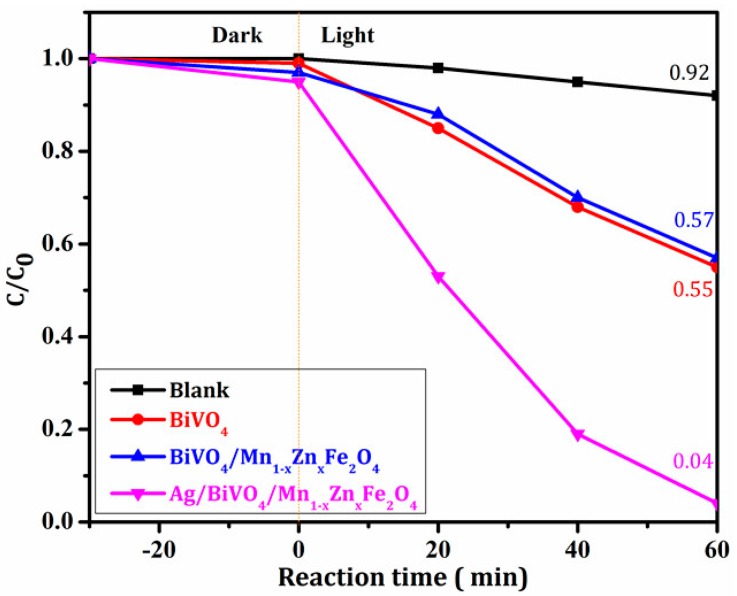
The photocatalytic performance of the samples under visible light irradiation was evaluated with the RhB photodegradation, and the result was shown in Figure 7. There was only a little degradation rate in the blank test (without any photocatalyst), indicating the poor self-degradation of RhB. The degradation rate for BiVO4 and BiVO4/Mn1−xZnxFe2O4 was approximately 45.0% after 60 min reaction. The same degradation rate proved that the introduction of Mn1−xZnxFe2O4 did not cause the activity loss of BiVO4. Figure 7 indicates that the degradation rate for Ag/BiVO4/Mn1−xZnxFe2O4 reached to 96.0% under the same condition. The setting time was only 60 min in this photodegradation test of RhB. Hence, the photocatalytic property of Ag/BiVO4/Mn1−xZnxFe2O4 was obviously higher than that of BiVO4 and BiVO4/Mn1−xZnxFe2O4. It meant that only 12.0 wt % Ag brought outstanding improvement in photocatalytic ability of BiVO4/Mn1−xZnxFe2O4.
Figure 7.
The degradation rates of RhB with the three photocatalysts.
In fact, most degradation tests are very slow (may take several hours) despite the improvements in visible light absorption of the photocatalyst. Here, the as-prepared Ag/BiVO4/Mn1−xZnxFe2O4 has a highly photocatalytic efficiency. This can be explained with the following three aspects: (1) Ag produced the surface plasmon resonance (plasma energy), which was transferred to BiVO4, leading to more formation of photo-excited electrons and holes. It was helpful to the enhancement of photocatalytic activity; (2) Ag particles acted as holes and accepted photo-produced electrons from the conduction band of BiVO4, extending the wavelength range and preventing the recombination of electrons and holes. The transformation or conversion of the charged particles in the interface was strengthened. In other words, the presence of Ag particles boosted the quantum efficiency for BiVO4/Mn1−xZnxFe2O4; (3) Owing to the nanostructure of Ag particles, Ag/BiVO4/Mn1−xZnxFe2O4 possessed a relatively large specific surface area, which increased the efficient sites and further yielded a high photocatalytic activity [19,25]. Thus, nanostructure Ag particles ensured high photocatalytic property of Ag/BiVO4/Mn1−xZnxFe2O4.
Ag/BiVO4/Mn1−xZnxFe2O4 was recovered with an external magnet in the end of the photocatalytic degradation test. 88~91 mg (after washing and drying) Ag/BiVO4/Mn1−xZnxFe2O4 could recover from initial dosage of 100 mg in each cycle. The average recovery rate of magnetic Ag/BiVO4/Mn1−xZnxFe2O4 was 89%, which was larger than the literature report value (85.0%) [28]. It is worth mentioning that the recovery method was quick with low energy consumption. The high recovery rate effectively avoided the leftover of catalysts in the water solution. Namely, Ag/BiVO4/Mn1−xZnxFe2O4 demonstrated itself as an environmentally friendly photocatalytic material and showed perspective industrial application in removal water-soluble contaminants.
The repeatability and stability were necessary in the practical photocatalytic application [27]. Cycling tests were employed to evaluate the photocatalytic stability of Ag/BiVO4/Mn1−xZnxFe2O4, and the degradation rate of RhB was described in Figure 8. It was clear that the degradation rate during the five cycles was severally 96.0%, 96.0%, 95.0%, 94.0% and 94.0%, which revealed photocatalyst efficiency of Ag/BiVO4/Mn1−xZnxFe2O4 barely decreased in the test process. Experiment results exhibited excellent photocatalytic stability. What is more, 94.0% of the degradation rate during the five cycles was very larger than that of the reference report [13]. So, magnetic photocatalyst Ag/BiVO4/Mn1−xZnxFe2O4 possessed promising prospect in the photo-decomposition organic dyes (industrial wastewater) field.
Figure 8.
Cycling tests of Ag/BiVO4/Mn1−xZnxFe2O4.
3.3.2. Photocatalytic Mechanism
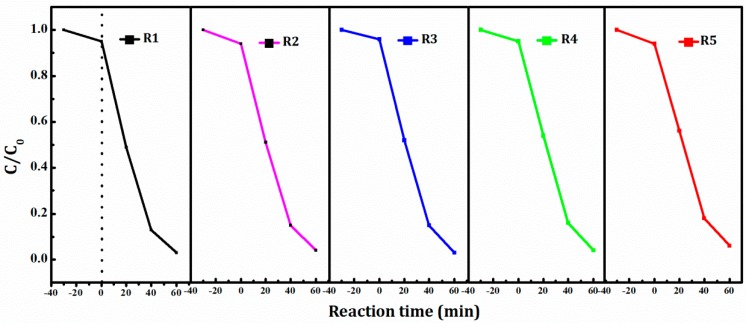
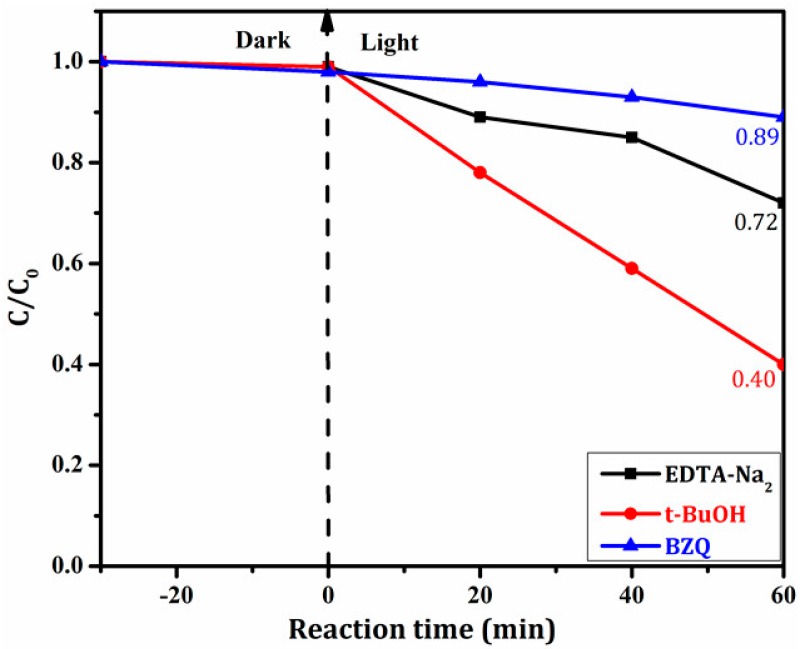
Radical scavengers were used to study active species in photocatalytic reaction, the result was displayed in Figure 9. In details, the degradation rate of RhB in Ag/BiVO4/Mn1−xZnxFe2O4-EDTA-Na2 (h+ scavenger) lowed and reached to 28.0%, which was significantly lower than that of Ag/BiVO4/Mn1−xZnxFe2O4. Under the same condition, the degradation rate steeply went down when BZQ (•O2− scavenger) in place of EDTA-Na2 was added into the reaction solution, the rate was only 11.0%. However, the introduction of t-BuOH (•OH scavenger) caused a large decrease in the degradation of about 60.0%. Namely, the change of the degradation rate in Ag/BiVO4/Mn1−xZnxFe2O4-t-BuOH was the smallest among three radical scavenger tests. The results illustrated that free radicals were major active species, and that •O2− played the domination role though •OH and h+ took part in the photocatalytic reaction.
Figure 9.
The photodegradation rate of RhB with Ag/BiVO4/Mn1−xZnxFe2O4 and different scavengers.
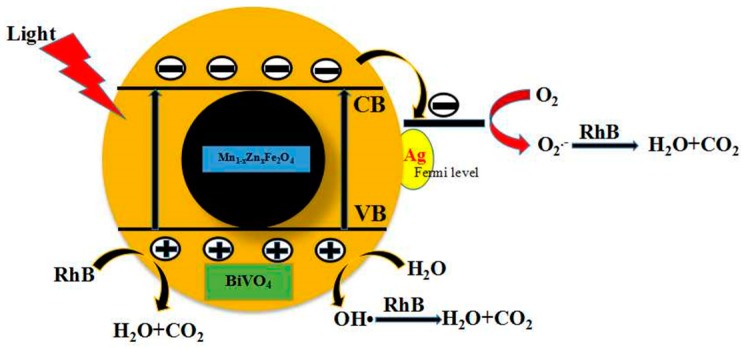
The electron transition occurred between valence band and conduction band, generating the photoelectrons and holes when the photon energy was higher than Eg of the semiconductor. The possible transition of photo-induced electron and hole was used to express the photocatalytic process under light irradiation. The photocatalytic mechanism of Ag/BiVO4/Mn1−xZnxFe2O4 was described in Figure 10. In detail, e- transferred to the surface of Ag particle, the dissolved oxygen (O2) could capture the electron and form the super oxygen radical (•O2− ) through the Fermi level surface resonance. The adsorbed H2O in the surface of Ag/BiVO4/Mn1−xZnxFe2O4 could be oxidized by holes (h+), yielding hydroxyl free radical (•OH). Both •O2− and •OH had a large oxidation ability and decomposed RhB into CO2 and H2O. At the same time, holes themselves prompted the degradation-oxidized of RhB [26]. Thus, the doping Ag was favorable to drive more •O2− and •OH radicals, strengthening the degradation of RhB in visible light irradiation.
Figure 10.
Photocatalytic mechanism scheme of Ag/BiVO4/Mn1−xZnxFe2O4.
In fact, we used Mn1−xZnxFe2O4 as magnetic substrate in order to simplify separation after photocatalytic reaction for BiVO4. The UV-vis DRS shown the incorporation of Mn1−xZnxFe2O4 did not enhance the optical properties of BiVO4. Noble metal-doping and graphene-loading were good ways to improve optical properties and enhance photocatalytic activity for single phase semiconductor. Here, we chose Ag-doping to boost the photocatalytic activity of BiVO4/Mn1−xZnxFe2O4. In addition, we will use graphene to modify BiVO4/Mn1−xZnxFe2O4. These studies will help to choose a better way (the above mentioned) for enhancing photocatalytic activity via comparing their photocatalytic activity and reaction kinetics, and then apply these findings to other signal semiconductors.
4. Conclusions
Ag/BiVO4/Mn1−xZnxFe2O4 was fabricated with the dip-calcination and in situ wet-chemistry synthesis method that was simple and environmentally-friendly. Element contents and their valence states in Ag/BiVO4/Mn1−xZnxFe2O4 were detected, indicating Ag granular particles dispersed in the spherical surface of BiVO4. The presence of Ag particles boosted the quantum efficiency, and further enhanced the photocatalytic activity. Under visible light irradiation (λ ≥ 400nm), the degradation rate of RhB using Ag/BiVO4/Mn1−xZnxFe2O4 after only 60 min reaction reached to 96.0%, which was greater than that of Mn1−xZnxFe2O4/BiVO4 and pure BiVO4. Most importantly, the degradation rate was close to 94.0% during the fifth recycle. We hope this research can promote the industrial application of BiVO4.
Author Contributions
Writing-Original Draft Preparation, H.L. and T.X.; Writing-Review & Editing, T.X. and C.L.; Supervision, C.L. and L.X.; Funding Acquisition, C.L.
Funding
This research was financially supported by the Fundamental and advanced research projects of Chongqing Science and Technology Commission (No. CSTC2015jcyjBX0015), and the Scientific & Technologic Program of Chongqing Land resources and Housing Authority (No. CQGT-KJ-2014012).
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- 1.Yu J.Q., Kudo A. Hydrothermal synthesis of nanofibrous bismuth vanadate. J. Chem. Lett. 2005;34:850–851. doi: 10.1246/cl.2005.850. [DOI] [Google Scholar]
- 2.Xia D.H., Hua L.L., Tan X.Q., He C., Pan W.Q., Yang T.S., Huang Y.L., Shu D. Immobilization of self-stabilized plasmonic Ag-AgI on mesoporous Al2O3 for efficient purification of industrial waste gas with indoor LED illumination. Appl. Catal. B: Environ. 2016;185:295–306. doi: 10.1016/j.apcatb.2015.12.019. [DOI] [Google Scholar]
- 3.Zhang L.S., Lian J.S., Wu L.Y., Duan Z.R., Jiang J., Zhao L.J. Synthesis of a Thin-Layer MnO2 Nanosheet-Coated Fe3O4 Nanocomposite as a Magnetically Separable Photocatalyst. Langmuir. 2014;30:7006–7013. doi: 10.1021/la500726v. [DOI] [PubMed] [Google Scholar]
- 4.Aiga N., Jia Q.X., Watanabe K., Kudo A., Sugimoto T., Matsumoto Y. Electron–Phonon Coupling Dynamics at Oxygen Evolution Sites of Visible-Light-Driven Photocatalyst: Bismuth Vanadate. J. Phys. Chem. C. 2013;117:9881–9886. doi: 10.1021/jp4013027. [DOI] [Google Scholar]
- 5.Chang X.X., Wang T., Zhang P., Zhang J.J., Li A., Gong J.L. Enhanced Surface Reaction Kinetics and Charge Separation of p–n Heterojunction Co3O4/BiVO4 Photoanodes. J. Am. Chem. Soc. 2015;137:8356–8359. doi: 10.1021/jacs.5b04186. [DOI] [PubMed] [Google Scholar]
- 6.Seabold J.A., Choi K.S. Efficient and Stable Photo-Oxidation of Water by a Bismuth Vanadate Photoanode Coupled with an Iron Oxyhydroxide Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2012;134:2186–2192. doi: 10.1021/ja209001d. [DOI] [PubMed] [Google Scholar]
- 7.Li H.Y., Sun Y.J., Cai B., Gan S.Y., Han D.X., Niu L., Wu T.S. Hierarchically Z-scheme photocatalyst of Ag@AgCl decorated on BiVO4(040) with enhancing photoelectrochemical and photocatalytic performance. Appl. Catal. B Environ. 2015;170:206–214. doi: 10.1016/j.apcatb.2015.01.043. [DOI] [Google Scholar]
- 8.Wang W.Z., Huang X.W., Wu S., Zhou Y.I., Wang L.J., Shi H.L., Liang Y.J., Zou B. Preparation of p–n junction Cu2O/BiVO4 heterogeneous nanostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2013;134:293–301. doi: 10.1016/j.apcatb.2013.01.013. [DOI] [Google Scholar]
- 9.Wang A.L., Shen S., Zhao Y.B., Wu W. Preparation and characterizations of BiVO4/reduced graphene oxide nanocomposites with higher visible light reduction activities. J. Colloid Intel. Sci. 2015;445:330–336. doi: 10.1016/j.jcis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Yu Q.Q., Tang Z.R., Xu Y.J. Synthesis of BiVO4 nanosheets-graphene composites toward improved visible light photoactivity. J. Energy Chem. 2014;23:564–574. doi: 10.1016/S2095-4956(14)60186-8. [DOI] [Google Scholar]
- 11.Abdi F.F., Dabirian A., Dam B., van de Krol R. Plasmonic enhancement of the optical absorption and catalytic efficiency of BiVO4 photoanodes decorated with Ag@SiO2 core-shell nanoparticles. Phys. Chem. Chem. Phys. 2014;16:15272–15277. doi: 10.1039/C4CP01583E. [DOI] [PubMed] [Google Scholar]
- 12.Li R.G., Han H.X., Zhang F.X., Wang D., Li C. Highly efficient photocatalysts constructed by rational assembly of dual-cocatalysts separately on different facets of BiVO4. Energy Environ. Sci. 2014;7:369–1376. doi: 10.1039/C3EE43304H. [DOI] [Google Scholar]
- 13.Xu L., Wei Y.G., Guo W., Guo Y.H., Guo Y.N. One-pot solvothermal preparation and enhanced photocatalyticactivity of metallic silver and graphene co-doped BiVO4ternarysystems. Appl. Surf. Sci. 2015;332:682–693. doi: 10.1016/j.apsusc.2015.01.235. [DOI] [Google Scholar]
- 14.Rismanchian A., Chen Y.W., Chuang S.S.C. In situ infrared study of photoreaction of ethanol on Au and Ag/TiO2. Catal. Today. 2016;264:16–22. doi: 10.1016/j.cattod.2015.07.038. [DOI] [Google Scholar]
- 15.Benedetti J.E., Bernardo D.R., Morais A., Bettini J., Nogueira A.F. Synthesis and characterization of a quaternary nanocomposite based on TiO2/CdS/rGO/Pt and its application in the photoreduction of CO2 to methane under visible light. RSC Adv. 2015;5:33914–33922. doi: 10.1039/C4RA15605F. [DOI] [Google Scholar]
- 16.Xue Y., Wang X.T. The effects of Ag doping on crystalline structure and photocatalytic properties of BiVO4. Int. J. Hydrogen Energy. 2015;40:5878–5888. doi: 10.1016/j.ijhydene.2015.03.028. [DOI] [Google Scholar]
- 17.Chen L., Huang R., Ma Y.J., Luo S.L., Au C.T., Yin S.F. Controllable synthesis of hollow and porous Ag/BiVO4 composites with enhanced visible-light photocatalytic performance. RSC Adv. 2013;3:24354–24361. doi: 10.1039/c3ra43691h. [DOI] [Google Scholar]
- 18.Chen F., Yang Q., Wang Y.L., Zhao J.W., Wang D.B., Li X.M., Guo Z., Wang H., Deng Y.C., Niu C.G., et al. Novel ternary heterojunction photcocatalyst of Ag nanoparticles and g-C3N4 nanosheets co-modified BiVO4 for wider spectrum visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2017;205:133–147. doi: 10.1016/j.apcatb.2016.12.017. [DOI] [Google Scholar]
- 19.Xie T.P., Liu C.L., Xu L.J., Li H. New Insights into MnxZn1−xFe2O4 via Fabricating Magnetic Photocatalyst Material BiVO4/MnxZn1−xFe2O4. Materials. 2018;11:335. doi: 10.3390/ma11030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi Y., Yuan Q., Li Y.J., Zhao L., Li N., Li X.T., Yan W.F. Magnetically separable Fe3O4@SiO2@TiO2-Ag microspheres withwell-designed nanostructure and enhanced photocatalytic. J. Hazard. Mater. 2013;262:404–411. doi: 10.1016/j.jhazmat.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z.D., Xu L.J., Liu C.L. Preparation and characterization of composite magnetic photocatalyst MnxZn1−xFe2O4/β-Bi2O3. RSC Adv. 2015;5:79997–80004. doi: 10.1039/C5RA11914F. [DOI] [Google Scholar]
- 22.Liu C.L., Li H., Ye H.P., Xu L.J. Preparation and Visible-Light-Driven Photocatalytic Performance of Magnetic SrFe12O19/BiVO4. J. Mater. Eng. Perform. 2015;24:771–777. [Google Scholar]
- 23.Laohhasurayotin K., Pookboonmee S., Viboonratanasri D., Kangwansupamonkon W. Preparation of magnetic photocatalyst nanoparticles TiO2/SiO2/Mn–Zn ferrite and its photocatalytic activity influenced by silica interlayer. Mater. Res. Bull. 2012;47:1500–1507. doi: 10.1016/j.materresbull.2012.02.030. [DOI] [Google Scholar]
- 24.Wu S.K., Shen X.P., Zhu G.X., Zhou H., Ji Z.Y., Chen K.M., Yuan A.H. Synthesis of ternary Ag/ZnO/ZnFe2O4 porous and hollownanostructures with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016;184:328–336. doi: 10.1016/j.apcatb.2015.11.035. [DOI] [Google Scholar]
- 25.Saravanakumar K., Ramjan M.M., Suresh P., Muthuraj V. Fabrication of highly efficient visible light driven Ag/CeO2 photocatalyst for degradation of organic pollutants. J. Alloys Comp. 2016;664:149–160. doi: 10.1016/j.jallcom.2015.12.245. [DOI] [Google Scholar]
- 26.Xie T.P., Liu C.L., Xu L.J., Yang J., Zhou W. Novel Heterojunction Bi2O3/SrFe12O19 Magnetic Photocatalyst with Highly Enhanced Photocatalytic Activity. J. Phys. Chem. C. 2013;117:24601–24610. doi: 10.1021/jp408627e. [DOI] [Google Scholar]
- 27.Yang J., Xu L.J., Liu C.L., Xie T.P. Preparation and photocatalytic activity of porous Bi5O7I nanosheets. Appl. Surf. Sci. 2014;319:265–271. doi: 10.1016/j.apsusc.2014.07.055. [DOI] [Google Scholar]
- 28.Xie T.P., Xu L.J., Liu C.L., Yang J., Wang M. Magnetic composite BiOCl–SrFe12O19: A novel p-n type heterojunction with enhanced photocatalytic activity. Dalt. Trans. 2014;43:2211–2220. doi: 10.1039/C3DT52219A. [DOI] [PubMed] [Google Scholar]