Abstract
HIV-1-infected cells can avoid cytotoxic T lymphocyte killing by Nef-mediated down-regulation of surface MHC I. Here, we show that HIV-1 Nef inhibits MHC II restricted peptide presentation to specific T cells and thus may affect the induction of antiviral immune responses. Nef mediates this effect by reducing the surface level of mature (i.e., peptide-loaded) MHC II while increasing levels of immature MHC II, which are functionally incompetent because of their association with the invariant chain. Nef was the only HIV-1 gene product to possess this capacity, which was also observed in the context of the whole HIV-1 genome. Other proteins of the endocytic pathway were not affected by Nef expression, suggesting that Nef effects on MHC II did not result from a general alteration of the endocytic pathway. Response patterns to previously characterized mutations of Nef differed for Nef-induced modulation of mature and immature MHC II. Furthermore, the doses of Nef required to observe each of the two effects were clearly different, suggesting that Nef could affect MHC II peptide presentation through distinct mechanisms. Cooperation between those mechanisms may enable Nef to efficiently inhibit MHC II function.
Keywords: class II-associated invariant chain, immune escape, adaptor complexes
Among the few proteins encoded by HIV, Nef seems to exhibit multiple functions. In vivo, it is required for efficient viral replication and pathogenicity (1). In vitro, Nef affects cellular signal transduction pathways and down-modulates surface expression of CD4, the receptor for HIV, by a posttranslational mechanism. Nef also down-regulates MHC I molecules (HLA-A and HLA-B) (2, 3), resulting in lower epitope presentation and possible escape from lysis by HIV-specific cytotoxic T cells (4). In contrast, Nef has no effect on HLA-C and HLA-E, thus protecting infected cells from natural killer cell-mediated lysis (5). The mechanisms underlying Nef-induced down-modulation of CD4 and MHC I are beginning to be unraveled. Nef induces CD4 degradation, first by promoting its accelerated endocytosis, connecting the tail of CD4 to complexes of the endocytic pathway [AP-2 and vacuolar H+-ATPase (v-ATPase)] (6–8) and second by addressing CD4 to lysosomes through interaction with COP I coatamers (9). Regarding MHC I, Nef would induce their relocalization at the trans-Golgi network (TGN) through interactions with coat complexes at this location, such as AP-1 and PACS 1 (3, 10, 11). Different functional domains of Nef have been ascribed to each of these two effects (1).
HIV is known to infect key players of the immune system: dendritic cells, activated CD4+ T cells, and macrophages. All of these cell types express surface MHC II molecules; consequently, HIV particles bear MHC II molecules at their surface (12). In monocytic cells chronically infected with HIV-1 in vitro, impairment of MHC II expression and antigen uptake was reported (13). MHC II molecules are specialized in the presentation of antigenic peptides to CD4+ T cells. This presentation is central to initiate immune responses as well as to establish efficient secondary immune responses. To fulfill their function, MHC II molecules follow a particular intracellular pathway. The α and β chains associate with the invariant chain chaperone (Ii) in the endoplasmic reticulum. After transfer to the Golgi apparatus, these complexes are sorted toward the endocytic pathway because of signals carried by Ii cytoplasmic tail. A fraction of the αβIi complexes travels first to the cell surface before internalization and access to the endocytic pathway. There, Ii is degraded in a sequential manner, allowing MHC II to bind antigenic peptides, generated in these acidic compartments, before reaching the cell surface.
HIV infection is known to target and affect the CD4+ T cell compartment. Here, we investigated the effect of HIV infection on the capacity of antigen-presenting cells to stimulate CD4+ T cells through their MHC II molecules. We show that the efficiency of peptide presentation was reduced by Nef, suggesting that MHC II presentation of viral antigens in infected antigen-presenting cells could be impaired by Nef expression. This resulted from Nef-induced increase of immature MHC II and a down-modulation of mature MHC II at the cell surface. Thus, Nef may affect the generation of the CD4+ Th activity specific for HIV, an activity shown to be central for the control of the infection (14).
Materials and Methods
Antibodies.
The following antibodies were used (see ref. 15 and references therein): anti-HLA-DR: TU36, L243, and 1B5; anti-Ii: a rabbit immune sera, PIN1 directed against the N terminus of Ii, BU45, and LN2 directed against the luminal part of Ii; anti-MHC I: W6/32; anti-transferrin receptor: 66Ig10; anti-Nef: MATG 020; and anti-myc tag: 9E10, anti-Lamp1 (PharMingen), and anti-CD4 (Becton Dickinson). Secondary antibodies were from Jackson ImmunoResearch.
Plasmids.
We used cytomegalovirus-promoter-driven plasmids; Nef-FT carrying the nef gene from LAI, Nef-A01 carrying the nef gene from A01, a control plasmid Nef-mock carrying the nef-A01 gene in an antisense orientation (3), Nef-G2A plasmid carrying the nef LAI gene with a point mutation G→A at position 2, and the Nef-FT-derived plasmid encoding the mutant NefLL165GG carrying the nef LAI gene with point mutation L→G at positions 164 and 165. Nef-HXB2 and its derived mutants, NefWL58AA, NefEEEE65AAAA, and NefPP75/78AA (16), were a gift of D. Trono (University of Geneva, Switzerland). We also used the plasmid carrying the cDNA of the provirus HIV-1NL43 and its counterpart deleted of the nef gene, HIV-1NL43ΔNef (17), the pcCD4 plasmid, a plasmid encoding class II transactivator (CIITA) (18), the pEGFP-N1 plasmid (CLONTECH), and the pSP72 plasmid (Promega). The DR1β*0101 cDNA was subcloned from the plasmid pSP72-DR1β (19) into the BglII/SacI sites of the NT-Hygro plasmid provided by C. Bonnerot (Institut Curie, France). The CD8stop plasmid carries a construct of CD8 lacking its cytoplasmic tail (20).
Transfections.
To establish HeLa-CIITA clones, HeLa cells were transfected with the CIITA plasmid. After selection and cloning, surviving clones were tested for high surface expression of MHC II and subcloned once. One of the clones was chosen and further analyzed. It showed large amounts of MHC II molecules accumulated in lysosome-like compartments positive for Lamp1, HLA-DM, and CD63 (not shown). As expected, HeLa-CIITA cells also expressed Ii and HLA-DM but not HLA-DO (not shown). For transient transfection experiments, 8 × 106 cells were electroporated by using a Bio-Rad gene pulser II (settings at 200 V and 975 μF) with 5–25 μg of the different expression vectors mixed with 1 μg of pEGFP-N1 and complemented with the pSP72 plasmid to a total of 30 μg of DNA per electroporation. By using green fluorescent protein (GFP) fluorescence as a read out, transfection rates consistently remained lower in MelJuSo cells (approximately 10%) than in HeLa-CIITA cells (approximately 20–30%). Therefore, the latter were used for all experiments except when indicated otherwise. Note that the transfection rates were underestimated because the pEGFP plasmid only represented 1/30 of the DNA mix used for electroporation.
Peptide Presentation.
HeLa-CIITA cells were found homozygous for HLA-DR*0102 and thus do not efficiently present the HA peptide (HA 306–318, PKYVKQNTLKLAT) to the HLA-DR1 restricted THA1–7 T cell line (21). HeLa-CIITA cells were transfected with a mix of plasmids containing CD8stop, DR1β*0101, and Nef-FT or Nef-mock. 24 h later, CD8+ cells were positively selected by MACS sorting following the instructions given by the supplier by using MicroBeads conjugated to an mAb anti-CD8 (LeuTM-2a) (Miltenyi Biotec, Auburn, CA). Isolated cells were then incubated with different concentrations of the HA peptide. Twenty-four hours later, cells were washed and fixed with 0.005% glutaraldehyde in PBS for 1 min. After quenching in complete medium, cells were washed, and 5 × 104 THA1–7 T cells were added per well; 24 h later, the supernatants were harvested, and the IL-2 content was measured by [3H]thymidine incorporation of the IL-2-dependent CTL-L2 cell line.
Flow Cytometry.
Cells were stained with various mAbs, by using as secondary reagent phycoerythrin-conjugated goat anti-mouse F(ab)′2 fragments. Flow cytometry analysis was performed with a FACScan (Becton Dickinson). Dead cells were excluded by a gate in FSC/SSC. Events corresponding to at least 3,000 live cells GFP+ were accumulated per sample. For security reasons, experiments with cells transfected with provirus were performed with fixed cells on a different site and therefore acquired with a different FACScan apparatus.
Confocal Immunofluorescence Microscopy.
Cells were stained as described (3), by using secondary antibodies coupled to Cy-3 or Cy-5, and analyzed by using a TCS4D scanning laser confocal microscope (Leica, Deerfield, IL).
Cell-Surface Biotinylation.
Cells were washed in ice-cold PBS before labeling with 1 mg/ml sulfo-NHS-LC-biotin in PBS for 30 min on ice. Biotinylation was stopped with 0.1 M glycine in PBS for 10 min. Cells were then lysed and subjected to immunoprecipitations. Samples were run on SDS/PAGE, transferred on Immobilon-P poly(vinylidene difluoride) membranes, exposed to streptavidin–peroxidase, and revealed by chemiluminescence. As a control for cell integrity at the time of surface biotinylation, immunoprecipitations of a cytosolic protein (Cbl1) were performed in parallel and generated no detectable signal (not shown). Results were quantified by analyzing scanned films with National Institutes of Health image software.
Results
Antigenic Peptide Presentation Is Reduced in Nef-Expressing Cells.
To study the effects of the HIV-1 protein Nef on MHC II molecules, we generated HeLa cells stably transfected with the transactivator CIITA, which induces the expression of genes required for MHC II presentation (including HLA-DR, HLA-DM, and Ii). This strategy was chosen to obtain an MHC II+ cell line, termed HeLa-CIITA, that can be used in transient transfection experiments, as Nef cytotoxicity makes it difficult to establish stable cell lines expressing high levels of this protein.
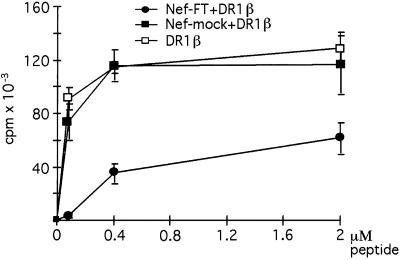
To evaluate potential effects of Nef expression on MHC II function, peptide presentation assays were performed by using the THA1–7 T cell line, which specifically recognizes HLA-DR1 molecules associated with the HA peptide (306) from the influenza hemagglutinin. HeLa-CIITA cells were transfected with DR*0101β in combination either with the Nef-FT vector carrying the nef LAI gene or with the control plasmid, Nef-mock. A small amount of a plasmid encoding a tailless form of CD8 (CD8stop) was added to the DNA used for electroporation to allow sorting of transfected cells by MACS on the basis of their CD8 expression. The resulting populations were tested for their capacity to present the HA peptide. Cells expressing Nef stimulated specific T cells clearly less efficiently than the control cells (Fig. 1), whereas nontransfected cells did not present the peptide at all (not shown).
Figure 1.
Peptide presentation is reduced by Nef expression. HeLa-CIITA cells were transfected with CD8stop in combination with the indicated plasmids. CD8+ sorted cells were assayed for presentation of the HA peptide to the THA1.7 T cell line specific for HLA-DR1/HA complexes. T cell responses, reflected by proliferation of the CTL-L2 cells, are plotted as a function of peptide concentration. Error bars are calculated for triplicates of one representative experiment.
To investigate how Nef mediates this phenomenon, we first looked at cell-surface expression of MHC II on the sorted cell populations used for the peptide presentation assays. A reduced surface level of mature MHC II was observed by cytofluorimetry in cells expressing Nef when compared with Nef-mock transfected cells or to cells only transfected with DR1β (not shown).
Nef Induces MHC II Down-Modulation and Increases Surface Expression of the Ii Chain.
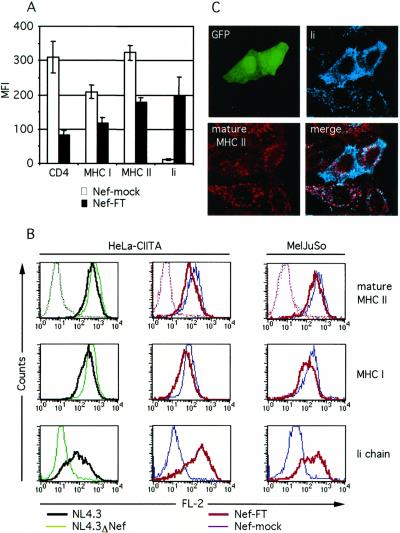
Because the procedure of isolating transfected cells by MACS only yielded 80% purity, another experimental system was set up to confirm and extend the results regarding surface MHC II expression. Cotransfection of a small amount of pEGFP plasmid into HeLa-CIITA cells together with CD4 and Nef-FT or Nef-mock allowed FACS analysis of transfected cells gated on the basis of GFP expression. Twenty-four hours after transfection, cells were analyzed by flow cytometry with different mAbs revealed with phycoerythrin-labeled secondary antibodies. As expected, transfection of Nef-FT induced a down-modulation of MHC I and CD4 (Fig. 2A). In addition, cells expressing Nef exhibited a decrease of mature HLA-DR molecules visualized with the mAb L243 of similar amplitude as the MHC I reduction (Fig. 2A). The mean fluorescence intensity (MFI) of transfected cells stained with the anti-mature MHC II antibody was on average 1.9 times lower than in control cells. Because Ii plays an important role for the trafficking of MHC II, surface expression of Ii was quantified on the same cell populations. Nef expression induced a striking increase of Ii surface expression (MFI mean increase of 16.7 times) compared with Nef-mock transfected cells (Fig. 2A), which resembled nontransfected cells regarding MHC II, MHC I, and Ii (not shown). Two different mAbs specific for the luminal side of Ii (LN2 and BU45) generated identical FACS profiles (not shown).
Figure 2.
Nef down-modulates surface-mature MHC II and induces an increase of surface Ii expression. (A) FACS analysis of HeLa-CIITA cells transfected with Nef-FT or Nef-mock in combination with the plasmids encoding CD4 and EGFP. MFI values of phycoerythrin fluorescence of GFP+ cells were subtracted of the negative control value and plotted for each indicated marker. The data shown represent mean values of four independent experiments. (B) FACS profiles of HeLa-CIITA or MelJuSo cells transiently transfected with the indicated plasmids, each in combination with the pEGFP plasmid (see Materials and Methods). Flow cytometry histograms of GFP+ gated cells are presented for phycoerythrin fluorescence. An irrelevant mAb, 9E10, was used as a negative control (dotted lines). (C) Localization of Ii and mature MHC II by confocal microscopy of HeLa-CIITA cells transfected with the Nef-FT and pEGFP plasmids. Transfected cells are identified by GFP expression.
To extend our results to a well studied model of MHC II trafficking, the same experiments were performed with the melanoma cell line MelJuSo (HLA-DR3). Typical FACS profiles of a representative experiment are given for HeLa-CIITA and MelJuSo cells. In this cell type, surface expression of MHC II, MHC I, and Ii were modulated by Nef in a manner similar to that observed in HeLa-CIITA cells (Fig. 2B). The use of a control mAb generated identical negative profiles for Nef-FT and Nef-mock transfected cells (Fig. 2B).
To test whether Nef would act the same way in the context of HIV-1 and whether other viral gene products might contribute to the Nef-induced effects or possess similar capacities, proviral DNA of HIV-1NL43 or HIV-1NL43ΔNef, from which the nef gene has been deleted, were electroporated into HeLa-CIITA cells. As before, cotransfection of the pEGFP plasmid was performed to allow FACS analysis of transfected cells. HIV-1NL43 expression induced a down-modulation of mature HLA-DR molecules while dramatically increasing levels of surface Ii (Fig. 2B). Again, a down-modulation of MHC I of similar amplitude to MHC II was observed in this experimental system (Fig. 2B). In contrast, the HIV-1NL43ΔNef provirus did not modify surface expression of MHC II, MHC I, and Ii (Fig. 2B), indicating that among the HIV-1 proteins, only Nef has this capacity. Cells transfected with a control plasmid (pSP72) gave results very similar to those transfected with HIV-1NL43ΔNef (not shown).
To further study the effects induced by Nef, HeLa-CIITA cells cotransfected with the Nef-FT and the pEGFP plasmids were analyzed by three-color confocal microscopy. Confirming our FACS data, in transfected cells identified by GFP expression, Ii staining was bright and diffuse, encompassing the cell surface, whereas the amount of surface-mature MHC II generally appeared reduced (Fig. 2C). In contrast, in nontransfected cells, Ii was exclusively localized in intracellular vesicles distinct from those containing mature MHC II; the latter were also present at the cell surface (Fig. 2C).
We conclude that among HIV-1 proteins, only Nef had the capacity to enhance Ii and reduce mature MHC II expression at the cell surface.
Selectivity of Nef Effects on MHC II.
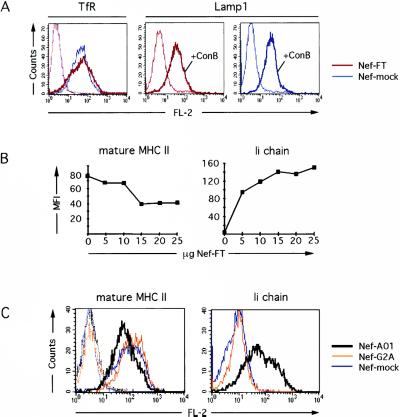
Because newly synthesized MHC II traffic through the endocytic pathway before acquiring peptides and reaching the cell surface, we tested whether Nef would modify surface expression of other protein constituents of this pathway. Transferrin receptor, a marker for early/recycling endosomes, and the lysosomal resident proteins Lamp1 and Lamp2 were examined. HeLa-CIITA cells transfected with Nef-FT or Nef-mock, and pEGFP expressed comparable surface levels of transferrin receptor and Lamp1 as judged by FACS analysis (Fig. 3A). Similarly, Lamp2 FACS profiles were identical in both cell populations (not shown). This suggested that Nef did not induce a redistribution of markers of the various endocytic compartments, but selectively affected MHC II trafficking.
Figure 3.
Characterization of Nef effects on MHC II and Ii surface expression. (A) HeLa-CIITA cells transfected with Nef-FT (red lines) or Nef-mock (blue lines) and pEGFP were either untreated (thin lines) or exposed to 20 nM ConB overnight (bold lines) and stained for the indicated markers. Analysis of GFP+ cells was performed as in Fig. 2. (B) Nef-FT dose-response curves. HeLa-CIITA cells transfected with pEGFP and increasing amounts of the Nef-FT plasmid were stained with anti-Ii and anti-mature MHC II mAbs. MFI values of GFP+ gated cells were determined for surface Ii and mature MHC II in FL-2. The background MFI value (obtained with a control mAb, PIN1) was subtracted from these MFI values, which were plotted as a function of the amount of the Nef-FT plasmid transfected. (C) HeLa-CIITA cells transfected with pEGFP and Nef-A01 (bold black line), Nef-G2A (orange line), or Nef-mock (blue line) were stained and analyzed by flow cytometry as in Fig. 2. The 9E10 mAb was used as a negative control (dotted lines).
Nef can bind the catalytic subunit of the v-ATPase, which is a proton pump required for the acidification of the vacuolar system (8). Exposure to concanamycin B (ConB), a potent inhibitor of the v-ATPase, results in an increased surface expression of Ii and Lamp1 but not Lamp2 in Epstein–Barr virus-transformed B cells (15), and we obtained similar results in HeLa-CIITA cells (Fig. 3A and data not shown). When ConB was added to Nef-FT or Nef-mock transfected HeLa-CIITA cells, a similar increase of Lamp1 surface expression was observed (Fig. 3A). Moreover, acidification of the endosomal pathway did not appear modified by Nef as tested by cytofluorimetry of cells exposed to lysotracker (not shown). These results indicated that Nef mediated its effects on MHC II without interfering with v-ATPase activity.
To further analyze the Nef-induced effects on MHC II and Ii, Nef expression was titrated. Induction of surface Ii increased with the quantity of Nef-FT plasmid transfected and was detected from 5 μg of DNA per electroporation approaching a plateau at 15 μg (Fig. 3B). In contrast, MHC II down-modulation required at least 15 μg of the Nef-FT plasmid and was barely detectable below that dose (Fig. 3B), suggesting a possible involvement of two distinct mechanisms. In agreement with previous studies, we observed that CD4 down-modulation was induced by lower doses of Nef than was down-modulation of MHC I (not shown). Interestingly, both MHC I and mature MHC II down-modulation required ≈15 μg of Nef-FT plasmid. Of note, HeLa cells transfected with similar amounts of the same Nef-FT plasmid expressed levels of Nef comparable to those measured in HIV-1 infected cells (3). This strongly suggests that the levels of Nef expressed in HeLa-CIITA cells were physiologically relevant.
We next tested whether membrane insertion of Nef was required for the effects on Ii and MHC II with the use of Nef-G2A, a mutant of Nef, which cannot be myristoylated. Expression of this mutant at levels similar to Nef wild type (as confirmed by Western blot, not shown) had no effect on Ii and MHC II surface expression, as FACS profiles were identical to the ones obtained with Nef-mock transfected cells (Fig. 3C). Two other HIV-1 Nef proteins, Nef-A01 derived from a primary isolate and Nef-HXB2, were tested in HeLa-CIITA cells and generated results similar to those obtained with Nef-FT (Fig. 3C and data not shown), suggesting that this capacity is common to various HIV-1 isolates. Nef effects on MHC II complexes were thus selective and dose dependent.
Nef Induces Surface Expression of Immature MHC II Complexes.
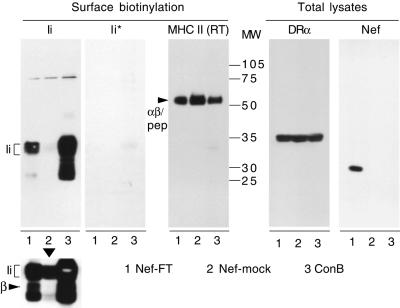
To test whether Ii that reaches the cell surface was associated with MHC II α and β chains, surface biotinylation experiments were carried out on HeLa-CIITA cells transfected with Nef-FT or Nef-mock. As a positive control for surface Ii, untransfected cells were exposed overnight to ConB, which induces expression of high levels of surface αβIi complexes (15). Following cell-surface biotinylation, cells were lysed and subjected to immunoprecipitations. The immunoprecipitates were analyzed by Western blot by using streptavidine–peroxidase. Confirming our FACS and confocal data, surface Ii was clearly detected with the anti-Ii mAb in cells expressing Nef in contrast to control cells (Fig. 4, Ii). In cells exposed to ConB, high amounts of cell surface Ii were detected as expected. On longer exposures (Fig. 4, Lower), the DRβ chain was clearly visible in cells expressing Nef, showing that surface Ii was associated with DRβ and therefore probably with DRα as well (comigration of Ii with the latter prevented clear identification). No Ii was detectable in an anti-Ii immunoprecipitation, when all HLA-DR complexes had been previously removed from the cell lysate by a TU36 immunoprecipitation (Fig. 4, Ii*). TU36 is specific for the DRαβ complex, with or without Ii, and cannot recognize any of the three chains alone (19). Therefore, all Ii molecules reaching the cell surface were most likely associated with α and β chains generating αβIi complexes, referred to as immature MHC II in the continuation of this study. Confirming our FACS data, surface SDS-stable HLA-DR complexes (indicative of a peptide-loaded state) were reduced in Nef expressing cells by 35–50% compared with control cells and by 30–45% in cells exposed to ConB [Fig. 4, MHC II (RT)]. Accordingly, levels of mature HLA-DR immunoprecipitated by L243 were reduced to similar extents (not shown). In contrast, total levels of HLA-DRα were not affected by Nef expression or by ConB exposure as judged by Western blot analysis of total cell lysate (Fig. 4), suggesting that biosynthesis of MHC II is not affected by Nef. It has to be taken into account that 100% of the cells were affected by ConB exposure, whereas only part of the electroporated cells expressed Nef, probably leading to an underestimation of the effects induced by Nef. Thus, Nef expression alters MHC II intracellular trafficking, leading to an increase of αβIi complexes and a decrease of peptide-loaded MHC II molecules at the cell surface.
Figure 4.
Biochemical characterization of surface MHC II complexes. Cell-surface biotinylation followed by parallel and sequential immunoprecipitations were performed with HeLa-CIITA cells transfected with Nef-FT, Nef-mock, or nontransfected cells exposed to 20 nM ConB overnight. Immunoprecipitates performed with mAbs specific for the indicated proteins were eluted at 95°C (except RT, which means eluted at room temperature) and revealed with streptavidine–peroxidase by Western blot. Below the Ii panel, a longer exposure of the same blot is presented. The Ii* panel corresponds to immunoprecipitates performed with the anti-Ii mAb PIN1 on lysates that had been precleared with the anti-HLA-DR mAb TU36. A short exposure of the MHC II panel obtained by elution at room temperature of immunoprecipitations performed with TU36 is presented to allow visualization of the different levels of SDS-stable complexes, but Ii bands clearly appeared on longer exposures (not shown). Total lysates from the same cells were analyzed by Western blot by using either the anti-HLA-DRα mAb 1B5 or the anti-Nef mAb MATG 020.
Nef Acts on Mature and Immature MHC II.
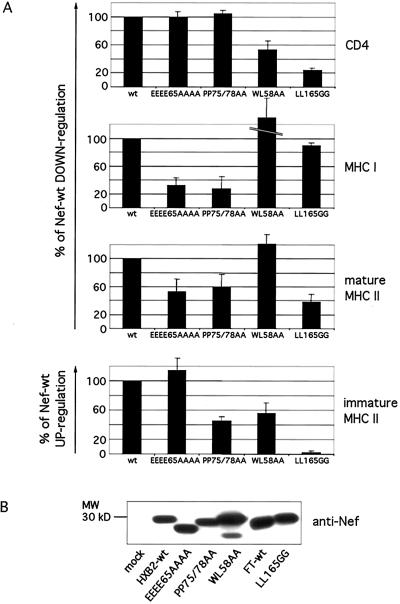
A mutational analysis of Nef was conducted to try to segregate Nef effects on mature and immature MHC II from each other and from Nef effects on CD4 and MHC I. Four mutants: NefWL58AA, NefLL165GG, NefEEEE65AAAA, and NefPP75/78AA, known to have differential effects on CD4 and MHC I down-modulation (16), were studied for their capacity to modulate surface-mature and immature MHC II complexes. This was followed by FACS analysis by using the anti-mature MHC II mAb L243 and the anti-Ii mAb LN2, respectively. The effects of the different mutants on MHC I and CD4 surface levels were tested in parallel. In our cellular system also, the two sets of mutations, WL58AA and LL165GG, did not affect Nef capacity to down-modulate MHC I but reduced the Nef-mediated down-regulation of CD4 (Fig. 5A). NefWL58AA remained fully active on mature MHC II and kept only 50% of its activity on immature MHC II (Fig. 5A, compare with its effect on CD4). In contrast, NefLL165GG had a reduced effect on mature MHC II and totally failed to induce surface Ii (Fig. 5A). NefPP75/78AA and NefEEEE65AAAA kept less than one-third of their activity on MHC I but still down-modulated CD4 efficiently (Fig. 5A). Whereas NefPP75/78AA retained a partial activity on both mature and immature MHC II complexes, the acidic stretch (EEEE65) proved important for down-modulating mature MHC II but dispensable for Nef's effect on immature MHC II (Fig. 5A). Thus, NefEEEE65AAAA and NefWL58AA had opposite effects on both types of MHC II complexes. Comparing the four mutants of Nef, it should be noticed that they were partly divergent in their activity on mature and immature MHC II. Finally, Nef modulation of each of the four surface markers (CD4, MHC I, and mature and immature MHC II) exhibited a different pattern of sensitivity to the four sets of mutations, suggesting that Nef effects on MHC II do not represent side effects of CD4 and MHC I down-modulation. Levels of expression of wild-type and mutant Nef proteins were comparable as tested by Western blot (Fig. 5B), except NefWL58AA, which probably had a longer half-life because it was expressed at a higher level as previously noticed (16).
Figure 5.
Mutational analysis of Nef. (A) HeLa-CIITA cells were cotransfected with the plasmids encoding Nef-wt, Nef-mock, or the different Nef mutants in combination with the plasmids encoding CD4 and EGFP. Cells were processed for cytofluorimetry, and GFP+ cells were analyzed as in Fig. 2. Immature MHC II complexes were followed with the anti-Ii mAb LN2 and mature MHC II complexes with the mAb L243. Relative activities of Nef mutants on CD4, MHC I, and mature and immature MHC II were calculated as percentage of Nef-wt activity in comparison to the activity of the Nef-mock control plasmid (0%). Nef from the LAI strain (FT-wt) was used as reference (100% of effect) for NefLL165GG, as this mutant has been derived from this allele. The other three mutants (NefWL58AA, NefEEEE65AAAA, and NefPP75/78AA) have been derived from the Nef allele HXB2, thus HXB2-wt was taken as reference (100% of effect) for these mutants. NefWL58AA induced a higher down-modulation of surface MHC I than Nef-wt (181%) as previously reported (16). The figure shows mean values and their standard deviations calculated from the data generated in four independent experiments. (B) Western blot analysis of lysates of cells transfected with the indicated wild type or mutants of Nef. The equivalent of 1 × 105 cells was loaded per lane.
To analyze a possible direct effect of Nef on mature MHC II, HeLa cells stably transfected with DR1 α and β chains (22), thereby lacking Ii, were analyzed in our experimental system. Nef also down-modulated mature MHC II independently of Ii expression, albeit to a lower extent than in HeLa-CIITA cells (reduction of 35% ± 2 in comparison to Nef-mock transfected cells). These data further suggest that Nef can distinctly act on both types of MHC II complexes.
Discussion
Recent studies have established the central role of the helper T cell activity for the control of HIV infection (23). Here, we show that Nef affects HLA-DR restricted peptide presentation most likely by modifying MHC II intracellular trafficking. First, Nef induced a 2-fold reduction of surface levels of peptide-loaded MHC II. Second, Nef induced a strong accumulation of surface-immature MHC II complexes made of Ii, α, and β chains. The level of total HLA-DRα chain remained similar upon Nef expression, suggesting that Nef does not directly affect MHC II biosynthesis. The effects on surface expression of mature and immature MHC II were dose dependent, obtained with Nef from three different HIV-1 isolates and in two cell lines expressing different HLA-DR alleles. Nef was the only HIV protein to mediate these effects, which also occurred in the presence of the other HIV gene products. Nef myristoylation and thus membrane anchorage were required to induce these modifications, as for the down-modulation of CD4 and MHC I (24). MHC I and mature MHC II surface expression were reduced to a similar extent by Nef.
Many surface markers remain unmodified by Nef expression (see ref. 24). This study showed that surface levels of transferrin receptor, Lamp1, and Lamp2 remained unchanged in the presence of Nef, demonstrating that Nef did not induce a general redistribution of protein constituents of the endocytic pathway but selectively affected MHC II trafficking.
Four sets of mutations of Nef previously characterized for their effects on Nef-induced MHC I and CD4 down-modulations were analyzed. Each of the Nef-induced modulations of surface CD4, MHC I, and immature and mature MHC II complexes displayed differential sensitivity to the four sets of mutations, strongly suggesting that Nef effects on MHC II do not simply represent a consequence of its activity on CD4 or on MHC I.
Substitutions WL58 to AA reduced the up-modulation of immature MHC II without affecting the down-modulation of mature MHC II, whereas an exact opposite situation was observed with the substitutions EEEE65 to AAAA. Therefore, Nef effects on mature MHC II are not a strict consequence of Nef effects on immature MHC II. Further, three times more Nef was required for the effects on mature MHC II than for the effects on immature MHC II. Moreover, even in the absence of Ii, mature MHC II complexes were down-modulated by Nef, albeit to a lower extent. Therefore, Nef can independently affect immature and mature MHC II.
The EEEE65 cluster of Nef mediates Nef interaction with PACS 1, a member of a new family of coat proteins, which in turn recruits AP-1 (11). Nef would bridge MHC I cytoplasmic tail to PACS 1 complexes, resulting in MHC I retention at the TGN (11). Whether a similar mechanism of Nef-induced retention of mature MHC II occurs remains to be established. Similarly, because Nef induces an acceleration of MHC I endocytosis (2), the same kind of mechanism could be proposed for mature MHC II. However, we did not detect any increase of MHC II endocytosis on Nef expression (not shown).
The Nef di-leucine motif (LL165), crucial for Ii surface expression, is also required for Nef-induced accelerated endocytosis of CD4. Nef would recruit, via this motif, AP-2 at the plasma membrane and link this complex to the CD4 cytoplasmic tail (10, 25). However, we could not demonstrate any binding of Nef to MHC II or to Ii (not shown). How can the same functional domain of Nef be involved in Nef-induced down-modulation of CD4 and in up-modulation of immature MHC II? The latter could result from a defect in sorting at the TGN or within the endocytic pathway, allowing immature MHC II to reach the plasma membrane. Note that the signals carried by the cytoplasmic tail of Ii, which target MHC II complexes from the TGN to the endocytic pathway, consist of two di-leucine-based motifs (LI7/8 and ML16/17). Like Nef, Ii can bind clathrin adaptor AP-1 complexes through its cytoplasmic tail (26, 27). However, it remains to be established whether αβIi sorting to endosomes indeed relies on AP-1 recruitment. Sorting of αβIi at the TGN occurs independently of both AP-1 and clathrin in I-cell disease B lymphoblasts (28). Expression of a dominant negative mutant of clathrin did not inhibit transport of αβIi from the TGN to lysosomes, suggesting that this transport step may occur independently of clathrin-coated vesicles and therefore of AP-1 (29). Consequently, it is unlikely that the capacity of Nef to recruit AP-1 modifies sorting of αβIi in a direct manner. Alternatively, surface-immature complexes might be internalized at slower rates because of a titration of available AP-2 complexes by Nef. This does not seem to be the case, as endocytosis of transferrin was not affected by Nef expression (30). Nevertheless, our data indicate that Nef expression affects—possibly in an indirect manner—the efficiency of the sorting signals carried by the cytoplasmic tail of Ii. Indeed, Nef effects on MHC II are similar to the modifications of MHC II trafficking and function reported in cells expressing a truncated version of Ii lacking its cytoplasmic tail (31). One could consider that Nef capacity to bind PACS 1, AP-1, and AP-2 might result in alterations of transport of MHC II between the TGN and the endocytic pathway.
The Nef proline-rich region (73) was necessary for both mature and immature MHC II modulations. It is required for MHC I down-modulation but not for CD4 (24) and constitutes a potential SH3 binding domain for the guanine nucleotide exchange factor Vav and for several kinases (see ref. 1). Therefore, our results with the NefPP75/78AA mutant emphasize the involvement of a signal transduction machinery (yet to be identified) for the observed effects of Nef on MHC II.
We observed that cells expressing Nef had a reduced capacity to activate T cells in a peptide-specific and HLA-DR restricted manner. Nef, which is expressed very early and at high levels throughout the viral cycle, may rapidly exert these effects in vivo during the infection. Our observations should be extended to macrophages and dendritic cells, which represent key targets for HIV infection and are also central for the initiation of the immune response. By impeding MHC II presentation in infected antigen-presenting cells, Nef would also impair the development of efficient HIV-specific Th response. Indeed, CD4 Th response plays a central role for the control of the HIV infection (14). An inverse relation between HIV-specific Th activity and HIV viremia has been established (32, 33). Thus, HIV seems to have selected several ways to lower the CD4+ T cell antiviral response including deletion by apoptosis, infection of specific Th cells, inefficient presentation of viral antigens by MHC II (13), and inhibition of MHC II transcription (34). Reports concerning viral blockage of MHC II antigen presentation are arising, as in the case of the human cytomegalovirus gene product US2, which targets HLA-DRα and HLA-DMα for degradation by the proteasome, thus inhibiting antigen presentation to CD4+ T cells (35). By affecting both MHC I and MHC II antigen presentation pathways, Nef would contribute to the ability of HIV to evade the ongoing immune response.
Acknowledgments
We thank M. J. Bijlmakers, A. Rodriguez, C. Thery, S. Amigorena, and C. Watts for helpful comments on the manuscript. This work was supported by grants (to P.B.) from Sidaction and the Agence Nationale de Recherche contre le Sida (which also supports P.S.-C.).
Abbreviations
- ConB
concanamycin B
- CIITA
class II transactivator
- Ii
invariant chain
- MFI
mean fluorescence intensity
- TGN
trans-Golgi network
- v-ATPase
vacuolar H+-ATPase
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Geyer M, Fackler O T, Peterlin B M. EMBO Rep. 2001;2:580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 3.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J M, Schwartz O. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 4.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. Nature (London) 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 5.Cohen G B, Gandhi R T, Davis D M, Mandelboim O, Chen B K, Strominger J L, Baltimore D. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piguet V, Chen Y L, Mangasarian A, Foti M, Carpentier J L, Trono D. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Yu H, Liu S H, Brodsky F M, Peterlin B M. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 9.Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier J L, Trono D. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 10.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 11.Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, Trono D. Nat Cell Biol. 2000;2:163–167. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay M J, Fortin J F, Cantin R. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 13.Polyak S, Chen H, Hirsch D, George I, Hershberg R, Sperber K. J Immunol. 1997;159:2177–2188. [PubMed] [Google Scholar]
- 14.Brander C, Walker B D. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 15.Benaroch P, Yilla M, Raposo G, Ito K, Miwa K, Geuze H J, Ploegh H L. EMBO J. 1995;14:37–49. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangasarian A, Piguet V, Wang J K, Chen Y L, Trono D. J Virol. 1999;73:1964–1973. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz O, Marechal V, Danos O, Heard J M. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steimle V, Siegrist C A, Mottet A, Lisowska-Grospierre B, Mach B. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 19.Bijlmakers M J, Benaroch P, Ploegh H L. EMBO J. 1994;13:2699–2707. doi: 10.1002/j.1460-2075.1994.tb06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 21.Hewitt C R A, Lamb J R, Hayball J, Hill M, Owen M J, O'Hehir R. J Exp Med. 1992;175:1493–1499. doi: 10.1084/jem.175.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busch R, Cloutier I, Sekaly R P, Hammerling G J. EMBO J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg E S, Walker B D. AIDS Res Hum Retroviruses. 1998;14, Suppl. 2:S143–S147. [PubMed] [Google Scholar]
- 24.Piguet V, Schwartz O, Le Gall S, Trono D. Immunol Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. Curr Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- 26.Salamero J, Le B R, Saudrais C, Goud B, Hoflack B. J Biol Chem. 1996;271:30318–30321. doi: 10.1074/jbc.271.48.30318. [DOI] [PubMed] [Google Scholar]
- 27.Rodionov D G, Bakke O. J Biol Chem. 1998;273:6005–6008. doi: 10.1074/jbc.273.11.6005. [DOI] [PubMed] [Google Scholar]
- 28.Glickman J N, Morton P A, Slot J W, Kornfeld S, Geuze H J. J Cell Biol. 1996;132:769–785. doi: 10.1083/jcb.132.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S H, Marks M S, Brodsky F M. J Cell Biol. 1998;140:1023–1037. doi: 10.1083/jcb.140.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foti M, Mangasarian A, Piguet V, Lew D P, Krause K H, Trono D, Carpentier J L. J Cell Biol. 1997;139:37–47. doi: 10.1083/jcb.139.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roche P A, Teleski C L, Karp D R, Pinet V, Bakke O, Long E O. EMBO J. 1992;11:2841–2847. doi: 10.1002/j.1460-2075.1992.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 33.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 34.Kanazawa S, Okamoto T, Peterlin B M. Immunity. 2000;12:61–70. doi: 10.1016/s1074-7613(00)80159-4. [DOI] [PubMed] [Google Scholar]
- 35.Tomazin R, Boname J, Hegde N R, Lewinsohn D M, Altschuler Y, Jones T R, Cresswell P, Nelson J A, Riddell S R, Johnson D C. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]