Abstract
Cellular senescence is a natural safeguard against cancer. Pharmacologically, it can be induced by drugs that inhibit the CDK4/6 kinases such as palbociclib, but the exact mechanism has never been dissected. Recent research by Miettinen et al (2018) reveals that senescence induced by this class of drugs is mediated by proteasome hyper‐activation.
Subject Categories: Cancer; Molecular Biology of Disease; Post-translational Modifications, Proteolysis & Proteomics
Abnormalities in cell cycle regulation are ubiquitous events that confer fitness advantages to cancer cells. Key components of the cell cycle machinery are cyclin‐dependent kinases (CDKs). CDKs complex with their cyclin regulatory partners and together control cell cycle transitions, leading to a commitment to cell division. Non‐selective CDK inhibitors showed disappointing results in the clinic due to lack of efficacy across different cancer types and excessive toxicity. However, more recent selective inhibitors such as palbociclib, a potent and selective CDK4/6 inhibitor, significantly prolong progression‐free survival in ER‐positive metastatic breast cancer when combined with oestrogen receptor antagonists. In addition to blocking cancer cell proliferation, palbociclib also induces senescence in multiple cell types (Ewald et al, 2010). Cellular senescence is a stable form of growth arrest that functions as a natural safeguard against oncogenic transformation. This protective mechanism actually operates in humans to prevent cancer, as melanocytic nevi often carry the very same activating BRAF mutations found in melanoma but are positive for many of the known senescence markers. Importantly, even some advanced cancer cells can be induced to enter a state of senescence by excessive oncogenic signalling or by chemotherapy treatment (Ewald et al, 2010; Sun et al, 2014). Senescent cells remain viable, but their cellular state is distinct from the proliferating counterparts and characterized by absence of proliferation markers, expression of tumour suppressor genes, senescence‐associated beta‐galactosidase activity and the presence of senescence‐associated heterochromatin foci (SAHFs; Narita et al, 2003). Senescent cells also secrete a collection of inflammatory cytokines, chemokines and proteinases, collectively referred to as the senescence‐associated secretory phenotype (SASP; Coppe et al, 2010). The SASP is one of the most profound features of senescence that facilitates immunosurveillance of senescent cells, as it recruits and activates distinct cells from the innate and adaptive immune system, such as macrophages, NK cells and T cells. In turn, these cells engage in a programme to specifically eliminate senescent cells (Burton & Stolzing, 2018). The induction of senescence by palbociclib is therefore an attractive feature of this drug, but the mechanism through which senescence is induced had until now not been studied.
To address this, Miettinen et al (2018) used an elegant thermal proteome profiling approach to shed light into the underlying mechanism of palbociclib‐induced senescence. They found that palbociclib elicits the dissociation of the proteasomal‐inhibitory protein ECM29 from the proteasome, unleashing the activation of the latter to a level incompatible with cell proliferation. This disturbance in proteasomal homeostasis is required for the cell cycle inhibition and senescence induced by palbociclib, as the co‐treatment with the proteasome inhibitor bortezomib abrogates these phenotypes.
This work uncovers additional forms of action of CDK4/6 inhibitors that may be translated in their rational application beyond ER‐positive breast cancer, the only indication in which this class of drugs is currently approved. The series of experiments focused on breast cancer lines representative of this subtype, yet it would be important to confirm whether increased proteasome activation upon palbociclib treatment/ECM29 loss also operates in other tumour types.
CDK4 and CDK6 phosphorylate and inactive the tumour suppressor RB1, and therefore, tumours that have lost this target of CDK4/6 tend to be insensitive to inhibitors of these kinases (Johnson et al, 2016). Consequently, RB1 wild‐type status remains the only significant biomarker of clinical response to palbociclib. Given that RB1 is a strong predictor of response to palbociclib, it would seem that proteasomal activation by itself is not sufficient to provoke a senescence response. Rather, it is more likely that the combination of cell cycle arrest invoked by prevention of RB1 phosphorylation combined with proteasomal activation strategy sensitizes RB1 wild‐type tumours to palbociclib‐induced senescence (Fig 1).
Figure 1. Model for palbociclib‐induced senescence.
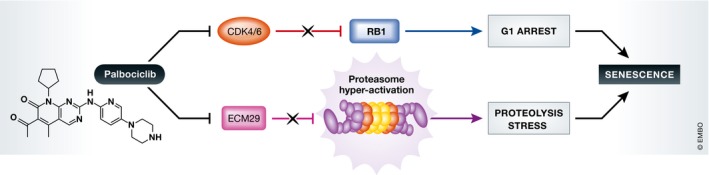
Palbociclib has two pro‐senescence effects on cancer cells. Through inhibition of CDK4 and CDK6, the tumour suppressor RB1 is maintained in an active hypophosphorylated form, which arrests cells in the G1 phase of the cell cycle. Palbociclib also activates the proteasome through decreasing its binding to the proteasome inhibitor ECM29. This in turn causes stress resulting from increased proteolysis. Together, these two effects cause a senescence response in cancer cells.
One reason why senescence induction may be a desirable outcome of a cancer therapy resides in the notion that senescence is a physiological response that operates to clear cells that have become obsolete or undesired in the body. In that sense, pro‐senescence therapy resembles the new and highly effective checkpoint immunotherapies for cancer, which also activate a normal physiological response to eradicate cancer cells. A caveat in this reasoning is that the SASP produced by senescent cancer cells potentially represents a double‐edged sword in tumour control (Coppe et al, 2010). While it is well‐established that the SASP can contribute to tumour eradication by recruitment of immune cells that result in clearance of senescent cells, recent data also indicate that a more chronic inflammatory response in a tumour mass can be deleterious. For instance, chronic inflammation can increase the metastatic rate of non‐senescent cells in a tumour population (Angelini et al, 2013). It is therefore well possible that induction of senescence by drugs such as palbociclib can have adverse effects also. Such effects can potentially be countered by using a follow‐up therapy such as a senolytic agent: a drug that selectively kills senescent cells. We have recently shown the feasibility of such a “one‐two punch” consecutive therapy for cancer in which senescent cancer cells are killed by ABT‐263 (Wang et al, 2017), a selective inhibitor of the anti‐apoptotic BCL‐2, BCL‐W and BCL‐XL proteins (Chang et al, 2016; Zhu et al, 2016). It is also a possibility to further enhance the activity of recruited immune cells to senescent cancer cells by combining pro‐senescence therapy with a checkpoint immunotherapy. Whether this will be sufficient to eradicate all cancer cells remains to be established. It does seem clear that the SASP created by different types of senescent cells is highly dissimilar (Hernandez‐Segura et al, 2017). Consequently, how the inflammatory responses act on senescent cancer cells is likely to be equally divergent and unpredictable. Potentially, the proverb “the only good cancer cell is a dead cancer cell” also applies here in that killing of senescent cancer cells through senolytic agents may be the only sure bet to take the sharp edges of the double‐edged sword named senescence.
The EMBO Journal (2018) 37: e99386 29669859
See also: https://doi.org/10.15252/embj.201798359 (May 2018)
References
- Angelini PD, Zacarias Fluck MF, Pedersen K, Parra‐Palau JL, Guiu M, Bernadó Morales C, Vicario R, Luque‐García A, Navalpotro NP, Giralt J, Canals F, Gomis RR, Tabernero J, Baselga J, Villanueva J, Arribas J (2013) Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res 73: 450–458 [DOI] [PubMed] [Google Scholar]
- Burton DGA, Stolzing A (2018) Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res Rev 43: 17–25 [DOI] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin‐Burns N, Krager K, Ponnappan U, Hauer‐Jensen M, Meng A, Zhou D (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence‐associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald JA, Desotelle JA, Wilding G, Jarrard DF (2010) Therapy‐induced senescence in cancer. J Natl Cancer Inst 102: 1536–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M (2017) Unmasking transcriptional heterogeneity in senescent cells. Curr Biol 27: 2652–2660 e2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Thijssen B, McDermott U, Garnett M, Wessels LF, Bernards R (2016) Targeting the RB‐E2F pathway in breast cancer. Oncogene 35: 4829–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen TP, Peltier J, Härtlova S, Gierliński M, Jansen VM, Trost M, Björklund M (2018) Thermal proteome profiling of breast cancer cells reveals proteasomal activation by CDK4/6 inhibitor palbociclib. EMBO J 37: e98359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW (2003) Rb‐mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113: 703–716 [DOI] [PubMed] [Google Scholar]
- Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C, Haanen J, Blank C, Wesseling J, Willems SM, Zecchin D, Hobor S, Bajpe PK, Lieftink C, Mateus C, Vagner S, Grernrum W, Hofland I, Schlicker A, Wessels LF et al (2014) Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 508: 118–122 [DOI] [PubMed] [Google Scholar]
- Wang L, Leite de Oliveira R, Wang C, Fernandes Neto JM, Mainardi S, Evers B, Lieftink C, Morris B, Jochems F, Willemsen L, Beijersbergen RL, Bernards R (2017) High‐throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep 21: 773–783 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann‐Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl‐2 family of anti‐apoptotic factors. Aging Cell 15: 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]


