Abstract
Key points
In aged rats, daily muscle stretching increases blood flow to skeletal muscle during exercise.
Daily muscle stretching enhanced endothelium‐dependent vasodilatation of skeletal muscle resistance arterioles of aged rats.
Angiogenic markers and capillarity increased in response to daily stretching in muscles of aged rats.
Muscle stretching performed with a splint could provide a feasible means of improving muscle blood flow and function in elderly patients who cannot perform regular aerobic exercise.
Abstract
Mechanical stretch stimuli alter the morphology and function of cultured endothelial cells; however, little is known about the effects of daily muscle stretching on adaptations of endothelial function and muscle blood flow. The present study aimed to determine the effects of daily muscle stretching on endothelium‐dependent vasodilatation and muscle blood flow in aged rats. The lower hindlimb muscles of aged Fischer rats were passively stretched by placing an ankle dorsiflexion splint for 30 min day–1, 5 days week–1, for 4 weeks. Blood flow to the stretched limb and the non‐stretched contralateral limb was determined at rest and during treadmill exercise. Endothelium‐dependent/independent vasodilatation was evaluated in soleus muscle arterioles. Levels of hypoxia‐induced factor‐1α, vascular endothelial growth factor A and neuronal nitric oxide synthase were determined in soleus muscle fibres. Levels of endothelial nitric oxide synthase and superoxide dismutase were determined in soleus muscle arterioles, and microvascular volume and capillarity were evaluated by microcomputed tomography and lectin staining, respectively. During exercise, blood flow to plantar flexor muscles was significantly higher in the stretched limb. Endothelium‐dependent vasodilatation was enhanced in arterioles from the soleus muscle from the stretched limb. Microvascular volume, number of capillaries per muscle fibre, and levels of hypoxia‐induced factor‐1α, vascular endothelial growth factor and endothelial nitric oxide synthase were significantly higher in the stretched limb. These results indicate that daily passive stretching of muscle enhances endothelium‐dependent vasodilatation and induces angiogenesis. These microvascular adaptations may contribute to increased muscle blood flow during exercise in muscles that have undergone daily passive stretch.
Keywords: endothelium, exercise hyperemia, nitric oxide synthase, hypoxia inducible factor‐1 alpha
Key points
In aged rats, daily muscle stretching increases blood flow to skeletal muscle during exercise.
Daily muscle stretching enhanced endothelium‐dependent vasodilatation of skeletal muscle resistance arterioles of aged rats.
Angiogenic markers and capillarity increased in response to daily stretching in muscles of aged rats.
Muscle stretching performed with a splint could provide a feasible means of improving muscle blood flow and function in elderly patients who cannot perform regular aerobic exercise.
Introduction
Ageing alters muscle blood flow during exercise, independent of changes in cardiac output, resulting in a mismatch between oxygen supply and demand (Rodeheffer et al. 1984; Delp et al. 1998; Behnke et al. 2012). Ageing‐induced alterations of muscle blood may be linked to the impairment of endothelium‐dependent vasodilatation of resistance arteries (Muller‐Delp et al. 2002) or changes in muscle capillarity, which are dependent on fibre size and oxidative capacity (Hepple & Vogell, 2004; Groen et al. 2014; Barnouin et al. 2017) in skeletal muscle. Exercise training, even initiated in late life, enhances endothelium‐dependent dilatation (Spier et al. 2004, 2007), increases capillarity (Charifi et al. 2004; Jensen et al. 2004), and improves blood flow distribution and capacity in the aged lower limb (Behnke et al. 2012). Despite the well‐known beneficial effects of exercise, the rate of compliance to exercise training programmes is low in elderly people (Chrisman et al. 2015), often as a result of the strenuous nature of exercise training. Reduced mobility and diminished muscular strength may decrease adherence to exercise programmes in the elderly (Morie et al. 2010); therefore, it is important to develop alternative therapies that mitigate the loss of muscle blood flow in the elderly.
Muscle stretching is widely performed as a warm‐up or cool‐down for patients undergoing physical therapy (Katalinic et al. 2011; Apostolopoulos et al. 2015). The intensity of muscle stretching is relatively light compared to aerobic exercise, such that even very old individuals can perform muscle stretching with minimal risk of injury. It has been reported that muscle stretching prevents muscle atrophy in immobilized lower limbs (Agata et al. 2009); however, little is known about the effects of daily muscle stretching on limb blood flow. Mechanical stimuli have a crucial role in keeping endothelial cells healthy (Fujiwara, 2003); endothelial cells sense stretch and shear stress, resulting in alterations of gene expression, morphology and function (Naruse et al. 1998; Kuebler et al. 2003; Thacher et al. 2010). Responses to the mechanical stimuli of stretch and shear stress also play important roles in maintaining normal vascular function and their impairment leads to various vascular diseases (Thijssen et al. 2009; Ando & Yamamoto, 2011). Circumferential stretch has been demonstrated to increase endothelial nitric oxide synthase (Awolesi et al. 1995) and to release nitric oxide (NO) from endothelial cells (Kuebler et al. 2003). Mechanical stretch/overload has been shown to increase levels of vascular endothelium‐derived growth factor (VEGF) and increase the capillarization of rat skeletal muscle (Rivilis et al. 2002). In healthy young humans, passive leg movement increases muscle blood flow and augments interstitial VEGF and endothelial nitric oxide synthase (eNOS) mRNA in muscle, independent of changes in metabolism or central haemodynamics (Hellsten et al. 2008; McDaniel et al. 2012); however, the effects of daily passive muscle stretching on adaptations of microvascular function and angiogenesis have not been studied.
We hypothesized that daily passive stretching of muscle using a splint (Baewer et al. 2004) would improve endothelium‐dependent vasodilatation and increase exercise‐induced hyperaemia in the skeletal muscle of aged rats. To determine whether acute changes in blood flow during daily muscle stretching provide the signal for microvascular adaptations as the muscle undergoes daily stretching, we evaluated blood flow responses during and immediately after an acute bout of static muscle stretching. After 4 weeks of daily muscle stretching, we evaluated muscle blood flow at rest and during exercise, and assessed endothelium‐dependent dilatation, capillarity and markers of angiogenesis in skeletal muscle.
Methods
Ethical approval
Twenty‐month‐old male Fisher 344 rats (n = 68) were obtained from National Institute of Aging. For performance of daily stretching, rats were assigned to stretch (n = 35), cage control (n = 8) or sham control (n = 8) groups. All animal procedures were approved by the Institutional Animal Care and Use Committee at University of Florida and Florida State University, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. All rats were housed in a temperature/light‐controlled environment and given access to standard rat chow and water ad libitum.
Muscle stretching protocol
The splints were made from Polyform (Smith & Nephew Rolyan, Inc., Menomonee Falls, WI, USA), as described previously (Baewer et al. 2004). The splint material was rendered malleable by heating in 70°C water and shaped by hand to fit onto the back of the leg and bottom of the foot. Muscle stretching was performed for 30 min, 5 days week−1, for 4 weeks by applying the splint to hold the left ankle joint at 30° of dorsiflexion (Fig. 1) as developed by Baewer et al. (2004). Placement of this splint produced stretch in muscles of the back of the lower leg: the soleus, plantaris, flexor hallucis longus and flexor digitorum longus muscles. Splint placement did not stretch muscles on the front of the lower leg: the red and white portion of tibialis anterior and extensor digitorum longus muscle. The right hindlimb served as a non‐stretched contralateral limb. For daily stretching, the rat was anaesthetized briefly (<1 min) with 1.5% isoflurane when the splint was secured to the left ankle using tape. During 30 min of muscle stretching, the rat was awake but immobilized in an acrylic restrainer. After stretching, the splint was taken off without anaesthesia. Sham control rats were briefly anaesthetized and immobilized in the restrainer for 30 min without a splint. Vascular responses, skeletal muscle blood flow measurements and harvesting of tissue for immunohistochemistry were performed at least 48 h after the last bout of muscle stretching.
Figure 1. Muscle stretch using splint.
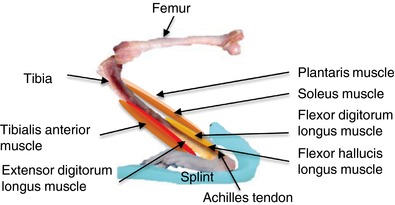
An ankle dorsiflexion splint was applied to hold the left ankle joint at 30° of dorsiflexion. The ankle dorsiflexion splint elongated ankle plantar flexor muscles (soleus, plantaris, flexor hallucis longus and flexor digitorum longus) and shortened ankle dorsiflexor muscles (tibialis anterior and extensor digitorum longus). [Color figure can be viewed at http://wileyonlinelibrary.com]
Surgical preparation for determination of blood flow
Rats were anaesthetized with 2.5% isoflurane and a catheter filled with heparinized saline was advanced into the right carotid artery to the aortic arch. The carotid catheter was externalized at the base of the neck and secured to the skin between the shoulder blades. A second catheter, placed in the caudal tail artery and externalized at the base of the tail, was used to monitor mean arterial pressure and to obtain a reference blood sample for calculating tissue flows. After closure of the incisions, the animals were given ≥4 h to recover because previous studies demonstrated that circulatory dynamics, regional blood flow, arterial blood gases and acid–base status are stable in the awake rat 1–6 h after gas anaesthesia (Flaim et al. 1984).
Skeletal muscle blood flow at rest and during treadmill exercise
At the end of the recovery period, the rat was placed on the treadmill and exercise was initiated (15 m min−1 at a 0° incline). After 3 min of total exercise time, blood withdrawal was begun from the caudal tail artery at 0.25 mL min−1. Radiolabelled microspheres were then infused into the carotid artery catheter (either 57Co and 85Sr, infused in random order; diameter 15 μm; ∼2.5 × 105 in number), as described previously (Flaim et al. 1984; Delp et al. 1998; Musch et al. 2004; Behnke et al. 2012). Blood withdrawal from the caudal tail artery continued for 45 s after microsphere infusion was complete. After a 30 min recovery period from exercise, infusion of the second microsphere (either 57Co and 85Sr) was performed to measure resting blood flow. After the second microsphere infusion, the rat was killed with pentobarbital sodium (>100 mg kg−1 i.p.). Prior to blood flow evaluations, rats were familiarized with the treadmill exercise during three sessions of walking (10 min day−1 at 15 m min−1, 0° incline).
Skeletal muscle blood flow during and after acute muscle stretching
Naïve rats (20 months old, male Fischer 344; n = 17) that did not undergo daily stretching via splint placement were used to determine the effects of acute splint placement (acute stretching). Naïve rats were acclimated to the splints and restrainers for 10 min day−1, on three consecutive days. At least 48 h elapsed between the last acclimation session and blood flow assessment during/after acute splint placement (acute stretching). Catheters were implanted as described above.
After a 4 h recovery period, the rat was placed in the restrainer and radiolabelled microspheres (either 57Co and 85Sr, infused in random order) were infused for determination of resting blood flow. In one subgroup of rats, skeletal muscle blood flow was measured during muscle stretching. After measuring resting blood flow, the rat was taken out of the restrainer, briefly anaesthetized with 1.5% isoflurane when the splint was placed on left ankle, and then returned to the restrainer. Ten minutes after splint placement, infusion of the second microsphere (either 57Co and 85Sr) was performed. In another subgroup of rats, skeletal muscle blood flow was measured immediately after acute muscle stretching. After measuring resting blood flow, the rat was taken out of the restrainer, briefly anaesthetized with 1.5% isoflurane when the splint was placed on left ankle, and then returned to the restrainer. The rat remained in the restrainer with the splint in place for 30 min. A second microsphere (either 57Co and 85Sr) was infused into the carotid catheter ∼30 s after the splint was removed. After the second microsphere infusion, the rat was killed with pentobarbital sodium.
Blood flow analysis
Plantar flexor muscles (soleus, plantaris, flexor digitorum longus and flexor hallucis longus) and dorsiflexor muscles (red and white portion of tibialis anterior and the extensor digitorum longus) were dissected from stretched and non‐stretched contralateral limbs and weighed. The radioactivity level of the individual muscles was determined using a gamma scintillation counter. Blood withdrawals from the caudal tail artery were utilized as reference samples. Blood flow to each muscle was calculated by reference sample method and expressed in mL min–1 100 g tissue–1 according to the formula:
Blood flow (mL min–1 100 g–1) = [(gamma counter counts of the tissue/gamma counter counts of reference sample) × 0.25 mL min–1]/[tissue wet weight (g)/100]
Kidney blood flows were used as an indicator of adequate mixing of microspheres; blood flow values were only considered valid if left and right kidney flows were within 15% of each other (Delp et al. 1998).
Microvessel preparation
Rats were weighed and anaesthetized with 3% isoflurane and killed by excision of the heart. The soleus‐plantaris‐gastrocnemius muscle group was removed from both the stretched and non‐stretched contralateral hindlimb, and immediately placed in cold (4°C) filtered physiological saline solution (PSS). First‐order arterioles were isolated from the soleus muscle and cannulated on pipettes and pressurized to 70 cmH2O in an organ chamber that contained warm (37°C) PSS. The chamber was then placed on an inverted microscope equipped with a video camera and micrometer to measure intraluminal diameter. Soleus muscle arterioles without leaks were allowed to equilibrate for ∼ 1 h until developing ≥20% spontaneous tone. At the end of all experiments, arterioles were placed in Ca2+‐free PSS with 100 μm of sodium nitroprusside to determine the maximal diameter. Development of spontaneous tone was expressed as percentage constriction relative to maximal diameter and calculated as:
Spontaneous tone (%) = (D max – D s)/D max × 100 where D max is the maximal inner diameter recorded at a pressure of 70 cmH2O under Ca2+‐free conditions and D s is the steady‐tone baseline diameter.
Evaluation of vasodilatory responsiveness
Vasodilatory responses to cumulative addition of the endothelium‐dependent vasodilator ACh (1 × 10−9 to 1 × 10−4 m) and the nitric oxide (NO) donor diethylamineNONOate (Dea‐NONOate) (1 × 10−9 to 1 × 10−4 m) were determined in soleus muscle arterioles, as described previously (Muller‐Delp et al. 2002).
Vasodilatory responses were expressed as percentage relaxation as calculated by the formula:
Relaxation (%) = [(D s – D b)/(D max – D b)] × 100 where D b is the steady baseline diameter before adding the first dose of the specific vasodilators, D is the steady diameter after addition of each dose of the vasodilators and D max is the maximal inner diameter recorded under Ca2+‐free conditions.
Immunohistochemical analysis of arteriolar proteins
Protein levels of eNOS and superoxide dismutase (SOD) were assessed in soleus muscle arterioles isolated from stretched and non‐stretched contralateral limbs. Arterioles were cannulated and pressurized to 70 cmH2O. The arterioles were incubated at 37°C in Ca2+‐free PSS with 100 μm sodium nitroprusside for 1 h, fixed in 50% Bouin's solution and frozen in OCT. Cross‐sections (5 μm) were cut on a cryostat. Sections were washed with PBS before adding a blocking solution of 0.3% Triton‐X and 10% normal donkey serum at room temperature for 1 h. Sections were then incubated with primary antibodies against either eNOS (anti‐eNOS antibody, dilution 1:200; Sigma, St Louis, MO, USA) or SOD (anti‐superoxide dismutase 1 antibody, dilution 1:50; Abcam, Cambridge, MA, USA) at 4°C overnight. After PBS washes, species‐specific anti‐IgG (FITC; Abcam) was added at a dilution of 1:100 for 1 h at room temperature. After washing, 4′,6‐diamidino‐2‐phenylindole (DAPI) was added and images were obtained using a fluorescence microscope. To exclude adventitial staining, a region of interest was established manually using ImageJ (NIH, Bethesda, MD, USA) after isolating the images for green fluorescence. For each section, the average pixel intensity in the region of interest was obtained. Background was determined by incubating sections in the absence of primary and secondary antibodies and then subtracted from positively stained images. The average of the pixel intensity values obtained from at least three adjacent sections (with a coefficient of variation ≤0.25) was calculated and used for statistical analysis.
Immunohistochemical analysis of skeletal muscle angiogenesis
Rats were weighed and anaesthetized with 2.5% isoflurane and killed by excision of the heart. A laparotomy was performed to isolate the abdominal aorta from the vena cava, and a catheter filled with warm, heparinized papaverine solution (37°C, 1000 U mL−1 heparin in 0.9% saline, 4 mg L−1 papaverine) was advanced into the abdominal aorta. The vena cava was nicked and the heparinized papaverine solution was infused into the abdominal aorta at a rate of 0.05 mL s−1. After clear saline emerged from the opening in the vena cava, 3.5 mL of saline containing 2% paraformaldehyde was infused into the abdominal aorta at the same rate. Soleus muscles from both stretched and non‐stretched contralateral limbs were dissected, weighed and frozen in OCT compound. Eight‐micron sections were cut on a cryostat. Sections were washed with PBS and incubated with rhodamine‐labelled Griffonia simplicifolia lectin I (15 μg mL−1 diluted with PBS; Vector Laboratories, Inc., Burlingame, CA, USA) for 1 h at room temperature in the dark (Hansen‐Smith et al. 1988). Sections were rinsed with PBS, visualized with a fluorescence microscope at 100× magnification and images were captured with a digital charge‐coupled device (CCD) camera. Muscle fibres were outlined by hand and colour and size thresholding was applied in MATLAB (MathWorks Inc., Natick, MA, USA) within the circumscribed area. The number of capillaries per field (one muscle fibre) was calculated automatically. The number of capillaries surrounding a muscle fibre was calculated for a minimum of five fields (fibres) per cross‐section, and at least three adjacent sections were analysed per muscle. Capillary‐to‐fibre ratio was determined by counting capillaries and fibres contained within a static grid overlaid on images with identical area, as described previously (Mathieu‐Costello et al. 1989). Image J was used to prevent multiple countings of fibres or capillaries.
To determine expression of angiogenic proteins, muscle sections were rinsed with PBS, incubated with blocking solution for 1 h at room temperature, and then incubated with primary antibodies [anti‐hypoxia‐induced factor‐1α (HIF‐1α), Abcam, dilution 1:100; anti‐VEGFA, Abcam, dilution 1:100; anti‐neuronal nitric oxide synthase (nNOS), Abcam, dilution 1:50] overnight at 4°C. After washing, secondary antibodies (Goat anti‐Rabbit IgG H & L; Abcam) were added at a dilution of 1:1000 for 1 h at room temperature. After washing with PBS, DAPI was applied, and images were visualized with a fluorescence microscope at 100× magnification, and captured with a digital CCD camera. Background was determined by incubating sections in the absence of primary and secondary antibodies, and was subtracted from positively stained images. Three adjacent sections were used for image analysis. Three skeletal muscle cells were randomly selected and imaged from each section, and the average pixel intensity was determined for the region of interest defined by outlining the entire skeletal muscle cell. The average of the pixel intensity values obtained from at least three adjacent sections (with a coefficient of variation ≤ 0.25) was calculated and used for statistical analysis.
Micro‐computed tomography (CT) scanning
Rats were weighed and anaesthetized with 2.5% isoflurane and killed by excision of the heart. A laparotomy was performed to isolate the abdominal aorta from the vena cava, and warm (37°C) heparinized papaverine solution (1000 U mL−1 of heparin in 0.9% saline, 4 mg L−1 papaverine) was infused into the abdominal aorta at a rate of 0.05 mL s−1. After clear saline emerged from the opening in the vena cava, 3.5 mL of warm contrast medium (40°C, Microfil®; Flow Tech, Carver, MA, USA) was infused at the same rate. After yellow contrast medium emerged from the opening in the vena cava, the rat was placed in refrigerator at 4°C overnight to allow polymerization. The next day, the soleus muscle was dissected from both stretched and non‐stretched contralateral limbs, weighed and saved in 2% paraformaldehyde. The soleus muscle vasculature was imaged using a high‐resolution (6 μm voxel size) micro‐CT imaging system (Scanco Medical, Basserdorf, Switzerland). Noise was eliminated using a low‐pass Gaussian filter. All vertical long axis tomograms were thresholded to render binarized 3D images of the vascular network separated from the surrounding tissues. Soleus muscles from stretched and non‐stretched contralateral limbs were evaluated individually to quantify 3D histomorphometric values, including absolute vascular volume, normalized vascular volume (vascular volume per unit muscle volume) and vessel connectivity (number of vascular bifurcations per unit muscle volume). To normalize vascular volume and connectivity, muscle volume was obtained by circumscribing the soleus muscle external margin and applying thresholding to the entire muscle 3D image.
Statistical analysis
Vessel responses to ACh and Dea‐NONOate were evaluated using two‐way ANOVA (stretch and dose/time) with repeated measurement (dose/time). Group differences in animal characteristics, vessel characteristics, muscle blood flow, capillarity, and protein levels in arterioles and muscles were assessed by a paired t test or a Wilcoxon signed‐rank test. In all data, the number of animals is indicated by ‘n’. P < 0.05 was considered statistically significant. All data are presented as the mean ± SE.
Results
Animals
Body weight and central haemodynamics (blood pressure and heart rate) for the groups are shown in Table 1. No differences between groups were observed for body mass or blood pressure at rest or during splinting (Table 1). Both heart rate and blood pressure were significantly elevated during exercise compared to rest (Table 1). Blood pressure was not altered during acute muscle stretching or immediately after acute muscle stretching compared to blood pressure at rest (Table 1). Body weight and blood pressure were not different in sham control rats compared to rats in which one leg had undergone daily stretching.
Table 1.
Body weight and central haemodynamics
| Sham control (n = 16) | Stretched (n = 35) | |
|---|---|---|
| BW (g) | 463 ± 5 | 454 ± 8 |
| SOL weight (mg) | ||
| Non‐stretched contralateral | 178 ± 7 | 166 ± 4 |
| Stretched | 178 ± 6 | 184 ± 6* |
| SOL weight/BW (mg g−1) | ||
| Non‐stretched contralateral | 0.38 ± 0.02 | 0.39± 0.01 |
| Stretched | 0.39 ± 0.02 | 0.43 ± 0.01*, † |
| MAP (mmHg) | ||
| At rest | 136 ± 5 | 137 ± 3 |
| During treadmill exercise | 145 ± 5 | 148 ± 3 |
| HR (beats min–1) | ||
| At rest | 346 ± 9 | 355 ± 9 |
| During treadmill exercise | 394 ± 11 | 401 ± 10 |
| Naïve rats (n = 17) | ||
| MAP (mmHg) | ||
| At rest | 140 ± 3 | |
| During acute muscle stretching | 130 ± 4 | |
| Immediately after acute muscle stretching | 133 ± 2 | |
| HR (beats min–1) | ||
| At rest | 350 ± 11 | |
| During acute muscle stretching | 352 ± 13 | |
| Immediately after acute muscle stretching | 366 ± 12 |
BW, body weight; SOL, soleus muscle; MAP, mean arterial pressure; HR, heart rate. * P < 0.05 vs. non‐stretched contralateral. † P < 0.05 vs. sham control; n, number of rats. Data are the mean ± SE.
Vessel and muscle characteristics
The characteristics of skeletal muscles and arterioles are shown in Table 2. Wall thickness, spontaneous tone and maximal diameter of soleus muscle arterioles were not altered by muscle stretching. Soleus and plantaris muscle weights were significantly higher in the stretched compared to the non‐stretched contralateral limb (P < 0.01, respectively) (Table 2); however, the weights of the flexor hallucis longus, flexor digitorum longus, and red and white portions of tibialis anterior and extensor digitorum longus muscles were not different between the stretched and the non‐stretched contralateral limb.
Table 2.
Vessel and muscle characteristics
| Non‐stretched | Stretched | P value | |
|---|---|---|---|
| (n = 35) | (n = 35) | ||
| Vessel wall thickness (μm) | 20 ± 1 | 21 ± 1 | 0.298 |
| Vessel tone (%) | 68 ± 4 | 69 ± 3 | 0.203 |
| Vessel diameter (baseline) (μm) | 82 ± 6 | 74 ± 4 | 0.348 |
| Maximum diameter (μm) | 120 ± 5 | 115 ± 7 | 0.246 |
| SOL weight (mg) | 166 ± 4 | 184 ± 6 | <0.001 |
| SOL weight/BW (mg g−1) | 0.39 ± 0.01 | 0.43 ± 0.01 | <0.001 |
| PLA weight (mg g−1) | 336 ± 13 | 362 ± 14 | 0.007 |
| PLA weight/BW (mg g−1) | 0.78 ± 0.04 | 0.89 ± 0.04 | 0.005 |
| FDL weight (mg) | 149 ± 23 | 176 ± 25 | 0.077 |
| FDL weight/BW (mg g−1) | 0.36 ± 0.06 | 0.41 ± 0.06 | 0.133 |
| FHL weight (mg) | 73 ± 10 | 75 ± 9 | 0.431 |
| FHL weight/BW (mg g−1) | 0.14 ± 0.02 | 0.16 ± 0.03 | 0.136 |
| TA weight (mg) | 338 ± 38 | 344 ± 32 | 0.452 |
| TA weight/BW (mg g−1) | 0.79 ± 0.09 | 0.79 ± 0.08 | 0.475 |
| EDL weight (mg) | 162 ± 8 | 168 ± 14 | 0.375 |
| EDL weight/BW (mg g−1) | 0.39 ± 0.03 | 0.37 ± 0.02 | 0.288 |
| Capillary to fibre ratio | 2.31 ± 0.09 | 3.08 ± 0.04 | <0.001 |
BW, body weight; SOL, soleus muscle; PLA, plantaris muscle; FDL, flexor digitorum longus muscle; FHL, flexor hallucis longus muscle; TA red, red portion of tibialis anterior muscle; TA white, white portion of tibialis anterior muscle; EDL, extensor digitorum longus muscle; n, number of rats. Data are the mean ± SE.
Blood flow at rest and during treadmill exercise
After 4 weeks of muscle stretching, resting blood flow to the soleus, plantaris, flexor hallucis longus, flexor digitorum longus, red and white portion of tibialis anterior, and extensor digitorum longus muscles was not different between the stretched and non‐stretched contralateral limbs (Fig. 2 A). During treadmill exercise, blood flow to the soleus, plantaris, flexor hallucis longus and flexor digitorum longus muscles was significantly higher in the stretched limb compared to the non‐stretched contralateral limb (P < 0.05, respectively) (Fig. 2 B). Blood flow to the red and white portion of tibialis anterior and extensor digitorum longus muscles of the stretched and non‐stretched contralateral limbs was not different during treadmill exercise.
Figure 2. Blood flow at rest and during exercise after 4 weeks of muscle stretching.
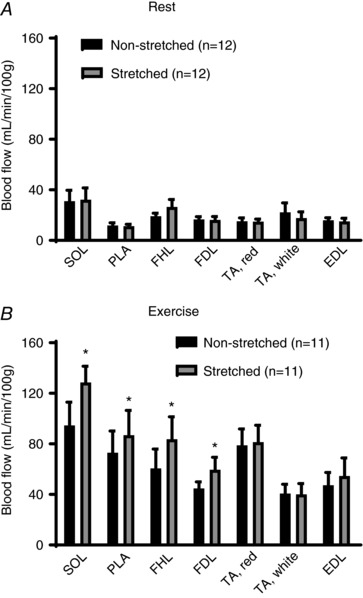
A, blood flow at rest was not different between any muscles from the stretched (grey) and non‐stretched contralateral limbs (black). B, blood flow to SOL, PLA, FDL and FHL during treadmill exercise was higher in the stretched limb (grey) compared to the non‐stretched contralateral limb (black); however, blood flow to TA and EDL was not different between the stretched and non‐stretched contralateral limb. SOL, soleus muscle; PLA, plantaris muscle; FDL, flexor digitorum longus muscle; FHL, flexor hallucis longus muscle; TA red, red portion of tibialis anterior muscle; TA white, white portion of tibialis anterior muscle; EDL, extensor digitorum longus muscle; n, number of rats. * P < 0.05 vs. non‐stretched contralateral limb. Values are the mean ± SE.
Blood flow during and immediately after acute muscle stretching
During acute muscle stretching, blood flow to the soleus, plantaris, flexor hallucis longus and flexor digitorum longus muscles was significantly lower in the stretched limb compared to the non‐stretched contralateral limb (P < 0.05, respectively) (Fig. 3 A); however, no differences in blood flow were observed between the red and white portion of tibialis anterior and extensor digitorum longus muscle of the stretched and the non‐stretched contralateral limb (Fig. 3 A). Immediately after acute muscle stretching, blood flow to skeletal muscle was not different between any muscles of the stretched and the non‐stretched contralateral limb (Fig. 3 B). Muscle blood flow and blood pressure were evaluated in rats placed in the restrainer without splint placement and did not differ significantly from resting values (data not shown).
Figure 3. Skeletal muscle blood flow during and immediately after acute muscle stretching.
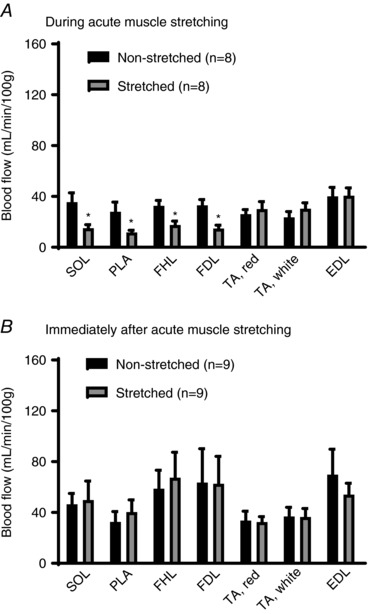
A, blood flow to SOL, PLA, FDL and FHL during acute muscle stretching was lower in the stretched limb (grey) compared to the non‐stretched contralateral limb (black); however, blood flow to TA and EDL was not different between the stretched and non‐stretched contralateral limbs. B, skeletal muscle blood flow was not different between any muscles from the stretched and non‐stretched contralateral limbs (B). SOL, soleus muscle; PLA, plantaris muscle; FDL, flexor digitorum longus muscle; FHL, flexor hallucis longus muscle; TA red, red portion of tibialis anterior muscle; TA white, white portion of tibialis anterior muscle; EDL, extensor digitorum longus muscle; n, number of rats; * P < 0.05 vs. non‐stretched contralateral limb. Values are the mean ± SE.
Vascular reactivity
Vasodilatory responses to ACh were significantly greater in skeletal muscle arterioles from the stretched limb compared to those from the non‐stretched contralateral limb (P < 0.001) (Fig. 4 A). ACh‐induced vasodilatation of soleus muscle arterioles from cage control and sham control rats was not different, as shown in Fig. 4 A (sham control). ACh‐induced vasodilatation was significantly higher in soleus muscle arterioles from the stretched limb compared to those from the non‐stretched contralateral limb, as well as those from cage and sham control rats (P < 0.001) (Fig. 4 A). Inhibition of eNOS with l‐NAME dramatically reduced ACh‐induced vasodilatation of soleus muscle arterioles and eliminated differences in responsiveness between groups (Fig. 4 B). Vasodilatory responses of soleus muscle arterioles to Dea‐NONOate were not altered by muscle stretching (Fig. 4 C).
Figure 4. Endothelium‐dependent and independent vasodilatation of soleus muscle arterioles.
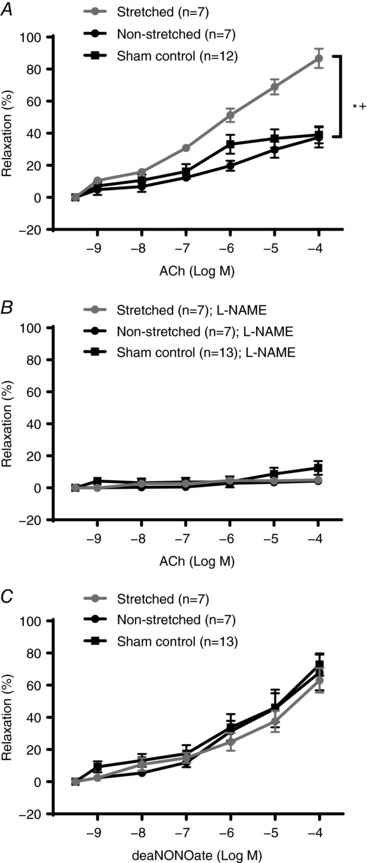
A, ACh‐induced vasodilatation was significantly greater in soleus muscle arterioles from the stretched limb (grey) compared to dilatation of arterioles from the non‐stretched contralateral limb (black) or limbs of sham control rats. B, inhibition with l‐NAME eliminated differences in ACh‐induced dilatation of soleus muscle arterioles from the stretched limb and the non‐stretched contralateral limb. C, Dea‐NONOate‐induced vasodilatation was not different between soleus muscle arterioles from the stretched limb and the non‐stretched contralateral limb stretch or limbs of sham control rats. n, number of rats; * P < 0.05 vs. non‐stretched contralateral limb; + P < 0.05 vs. sham control.
Arteriolar protein levels
Figure 5 A shows representative images of skeletal muscle arterioles stained with primary anti‐eNOS or anti‐SOD. Average pixel intensity of eNOS was higher in arterioles from the stretched limb compared to those from the non‐stretched contralateral limb (P < 0.05) (Fig. 5 B); however, SOD was not different between arterioles from the stretched and non‐stretched contralateral limb.
Figure 5. eNOS and SOD proteins in soleus muscle arterioles after 4 weeks of muscle stretching.
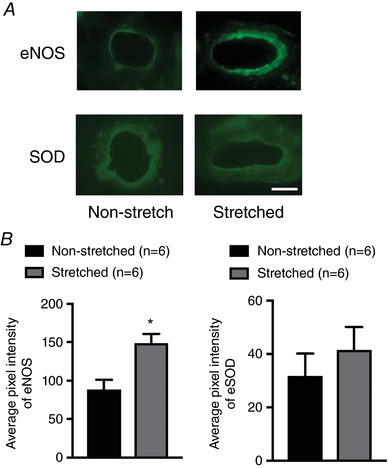
A, representative images of soleus muscle arterioles from the stretched and non‐stretch contralateral limbs. B, average pixel intensity of eNOS staining was higher in soleus muscle arterioles from the stretched limb. SOD staining in soleus muscle arterioles was not different between the stretched and the non‐stretched contralateral limb. n, number of rats; * P < 0.05 vs. non‐stretched contralateral limb. Scale bar = 25 μm. [Color figure can be viewed at http://wileyonlinelibrary.com]
Capillarity
Figure 6 A shows representative images of soleus muscle stained with G. simplicifolia lectin I, anti‐HIF‐1α, anti‐VEGFA or anti‐nNOS. The number of capillaries surrounding each skeletal muscle fibre was significantly higher in the soleus muscle from the stretched limb compared to that from the non‐stretched contralateral limb (P < 0.05) (Fig. 6 B). The capillary‐to fibre ratio was also increased in the soleus muscle from the stretched limb compared to that from the non‐stretched contralateral limb (Table 2). Levels of HIF‐1α and VEGFA were significantly higher in the soleus muscle from the stretched limb compared to the soleus muscle of the non‐stretched contralateral limb (P < 0.05) (Fig. 6 B). The level of nNOS was not different between the soleus muscle of the stretched limb and the soleus muscle of the non‐stretched contralateral limb.
Figure 6. Capillarity and angiogenic proteins in soleus muscles after 4 weeks of muscle stretching.
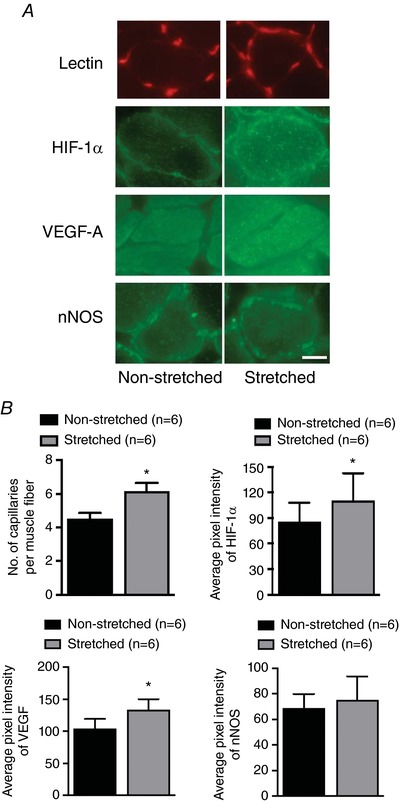
A, representative images of staining with lectin, HIF‐1α, VEGF‐A and nNOS in soleus muscles from stretched and non‐stretched contralateral limbs. B, the number of capillaries per muscle fibre was higher in the soleus muscle from the stretched limb. Levels of HIF‐1α and VEGF‐A were higher in the soleus muscle from the stretched limb compared to the non‐stretched contralateral limb; however, the level of nNOS was not different between the stretched limb and the non‐stretched contralateral limb. n, number of rats; * P < 0.05 vs. non‐stretched contralateral limb. Scale bar = 25 μm. [Color figure can be viewed at http://wileyonlinelibrary.com]
Muscle vascularity
Micro‐CT analysis (Fig. 7 A) revealed that vascular volume (normalized to total muscle volume) increased in the soleus of the stretched limb compared to that of the non‐stretched contralateral limb (Fig. 7 B). Absolute vessel volume and vascular connectivity tended to increase (P = 0.098 and 0.056, respectively), although the increase did not reach statistical significance (Fig. 7 B).
Figure 7. Micro‐CT analysis of microvascular parameters after 4 weeks of muscle stretching.
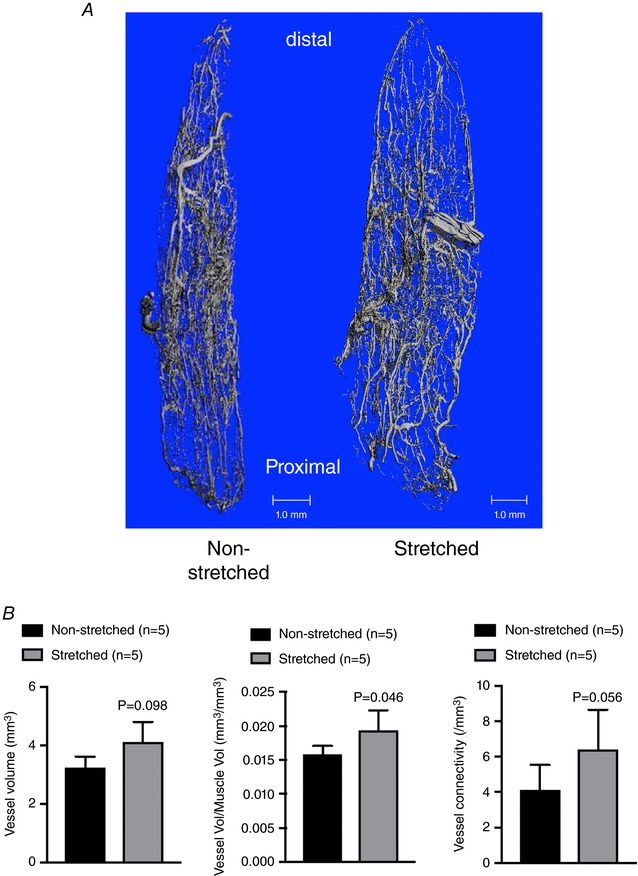
A, representative images of the soleus muscle microvasculature from the stretched and non‐stretched contralateral hindlimb. B, absolute vessel volume (mm3), normalized vascular volume (vascular volume/total muscle volume; mm3/mm3 and vessel connectivity in soleus muscles from stretched and non‐stretched contralateral limbs. n, number of rats; * P < 0.05 vs. non‐stretched contralateral limb. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
The main findings of the present study are that 4 weeks of daily muscle stretching (i) increased blood flow to skeletal muscle during exercise; (ii) enhanced endothelium‐dependent vasodilatation of resistance arterioles; and (iii) increased several morphological indices of O2 delivery capacity in stretched muscle of old rats. To our knowledge, this is the first report of the effect of daily muscle stretching on exercise hyperaemia in aged skeletal muscle. Our ankle dorsiflexion splint elongated ankle plantar flexor muscles and shortened dorsiflexor muscles; after 4 weeks of daily splint placement, exercise‐induced hyperaemia in the muscles elongated during daily splint placement (soleus, plantaris, flexor hallucis longus and flexor digitorum longus muscles) was significantly augmented in the stretched limb. By contrast, blood flow during exercise was not altered in the tibialis anterior and the extensor digitorum longus, ankle dorsiflexor muscles which were shortened during daily splint placement. During acute muscle stretching, blood flow to the ankle plantar flexor muscles of the stretched limb was reduced by ∼60% compared to the same plantar flexor muscles of the non‐stretched contralateral limb. By contrast, blood flow to dorsiflexor muscles of the stretched limb was not altered during acute muscle stretching. These results suggest that acute stretching produces a mechanical lengthening and local ischaemia within muscle, both of which may trigger vascular adaptations that contribute to increased blood flow during exercise in muscles that are stretched daily over 4 weeks.
Daily muscle stretching enhanced endothelium‐dependent vasodilatation of soleus muscle arterioles (Fig. 4 A). Additionally, eNOS protein levels were significantly higher in soleus muscle arterioles from the stretched limb compared to those from the non‐stretched contralateral limb (Fig. 5 B). Awolesi et al. (1995) reported increased eNOS in cultured endothelial cells after 24 h of cyclic stretch stimuli. Thacher et al. (2010) reported blunting of endothelium‐dependent vasodilatation of isolated carotid arteries maintained without stretch for 24 h compared to 24 h of longitudinal cyclic stretch. In humans, Hotta et al. (2013) reported enhanced vascular endothelial function after a single session of muscle stretching in patients with acute myocardial infarction. eNOS mRNA increases in vastus lateralis muscle of young male subjects after a single 90 min bout of passive knee movement (Hellsten et al. 2008) and after 4 weeks of passive knee movement for 90 min day−1, 4 days week−1 (Hoier et al. 2010). Consistent with these reports, the results of the present study indicate that passive muscle stretching increases eNOS expression in intramuscular arterioles. By contrast to passive knee movements that increase muscle length at the same time as increasing muscle blood flow, our data indicate that static muscle stretching stimulates an increase in vascular eNOS expression without an increase in blood flow. By contrast, SOD levels were not changed by daily stretching, which is different from the increase in SOD content that occurs in muscle arterioles from old rats in response to treadmill exercise training (Sindler et al. 2013).
We also found that capillarity was higher in the soleus muscle of the stretched limb compared to the soleus muscle of the non‐stretched contralateral limb, which is consistent with the notion that stretching‐induced angiogenesis is a mechanism that contributes to enhanced exercise hyperaemia in the muscles of the stretched limb. Micro‐CT analysis further indicated that stretching increased the number of microvascular connections and microvascular volume of the soleus muscle. Increased expression of HIF‐1α, VEGF and angiogenesis can be triggered by several stimuli including mechanical forces (Holly et al. 1980; Milkiewicz et al. 2001; Rivilis et al. 2002), as well as exposure of vascular endothelial cells to a decreased or ischaemia (Levy et al. 1995; Westvik et al. 2009). HIF‐1α and VEGF both increase in skeletal muscle after a single bout of blood flow restricted exercise (Gustafsson et al. 1999) or with sustained stretch (Milkiewicz et al. 2007). Furthermore, there is abundant evidence to suggest that HIF‐1A can promote the transcription of VEGFA (Forsythe et al. 1996) and drive angiogenesis (Pajusola et al. 2005), although the precise mechanisms for the increased HIF‐1α protein expression in the soleus observed in the present study are less clear. Consistent with reports of reduced skeletal muscle blood flow at extended sarcomere lengths (Poole et al. 1997; Kindig & Poole, 2001), we found that blood flow decreased during splint placement in muscles that were elongated by the dorsiflexion positioning of the ankle joint. According to the Fick principle, to maintain a given oxygen uptake in the face of reduced blood flow, there is an obligatory increase in muscle fractional O2 extraction. Assuming that vascular diffusing capacity () is either unchanged or reduced, Fick's law dictates that a widening of the capillary‐to‐intramyocyte gradient is requisite to increase extraction which, in the absence of an enhanced capillary or microvascular , would require a lowering of intramyocyte (Poole & Ferreira, 2007). However, during hypoxic gas breathing intracellular values (∼ 20 mmHg) (Richardson et al. 2006) are still four‐ to five‐fold higher than that reported during even moderate intensity exercise (∼5 mmHg) (Richardson et al. 2001). Thus, notwithstanding the intracellular hypoxia associated with reduced muscle blood flow during exercise, the reduction in blood flow observed during passive stretch in the soleus would probbaly not induce significant intracellular hypoxia. However, we cannot rule out local areas of ischaemia and hypoxia contributing, in part, to an increased HIF‐1α signalling. A more probable mechanism driving the upregulation of HIF‐1α in the present study is the prolonged mechanical deformation of the blood vessels associated with sustained muscle stretch. Specifically, HIF‐1α expression is increased with cyclical mechanical stretch in vascular smooth muscle cells (Chang et al. 2003) and prolonged stretch in isolated vessels (Lim et al. 2011). Vascular endothelial cells sense extracellular mechanical stimuli such as shear stress and stretch and then transduce these signals into intracellular signalling cascades that trigger angiogenesis even under nonhypoxic conditions (Holly et al. 1980; Hoier et al. 2010). Thus, based upon current evidence, the repeated prolonged vascular deformation and stretch in muscles of the splinted limb is probably the main mechanism for the upregulated HIF‐1α and VEGF vs. hypoxia, per se.
The involvement of nNOS in the angiogenic response to exercise training in skeletal muscle has been demonstrated previously (Huber‐Abel et al. 2012). Tidball et al. (1998) reported that nNOS levels in skeletal muscle decreased after 10 days of hindlimb unloading, and returned to baseline levels after 2 days of reloading. Gavin et al. (2000) investigated the link between NO and angiogenic proteins using systemic NOS inhibition and found that NOS inhibition attenuates any exercise‐induced increase of VEGF mRNA in rat skeletal muscle. Similarly, in humans, exercise training increases nNOS mRNA and protein, which is correlated with angiogenesis in vastus lateralis muscle (Huber‐Abel et al. 2012). By contrast to these results but consistent with results reported by Williams et al. (2006), in which angiogenesis induced by unilateral extirpation was unaffected by deletion of nNOS, the results of the present study showed no difference in the level of nNOS between soleus muscles of the stretched and non‐stretched contralateral limb.
A key mechanism for the old age‐related reduction in exercise capacity is an oxygen delivery‐to‐demand mismatching (Behnke et al. 2005) resulting from alterations in skeletal muscle blood flow. The most common and arguably efficacious paradigm used to improve exercising muscle hyperaemia with old age is strenuous aerobic exercise training, which improves blood flow distribution during exercise (Behnke et al. 2012). The findings of the present study provide a novel method (via daily dorsiflexion splinting) to improve exercise hyperaemia in aged skeletal muscle. There are several important similarities between the effects of daily muscle stretching and those associated with chronic aerobic exercise training in old age. First, neither daily muscle stretching (Fig. 2 A), nor exercise training (Behnke et al. 2012) affected skeletal muscle blood flow at rest. Second, both daily muscle stretching (Fig. 2 A) and aerobic exercise training (Behnke et al. 2012) increased skeletal muscle blood flow during moderate intensity exercise. Importantly, the increase in exercise hyperaemia between the two paradigms was quantitatively similar (∼30% increase in blood flow during exercise) with daily muscle stretching (Fig. 2 B) and aerobic exercise training (Behnke et al. 2012). Third, daily muscle stretching increased muscle capillarity (Fig. 6) and VEGF expression (Fig. 6) similar to that occurring in aged skeletal muscle after exercise training (Gavin et al. 2015). An important difference between the two paradigms is that the beneficial effects of daily stretching were confined to muscles displaying an ischaemic response during stretch (Fig. 3 A), whereas aerobic exercise training can induce systemic cardiovascular improvements (McCullough et al. 2011). We would not expect systemic cardiovascular benefits, nor local improvements in the oxidative capacity of the muscle as observed with chronic aerobic exercise training (Spier et al. 2004); however, we did not measure muscle oxidative capacity. It is possible that oxidative capacity was reduced in the muscles demonstrating an increased expression of HIF‐1α because this protein has been implicated in the suppression of mitochondrial biogenesis (Mason et al. 2004).
In summary, the results of the present study suggest that daily muscle stretching induces enhanced endothelium‐dependent vasodilatation and angiogenesis, enhancing exercise‐induced hyperaemia in the skeletal muscles of aged rats. Local ischaemia and/or mechanical stretching of intramuscular blood vessels are probable triggers of these vascular adaptations in chronically stretched skeletal muscle. Thus, a programme of passive muscle stretching, in the absence of pharmacological or surgical intervention, is sufficient to induce angiogenesis and augment exercise hyperaemia in aged skeletal muscle. Skeletal muscle blood flow is clinically important in elderly patients who have frailty, heart failure or peripheral artery disease. Muscle stretching performed with a splint could provide a feasible means of improving muscle blood flow and function in patients that cannot perform regular aerobic exercise. Clinical studies of the effects of muscle stretching on skeletal muscle blood flow in elderly patients are needed.
Additional information
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
KH and JMD designed the study, including the muscle stretch using splints, blood flow measurements, vessel experiments and immunohistochemistry. KH, BA, MLE and JMD designed the micro‐CT analysis. KH, BJB and JMD contributed to writing the manuscript. KH performed the statistical analyses and prepared the figures. KH, BJB, PM, DK and JMD contributed to the blood flow measurements. KH, PG, BC and JMD contributed to the vessel experiments. KH, PG, BC and RB contributed to daily muscle stretch training. KH, RB, MK and AC performed the immunohistochemistry. KH and JJM contributed to MATLAB pogramming and image analysis of immunohistochemistry. BJB, DDC and JMD revised the manuscript. KH, JMD and DDC obtained financial support. All authors checked and approved final version of manuscript submitted for publication.
Funding
This work was supported by National Institutes of Health Grants R21 AG‐044858 (to Judy M. Muller‐Delp and Demetra D. Christou) and The 27th Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad (to Kazuki Hotta).
Biography
Kazuki Hotta received his Master's Degree in Medical Science and his Doctorate in Medicine from Kitasato University in Sagamihara, Japan. Dr Hotta trained as a postdoctoral fellow in the Department of Biomedical Sciences at Florida State University. He is currently a Research Scientist in the Department of Engineering Science at the University of Electro‐communications in Tokyo, Japan, where he is working on developing in vivo skeletal muscle microvascular imaging using two‐photon microscopy.

Edited by: Michael Hogan & Ylva Hellsten
This is an Editor's Choice article from the 15 May 2018 issue.
References
- Agata N, Sasai N, Inoue‐Miyazu M, Kawakami K, Hayakawa K, Kobayashi K & Sokabe M (2009). Repetitive stretch suppresses denervation‐induced atrophy of soleus muscle in rats. Muscle Nerve 39, 456–462. [DOI] [PubMed] [Google Scholar]
- Ando J & Yamamoto K (2011). Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal 15, 1389–1403. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos N, Metsios GS, Flouris AD, Koutedakis Y & Wyon MA (2015). The relevance of stretch intensity and position – a systematic review. Front Psychol 6, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolesi MA, Sessa WC & Sumpio BE (1995). Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest 96, 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baewer DV, Hoffman M, Romatowski JG, Bain JL, Fitts RH & Riley DA (2004). Passive stretch inhibits central corelike lesion formation in the soleus muscles of hindlimb‐suspended unloaded rats. J Appl Physiol 97, 930–934. [DOI] [PubMed] [Google Scholar]
- Barnouin Y, McPhee JS, Butler‐Browne G, Bosutti A, De Vito G, Jones DA, Narici M, Behin A, Hogrel JY & Degens H (2017). Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J Cachexia Sarcopenia Muscle 8, 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke BJ, Delp MD, Dougherty PJ, Musch TI & Poole DC (2005). Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146, 259–268. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Ramsey MW, Stabley JN, Dominguez JM 2nd, Davis RT 3rd, McCullough DJ, Muller‐Delp JM & Delp MD (2012). Effects of aging and exercise training on skeletal muscle blood flow and resistance artery morphology. J Appl Physiol 113, 1699–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Shyu KG, Wang BW & Kuan P (2003). Regulation of hypoxia‐inducible factor‐1alpha by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin Sci (Lond) 105, 447–456. [DOI] [PubMed] [Google Scholar]
- Charifi N, Kadi F, Feasson L, Costes F, Geyssant A & Denis C (2004). Enhancement of microvessel tortuosity in the vastus lateralis muscle of old men in response to endurance training. J Physiol 554, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisman M, Daniel CR, Chow WH, Wu X & Zhao H (2015). Acculturation, sociodemographic and lifestyle factors associated with compliance with physical activity recommendations in the Mexican‐American Mano A Mano cohort. BMJ Open 5, e008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Evans MV & Duan C (1998). Effects of aging on cardiac output, regional blood flow, and body composition in Fischer‐344 rats. J Appl Physiol 85, 1813–1822. [DOI] [PubMed] [Google Scholar]
- Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K & Newman ED (1984). Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods 11, 1–39. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD & Semenza GL (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia‐inducible factor 1. Mol Cell Biol 16, 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K (2003). Mechanical stresses keep endothelial cells healthy: beneficial effects of a physiological level of cyclic stretch on endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 285, L782–L784. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Kraus RM, Carrithers JA, Garry JP & Hickner RC (2015). Aging and the skeletal muscle angiogenic response to exercise in women. J Gerontol A Biol Sci Med Sci 70, 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin TP, Spector DA, Wagner H, Breen EC & Wagner PD (2000). Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol 88, 1192–1198. [DOI] [PubMed] [Google Scholar]
- Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H & van Loon LJ (2014). Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol 116, 998–1005. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Puntschart A, Kaijser L, Jansson E & Sundberg CJ (1999). Exercise‐induced expression of angiogenesis‐related transcription and growth factors in human skeletal muscle. Am J Physiol Heart Circ Physiol 276, H679–H685. [DOI] [PubMed] [Google Scholar]
- Hansen‐Smith FM, Watson L, Lu DY & Goldstein I (1988). Griffonia simplicifolia I: fluorescent tracer for microcirculatory vessels in nonperfused thin muscles and sectioned muscle. Microvasc Res 36, 199–215. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P & Bangsbo J (2008). Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294, R975–R982. [DOI] [PubMed] [Google Scholar]
- Hepple RT & Vogell JE (2004). Anatomic capillarization is maintained in relative excess of fiber oxidative capacity in some skeletal muscles of late middle‐aged rats. J Appl Physiol (1985) 96, 2257–2264. [DOI] [PubMed] [Google Scholar]
- Hoier B, Rufener N, Bojsen‐Moller J, Bangsbo J & Hellsten Y (2010). The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol 588, 3833–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly RG, Barnett JG, Ashmore CR, Taylor RG & Mole PA (1980). Stretch‐induced growth in chicken wing muscles: a new model of stretch hypertrophy. Am J Physiol Cell Physiol 238, C62–C71. [DOI] [PubMed] [Google Scholar]
- Hotta K, Kamiya K, Shimizu R, Yokoyama M, Nakamura‐Ogura M, Tabata M, Kamekawa D, Akiyama A, Kato M, Noda C, Matsunaga A & Masuda T (2013). Stretching exercises enhance vascular endothelial function and improve peripheral circulation in patients with acute myocardial infarction. Int Heart J 54, 59–63. [DOI] [PubMed] [Google Scholar]
- Huber‐Abel FA, Gerber M, Hoppeler H & Baum O (2012). Exercise‐induced angiogenesis correlates with the up‐regulated expression of neuronal nitric oxide synthase (nNOS) in human skeletal muscle. Eur J Appl Physiol 112, 155–162. [DOI] [PubMed] [Google Scholar]
- Jensen L, Bangsbo J & Hellsten Y (2004). Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol 557, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katalinic OM, Harvey LA & Herbert RD (2011). Effectiveness of stretch for the treatment and prevention of contractures in people with neurological conditions: a systematic review. Phys Ther 91, 11–24. [DOI] [PubMed] [Google Scholar]
- Kindig CA & Poole DC (2001). Sarcomere length‐induced alterations of capillary hemodynamics in rat spinotrapezius muscle: vasoactive vs passive control. Microvasc Res 61, 64–74. [DOI] [PubMed] [Google Scholar]
- Kuebler WM, Uhlig U, Goldmann T, Schael G, Kerem A, Exner K, Martin C, Vollmer E & Uhlig S (2003). Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med 168, 1391–1398. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S & Goldberg MA (1995). Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 270, 13333–13340. [DOI] [PubMed] [Google Scholar]
- Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, Davies AH & Khalil RA (2011). Prolonged mechanical stretch is associated with upregulation of hypoxia‐inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg 53, 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SD, Howlett RA, Kim MJ, Olfert IM, Hogan MC, McNulty W, Hickey RP, Wagner PD, Kahn CR, Giordano FJ & Johnson RS (2004). Loss of skeletal muscle HIF‐1alpha results in altered exercise endurance. PLoS Biol 2, e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu‐Costello O, Hoppeler H & Weibel ER (1989). Capillary tortuosity in skeletal muscles of mammals depends on muscle contraction. J Appl Physiol (1985) 66, 1436–1442. [DOI] [PubMed] [Google Scholar]
- McCullough DJ, Davis RT 3rd, Dominguez JM 2nd, Stabley JN, Bruells CS & Behnke BJ (2011). Effects of aging and exercise training on spinotrapezius muscle microvascular PO2 dynamics and vasomotor control. J Appl Physiol 110, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel J, Ives SJ & Richardson RS (2012). Human muscle length‐dependent changes in blood flow. J Appl Physiol 112, 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkiewicz M, Brown MD, Egginton S & Hudlicka O (2001). Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation 8, 229–241. [DOI] [PubMed] [Google Scholar]
- Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M & Haas TL (2007). HIF‐1alpha and HIF‐2alpha play a central role in stretch‐induced but not shear‐stress‐induced angiogenesis in rat skeletal muscle. J Physiol 583, 753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie M, Reid KF, Miciek R, Lajevardi N, Choong K, Krasnoff JB, Storer TW, Fielding RA, Bhasin S & Lebrasseur NK (2010). Habitual physical activity levels are associated with performance in measures of physical function and mobility in older men. J Am Geriatr Soc 58, 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller‐Delp JM, Spier SA, Ramsey MW & Delp MD (2002). Aging impairs endothelium‐dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283, H1662–H1672. [DOI] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS & Poole DC (2004). Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96, 81–88. [DOI] [PubMed] [Google Scholar]
- Naruse K, Yamada T & Sokabe M (1998). Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol Heart Circ Physiol 274, H1532–H1538. [DOI] [PubMed] [Google Scholar]
- Pajusola K, Kunnapuu J, Vuorikoski S, Soronen J, Andre H, Pereira T, Korpisalo P, Yla‐Herttuala S, Poellinger L & Alitalo K (2005). Stabilized HIF‐1alpha is superior to VEGF for angiogenesis in skeletal muscle via adeno‐associated virus gene transfer. FASEB J 19, 1365–1367. [DOI] [PubMed] [Google Scholar]
- Poole DC & Ferreira LF (2007). Oxygen exchange in muscle of young and old rats: muscle‐vascular‐pulmonary coupling. Exp Physiol 92, 341–346. [DOI] [PubMed] [Google Scholar]
- Poole DC, Musch TI & Kindig CA (1997). In vivo microvascular structural and functional consequences of muscle length changes. Am J Physiol Heart Circ Physiol 272, H2107–H2114. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Duteil S, Wary C, Wray DW, Hoff J & Carlier PG (2006). Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Newcomer SC & Noyszewski EA (2001). Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol (1985) 91, 2679–2685. [DOI] [PubMed] [Google Scholar]
- Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O & Haas TL (2002). Differential involvement of MMP‐2 and VEGF during muscle stretch‐ versus shear stress‐induced angiogenesis. Am J Physiol Heart Circ Physiol 283, H1430–H1438. [DOI] [PubMed] [Google Scholar]
- Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML & Lakatta EG (1984). Exercise cardiac‐output is maintained with advancing age in healthy‐human subjects – cardiac dilatation and increased stroke volume compensate for a diminished heart‐rate. Circulation 69, 203–213. [DOI] [PubMed] [Google Scholar]
- Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD & Muller‐Delp JM (2013). Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW & Muller‐Delp JM (2004). Effects of ageing and exercise training on endothelium‐dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556, 947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Stallone JN, Dominguez JM 2nd & Muller‐Delp JM (2007). Exercise training enhances flow‐induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292, H3119–H3127. [DOI] [PubMed] [Google Scholar]
- Thacher TN, Silacci P, Stergiopulos N & da Silva RF (2010). Autonomous effects of shear stress and cyclic circumferential stretch regarding endothelial dysfunction and oxidative stress: an ex vivo arterial model. J Vasc Res 47, 336–345. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Dawson EA, Tinken TM, Cable NT & Green DJ (2009). Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53, 986–992. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT & Wehling M (1998). Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol Cell Physiol 275, C260–C266. [DOI] [PubMed] [Google Scholar]
- Westvik TS, Fitzgerald TN, Muto A, Maloney SP, Pimiento JM, Fancher TT, Magri D, Westvik HH, Nishibe T, Velazquez OC & Dardik A (2009). Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg 49, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Cartland D, Hussain A & Egginton S (2006). A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol 570, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]


