Abstract
Toll-like receptor 4 (TLR4), the principal signaling receptor for lipopolysaccharide (LPS) in mammals, requires the binding of MD-2 to its extracellular domain for maximal responsiveness. MD-2 contains a leader sequence but lacks a transmembrane domain, and we asked whether it is secreted into the medium as an active protein. As a source of secreted MD-2 (sMD-2), we used culture supernatants from cells stably transduced with epitope-tagged human MD-2. We show that sMD-2 exists as a heterogeneous collection of large disulfide-linked oligomers formed from stable dimeric subunits and that concentrations of sMD-2 as low as 50 pM enhance the responsiveness of TLR4 reporter cells to LPS. An MD-2-like activity is also released by monocyte-derived dendritic cells from normal donors. When coexpressed, TLR4 indiscriminately associates in the endoplasmic reticulum/cis Golgi with different-sized oligomers of MD-2, and excess MD-2 is secreted into the medium. We conclude that normal and transfected cells secrete a soluble form of MD-2 that binds with high affinity to TLR4 and that could play a role in regulating responses to LPS and other pathogen-derived substances in vivo.
Mammalian cells respond to lipopolysaccharide (LPS) (1), a major component of the outer membrane of Gram-negative bacterial cell walls, by activating Toll-like receptor 4 (TLR4) (1–5). TLR4 was first described as a member of a family of type I integral membrane proteins that contain numerous leucine-rich motifs on their extracellular portions and an intracellular signaling domain homologous to that of the IL-1 receptor (6, 7). In response to LPS, TLR4 initiates a cascade of serine/threonine kinases that eventually leads to the transcription of genes involved in inflammation (8, 9). To respond efficiently to LPS, TLR4 requires an accessory protein, MD-2 (10). MD-2 is a 20–30-kDa glycoprotein that was originally discovered by sequence homology with MD-1, a protein found associated with a B cell homologue of TLR4, RP105 (11, 12). MD-1 binds to the extracellular domain of RP105 and enhances its surface expression. Similarly, MD-2 binds to the extracellular domains of both TLRs 2 and 4 and causes their surface expression levels to increase (10, 13). Photoaffinity labeling studies have recently shown that LPS binds directly to the TLR4/MD-2 complex and that both molecules are in close proximity to the bound LPS (14).
MD-2, which contains a leader sequence but lacks a transmembrane domain, is secreted into the medium by transfected kidney epithelial cells (15). However, the structure and function of secreted MD-2 (sMD-2) have not been defined, nor is it known whether MD-2 is released into the medium by normal cells. To examine the properties of sMD-2, we have stably transfected HEK293 cells with an epitope-tagged version of MD-2 and have examined the secreted protein in culture supernatants. Based on previous findings from this laboratory that immature dendritic cells (iDC) express elevated levels of MD-2 message and decreased levels of TLRs 2 and 4 relative to monocytes (16), we have also examined culture supernatants from iDC for MD-2-like activity. In the present report, we show that sMD-2 consists of large disulfide-linked oligomers of stable, dimeric subunits. Addition of picomolar concentrations of sMD-2 to TLR4 positive, MD-2 negative cells greatly enhances their responsiveness to LPS, as do supernatants from cultured iDC. These findings suggest that sMD-2 might play a physiological role in controlling TLR-mediated responses to pathogens.
Materials and Methods
Cells and Reagents.
Human embryonic kidney 293, 293T, and 293TLR4 (293 cells stably transfected with human TLR4, a kind gift from Dr. Jesse Chow, Eisai Research Institute, Andover, MA; ref. 17) cell lines were maintained in complete medium (DMEM/10% FCS/2 mM glutamine/100 μg/ml penicillin/streptomycin) at 37°C in a humidified 5% CO2 incubator. Culture supernatants from human iDC (16) were centrifuged, filtered through a 22-μm membrane, and used immediately.
Expression Vectors and Transient Transfections.
TLR4-GFP was generated by PCR amplification of a full-length human TLR4 clone provided by Dr. Ruslan Medzhitov (Yale University School of Medicine, New Haven, CT) using Vent DNA polymerase (New England Biolabs) and inserting the PCR product into the XhoI (5′) and BamHI (3′) sites of pEGFP-N1 (CLONTECH). The resulting fusion protein was fluorescent and activated NF-κB in an LPS and MD-2-dependent fashion (not shown). The retroviral construct, rMD-2, was obtained by subcloning MD-2-FLAG cDNA (provided by Dr. Kensuke Miyake, Saga Medical School, Nabeshima, Japan) (10) in the XhoI–NotI sites of the CLRCX− retroviral vector (18). The vectors encoding dominant negative versions of MyD88 (19) and TLR4 (8) were provided by Dr. Marta Muzio (Amersham Pharmacia). The Fas-GFP construct was a gift from Dr. Richard Siegel (National Institute of Allergy and Infectious Diseases, Bethesda).
Production and Characterization of sMD-2.
A cell line that stably secretes MD-2-FLAG was generated by transducing 293 cells with the rMD-2 retroviral construct. Culture supernatants from confluent 293MD-2 cells were centrifuged, passed through 22-μm nitrocellulose filters, and concentrated with Centriprep YM-10 centrifugal filter units (Millipore). Concentrated supernatants (200 μl) were fractionated by FPLC on a Superdex 200 HR 10/30 gel filtration column (Amersham Pharmacia) in PBS, and 1 ml fractions were collected. Mild reduction of MD-2 was achieved by incubating immunoprecipitated MD-2 in 5 mM DTT/10 mM Tris, pH 8.0, for 1 h at room temperature followed by addition of 25 mM iodoacetamide.
Immunoprecipitation and Western Blotting.
Transfected cells from 10-cm dishes were lysed for 10 min at 0°C in 1 ml of lysis buffer (137 mM NaCl/20 mM Tris⋅HCl, pH 7.4/50 mM NaF/1 mM EDTA/1% Triton X-100) containing 60 mM n-octyl glucoside and protease inhibitors (10 μg/ml leupeptin and aprotinin, 1 mM PMSF). Lysates were centrifuged and immunoprecipitated with 4 μg of either M2 anti-FLAG mAb (Sigma) or anti-green fluorescent protein (GFP) polyclonal antibody (Molecular Probes) and 20 μl of packed protein A Sepharose (Amersham Pharmacia) for at least 16 h at 4°C. Alternatively, 293MD-2 supernatants (10 ml) were immunoprecipitated with 10 μg of M2 mAb overnight at 4°C. In some experiments, resin-bound protein was treated with endoglycosidase H (endo H) or peptide:N-glycosidase F (PNGase F, New England Biolabs) according to the manufacturer's instructions. Pellets were washed four times in lysis buffer and boiled in SDS sample buffer under reducing or nonreducing conditions. Samples were resolved by SDS/PAGE, transferred to nitrocellulose membranes, and immunoblotted with HRP-M2 mAb (Sigma) or anti-GFP mAb (CLONTECH) followed by a horseradish peroxidase (HRP)-conjugated secondary antibody. To assess surface expression, calcium phosphate-transfected 293T cells were biotinylated for 30 min at 4°C with 5 ml of 1 mg/ml Sulfo-NHS-biotin (Pierce) in Hanks' balanced salt solution and washed twice with 25 mM lysine in Hanks' balanced salt solution. Cell lysates were immunoprecipitated as described above and blotted with avidin-HRP Southern Biotech (Amersham Pharmacia).
NF-κB Assay.
A total of 5 × 104 293TLR4 cells per well were plated in 96-well flat-bottom microtiter plates, allowed to adhere, and transfected using calcium phosphate 1 day before treatment. Every well received 10 ng of each reporter vector (Ig-kB-Luciferase and pRSV-β-Gal, provided by Dr. Ulrich Siebenlist, National Institute of Allergy and Infectious Diseases), and in addition some wells received 10 ng of a vector encoding MyD88DN, NR4DW or 5 ng of MD-2 vector. Total amounts of DNA were kept constant by adding empty expression plasmid as needed. To test the activity of sMD-2, dilutions of concentrated supernatant from 293MD-2 cells were added to 293TLR4 reporter cells in the presence or absence of 5 mg/ml LPS (phenol-extracted S. minnesota Re595, Sigma) for 16 h. Samples were lysed, and luciferase and β-galactosidase (β-Gal) activity quantified with a luminometer. Data are presented as means ± SD of luciferase units divided by β-Gal units from duplicate wells (relative luciferase units).
Pulse–Chase Experiment.
Adherent cells were grown to 80% confluence and incubated with 35S-Met (ICN) in Met-free DMEM supplemented with 10% dialyzed FCS, glutamine, and antibiotics for 16 h in 10 ml of medium, or for 1 h in 5 ml of medium. After labeling, adherent cells were washed twice in cold PBS and lysed immediately or chased for varying times before lysis. Centrifuged lysates were precleared with protein A Sepharose, immunoprecipitated with anti-FLAG, and analyzed by SDS/PAGE. Autoradiograms were obtained after exposure of the dried gels to Kodak X-Omat AR films (Eastman Kodak).
Results
Production of sMD-2.
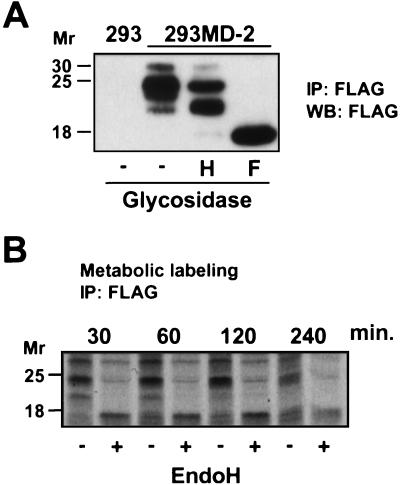
The capacity of MD-2 to enhance TLR4 responses has been demonstrated in transfected cells (10), but it is not known whether a soluble form of MD-2 could bind and activate TLR4 at the cell surface. To answer this question, sMD-2 was generated by stably transducing 293 cells with MD-2-FLAG and collecting supernatants from cultured cells; the secreted product is characterized in Fig. 1A. As seen, sMD-2 consists of three glycoforms, 30, 25, and 23 kDa, that are decreased to 18 kDa, the calculated molecular mass of MD-2 polypeptide, when N-linked sugars are removed with PNGase F. Endo H, which is specific for high-mannose N-linked carbohydrate, converted a portion of the 25-kDa species to 23 kDa, suggesting that sMD-2 contains a high mannose glycan. By contrast, most newly synthesized MD-2 is completely digested to the unglycosylated peptide by endo H (Fig. 1B), indicating that it contains only high-mannose carbohydrate, which is characteristic of proteins located in the ER and cis Golgi compartments (20). The loss of MD-2 from the cells seen after 2 h presumably reflects the entry of MD-2 into the secretory pathway with concomitant processing of N-linked glycans to the endo H-resistant form seen in Fig. 1A.
Figure 1.
Detection and characterization of sMD-2. (A) sMD-2-FLAG was immunoprecipitated (IP) from 10 ml of 293MD-2 culture supernatant with anti-FLAG mAb, and immunoprecipitates were left untreated (−) or treated with endo H (H) or PNGase F (F). Immunoprecipitates from supernatants of untransfected 293 cells served as a negative control (left lane). Samples were resolved by 15% SDS/PAGE, Western blotted (WB) with anti-FLAG, and detected by enhanced chemiluminescence. (B) Kinetics of maturation of MD-2. 293MD-2 cells were pulse labeled for 1 h with 35S-Met and chased for the indicated times. Cells were lysed, immunoprecipitated with an anti-FLAG mAb, and either left untreated (−) or treated with endo H (+). Samples were resolved by SDS/15% PAGE under reducing conditions and subjected to autoradiography for 10 days.
sMD-2 Confers LPS Sensitivity to TLR4-Expressing Cells.
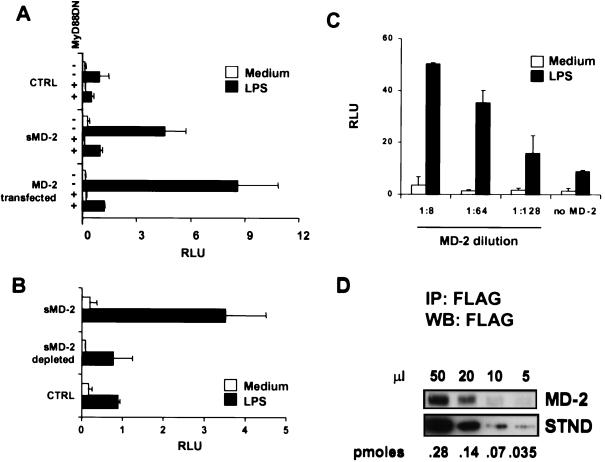
Supernatants from 293 and 293MD-2 cells were next added with or without LPS to 293TLR4 cells containing NF-κB and β-Gal reporter plasmids (reporter cells); cells transfected with a dominant negative version of MyD88 (MyD88DN) were used to test for the specificity of the signaling pathway. As shown in Fig. 2A, supernatants from 293MD-2, but not from control 293 cells, conferred LPS responsiveness to the reporter cells, and the response was efficiently abrogated by the MyD88DN construct. A TLR4DN construct lacking the cytoplasmic domain also blocked the response (data not shown). The sMD-2 was nearly as efficient as transfected MD-2 at enhancing the LPS response, and anti-FLAG depletion experiments (Fig. 2B) confirmed that this activity was in fact mediated by sMD-2. To determine the amount of sMD-2 required for LPS responsiveness, we titrated the reporter cells with MD-2-containing culture medium; as seen in Fig. 2C, a significant response was detected at 1:128 dilution. By comparing Western blot intensities of sMD-2 with a FLAG-tagged standard (Fig. 2D), we estimated that this represented a concentration of ≈50 pM. Thus, TLR4 behaves as a high affinity receptor for sMD-2.
Figure 2.
sMD-2 confers LPS responsiveness to TLR4+/MD-2− reporter cells. (A) 293TLR4 cells were cotransfected with NF-κB and β-Gal reporter vectors and either dominant negative MyD88 (MyD88DN) (+) or empty vector (−). After 24 h, each group of transfectants was treated with culture medium from 293 (CTRL) or 293MD-2 cells (sMD-2) in the presence or absence of LPS. As a comparison, 293TLR4 cells that had been transfected with MD-2 were treated with or without LPS (MD-2 transfected) in fresh medium. Bars indicate relative NF-κB activity. Data are representative of six separate experiments. (B) The LPS-enhancing activity is depleted by anti-FLAG mAb. 293TLR4 cells were left untreated (open bars) or treated with LPS (filled bars) in medium alone (CTRL), in medium containing supernatant from 293MD-2 cells (sMD-2), or in 293MD-2 cell medium adsorbed with anti-FLAG mAb (sMD-2 depleted). (C) Dose-response of MD-2. 293TLR4 cells were incubated with the indicated dilutions of concentrated 293MD-2 culture medium or with medium alone (no MD-2), and the LPS-dependent activation of NF-κB was followed as in A. (D) Quantitation of sMD-2. Serial dilutions of concentrated 293MD-2 culture medium (MD-2) were immunoprecipitated with the anti-FLAG mAb, resolved by SDS/10% PAGE, blotted with HRP-anti-FLAG, and developed by enhanced chemiluminescence. Intensities were compared with those from known concentrations of a standard protein (STND, carboxyl-terminal FLAG-BAP fusion protein, Sigma) shown in the lower panel. By comparing intensities, we estimate that 20 μl of the MD-2 concentrate contained ≈0.1 pmol of MD-2.
Human iDC Secrete an MD-2-Like Factor.
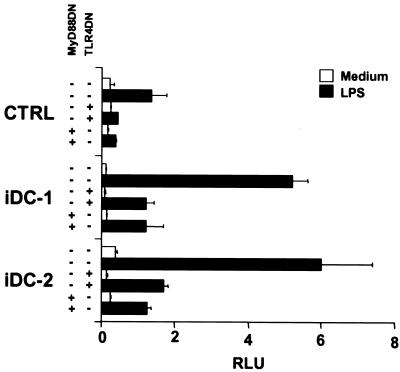
It was important to demonstrate that normal cells secrete an MD-2-like activity. We previously showed that human monocyte-derived iDC express high amounts of MD-2 message relative to TLR4 (16), suggesting that iDC might normally secrete MD-2. To test this hypothesis, reporter cells were incubated with supernatants from several iDC preparations in the presence or absence of LPS. Fig. 3 shows that supernatants from two representative iDC cultures (iDC-1, iDC-2) confer LPS sensitivity to the reporter cells, indicating that iDC do secrete an MD-2-like activity. The iDC-reconstituting activity was blocked by cotransfecting the reporter cells with MyD88DN or TLR4DN constructs, indicating that the factor was acting directly on the TLR4/IL-1R signaling pathway. However, because anti-MD-2 antibodies are not currently available, we were unable to establish definitively that the MD-2-like activity was mediated by MD-2 itself.
Figure 3.
iDC secrete MD-2-like factors. 293TLR4 cells were treated with supernatants from two human iDC preparations (iDC-1, iDC-2) in the absence (open bars) or presence (filled bars) of LPS. Where indicated, cells were also transfected with dominant negative versions of either TLR4 or MyD88. Histograms represent relative NF-κB activation as in Fig. 2A. Results are representative of 12 iDC supernatants tested.
sMD-2 Consists of Large, Disulfide-Linked Oligomers.
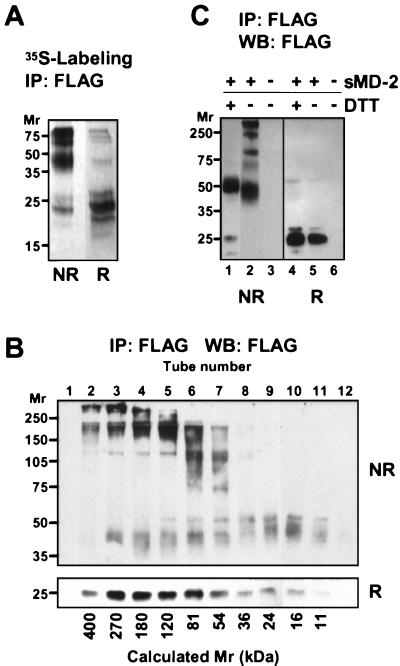
Because MD-2 contains an odd number of Cys residues, we hypothesized that at least one sulfhydryl group might be involved in intermolecular crosslinking. To test this, supernatants from metabolically labeled MD-2 transfectants were immunoprecipitated, resolved by SDS/PAGE under nonreducing and reducing conditions, and subjected to autoradiography. As seen in Fig. 4A, sMD-2 in the unreduced state consists of mainly dimers (band between 35 and 50 kDa) and larger oligomers, all of which migrate as monomeric MD-2 under reducing conditions. Moreover, no other protein of cellular origin was detected in MD-2 immunoprecipitates under reducing conditions, suggesting that the high molecular weight material contained only the MD-2 glycoprotein. To determine the size distribution of disulfide-linked species under nondenaturing conditions, we subjected sMD-2 to gel filtration chromatography in PBS, then analyzed the column fractions by SDS/PAGE under reducing and nonreducing conditions (Fig. 4B). As seen in the reducing gel (Lower, R), MD-2 eluted from the gel filtration column as a broad peak, mostly in the 50–400-kDa molecular mass range. Under nonreducing conditions (Upper, NR), species of ≈40, 80, 120, 240, and >300 kDa were resolved, corresponding roughly to dimers, tetramers, hexamers, octamers, and larger oligomers of the MD-2 monomer. In general, oligomers eluted from the gel filtration column at positions consistent with their molecular weights as determined by nonreducing SDS/PAGE. However, SDS treatment did dissociate some small molecular weight species from larger complexes, suggesting that noncovalent interactions, in addition to disulfide bonding, might be involved in oligomer formation. To determine whether all interchain disulfide bonds were equally stable, sMD-2 was exposed to mild reduction and alkylation under nondenaturing conditions. As shown in Fig. 4C, this treatment produced nearly homogeneous dimers of MD-2. Thus, the most stable subunit of MD-2 is a disulfide-linked dimer, and sMD-2 oligomers result from the formation of additional, more labile, disulfide bonds between dimers.
Figure 4.
sMD-2 forms large, disulfide-bonded aggregates. (A) 293T cells transfected with MD-2 were labeled overnight with 35S-Met. MD-2 was immunoprecipitated from the supernatant with an anti-FLAG mAb and subjected to SDS/PAGE under nonreducing (NR) or reducing (R) conditions. Shown is an autoradiogram after 2 days of exposure. (B) Concentrated supernatant (200 μl) from 293MD-2 cells was passed over a Superdex 200 HR 10/30 gel filtration column, and 1-ml fractions were collected immediately after the void volume. Fractions were immunoprecipitated, separated by SDS/10% PAGE under nonreducing (NR) and reducing (R) conditions, and blotted with HRP-anti-FLAG. No FLAG immunoreactivity was detected elsewhere in either membrane. Calculated molecular weights based on the elution of standard proteins are indicated at the bottom of the panel. (C) Mild reduction of sMD-2 yields disulfide-linked dimers. Immunoprecipitated sMD-2 was reduced (lanes 1 and 4) or not (lanes 2 and 5) with 5 mM DTT under nondenaturing conditions and resolved by SDS/10% PAGE under nonreducing (NR) and reducing (R) conditions. The gel was transferred and blotted with the anti-FLAG mAb as in 1B.
MD-2 Multimers Associate with TLR4.
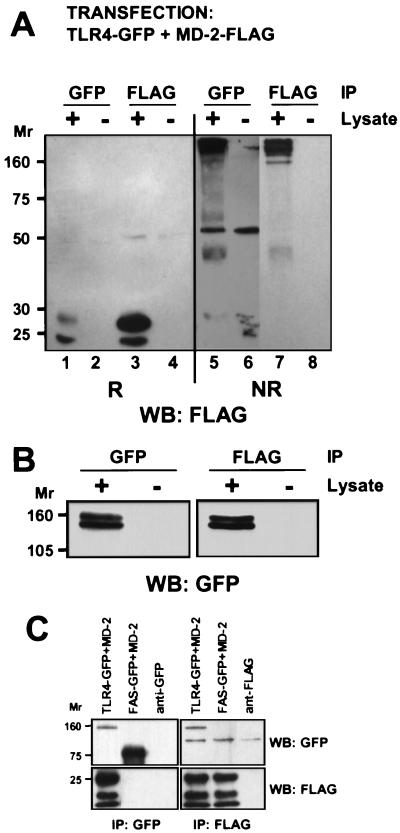
Because MD-2 forms a heterogeneous array of multimers, experiments were performed to determine which MD-2 species bind to TLR4. 293T cells were cotransfected with TLR4-GFP and MD-2-FLAG and immunoprecipitated with either anti-GFP or anti-FLAG antibodies. The immunoprecipitates were then analyzed by SDS/PAGE under reducing and nonreducing conditions and blotted for MD-2 with the anti-FLAG mAb (Fig. 5A). Analysis of the nonreduced samples indicated that MD-2 oligomers of many different sizes associated with TLR4 (lane 5), comparable to the molecular weight distribution of MD-2 in the total cell lysate (lane 7). Identical results were obtained when 10 mM iodoacetamide was included in the lysis buffer (data not shown), indicating that oligomerization did not result from sulfhydryl oxidation or exchange in the lysates. Anti-GFP blots of the same immunoprecipitates (Fig. 5B) confirm that TLR4 was present and suggest that most TLR4 molecules had associated with MD-2. By contrast, most MD-2 molecules did not coprecipitate with TLR4 (Fig. 5A, lanes 1 and 3), suggesting that MD-2 was produced in excess to TLR4. The interaction between TLR4 and MD-2 was specific, as shown by the fact that MD-2 failed to coprecipitate with Fas-GFP, a type I integral membrane receptor used as a negative control (Fig. 5C). We conclude that TLR4 indiscriminately binds to different-sized MD-2 oligomers.
Figure 5.
Oligomers of MD-2 bind to TLR4. (A) 293T cells were cotransfected with TLR4-GFP and MD-2-FLAG, lysed after 48 h, and immunoprecipitated with either anti-GFP or anti-FLAG mAbs. TLR4-associated MD-2 (lanes 1 and 5) and total MD-2 (lanes 3 and 7) were detected by Western blotting with anti-FLAG. Shown is a blot from a SDS/10% PAGE gel run under reducing (lanes 1–4) and nonreducing (lanes 5–8) conditions. The TLR4-associated, nonreduced MD-2 (lane 5) is shown at a higher exposure to facilitate comparison with total nonreduced MD-2 (lane 7) and indicates that various-sized oligomers of MD-2 bind to TLR4. (B) Immunoprecipitates from lysates of cells cotransfected with TLR4-GFP and MD-2-FLAG were subjected to SDS/7.5% PAGE under reducing conditions, transferred, and blotted for TLR4 expression with anti-GFP. Note that the total amount of TLR4 expressed (lane 1) was approximately the same as the amount bound to MD-2 (lane 3), in contrast to MD-2, which was expressed in excess to TLR4 (A, lanes 1 and 3). (C) As a specificity control, cells were cotransfected with MD-2 and either TLR4-GFP or Fas-GFP. As seen, MD-2 coprecipitated with TLR4-GFP but not with Fas-GFP.
MD-2 Binds to TLR4 in the ER.
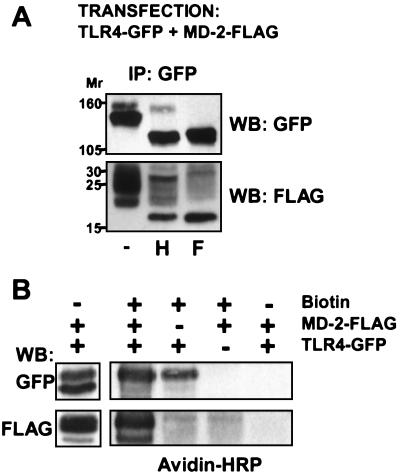
The observation that sMD-2 interacts functionally with TLR4 posed the question of whether MD-2 normally associates with TLR4 inside the cell when both are expressed together, or whether it must be first secreted into the medium. To address this question, cells were cotransfected with MD-2-FLAG and TLR4-GFP, and the cellular localization of both proteins was monitored by following the endo H sensitivity of anti-GFP immunoprecipitates. As shown in Fig. 6A (center lane), both TLR4 and TLR4-associated MD-2 contained endo H-sensitive and resistant species, with the endo H-sensitive fractions predominating for both proteins. This indicates that MD-2 binds TLR4 in the ER/cis Golgi and that major fractions of both proteins are located in these compartments at steady state. To test whether the TLR4/MD-2 complex could reach the cell surface, transient transfectants were externally labeled with biotin, and TLR4 immunoprecipitates were blotted with HRP avidin (Fig. 6B). As seen, TLR4 and the associated MD-2 stained positive in avidin blots, confirming that the complex did reach the cell surface.
Figure 6.
TLR4 and MD-2 associate in the cytoplasm. (A) 293T cells were cotransfected with TLR4-GFP and MD-2-FLAG, and lysates were immunoprecipitated after 48 h with the anti-GFP pAb and protein A Sepharose. Beads were left untreated (−) or treated with endo H (H) or PNGase F (F), solubilized in SDS reducing buffer, and analyzed by Western blotting after 6% (upper membrane) or 13% (lower membrane) SDS/PAGE. The upper membrane was blotted for TLR4, the lower membrane for MD-2. This experiment has been repeated twice with similar results. (B) 293T cells were transfected with either TLR4-GFP or MD-2-FLAG or both, as indicated, and cell-surface proteins were modified (or not) with sulfosuccinimidobiotin. Cells were lysed, immunoprecipitated with the anti-GFP, and analyzed by SDS/PAGE and Western blotting using either anti-GFP or anti-FLAG antibodies (left lane) or HRP-avidin (right lanes).
Discussion
In mammals, the recognition of LPS requires at least three proteins, CD14, TLR4, and MD-2 (10, 14, 21). Of these, TLR4 and MD-2 form a complex in which MD-2 binds to the extracellular domain of TLR4; together, the two proteins transduce a signal upon binding LPS. In addition, a recent report indicates that MD-2 also binds to TLR2, although less avidly than to TLR4, and enhances the TLR2 response to a number of pathogen-derived substances (13). The immature form of MD-2 contains a leader sequence that targets the molecule to the ER, but lacks a transmembrane domain that would anchor the protein to the cell membrane. This suggests that MD-2 is a soluble protein that could potentially bind to TLRs 4 and 2 in the ER or, alternatively, might first be secreted into the medium and then bind to TLRs 4 and 2 on the cell surface. In this study, we characterized sMD-2 produced by cells that stably express epitope-tagged MD-2 but not TLR4 or TLR2. Supernatants from these cells contained MD-2 in a form that was relatively resistant to endo H digestion, indicating that it had passed through the Golgi apparatus before secretion. The sMD-2 exhibited a remarkable capacity for restoring LPS responsiveness to reporter cells that expressed TLR4 but not MD-2. Even at concentrations as low as 50 pM, MD-2 significantly enhanced LPS reactivity, suggesting that TLR4 has a functional affinity constant for MD-2 in the range of 50–500 pM. This represents a high affinity interaction, typical of the affinities with which cytokines and hormones bind their receptors (22).
Although the calculated molecular weight of the MD-2 polypeptide is 18,400, the secreted form is much larger. Part of the increase in molecular weight is because of glycosylation. MD-2 contains two N-linked glycosylation sites, and when N-linked sugars were removed with PNGase F, the relative molecular mass of MD-2 under reducing conditions decreased from ≈25 kDa to that expected for the unmodified polypeptide. The molecular weight of MD-2 is also increased by interchain disulfide bond formation. MD-2 contains seven Cys residues, suggesting that the unpaired sulfhydryl residue might participate in the formation of MD-2 dimers. However, we unexpectedly found that MD-2 polymerizes into large, disulfide-linked, oligomers. This implies that at least two sulfhydryl groups in MD-2 participate in interchain disulfide bonding, leaving a maximum of five sulfhydryls available for intrachain disulfide loop formation. Thus, MD-2 contains, at most, two intrachain disulfide bonds and may use three or more sulfhydryls in the formation of MD-2 oligomers. MD-2 also forms stable dimers that resist mild reduction in nondenaturing solution, implying that one intermolecular disulfide bridge is less exposed than the others and suggesting that the large oligomers of MD-2 consist of polymerized dimers. As we have demonstrated, TLR4 associates with MD-2 oligomers of all sizes. This raises the possibility that each MD-2 oligomer might bind to several TLR4 molecules, leading to the formation of large clusters on the cell surface before activation. Moreover, because MD-2 also binds to TLR2, these clusters could contain both TLR2 and TLR4 and possibly other MD-2 binding proteins.
When MD-2 and TLR4 are expressed in the same cell, they associate in the ER/cis Golgi as shown by the fact that both proteins in coprecipitates are susceptible to endo H digestion. However, cells such as double transfectants and iDC that express both MD-2 and TLR4 secrete MD-2 (or an MD-2-like activity) into the medium, implying that not all MD-2 is bound to TLR4. This was also demonstrated directly in coprecipitation experiments (Fig. 5), which showed that essentially all TLR4 was associated with MD-2, whereas only a fraction of total MD-2 was bound to TLR4. We conclude that at least in some cells MD-2 is synthesized in large excess to TLR4 and that it saturates all available TLR4 molecules in the ER, with the excess MD-2 being secreted into the medium. Thus, the MD-2 that is associated with TLR4 on the cell surface could potentially derive from either the MD-2 that binds TLR4 in the ER or from sMD-2 that exchanges with the ER-derived MD-2, similar to the situation with MHC-I molecules and β-2 microglobulin (23).
An important question raised by the current study is whether sMD-2 serves a physiological function in the innate recognition of LPS and other pathogen-associated molecules. The observations that normal cells secrete an MD-2-like activity and that sMD-2 binds to TLR4 with very high affinity suggest that sMD-2 could play an important role in controlling the physiological responses of TLR4, and perhaps TLR2, to pathogen-derived substances. sMD-2 would be most effective, however, in cells that express TLR4 but little or no MD-2, and it will be interesting to see whether any cells have this property. Finally, sMD-2 (ESOP-1) is secreted at various stages of development in tissues of the hematopoietic, nervous, and reproductive systems (15), suggesting that it may have some broader function than serving as a cofactor for TLRs.
After submission of this manuscript, Schromm et al. (24) reported that supernatants from MD-2-transfected cells could confer signaling function on TLR4-expressing cells.
Acknowledgments
We thank Drs. Jesse Chow, Kensuke Miyake, Marta Muzio, and Richard Siegel for kindly providing several of the expression vectors used in this report, and Dr. Paul Roche for thoughtful comments during the preparation of the manuscript.
Abbreviations
- β-Gal
β-galactosidase
- endo H
endoglycosidase H
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- iDC
immature dendritic cells
- LPS
lipopolysaccharide
- PNGase F
peptide:N-glycosidase F
- sMD-2
secreted MD-2
- TLR
Toll-like receptor
References
- 1.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 2.Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 3.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 4.Aderem A, Ulevitch R J. Nature (London) 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 6.Rock F L, Hardiman G, Timans J C, Kastelein R A, Bazan J F. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr Nature (London) 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 8.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C A., Jr Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 10.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake K, Shimazu R, Kondo J, Niki T, Akashi S, Ogata H, Yamashita Y, Miura Y, Kimoto M. J Immunol. 1998;161:1348–1353. [PubMed] [Google Scholar]
- 12.Miura Y, Shimazu R, Miyake K, Akashi S, Ogata H, Yamashita Y, Narisawa Y, Kimoto M. Blood. 1998;92:2815–2822. [PubMed] [Google Scholar]
- 13.Dziarski R, Wang Q, Miyake K, Kirschning C J, Gupta D. J Immunol. 2001;166:1938–1944. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- 14.da Silva Corrcia J, Soldau K, Christen U, Tobias P S, Ulevitch R J. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Morrison A M, Nakano T, Tashiro K, Honjo T. Blood. 2000;96:362–364. [PubMed] [Google Scholar]
- 16.Visintin A, Mazzoni A, Spitzer J H, Wyllie D H, Dower S K, Segal D M. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Young D W, Gusovsky F, Chow J C. J Biol Chem. 2000;275:20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- 18.Wang R F, Wang X, Johnston S L, Zeng G, Robbins P F, Rosenberg S A. Cancer Res. 1998;58:3519–3525. [PubMed] [Google Scholar]
- 19.Muzio M, Ni J, Feng P, Dixit V M. Science. 1997;278:1612–1615. [Google Scholar]
- 20.Helenius A, Aebi M. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 21.Wright S D. J Exp Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barclay A N, Brown M H, Law S K A, McKnight A J, Tomlinson M G, van der Merwe P A. The Leucocyte Antigen Facts Book. London: Academic; 1997. [Google Scholar]
- 23.Hyafil F, Strominger J L. Proc Natl Acad Sci USA. 1979;76:5834–5838. doi: 10.1073/pnas.76.11.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schromm A B, Lien E, Henneke P, Chow J C, Yoshimura A, Heine H, Latz E, Monks B G, Schwartz D A, Miyake K, et al. J Exp Med. 2001;194:79–88. doi: 10.1084/jem.194.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]