Abstract
The introduction of dry electrodes for EEG measurements has opened up possibilities of recording EEG outside of standard clinical environments by reducing required preparation and maintenance. However, the signal quality of dry electrodes in comparison with wet electrodes has not yet been evaluated under activities of daily life (ADL) or high motion tasks. In this study, we compared the performances of foam-based and spring-loaded dry electrodes with wet electrodes under three different task conditions: resting state, walking, and cycling. Our analysis showed signals obtained by the 2 types of dry electrodes and obtained by wet electrodes displayed high correlation for all conditions, while being prone to similar environmental and electrode-based artifacts. Overall, our results suggest that dry electrodes have a similar signal quality in comparison to wet electrodes and may be more practical for use in mobile and real-time motion applications due to their convenience. In addition, we conclude that as with wet electrodes, post-processing can mitigate motion artifacts in ambulatory EEG acquisition.
I. INTRODUCTION
Electroencephalography (EEG) is a common method used to measure and compare electrical activity from different regions of the human brain, serving as a fundamental tool in the study of the brain to diagnose neuropsychiatric and psychophysical disorders and more recently, in brain-computer interfaces (BCI). The ability to record EEG without the need for time consuming preparation will allow applications outside of static, clinical environments into mobile, real-time environments [1]–[4].
The common practice of using wet EEG electrodes in controlled clinical environments requires a conductive solution to transfer electrolyte between the electrode and the scalp. This requirement is inconvenient for both researchers and users, increasing preparation time and often creating discomfort. Moreover, improper use of the conductive solution may result in inaccurate measurements. Applying too much conductive solution onto the scalp risks creating electrolyte bridges that may short circuit adjacent EEG electrodes [5]. In contrast, too little conductive solution may dry and result in poor EEG quality over time [4].
Recent developments in dry and non-contact EEG electrodes have shown promise in significantly reducing preparation time, and thereby potentially enabling non-clinical applications. Dry electrodes, in particular, do not require the use of conductive solution typical of wet electrodes, while promising similar performances. Commercially available dry electrodes include Flex Sensors (Cognionics, Inc., San Diego, CA), g.SAHARA (g.tec medical engineering, Schiedlberg, Austria), bristle-sensors by [6], and foam-based and spring-loaded dry electrodes by Mindo (National Chiao Tung University - Brain Research Center, Hsinchu, Taiwan).
In this study, we investigated the performance of Mindo foam-based dry electrodes and spring-loaded dry electrodes due to several advantageous design features. These features include the use of a flexible material that provides a high degree of comfort even when force is applied, low manufacturing cost for the foam-based dry electrodes [7], and high reusability for the spring-loaded dry electrodes [8]. Although these electrodes have been successfully implemented into mobile EEG systems for BCI applications, these systems were designed primarily for use in low motion conditions [9], [10].
The aim of this study was to evaluate the broadband EEG signal recorded from the 3 types of electrodes by comparing the quality of the EEG signals under 3 task conditions: resting state, walking on a treadmill, and cycling on a stationary bicycle.
II. MATERIALS AND METHODS
A. Participants
Ten healthy adults (7 male, 3 female) with a mean age of 22.2 ± 1.9 years participated in this study. Subjects were excluded from the study if they had any reported history of neurological disorders. All participants provided assent to the study and signed an informed consent form that was approved by the University of British Columbia.
B. Materials
A custom head cap incorporating 9 wet EEG electrodes and 8 dry EEG electrodes was constructed for this study. The wet electrodes used were silver-chloride HydroCel Geodesic SensorNets (HCGSN) electrodes by Electrical Geodesics, Inc. (EGI, Eugene, OR), used mainly for research and clinical diagnoses. Four Mindo foam-based electrodes and 4 Mindo spring-loaded electrodes were used as dry electrodes.
The foam-based electrode was made of conductive urethane polymer foam, covered with a thin conductive polymer fabric. A Ni/Cu coating established proper electrical contact between the scalp and the electrode [7]. For this study, all foam-based electrodes were placed on the frontal regions of the scalp where no hair was present.
Each spring-loaded electrode was composed of 16 gold-coated BeCu pins attached to a flexible copper plate. When placed on the scalp in an area covered with hair, the retractable pins pass through hair to maintain proper electrical conductivity in the electrode-scalp interface [8]. For this study, all spring-loaded electrodes were placed over the occipital and central regions of the scalp.
Each of the 4 foam-based electrodes was paired with a HCGSN electrode and placed in one of the following positions, in accordance with the International 10–20 System: Fp1, Fp2, F7, and F8. Each of the 4 spring-loaded electrodes was also paired with a HCGSN electrode at the following positions: O1, O2, FCz, and CPz. Each electrode was within 10 mm from its paired electrode. Wet electrodes were positioned below dry electrodes to prevent conductive solution from dripping onto the dry electrodes. The remaining HCGSN electrode was positioned at Cz and acted as reference for both the wet and dry electrodes.
All electrodes were connected to the EGI Net Amps 300 Amplifier system (EGI, Eugene, OR) via a 140-pin Hypertronics connector (Smith Connectors, Hudson, MA).
The conductive solution used for the HCGSN wet electrodes was a solution of water, potassium chloride, and baby shampoo, as instructed by EGI [11].
C. Data Acquisition and Processing
After fitting the head cap onto the subjects head, conductive solution was applied to the wet electrodes with care, to ensure that the dry electrodes remain solution-free. Net Station Acquisition software (EGI, Eugene, OR) was used to measure the impedances of all electrodes with a 20 Hz, 400 μV signal [12]. If the impedance of a wet electrode was above 50 kΩ (recommended threshold by EGI), additional conductive solution was applied to the electrode or the hair underneath was displaced [11]. If the impedance of a dry electrode was above 50 kΩ, the head cap was tightened to increase the electrode-scalp interface area. This was repeated until the measured impedance was below 50 kΩ. Although no immediate discomfort was reported, several subjects reported slight discomfort by the end of the study due to the necessary tightness of the head cap.
Once all attached electrodes had achieved impedances below 50 kΩ, EEG signals were recorded at a sampling rate of 250 Hz for the following 3 tasks: eyes-closed sitting (resting) for 5 minutes, eyes-open walking (1 m/s) on a treadmill for 5 minutes, and eyes-open cycling (speed based on subject’s preference) on a stationary bicycle for 10 minutes. After the completion of each task, the impedances were measured again and the required actions were taken to decrease the impedances to below 50 kΩ before starting the next assigned task. In total, 3 tasks were completed by each subject with a total recording time of 20 minutes.
Data obtained from the Net Station Acquisition software was transferred to MATLAB (MathWorks, Natick, MA) using the EEGLab toolbox [13]. The signals were then passed through a 0.5 to 45 Hz FIR filter to mitigate ambient electrical noise from nearby electronics that are above 45 Hz, and sweat artifacts, which are generally below 0.5 Hz [5], [14].
D. Artifact Rejection with ICA
Motion artifacts present in the walking and cycling states have relatively higher amplitude than the background EEG brain signals, and appeared in both the dry and wet electrodes. As a consequence, these artifacts would create a large bias towards high correlation if linear correlation analysis were to be applied directly to the raw EEG data. To accurately assess the quality of signals measured from dry electrodes during motion, we first removed artifacts using independent component analysis (ICA) [15].
Due to the non-stationarity of the observed artifacts over the whole recording, Extended Infomax ICA [13] was performed on moving 10-second windows. Independent components (IC’s) with the largest variances corresponded to motion-related artifacts. The IC’s to be rejected within each window was determined by calculating the minimum number of IC’s to be removed from the contaminated signal such that the resulting cleaned signal has comparable power to the resting state EEG signals. This method cleaned contaminated EEG data from motion artifacts and other artifacts, such as eye movements, EMG, and environmental artifacts.
E. Correlation Analysis
To determine the correlation of the wet and dry electrodes, signals from each pair of wet and dry electrodes were divided into 1-second windows. The Pearson’s linear correlation coefficient was then calculated for each window.
Pairs of electrodes which did not reach impedances of less than 50 kΩ and which did not pick up any signal were excluded from analysis. In total 12 out of 40 pairs of resting datasets, 11 out of 40 pairs of walking datasets, and 10 out of 40 pairs of cycling datasets were excluded.
The statistical significance for each correlation coefficient was then determined using the Benjamini-Hochberg false discovery rate (FDR) method with a q-value < 0.05.
III. RESULTS AND DISCUSSION
Differences are expected in EEG signals acquired with similar electrodes in close proximity, due to volume conduction. We analyzed these differences using a 256-channel EEG dataset acquired with Biosemi wet electrodes (Biosemi, Amsterdam, Netherlands). We found that electrode pairs that are less than 12 mm apart had a median correlation of 0.9509. In this section, we present results of the correlations between the HCGSN wet and Mindo dry electrodes in this context.
A. Correlation of Foam-Based Electrodes
For all tasks, correlations obtained from the foam-based dry electrodes and the wet electrodes (Fp1, Fp2, F7, F8) displayed negative skewness, indicating a bias towards high correlation (see Fig. 1). The median correlation coefficients for the resting, walking, and cycling states were 0.8857, 0.9176, and 0.8971 respectively (see Table I).
Fig. 1.
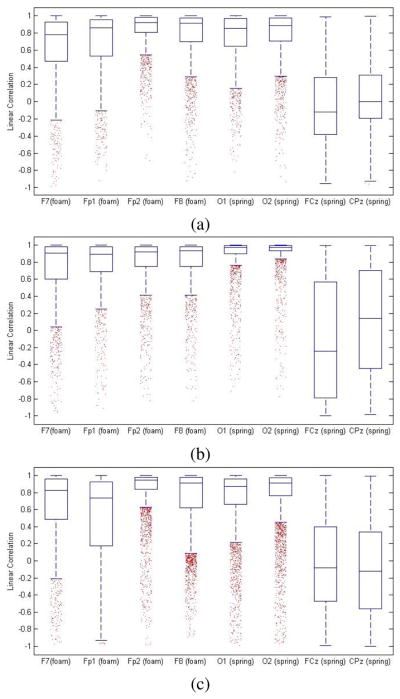
Distribution of correlation coefficients for (a) the resting state, (b) the walking state, and (c) the cycling state.
TABLE I.
Correlation Coefficients of Foam-Based Dry Electrodes and Wet Electrodes
| Resting State | Walking State | Cycling State | ||||
|---|---|---|---|---|---|---|
| Position | Median | Skewness | Median | Skewness | Median | Skewness |
| F7 | 0.7791 | −1.600 | 0.9079 | −1.8015 | 0.8259 | −1.6014 |
| Fp1 | 0.8578 | −1.5955 | 0.8967 | −2.2727 | 0.7368 | −1.0889 |
| Fp2 | 0.9209 | −2.5291 | 0.9213 | −2.4961 | 0.9489 | −3.3228 |
| F8 | 0.9118 | −2.1439 | 0.9358 | −2.3839 | 0.9156 | −1.615 |
| Combined | 0.8857 | −2.0367 | 0.9176 | −2.2427 | 0.8971 | −1.7877 |
For a q-value < 0.05, the overall percentages of windows showing significant correlation for the foam-based dry electrode and wet electrode pairs were 93.49, 93.96, and 87.88 for the resting, walking, and cycling states respectively.
These results showed high correlation between the foam-based dry electrodes and the wet electrodes in the resting state. Furthermore, high correlation in the walking and cycling states suggested that there were no significant differences between foam-based dry electrodes and wet electrodes under the conditions of motion. Because hair in the electrode-scalp interface causes poor contact, foam-based electrodes are only suitable for hairless locations of the scalp [8], [10].
B. Correlation of Spring-Loaded Electrodes
Results for all tasks for the spring-loaded electrodes and the wet electrodes (O1, O2, FCz, CPz) displayed negative skewness, indicating a bias towards high correlation (see Fig. 1). The median correlation coefficients for the resting, walking, and cycling states were 0.7800, 0.9020, and 0.7226 respectively (see Table II).
TABLE II.
Correlation Coefficients of Spring-Loaded Dry Electrodes and Wet Electrodes
| Resting State | Walking State | Cycling State | ||||
|---|---|---|---|---|---|---|
| Position | Median | Skewness | Median | Skewness | Median | Skewness |
| O1 | 0.8518 | −1.8037 | 0.9729 | −3.9013 | 0.87044 | −2.2614 |
| O2 | 0.8871 | −2.1320 | 0.9763 | −4.9104 | 0.9153 | −2.7302 |
| FCz | −0.1194 | 0.4269 | −0.2395 | 0.2076 | −0.0789 | 0.1705 |
| CPz | 0.0025 | 0.2376 | 0.1434 | −0.1450 | −0.1218 | 0.1969 |
| Combined | 0.7800 | −1.1641 | 0.9020 | −1.2281 | 0.7226 | −0.9360 |
From the overall distribution of correlation coefficients from the 3 states, we observed negative skewness at O1 and O2 (−2.3924 and −2.8517), but slightly positive skewness at FCz and CPz (0.1516 and 0.0924). Furthermore, analysis indicated median correlation coefficients of 0.9034 and 0.9355 respectively at O1 and O2. However, median correlation coefficients at FCz and CPz were significantly lower at −0.1121 and −0.0465 respectively.
For a q-value < 0.05, 95.63 (resting), 98.14 (walking), and 94.43 (cycling) percent of windows had statistically significant correlation for positions O1 and O2. For the same q-value, 35.28 (resting), 44.72 (walking), and 36.99 (cycling) percent of windows had significant correlation for positions FCz and CPz.
The clear differences between the median correlation coefficients and the skewness at positions O1 and O2 and positions FCz and CPz suggested abnormalities in the measured EEG signals at one of these subsets of positions. Inspection of the EEG signals at FCz and CPz indicated consistent low amplitudes (< 2 μV) in the wet electrode at FCz and the spring-loaded dry electrode at CPz for the majority of trials. Comparison of these low amplitudes to the expected amplitudes of EEG signals (10 to 100 μV) implied the influence of artifacts [5], [14], [16]. Due to the proximity of positions FCz and CPz with the reference Cz, the low amplitude EEG signals were suspected to be caused by an electrolyte bridge, resulting in the transfer of ions between electrodes without passing through the scalp [14]. Therefore, the correlation coefficients obtained at FCz and CPz should be considered unreliable in comparing the performances of the spring-loaded dry electrodes and the wet electrodes.
C. Artifacts in the EEG
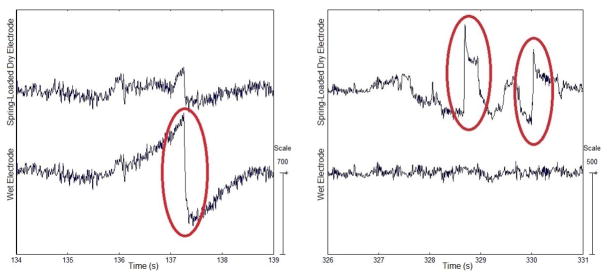
Visual inspection of the raw signals obtained in the resting and cycling states displayed occasional artifacts which were present in either the dry electrodes or the wet electrodes. Inconsistent artifact appearances between the corresponding signals obtained from electrode pairs implied that these artifacts may be from electrode-based sources and therefore, they appeared in one electrode, but not its paired electrode. Characteristics of these artifacts include sudden magnitude changes in the amplitude of the recorded signal followed by either further fluctuations or decay back to normal amplitude range after a few seconds. Artifacts featured with a steep amplitude increase followed by a gradual fall may be distinguished as electrode pop artifacts, caused by capacitive properties between the electrode-scalp interface [5]. Artifacts exhibiting fast or slow waves and with less stable forms suggest either electrode or cable movement [5], [14]. These artifacts were not visible in the signals obtained in the walking state.
The presence of these artifacts suggests poor or shifting contact in the electrode-scalp interface. Along with environment-based artifacts, both electrode pop artifacts and movement artifacts were seen in signals obtained from all 3 types of electrodes (see Fig. 2). Possible causes of these artifacts may include head or body movement, reduced friction due to conductive solution, or shear stress in the electrode-scalp interface due to the tightness of the cap.
Fig. 2.
Electrode pop artifacts recorded by (left) the wet electrode and (right) the spring-loaded dry electrode at O2 during the cycling state.
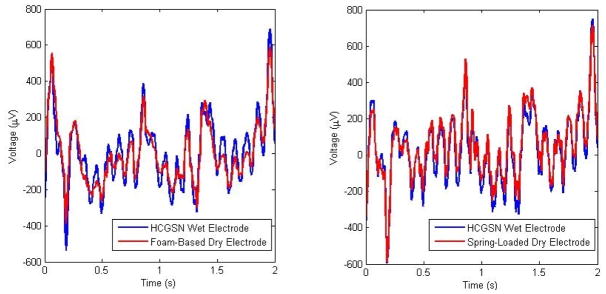
A comparison of the amplitudes recorded during the walking state indicated that the signals obtained from the foam-based dry electrodes had slightly lower amplitude than those of the wet electrodes (see Fig. 3). The lower amplitudes may indicate that the foam-based dry electrodes are less susceptible to motion-related artifacts than wet electrodes.
Fig. 3.
Comparison of gait-related artifacts recorded by the wet electrodes and the dry electrodes: (left) foam-based electrode at Fp2 and (right) spring-loaded electrode at O1.
IV. CONCLUSION
Linear correlation analyses of both types of Mindo dry electrodes with HCGSN wet electrodes suggest that dry electrodes may be a viable alternative for traditional wet electrodes. The high correlation observed in the resting state supports previous results obtained by Mindo [7], [8]. Moreover, we found high correlation between signals obtained from the dry and wet electrodes after ICA-based motion artifact removal in the walking and cycling conditions. These results suggest that dry electrodes perform similarly to wet electrodes under high motion tasks. The use of wireless EEG acquisition systems may further reduce artifacts that often results from cable or electrode movement. A combination of these artifact reduction methods offers the possibility of incorporating Mindo foam-based and spring-loaded dry electrodes into mobile EEG headsets specifically aimed at high motion conditions.
Footnotes
Research was supported by the NSERC Engage Grants program.
References
- 1.Barr W, Prichep L, Chabot R, Powell M, McCrea M. Measuring brain electrical activity to track recovery from sport-related concussion. Brain Injury. 2012;26:58–66. doi: 10.3109/02699052.2011.608216. [DOI] [PubMed] [Google Scholar]
- 2.D’Arcy R, Hajra S, Liu C, Sculthorpe L, Weaver D. Towards Brain First-Aid: A Diagnostic Device for Conscious Awareness. IEEE Trans Biomed Eng. 2011 Mar;58(3):750–754. doi: 10.1109/TBME.2010.2090880. [DOI] [PubMed] [Google Scholar]
- 3.Thompson T, Steffert T, Ros T, Leach J, Gruzelier J. EEG applications for sport and performance. Methods. 2008;45:279–288. doi: 10.1016/j.ymeth.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Reis P, Hebenstreit F, Gabsteiger F, von Tscharner V, Lochmann M. Methodological aspects of EEG and body dynamics measurements during motion. Front Hum Neurosci. 2014 Mar;8(156) doi: 10.3389/fnhum.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern JM. Atlas of EEG Patterns. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 6.Grozea C, Voinescu C, Fazli D. Bristle-sensors - Low-cost Flexible Passive Dry EEG Electrodes for Neurofeedback and BCI Applications. J Neural Eng. 2011;8(2):025008. doi: 10.1088/1741-2560/8/2/025008. [DOI] [PubMed] [Google Scholar]
- 7.Lin C-T, Liao L-D, Liu Y-H, Wang I-J, Lin B-S, Chang J-Y. Novel Dry Polymer Foam Electrodes for Long-Term EEG Measurement. IEEE Trans Biomed Eng. 2011 May;58:1200–1207. doi: 10.1109/TBME.2010.2102353. [DOI] [PubMed] [Google Scholar]
- 8.Liao L-D, Wang I-J, Chen S-F, Chang J-Y, Lin C-T. Design, Fabrication and Experimental Validation of a Novel Dry-Contact Sensor for Measuring Electroencephalography Signals without Skin Preparation. Sensors. 2011 May;11:5819–5834. doi: 10.3390/s110605819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C-T, Chuang C-H, Huang C-S, Chen Y-H. Real-time Assessment of Vigilance Level using an Innovative Mindo4 Wireless EEG System. Proc IEEE Int Symp Circuits and Systems. 2013:1528–1531. [Google Scholar]
- 10.Ko L-W, Chuang C-H, Huang C-S, Chen Y-H, Lu S-W, Liao L-D, Chang W-T, Lin C-T. Real-Time Vigilance Estimation Using Mobile Wireless Mindo EEG Device with Spring-Loaded Sensors. Foundations of Augmented Cognition. 2013;8027:450–458. [Google Scholar]
- 11.Geodesic Sensor Net Technical Manual. Electrical Geodesics, Inc; Eugene, OR: 2007. [Google Scholar]
- 12.Net Station Acquisition Technical Manual. Electrical Geodesics, Inc; Eugene, OR: 2003. [Google Scholar]
- 13.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004 Mar;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Tatum WO, Dworetzky BA, Schomer DL. Artifact and Recording Concepts in EEG. J Clin Neurophysiol. 2011 Jun;28:252–263. doi: 10.1097/WNP.0b013e31821c3c93. [DOI] [PubMed] [Google Scholar]
- 15.Gwin JT, Gramann K, Makeig S, Ferris DP. Removal of movement artifact from high-density EEG recorded during walking and running. J Neurophysiol. 2010 Jun;103:3526–3534. doi: 10.1152/jn.00105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niedermeyer E. The Normal EEG of the Waking Adult. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 167–192. [Google Scholar]