Abstract
Background
Over 130 000 patients in the United States alone need a life-saving organ transplant. Genetically modified porcine organs could resolve the donor organ shortage, but human xenoreactive antibodies destroy pig cells and are the major barrier to clinical application of xenotransplantation. The objective of this study was to determine whether waitlisted patients possess preformed antibodies to swine leukocyte antigen (SLA) class II, homologs of the class II human leukocyte antigens (HLA).
Methods
Sera from people currently awaiting solid organ transplant were tested for IgG binding to class II SLA proteins when expressed on mammalian cells. Pig fibroblasts were made positive by transfection with the class II transactivator (CIITA). As a second expression system, transgenes encoding the alpha and beta chains of class II SLA were transfected into Human embryonic kidney (HEK293) cells.
Results
Human sera containing IgG specific for class II HLA molecules exhibited greater binding to class II SLA positive cells than to SLA negative cells. Sera lacking antibodies against class II HLA showed no change in binding regardless of the presence of class II SLA. These antibodies could recognize either SLA-DR or SLA-DQ complexes.
Conclusions
Class II SLA proteins may behave as xenoantigens for people with humoral immunity towards class II HLA molecules.
Introduction
Xenoreactive antibodies have been a significant barrier to implementation of clinical xenotransplantation (1,2). Recent advances in genetic engineering are making it possible to delete multiple xenoantigens in a single reaction (3,4). The creation of the GGTA1/CMAH/B4GALNT2 (triple KO) knockout pig has eliminated the xenoreactive antibody barrier for many but not all waitlisted patients (5).
Major histocompatibility antigens have been recognized targets of humoral rejection in allotransplantation for more than 50 years (6,7). The development of single Human Leukocyte Antigen (HLA) beads has simplified the analysis of a broad-spectrum of HLA antibodies in clinical allotransplantation, and facilitated the detection of donor specific antibodies (DSA) directed against class I and class II HLA proteins (8,9). The sensitivity of single antigen beads also helped determine the importance of class II antibodies on long term graft survival, something that was previously difficult to determine when relying on CDC and flow cytometry (FCM) using donor cells. Previous studies suggested that HLA-specific antibodies cross-react with the homologous class I and class II swine leukocyte antigens (SLA) (10,11).
Our recent work using PBMCs from pigs deficient in SLA class I shows that some class I HLA-specific antibodies cross-react with SLA class I molecules explains the positive crossmatch that some people continue to have against the triple KO pig (12). Whether humans have antibodies to SLA class II is less well established. Class II SLA reactivity was indicated by the inability to fully deplete binding with class I HLA positive/class II HLA negative pooled human platelets (10,11). Insufficient platelet material used for depletion or sera containing HLA specificities not expressed on the platelets could also explain the appearance of antibodies cross-reacting with class II HLA and SLA. Here we compared human IgG binding a pig cell line made to express a human class II transactivator (CIITA) transgene which drives class II SLA expression (13). We also examined human immunoglobulin binding to a human cell line expressing functional SLA-DR or SLA-DQ molecules. These assays enabled SLA antibody-reactivity to be tested without relying on platelet depletion of the antibodies in question and demonstrated that class II SLA can be xenoantigens.
Materials and Methods
Culture of Parent Cell Line
A SV40 T antigen immortalized fibroblast cell line derived from a SLA class I and galactose-α1,3-galactose deficient pig (14) was cultured in minimum essential media (MEM-α) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan UT) and Amphotericin B (Thermo Fisher Scientific, Waltham, MA) in collagen-I-coated plates (Becton Dickinson, Bedford, MA) at 37°C and 5% CO2. Cells were confirmed to be SLA class II negative by incubation with anti-SLA-DR-FITC Ab or with anti-SLA-DQ-FITC (AbD Serotec, Raleigh, NC) and analyzed using BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA).
Creation of Pig Cells Expressing Class II SLA Molecules
Parent cells were grown to 90% confluency in a 10 cm culture plate and transfected with Lipofectamine 2000CD (Invitrogen, Carlsbad, CA) as specified by company protocol. A transgene encoding human class II transactivator was used to drive SLA class II expression in the parent cell line. The donor plasmid, pCDNA3 myc CIITA was a gift from Matija Peterlin (Addgene plasmid #14650) (15). Three-days posttransfection cells were screened on a BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA) using anti-SLA class II DR-FITC Ab (AbD Serotec, Raleigh, NC). Cells with high levels of class II DR expression were sorted 1 cell per well into 96-well plates by the FACS Aria flow cytometer. The cells were placed into selection against Geneticin, G418 (Invitrogen, Carlsbad, CA). Expanded clonal cultures were then analyzed for presence or absence of SLA class II DR using the previously mentioned anti-SLA class II DR antibody. Clones with a high level of SLA class II DR Ab binding were then evaluated for SLA class II DQ (AbD Serotec, Raleigh, NC). Finally, 2 clones were selected, 1 that demonstrated a stable class II positive (DR+/DQ+) phenotype and another with a class II negative (DR-/DQ-) phenotype, both resistant to G418 selection. These cells contained the following class II SLA genes: DRα (*02102/*w04re01), DRβ (1*1001/1*0403), DQα (*0204/*0101), DQβ (1*0601/1*0303) (16).
Creation of Human Cells Expressing Class II SLA Molecules
The expression vector, pBudCE4.1 (Thermo Fisher Scientific, Waltham, MA), was engineered to simultaneously express cDNA encoding SLA-DRA1-DRB1, -DRA1--DRB2, -DRA2-DRB1, -DRA2-DRB2, -DQA1-DQB1, -DQA1-DQB2, -DQA2-DQB1, -DQA2-DQB2. The alpha chains of the alleles were inserted into the CMV promoter site and the beta chains were inserted into the EF-1a site of pBudCE4.1 using restriction enzyme digestion. These plasmids were introduced into HEK 293 cells that had been made deficient in class I HLA expression by using the gRNA (Forward: 5′-CTACTCTCTCTTTCTGGC-3′ and Reverse: 5′-GGCCAGAAAGAGAGAGTAG-3′) to disrupt the b2-microglobulin gene which is critical for cell surface expression of class I HLA. Class I HLA-deficient HEK cells were isolated by staining with anti-HLA class I monoclonal antibody (Clone W6/32, Thermo Fisher Scientific, Waltham, MA) and sorting on a BD FCSAria II at the UAB Comprehensive Flow Cytometry Core. Once a HLA class I negative population was obtained, the cells were transfected using a calcium phosphate protocol. Briefly, 1E6 cells were plated into a 6-well dish and transfected by adding a cocktail of 214 uL water, 31 uL 2 M CaCl2, 2.5 ug DNA, and 250 uL of 2x HBS. The cells were grown in MEM-a + 10% FBS + Amphotericin and 3 days posttransfection were placed in selection with the antibiotic Zeocin (Thermo Fisher Scientific, Waltham, MA). The cells were sorted again at the UAB Comprehensive Flow Cytometry Core on a BD FACSAria II for SLA class II expression as described above.
Sequencing
Polymerase chain reaction (PCR) amplification of the class II alleles was performed using the primers and conditions as described by Reyes et al, 10 PCR products were ligated into pCR2.1-TOPO plasmid using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA) and analyzed by Sanger sequencing (Genewiz Inc., South Plainfield, NJ).
Analysis of Human Antiporcine IgG Binding
104 sera samples were discarded and deidentified material from the University of Alabama-Birmingham Histocompatibility Lab that had been selected based on the presence or absence of class II HLA-specific antibodies. This activity was determined to meet the definition of nonhuman subjects research by the UAB IRB. These samples were heat inactivated at 57°C for 30 min and the sera was absorbed for 30 minutes with an equal volume of packed WT pig RBCs to reduce background binding by removing any antipig glycan antibodies. Pig use was approved by the IACUC. This protocol removes human antipig glycan antibodies to lower the background when evaluating anti-SLA reactivity (12).
25 uL of absorbed sera was incubated for 30 min at 4°C with 1 × 105 cells in EX-CELL 610-HSF Serum-Free Medium (Sigma, St. Louis, MO, USA) with 0.1% sodium azide on either the class II positive or negative cell lines. Cells were washed 3 times with EX-CELL + sodium azide and then stained with goat antihuman IgG Alexa Fluor 647 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 30 min at 4°C. Cells were washed 3 times using EX-CELL medium as above and flow cytometric analysis was completed on BD Accuri C6 flow cytometer. Samples were gated on FSC-A by SSC-A.
Statistical Analysis of Antibody Binding
Antibody binding results were reported as means of median fluorescence intensity (MFI) after subtracting out fluorescence values obtained with secondary antibodies alone. Graph and data analyses were completed using Prism 7 for Macintosh (GraphPad Software Inc., La Jolla, CA, USA). The resulting data did not approximate normal distributions even after logarithmic transformation. Therefore, the Kruskal-Wallis test was used to compare group means with correction by the Dunn’s multiple comparison test.
Results
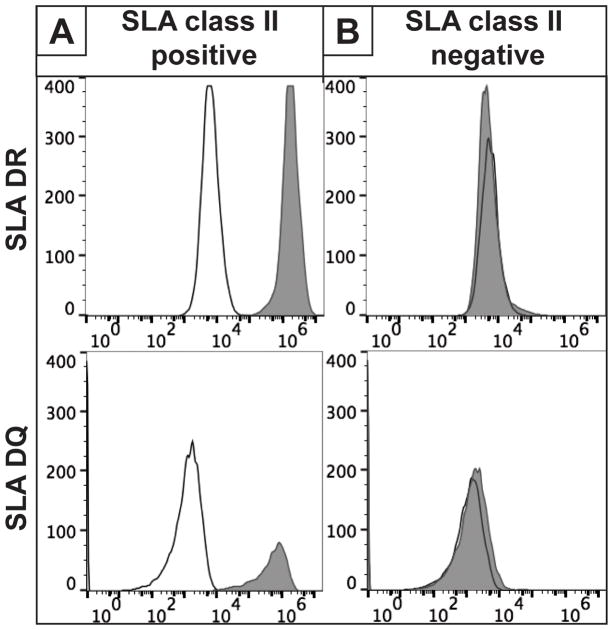
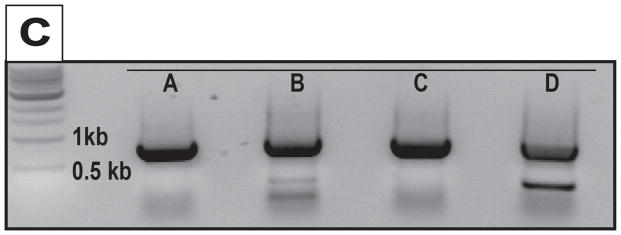
An immortal fibroblast cell line was derived from an engineered knockout pig lacking expression of the GGTA1 and all 3 classical class I SLA genes (14). Because this line does not express cell surface class II SLA (Figure 1B), a human CIITA transgene was introduced to drive transcription of the swine class II major histocompatibility complex (MHC) genes, SLA-DRα/β and SLA-DQα/β (13). Cells expressing the CIITA transgene were identified by their cell surface display of class II SLA (Figure 1A). Sanger sequence analysis of PCR products generated from the class II loci confirmed expression of 2 alleles each of the DRα, DRβ, DQα and DQβ genes. (Figure 1C).
Figure 1.
Human Antibody Binding to SLA Class II
The target cells described above were incubated with human sera previously absorbed with wild type red blood cells which lack SLA proteins. This absorption eliminates antibodies recognizing the 3 known carbohydrate xenoantigens (alpha galactose, Neu5GC, and B4GalNT2-derived glycans) which enhances the detection of additional xenoantigens such as class I SLA proteins (12).
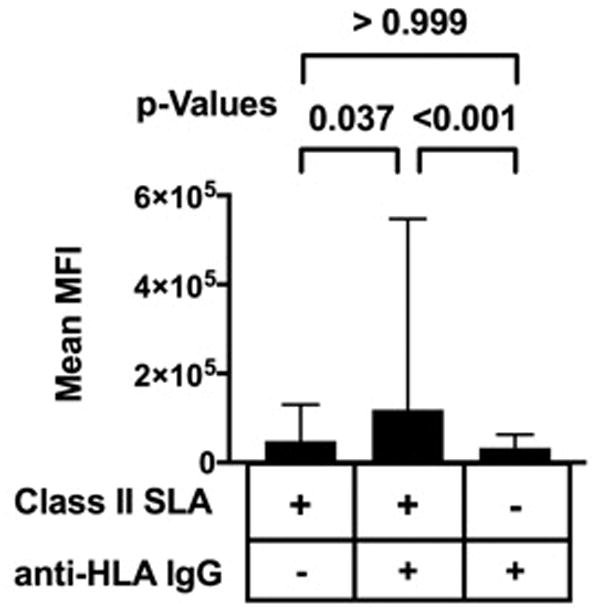
Of the 104 tested sera, 19 lacked antibodies specific for class II HLA proteins. The remaining 85 contained class II HLA-specific IgG. These 85 sera showed elevated binding to cells expressing SLA-DR and -DQ when compared to cells which did not express SLA. Sera containing class II HLA reactive antibodies also exhibited elevated binding to SLA-DR and –DQ positive cells relative to human sera lacking class II HLA specific antibodies (Figure 2). These data indicate that HLA-specific antibodies may cross-react with class II SLA proteins.
Figure 2.
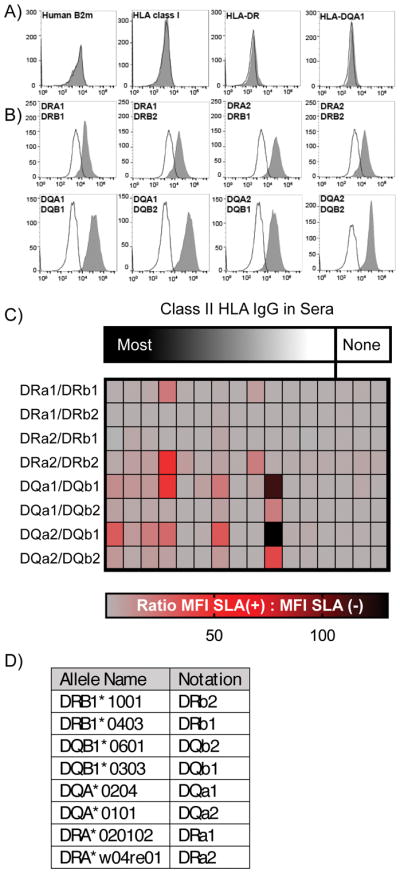
Because the CIITA transcription activator regulates several genes in addition to -DR and –DQ, it is possible that antibody binding may be influenced by antigens other than the SLA proteins (17). Therefore, we developed an additional class II SLA expression system which avoided the use of CIITA. Transgenes encoding SLA-Drα/βand -DQα/β were expressed in immortalized HEK293 human cells. Antibody binding to HLA proteins on these human cells because under the conditions used, these cells also do not express class II HLA, and Class I HLA protein expression was eliminated by CRISPR/Cas9-targeted disruption of the β2-microglobulin gene (Figure 3A). These transfectants exhibited stable expression of all combinations of SLA-DRα/β and SLA-DQα/β heterodimers (Figure 3B). While sera lacking class II HLA-specific antibodies did not bind these cells (Figure 3C), IgG from sera containing class II HLA reactive immunoglobulin revealed multiple patterns of reactivity. Some did not cross-react with either SLA-DR or –DQ (Figure 3C). Other samples contained antibodies that bound to SLA-DR, SLA-DR and –DQ, or to SLA–DQ only. One sera only bound cells expressing SLA-DQ (Figure 3C) and generated MFI 130-fold over HEK293 cells that lacked any class II SLA proteins. The DQβ1 allele appears to be the primary target of the antibodies in this serum as its pairing with either DQα allele has minimal impact on the level of antigenicity.
Figure 3.
Discussion
Preformed antibodies have been recognized as a significant barrier to kidney transplantation since 1964 (18). Allografts and xenografts each initially had glycan antigens to which recipients had antibodies. In the case of the allograft the ABO blood group antigens were a barrier, while in xenotransplantation, α-gal, Neu5Gc, and the B4GalNT2-derived antigen(s) were the barriers (1,2). The longstanding strategy to avoid antibody-mediated rejection (AMR) in clinical allotransplantation has been to refrain from transplanting patients in situations where they had preformed antibody to a donor antigen (6,18,19). Preclinical studies in the pig-to-primate model confirm that lowered antibody levels are important in xenotransplantation as well, with gal KO pig kidneys surviving for greater than 6 months if recipients were prescreened and selected based on having low xenoreactive antibody levels (20). Genome editing using CRISPR/Cas has facilitated the creation of triple-xenoantigen KO pigs (GGTA1/CMAH/B4GALNT2) that have a favorable crossmatch for xenotransplantation of many allounsensitized patients. Examination of the pathology of the long surviving kidneys shows that AMR with thrombotic microangiopathy is the cause of graft failure, highlighting the continued importance of identifying and developing genome engineering strategies to eliminate all xenoantigens. Cross reactivity of human HLA and SLA may also have implications in settings where a patient first receives a xenotransplant followed by an allotransplant (21).
SLA and HLA proteins have extensive sequence identity and structural identity suggesting that they also may share cross-reactive humoral epitopes. Consequently, anti-HLA antibodies in highly sensitized patients may cross-react with SLA, and pose a barrier to these patients from participating in initial clinical trials. In support of this concept, we used PBMCs from SLA I deficient pigs to evaluate class I SLA as a xenoantigen, and showed that there were some epitopes shared between class I HLA and class I SLA (12). These antigenic determinants were likely responsible for the anti-HLA binding to the class I SLA proteins.
Alternative approaches to determine the existence of anticlass II SLA antibodies were attempted but presented a few confounding issues. Swine B cell versus T crossmatches cannot be used to provide insight into the presence of class II SLA antibodies in human sera. Pig CD8+ T cells constitutively express class II SLA. Given that pigs contain abundant circulating CD4+/CD8+ double positive T cells, isolating a specific population of lymphocytes lacking class II MHC poses significant challenges (22,23). Additionally, there are other unknown xenoantigens and a marked variability in xenoreactivity from serum to serum. A crossmatch of WT RBC absorbed sera on swine PBMCs would remove the unknown antiglycan xenoantibodies but leave anticlass I SLA antibodies, making interpretation difficult. To overcome these challenges, we developed the assays described in this report, namely the direct antibody binding to cells forced to express class II SLA proteins by the insertion of transgenes.
The described lack of available reagents to examine class II SLA previously forced indirect evaluations of class II HLA antibody cross reactivity with class II SLA. Class II SLA is like HLA in that it expresses DR and DQ, but is distinct from HLA in that there is no DP (24,25). The importance of anticlass II antibodies in allotransplantation has been appreciated more recently, as class II antibodies are now recognized as an important contributor to transplant glomerulopathy and graft loss (26). Our CIITA positive pig cells express both SLA-DR and DQ at the cell surface and demonstrate increased reactivity with human sera containing class II HLA antibodies (Figure 2). CIITA modifies expression of several genes in addition to class II MHC molecles (17). Consequently, it may alter the production of non-SLA xenoantigens, causing human IgG binding to increase independently of –DR and –DQ expression. The fact that human immunoglobulin binding also increases on human cell lines expressing either SLA-DR or SLA-DQ (Figure 3) supports the idea that these molecules are xenoantigens recognized in CIITA positive pig cells. Both SLA-DR and –DQ appear to be xenoantigens. Anti-DQ antibodies are among the most frequent HLA antibodies found in recipients with a failed renal allograft, so it may be important to address the SLA DQ in xenotransplantation (27). Genome editing provides several potential strategies, which include either deleting DQ from the pig using CRISPR/Cas, or altering the sequence to eliminate epitopes present in our pig’s DQ genes. We are performing broader analyses to gain insight into the epitopes and frequency of –DR and –DQ related cross-reactive antibodies.
In summary, in highly sensitized patients the xenoreactive antibody barrier to clinical application of xenotransplantation is still significant. The studies described in this paper show the presence of anti-SLA class II antibodies that bind to SLA DR and/or DQ. Further studies are required to determine if this response is due to SLA specific or cross-reactive anti-HLA class II antibodies. Genome editing with CRISPR/Cas can be used to eliminate either the class II antigen or its epitope in the donor pig. The prospects of creating donor pigs that have a negative crossmatch for every patient with renal failure regardless of their degree of HLA sensitization are bright.
Acknowledgments
Funding
This publication was made possible in part by a grant from United Therapeutics Corp.
Abbreviations
- B4GALNT2
beta-1,4-N-acetyl-galactosaminyltransferase 2
- CDC
complement dependent cytotoxicity
- CMAH
cytidine monophosphate-N-acetylneuraminic acid hydroxylase
- cPRA
calculated panel reactive antibody
- GGTA1
α1, 3-galactosyltransferase
- KO
knockout
- HLA
human leukocyte antigen
- KO
knockout
- SLA
swine leukocyte antigen
- PBMC
peripheral blood mononuclear cells
Footnotes
Authorship
This manuscript has been revised and approved by all authors. J.M.L., D.E.E., M.T., and A.J.T. drafted this article and developed and refined study concepts. L.M.R. and J.M.L. performed SLA sequencing and gene target analysis. J.M.L. J.R.B., G.R.M., and Z.Y.W, performed data collection, sample selection, cell immortalization and data analysis.
Disclosure
A.J.T. is the founder of Xenobridge LLC, has United Therapeutics funding and applied for patents. The other authors declare no conflicts of interest.
References
- 1.Byrne GW, McGregor CG, Breimer ME. Recent investigations into pig antigen and anti-pig antibody expression. Int J Surg. 2015;23(Pt B):223–228. doi: 10.1016/j.ijsu.2015.07.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DK. Xenoantigens and xenoantibodies. Xenotransplantation. 1998;5(1):6–17. doi: 10.1111/j.1399-3089.1998.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 3.Butler JR, Ladowski JM, Martens GR, Tector M, Tector AJ. Recent advances in genome editing and creation of genetically modified pigs. Int J Surg. 2015;23(Pt B):217–222. doi: 10.1016/j.ijsu.2015.07.684. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Estrada JL, Burlak C, et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation. 2015;22(1):20–31. doi: 10.1111/xen.12131. [DOI] [PubMed] [Google Scholar]
- 5.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22(3):194–202. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachary AA, Leffell MS. Desensitization for solid organ and hematopoietic stem cell transplantation. Immunol Rev. 2014;258(1):183–207. doi: 10.1111/imr.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363(15):1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 8.Pei R, Lee J, Chen T, Rojo S, Terasaki PI. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum Immunol. 1999;60(12):1293–1302. doi: 10.1016/s0198-8859(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 9.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75(1):43–49. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Diaz Varela I, Sanchez Mozo P, Centeno Cortes A, Alonso Blanco C, Valdes Canedo F. Cross-reactivity between swine leukocyte antigen and human anti-HLA-specific antibodies in sensitized patients awaiting renal transplantation. J Am Soc Nephrol. 2003;14(10):2677–2683. doi: 10.1097/01.asn.0000088723.07259.cf. [DOI] [PubMed] [Google Scholar]
- 11.Taylor CJ, Tang KG, Smith SI, White DJ, Davies HF. HLA-specific antibodies in highly sensitized patients can cause a positive crossmatch against pig lymphocytes. Transplantation. 1998;65(12):1634–1641. doi: 10.1097/00007890-199806270-00016. [DOI] [PubMed] [Google Scholar]
- 12.Martens GR, Reyes LM, Butler JR, et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation. 2017;101(4):e86–92. doi: 10.1097/TP.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14(1):301–31. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 14.Reyes LM, Estrada JL, Wang ZY, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193(11):5751–5757. doi: 10.4049/jimmunol.1402059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanazawa S, Okamoto T, Peterlin BM. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity. 2000;12(1):61–70. doi: 10.1016/s1074-7613(00)80159-4. [DOI] [PubMed] [Google Scholar]
- 16.Reyes LM, Blosser RJ, Smith RF, et al. Characterization of swine leucocyte antigen alleles in a crossbred pig to be used in xenotransplant studies. Tissue Antigens. 2014;84(5):484–488. doi: 10.1111/tan.12430. [DOI] [PubMed] [Google Scholar]
- 17.Krawczyk M, Leimgruber E, Seguín-Estévez Q, Dunand-Sauthier I, Barras E, Reith W. Expression of RAB4B, a protein governing endocytic recycling, is co-regulated with MHC class II genes. Nucleic Acids Res. 2007;35(2):595–605. doi: 10.1093/nar/gkl980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3(6):665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 19.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280(14):735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 20.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221–230. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper DK, Dou KF, Tao KS, Yang ZX, Tector AJ, Ekser B. Pig liver xenotransplantation: a review of progress toward the clinic. Transplantation. 2016 Oct 1;100(10):2039–47. doi: 10.1097/TP.0000000000001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunney JK, Pescovitz MD. Phenotypic and functional characterization of pig lymphocyte populations. Vet Immunol Immunopathol. 1987;17(1–4):135–44. doi: 10.1016/0165-2427(87)90134-6. [DOI] [PubMed] [Google Scholar]
- 23.Saalmüller A, Weiland F, Reddehase MJ. Resting porcine T lymphocytes expressing class II major histocompatibility antigen. Immunobiology. 1991;183(1–2):102–14. doi: 10.1016/S0171-2985(11)80190-7. [DOI] [PubMed] [Google Scholar]
- 24.Smith DM, Lunney JK, Ho CS, et al. Nomenclature for factors of the swine leukocyte antigen class II system, 2005. Tissue Antigens. 2005;66(6):623–639. doi: 10.1111/j.1399-0039.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 25.Chardon P, Renard C, Vaiman M. The major histocompatibility complex in swine. Immunol Rev. 1999;167:179–192. doi: 10.1111/j.1600-065x.1999.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiebe C, Pochinco D, Blydt-Hansen TD, et al. Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant. 2013;13(12):3114–3122. doi: 10.1111/ajt.12478. [DOI] [PubMed] [Google Scholar]
- 27.Tambur AR. HLA-DQ antibodies: are they real? Are they relevant? Why so many? Curr Opin Organ Transplant. 2016;21(4):441–446. doi: 10.1097/MOT.0000000000000325. [DOI] [PubMed] [Google Scholar]