Abstract
The development of an effective AIDS vaccine has been challenging due to viral genetic diversity and the difficulty in generating broadly neutralizing antibodies (bnAbs). Here, we engineered trispecific antibodies (Abs) that allow a single molecule to interact with three independent HIV-1 envelope determinants: 1) the CD4 binding site, 2) the membrane proximal external region (MPER) and 3) the V1V2 glycan site. Trispecific Abs exhibited higher potency and breadth than any previously described single bnAb, showed pharmacokinetics similar to human bnAbs, and conferred complete immunity against a mixture of SHIVs in non-human primates (NHP) in contrast to single bnAbs. Trispecific Abs thus constitute a platform to engage multiple therapeutic targets through a single protein, and could be applicable for diverse diseases, including infections, cancer and autoimmunity.
INTRODUCTION
A variety of broadly neutralizing antibodies (bnAbs) have been isolated from HIV-1 infected individuals (1,2,3), but their potential to treat or prevent infection in humans may be limited by the potency or breadth of viruses neutralized (4,5). The targets of these antibodies have been defined based on an understanding of the HIV-1 envelope structure (6–9). While bnAbs occur in selected HIV-1 infected individuals, usually after several years of infection, it remains a challenge to elicit them by vaccination because broad and potent HIV-1 neutralization often requires unusual antibody characteristics, such as long hypervariable loops, interaction with glycans, as well as a substantial level of somatic mutation. Strategies have thus shifted from active to passive immunization to both protect against infection and to target latent virus (10–14). We and others have begun to explore combinations of bnAbs that optimize potency and breadth of protection, thus reducing the likelihood of resistance and viral escape (15–17). Antibodies directed to the CD4bs, MPER, and variable region glycans are among the combinations that so far provide optimal neutralization (18). In addition, alternative combinations have also been investigated for the immunotherapy of AIDS, by directing T lymphocytes to activate latent viral gene expression and enhance lysis of virally-infected cells (19,20). Given that multiple antibodies may help to reduce the viral replication that sustains chronic HIV-1 infection, we report here the generation of multi-specific antibodies designed to increasing the efficacy of HIV therapy.
RESULTS
Design of Bispecific Antibodies and Evaluation of Neutralization Breadth
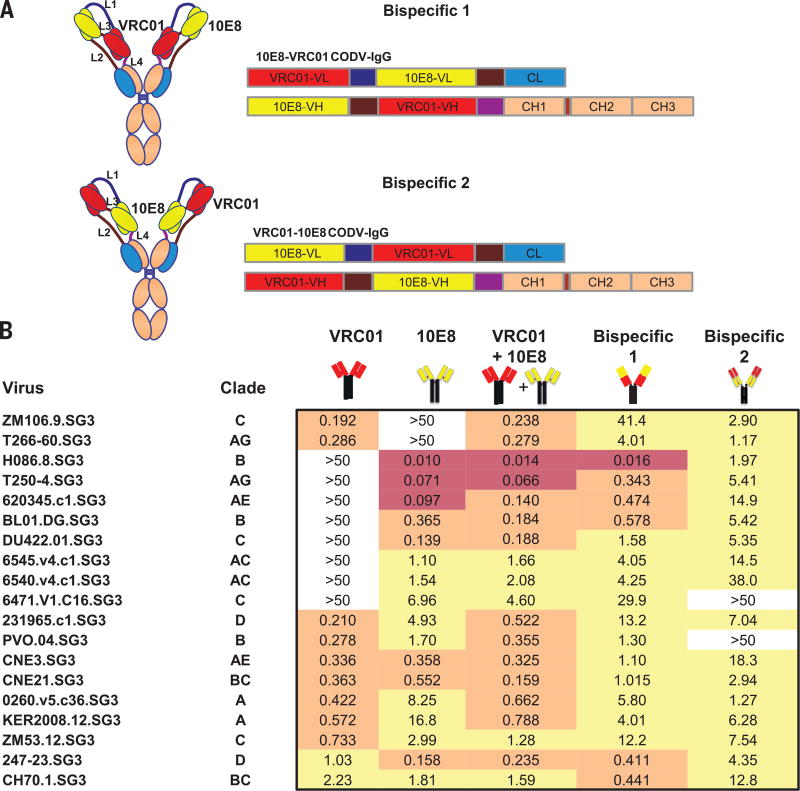
Although individual anti-HIV-1 bnAbs can neutralize naturally occurring viral isolates with high potency, the percentage of strains inhibited by these mAbs varies (21,22). In addition, resistant viruses can be found in the same patients from whom bnAbs were isolated, suggesting that immune pressure against a single epitope may not optimally protect or treat HIV-1 infection. We hypothesized that the breadth and potency of HIV-1 neutralization by a single antibody could be increased by combining the specificities against different epitopes into a single molecule. This strategy would be expected to not only improve efficacy, but also simplify both treatment regimens and the regulatory issues required for clinical development. To test this concept, we initially incorporated prototype bnAbs to the CD4bs and MPER sites into a modified bispecific Ab. When two variable regions are linked in tandem, the distal site typically retains its ability to bind antigen while the proximal binding is markedly diminished. We therefore utilized an alternative configuration, termed CODV-Ig, which introduced linkers and inverted the order of the antibody binding site in light and heavy chains to alter the orientation of the variable regions, allowing each region to interact with their target (23). Several known bnAbs were evaluated, including VRC01, 10E8, PGT121, and PGT128 (reviewed in ref. 1) for their ability to neutralize a select panel of viruses with known resistance or sensitivity to these antibodies (Fig. S1). Initially, we determined whether the position of the variable regions from VRC01 and 10E8 in the proximal or distal positions (Fig. 1A) could affect neutralization breadth and potency. Inclusion of both variable regions in either orientation in the bispecific antibody reduced the number of resistant strains compared to the parental antibodies alone (Fig. 1B). Better potency was observed when VRC01 was proximal and 10E8 distal, though neither bispecific antibody was as potent as a mixture of the two antibodies alone.
Fig. 1. CODV-Ig bispecific antibody design and neutralization titers of the VRC01/10E8 bispecific antibodies.
(A) CODV-Ig bispecific antibody design with two different orientations of 10E8 and VRC01. (B) Neutralization titers (IC50) in µg/ml of VRC01/10E8 bispecific Abs and parental Abs against a select panel of 19 previously circulating HIV-1 strains highlighted in red, yellow and grey indicating highest, medium and lowest potency respectively.
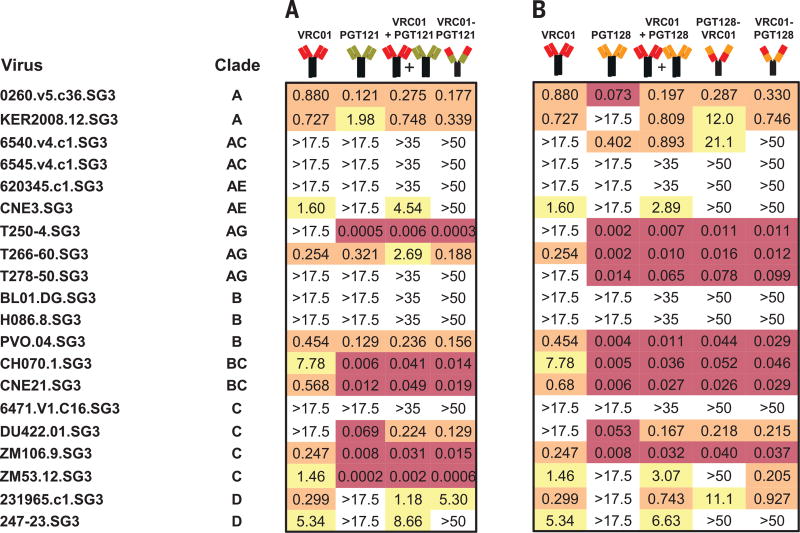
To explore whether other bnAbs could perform better in the bispecific format, we evaluated two different combinations, namely VRC01 plus PGT121, or VRC01 plus PGT128. For PGT121, expression was observed only with VRC01 in the distal position. When this antibody was compared to the parental antibodies alone, it provided marginally better neutralization (Fig. 2A). In contrast, VRC01 could be expressed with PGT128 in both positions, with greater breadth observed when VRC01 was distal (Fig. 2B). Together, these data indicated that improvements in breadth could be achieved with a bispecific format; however, the potency was not consistently improved compared to each Ab alone. We therefore sought an alternative format to improve the potency and breadth of neutralization.
Fig. 2. Neutralization titers of VRC01/PGT121 and VRC01/PGT128 based bispecific antibodies.
Neutralization titers (IC50) in µg/ml of the VRC01/PGT121 (A) and VRC01/PGT128 (B) bispecific Abs against a select panel of 20 circulating HIV-1 strains, with highlights as in Fig.1.
Generation and Comparison of Broad and Potent Trispecific Antibodies
To achieve our goal, we used a previously undescribed trispecific Ab format. Three specificities were combined by using knob-in-hole heterodimerization (24) to pair a single arm derived from a normal immunoglobulin (IgG) with a double-arm generated in the CODV-Ig. A panel of bnAbs was evaluated, including those directed against the CD4bs that included VRC01 and N6, as well as PGT121, PGDM1400 and 10E8 (fig. S1). A modified version of the latter, termed 10E8v4, was used because of its greater solubility (25). We first determined which bispecific arms showed the best potency, breadth and yield. This screening analysis revealed that combinations which contained PGDM1400, CD4bs, and 10E8v4 showed the highest level of production and greatest potency of neutralization (fig. S2).
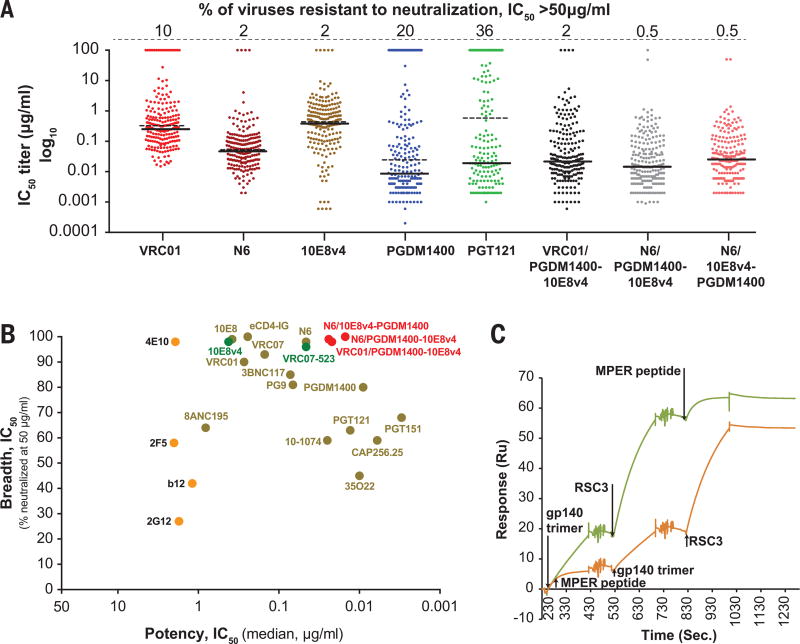
We then evaluated different combinations of single arm and double arm specificities from PGDM1400, CD4bs, and 10E8v4 Abs for their expression levels and activity against a small panel of viruses (fig. S3), leading ultimately to the identification of trispecific antibodies VRC01/PGDM1400-10E8v4 and N6/PGDM1400-10E8v4 as lead candidates. When analyzed against a panel of 208 viruses (18) and compared to the parental antibodies alone, the highest neutralization potency and breadth was observed with N6/PGDM1400-10E8v4, with only 1 of the 208 viruses showing neutralization resistance and a median IC50 of less than 0.02 µg/ml (Fig. 3A). VRC01/PGDM1400-10E8v4 also displayed high potency and breadth, and only 4 resistant viruses were found. While some parental mAbs displayed either high breadth (e.g. 10E8, N6) or high potency (PGDM1400), none displayed a combination of breadth and potency as optimal as the trispecific Abs (Fig. 3B). For example, the most potent and broad parental mAb, N6, was around 5-fold less potent than the N6/PGDM1400-10E8v4 trispecific Ab and targeted only a single epitope, which could increase the chance of viral escape mutations. Importantly, as a single recombinant protein, the trispecific Abs demonstrated potency and breath superior to any single antibody yet defined (Fig. 3 and fig. S4). We also determined the binding affinity of each component of the trispecific Ab and compared each to its parental Fab. The equilibrium binding constant, Kd, of each binding site in the trispecific Ab, determined by surface plasmon resonance (SPR), was comparable to the affinity of the parental Fab, with PGDM1400 showing a slight decrease (~3-fold), and VRC01 and 10E8v4 exhibiting approximately a log increase in affinity (fig. S5). In addition, the trispecific Ab was able to bind sequentially to each of the three antigens (Fig. 3C), indicating that there is independent binding of each epitope.
Fig. 3. Neutralization titers of trispecific antibodies and broadly neutralizing antibodies, and sequential binding of alternative Env epitopes.
(A) The neutralization titers (IC50) of different bnAbs and trispecific Abs against a genetically diverse panel of 208 circulating HIV-1 strains. The solid line denotes the median IC50 neutralization titer of sensitive viruses while the dotted line indicates median titers of all 208 viral strains. The percentage of resistant viruses are shown in the top line. (B) The breadth and potency of the trispecific Abs compared to other bnAbs. (C) Sequential binding of three antigens to the trispecific Ab, VRC01/PGDM1400-10E8v4 in the indicated order. The RSC3 (44) antigen represents monomeric gp120 optimized for the CD4 binding site ab VRC01. MPER peptide interacts with 10E8 (7), and gp140 trimer for PGDM1400 was derived from the gp140ΔN6 (BG505) protein.
The N6 trispecific Ab also showed greater potency and breadth compared to three related bispecific Abs when tested against a panel of 20 viruses that were selected for resistance to bnAbs (table S1). This finding is consistent with previous studies comparing the efficacy of mixtures of two vs. three bnAbs (18) and provides additional support for the multi-targeting concept. In addition to their greater efficacy, the trispecific Abs also yielded higher protein levels and greater solubility than the bispecific model (see fig. S2A vs. S3), facilitating large scale production and clinical translation.
Fc Modification to Extend Half-Life and Crystal Structure
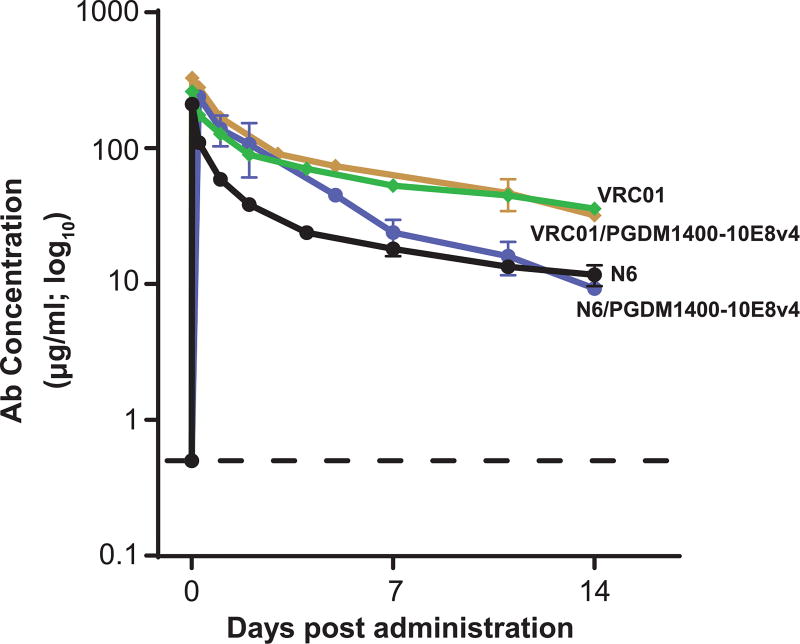
To identify the optimal candidate for further development, we determined the half-life of the trispecific Abs in NHP. We previously showed that, in context of the VRC01 mAb, mutations that increased binding to the neonatal Fc receptor (FcRn), which recycles IgG in intestinal epithelial cells and increases levels in the serum, extended half-life enhanced mucosal localization and conferred more efficient protection against lentivirus infection compared to wild type antibody (26). One such mutation was incorporated into the trispecific Abs as well as the parental VRC01 and N6 Abs. Abs were then infused into rhesus macaques and serum levels analyzed over a 14 day time frame. Ab VRC01 displayed a longer half-life over the more broad and potent N6, which was also directed to the CD4bs (Fig. 4, VRC01 vs. N6). Similarly, the trispecific Ab containing VRC01 showed greater persistence and a longer half-life (7.43 days, based on day 1-14 serum concentrations) than the N6 trispecific (4.79 days) in vivo (Fig. 4, VRC01/PGDM1400-10E8v4 and N6/PGDM1400-10E8v4). For this reason, and because the N6 trispecific Ab yielded less product with decreased solubility, we studied the VRC01/PGDM1400-10E8v4 trispecific Ab further.
Fig. 4. Serum antibody levels in rhesus macaques infused with parental and trispecific antibodies.
The concentration of VRC01, N6 and the two trispecific Abs containing a Fc mutation to extend half-life, were measured in serum over the course of 14 days after intravenous administration of a single 10 mg/kg dose of each antibody. Each data point represents the mean +/− SEM of the values from 2–6 animals per group (VRC01, n=6; N6, n=4; each trispecific Ab, n=2) and determined in replicates from two independent experiments.
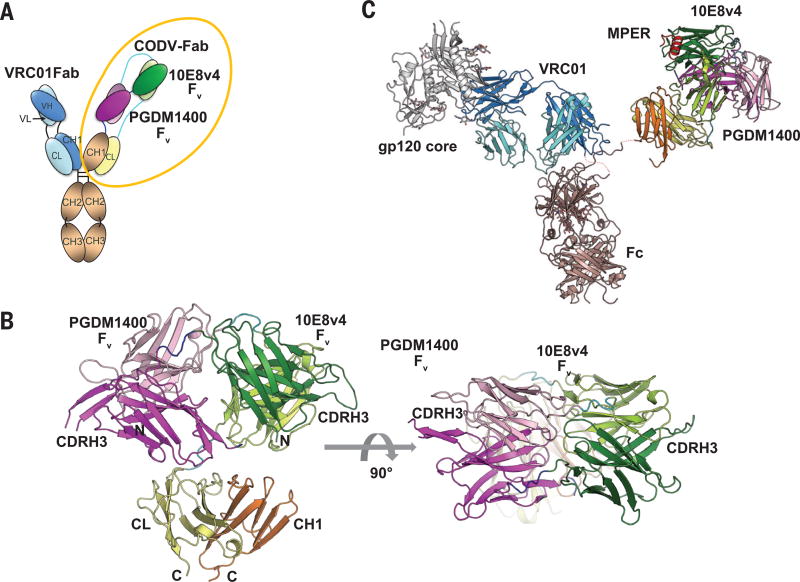
Further characterization was performed by solving the crystal structure of the bispecific arm of the trispecific Ab, PGDM1400-10E8v4 CODV Fab, at 3.55Å resolution (Fig. 5 A and B). While the light chain was well resolved in the electron density (with the exception of the two most C-terminal residues), the heavy chain showed some regions of dynamic disorder. The most notable region consisted of part of PGDM1400 CDRH3 and the linker between PGDM1400 Fv and the heavy chain constant domain (residues 280-305). Similar to the anti-IL4/IL13 CODV Fab crystal structures (23), PGDM1400 and 10E8v4 Fvs opposed one another with the CDRs well exposed to the solvent. The distance between the CDRH3s of PGDM1400 and 10E8v4 is over 100Å. The PGDM1400 and 10E8v4 Fvs superposed very well with their respective parental Fv structures with RMSD(Cα) around 1 Å (fig. S6; see also refs. 25,27), confirming that their antigen binding properties have been well preserved in the CODV format. Most importantly, the orientations of the CDRs in two Fv’s were 180 degrees from each other, suggesting that each antibody combining site can independently engage it antigen without obstructing the other Fv structure. A model for the trispecific Ab was constructed by combining the PGDM1400-10E8v4 CODV Fab with VRC01 (6) and the intact b12 (28) IgG crystal structures (Fig. 5C). Similar to a natural IgG, the distance between the monovalent fragment of antigen binding (Fab) and CODV Fab is about 150Å. Two out of three antigens (gp120 core and gp41 MPER) were also included in the model, though we do not have direct evidence that all three HIV epitopes can be engaged simultaneously by a single trispecific Ab.
Fig. 5. Crystal structure of the CODV Fab and a structure model of the trispecific antibody.
(A) Configuration of the trispecific antibody, color-coded by parental antibody. Dark shades (red or green) refer to heavy chain while pastels indicate light chain peptides. (B) Crystal structure of the PGDM1400-10E8v4 CODV Fab in side and top views. CDRH3s from the two Fvs are labeled to highlight the antigen binding region gp41 MPER was modeled in by superposing PDB 5IQ9 on to the 10E8v4 Fv. (C) VRC01/gp120 structure (PDB 4LST) and the CODV Fab were modeled onto the b12 structure (PDB 1HZH) by overlaying the CH1-CL domains. Color codes are matched in A, B, and C.
Enhanced Cross-Protection and Decreased Viral Escape In Vivo
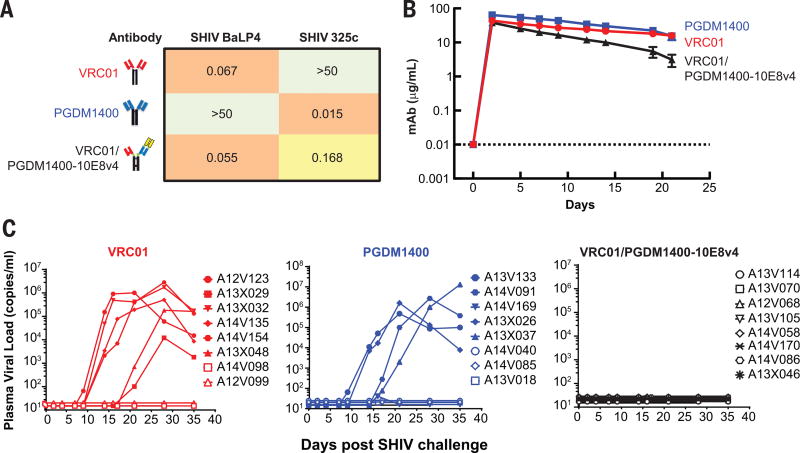
The VRC01/PGDM1400-10E8v4 trispecific Ab was evaluated for its ability to protect against infection, using a mixture of two SHIVs that each differed in neutralization sensitivity to the parental bnAbs. In vitro assessment of the replication competent SHIV challenge stocks showed that SHIV BaL P4 was sensitive to VRC01 and the trispecific antibody, however was resistant to PGDM1400 (Fig. 6A). In contrast, SHIV 325C virus was sensitive to PGDM1400 and the trispecific Ab, yet resistant to VRC01 (Fig. 6A). In a neutralization assay with an equal mixture of SHIV BalP4 and SHIV 325c, we observed only the trispecific Ab could achieve complete neutralization of the viral mixture compared to either VRC01 or PGDM1400 (fig. S7). When naïve rhesus macaques were infused with the half-life extended VRC01, PGDM1400 or VRC01/PGDM1400-10E8v4 (5 mg/kg) respectively, serum concentrations were maintained at levels of ≥1 µg/ml for more than 14 days for all Abs (Fig. 6B). A decrease in serum levels at later time points for the trispecific Ab correlated with the development of monkey anti-human Abs but arose almost two weeks after the SHIV challenge.
Fig. 6. Trispecific and broad neutralizing antibody sensitivity of SHIVs, plasma antibody levels and viremia in rhesus macaques.
(A) The IC50 neutralizing titers (ug/ml) of VRC01, PGDM1400, and VRC01/10E8v4-PGDM1400 against replication competent SHIV BaLP4 or SHIV 325c. (B) Plasma levels of VRC01, PGDM1400 and VRC01/PGDM1400-10E8v4 in rhesus macaques (n=8 on each arm, done in two separate experiments with 4 animals each). All animals were administered 5 mg/kg of the indicated antibody intravenously. Each data point represents the mean +/− SEM of the values from all 8 animals per group. (C) Plasma viral loads in rhesus macaques (n=8 per group) challenged with a mixture of SHIV BaLP4 and SHIV 325c, 5 days after intravenous administration of either VRC01, PGDM1400 or VRC01/PGDM1400-10E8v4.
To ensure an adequate challenge dose, naïve animals were first challenged with each virus independently. For SHIV 325c, 4 naïve rhesus macaques were inoculated one time intrarectally with 1 ml of undiluted viral stock. All four animals were infected and showed persistent viremia for up to 90 days (fig. S8). For SHIV BaLP4, the same stock and dose of virus were used as described in several of our prior publications (26,29,30). In total, 30 control animals were previously challenged with a single 1ml intrarectal inoculation of SHIV BaLP4 and all became infected.
To assess in vivo protection, NHP were challenged mucosally with a mixture of these differentially sensitive SHIVs, 5 days after Ab infusion in two separate experiments, with 4 animals in each group. In total, 6 of 8 macaques (75%) infused with VRC01 alone and 5 of 8 (62%) animals treated solely with PGDM1400 became infected. In contrast, none of the 8 animals in the trispecific-treated group were infected (Fig. 6C; p=0.0058 by two-tailed Fisher exact test). These data confirm that the improved breath and potency of the trispecific Ab conferred protection against viruses that otherwise show resistance to single bnAbs alone.
DISCUSSION
Next Generation HIV bnAbs
A hallmark of HIV infection is the remarkable genetic diversity of the virus. Since 2010, significant progress has been made in the identification of bnAbs that show exceptional breadth and potency (reviewed in ref. 1). Several of these antibodies have progressed into clinical trials for prevention or treatment, and there is renewed interest in exploring their potential in the clinical management of HIV infection (5,12,14). Here, we explored the potential of different bnAbs to combine into a single protein that confers protection against diverse HIV strains. Among the classes of bnAbs, we found that trispecific Abs derived from bnAbs with CD4bs, MPER, and V1V2 glycan specificities had broad specificity, were potent and could be produced in sufficient quantities to allow evaluation in NHP, and eventually in humans. When tested in NHPs with viruses resistant to individual parental bnAbs, the trispecific Ab demonstrated complete protection against both viruses whereas infection was established in most animals treated with individual parental antibodies VRC01 and PDGM1400. In addition, the ability of this trispecific Ab to target three independent epitopes may improve treatment efficacy in humans.
In HIV-1 infected patients, reductions in viral load have been observed after one infusion of a single bnAb, thus demonstrating biological activity of HIV bnAbs (31–34). A modest extension of viral rebound was also observed when individual bnAbs were infused after antiretroviral drugs were discontinued in previously suppressed HIV-infected subjects (32,33). NHP and human passive transfer studies have also suggested that such bnAbs can enhance anti-viral immunity that may contribute to improved viral control (35,36). In addition, NHP studies demonstrate the importance of mAb potency and prolonged antibody half-life in mediating protection against infection (26,29). The generation of trispecific Abs with improved potency and breadth may further enhance the efficacy of either passive immunity or passive-active immunization strategies.
Although bnAbs show exceptional breadth and potency, resistant viral strains have been detected in patients who make these Abs (6,37) and among natural viral isolates (38–40), raising the concern that resistance and escape mutations may arise. Such escape mutations are produced frequently with antiviral drug therapy (41), and countermeasures to reduce the likelihood of escape would increase the likelihood of developing a globally relevant therapy. While such breadth of coverage might alternatively be generated by administering multiple bnAbs, increased complexity, cost and regulatory burdens of combination treatments, in contrast to the simplicity and reduced expense of administering a single biologic therapy provides an additional advantage to this approach. The potency of the trispecific Abs described here also exceeds that of a broad and potent recombinant form of CD4 (42), termed eCD4-Ig (fig. S4), and this latter molecule is also directed to a single, albeit highly conserved, HIV Env epitope. The availability of a single protein that targets multiple independent epitopes on virus also reduces the potential generation of escape mutations. This advantage in part could relate to the presence of three independent binding specificities at all times in contrast to mixtures of antibodies where selective pressure by individual mAbs with shorter half-lives may wane.
Clinical Translation
The trispecific Abs have not yet been evaluated for safety and efficacy in humans. While initial characterization of their half-life in NHPs suggests that they behave similarly to conventional antibodies, the question remains as to whether they could be immunogenic in vivo. The administration of a bispecific antibody to the human cytokines IL-4 and IL 13, which uses a related format and linkers (43), may provide guidance in this regard. This bispecific antibody has been evaluated in humans where single subcutaneous doses of SAR156597, ranging from 10-300 mg/kg, were well tolerated in healthy subjects, with low titers of ADA in only 4 of 36 subjects (43). Importantly, it showed a mean half-life of about two weeks (43), similar to natural monoclonal antibodies. While further human trials are needed to assess the full potential of the trispecific Ab platform, the data from the NHP challenge study described here, as well as the previous experience in humans with bispecific Abs (43), suggests that the approach merits further clinical investigation. Studies in HIV-infected subjects, alone or in combination with other immune interventions, will address the potential of trispecific Abs to provide durable protective immunity against infection or sustained viral control in HIV infected subjects during drug holidays or in the absence of antiretroviral therapy. The recognition of independent target sites with multi-specific antibodies can also be applied to other infectious diseases, cancer, and autoimmunity. These antibodies can promote recognition and binding to critical antigenic determinants on target cells and simultaneously allow engagement of immune cells that can stimulate relevant effector function without the complications and expense of delivering multiple recombinant proteins.
Supplementary Material
Acknowledgments
We thank C. Lawendowski for excellent program management; A. E. Schroeer and B. DelGiudice for graphic arts support; K. Radošević, C.J. Wei, M. Hollis, S. Rao and B. Zhang for organizational support; S-Y. Ko for pharmacodynamics analysis, H. Qiu and B. Brondyk for technical advice, and L. Hou and A. Park from Sanofi for expressing and purifying the 10E8v4-PGDM1400 CODV Fab used in the crystallization. The reported are tabulated in the main text and supplementary materials. The coordinates and crystal structure factors were deposited in the Protein Data Bank (PDB) under code: 5WHZ. L.X., Z-y.Y., G.J.N., R.R.W., J.B., J.K., E.R., W.D.L., C.B., C.L., M.C., J.R.M., R.A.K., N.D-R., T.Z., P.D.K., Y.D.K., and A.P. are inventors on patent application WO 2017/074878 submitted by Sanofi, The United States of America, as represented by the Secretary, Department of Health and Human Services, and the National Institutes of Health, that discloses the use of anti-HIV antibodies. The work of L.X. L.W., E.R., J.B., J.K., D.M.L., R.R.W., G.D., C.B., C.L., W.D.L., R.S., Z-Y. Y., G.J.N. was supported by funding from Sanofi Global R&D. Support for this work for A.P., N.D.R., K.M., M.L., M.A., Y.D.K., T.Z., S.D.S., R.B., K.W.,M.C., Z.M., S.O., J-P.T. X.J.C., M.R., M.C., P.D.K., R.A.K., J.R.M. came from the Division of intramural Research and the Vaccine Research Center, NIAID, NIH. Funding for D.H.B. and L.J.T. includes NIH grants AI096040, AI124377, AI126603 (D.H.B.); D.R.B. is supported by UM1AI100663 and IAVI.
Footnotes
Author contributions: Z-y.Y., M.R., P.D.K., R.A.K., J.R.M. D.B., and G.J.N. designed the research. Z-y.Y., A.P., N.D.R., E.R., J.B., J.K., R.S., R.B.T. carried out the research. Z-y.Y., L.X.,A.P.,L.W., M.A., D.M.L.,R.W., G.J.D., K.M., M.L., C.B., C.L., W.D.L., S.D.S., R.B., K.W.,M.C., Z.M., S.O., J-P.T. X.J.C. performed the experiments; Y.K., T.Z., D.R.B. M.R., M.C. contributed new reagents/viral strains; Z-y.Y., L.X., A.P.,N.D.R.,R.W. ,G.J.D., P.D.K., J.R.M., and G.J.N. analyzed the data; Z-y.Y., A.P.,N.D.R., R.W., J.R.M. and G.J.N. wrote the paper. All authors reviewed the paper. VRC01, VRC07, VRC13, and CAP256-VRC26.25 antibodies are available from J.R.M. and 10E8 and N6 antibodies are available from M.C. under a material transfer agreement with the NIH. All other requests for data and further information should be directed to G.J.N. at gary.nabel@sanofi.com or J.R.M. at jmascola@mail.nih.gov.
References
- 1.Burton DR, Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol. 2016;34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev. 2017;275:11–20. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolis DM, Koup RA, Ferrari G. HIV antibodies for treatment of HIV infection. Immunol Rev. 2017;275:313–323. doi: 10.1111/imr.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou T, Zhu J, Wu X, Moquin S, Zhang B, Acharya P, Georgiev IS, Altae-Tran HR, Chuang GY, Joyce MG, Kwon YD, Longo NS, Louder MK, Luongo T, McKee K, Schramm CA, Skinner J, Yang Y, Yang Z, Zhang Z, Zheng A, Bonsignori M, Haynes BF, Scheid JF, Nussenzweig MC, Simek M, Burton DR, Koff WC, NISC Comparative Sequencing Program. Mullikin JC, Connors M, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39(2):245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang Z-y, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward AB, Wilson IA. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev. 2017;275:21–32. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes BF, Mascola Jr. The quest for an antibody-based HIV vaccine. Immunol Rev. 2017;275:5–10. doi: 10.1111/imr.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauci AS. An HIV vaccine: mapping uncharted territory. JAMA. 2016;316:143–144. doi: 10.1001/jama.2016.7538. [DOI] [PubMed] [Google Scholar]
- 12.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev. 2017;275:296–312. doi: 10.1111/imr.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady Jm, Baltimore D, Balazs AB. Antibody gene transfer with adeno-associated viral vectors as a method for HIV prevention. Immunol Rev. 2017;275:324–333. doi: 10.1111/imr.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing antibodies for HIV-1 prevention or immunotherapy. N Engl J Med. 2016;375:2019–2021. doi: 10.1056/NEJMp1613362. [DOI] [PubMed] [Google Scholar]
- 15.Asokan M, Rudicell RS, Louder MK, McKee K, O'Dell S, Stewart-Jones G, Wang K, Xu L, Chen X, Choe M, Chuang G, Georgiev IS, Joyce MG, Kirys T, Ko S, Pegu A, Shi W, Todd J-P, Yang Z-y, Bailer RT, Rao S, Kwong PD, Nabel GJ, Mascola JR. Bispecific antibodies targeting different epitopes on the HIV-1 envelope exhibit broad and potent neutralization. J Virol. 2015;89:12501–12512. doi: 10.1128/JVI.02097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. Bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell. 2016;165:1609–1620. doi: 10.1016/j.cell.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, Tsai L, Gajjar MR, Sun M, Seaman MS, Padte NN, Ho DD. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016;165:1621–1631. doi: 10.1016/j.cell.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong R, Louder MK, Wagh K, Bailer RT, deCamp A, Greene K, Gao H, Taft JD, Gazumyan A, Liu C, Nussenzweig MC, Korber B, Montefiori DC, Mascola JR. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol. 2015;89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegu P, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, Guo X, Shi W, Georgiev I, Zhou T, Chen X, O'Dell S, Todd J-P, Kwong PD, Rao S, Yang Z-y, Koup RA, Mascola JR, Nabel GJ. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun. 2015;6:8447. doi: 10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloan DD, Lam CY, Irrinki A, Liu L, Tsai A, Pace CS, Kaur J, Murry JP, Balakrishnan M, Moore PA, Johnson S, Nordstrom JL, Cihlar T, Koenig S. Targeting HIV reservoir in infected CD4 T cells by dual-affinity re-targeting molecules (DARTs) that bind HIV envelope and recruit cytotoxic T cells. PLoS Pathog. 2015;11:e1005233. doi: 10.1371/journal.ppat.1005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, Lu X, Cai F, Hwang KK, Song H, Zhou T, Lynch RM, Alam SM, Moody MA, Ferrari G, Berrong M, Kelsoe G, Shaw GM, Hahn BH, Montefiori DC, Kamanga G, Cohen MS, Hraber P, Kwong P/D, Korber BT, Mascola JR, Kepler TB, Haynes BF. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell. 2014;158:481–91. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, Altae-Tran HR, Bailer RT, Crooks ET, Cupo A, Druz A, Garrett NJ, Hoi KH, Kong R, Louder MK, Longo NS, McKee K, Nonyane M, O'Dell S, Roark RS, Rudicell RS, Schmidt SD, Sheward DJ, Soto C, Wibmer CK, Yang Y, Zhang Z, NISC Comparative Sequencing Program. Mullikin JC, Binley JM, Sanders RW, Wilson IA, Moore JP, Ward AB, Georgiou G, Williamson C, Abdool Karim SS, Morris L, Kwong PD, Shapiro L, Mascola JR. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmetz A, Vallée F, Beil C, Lange C, Baurin N, Beninga J, Capdevila C, Corvey C, Dupuy A, Ferrari P, Rak A, Wonerow P, Kruip J, Mikol V, Rao E. CODV-Ig, a universal bispecific tetravalent and multifunctional immunoglobulin format for medical applications. MAbs. 2016;8:867–878. doi: 10.1080/19420862.2016.1162932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific IgG. Nat Biotechnol. 1998;16:677–681. doi: 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 25.Kwon YD, Georgiev IS, Ofek G, Zhang B, Asokan M, Bailer RT, Bao A, Caruso W, Chen X, Choe M, Druz A, Ko SY, Louder MK, McKee K, O'Dell S, Pegu A, Rudicell RS, Shi W, Wang K, Yang Y, Alger M, Bender MF, Carlton K, Cooper JW, Blinn J, Eudailey J, Lloyd K, Parks R, Alam SM, Haynes BF, Padte NN, Yu J, Ho DD, Huang J, Connors M, Schwartz RM, Mascola JR, Kwong PD. Optimization of the solubility of HIV-1-neutralizing antibody 10E8 through somatic variation and structure-based design. J Virol. 2016;90:5899–5914. doi: 10.1128/JVI.03246-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko SY, Pegu A, Rudicell RS, Yang Z-y, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, Zeng M, Schmidt SD, Todd J-P, Penzak SR, Saunders KO, Nason MC, Haase AT, Rao SS, Blumberg RS, Mascola JR, Nabel GJ. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sok D, van Gils MJ, Pauthner M, Julien JP, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, Hua Y, Seaman MS, Moore JP, Ward AB, Wilson IA, Sanders RW, Burton DR. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. PNAS. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- 29.Pegu A, Yang Z-y, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, Todd J-P, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 2014;6:243ra88. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders KO, Pegu A, Georgiev IS, Zeng M, Joyce MG, Yang Z-y, Ko SY, Chen X, Schmidt SD, Haase AT, Todd J-P, Bao S, Kwong PD, Rao SS, Mascola JR, Nabel GJ. Sustained delivery of a broadly neutralizing antibody in nonhuman primates confers long-term protection against simian/human immunodeficiency virus infection. J Virol. 2015;89:5895–903. doi: 10.1128/JVI.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O'Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE VRC 601 Study Team. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, Learn GH, Proschan MA, Kreider EF, Blazkova J, Bardsley M, Refsland EW, Messer M, Clarridge KE, Tustin NB, Madden PJ, Oden K, O'Dell S, Jarocki B, Shiakolas AR, Tressler RL, Doria-Rose NA, Bailer RT, Ledgerwood JE, Capparelli EV, Lynch RM, Graham BS, Moir S, Koup RA, Mascola JR, Hoxie JA, Fauci AS, Tebas P, Chun TW. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med. 2016;375:2037–2050. doi: 10.1056/NEJMoa1608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP, Jr, Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC, Caskey M. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, Learn GH, Cohen YZ, Lehmann C, Gillor D, Shimeliovich I, Unson-O'Brien C, Weiland D, Robles A, Kümmerle T, Wyen C, Levin R, Witmer-Pack M, Eren K K, Ignacio C, Kiss S, West AP, Jr, Mouquet H, Zingman BS BS, Gulick RM, Keler T, Bjorkman PJ, Seaman MS, Hahn BH, Fätkenheuer G, Schlesinger SJ, Nussenzweig MC MC, Klein F. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23:185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, Oliveira T, Lorenzi JC, Parrish EH, Learn GH, West AP, Jr, Bjorkman PJ, Schlesinger SJ, Seaman MS, Czartoski J, McElrath MJ, Pfeifer N, Hahn BH, Caskey M, Nussenzweig MC. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science. 2016;352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, Klein F, Gazumyan A, Golijanin J, Donaldson M, Donau OK, Plishka RJ, Buckler-White A, Seaman MS, Lifson JD, Koup RA, Fauci AS, Nussenzweig MC, Martin MA. Nature. 2017;543:559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Zhang Z, Schramm CA, Joyce MG, Kwon YD, Zhou T, Sheng Z, Zhang B, O'Dell S, McKee K, Georgiev IS, Chuang GY, Longo NS, Lynch RM, Saunders KO, Soto C, Srivatsan S, Yang Y, Bailer RT, Louder MK, NISC Comparative Sequencing Program. Mullikin JC, Connors M, Kwong PD, Mascola JR, Shapiro L. Maturation and diversity of the VRC01-antibody lineage over 15 years of chronic HIV-1 infection. Cell. 2015;161:470–485. doi: 10.1016/j.cell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao H-X, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program. Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore PL, Sheward D, Nonyane M, Ranchobe N, Hermanus T, Gray ES, Abdool Karim SS, Williamson C, Morris L. Multiple pathways of escape from HIV broadly cross-neutralizing V2-dependent antibodies. J Virol. 2013;87:4882–4894. doi: 10.1128/JVI.03424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhiman JN, Anthony C, Doria-Rose NA, Karimanzira O, Schramm CA, Khoza T, Kitchin D, Botha G, Gorman J, Garrett NJ, Abdool Karim SS, Shapiro L, Williamson C, Kwong PD, Mascola JR, Morris L, Moore PL. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med. 2015;21:1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HIV/AIDS Programme World Health Organization. WHO HIV Drug Resistance Report 2012. 2012 [Google Scholar]
- 42.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, Neale ES, Jr, Fellinger CH, Joshi VR, Fuchs SP, Martinez-Navio JM, Quinlan BD, Yao AY, Mouquet H, Gorman J, Zhang B, Poignard P, Nussenzweig MC, Burton DR, Kwong PD, Piatak M, Jr, Lifson JD, Gao G, Desrosiers RC, Evans DT, Hahn BH, Ploss A, Cannon PM, Seaman MS, Farzan M. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soubrane C, et al. International Colloquium on Lung and Airway Fibrosis. 2014. [Google Scholar]
- 44.Wu X, Yang Z-y, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose NA, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 47.Bolton DL, Pegu A, Wang K, McGinnis K, Nason M, Foulds K, Letukas V, Schmidt SD, Chen X, Todd J-P, Lifson JD, Rao SS, Michael NL, Robb ML, Mascola JR, Koup RA. Human immunodeficiency virus type 1 monoclonal antibodies suppress acute simian-human immunodeficiency virus viremia and limit seeding of cell-associated viral reservoirs. J Virol. 2015;90:1321–1332. doi: 10.1128/JVI.02454-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.