Abstract
Background
Impulsive aggressive behavior is thought to be facilitated by activation of the limbic brain, particularly the amygdala and hippocampus., Functional imaging studies suggest abnormalities in limbic brain activity during emotional information processing in impulsively aggressive subjects with Intermittent Explosive Disorder (IED). It is not known if IED is associated with altered amygdala and hippocampus volume and shape.
Methods
We examined the volume and shape of the amygdala-hippocampal complex, using morphometric analysis of high resolution structural 3T MR scans in healthy control (HC: n = 73) subjects without history of Axis I or II psychiatric conditions and in subjects with IED (n = 67).
Results
While no volume differences were observed between HC and IED subjects, a significant level of morphometric deformation, suggestive of cell loss, in both amygdala and hippocampal structures was observed bilaterally in IED subjects. Analysis of a canonical variable that used the first 10 eigenvectors from both sides of the brain revealed that these morphometric deformations in the IED subjects were not due the presence of confounding variables or to comorbidities among IED subjects.
Conclusions
These data reveal that IED is associated with a significant loss of neurons in both the amygdala and hippocampus. These changes may play a role in the functional abnormalities observed in previous fMRI studies and in the pathophysiology of impulsive aggressive behavior.
Keywords: IED, Aggression, Amygdala, Hippocampus
INTRODUCTION
Intermittent Explosive Disorder (IED) is an impulse control disorder that describes individuals with recurrent, problematic, impulsive aggression (1). Using DSM-IV criteria, the lifetime prevalence of IED is between 5-8% (2). Its incidence peaks in the teenage years but can first be observed in children before the age of ten. Both environmental and genetic factors are linked to impulsive aggression (3)., On the molecular level, functional abnormalities of the neurotransmitter serotonin have been repeatedly associated with impulsive aggression (4).
Impulsive aggression, the core behavior in IED, is modulated by limbic structures in the brain, particularly amygdala and hippocampus(5). In human imaging studies of borderline personality disorder (BPD), a closely related disorder also characterized by impulsive aggression, reductions in the grey matter volume of amygdala and hippocampus have been reported (6). In two studies of BPD subjects, hippocampal volume was correlated inversely with the age of onset of aggression in one (i.e., the earlier the onset of aggression, the greater the reduction in hippocampal volume (7). In the other study, hippocampal volume correlated inversely with aggression severity (8). In addition, individuals with Antisocial Personality Disorder (AsPD), and/or psychopathy, who also exhibit aggressive behavior have been reported to have reduced amygdala and hippocampal volume (9).
In contrast to BPD, AsPD, and psychopathy, little has been reported regarding the morphometry of brain structures in individuals with IED. In the one published study to date in temporal lobe epilepsy patients with and without IED, the IED subgroup exhibited severe amygdala atrophy (10). Thus, the current study was undertaken to explore the possibility of volumetric and morphometric shape differences in amygdala and hippocampus in the brains of IED subjects compared with healthy controls. Given the complex comorbidity relationship between IED and personality disorder, secondary analyses were also performed to see if IED subjects with and without BPD/AsPD differed from each other.
METHODS
Subjects
All subjects gave informed consent and signed the informed consent document approved by our Institutional Review Board. One-hundred-forty right handed individuals were studied with structural magnetic resonance imaging (MRI). All subjects were medically healthy and were systematically evaluated with regards to aggressive, anxiety and other behaviors as part of a larger program designed to study correlates of impulsive aggressive, and other personality-related behaviors in human subjects. Subjects were recruited through public service announcements, newspaper, and other media, advertisements seeking out individuals who: a) reported psychosocial difficulty related to one or more syndromal and/or personality disorders or, b) had little evidence of psychopathology. Inclusion criteria required subjects to be medically healthy, right-handed, and to meet DSM-5 criteria for Intermittent Explosive Disorder (IED) or be free of a life history of any DSM-5 disorder. Subjects who had a life history of bipolar disorder, psychosis, or developmental disorder, or who had a current DSM-5 substance use disorder were excluded from study.
Diagnostic Assessment
Syndromal and personality disorder diagnoses were made according to DSM-5 criteria (11). Diagnoses were made using information from: (a) the Structured Clinical Interview for DSM Diagnoses (SCID-I) (12) for syndromal disorders and the Structured Interview for the Diagnosis of DSM Personality Disorder (SIDP) (13) for personality disorders; (b) clinical interview by a research psychiatrist; and, (c) review of all other available clinical data. The research diagnostic interviews were conducted by individuals with a masters, or doctorate, degree in Clinical Psychology. All diagnostic raters went through a rigorous training program that included lectures on DSM diagnoses and rating systems, videos of expert raters conducting SCID/SIDP interviews, and practice interviews and ratings until the rater were deemed reliable with the trainer. This process resulted in good to excellent inter-rater reliabilities (mean kappa of .84 ± .05; range: .79 to .93) across anxiety, mood, substance use, impulse control, and personality disorders. Final diagnoses were assigned by team best-estimate consensus procedures involving research psychiatrists and clinical psychologists as previously described (14). Medical health of all subjects was documented by medical history and examination, and urine screen for illicit drugs. Syndromal and personality disorder diagnoses are listed in Table I. Of the 67 subjects with DSM-5 IED, most (82%) reported: a) history of formal psychiatric evaluation and/or treatment (54%) or, b) history of behavioral disturbance during which the subject, or others, thought they should have sought mental health services but did not (28%).
TABLE I.
Syndromal and Personality Disorder Diagnoses in the Sample
| IED (N = 67) | |
|---|---|
| Current Syndromal Disorders: | |
| Intermittent Explosive Disorder | 55 (82.1%) |
| Any Depressive Disorder | 15 (22.4%) |
| Any Anxiety Disorder | 19 (28.4%) |
| Any Substance Use Disorder | 0 (0.0%) |
| Any Stress and Trauma Disorder | 10 (14.9%) |
| Any Eating Disorder | 3 (4.5%) |
| Any Obsessive-Compulsive Disorder | 1 (1.5%) |
| Any Somatoform Disorder | 1 (1.5%) |
| Non-IED Impulse Control Disorder | 1 (1.5%) |
| Lifetime Syndromal Disorders: | |
| Intermittent Explosive Disorder | 67 (100.0%) |
| Any Depressive Disorder | 45 (67.2%) |
| Any Anxiety Disorder | 23 (34.3%) |
| Any Substance Use Disorder | 31 (46.3%) |
| Any Stress and Trauma Disorder | 15 (22.4%) |
| Any Eating Disorder | 4 (6.0%) |
| Any Obsessive-Compulsive Disorder | 2 (3.0%) |
| Any Somatoform Disorder | 1 (1.5%) |
| Non-IED Impulse Control Disorder | 3 (4.5%) |
| Personality Disorders: | |
| Any Personality Disorder | 63 (94.0%) |
| Specific Personality Disorder Cluster | |
| Cluster A (Odd) | 9 (13.4%) |
| Cluster B (Dramatic) | 30 (44.8%) |
| Cluster C (Anxious) | 21 (31.3%) |
| PD-NOS | 21 (31.3%) |
| Specific Personality Disorders: | |
| Borderline Personality Disorder | 27 (40.3%) |
| Antisocial Personality Disorder | 12 (17.9%) |
| PCL-SV Psychopathic Personality | 10 (14.9%) |
Measures of Other-Directed Aggression, Impulsivity, Self-Directed Aggression, and Psychopathic Personality
Aggression was assessed with the Aggression score from the Life History of Aggression (LHA) (15) assessment and the Aggression (Physical and Verbal) score from the Buss-Perry Aggression Questionnaire (BPA) (16). The LHA assesses history of actual aggressive behavior and BPA assesses aggressive tendencies as a personality trait. Impulsivity was assessed using the Life History of Impulsive Behavior (LHIB) (17) and the Barratt Impulsiveness Scale (BIS-11) (18). The LHIB assesses history of actual impulsive behavior and BIS-11 assesses impulsive tendencies as a personality trait. Aggression and impulsivity source variables were combined into composite variables by taking the average of the z-scores for the source variables (i.e., LHA and BPA for Composite Aggression; LHIB and BIS-11 for Composite Impulsivity). Life history of suicidal, and self-injurious, behavior were assessed during the diagnostic assessment. A suicidal act was considered present if it involved behavior with the conscious (even if ambivalent) intent to die by means the subject believed could end his or her life; a self-injurious act was considered present if it involved behavior with the conscious (even if ambivalent) intent to physically harm, but not kill, the subject. The Psychopathy Checklist Screening Version (PCL-SV) (19) was used to assess for presence of Psychopathic Personality (PP) using a threshold of PCL-SV score ≥ 13.
Assessments of Other Relevant Variables
The Beck Depression Inventory (BDI-II) (20) and the Beck Anxiety Inventory (BAI) (21) were used to assess state depression and state anxiety, respectively. History of head injury, and loss of consciousness, was assessed during the diagnostic assessment. Head injury was defined as any injury to the head, regardless of severity. Loss of consciousness was defined as any period of unconsciousness after a head injury. Subjects with any history of head injury with loss of consciousness greater than one hour in duration were excluded from this study. Global Assessment of Function (GAF) scale was used as a measure of psychosocial functioning. Racial data, collected during the diagnostic assessment, reflected self-identified racial characteristics of subjects.
MR Scanning Procedures
After informed consent, each subject underwent one MR Scanning session to obtain high resolution structural data. All MR scanning was performed on a 3.0 Tesla GE Signa MRI System (General Electric, Waukesha, WI) at The University of Chicago Brain Research Imaging Center (BRIC). Prior to each imaging session, subjects were confirmed to be negative for recent drug use and for females, pregnancy, by urinalysis. Once positioned, head movement was minimized through: a) instructions to participants; b) packing the head inside the head coil with foam padding and pillows that limit head motion. A high-resolution T1-weighted 3D MP-RAGE scan was acquired to provide precise anatomical information. The parameters for MP-RAGE were: TE = 3.2ms, TR = 8ms, preparation time = 725 ms, flip angle 6°, field of view 24 cm × 24 cm, 124 sagittal slices, 1.5 mm slice thickness, 192 × 256 image matrix reconstructed to 256 × 256.
Brain Mapping of Surface Deformations
All scans were processed through the atlas-based FSLDDMM (FreeSurfer + Large-Deformation Diffeomorphic Metric Matching) pipeline (24). The atlas scan was taken from previously published studies but was not otherwise included in the data analysis. The left and right amygdala and hippocampus in this atlas have been previously segmented and validated; and surfaces were tessellated to be used as anatomic templates (22, 23). The FSLDDMM pipeline consisted of the following three stages: 1) Initial, automated segmentation of the template and each target image using FreeSurfer (24), which generated structural labels including the hippocampus and amygdala, formed the first stage in the FSLDDMM pipeline. These labels, while largely correct, were coarse and often over-estimating, unsuitable for shape analysis. 2) Alignment of regions of interest (ROIs) subvolumes surrounding the hippocampal and amygdala FreeSurfer labels between the atlas scan and each subject. 3) Lastly, LDDMM (25) was performed on the aligned ROIs for fine mapping between the atlas scan and each subject. The template surfaces were propagated to each subject under these LDDMM transformations to form the delineations of target structure.
Structural Volume and Shape of Amygdala and Hippocampus
Left and right hippocampal and amygdala volumes in each subject were calculated as the volumes enclosed by the surfaces. Shape was represented by principal component analysis (PCA) and subsequent principal component (PC) scores for each structure using previously described methods (22). Briefly, a population average surface was generated to which all subject surfaces were first rigidly aligned. Then a vertex-wise deformation vector was computed from each subject's aligned surface to the average, and a PCA was performed on these vectors for dimensionality reduction. The subset of PC scores that accounted for at least 75% of total variance were retained for statistical analysis. Intracranial volume (ICV) was provided by FreeSurfer segmentation.
Statistical Analysis
Comparisons between groups with respect to demographic information were performed by Fishers Exact Test (for categorical variables), t-test (for dimensional variables), with correction for unequal variances as appropriate, ANOVA and MANOVA were included in statistical models, Pearson/partial correlation, and multiple regression analysis. All reported p-values are two-tailed. The first analysis involved comparing the total volumes of amygdala and hippocampus. For volume comparison, we included left and right structural volumes in a repeated-measures analysis of variance (ANOVA) with group status as main effect and hemisphere as repeated factor. To test the central hypothesis that IED is associated with deformation of limbic brain structures, PC scores were entered into repeated-measures ANOVAs, with group status as main effect, hemisphere and PCs as repeated measurements. PCs were listed as “identity” in the repeated statement in SAS. In both volume and shape PC analyses, the models were tested with and without ICV as a covariate to control for head size. To determine post-hoc whether structural volume and shape could be used for discriminant purposes, logistic regression procedures were used to determine odds ratios, significance (95% confidence limits) and the C-statistics for each variable (i.e., volume and PC scores). The odds ratio is similar to regression coefficients, whereas the C-statistic is similar to area under the ROC curve which can be interpreted as probability of correct classification. An alpha of .05 was maintained for all analyses. The next set of analysis used CAN morphometric variables. CAN is a canonical variable (based on canonical discriminant analysis) that uses all of the first 10 eigenvectors from both sides (by averaging left and right), value from the imaging analyses. A positive CAN value indicates an outward formation, while a negative CAN value indicates an inward deformation, from the surface of the structure. Statistical analysis of the extracted CAN variables allows for exploratory testing of relationship between morphometric parameters and important diagnostic and behavioral constructs relevant to IED such as personality disorder, impulsivity, history of aggressive behavior, mood, and psychopathy. These follow-up analyses focused on CAN values as a function of: a) IED with and without Composite Aggression and Composite Impulsivity scores; b) IED with and without comorbid Borderline (BPD), Antisocial (AsPD) Personality Disorder, and PCL-SV defined “psychopathic personality” (PP), c) IED and other current and lifetime psychiatric and personality disorders, d) state depression and state anxiety, e) history of head injury and loss of consciousness, e) history of suicide attempt (SA) and self-injurious behavior (SIB), and f) dimensional scores of impulsivity, aggression, and psychopathy
RESULTS
Characteristics of the Sample (Table II)
TABLE II.
DEMOGRAPHIC AND PSYCHOMETRIC CHARACTERISTICS OF SAMPLE
| HC (N = 73) | IED (N = 67) | p = | |
|---|---|---|---|
| Demographic Variables | |||
| Age | 30.0 ± 7.5 | 34.1 ± 8.6 | .003a |
| Gender (%Male) | 40 | 49 | NSb |
| Race (%W/AA/Other) | 78 / 11 / 11 | 42 / 30 / 28 | < .001b |
| SES Score | 46.9 ± 11.0 | 37.9 ± 12.8 | < .001a |
| Psychometric Variables | |||
| LHA Aggression | 5.7 ± 3.1 | 18.6 ± 4.1 | <.001a |
| BPA Aggression | 30.1 ± 9.7 | 48.5 ± 11.0 | < .001a |
| LHIB Impulsivity | 28.5 ± 19.8 | 53.6 ± 18.3 | < .001a |
| BIS-11 Impulsivity | 55.7 ± 9.6 | 69.7 ± 10.0 | < .001a |
| BDI-2 State Depression | 3.1 ± 7.4 | 12.6 ± 11.2 | <.001a |
| BAI State Anxiety | 23.4 ± 3.1 | 27.7 ± 5.9 | < .001a |
| PCL-SV Psychopathy | 0.6 ± 1.2 | 7.7 ± 4.9 | < .001a |
| Full Scale IQ | 104.5 ± 13.8 | 110.2 ± 13.2 | NS a |
Notes:
by t-test
by Fisher Exact Test.
HC and IED groups differed in age, socioeconomic status (SES) score and in distribution of ethnicity; there was no difference in gender or in Full Scale IQ. None of these variables affected the results reported below. As expected, IED subjects scored higher than HC subjects in measures of aggression, impulsivity, psychopathy, state depression and anxiety scores.
Volume and Morphometry for Amygdala and Hippocampus
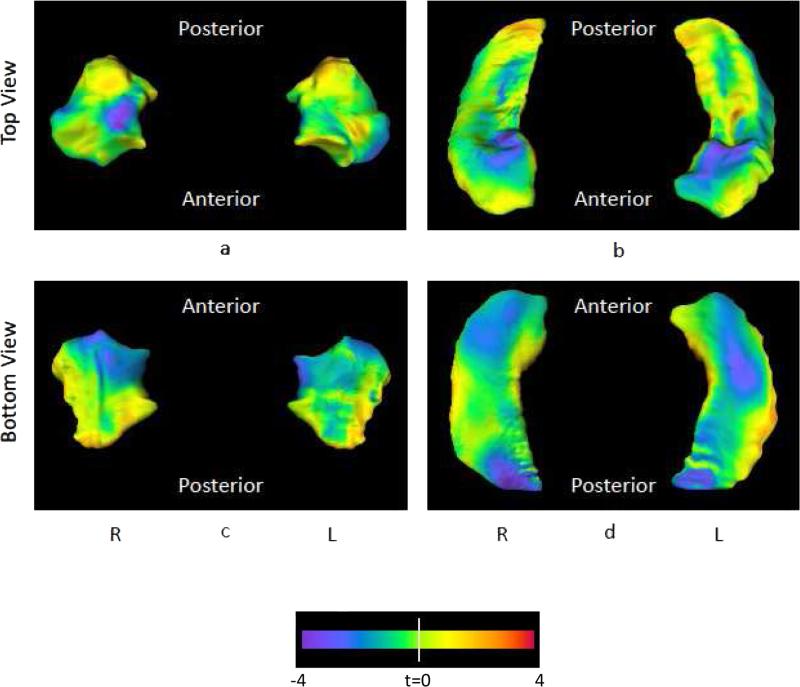
HC and IED subjects did not differ in mean amygdala volume (F[1,138] = 0.45, p = .50) but did differ significantly in surface shape deformations of the amygdala (PC scores: F[10,129] = 2.3, p = .018). The first 10 PCs accounted for at least 75% of the total variance. Controlling for ICV did not significantly alter either the volume or shape analysis (Volume: F[1,137] = 0.10, p = 0.80; Shape PC scores: F[10,137] = 2.10, p = 0.028). The surface shape difference between the IED group and the control group is visualized as t-maps in Figure 1 (left). A blue flame scale indicates regions of the mean IED surface showing inward deformity relative to the controls. Areas showing the greatest inward deformities are concentrated in the superior and inferior aspects of the medial-anterior parts of the amygdala. To assess the outward deformation areas, we first determined whether both the inward and outward deformations were statistically significant at p < 0.05 level vertex-wise. The areas of significant outward deformation occupied a negligible 1.3% of the total surface area, likely due to noise in the data. The areas of significant inward deformation occupied 6.9% of the total surface area. Finally, amygdala PC scores were entered into a logistic regression procedure to examine its ability to discriminate the two groups. This procedure yielded a sensitivity of 63% and specificity of 71% with an area under the ROC curve of 0.724.
Figure 1.
Panel (a) shows the left and right amygdala from the top (dorsal surface), panel (c) shows the left and right amygdala from the bottom (ventral surface). Panel (b) shows the left and right hippocampus from the top (dorsal surface), and panel (d) shows the left and right hippocampus from the bottom (ventral surface). The flame coloring represents the difference between the mean surface of the IED subjects the mean surface of controls. Inward variation of the surface is represented by cooler colors (i.e., blue to purple), while outward variation is represented by warmer colors (i.e., orange to red). For the amygdala, areas showing the greatest inward deformities are concentrated in the superior and inferior aspects of the medial-anterior parts of the amygdala. For the hippocampus, areas showing the greatest inward deformities are concentrated in the superior and inferior aspects of the head as well as the inferior aspect of the tail.
As in amygdala, HC and IED subjects did not differ in mean hippocampal volume (F[1,138] = 2.3, p = .13) but did differ significantly in surface shape deformations (PC scores: F[10,129], p = .011). Controlling for ICV did not significantly alter either the volume or shape analysis (Volume: F[1,137] = 0.80, p = 0.38; Shape PC scores: F[10,137] = 2.20, p = 0.021). The surface shape difference between the IED group and the control group is visualized as t-maps in Figure 1 (right). A blue flame scale indicates regions of the mean IED surface showing inward deformity relative to the controls. Areas showing the greatest inward deformities are concentrated in the superior and inferior aspects of the hippocampus head as well as the inferior aspect of the hippocampus tail. Similar to the amygdala, the areas of significant outward and inward deformation occupied 1.8% and 9.8% of the total surface area, respectively. Finally, hippocampal PC scores were entered into a logistic regression procedure to examine its ability to discriminate the two groups. This procedure yielded a sensitivity of 61% and specificity of 75% with an area under the ROC curve of 0.715.
CAN Variables in HC and IED Subjects
For both amygdala and hippocampus, mean (± sd) CAN values were lower in IED compared with HC subjects (MANOVA: Wilks ƛ = 0.80, F[2,137] = 17.66, p < .001; Amygdala: F[1,138] = 24.27, p < .001; IED = −0.44 ± 1.00 vs. HC = 0.40 ± 1.50; Hippocampus: F[1,138] = 25.88, p < .001; IED = −0.45 ± 1.00 vs. HC = 0.41 ± 1.50). Adding age, sex, race, ses, and IQ scores to this statistical model did not change these results. Binary logistic regression revealed a highly statistically significant relationship between CAN values and IED status (Amygdala: B = −0.83 ± 0.20, Wald = 18.28, df = 1, p < .001; Hippocampus: B = −0.88 ± 0.20, Wald = 18.90, df = 1, p < .001) that was eliminated by adding Composite Aggression and Composite Impulsivity scores to the statistical model (Amygdala: B = −0.51 ± 0.45, Wald = 1.29, df = 1, p = .256; Hippocampus: B = −0.63 ± 0.50, Wald = 1.62, df = 1, p = .204).
Relationship Between CAN and Clinical Variables in IED Subjects
CAN variables did not correlate significantly with age of onset of IED (Amygdala: r = .13, p > .27; Hippocampus: r = −.04, p = .75), or duration of IED (Amygdala: r = .03, p = .80; Hippocampus: r = .19, p = .134). Neither were differences in mean CAN values accounted for by lifetime exposure of psychotropic medication [Wilks ƛ = 0.96, F[2,64] = 1.19, p = .31) in amygdala [Psychotropic Hx+ (n = 19): −0.20 ± 1.01 vs. Psychotropic Hx- (n = 48): −0.53 ± 1.05, p > .24) or in hippocampus (Psychotropic Hx+: −0.17 ± 1.02 vs. Psychotropic Hx-: −0.56 ± 1.03, p > .16]. Finally, these differences were not accounted for by individual differences in: a) state depression score, b) state anxiety score, c) history of mild to moderate head injury with, or without, loss of consciousness or, d) presence, or absence, of comorbid current, or lifetime, DSM-5 syndromal or DSM-5 personality disorder, in IED subjects (see Supplemental Materials).
CAN Variables as a Function of Borderline (BPD), Antisocial (AsPD) Personality Disorder, and PCL-SV Defined Psychopathic Personality (PP)
The presence or absence of comorbid BPD, AsPD, or PP, did not affect these results for the amygdala [Mean (± sd) CAN values: “IED Alone”: −0.55 ± 1.04 vs. “IED with BPD”: −0.27 ± 1.06; “IED Alone”: −0.43 ± 1.04 vs. “IED with AsPD”: −0.47 ± 1.17; “IED Alone”: −0.46 ± 1.04 vs. “IED + PP”: −0.28 ± 1.18] or the hippocampus [Mean (± sd) CAN values: “IED Alone”: −0.36 ± 1.14 vs. “IED with BPD”: −0.58 ± 0.84; “IED Alone”: −0.43 ± 1.04 vs. “IED with AsPD”: −0.47 ± 1.17; “IED Alone”: −0.43 ± 1.00 vs. “IED + PP”: −0.55 ± 1.24]. Mean (± sd) CAN values for both amygdala and hippocampus, for each of these IED subgroups, were significantly lower than that of HC subjects for amygdala (0.40 ± 0.95; p < .05 in each case) and for hippocampus −0.41 ± 0.97, p < .05 in each case). Removal of the 32 of 67 IED subjects with comorbid BPD or AsPD or PP (Wilks ƛ = 0.78, F[2,105] = 14.49, p < .001) did not change the results for amygdala (“IED Alone”: −0.63 ± 1.02 vs. HC: 0.40 ± 0.95, F[1,106] = 26.65, p < .001) or for hippocampus (“IED Alone”: −0.34 ± 1.13 vs. HC: 0.41 ± 0.97, F[1,106] = 12.59, p = .001).
CAN Variables as a Function of as a Function of History of Suicide Attempt (SA+) and of Self-Injurious Behavior (SIB+)
MANOVA revealed no significant influence of either SA+ or SIB+ on mean (± sd) CAN values across all subjects (Wilks ƛ = 1.00, F[2,136] = 0.31, p = .737) for Amygdala (SA+: −0.13 ± 1.12 vs. SA-: −0.17 ± 2.75, F[1,137] = 0.02, p = .894; SIB+: −0.33 ± 1.09 vs. SIB-: 0.03 ± 1.89, p = .440, F[1,137] = 0.60, p =.086) or Hippocampus (SA+: −0.60 ± 1.19 vs. SA-: −0.05 ± 2.73, F[1,137] = 0.25, p = .619; SIB+: −0.44 ± 1.08 vs. SIB-: −0.21 ± 1.88, F[1,137] = 0.25, p = .619).
Relationship Between CAN Variables and Aggression, Impulsivity, and Psychopathy Scores
Because of the conceptual overlap between aggression, impulsivity, and psychopathy, multiple regression with CAN values as the dependent variables and with Composite Aggression, Composite Impulsivity, and PCL-SV Psychopathy scores as predictor variables were performed. This revealed that Composite Aggression, but not Composite Impulsivity or PCL-SV Psychopathy, scores related uniquely to CAN values for amygdala (β for Composite Aggression = −0.45, p = .006; β for Composite Impulsivity = 0.00, p = .971; PCL-SV Psychopathy = 0.14, p = .383). Composite Aggression and Impulsivity, but not PCL-SV Psychopathy, scores related uniquely to CAN values for hippocampus (β for Composite Aggression = −0.30, p = .05; β for Composite Impulsivity = −0.25, p < 0.05; β for PCL-SV Psychopathy = 0.04, p = .778).
DISCUSSION
The results of this study demonstrate that adults with DSM-5 IED have a localized, inwardly directed deformation in both the amygdala and hippocampus compared with healthy control subjects. The relationship between deformation values and IED status was highly statistically significant and almost entirely accounted for by dimensionally measured aggression and impulsivity, as would be expected for a disorder defined by impulsive aggressive behavior. This finding is consistent with previous work discovering dysfunction in fronto-limbic circuits, particularly in the amygdala, in aggressive humans and other primates (5). Despite the generally positive association between other-directed and self-directed aggressive behaviors in IED subjects, CAN values were not reduced as a function of life history of suicidal, or self-injurious, behavior. The number of subjects in these groups was small, however, and the present study had limited statistical power to detect differences in this regard.
The group differences in CAN values, suggesting inward deformations of surface aspects of amygdala and hippocampus, were not likely to have been accounted for by potentially confounding demographic and clinical such as age of onset, or duration, of IED, lifetime history of exposure to psychotropic medication, levels of state depression or state anxiety, current/lifetime psychiatric, or personality, disorder or, lifetime history of head injury or loss of consciousness (see Supplemental Material). Nor were the group differences accounted for by comorbidity with borderline, or antisocial, personality disorder or by comorbidity with PCL-SV defined psychopathic personality. In each case, IED subjects without these comorbidities still demonstrated inward deformations (i.e., lower CAN values) compared with healthy control subjects. In addition, removing all IED subjects with any of these specific personality disorders yielded the same result, demonstrating that inward deformations of both amygdala and hippocampus, in IED, is present even in the absence of factors previously reported to have reduced amygdala/hippocampal volume (6-9, 26).
To our knowledge, this is the first study examining morphometric shape of amygdala and hippocampus in aggressive subjects of any kind, let alone subjects in whom impulsive aggression is defined by fully operationalized DSM-5 IED diagnostic criteria. While several studies have reported differences in grey matter volume of these structures in subjects with “aggressive” features, none have examined the external shape of these structures as a function of aggression. Subtle or localized structural abnormalities in the absence of volume difference may be detected by morphometric or morphological assessments, as previously demonstrated in studies of schizophrenia using structural shape features (22, 27).
The inward deformities on surfaces of the amygdala and hippocampus imply the presence of a deleterious effect on neuronal processes within these structures. Evidence for this possibility comes from animal studies demonstrating that chronic stress, which is associated with reductions in size of limbic structures, is associated with an increase in the packing density of glia and neurons (28) and with reduction in the arborization of neuronal processes (29). For the amygdala, the areas affected include the extended amygdala (ExA), including the basolateral amygdala (BLA), and central nucleus (CeA), all of which may be associated with the pathophysiological processes that occur in IED. The BLA is involved in perception and in regulation of emotionally significant events via interactions with multiple brain systems, including sensory/perceptual association cortices, limbic-paralimbic affective systems, frontoparietal attentional network, and medial prefrontal emotion regulation system (30). The CeA, is involved in controlling automatic expressions of emotion, such as fear and freezing, through projections to brainstem, cerebellum, and sensorimotor system (30). Therefore, cellular abnormalities in the BLA/CeA could lead to dysregulation of emotional reactivity (e.g. increased amygdala response to emotional stimuli (31). While the hippocampus is less commonly conceived of as a neural substrate of aggression, , it is known that tumors and infections in the hippocampus are associated with changes in aggressive behavior in humans (32) and that regional stimulation of the hippocampus stimulate or inhibits aggression in cats (33). In addition, hippocampal CA1 neurons send projections to the medial prefrontal cortex and are predominantly found in this region of the hippocampus (34, 35). Thus, cellular abnormalities in the hippocampus could be associated with the cognitive schema and emotional difficulties observed in IED subjects (36).
Areas of significant outward deformation occupied a negligible 1.8% of the total surface area for the hippocampus and 1.3% for the amygdala (left and right combined). Thus, we posit that this result is due to “noise” in the data. Areas of significant inward deformation, in contrast, occupied 9.8% of the total surface area for the hippocampus and 6.9% for the amygdala (left and right combined) and, likely, represent the more relevant finding.
The cause of such inwardly directed deformations in IED subjects is not known. The results of exploratory analyses examining potentially confounding demographic and clinical factors confirmed that the observed shape deformities were most likely related to the fundamental neurobiology of IED. Given that problematic impulsive aggressive behavior begins early in childhood and persists throughout life, it is likely that the morphologic abnormalities detected in this study are due to genetic and/or gene-environment interactions affecting the developing brain and fronto-limbic circuits. The relevance of genetic factors in impulsive aggression has been appreciated for some time. Evidence of substantial genetic influence has been found for both aggression and impulsivity, although the identity of the genes remain unknown (3). Environmental factors associated with the development and maintenance of impulsive aggression include evidence demonstrating that history of childhood trauma (e.g., chronic is associated with both aggression (37) and IED (38), and with reductions in fronto-limbic volumes and grey matter volume (39-41).
The strengths of this study include a well characterized sample of impulsive aggressive subjects, validated measures of aggression and impulsivity, and the assessment of several relevant variables that could have confounded these findings. Limitations include the fact that this is a cross-sectional study and no causal conclusions can be made from associative analyses. Second, ascertainment of volunteer subjects from the community may limit the generalizability of these findings to the clinic. However, more than eighty percent of the IED subjects reported a past history of psychiatric treatment (or of having episodes of behavioral disturbance for which they, or others, thought they should have sought mental health services but did not) and, thus, most of these subjects are likely similar to individuals who would have been recruited from a clinical setting. Third, our methodology did not allow for similar analysis of orbito-frontal cortex. Thus, despite the importance of this structure for aggression (5), we cannot discuss the matter of potential surface deformities in this region. Lastly, because we are not able to parcellate (42) the hippocampus or amygdala into separate anatomic components, information about differential deformations and function of these structures cannot be analyzed and presented.
In summary, we report an inwardly directed deformation in the shape of both the amygdala and hippocampus in IED, compared with healthy control, subjects. This relationship was not accounted for by any of potentially confounding factors studied or by any comorbidity with other psychiatric/personality disorders.
Supplementary Material
Highlights.
Intermittent Explosive Disorder (IED) and healthy controls (HC) were studied.
IED subjects had significantly greater inward deformations in the shape of the amygdala and hippocampus compared with HC subjects regardless of any intervening factors.
This finding is suggestive of increased packing density of glia and neurons and/or changes in arborization of neuronal processes in these structures, possibly accounting for differences in aggressive behavior between IED and HC subjects.
ACKNOWLEDMENTS
This work was supported in part by grants from the National Institute of Mental Health: RO1 MH60836, RO1 MH63262, RO1 MH66984 (Dr. Coccaro) and RO1 MH056584 (Dr. Csernansky).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
Dr. Coccaro reports being on the Scientific Advisory Board of Azevan Pharmaceuticals, Inc.; Dr. Lee reports being the recipient of a research grant from Azevan Pharmaceuticals, Inc. Drs. Csernansky, McCloskey and Wang have nothing to disclose.
REFERENCES
- 1.Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. The American Journal of Psychiatry. 2012;169:577–588. doi: 10.1176/appi.ajp.2012.11081259. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Coccaro EF, Fava M, Jaeger S, Jin R, Walters E. The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006;63:669–678. doi: 10.1001/archpsyc.63.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seroczynski AD, Bergeman CS, Coccaro EF. Etiology of the impulsivity/aggression relationship: genes or environment? Psychiatry Research. 1999;86:41–57. doi: 10.1016/s0165-1781(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 4.Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: Serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology. 2010;35:435–444. doi: 10.1038/npp.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biological Psychiatry. 2011;69:1153–1159. doi: 10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Nunes PM, Wenzel A, Borges KT, Porto CR, Caminha RM, de Oliveira IR. Volumes of the hippocampus and amygdala in patients with borderline personality disorder: A meta-analysis. Journal of Personality Disorder. 2009;23:333–345. doi: 10.1521/pedi.2009.23.4.333. [DOI] [PubMed] [Google Scholar]
- 7.Zetzsche T, Preuss UW, Frodl T, Schmitt G, Seifert D, Münchhausen E, et al. Hippocampal volume reduction and history of aggressive behaviour in patients with borderline personality disorder. Psychiatry Research. 2007;154:157–170. doi: 10.1016/j.pscychresns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Sala M, Caverzasi E, Lazzaretti M, Morandotti N, De Vidovich G, Marraffini E, et al. Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. Journal of Affective Disorders. 2011;131:417–421. doi: 10.1016/j.jad.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. Journal of Abnormal Psychology. 2012;121:649–658. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Elst LT, Woermann FG, Lemieux L, Thompson PJ, Trimble MR. Affective aggression in patients with temporal lobe epilepsy: a quantitative MRI study of the amygdala. Brain. 2000;123:234–243. doi: 10.1093/brain/123.2.234. [DOI] [PubMed] [Google Scholar]
- 11.Association Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition. 5th ed. American Pyschiatric Press., Inc.; Washington, DC: 2013. [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Psychiatric Institute, Biometrics Research; New York: 1997. [Google Scholar]
- 13.Pfohl B, Blum N, Zimmerman M. Structured interview for DSM-IV Personality Disorder: SIDP-IV. American Psychiatric Press; Washington D.C.: 1997. [Google Scholar]
- 14.Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71:158–165. doi: 10.1001/jamapsychiatry.2013.3297. [DOI] [PubMed] [Google Scholar]
- 15.Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Research. 1997;73:147–57. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 16.Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 17.Coccaro EF, Schmidt-Kaplan CA. Life history of impulsive behavior: development and validation of a new questionnaire. Journal of Psychiatric Research. 2012;46:346–352. doi: 10.1016/j.jpsychires.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Patton J, Stanford M, Barratt E. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Hart SD, Cox DN, Hare RD. Hare Psychopathy Checklist: Screening Version (PCL-SV) MHS; Totonto, Ontario, Canada: 2003. [Google Scholar]
- 20.Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: Manual. 2nd ed. Psychological Corp., Harcourt Brace; San Antonio, Tex.: 1996. [Google Scholar]
- 21.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 22.Csernansky JG, Wang L, Joshi SC, Ratnanather JT, Miller MI. Computational anatomy and neuropsychiatric disease: probabilistic assessment of variation and statistical inference of group difference, hemispheric asymmetry, and time-dependent change. Neuroimage. 2004;23:S56–S68. doi: 10.1016/j.neuroimage.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, et al. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biological Psychiatry. 2008;64:1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 25.Beg MF, Miller MI, Trouve A, Younes L. Computing Large Deformation Metric Mappings via Geodesic Flows of Diffeomorphisms. International Journal of Computer Vision. 2005;61:139. [Google Scholar]
- 26.Tiihonen J, Hodgins S, Vaurio O, Laakso M, Repo E, Soininen H, et al. Amygdaloid loss in psychopathy. Society For Neuroscience Abstracts. 2000;26:754, 6. [Google Scholar]
- 27.Qiu A, Wang L, Younes L, Harms MP, Ratnanather JT, Miller MI, et al. Neuroanatomical Asymmetry Patterns in Individuals with Schizophrenia and their Non-psychotic Siblings. Neuroimage. 2009;47:1221–1229. doi: 10.1016/j.neuroimage.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biological Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin KJ, Baran SE, Conrad CD. Chronic stress-and sex-specific neuromorphological and functional changes in limbic structures. Molecular Neurobiology. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- 30.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Malamud N. Psychiatric disorder with intracranial tumors of limbic system. Archives of Neurology. 1967;17:113–123. doi: 10.1001/archneur.1967.00470260003001. [DOI] [PubMed] [Google Scholar]
- 33.Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Progress in Neuropsychopharmacology and Biological Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 34.Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 36.Coccaro EF, Noblett KL, MS M. Attributional and emotional responses to socially ambiguous cues: validation of a new assessment of social/emotional information processing in healthy adults and impulsive aggressive patients. J Psychiatric Research. 2009;43:915–925. doi: 10.1016/j.jpsychires.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Crick NR, Dodge KA. Social information-processing mechanisms in reactive and proactive aggression. Child Development. 1996;67:993–1002. [PubMed] [Google Scholar]
- 38.Fanning JR, Meyerhoff JJ, Lee R, Coccaro EF. History of childhood maltreatment in Intermittent Explosive Disorder and suicidal behavior. Journal of Psychiatric Research. 2014;56:10–17. doi: 10.1016/j.jpsychires.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. Journal of Psychiatric Research. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson R, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomoda A, Suzukim H, Rabi K, Sheu YS, Polcari A, Teicher MH. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47:T66–T71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopal A, Clark E, Allgair A, D'Amato C, Furman M, Gansler D, et al. Dorsal/central parcellation of the amygdala: Relevance to impulsivity and aggression. Psychiatry Research. 2013;211:24–40. doi: 10.1016/j.pscychresns.2012.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.