Abstract
Genetically-engineered pig organs could provide transplants to all patients with end-stage organ failure, but antibody-mediated rejection remains an issue. This study examines the class II swine leukocyte antigen (SLA) as a target of epitope-restricted antibody binding.
Transfection of individual alpha or beta heavy chains into human embryonic kidney cells resulted in both traditional and hybrid class II SLA molecules. Sera from individuals on the solid organ transplant waiting list were tested for antibody binding and cytotoxicity to this panel of class II SLA single antigen cells. A series of elution studies from a SLA-DQ cell line were performed.
Our results indicate human sera contain antibodies specific for and cytotoxic against class II SLA. Our elution studies revealed sera bind the SLA-DQ molecule in an epitope restricted pattern. Site-specific mutation of one of these epitopes resulted in statistically decreased antibody binding.
Humans possess preformed, specific, and cytotoxic antibodies to class II SLA that bind in an epitope-restricted fashion. Site-specific epitope mutagenesis may decrease the antibody binding of highly-sensitized individuals to pig cells.
1. Introduction
Xenotransplantation, the use of genetically-engineered pigs as organ donors, could allow all patients with end-stage organ failure to receive a life-saving transplant. However, strong human antibody responses against pig tissue prevent this goal from being realized(1, 2). Recent advances in genetic engineering have made it possible to inactivate three carbohydrate-modifying genes which produce xenoantigens on pig tissues. The low xenoantigenicity of these animals may have eliminated the humoral immune barrier for many people. For others, additional targets of humoral immunity remain(3, 4). Swine leukocyte antigens (SLA) also contribute to xenoantigenicity(4, 5). Swine major histocompatibility complex (MHC) genes encode these molecules, which are homologs of human leukocyte antigens (HLA). Class I HLA and SLA proteins consist of a polymorphic membrane-bound MHC protein associated with the invariant β2-microglobulin and a short peptide fragment. Class II proteins contain MHC-encoded polymorphic membrane-bound alpha and beta chains and a short peptide(6). Multiple class I and class II MHC genes, each locus containing many alleles, contribute to significant variation among HLA and SLA proteins. The strong antigenicity of HLA in allotransplantation originates from this polymorphism. Despite the variability of SLA and HLA, they contain significant structural and amino acid sequence identity. Consequently, we hypothesize that homologous proteins share epitopes, which enable cross-reactivity of HLA-specific human antibodies with pig cells.
To examine whether or not human antibody binds to class II SLA proteins, we previously created cell lines expressing unique combinations of class II alpha and beta chains(5). These transgenes encoded open reading frames of the class II proteins, SLA-DR and SLA-DQ, homologs of HLA-DR and HLA-DQ. Pigs do not contain genes homologous to the human HLA-DP loci(7). We used these reagents to show that SLA-DR and -DQ proteins can be xenoantigens(5). Here we extend these analyses by expressing class II SLA heterodimers with swine CD74, also known as the invariant chain. This chaperone facilitates biosynthesis of class II MHC proteins, raising their cell surface abundance (6).
This report demonstrates: 1) that co-expressing pig CD74 and class II SLA alpha/beta heterodimers improved detection of xeno-reactive human antibodies. 2) Human antibody binding to class II SLA proteins initiates complement mediated cell killing. 3) Though prior sensitization to class II HLA contributes to the generation of class II SLA antibodies it is not essential. 4) Human antibody binding to a specific class II epitope can be reduced by mutagenizing amino acids which contribute to the antigenic determinant.
2. Materials and Methods
2.1 Research Oversight
All human sera were collected and used in accordance with IRB-approved protocols.
2.2 Culture of Parent HEK 293T Cell Line
Human embryonic kidney (HEK) 293T cells (ATCC CRL-3216) were cultured in minimum essential media (MEM-α) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan UT) in collagen-I-coated plates (Becton Dickinson, Bedford, MA) at 37°C and 5% CO2. Cells were confirmed to be class II HLA negative by incubation with an anti-HLA-DR Ab (Clone L243) and anti-HLA-DQA1 (Clone DQA1) and analyzed using BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA).
2.3 Creation of β2m Deficient HEK 293T Cells Expressing Class II SLA Molecules
Previously we sequenced the SLA-DRα/β and -DQα/β genes in a single pig(8). The bicistrionic expression vector, pBudCE4.1 (Thermo Fisher Scientific, Waltham, MA), was engineered to express cDNA encoding 16 potential class II SLA heterodimers arising from the haplotypes present in that animal (Tables 1 and 2). The alpha chains of the alleles were inserted behind the CMV promoter and the beta chains were inserted behind EF-1α promoter of pBudCE4.1 using restriction enzyme digestion and DNA ligation. A second plasmid, pCDNA3.1+/Hygro (Thermo Fisher Scientific, Waltham, MA) encoding the swine invariant chain was also created using restriction enzyme digestion and DNA ligation. These plasmids were introduced into HEK 293T cells made deficient in class I HLA expression previously described by Ladowski et al(5).
Table 1. Class II SLA Alleles.
These alleles represent two haplotypes from a single pig(8).
| SLA Allele | Accession # |
|---|---|
| DRα*020102 | AIH07184.1 |
| DRα*w04 | AIH07183.1 |
| DRβ1*0403 | AIH07189.1 |
| DRβ1*1001 | AIH07190.1 |
| DQα1*0204 | AIH07186.1 |
| DQα1*0101 | AIH07185.1 |
| DQβ1*0303 | AIH07187.1 |
| DQβ2 *0601 | AIH07188.1 |
Table 2. Allele combination nomenclature.
The various pairings of the alpha and beta chains of DR and DQ are indicated. Pairing numbers will be used to simplify the labeling of figures.
| SLA Allele combination | Pairing |
|---|---|
| DRα*020102 + DRβ1 *0403 | 1 |
| DRα*020102 + DRβ1*1001 | 2 |
| DRα*w04 + DRβ1*0403 | 3 |
| DRα*w04 + DRβ1*1001 | 4 |
| DQα1*0204 + DQβ1*0303 | 5 |
| DQα1*0204 + DQβ2*0601 | 6 |
| DQα1*0101 + DQβ1*0303 | 7 |
| DQα1*0101 + DQβ2*0601 | 8 |
| DRα*020102 + DQβ1*0303 | 9 |
| DRα*w04 + DQβ1*0303 | 10 |
The class I HLA deficient cells were transfected using a calcium phosphate protocol: 1 × 106 cells were plated into a 6-well dish and transfected by adding a cocktail of 214 uL water, 31 uL 2 M CaCl2, 2.5 ug DNA, and 250 uL of 2x HBS. The cells were placed into selection with either Zeocin alone or Zeocin and Hygromycin for swine invariant chain transfectants, grown in MEM-α+ 10% FBS and sorted at the UAB Comprehensive Flow Cytometry Core on a BD FACSAria II for class II SLA expression as described above. The sorted cells were analyzed by a monoclonal anti-CD74 (Cerclip.1) antibody (Novus Biologicals, Littleton, Colorado, USA).
2.4 Human Antibody Binding to Class II SLA Single Antigen Cells
64 human sera samples, 26 sensitized towards class II HLA and 38 with no sensitization, were chosen for study of haplotype specific antibody binding on the panel of class II SLA single antigen cells. Sera treatment and methods of flow cytometric analyses were described previously (5).
2.5 CDC Assay on Class II SLA Single Antigen Cells
14 of the 64 flow cytometry samples were screened by complement-dependent cytotoxicity. 7 had class II HLA sensitization and 7 lacked allosensitization. The CDC assay was performed as described by Diaz et al., with minor modifications(9). Briefly, Cells were added to each well with 1×105 in 25 ul HBSS of Nunc™ 96-Well Polypropylene MicroWell™ Plate (Thermo Fisher Scientific, Waltham, MA), and incubated at 4°C for 30min with 25 ul/well of human sera treated with or without DTT (2.5mM in final) for 30min at 37°C. The cells were washed at 3 times with HBSS, then treated with 50ul/well of 11-fold dilution of Low-Tox®-H Rabbit Complement (Cedarlane, Burlington, NC) for 90min at 37°C. The cells were stained with FDA (0.5 ug/ml)/PI (2.5mg/ml, Sigma-Aldrich) at 4°C for 15min. Data were collected using a BD Accuri C6 Flow Cytometer and software (BD Accuri, Ann Arbor, MI, USA).
2.6 Antibody Elution from Cells Expressing SLA-DQα1*0101 + DQβ1*0601
Of the 64 flow cytometry samples, 6 were chosen for antibody binding and elution from a target cell. Briefly, 20 × 106 HEK 293T cells expressing SLA-DQα1*0101, SLA-DQβ1*0601, and swine invariant chain were incubated with 100 uL of serum for 30 minutes at 4°C. Unbound antibodies were removed by washing twice with phosphate buffered saline. Citric acid/phosphate buffer (pH 3.3) was added for 2 minutes to elute bound antibodies. Cells were pelleted by centrifugation and the supernatant was neutralized with Tris-buffered saline, pH 8.0, and concentrated to the original 100 uL serum volume using Vivaspin 6 30,000 MWCO centrifugal concentrators (Sartorius, New York, NY). These samples were screened on a class II HLA Luminex bead panel (One Lamda, Canoga Park, CA). In each case, antibody binding and elution from the HEK β2m KO parent cell, and re-binding to the class II HLA Luminex bead panel was performed as a negative control.
2.7 Antibody Binding of HLA-DQ4,5,6 Sensitized Sera on the SLA-DQ Expressing Cell Line
63 class II HLA sensitized sera samples of discarded and de-identified material from the University of Alabama-Birmingham Histocompatibility Lab were provided based on the presence (42 samples) or absence (21 samples) of sensitization to HLA-DQ4,5,6. Assays evaluating human antibody binding to cells expressing SLA-DQα1*0101, SLA-DQβ1*0601, and CD74 were performed as previously described (5).
2.8 Creation of the Cell Line Expressing SLA-DQα1*0101 and Mutated SLA-DQβ1*0601
The 55Arg residue of SLA-DQβ1*0601 was converted to 55Pro in the pBUDCE4.1 SLA-DQα1*0101/SLA-DQβ1*0601 plasmid using the QuickChange XL Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA) and the following primers: Forward: 5′-GTGAGTGACCCCGCTGGGGCCGCCGGACGCCGACTAC-3′ and Reverse: 5′-GTAGTCGGCGTCCGGCGGCCCCAGCGGGGTCAC-3′. This plasmid encoding the R55P mutant was transfected with pCDNA3.1+/Hygro encoding swine invariant chain the HEK 293T β2m KO cells with the calcium phosphate protocol described above. Transfected cells were placed into selection with Zeocin and Hygromycin, grown in MEM-α + 10% FBS, and sorted at the UAB Comprehensive Flow Cytometry Core on a BD FACSAria II for SLA class II expression with the CerCLIP.1 monoclonal antibody labeled with PE (Clone: CerCLIP.1, Thermo Fisher Scientific, Waltham, MA). A second monoclonal SLA-DQ antibody was also used to evaluate the SLA-DQ 55Pro mutant expression (Clone TH81A5, Washington State Monoclonal Antibody Center, Pullman, WA)
2.9 Human Antibody Binding to Cells Expressing SLA-DQα1*0101, 55P Mutant SLA-DQβ1*0601and CD74
21 sera samples from the cytotoxicity, elution, and the HLA-DQ4,5,6 sensitization studies suspected to have anti-SLA-55Arg antibodies with sufficient volume for further analysis were tested for immunoglobulin binding to the parent β2m KO HEK 293T cell expressing SLA-DQα1*0101, 55P Mutant SLA-DQβ1*0601 and CD74. Flow cytometry was performed as previously described (5).
2.10 Statistical Analysis of Antibody Binding
Flow cytometry files were analyzed in FlowJo V10 (FlowJo LLC, Ashland, GA). Antibody binding results were reported as median fluorescence intensity (MFI) (Figures 1, 4, and 5) or ratio of MFI compared to MFI of sera on parent cell (Figures 2 and 6). Cytotoxicity killing and SLA class II expression were determined by placing a fluorescence gate on the negative control. Graph and data analyses were completed using Prism 7 for Macintosh (GraphPad Software Inc., La Jolla, CA, USA). The resulting data did not approximate normal distributions even after logarithmic transformation. Therefore, the Wilcoxon and Mann-Whitney with correction by the Dunn’s multiple comparison tests were used to compare group means. SLA class II molecule structure predictions were created in SWISS-MODEL (University of Basel, Basel, Switzerland) and visualized in UCSF Chimera (University of California, San Francisco, CA).
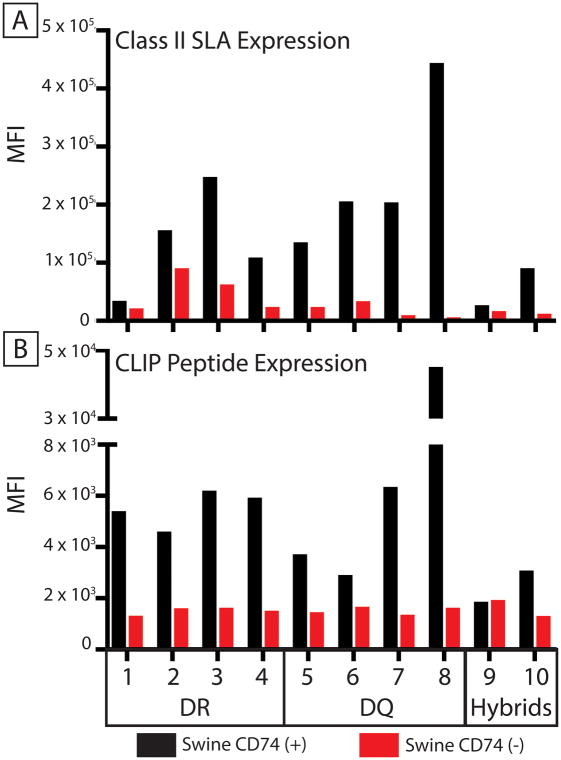
Figure 1. Pig CD74 increases cell surface expression of class II SLA proteins.
HEK 293T cells expressing 10 different class II SLA α and β heterodimers in the presence (black bars) or absence (red bars) of pig CD74 were tested for: (A) Binding to a monoclonal antibody, specific for class II SLA and (B) Binding of CerCLIP.1, a monoclonal antibody specific for invariant chain peptides associated with class II SLA. CD74 expression significantly increased antibody binding (p=0.002 for both assays, Wilcoxon matched-pairs sign rank test). Specific alleles making up each alpha-beta combination are indicated in table 2. DR indicates SLA-DRα/DRβ pairs, DQ represents SLA-DQα/DQβ pairs, and hybrid represent SLA-DRα/DQβ pairs.
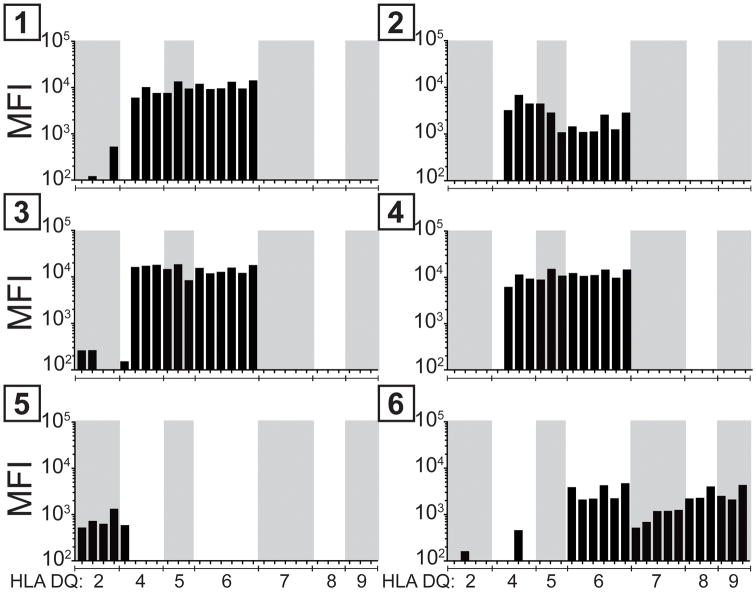
Figure 4. Examining cross-reactivity of human antibodies with class II SLA and class II HLA proteins.
6 sera containing class II HLA specific antibodies were incubated with a cell line expressing pig CD74, SLA-DQα1*0101, and -DQβ1*0601. IgG eluted from this cell line cross-reacted with beads containing HLA-DQ antigens but not HLA-DR or HLA-DP. The median fluorescence of antibody binding to beads containing the different HLA-DQ proteins are shown.
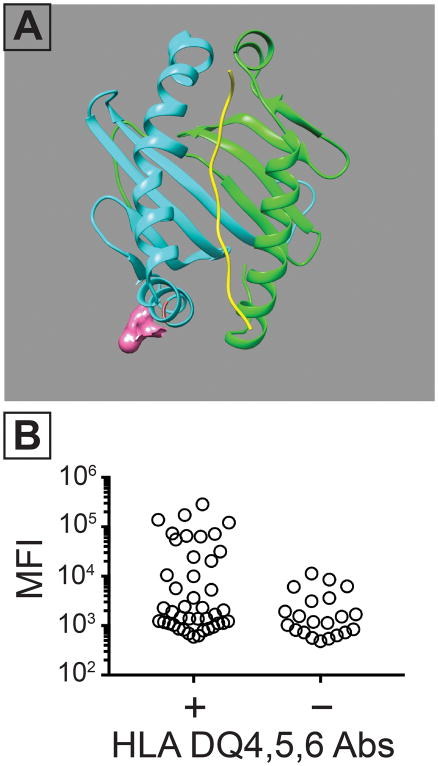
Figure 5. Amino acid 55 contributes to a class II SLA epitope which cross-reacts with human IgG.
Following the results in Figure 4, sequence alignments of HLA-DQ4,5,6, SLA- DQα1*0101/DQβ1*0601 were performed. These suggested that arginine at position 55 contributed to the cross-reactive epitope in DQβ and in the HLA beta chains in HLA-DQ4,5,6. (A) Structural prediction highlighting the putative location of the 55R residue in the SLA- DQα1*0101/DQβ1*0601 molecule. This residue was mutated to a proline (R55P) which is found in class II HLA molecules other than -DQ4,5,6 HLA molecules. (B) Human IgG binding to a cell line expressing CD74, SLA-DQα1*0101, and SLA-DQβ1*0601. All tested sera contained some class II HLA-binding IgG. The samples were split into two groups based on the presence (n = 42) or absence (n = 21) of HLA-DQ4,5,6 reactivity. The presence of antibodies against HLA-DQ4,5,6 increased binding to SLA-DQα1*0101, and DQβ1*0601(Mann-Whitney test, p= 0.030).
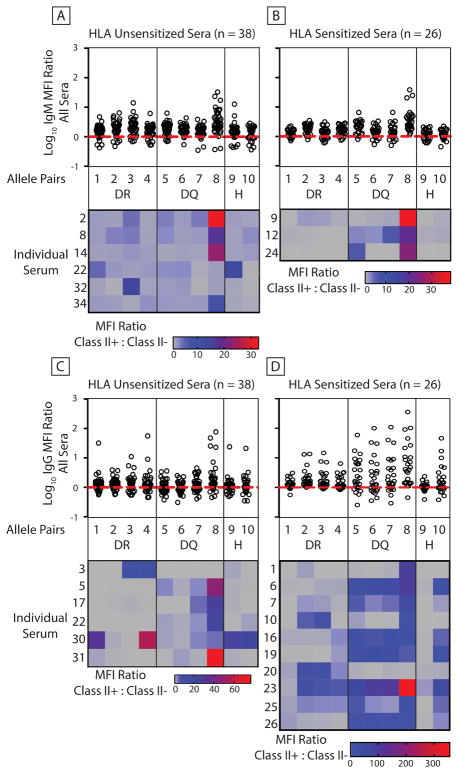
Figure 2. Evaluation of human antibody binding to class II SLA co-expressed with swine CD74.
Human sera were incubated with cells expressing pig CD74 and various class II SLA proteins or with parent cells lacking both class II SLA and CD74. 26 sensitized sera contained antibodies specific for class II HLA. 38 un-sensitized sera lacked anti-HLA antibodies. Fluorescent antibodies detected human immunoglobulin binding to cells (panel A, IgM binding-unsensitized sera; panel B, IgM binding-sensitized sera; panel C, IgG binding unsensitized sera; panel D, IgG binding-sensitized sera). Dot plots show the Log10 of MFI ratios comparing immunoglobulin binding to SLA positive cells versus SLA-deficient parent cells. Values greater than 0 indicate more binding to class II positive cells. Log10 values less than 0 represent greater immunoglobulin binding to the parent cell line. When antibodies bind equally to both cell types the Log10 = 0. Heatmaps below the dot plots show binding results of sera having at least a single Log10 ≥ 1. Each row represents an individual serum tested against every class II SLA heterodimer. Table 2 describes the class II allele-pair combinations. Gray squares represent class II (+) vs class II(−) MFI ratios ≤ 1. All other squares indicate increased binding to class II expressing cells. DR indicates SLA-DRα/DRβ pairs, DQ represents SLA-DQα/DQβ pairs, and H represent SLA-DRα/DQβ pairs.
Figure 6. Mutation of arginine 55 in SLA-DQ beta reduces class II SLA-based xenoantigenicty.
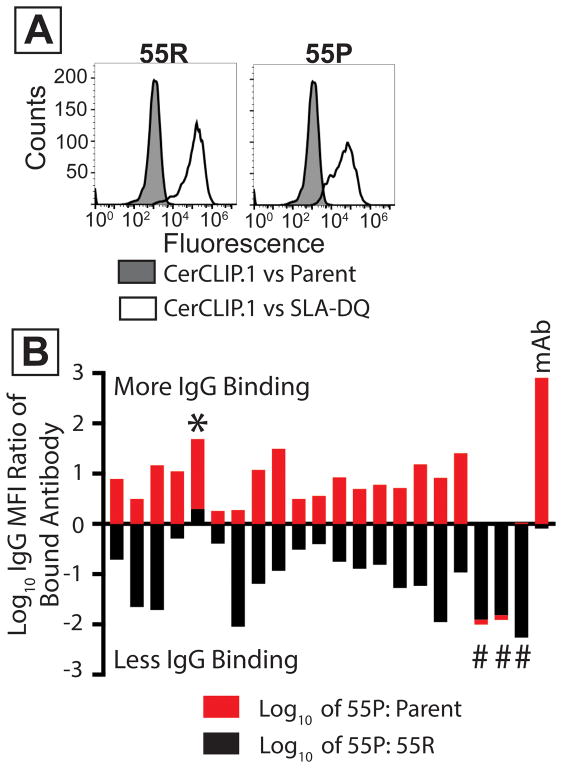
Cell lines expressed CD74, SLA-DQα1*0101, and mutant or unmodified SLA-DQβ1*0601. Mutant molecules contained a β chain having proline at position 55 (55P). Wild type class II contained arginine at position (55R). (A) White histograms show binding of the CerCLIP.1 antibody to cells expressing either 55R or 55P. Gray histograms show CerCLIP.1 binding to CD74/SLA-deficient HEK 293T cells. (B) 21 human sera were incubated with cells expressing either the 55R or 55P variants of SLA-DQ. These sera were selected from prior experiments suggesting they reacted with SLA-DQα1*0101/SLA-DQβ1*0601. Data from each sample is represented by one red and one black bar (stacked on top of each other). MFI ratios of fluorescent anti-human IgG were calculated to compare binding to 55P versus 55R molecules. The log10 values of these ratios were plotted for each serum (black bars). The same analysis compared IgG binding to the 55P cells versus SLA-negative parent cells (red bars). The 55P mutation partially reduced IgG binding to the SLA-DQ protein in 17 samples. Three samples had no detectable antibody staining of 55P mutants (#). One sample showed greater binding to the 55P mutant than to 55R (*). The mAB sample indicates the binding of a monoclonal antibody specific for SLA-DQ. This antibody recognized SLA-DQ 55P variants (red bar) almost identically to 55R variants (black bar, Log10 of 55P/55R = −0.09).
3. Results
3.1 Pig CD74 increases cell surface expression of class II SLA proteins
HEK293T cells were used to express pig transgenes. These cells do not transcribe class II HLA genes and were engineered to lack expression of class I HLA proteins. Consequently, these cells exhibit low background binding to human sera, including those which contain HLA-specific immunoglobulin(5). Transgenes encoding various combinations of class II SLA alpha and beta chains were inserted in the HEK cells. Table 1 contains the name, abbreviation, and accession number of each allele studied. Table 2 contains the specific alpha-beta alleles making up the various SLA molecules examined in every figure. Some cells were also transfected with a transgene encoding pig CD74 to examine class II SLA expression in its presence (Figure 1). The invariant chain increased cell-surface density of all pairs a class II SLA-α/β heterodimers (Figure 1A and Supplemental Figure 1).
In humans and mice, some cross-pairings of class II α proteins with class II β proteins from different gene loci can give rise to “hybrid” class II proteins(10, 11). In pigs, this could result in SLA-DRα paired with SLA-DQβ and SLA-DQα paired with SLA-DRβ. All combinations of SLA-DRα/DQβ and SLA-DQα/DRβ were expressed in the presence or absence of pig CD74. Two hybrid molecules (pairs 9 and 10, table 2), containing the same DQβ chain, accumulated on the cell surface (Figure 1A, Supplemental Figure 1 and Supplemental Figure 2). Their DRα chains had 99% amino acid identity. Other hybrids did not express at the cell surface even in the presence of CD74 (supplementary data 2).
As an independent measure of class II SLA expression, these cell lines were also stained with the monoclonal antibody CerCLIP.1. This antibody recognizes the “clip” fragment of human invariant chain that associates with the peptide binding groove of class II HLA molecules(12). In the absence of CD74, every class II positive cell line yielded a background median fluorescence intensity (MFI) of approximately 1,000 (Figure 1B). Co-expression of invariant chain and class II SLA increased CerCLIP.1 binding in all SLA molecules tested except for one hybrid (pair 9, Figure 1B). This data indicates that CerCLIP.1 cross-reacts with pig CD74 and can be used to probe for cell surface expression of class II SLA molecules.
3.2 Evaluation of human antibody reactivity towards class II SLA
Our previous work demonstrated that human IgG specific for class II HLA also cross-reacts with SLA-DR and -DQ(5). The role of the invariant chain was not evaluated. Here we extended these analyses to determine if CD74 affected the xenoantigenicity of class II SLA. We also tested greater numbers of human sera than in our previous report and included evaluations of IgM binding. In the presence of invariant chain, IgG from sera containing class II HLA antibodies frequently cross-reacted with class II SLA (Figure 2D, dot plots). In contrast to our prior results, sera lacking anti-HLA antibodies occasionally contained IgG capable of recognizing class II SLA (Figure 2C dot plots). Multiple samples also contained IgM capable of binding class II SLA molecules regardless of the presence of class II HLA antibodies (Figures 2A and 2B). Hybrid SLA-DRα and DQβ molecules did not yield obvious IgM binding, but they were recognized by IgG (Figures 2A and 2B). Heatmaps below the dot plots show individual sera reactivity towards each class II SLA molecule (Figures 2A, and 2B). Sera displayed in the heatmaps, were chosen based on robust interaction with at least one class II SLA protein (MFI ratio of class II SLA positive to class II SLA negative control cell ≥ 10). Some samples contained antibodies reactive with most class II SLA proteins, while others exhibited a more restricted pattern.
3.3 Class II SLA antibodies drive complement-mediated cytotoxicity
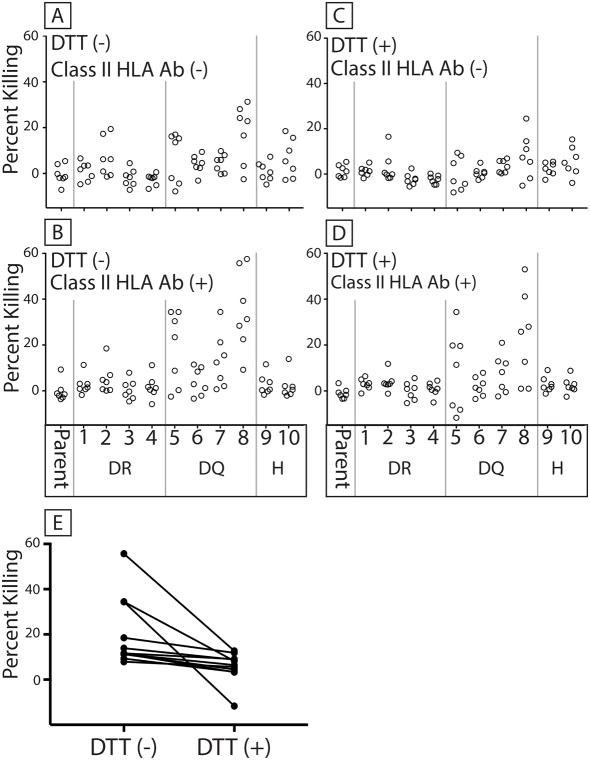
Human sera were incubated with the panel of cells expressing various class II SLA molecules and pig invariant chain (Figure 3). We examined the cytotoxic activity of human sera lacking (Figure 3A) or containing (Figure 3B) class II HLA-specific antibodies in the presence of rabbit complement. Residual lytic activity, despite DTT-mediated inactivation of IgM, showed that IgG specific for class II SLA drove cytotoxicity in multiple samples. (Figures 3C and 3D). IgM specific for class II SLA also killed cells as shown by the ability of DTT to reduce cell death (Figure 3E).
Figure 3. Human antibodies specific for class II SLA can initiate complement mediated cytotoxicity.
7 sera lacking class II HLA antibodies and 7 sera containing class II HLA antibodies were incubated with cells expressing pig CD74 and individual combinations of class II SLA alleles. Table 2 describes the class II allele combinations. Parent cells were HEK 293T cells devoid of CD74 and class II SLA. Rabbit complement was added and dead cells counted as an indicator of antibody initiated complement cytotoxicity (percent killing). (A) Cytotoxicity of sera lacking class II HLA antibodies. (B) Cytotoxicity of sera containing class II HLA antibodies. DTT was added to inactivate IgM in panels C (sera lacking class II HLA antibodies) and D (sera containing class II HLA antibodies). DR indicates SLA-DRα/DRβ pairs, DQ represents SLA-DQα/DQβ pairs, and H represent SLA-DRα/DQβ pairs. Parent represents killing against cells lacking both class II SLA proteins and CD74. Panel E shows the reduction of cell killing for a single serum against cells expressing all 10 class II SLA proteins before and after DTT treatment (±DTT comparison p = 0.001, Wilcoxon test).
3.4 Examining antibody cross-reactivity with class II HLA and SLA proteins
IgG specific for class II HLA proteins were tested for cross-reactivity with class II SLA. Six human sera were incubated with class II SLA positive Hek cells. Cells expressing pig CD74, SLA-DQα1*0101, and DQβ1*0601 were used because they contained abundant cell surface class II SLA protein. After washing, bound antibodies were eluted and used to probe beads containing individual class II HLA molecules (Figure 4). Four of the six sera contained cross-reactive antibodies primarily recognizing HLA-DQ4,5,6. The other sera recognized either HLA-DQ2,4 or HLA-DQ2,4,6,7,8,9. No cross-reactivity occurred between SLA-DQα1*0101/DQβ1*0601 molecules and HLA-DR or HLA-DP (not shown).
Comparing amino acid sequences of the cross-reactive molecules (SLA-DQα1*0101/DQβ1*0601, and HLA-DQ4,5,6) identified a common arginine at position 55 (55R) in the β chains from these particular HLA and SLA molecules. In addition, 55R contributes to an epitope found in HLA-DQ4,5,6(13), and is located at the surface of the β chain (Figure 5A). These observations form the hypothesis that 55R resides in a cross-reactive epitope found in HLA-DQ4,5,6 and class II SLA.
To test this idea, cells expressing pig CD74, SLA-DQα1*0101, and SLA-DQβ1*0601 were incubated with human sera containing antibodies specific for class II HLA. Samples with HLA-DQ4,5,6 reactivity exhibited greater binding to the SLA-DQ positive cells than did allosensitized sera lacking antibodies specific for HLA-DQ4,5,6 (Figure 5B). Next, we produced a mutant SLA-DQβ1*0601, having a proline at residue 55 (55P) instead of arginine. We reasoned this change would not impair biosynthetic competence of the SLA-DQ variant because several class II HLA-β chain alleles contain 55P(13). Compared to the non-mutant SLA-DQ 55R variants, the 55P molecules showed similar cell surface expression (Figure 6B) and binding of the invariant chain clip peptide (Figure 6A).
Lastly, 21 human sera were tested against cells expressing either the 55P or the 55R SLA-DQ variants (Figure 6B). Again, these sera contained HLA-DQ4,5,6 reactive antibodies. In a single sample, IgG binding increased on the 55P mutant compared to 55R (Figure 5B, *). In three samples, the 55P mutation reduced antibody interaction (Figure 5B, #; black bar values < 0) to background levels (Figure 5B, #; no red bar values > 0). For the remaining 17 sera, the 55P mutant also diminished immunoglobulin binding relative to the 55R variant (Figure 5B, black bar values <0). Antibody reactivity towards 55P was not completely eliminated in these samples as residual binding exceeded levels observed with SLA-deficient cells (Figure 5B, red bar values > 0).
4. Discussion
Initial pig-to-human transplantation experiments failed because preformed antibodies recognized glycan antigens on the donor tissue. Consequently, pigs have been engineered to express human complement regulatory proteins and lack expression of a single gene(14). This gene, GGTA1, produces an enzyme which transfers galactose to cell surface carbohydrates in an α1–3 linkage (αGal). Though pigs deficient in αGal expressed additional xenoantigens, the hope was that human complement regulators would inhibit antibody-mediated tissue damage. Inactivating other pig genes, CMAH and β4GalNT2, have further reduced xenoantigen expression when evaluated against most humans. The carbohydrates produced by the pig GGTA1/CMAH behave as antigens in humans because people lack these glycans. Their deficiency arises as a consequence of evolutionary inactivation of GGTA1/CMAH in people(15, 16). The reason for β4GalNT2 creating pig xenoantigens remains less clear because this gene remains functional in most humans(17).
In vitro analyses suggest that elimination of multiple xenoantigens may bypass antibody-mediated rejection in the absence of human complement regulatory transgenes(18). Our preliminary work using donor pigs with deficient GGTA1 and β4GalNT2 genes, and no human transgenes, supports this concept. A rhesus macaque has survived over 400 days with a life-supporting kidney from this double knockout pig [manuscript in preparation]. These results suggest that avoiding pig antigens, targeted by pre-existing human antibodies, may prevent donor-specific antibodies (DSA) from rejecting a xenograft.
This report shows that class II SLA molecules are xenoantigens. Human antibodies recognizing these proteins initiate complement-mediated cell lysis (Figure 3) and cross-react with shared epitopes found on HLA and SLA (Figures 4–6). Mutating an arginine in the SLA-DQ beta chain (55R) to proline (55P) eliminated SLA reactivity of three human sera. Cell surface expression of this mutant was minimally affected (Figure 6). This proof-of-principle shows that it is possible to eliminate MHC-driven xenoantigenicity without creating SLA-deficient animals. The 55P mutation reduced, but did not eliminate, IgG binding to the SLA-DQα1*0101/DQβ1*0601 protein in 17 of 21 human sera. This may result from incomplete disruption of a single antigenic determinant or from sera having IgG which recognize multiple epitopes. Further mutagenesis of the class II molecule could resolve this issue.
Careful histocompatibility analyses that match donors and recipients provide an engineering-independent method of bypassing DSA. The practice of looking for HLA-specific antibodies which may attack donor tissue has been key to the success of human allotransplantation. Recent data in pig to NHP xenotransplant models indicate careful cross-matching may improve xenotransplant outcomes(19). Rather than performing complex genome editing, it may be possible to avoid class II SLA xenoantigenicity by careful donor-recipient pairing. However, in the case of people who have IgG that recognize HLA-DQ4,5,6 this may not be possible. These alloantibodies frequently target 55R in the SLA-DQβ chain (Figure 6B), and most, if not all, SLA-DQβ contain 55R. We found 100 SLA-DQβ protein sequences in the NCBI protein database. Of these, 94 contained arginine at position 55(20). A second analysis showed that all 43 SLA-DQβ protein sequences in the IMGT database contained 55R(21). Therefore, switching animals to find a non-reactive SLA-DQ genotype for patients with antibodies recognizing the 55R epitope may be difficult.
Reagents created in this study have improved detection of humoral responses against class II SLA. As with other species the invariant chain improves cell surface expression of pig class II SLA proteins. These tools permitted the identification of amino acids having partial or major contributions to a xenoreactive epitope. Despite identifying SLA-DR, -DQ, and hybrid (DRα/DQβ) molecules as targets of xenoreactive human immunoglobulin, further refinements will be valuable. Though powerful, expressing various combinations of alpha and beta chains generates different molecules with variable levels of cell surface expression. This may skew which SLA appear to be the target of human antibodies towards highly expressed molecules. As an example, allele pair 8 (Table 2) yields abundant SLA-DQ at the cell surface (Figure 1). Though we detected antibodies against every allele combination, pair 8 generated the most frequent and robust positive responses (Figures 2 and 3). Elevating the expression of the other class II SLA molecules may be needed to define their true role as xenoantigens.
As our understanding of pig antigens matures, issues of humoral xenoantigenicity appear increasingly similar to those facing alloantigenicity. Carbohydrates can be targets of DSA with α-Gal, Neu5Gc, and the β4GalNT2-derived glycans forming pig antigens(3, 4). Carbohydrates of the ABO blood group comprise antigens on human tissue(22). DSA against carbohydrate-based xeno- and allo-antigens exist before exposure to the donor tissue(23). In addition, pig and human MHC synthesize protein antigens which can be targets of DSA(4, 5, 24). Generating HLA-specific antibodies typically requires exposure to foreign human tissue through transfusions, pregnancy, or organ transplantation. This report suggests that while HLA/SLA cross-reactivity may increase by allosensitization, human IgG and IgM can also arise in the absence of HLA-specific antibodies (Figures 2 and 3). Perhaps SLA antibodies generated in the absence of HLA reactivity reflect exposure to MHC-derived proteins from other species either through the food supply or vaccination. The cross-reactivity of HLA/SLA suggests that receipt of a xenograft will result in generation of SLA-specific antibodies that also bind HLA. This could reduce the opportunity of a patient to benefit from future allografts. This concern may be minimized in the first xenotransplant trials which will likely include individuals who already have high levels of HLA antibodies which prevent them from receiving allotransplants.
Supplementary Material
Acknowledgments
Grant Support:
This publication was made possible in part by a grant from United Therapeutics Corp.
Abbreviations
- B4GALNT2
β-1,4-N-acetyl-galactosaminyltransferase 2
- CDC
complement dependent cytotoxicity
- CMAH
cytidine monophosphate-N-acetylneuraminic acid hydroxylase
- cPRA
calculated panel reactive antibody
- DSA
donor-specific antibodies
- HEK
human embryonic kidney
- GGTA1
α1, 3-galactosyltransferase
- HLA
human leukocyte antigen
- KO
knockout
- MHC
major histocompatibility complex
- NHP
non-human primate
- PBMC
peripheral blood mononuclear cells
- SLA
swine leukocyte antigen
Footnotes
Authorship:
A.J.T. is the founder of Xenobridge LLC, has United Therapeutics funding and applied for patents.
The other authors declare no conflicts of interest.
This manuscript has been revised and approved by all authors. J.M.L., D.E.E., M.T., and A.J.T. drafted this article and developed and refined study concepts. L.M.R. and J.M.L. performed SLA sequencing and gene target analysis. J.M.L. G.R.M., and Z.Y.W, performed data collection, sample selection, cell immortalization and data analysis.
References
- 1.Platt JL, Lin SS, McGregor CG. Acute vascular rejection. Xenotransplantation. 1998;5:169–175. doi: 10.1111/j.1399-3089.1998.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 2.Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, NAJARIAN JS, Bach FH. Transplantation of discordant xenografts: a review of progress. Immunology Today. 1990;11:450–6. doi: 10.1016/0167-5699(90)90174-8. discussion 456–7. [DOI] [PubMed] [Google Scholar]
- 3.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, Butler JR, Sidner R, Tector M, Tector J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martens GR, Reyes LM, Butler JR, Ladowski JM, Estrada JL, Sidner RA, Eckhoff DE, Tector M, Tector AJ. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation. 2017;101:e86–e92. doi: 10.1097/TP.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladowski JM, Reyes LM, Martens GR, Butler JR, Wang ZY, Eckhoff DE, Tector M, Tector AJ. Swine Leukocyte Antigen (SLA) Class II is a Xenoantigen. Transplantation. 2017:1. doi: 10.1097/TP.0000000000001924. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunney JK, Ho CS, Wysocki M, Smith DM. Molecular genetics of the swine major histocompatibility complex, the SLA complex. Developmental & Comparative Immunology. 2009;33:362–374. doi: 10.1016/j.dci.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Reyes LM, Blosser RJ, Smith RF, Miner AC, Paris LL, Blankenship RL, Tector MF, Tector AJ. Characterization of swine leucocyte antigen alleles in a crossbred pig to be used in xenotransplant studies. Tissue Antigens. 2014;84:484–488. doi: 10.1111/tan.12430. [DOI] [PubMed] [Google Scholar]
- 9.Díaz TM, Pértega S, Ortega D, López E, Centeno A, Mañez R, Doménech N. FDA/PI flow cytometry assay of complement-mediated cytotoxicity of antibodies generated during xenotransplantation. Cytometry A. 2004;62:54–60. doi: 10.1002/cyto.a.20076. [DOI] [PubMed] [Google Scholar]
- 10.Germain RN, Quill H. Unexpected expression of a unique mixed-isotype class II MHC molecule by transfected L-cells. Nature. 1986;320:72–75. doi: 10.1038/320072a0. [DOI] [PubMed] [Google Scholar]
- 11.Lotteau V, Teyton L, Burroughs D, Charron D. A novel HLA class II molecule (DR alpha-DQ beta) created by mismatched isotype pairing. Nature. 1987;329:339–341. doi: 10.1038/329339a0. [DOI] [PubMed] [Google Scholar]
- 12.Denzin LK, Robbins NF, Carboy-Newcomb C, Cresswell P. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994;1:595–606. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 13.El-Awar N, Nguyen A, Almeshari K, Alawami M, Alzayer F, Alharbi M, Sasaki N, Terasaki PI. HLA class II DQA and DQB epitopes: Recognition of the likely binding sites of HLA-DQ alloantibodies eluted from recombinant HLA-DQ single antigen cell lines. Human Immunology. 2013;74:1141–1152. doi: 10.1016/j.humimm.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 14.McGregor CGA, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, Tazelaar HD, Byrne GW. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. PNAS. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galili U. The α-gal epitope (Galα1–3Galβ1–4GlcNAc-R) in xenotransplantation. Biochimie. 2001;83:557–563. doi: 10.1016/s0300-9084(01)01294-9. [DOI] [PubMed] [Google Scholar]
- 17.Byrne GW, Du Z, Kogelberg H, McGregor C. Porcine B4GALNT2 a Source of New Xenogenic Glycan. The Journal of Heart and Lung Transplantation. 2015;34:S150–S151. [Google Scholar]
- 18.Butler JR, Martens GR, Estrada JL, Reyes LM, Ladowski JM, Galli C, Perota A, Cunningham CM, Tector M, Joseph Tector A. Silencing porcine genes significantly reduces human-anti-pig cytotoxicity profiles: an alternative to direct complement regulation. Transgenic Res. 2016;25:751–759. doi: 10.1007/s11248-016-9958-0. [DOI] [PubMed] [Google Scholar]
- 19.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, Larsen CP, Ford ML, Lutz AJ, Tector M, Newell KA, Tector AJ, Adams AB. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D, Astashyn A, Badretdin A, Bao Y, Blinkova O, Brover V, Chetvernin V, Choi J, Cox E, Ermolaeva O, Farrell CM, Goldfarb T, Gupta T, Haft D, Hatcher E, Hlavina W, Joardar VS, Kodali VK, Li W, Maglott D, Masterson P, McGarvey KM, Murphy MR, O’Neill K, Pujar S, Rangwala SH, Rausch D, Riddick LD, Schoch C, Shkeda A, Storz SS, Sun H, Thibaud-Nissen F, Tolstoy I, Tully RE, Vatsan AR, Wallin C, Webb D, Wu W, Landrum MJ, Kimchi A, Tatusova T, DiCuccio M, Kitts P, Murphy TD, Pruitt KD. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–45. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccari G, Robinson J, Ballingall K, Guethlein LA, Grimholt U, Kaufman J, Ho CS, de Groot NG, Flicek P, Bontrop RE, Hammond JA, Marsh SGE. IPD-MHC 2.0: an improved inter-species database for the study of the major histocompatibility complex. Nucleic Acids Res. 2017;45:D860–D864. doi: 10.1093/nar/gkw1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stussi G, West L, Cooper DKC, Seebach JD. ABO-incompatible allotransplantation as a basis for clinical xenotransplantation. Vol. 13. Blackwell Publishing Ltd; 2006. pp. 390–399. [DOI] [PubMed] [Google Scholar]
- 23.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 24.Peterson M, Miller J. Invariant chain influences the immunological recognition of MHC class II molecules. Nature. 1990;345:172–174. doi: 10.1038/345172a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.