Abstract
Purpose
Stereotactic body radiotherapy (SBRT) may stimulate innate and adaptive immunity to augment immunotherapy response. Multisite SBRT is an emerging paradigm for treating metastatic disease. Anti-PD-1–treatment outcomes may be improved with lower disease burden. In this context, we conducted a phase I study to evaluate the safety of pembrolizumab with multisite SBRT in patients with metastatic solid tumors.
Patients and Methods
Patients progressing on standard treatment received SBRT to two to four metastases. Not all metastases were targeted, and metastases > 65 mL were partially irradiated. SBRT dosing varied by site and ranged from 30 to 50 Gy in three to five fractions with predefined dose de-escalation if excess dose-limiting toxicities were observed. Pembrolizumab was initiated within 7 days after completion of SBRT. Pre- and post-SBRT biopsy specimens were analyzed in a subset of patients to quantify interferon-γ–induced gene expression.
Results
A total of 79 patients were enrolled; three patients did not receive any treatment and three patients only received SBRT. Patients included in the analysis were treated with SBRT and at least one cycle of pembrolizumab. Most (94.5%) of patients received SBRT to two metastases. Median follow-up for toxicity was 5.5 months (interquartile range, 3.3 to 8.1 months). Six patients experienced dose-limiting toxicities with no radiation dose reductions. In the 68 patients with imaging follow-up, the overall objective response rate was 13.2%. Median overall survival was 9.6 months (95% CI, 6.5 months to undetermined) and median progression-free survival was 3.1 months (95% CI, 2.9 to 3.4 months). Expression of interferon-γ–associated genes from post–SBRT tumor biopsy specimens significantly correlated with nonirradiated tumor response.
Conclusion
Multisite SBRT followed by pembrolizumab was well tolerated with acceptable toxicity. Additional studies exploring the clinical benefit and predictive biomarkers of combined multisite SBRT and PD-1–directed immunotherapy are warranted.
INTRODUCTION
Immunotherapy has changed treatment paradigms in oncology, particularly with the addition of PD-1 inhibition to the armamentarium. However, despite the optimism surrounding treatment with immune-checkpoint blockade, most patients do not respond.1 Predictive biomarkers, such as programmed death-ligand 1 (PD-L1) expression, tumor-infiltrating lymphocytes, and interferon (IFN)-γ–induced gene expression are under investigation.2-4
An approach to expand the benefit of PD-1 immunotherapy may involve combinations with treatments that induce IFN-associated T-cell inflammation, such as ionizing radiation. Modeling of high-dose ablative radiation in murine systems suggests activation of innate immune signaling pathways, potentially leading to adaptive immune responses within tumors in the radiation field as well as in distant tumors.3,4 Mechanisms facilitating the augmentation of immunotherapy by radiation include increased tumor antigen release, activation of innate immune pathways, increased T-cell infiltration, augmented antigen presentation, and modulation of immunosuppressive cells.5,6
SBRT precisely delivers high radiation doses in a limited number of treatments to a target. This approach is effective in early-stage non–small-cell lung cancer and is deployed for localized pancreatic and prostate cancer, as well as for metastatic disease, with control rates between 70% and 90%.7 This finding led to multiple, ongoing randomized trials in patients with limited metastases to determine if long-term survival is achievable after SBRT and systemic therapy.8
Increased tumor burden correlates with decreased efficacy of PD-1 immunotherapy.9 Tumor debulking by SBRT may enhance immunotherapy response. Moreover, preclinical observations demonstrate tumor infiltration of T cells after radiation, suggesting the potential for synergy with immunotherapies.10-12
Although there is potential for overlapping toxicities, radiation is commonly used with immunotherapy, even with a paucity of prospective data. To our knowledge, there are no reports evaluating the tolerability of SBRT to multiple tumors within the same patient when combined with PD-1 blockade. Herein, we present our phase I study of pembrolizumab immunotherapy with multisite SBRT delivered to at least two distinct metastases in patients with advanced solid tumors.
PATIENTS AND METHODS
Study Design
The primary objective was to determine the recommended SBRT dose to anatomic locations before pembrolizumab. Secondary objectives included grade 3 or higher adverse events, response rate (RR), irradiated tumor control, progression-free survival (PFS), overall survival (OS), and immune score gene-expression analysis. A cohort with reduced radiation dose was planned depending on rates of dose-limiting toxicity (DLT). Initially, six evaluable patients were to be enrolled per cohort and analyzed for ≥ 6 months of follow-up, with patients who dropped out of the study replaced to ensure at least six evaluable patients per cohort. However, the typical PFS time was 3 months and data arose during the trial that demonstrated most toxicity associated with PD-1 inhibition occurred within 90 days.13 We amended the primary end point (ie, toxicity) of the study from 6 months follow-up to 3 months. The study and amendments were approved by the University of Chicago institutional review board. This trial is registered on www.clinicaltrials.gov (ClinicalTrials.gov identifier: NCT02608385).
Toxicity data were collected using Common Terminology Criteria for Adverse Events, version 4.0, with assessment to determine attribution. DLT was defined as treatment-related grade 3 or higher toxicity (excluding asymptomatic biochemical abnormalities) within 3 months, starting from the first day of radiotherapy. Toxicity attribution was reviewed weekly.
Patients were placed in a single cohort according to location of irradiated metastases. The seven anatomic cohorts were peripheral lung, central lung, mediastinum/thoracic, liver, spinal, osseous, and abdominal/pelvic, as used in ongoing studies.14 Larger size and morbidity of the metastasis were prioritized when determining which irradiated metastasis would dictate cohort assignment.
Patients
Patients with Eastern Cooperative Oncology Group performance status ≤ 1 were eligible for enrollment if they were ≥ 18 years old with metastatic solid tumor previously treated with standard-of-care therapy. Measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, was required with at least two tumors ranging from 0.25 mL to 65 mL that were amenable to SBRT. Metastatic tumors > 65 mL were partially treated with radiation. A prospectively followed nonirradiated target metastasis was not required.
Patients were required to have adequate organ function, including absolute neutrophil count ≥1,500/μL, serum creatinine level ≤ 1.5 times the upper limit of normal (ULN), AST and ALT ≤ 2.5 times ULN (or ≤ 5 times ULN for patients with liver metastases), and albumin level ≥ 3.5 mg/dL. Notable exclusions included uncontrolled CNS metastases and history of noninfectious pneumonitis requiring steroids. Prior radiation therapy was permitted if the metastases to be targeted received minimal radiation (ie, < 10% of prior prescription dose). Patients previously treated with immunotherapies, including anti-PD-1, were eligible.
Radiation Technique
Patients were treated with multisite SBRT, as previously defined.14 For multisite SBRT, consensus has been established to limit the target metastasis size to 5 cm (ie, a 65-mL sphere) and the number of metastases targeted in a patient to four.14 Doses to organs at risk were stipulated in the protocol and two to four metastases were targeted. Owing to limitations of SBRT planning, not all metastases were targeted. When selecting metastases to be irradiated, symptomatic or clinically relevant metastases were prioritized. For tumors > 65 mL, a target volume was created within the gross tumor volume to limit the treated tumor to < 65 mL. SBRT dose varied by anatomic site: 45 Gy in three fractions for peripheral lung, liver, and abdominal/pelvic; 50 Gy in five fractions for central lung and mediastinal/cervical; 30 Gy in three fractions for osseous and spinal/paraspinal). If excess toxicity was observed, a reduced dose was specified.14 Treatment using photons with linear accelerators was delivered once daily on an every-other-day basis within 14 days.
Study Medication
Pembrolizumab 200 mg intravenously every 3 weeks was initiated within 7 days after the final SBRT fraction. Treatment continued until clinical or radiographic disease progression, dose-limiting toxicity, withdrawal from study, or death. Treatment beyond progression was allowed. No additional anticancer therapeutics were permitted during enrollment.
Follow-Up
Evaluation of study end points began from the start date of SBRT. Patients were evaluated clinically during radiotherapy and with each cycle of pembrolizumab. Computed tomography scans were scheduled after every fourth cycle of pembrolizumab, or earlier if clinically indicated. RECIST 1.1 was used to assess overall response. Irradiated, partially irradiated, and nonirradiated metastasis response was calculated on a per-patient basis, using RECIST techniques, by aggregating the largest axial diameter of the irradiated metastases and nonirradiated metastases separately. Tumor control was defined as any response other than progressive disease.
Gene-Expression Analysis
Pre–SBRT and post–SBRT tumor biopsy specimens from the same metastasis were collected within 7 days before or after SBRT. Total RNA was extracted using previously published techniques.4,15 At the time of study conception, we reviewed the literature for candidate genes that were consistently upregulated in tumors responding to immunotherapies. Treatment with ipilimumab16 was reported to increase expression of granzyme K (GZMK), lymphocyte-specific protein tyrosine kinase (LCK), toll-like receptor 8 (TLR8), and G-protein-coupled receptor 171 (GPR171). These were selected for analysis in our patient cohort. Gene expression values for GMZK, LCK, TLR8, and GPR171 were normalized to the geometric mean of β-actin and 18S ribosomal RNA. An immune score was defined as the median expression value of the normalized mRNA values for the four genes. Gene-expression measurements were correlated to treatment response for eight patients with pre– and post–SBRT biopsy tissue and at least one imaging follow-up.
Statistical Analysis
For each of the assigned seven anatomic cohorts, DLTs were assessed using continued toxicity assessment. Newly enrolled patients received radiation to their respective sites at the dose level deemed safe at the time of enrollment. If the cumulative DLT rate at 3 months was ≥ 33% in six evaluable patients (ie, at least two of six) assigned to a particular cohort, the radiation dose would be reduced. Exact logistic regression analyses were planned to model the probability of DLTs; because no dose reductions occurred, however, these were not performed.
Descriptive statistics were used to summarize patient characteristics. Investigator-determined grade 3 or higher toxicity deemed possibly, probably, or definitely related to treatment was summarized.
Separate summaries of grade 2+ treatment-related toxicity and all adverse events by Common Terminology Criteria for Adverse Events category were tabulated. A paired t test was used to compare mean percent tumor-burden changes between irradiated and nonirradiated tumors within the same patient. The distributions of the percent changes and the within-patient differences were examined using histograms and normal quantile plots, and no strong departure from normality was observed. McNemar test was performed to compare control of irradiated versus nonirradiated metastases. PFS and OS curves were estimated using the Kaplan-Meier method. OS was calculated from date of first fraction of SBRT to date last known alive or death. PFS was calculated from date of first fraction of SBRT to date of progressive disease, death, or censored at last clinical follow-up. Preplanned assessment was performed on four genes (TLR8, GPR171, LCK, and GZMK). The association of gene expression with treatment response was assessed using the Spearman correlation coefficient. JMP software, version 9.0 (SAS Institute, Cary, NC) and Stata, version 14.0 (StataCorp, College Stations, TX) were used for statistical analysis.
RESULTS
Patient Characteristics
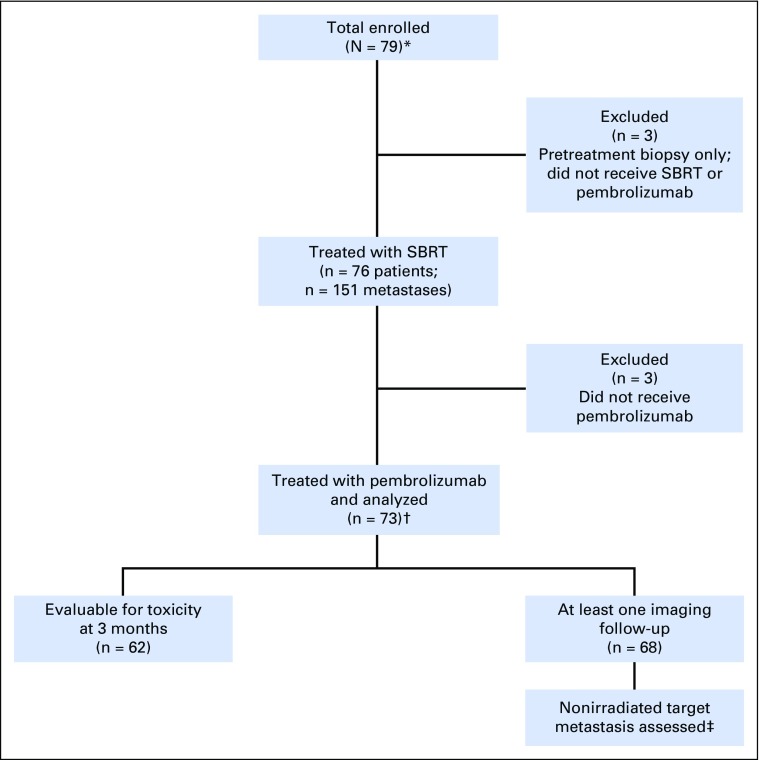
Seventy-nine patients were enrolled between January 6, 2016, and March 3, 2017 (Fig 1). Three patients underwent pretreatment biopsies but declined clinically owing to disease and were not treated with SBRT or pembrolizumab. Three other patients were treated with SBRT alone (these patients experienced no treatment-related toxicity); therefore, they were excluded from this analysis.
Fig 1.
Protocol enrollment and analysis diagram. (*) A total of 12 patients had paired (both pre- and postradiation) biopsy samples, six patients had preradiation biopsy samples, and six patients had postradiation biopsy samples. Of the patients with paired samples, eight had adequate follow-up to be included in this analysis. (†) Five patients had prior PD-1–axis blockade. (‡) A total of 52 patients had at least one measurable RECIST target metastasis that was not irradiated. SBRT, stereotactic body radiotherapy.
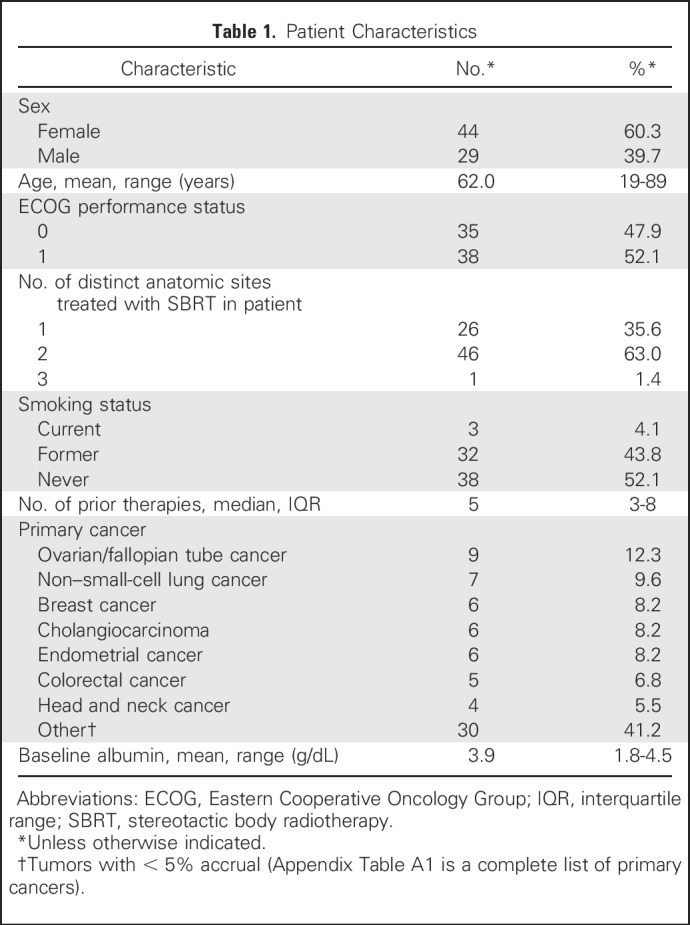
Baseline characteristics of the 73 patients included are outlined in Table 1. Patients had a median number of five prior therapies (interquartile range [IQR], three to eight therapies). All patients were diagnosed with a stage IV metastatic solid tumor (Appendix Table A1 lists primary tumors).
Table 1.
Patient Characteristics
Treatment
Nearly all patients (n = 69 of 73; 94.5%) had two metastases treated with SBRT, three of 73 (4.1%) had three metastases treated, and one of 73 (1.3%) had four metastases treated. Twenty-six (35.6%) patients had irradiated metastases confined to one anatomic location and 47 patients (64.4%) had irradiated metastases in two or more locations. Overall, there were 151 total metastases treated with SBRT (n = 30 peripheral lung, n = 23 central lung, n = 15 mediastinum/thoracic, n = 24 liver, n = 15 spinal, n = 16 osseous, and n = 28 abdominal/pelvic). Within 7 days of finishing radiotherapy, all 73 patients received at least one cycle of intravenous pembrolizumab (median, four cycles; IQR, two to seven cycles).
Toxicity
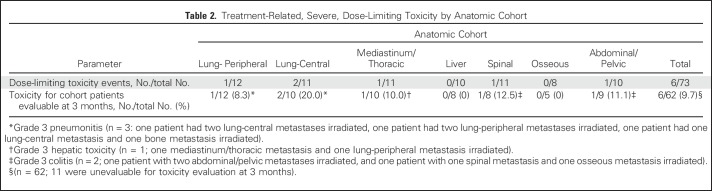
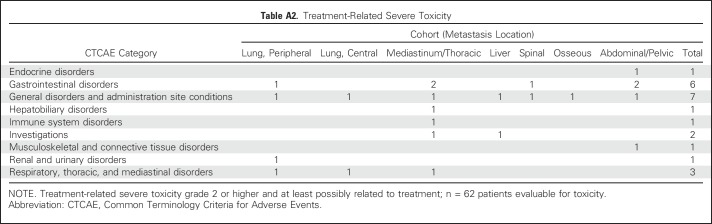
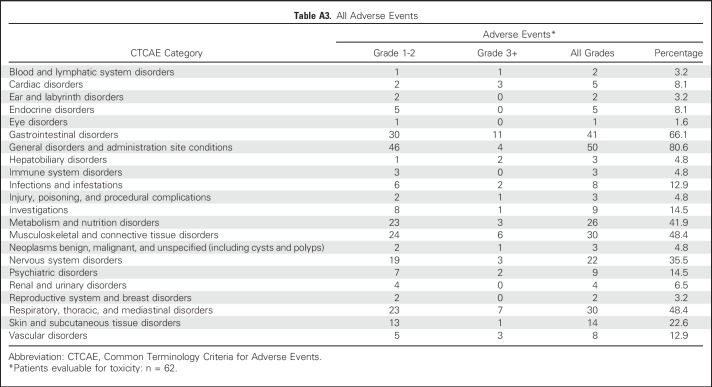
The primary end point of treatment-related DLT on the basis of anatomic cohort assignment is depicted in Table 2. Median follow-up for treatment-related toxicity was 5.5 months (IQR, 3.3 to 8.1 months) for all patients and 7.1 months (IQR, 4.8 to 10.2 months) for living patients. Overall, there were no radiation-dose reductions, but six patients experienced severe treatment-related toxicity (n = 3 grade 3 pneumonitis, n = 2 grade 3 colitis, and n = 1 grade 3 hepatic toxicity). Sixty-two patients had at least 3 months of follow-up, corresponding to a DLT rate of 9.7%. All patients with a DLT had two metastases treated with SBRT. All planning constraints (including < 15% normal lung volume receiving 20 Gy) were met. Three patients had both metastases in the same anatomic location, and three patients had metastases in separate anatomic locations. Grade 2 and higher treatment-related toxicity by cohort and all adverse event data for the 62 patients with at least 3 months of follow-up are included in the Appendix Tables A2 and A3 (online only).
Table 2.
Treatment-Related, Severe, Dose-Limiting Toxicity by Anatomic Cohort
Treatment Response
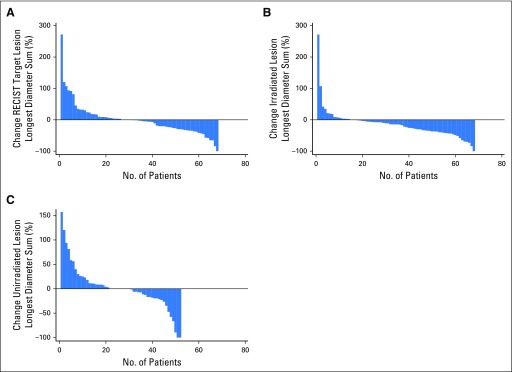
To evaluate secondary efficacy end points, at least one imaging follow-up was available for each of 68 patients. The best RECIST responses were one complete response, eight partial responses, 21 patients with stable disease, and 38 with progressive disease, yielding an objective RR of 13.2% (nine of 68 patients). Waterfall plots for best overall RR using aggregate tumor diameter for RECIST target metastases, irradiated metastases, and nonirradiated RECIST target metastases are depicted in Figure 2. Out-of-field response using aggregate diameter of nonirradiated RECIST target metastases was 13.5% (seven of 52); however, using response defined by 30% reduction in any single, nonirradiated RECIST target metastasis, the nonirradiated RR was 26.9%.
Fig 2.
Best overall response waterfall plots. (A) Maximum percent change in the aggregate diameter of Response Evaluation Criteria in Solid Tumors (RECIST) target metastases. (B) Maximum percent change in aggregate diameter of irradiated metastases. (C) Maximum percent change in aggregate diameter of unirradiated RECIST target metastases.
The mean percent change in tumor diameter was −21.7% (standard deviation, 24.3%) for irradiated metastases versus 1.7% (standard deviation. 46.3%) for nonirradiated metastases (P = .0008). Tumor control (lack of progression at last follow-up) was compared between irradiated and nonirradiated metastases for the 52 patients with paired data. In 36 patients, neither the irradiated nor the nonirradiated metastases progressed; in one, the irradiated metastases progressed but the nonirradiated did not; in 15 patients, the nonirradiated metastases progressed but the irradiated did not; and in none did both progress (P = .0005).
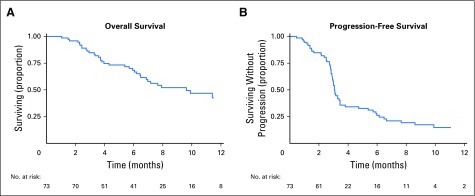
Seventeen (25%) of the 68 patients with imaging follow-up had at least one metastasis measuring > 65 mL that was partially treated with SBRT. Median, initial, single-metastasis gross tumor volume for partial tumor irradiation was 116.6 mL (IQR, 90.7 to 219.7 mL) versus 7.2 mL (IQR, 2.6 to 14.8 mL) for the metastases treated with complete tumor irradiation (P = .0001). Comparison of patients with at least one tumor partially irradiated with patients whose tumors were completely irradiated showed no statistically significant difference in control at 3 months (88% for the partially irradiated patients v 95% for the completely irradiated group; P = .108). Median OS was 9.6 months (95% CI, 6.5 months to undetermined) and median PFS was 3.1 months (95% CI, 2.9 to 3.4 months), as shown in Figure 3.
Fig 3.
Kaplan-Meier curves of (A) overall and (B) progression-free survival.
Immunologic Change Correlation with Treatment Response
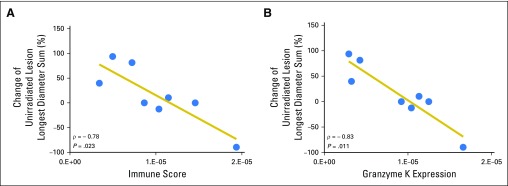
To assess whether SBRT might result in favorable immunologic changes in the tumor microenvironment, expression of four preselected IFN-γ–associated genes was analyzed in post irradiation biopsy specimens. Increased gene expression was significantly correlated with responses in nonirradiated tumors (P = .023; Fig 4). Elevated immune scores were primarily driven by GZMK overexpression (P = .011; Fig 4B) and predicted greater decreases in the nonirradiated lesion size. By contrast, no association was observed of preirradiation immune gene expression with irradiated or nonirradiated tumor responses. In addition, SBRT did not consistently increase IFN-γ–associated gene expression across patients.
Fig 4.
Posttreatment biopsy-specimen immune score correlation with unirradiated metastasis response. (A) Spearman rank analysis of poststereotactic body radiotherapy–irradiated tumor gene expression demonstrated significant correlations between the four-gene interferon-γ–related immune score and percent change in the nonirradiated Response Evaluation Criteria in Solid Tumors target metastasis size in relation to baseline measurements. (B) Of the four genes, granzyme K expression in the irradiated metastasis demonstrated the strongest correlation with unirradiated metastasis response. Each dot represents an individual patient sample.
DISCUSSION
In this study of multisite SBRT followed by pembrolizumab, similar rates of toxicity to SBRT or pembrolizumab monotherapy occurred, with high control rates of irradiated metastases and responses in nonirradiated metastases.7 The paradigm was well tolerated across anatomic sites with overall DLT < 10%. This rate is similar to the post hoc analysis of KEYNOTE-001 that showed 13% treatment-related toxicity in patients who previously received radiotherapy.17 Toxicity was generally low in our study; however, when toxicity was observed, it appeared to be in the region that was irradiated. This made it difficult to distinguish between toxicity of combination therapy versus radiation alone. A retrospective review of patients treated with palliative radiotherapy (RT) and anti–PD-1 showed a 4% grade 3 or higher immune-related adverse event rate, and toxicity was not related to anatomic location of RT.18 Clear distinctions of our study are the prospective design, higher radiation doses, and the consistent timing of radiation followed by PD-1 therapy. Our trial also used strict, normal, tissue-radiation planning parameters adopted from ongoing National Cancer Institute–sponsored trials to inform future analysis comparing our results with those of multisite SBRT alone.14
Our RECIST-based RR was 13.2% in a population of heavily pretreated patients. This population was unselected for PD-L1 expression, consisted of 27 cancer types, and was enriched in histologies not associated with significant RR to pembrolizumab. Our RR is similar to that of the multitumor basket study KEYNOTE-028 (range, 11% to 13% RR)19 in which patients were selected for high PD-L1 expression. We found no consistent clinical characteristics that were associated with treatment response. These results build on initial studies that incorporated SBRT and interleukin-2–based immunotherapy.20 Our findings support clinical and animal models demonstrating synergistic antitumor activity of RT and immunotherapy.21-23 To our knowledge, this is the most robust prospective evaluation to demonstrate safety of SBRT combined with PD-1 immunotherapy and the first report of multisite SBRT combined with immunotherapy.
We hypothesized that radiation may augment PD-1 immunotherapy and we observed marked control of tumors. No significant changes from pre– to post–SBRT tumor biopsy specimens were seen in our predefined set of IFN-γ–associated genes. We noted, however, a significant correlation between the post–SBRT expression of the four-gene IFN-γ–related immune score and distant metastatic responses. We acknowledge our sample size was limited, the timing of a single biopsy procedure within 7 days after radiation was arbitrary, and only a limited set of genes has been analyzed. Nonetheless, our results indicate that additional studies characterizing the immune-activating effects of SBRT predictive of immunotherapy response in clinical metastases are warranted.
The effects of SBRT and PD-1 blockade may be away from the radiation target (ie, abscopal) or close to the radiation target (ie, adscopal). Despite increased interest in the abscopal effect, no consensus definition exists.24 Formenti24 and others have defined an abscopal response as a reduction in the size of one nonirradiated metastasis by ≥ 30%.25 We assessed abscopal response by aggregating the sum of the largest diameter for all of the nonirradiated RECIST target metastases (≥ 30% reduction indicating response), constituting a RR of 13%. When assessing a single nonirradiated RECIST target metastasis response ≥ 30%, the abscopal RR was 27%, similar to combined RT and granulocyte macrophage colony-stimulating factor.25 These results are consistent with preclinical models of the abscopal response and support additional investigation of SBRT to improve checkpoint blockade.5,6
Although some have combined nonablative RT doses with immunotherapy,26 we used ablative SBRT doses developed through a US National Cancer Institute consensus working group.14 We anticipated an enhanced immune response with dose-escalated radiation.27,28 Also, there may be improved clinical outcomes to RT29 and immunotherapy30 with lower tumor burden. Although ongoing SBRT trials have limited patient enrollment to tumors with a 5-cm maximum diameter (ie, 65-mL sphere),14 we chose to partially irradiate larger tumors. We speculate that delivering SBRT to portions of tumors > 65 mL could provide cytoreduction while inducing effector T-cell trafficking throughout the remaining unirradiated portion of the lesion to enhance the local effects of PD-1 blockade. An exploratory subset analysis of partially irradiated tumors showed similar tumor control compared with total tumor irradiation, suggesting an adscopal immune effect of nontarget radiotherapy and pembrolizumab. Another consideration leading to this local control finding is that the low dose of radiation received to the nontargeted portion of the tumor may have been sufficient to elicit an immune response.
We acknowledge that our study has limitations. We treated a heterogeneous patient population with a variety of primary cancers not screened for PD-L1 status. Moreover, patients were exposed to a high number of prior therapies and some patients died before the 3-month threshold for toxicity end point. Because there was no comparison of different SBRT doses, our study does not determine the radiation dose required to stimulate an optimal immune response.
In conclusion, the treatment paradigm was well tolerated, with clinical activity supporting a complementary role of SBRT and PD-1 immunotherapy. Expansion cohorts including large, partially irradiated tumors and additional analyses of tumor biopsy specimens are planned. Randomized studies are needed to determine whether combining pembrolizumab and SBRT potentiates efficacy.
ACKNOWLEDGMENT
We thank the patients and their families who participated in this study. We thank Robyn Hseu, Bernadette Libao, Jill Stetkevych, and Lauren Wall for logistical support of the trial and Amy K. Huser for editorial assistance.
Appendix
Table A1.
Primary Cancers
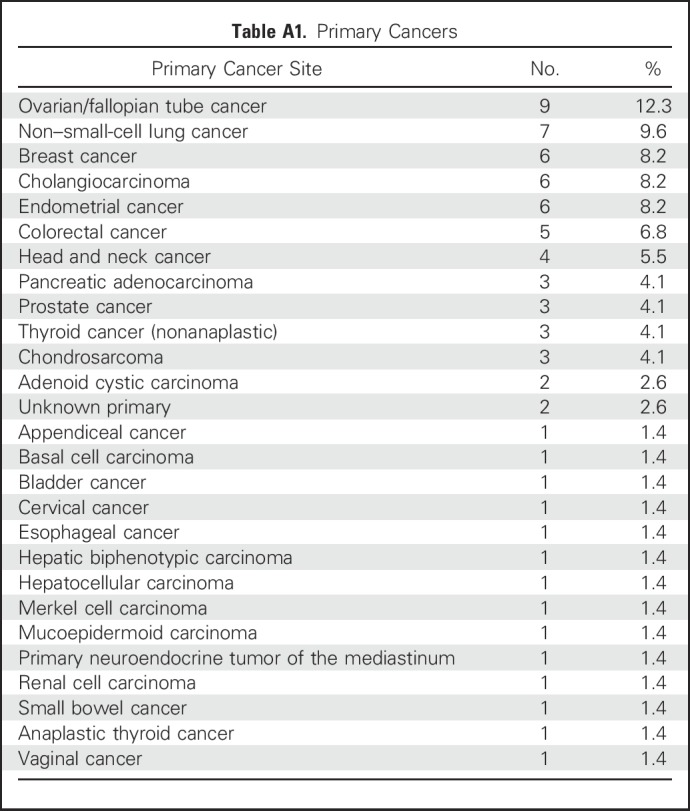
Table A2.
Treatment-Related Severe Toxicity
Table A3.
All Adverse Events
Footnotes
Supported by The Ludwig Center for Metastasis Research at The University of Chicago, The Human Immune Monitoring Core of The University of Chicago Biological Science Division, The Institute for Translational Medicine/CTSA (National Institutes of Health [NIH] Grant No. UL1 RR024999), and a University of Chicago Cancer Center Early Stage Grant (P30 CA014599-41); Merck; and Paul Calabresi Career Development in Clinical Oncology Award (NIH Grant No. 1K12CA139160-05), Young Investigator Award from the Cancer Research Foundation and the Arthur J Schreiner Family Melanoma Research Fund, the J. Edward Mahoney Foundation Research Fund, and the Brush Family Immunotherapy Research Fund (J.J.L.).
AUTHOR CONTRIBUTIONS
Conception and design: Jason J. Luke, Jeffrey M. Lemons, Theodore G. Karrison, Hania A. Al-Hallaq, Julia R. White, Mark J. Ratain, Thomas F. Gajewski, Ralph R. Weichselbaum, Steven J. Chmura
Administrative support: Mark J. Ratain
Provision of study materials or patients: John Moroney, Mark J. Ratain
Collection and assembly of data: Jason J. Luke, Jeffrey M. Lemons, Sean P. Pitroda, James M. Melotek, Yuanyuan Zha, Ainhoa Arina, Linda Janisch, Gini F. Fleming, Steven J. Chmura
Data analysis and interpretation: Jason J. Luke, Jeffrey M. Lemons, Theodore G. Karrison, Sean P. Pitroda, James M. Melotek, Yuanyuan Zha, Ainhoa Arina, Nikolai N. Khodarev, Paul Chang, Jyoti D. Patel, Gini F. Fleming, John Moroney, Manish R. Sharma, Julia R. White, Mark J. Ratain, Thomas F. Gajewski, Ralph R. Weichselbaum, Steven J. Chmura
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jason J. Luke
Honoraria: Intellisphere
Consulting or Advisory Role: Merck
Research Funding: Merck
Travel, Accommodations, Expenses: Merck
Jeffrey M. Lemons
No relationship to disclose
Theodore G. Karrison
No relationship to disclose
Sean P. Pitroda
No relationship to disclose
James M. Melotek
No relationship to disclose
Yuanyuan Zha
No relationship to disclose
Hania A. Al-Hallaq
Honoraria: Reflexion Medical
Patents, Royalties, Other Intellectual Property: Royalties for computer-aided diagnosis software for breast cancer detection, licensed from The University of Chicago and outside the scope of the current work.
Ainhoa Arina
No relationship to disclose
Nikolai N. Khodarev
Stock or Other Ownership: Catherex
Patents, Royalties, Other Intellectual Property: Antitumor therapy; UCHI2165a, pending (Inst); antitumor therapy, UCHI 2259/2320, pending (Inst); RIG-I oligonucleotide stimulators, UCHI 2519, pending (Inst); antitumor therapy, UCHI 2165b, pending (Inst)
Linda Janisch
No relationship to disclose
Paul Chang
Research Funding: Philips Healthcare
Jyoti D. Patel
Consulting or Advisory Role: ARIAD, Abbvie
Gini F. Fleming
Research Funding: Corcept Therapeutics (Inst)
John Moroney
No relationship to disclose
Manish R. Sharma
Consulting or Advisory Role: Ipsen, Bayer, Abbvie, Taiho Pharmaceutical
Julia R. White
Honoraria: Qfix
Mark J. Ratain
Stock or Other Ownership: Biscayne Pharmaceuticals
Consulting or Advisory Role: Abbvie, Biscayne Pharmaceuticals, Cyclacel, Shionogi Pharma, Portola Pharmaceuticals, Amgen
Research Funding: OncoTherapy Science (Inst), Dicerna (Inst), Abbvie (Inst)
Patents, Royalties, Other Intellectual Property: Royalties related to UGT1A1 genotyping for irinotecan; royalties related to UGT1A1 genotyping for irinotecan (Inst); pending patent related to a genomic prescribing system, pending patent related to a genomic prescribing system (Inst)
Expert Testimony: Multiple companies
Thomas F. Gajewski
Stock or Other Ownership: Jounce Therapeutics
Consulting or Advisory Role: Merck, Roche, Jounce Therapeutics, Bayer, Abbvie, Amgen, Aduro Biotech, Adaptimmune
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Roche (Inst), Incyte (Inst), Seattle Genetics (Inst), Ono Pharmaceutical (Inst), Bayer (Inst)
Patents, Royalties, Other Intellectual Property: Licensing to Evelo (Inst)
Ralph R. Weichselbaum
Stock or Other Ownership: Catherex
Honoraria: Merck, AstraZeneca
Consulting or Advisory Role: Merck KGaA, AstraZeneca
Research Funding: Regeneron, Varian Medical Systems
Steven J. Chmura
No relationship to disclose
REFERENCES
- 1.Khoja L, Butler MO, Kang SP, et al. : Pembrolizumab. J Immunother Cancer 3:36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Soria JC, Kowanetz M, et al. : Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563-567, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weichselbaum RR, Liang H, Deng L, et al. : Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol 14:365-379, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Deng L, Liang H, Xu M, et al. : STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41:843-852, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demaria S, Formenti SC: Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front Oncol 2:153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng L, Liang H, Burnette B, et al. : Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687-695, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick JP, Kelsey CR, Palta M, et al. : Stereotactic body radiotherapy: A critical review for nonradiation oncologists. Cancer 120:942-954, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Wong AC, Watson SP, Pitroda SP, et al. : Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 122:2242-2250, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Huang AC, Postow MA, Orlowski RJ, et al. : T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545:60-65, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugade AA, Moran JP, Gerber SA, et al. : Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 174:7516-7523, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Schaue D, Comin-Anduix B, Ribas A, et al. : T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res 14:4883-4890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dovedi SJ, Cheadle EJ, Popple AL, et al. : Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res 23:5514-5526, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Weber JS, Hodi FS, Wolchok JD, et al. : Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 35:785-792, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Al-Hallaq HA, Chmura S, Salama JK, et al. : Rationale of technical requirements for NRG-BR001: The first NCI-sponsored trial of SBRT for the treatment of multiple metastases. Pract Radiat Oncol 6:e291-e298, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlin H, Meng Y, Peterson AC, et al. : Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 69:3077-3085, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji RR, Chasalow SD, Wang L, et al. : An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 61:1019-1031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaverdian N, Lisberg AE, Bornazyan K, et al. : Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 18:895-903, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang A, Wilhite TJ, Pike LRG, et al. : Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys 98:344-351, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Ott PA, Bang YJ, Berton-Rigaud D, et al. : Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J Clin Oncol 35:2535-2541, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Seung SK, Curti BD, Crittenden M, et al. : Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med 4:137ra74, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Formenti SC, Demaria S: Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 84:879-880, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharabi AB, Lim M, DeWeese TL, et al. : Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 16:e498-e509, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Vanpouille-Box C, Pilones KA, Wennerberg E, et al. : In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 33:7415-7422, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Formenti SC, Demaria S: Systemic effects of local radiotherapy. Lancet Oncol 10:718-726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden EB, Chhabra A, Chachoua A, et al. : Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol 16:795-803, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Vanpouille-Box C, Formenti SC, Demaria S: Toward precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res doi: 10.1158/1078-0432.CCR-16-0037epub ahead of print on July 27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaue D, Ratikan JA, Iwamoto KS, et al. : Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 83:1306-1310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y, Auh SL, Wang Y, et al. : Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 114:589-595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allibhai Z, Taremi M, Bezjak A, et al. : The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 87:1064-1070, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Joseph RW, Elassaiss-Schaap J, Wolchok JD, et al. : Baseline tumor size as an independent prognostic factor for overall survival in patients with metastatic melanoma treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol 32:3015, 2014. (suppl) [Google Scholar]