Abstract
The diagnosis of pneumonia is challenging. Our objective was to assess whether low-dose computed tomography (LDCT) modified the probability of diagnosing pneumonia in elderly patients.
We prospectively included patients aged over 65 years with a suspicion of pneumonia treated with antimicrobial therapy (AT). All patients had a chest radiograph and LDCT within 72 h of inclusion. The treating clinician assessed the probability of pneumonia before and after the LDCT scan using a Likert scale. An adjudication committee retrospectively rated the probability of pneumonia and was considered as the reference for diagnosis. The main outcome was the difference in the clinician's pneumonia probability estimates before and after LDCT and the proportion of modified diagnoses which matched the reference diagnosis (the net reclassification improvement (NRI)).
A total of 200 patients with a median age of 84 years were included. After LDCT, the estimated probability of pneumonia changed in 90 patients (45%), of which 60 (30%) were downgraded and 30 (15%) were upgraded. The NRI was 8% (NRI event (−6%) + NRI non-event (14%)).
LDCT modified the estimated probability of pneumonia in a substantial proportion of patients. It mostly helped to exclude a diagnosis of pneumonia and hence to reduce unnecessary AT.
Short abstract
Low-dose CT modified the estimated probability of pneumonia in a substantial proportion (45%) of elderly patients http://ow.ly/V1ha30jvOMk
Introduction
Pneumonia is ranked highly amongst causes of hospitalisation in the elderly and is the leading cause of death from infection in patients aged 65 years or older [1]. The incidence of community-acquired pneumonia (CAP) increases with age, from 2.81 episodes per 1000 person-years for those aged 65–69 years to 21.81 episodes per 1000 person-years for those aged 85–89 years [2]. In a US cohort of 6205 patients with a mean age of 67 years, 30-day mortality from CAP was 8.2% for all patients and 16.1% for patients aged over 80 [3].
According to international recommendations, the standard criteria for the diagnosis of pneumonia are the presence of acute respiratory symptoms and/or fever associated with newly identified or modified infiltrates on a chest radiograph [4, 5]. However, particularly in the elderly, clinical symptoms and signs of pneumonia are often atypical and chest radiograph abnormalities may be absent, delayed or non-specific [6–10]. Both performing and interpreting high-quality chest radiographs in this population has multiple limitations [11]. Furthermore, there is significant inter-observer variability in the interpretation of chest radiographs, even amongst experienced radiologists [12–14]. Correctly diagnosing pneumonia is essential to avoid both under-diagnosis, with the risk that late initiation of treatment might harm the patient, and over-diagnosis which might lead to over-consumption of antibiotics [15]. Previous data have shown the superiority of low-dose computed tomography (LDCT) over chest radiography for the diagnosis of pneumonia in different populations [11, 16–18]. The potential impact of a strategy to systematically carry out thoracic computed tomography (CT) scans for diagnosing pneumonia in elderly patients merits further analysis. We aimed to analyse how LDCT chest scanning modifies the probability of a diagnosis of pneumonia in elderly patients.
Material and methods
Ethics statement
The Geneva institutional review board approved the study protocol (CER 14-250), which was also registered in www.clinicaltrials.gov (NCT02467192). All enrolled patients provided written informed consent before inclusion and consent from next of kin was obtained for those patients for whom the ability to make decisions was impaired.
Setting
This study was conducted in Switzerland at the Department of Internal Medicine, Rehabilitation and Geriatrics at Geneva University Hospitals, a 1800-bed tertiary care institution serving a population of about 500 000 inhabitants.
Study population
Between May 01, 2015 and April 30, 2016, we enrolled consecutive hospitalised patients aged 65 years or older with a suspicion of CAP or hospital-acquired pneumonia, who were being treated with antimicrobial therapy (AT) for that indication. A clinical suspicion of pneumonia required the presence of at least one respiratory symptom (new or increasing cough, purulent sputum, pleuritic chest pain, new or increasing dyspnoea, respiratory rate >20 breaths·min–1, focal auscultatory findings or oxygen saturation <90% on room air) and at least one symptom or laboratory finding compatible with an infection (temperature >38.0 °C or <35.0 °C, C-reactive protein (CRP) >10 mg·L−1, leukocyte count >10 g·L−1 with >85% neutrophils or band forms) [4]. A chest radiography image suggestive of pneumonia was not necessary for inclusion. Patients who had been treated for pneumonia during the previous 6 months, who had already undergone a CT scan during the present episode or needed a contrast-enhanced CT, who had been admitted to the intensive care unit (ICU), or who had already been treated with AT for more than 48 h before inclusion were excluded, as well as those patients for whom the ability to make decisions was impaired and for whom the family could not provide consent. CAP and nursing home acquired pneumonia were defined according to where patients lived; hospital-acquired pneumonia was defined as pneumonia developing two or more days after hospitalisation.
Patient management and data collection
Patients were managed according to local guidelines and screened by the study nurse. Both the choice and duration of AT were left to the discretion of the treating clinician with the help of institutional recommendations (see appendix). Demographic data, comorbidities, vital signs, clinical findings, severity scores and the results of standard laboratory tests (including CRP, procalcitonin (PCT), PCR assay for respiratory viruses on nasopharyngeal swabs, blood, sputum, urine cultures and urinary antigens) were prospectively recorded at baseline.
Radiological data
Chest radiographs were obtained for all patients, preferably while standing and with postero-anterior and lateral incidence if possible. The clinician assessed the probability of pneumonia by integrating the results of the clinical examination, biological data and the results of the chest radiograph, and then graded the probability of pneumonia on a five-level Likert scale (excluded, low, intermediate, high and certain). An LDCT scan (without administration of intra-venous contrast) was performed as soon as possible after the first Likert-scale estimate, ideally within 24 h of inclusion in the study but no later than 72 h from inclusion. A new chest radiograph was performed if the delay between the first chest radiograph and the CT scan was more than 12 h. LDCT images were interpreted by the attending radiologist, who was informed of the clinical suspicion of pneumonia but had no other medical information and was not directly involved in the study. In addition to the usual description of the image, the radiologist graded the probability of pneumonia on the previously mentioned five-level Likert scale. The clinician then made a new evaluation of the probability of pneumonia by incorporating the results of the LDCT and the radiologist's interpretation. Finally, the clinician decided whether to continue or withdraw the AT. The investigators had no influence over decisions regarding AT.
Adjudication committee
After completion of the study, an adjudication committee (see appendix), blinded to the results of the LDCT scan, retrospectively rated the probability of pneumonia according to patient records and based on international guidelines for diagnosis of pneumonia [4, 5]. Their final decision was considered as the reference diagnosis.
Outcomes
The study's primary endpoint was the proportion of patients whose probability of pneumonia changed (upgraded or downgraded) before and after LDCT and the proportion of modified diagnoses which matched the reference diagnosis. Secondary endpoints were: 1) the test characteristics of LDCT (sensitivity, specificity, likelihood ratios, positive predictive values (PPVs) and negative predictive values (NPVs), and receiver operating characteristic (ROC) curve) in comparison to the reference diagnosis; 2) the rate of agreement between the adjudication committee's experts; 3) the proportion of patients for whom the prescribing of AT for pneumonia was discontinued after the LDCT (for all patients and for those with a low post-LDCT probability); 4) the prevalence of ancillary findings on LDCT.
Statistical analyses
Baseline characteristics were described using means (with standard deviation), medians (with interquartile ranges) and proportions (with 95% confidence intervals) as appropriate. The estimated probabilities of pneumonia before and after LDCT were compared and the proportion of modified diagnoses (with 95% CI) was calculated. For clarifying the comparison of pre-LDCT and post-LDCT probability, high and certain probabilities (as well as low and excluded probabilities) were regrouped, leading to a three-level Likert scale. To assess whether LDCT helped the clinicians to properly reclassify patients in agreement with the adjudication committee's reference diagnoses, we calculated the net reclassification improvement (NRI). The absolute NRI calculates the absolute number of patients correctly reclassified (the net reclassification of patients with pneumonia according to the adjudication committee (NRI event) + the net reclassification of patients without pneumonia according to the adjudication committee (NRI nonevent)) based on the total number of patients [19, 20]. Patients with intermediate or high probability of pneumonia according to the adjudication committee were considered as having pneumonia, while patients with low probability were considered as not having pneumonia.
We calculated the ROC curve of the true-positive rate (sensitivity) against the false-positive rate (1–specificity) of LDCT compared to the reference diagnosis (with pneumonia if of intermediate or high probability, or without pneumonia if of low probability) (figure 1). A second curve showing the accuracy of the post-LDCT probability against the reference diagnosis can be found in the appendix as supplementary figure S1. Also illustrated in the appendix is the calculation of the rate of agreement between the adjudication committee members. Analyses were performed using the R statistical software package, version 3.1.1 (www.cran.r-project.org).
FIGURE 1.
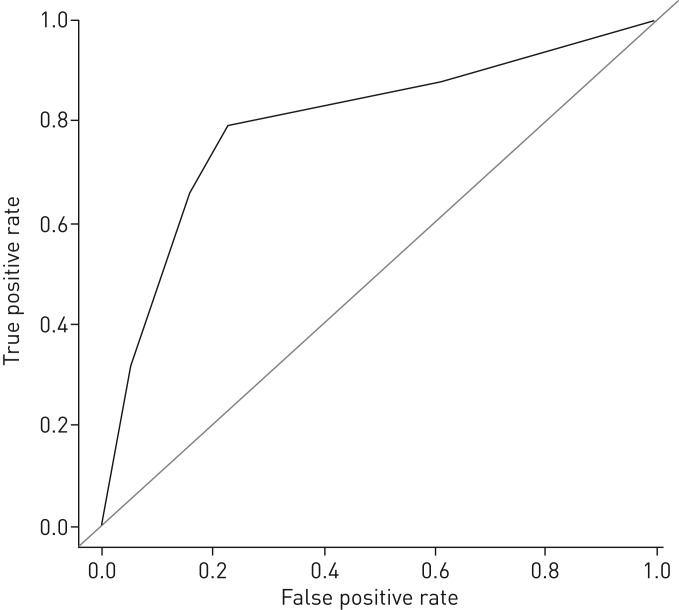
Receiver operating characteristic (ROC) curve of the low-dose computed tomography (LDCT) scan probabilities of a diagnosis of pneumonia compared with the reference diagnosis (area under the curve (AUC) 0.79 (95% CI 0.73–0.86)).
Sample size
In a previous study [21], a CT scan modified the diagnostic classification of CAP in 59% of cases (95% CI 53.2–64.0), with an upgraded probability of diagnosis in 19% of cases. Demonstrating an improvement in the pneumonia detection rate by using CT would require 46 patients (p=0.05; power: 90%). Thus, considering a true incidence of pneumonia among patients hospitalised for pneumonia of 45% (according to the adjudication committee's reference diagnosis), we calculated that at least 100 patients would be needed to allow the estimation of any changes in a diagnosis of pneumonia. Moreover, the present study took place over a period of one year in order to avoid any seasonal bias and thus allow for the inclusion of a broad range of pneumonias, including those seen during winter flu epidemics.
Results
Patients’ characteristics
Of 899 patients screened, 200 were included (figure 2). Characteristics are provided in tables 1 and 2. The median delay of LDCT was 2.2 h after inclusion.
FIGURE 2.
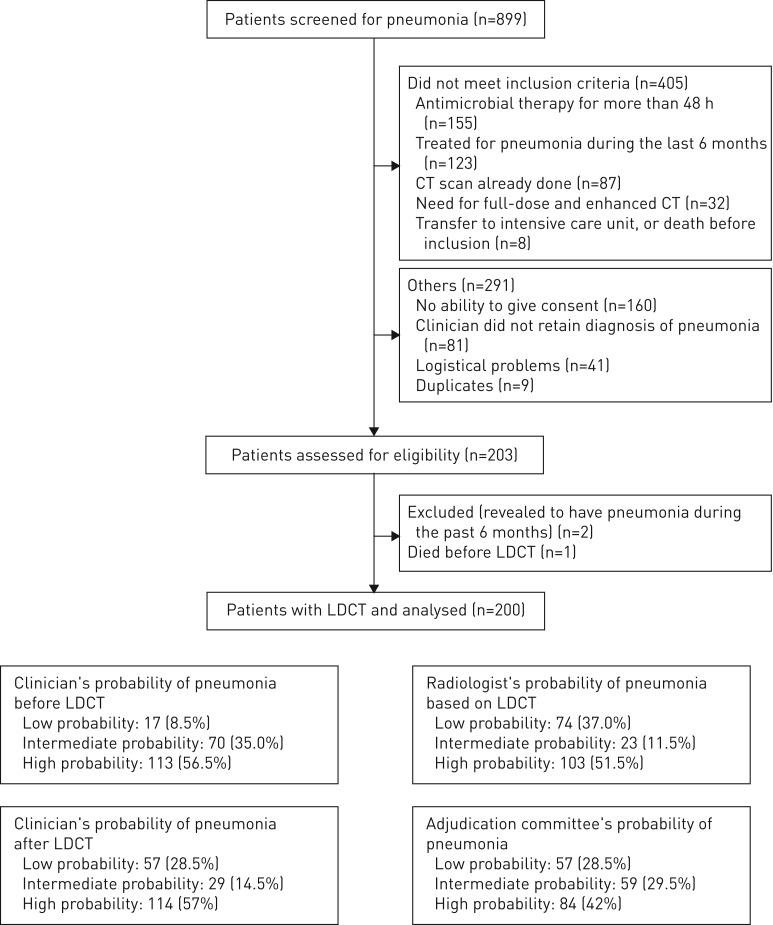
Flowchart for patient pneumonia screening. CT: computed tomography; LDCT: low-dose CT.
TABLE 1.
Demographic and clinical characteristics of the 200 patients included in the PneumOLD-CT study at baseline
| Characteristic# | n (%) | Median (IQR) |
| Age years | 84.0 (78.6–90.2) | |
| ≥85 years old | 93 (46.5) | |
| Female | 98 (49.0) | |
| Nursing home residents | 28 (14.0) | |
| BMI kg·m−2 | 24.5 (21.5–28.6) | |
| Mini mental state examination score (N=162) | 24 (19–27) | |
| Mini nutritional assessment (N=178) | 8 (6–11) | |
| Functional independence measure score (N=171) | 69 (50–97) | |
| Influenza vaccination within the past year (N=182) | 103 (56.6) | |
| Pneumococcal vaccination within the past 5 years (N=177) | 7 (4.0) | |
| Comorbidities | ||
| Hospitalisation during the past 6 months | 70 (35) | |
| Chronic cardiac disease | 103 (51.5) | |
| COPD | 35 (17.5) | |
| Kidney disease (N=199) | 60 (30.2) | |
| Liver disease | 11 (5.5) | |
| Neoplasia | 17 (8.5) | |
| Smoking (past and present) | 100 (50.0) | |
| History of stroke | 33 (16.5) | |
| Cognitive disorders¶ | 66 (33.0) | |
| Swallowing disorders+ | 28 (14.0) | |
| Poor oral hygiene§ | 38 (19.0) | |
| Immunosuppressive treatment (N=199)f | 15 (7.5) | |
| Clinical characteristics of pneumonia | ||
| Type of pneumonia | ||
| Community-acquired pneumonia | 162 (81.0) | |
| Nursing home-acquired pneumonia | 22 (11.0) | |
| Hospital-acquired pneumonia (>72 h after hospitalisation) | 16 (8.0) | |
| Bronchoaspiration (N=161)## | 12 (7.5) | |
| Temperature ≥38.0 °C | 116 (58.0) | |
| Cough | 170 (85.0) | |
| Dyspnoea | 145 (72.5) | |
| Sputum production | 74 (37.0) | |
| Chest pain | 35 (17.5) | |
| Crackles | 171 (85.5) | |
| Decrease in respiratory sounds | 51 (25.5) | |
| Peripheral oxygen saturation (SpO2 <90% on admission) | 102 (51.0) | |
| Respiratory rate >20 per min on admission | 143 (71.5) | |
| Delirium | 92 (46.0) | |
| Fall | 71 (35.5) | |
| CURB-65 score¶¶ | ||
| 1 | 36 (18.0) | |
| 2 | 75 (37.5) | |
| 3 | 68 (34.0) | |
| 4 | 21 (10.5) | |
| Fine score | 102 (89–123) |
Laboratory values and vital signs were obtained at hospital admission. IQR: interquartile range; BMI: body mass index; COPD: chronic obstructive pulmonary disease; SpO2: arterial oxygen saturation measured by pulse oximetry. #: the number of patients (N) is 200 unless otherwise stated; ¶: cognitive disorders were diagnosed after a cognitive consultation (at least clinical dementia rating (CDR) 1 dementia); +: swallowing disorders were observed during the hospitalisation; §: oral hygiene was defined as good, medium or poor; f: immunosuppressive treatment means that patient is under prednisone during more than 15 days or other immunosuppressive drugs; ##: bronchoaspiration was defined by the clinician according to usual definition; ¶¶: CURB-65 is a pneumonia severity score taking into account various factors (confusion, urea >7 mmol·L−1, respiratory rate ≥30 breaths·min−1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic), age ≥65 years).
TABLE 2.
Biological, microbiological and radiological characteristics at baseline, and treatment and outcome of the 200 patients included in the PneumOLD-CT study
| Characteristics# | n (%) | Median (IQR) |
| Biological | ||
| White blood cell count 103 per mm3 (on admission) | 11.0 (8.2–14.0) | |
| proBNP ng·L−1 (range <300 ng·L−1) (N=170) | 1836 (667–3801) | |
| CRP mg·L−1 (range 0–10 mg·L−1) | 84.0 (45.8–159.6) | |
| PCT µg·L−1 (range <0.25 µg·L−1) (N=185) | 0.33 (0.13–1.30) | |
| Urea mmol·L−1 | 7.9 (6.0–11.9) | |
| Creatinine µmol·L−1 | 97.0 (77.0–133.0) | |
| Albumin g·L−1 (N=193) | 35.0 (32.0–38.0) | |
| Prealbumin mg·L−1 (N=193) | 122.0 (95.0–162.0) | |
| Microbiological | ||
| Positive culture | ||
| Blood (N=192) | 11 (5.7) | |
| Urinary (N=177) | 82 (46.3) | |
| Sputum (N=81) | 36 (44.4) | |
| Pleural effusion (N=6) | 0 | |
| Positive urinary antigen | ||
| Legionella (N=183) | 0 | |
| Pneumococcal (N=178) | 8 (4.5) | |
| Positive serology (IgM) | ||
| Legionella pneumophila | 2 (1) | |
| Chlamydophila pneumoniae | 4 (2) | |
| Mycoplasma pneumoniae | 3 (1.5) | |
| Radiological | ||
| Chest radiography | ||
| Standing | 66 (33) | |
| Two incidences | 63 (31.5) | |
| Delay of LDCT chest scan h | 2.2 (0.9–15.4) | |
| Radiologist's opinion on quality (good/satisfactory) | 127 (63.5) | |
| Radiologist's estimate of pneumonia probability | ||
| High | 103 (51.5) | |
| Intermediate | 23 (11.5) | |
| Low | 74 (37.0) | |
| Treatment | ||
| Duration of AT days | 7 (6–9) | |
| Outcome | ||
| Transfer to intermediate care unit or ICU | 13 (7.0) | |
| 30-day mortality | 11 (5.4) | |
| 90-day mortality | 30 (15.0) |
IQR: interquartile range; proBNP: pro-brain natriuretic peptide; CRP: C-reactive protein; PCT: procalcitonin; IgM: immunoglobulin M; LDCT: low-dose computed tomography; AT: antimicrobial therapy; ICU: intensive care unit. #: the number of patients (N) is 200 unless otherwise stated.
The initial probability of pneumonia was high in 56.5% of cases (113 patients), intermediate in 35% of cases (70 patients) and low in 8.5% of cases (17 patients). After the LDCT scan, these probabilities became high in 57% of cases (114 patients), intermediate in 14.5% of cases (29 patients) and low in 28.5% of cases (57 patients). The LDCT results changed the probability of pneumonia in 45% of cases (90 patients). The probability was upgraded in 15% of cases (30 patients) and downgraded in 30% of cases (60 patients) (table 3). More than 80% of patients with intermediate pre-LDCT probability had their probability changed after LDCT. The changes in clinician's probability subsequently matched the reference diagnoses in 67.8% of modifications (61 out of 90 patients) and in 30.5% of all patients (61 out of 200 patients). The absolute number of patients correctly reclassified according to the diagnosis of the adjudication committee is –12 patients among those with pneumonia and +28 patients among those without pneumonia. Overall, the absolute number of patients correctly reclassified is thus +16 patients, corresponding to 8.0% of all patients in our sample (table 4).
TABLE 3.
Clinician's estimates of the probability of pneumonia in 200 patients before and after low-dose computed tomography (LDCT) chest scans
| Clinician's estimates of the probability of pneumonia after LDCT | ||||||
| Low | Intermediate | High | Total | Change of probability | ||
| n | % (95% CI) | |||||
| Clinician's estimates of the probability of pneumonia before LDCT | ||||||
| Low | 10 | 3 | 4 | 17 | 7 | 41 (18–24) |
| Intermediate | 34 | 13 | 23 | 70 | 57 | 81 (72–90) |
| High | 13 | 13 | 87 | 113 | 26 | 23 (15–31) |
| Total | 57 | 29 | 114 | 200 | 90 | 45 (38–52) |
Values in bold are for upgraded probability, while values in italic are for downgraded probability.
TABLE 4.
The net reclassification improvement (NRI) amongst 200 patients
|
Clinician's estimates of the probability of pneumonia after LDCT |
Net reclassification | |||
|
Excluded/ low |
Intermediate/ high/certain |
Total | ||
| Patients with pneumonia according to the adjudication committee (n=143) | ||||
| Clinician's estimates of the probability of pneumonia before LDCT | Net reclassification=6–18 = –12 patients (–8.4% of the 143 patients with pneumonia) | |||
| Excluded/low | 2 | 6 | ||
| Intermediate/high/certain | 18 | 117 | ||
| Total | 143 | |||
| Patients without pneumonia according to the adjudication committee (n=57) | ||||
| Clinician's estimates of the probability of pneumonia before LDCT | Net reclassification=29–1 = +28 patients (+49.1% of the 57 patients without pneumonia) | |||
| Excluded/low | 8 | 1 | ||
| Intermediate/high/certain | 29 | 19 | ||
| Total | 57 | |||
| Total patients | 200 | Absolute NRI=28–12 = +16 patients (8.0% of all 200 patients) | ||
The values in bold show patients correctly reclassified by the new model, while the values in italic show patients incorrectly reclassified by the new model. The absolute number of patients correctly reclassified is –12 patients among those with pneumonia and +28 patients among those without pneumonia. Overall, the absolute number of patients correctly reclassified is thus +16 patients, which corresponds to 8.0% of all patients in the sample. LDCT: low-dose computed tomography.
Comparing the probability of a pulmonary infiltrate on the LDCT interpreted by a radiologist and the diagnostic probability of pneumonia determined by the adjudication committee, we identified 113 true-positive, 44 true-negative, 13 false-positive and 30 false-negative cases. This provided the following measures of accuracy: sensitivity=0.79, specificity=0.77, PPV=0.90, NPV=0.59, positive likelihood ratio=3.46, negative likelihood ratio=0.27 and the area under the curve (AUC)=0.79 (95% CI 0.73–0.86) (figure 1). When using the adjudication committee as reference, the diagnostic performance of the clinician with access to the LDCT, expressed as the AUC of an ROC curve was 0.847 (95% CI 0.7907–0.9032) (see supplementary figure S1 in the appendix). Among the 30 false-negative cases (low probability of diagnosis of pneumonia on LDCT and intermediate or high probability according to the members of the adjudication committee), 23 cases (77%) were of intermediate probability according to the adjudication committee and 7 were judged to have a high probability of pneumonia.
The initial agreement rate for the probability of pneumonia between the experts of the adjudication committee was 31.5% using a three-level Likert scale. The prevalence of findings other than pneumonia was 38%. Pulmonary nodules requiring further examination were present in 19 cases (9.5%). Other findings identified are provided in table 5. The LDCT scan results led the clinician to withdraw AT in 8.5% of all patients and in 30% of the patients with a low post-LDCT probability without any intervention from the study team. It is of note that the presence of an infectious differential diagnosis justified the continuation of AT in 29 patients (51%) with a low post-LDCT probability (see supplementary table S1 in the appendix).
TABLE 5.
Additional findings following analysis of low-dose computed tomography (LDCT) chest scans
| Additional findings | n |
| Total findings | 76 |
| Pulmonary findings | 51 |
| Pleural effusion | 22 |
| Pulmonary nodules | 19 |
| Lung mass | 1 |
| Lung metastases | 1 |
| Emphysema | 5 |
| Lung fibrosis | 1 |
| Asbestosis | 1 |
| Tuberculosis sequelae | 1 |
| Other findings | 28 |
| Adenomegalies | 7 |
| Hepatic lesions | 5# |
| Adrenal nodules | 4 |
| Pericardial effusion | 3 |
| Abdominal aortic aneurysm | 3 |
| Renal cysts | 2 |
| Thyroid nodules | 1 |
| Fractures | 3 |
The same patient could have more than one finding. #: including one hepatic abscess.
Discussion
To the best of our knowledge, this is the largest prospective study to date assessing the utility of LDCT chest scans in a cohort of elderly people with suspected pneumonia. LDCT results changed the probability of a diagnosis of pneumonia in a high proportion of patients (45%), upgrading the probability in 15% of cases and downgrading it in 30%. These changes matched the reference diagnoses in 30.5% of all patients. The absolute NRI was 8%, with NRI nonevents superior to NRI events, meaning that LDCT mostly helped to exclude a diagnosis of pneumonia. We found fair positive-likelihood and negative-likelihood ratios for CT and a low agreement rate for the probability of pneumonia among the members of the adjudication committee. LDCT allowed supplementary findings in 38% of cases and led the clinician to withdraw antibiotics in 8.5% of all patients and in 30% of the patients with a low post-LDCT probability.
A recent prospective study by Claessens et al. [21] on of the impact of CT on the diagnosis of CAP in 319 adults (mean age: 65 years old), consulting in the emergency department, reported similar results. In this study, chest CT led to a change of diagnosis in 59% of cases (probability of pneumonia was lowered in 40% of cases and raised in 19% of cases). The NRI reported in the study was 18.8% (NRI event: −0.6%; NRI nonevent: 19.4%). Interestingly, both in our study and in the study by Claessens et al. [21], correct reclassification was mainly observed in patients not having pneumonia according to the reference diagnosis. This means that the potential benefit of LDCT would mainly be in reducing the overdiagnosis of pneumonia, an important issue in terms of cost and inappropriate antibiotic use (which might potentially lead to complications and selection of resistant strains). Some studies have reported that less than half of patients treated for pneumonia in US hospitals satisfied diagnostic criteria for pneumonia [15]. Moreover, chest radiography lacks the sensitivity required for the diagnosis of pneumonia and LDCT may allow the detection of pulmonary infiltrates not diagnosed on the chest radiograph, especially among elderly patients. In a previous study, chest radiography and full-dose CT scans were performed on 58 bedridden patients with a mean age of 84 years and an intermediate or high probability of pneumonia. The use of CT scanning allowed a positive diagnosis of pneumonia in 53% of cases compared with 21% of cases with a chest radiograph. Of the 11 patients with a normal chest radiograph, six (55%) had CT scans showing bilateral infiltrates [11]. Nevertheless, this study did not include comparison with a reference diagnosis. In our study, eight of the 17 patients whom clinicians had initially considered as having a low probability of pneumonia had an intermediate or high probability according to the reference diagnosis. Similarly, Claessens et al. [21] found infiltrates on the CT scans of 40 out of 120 patients considered as having a low probability of pneumonia based on chest radiography. Unfortunately, we were not able to evaluate the benefit of LDCT in terms of sensitivity improvement, since patients judged not to have pneumonia based on the initial clinical and radiological assessment, and hence the potential false-negatives from patients in the initial evaluation that could have been identified by a LDCT, were not included in the study.
The overall performance of LDCT was higher in the study by Claessens et al. [21] than in the present study. For example, reclassification matched the reference diagnosis of Claessens et al. [21] in 80% of cases, compared to 67.5% in the present study. This discrepancy may be explained by the use of different reference diagnosis definitions. Furthermore, in the study by Claessens et al. [21], the adjudication committee had access to the LDCT scan results, which may have led to an overestimation of LDCT diagnostic performance due to the incorporation bias of using the LDCT data for the reference diagnosis. We instead chose to blind our adjudication committee to the LDCT results in order to remain conservative in the evaluation of LDCT diagnostic improvement.
The present study only found fair positive-likelihood and negative-likelihood ratios for CT; however, these evaluations are set against an arguable reference diagnosis. Some patients without infiltrate on the LDCT scan and considered as LDCT false-negatives may rather be false-positives of our reference diagnosis (diagnosis of pneumonia established by the expert committee blinded to the CT results). Of note, five of these patients had heart failure, a diagnosis that can confound the interpretation of a chest radiograph. The rate of agreement for the diagnosis of pneumonia among experienced clinicians was low, even though they had access to all the clinical information (including patient outcomes). This highlights the difficulties in making unambiguous diagnoses based on international recommendations: there is an even greater lack of sensitivity and specificity in the clinical and radiological examination of elderly patients [8, 11, 22].
The diagnosis of pneumonia in current clinical practice is likely flawed by both overdiagnosis and underdiagnosis. Underdiagnosis exposes patients to delays in the initiation of antibiotic treatment, which might worsen their prognosis. On the other hand, in addition to the failure to identify the real cause of a patient's symptoms, overdiagnosis can lead to excessive use of antimicrobials [23]. In our study, the clinicians stopped AT in 8.5% of patients after the results of LDCT were known, with no intervention from the study team. This should be compared with 28% of patients diagnosed with a low post-CT probability of pneumonia. Failure to withdraw antibiotics, even when the probability of pneumonia is judged to be low, may be the result of the clinicians' lack of confidence in the diagnosis, overestimation of the benefits of antibiotics in respiratory tract infections other than pneumonia, or underestimation of the adverse consequences of their overuse. Moreover, patients may present with another source of infection requiring AT, as was the case for 29 patients (14.5%) in our study.
Our study demonstrates the feasibility of using LDCT scanning for elderly people hospitalised in our institution while only one third of them received standing and profile chest radiography. LDCT scans took 10 min to perform and were well-tolerated with a mean radiation exposure of 1.5±0.47 mSv. This can be compared to a mean exposure of 0.05±0.03 mSv for a conventional chest radiograph, to Switzerland's natural background radiation level of 4 mSv·year–1 and to 7 mSv for a full-dose CT scan [24]. However, CT scanners are not available everywhere and scans are more expensive than chest radiography. In Switzerland, the cost of a chest CT scan is about three times that of a chest radiograph, but the total costs and ratios of these examinations vary widely from one country to another.
Moreover, additional radiological findings were observed in about one third of patients and pulmonary nodules in about 10% of patients, which is similar to data obtained from the literature [25, 26]. These findings may represent an opportunity to diagnose and treat unexpected diseases but may also lead to further investigations, increasing costs and potential risks in elderly patients [27]. As it is possible for chest radiography findings to be delayed relative to symptoms and disease onset, we performed a subgroup analysis to determine if LDCT had a diagnostic advantage in the “short duration of symptoms” group and compared patients presenting within 24 h of symptoms onset with patients presenting later. There was no significant diagnostic advantage of LDCT over chest radiography in the “short duration of symptoms”.
The strengths of our study include the prospective inclusion of 200 consecutive elderly patients with pneumonia in a large teaching hospital. By evaluating their pre-LDCT scan and post-LDCT scan probability of having the disease according to the clinician, we could assess the contribution of LDCT in the diagnosis of pneumonia. A rigorous reference diagnosis was obtained according to international guidelines and the adjudication committee was blinded to LDCT results in order to avoid incorporation bias. Conversely, there are some limitations to this work. First, our study was performed in a single centre limiting the degree to which our results could be generalised. Secondly, our inclusion criteria did not allow an accurate estimate of underdiagnosis of pneumonia, as only patients with a pneumonia probability warranting an AT were included. Thirdly, a health economic assessment of the diagnostic imaging tests would have been interesting. The cost of tests is an important aspect and it should be balanced with the consequences of a better classification/diagnosis of patients (not only in terms of clinical outcomes, but also in terms of economic burden). However, our study was not designed for this purpose as the patients were not randomised and therapeutic decisions were taken by the clinicians with knowledge of the results of both diagnostic tests. Finally, our choice of a consensus reference diagnosis could be criticised because of its low concordance. However, we used widely accepted definitions of pneumonia and used a systematic approach including independent experts in respiratory medicine, infectious diseases and internal medicine to obtain a consensual reference diagnosis for each case.
Significance of the study and implications for clinicians
The diagnosis of pneumonia is difficult, even more so in elderly patients because of atypical symptoms, alternative diagnoses and because the reference diagnosis according to international recommendations is debatable and poorly reproducible. The use of LDCT scanning modifies the diagnosis in patients hospitalised for pneumonia even if its availability and cost are limitations, and despite the fact that it is almost impossible to determine if the changes in diagnosis are appropriate in the absence of a gold standard. The present results should encourage clinicians to consider performing LDCT in pneumonia diagnostic recommendations in elderly patients, especially in patients with intermediate probability. It can help to exclude a diagnosis of pneumonia, encourage the search for an alternate diagnosis and reduce unnecessary AT. These results should be confirmed in a randomised clinical trial including the discontinuation of antimicrobials in patients with negative LDCT scans.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix ERJ-02375-2017_Appendix (36.2KB, pdf)
Acknowledgements
We would like to thank our funders, the patients and their families who volunteered to participate in this study, Valentine Loustau (Hôpital des Trois-Chêne, Geneva, Switzerland) and all the clinicians, our research nurses Marie-Pierre Meynet and Véronique Lachat (Hôpital des Trois-Chêne, Geneva, Switzerland), the case managers who helped us enrol our participants, Enrique Maturana (Hôpital des Trois-Chêne, Geneva, Switzerland) and the radiology technicians (Geneva University Hospitals) and our translator Darren Hart (Publish or Perish). The contribution of the ESCMID Study Group for Infections in the Elderly (ESGIE; www.escmid.org/esgie) to this study is also acknowledged.
Footnotes
This article has supplementary material available from erj.ersjournals.com
This study is registered at www.clinicaltrials.gov with identifier number NCT02467192.
Author contributions: V. Prendki and J. Stirnemann conceived the study, wrote the grant to obtain the funding, obtained ethical approval, participated in recruitment, followed the subjects, analysed the results and wrote the manuscript. M. Scheffler, X. Montet, B. Huttner, N. Garin, J.P. Janssens, C. Marti, S. Carballo, X. Roux, C. Serratrice and J. Serratrice were among the adjudication committee and contributed to the manuscript. F. Herrmann and T. Agoritsas helped with the statistical plan and the analyses. J.L. Reny, A. Perrier, C. Becker and L. Kaiser helped design the study and contributed to the manuscript. S. Rosset-Zufferey and V. Soulier helped recruit the patients, collect data and assisted with patient follow-up. J. Stirnemann created the statistical plan and performed the analyses.
Support statement: The study was supported by a Research & Development Grant from the Medical Directorate of Geneva University Hospitals (HUG) and a grant from the Ligue Pulmonaire Genevoise, a non-profit association involved in the home care of patients with respiratory diseases. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: None declared.
References
- 1.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67: 71–79. [DOI] [PubMed] [Google Scholar]
- 2.Millett ER, Quint JK, Smeeth L, et al. . Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One 2013; 8: e75131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luna CM, Palma I, Niederman MS, et al. . The impact of age and comorbidities on the mortality of patients of different age groups admitted with community-acquired pneumonia. Ann Am Thorac Soc 2016; 13: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 4.Mandell LA, Wunderink RG, Anzueto A, et al. . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44: Suppl. 2, S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim WS, Baudouin SV, George RC, et al. . BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009; 64: Suppl. 3, iii1–iii55. [DOI] [PubMed] [Google Scholar]
- 6.Janssens J-P, Krause K-H. Pneumonia in the very old. Lancet Infect Dis 2004; 4: 112–124. [DOI] [PubMed] [Google Scholar]
- 7.Faverio P, Aliberti S, Bellelli G, et al. . The management of community-acquired pneumonia in the elderly. Eur J Intern Med 2014; 25: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrie TJ, File TM Jr. Bacterial pneumonia in older adults. Clin Geriatr Med 2016; 32: 459–477. [DOI] [PubMed] [Google Scholar]
- 9.Basi SK, Marrie TJ, Huang JQ, et al. . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004; 117: 305–311. [DOI] [PubMed] [Google Scholar]
- 10.Hagaman JT, Rouan GW, Shipley RT, et al. . Admission chest radiograph lacks sensitivity in the diagnosis of community-acquired pneumonia. Am J Med Sci 2009; 337: 236–240. [DOI] [PubMed] [Google Scholar]
- 11.Esayag Y, Nikitin I, Bar-Ziv J, et al. . Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med 2010; 123: 88.e1–88.e5. [DOI] [PubMed] [Google Scholar]
- 12.Albaum MN, Hill LC, Murphy M, et al. . Interobserver reliability of the chest radiograph in community-acquired pneumonia. PORT Investigators. Chest 1996; 110: 343–350. [DOI] [PubMed] [Google Scholar]
- 13.Loeb MB, Carusone SB, Marrie TJ, et al. . Interobserver reliability of radiologists’ interpretations of mobile chest radiographs for nursing home-acquired pneumonia. J Am Med Dir Assoc 2006; 7: 416–419. [DOI] [PubMed] [Google Scholar]
- 14.Hopstaken RM, Witbraad T, van Engelshoven JM, et al. . Inter-observer variation in the interpretation of chest radiographs for pneumonia in community-acquired lower respiratory tract infections. Clin Radiol 2004; 59: 743–752. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar M, Brar N, Khatib R, et al. . Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4-h antibiotic administration rule. Chest 2007; 131: 1865–1869. [DOI] [PubMed] [Google Scholar]
- 16.Syrjala H, Broas M, Suramo I, et al. . High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis 1998; 27: 358–363. [DOI] [PubMed] [Google Scholar]
- 17.Self WH, Courtney DM, McNaughton CD, et al. . High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med 2013; 31: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haga T, Fukuoka M, Morita M, et al. . Computed tomography for the diagnosis and evaluation of the severity of community-acquired pneumonia in the elderly. Intern Med 2016; 55: 437–441. [DOI] [PubMed] [Google Scholar]
- 19.Pencina KM, Pencina MJ, D'Agostino RB Sr. What to expect from net reclassification improvement with three categories. Stat Med 2014; 33: 4975–4987. [DOI] [PubMed] [Google Scholar]
- 20.Alba A, Agoritsas T, Walsh M, et al. . Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA 2017; 318: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 21.Claessens YE, Debray MP, Tubach F, et al. . Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015; 192: 974–982. [DOI] [PubMed] [Google Scholar]
- 22.Metlay JP, Schulz R, Li YH, et al. . Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997; 157: 1453–1459. [PubMed] [Google Scholar]
- 23.Scholze K, Wenke M, Schierholz R, et al. . The reduction in antibiotic use in hospitals. Dtsch Arztebl Int 2015; 112: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larke FJ, Kruger RL, Cagnon CH, et al. . Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol 2011; 197: 1165–1169. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs PC, Mali WP, Grobbee DE, et al. . Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. J Comput Assist Tomogr 2008; 32: 214–221. [DOI] [PubMed] [Google Scholar]
- 26.Hall WB, Truitt SG, Scheunemann LP, et al. . The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009; 169: 1961–1965. [DOI] [PubMed] [Google Scholar]
- 27.Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imag 2012; 12: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix ERJ-02375-2017_Appendix (36.2KB, pdf)


