Abstract
The invariant chain (Ii) binds to newly synthesized MHC class II molecules with the CLIP region of Ii occupying the peptide-binding groove. Here we demonstrate that recombinant Ii proteins with the CLIP region replaced by antigenic self-epitopes are highly efficient in activating and silencing specific T cells in vitro and in vivo. The Ii proteins require endogenous processing by antigen-presenting cells for efficient T cell activation. An Ii protein encompassing the epitope myelin basic protein amino acids 84–96 (Ii-MBP84–96) induced the model autoimmune disease experimental allergic encephalomyelitis (EAE) with a higher severity and earlier onset than the peptide. When applied in a tolerogenic manner, Ii-MBP84–96 abolished antigen-specific T cell proliferation and suppressed peptide-induced EAE more effectively than peptide alone. Importantly, i.v. administration of Ii proteins after EAE induction completely abrogated the disease, whereas peptides only marginally suppressed disease symptoms. Ii fusion proteins are thus more efficient than peptide in modulating CD4+ T cell-mediated autoimmunity, documenting their superior qualities for therapeutic antigen delivery in vivo.
CD4+ T cells recognize peptides presented in association with MHC class II molecules on the surface of antigen-presenting cells. Peptide loading of MHC class II molecules is regulated by the chaperone molecule invariant chain (Ii). Trimers of Ii form nonameric complexes with newly synthesized MHC class II αβ-heterodimers within the endoplasmic reticulum. In these complexes, the CLIP core region (amino acids 90–98 in mice) of Ii occupies the peptide-binding groove of the class II molecule irrespective of the MHC haplotype (1, 2). In addition, sequences on both sides of the CLIP core region bind to the class II molecule (3–5).
Genetically modified Ii proteins, in which the CLIP core region has been replaced by a T helper epitope, can be processed and presented in vitro to T cell clones by HLA DR-expressing cell lines (6, 7). Here we show that such genetically modified Ii proteins are superior to the peptide epitopes in triggering polyclonal, antigen-specific T cell populations in vitro and in vivo and, most importantly, in modulating autoimmunity in vivo. Our model system uses the peptides MBP84–96 and PLP139–151, immunodominant epitopes of the self-antigens myelin basic protein (MBP) and myelin proteolipid protein (PLP). MBP84–96 and PLP139–151 induce experimental allergic encephalomyelitis (EAE), a CD4+ T cell-mediated experimental autoimmune disease, in immunized SJL mice (8, 9). We show that Ii proteins, wherein the CLIP core region has been replaced by MBP84–96 or PLP139–151, are more potent than the peptides in activating specific T cells in vitro and in vivo. Ii-MBP84–96, but not the peptide, required endogenous antigen processing by antigen-presenting cells to stimulate MBP84–96-specific T cells. Lower amounts of the Ii proteins were sufficient to break self-tolerance and induce autoimmunity in mice. On the other hand, i.p. injections of Ii-MBP84–96 emulsified with incomplete Freund's adjuvant (IFA) were more efficient in inducing antigen-specific tolerance and preventing EAE. Importantly, i.v. injection of Ii-MBP84–96 or Ii-PLP139–151 after disease induction completely suppressed EAE, demonstrating the qualities of recombinant Ii proteins as potent antigen delivery systems for the treatment of autoimmunity.
Materials and Methods
Mice.
SJL mice were bred under specific pathogen-free conditions at the Netherlands Cancer Institute. In all experiments, 8- to 12-week-old female mice were used according to approved protocols.
Antigens and Recombinant Proteins.
MBP84–96 (VHFFKNIVTPRTP) and PLP139–151 (HCLGKWLGHPDKF) were synthesized by using standard g-fluorenylmethoxycarbonyl (Fmoc) chemistry and then purified by HPLC. The cDNA-encoding mouse Ii was obtained by reverse transcription of total RNA from the mouse B cell line L243. The full-length cDNA of Ii was assembled from two PCR fragments that were separately amplified by PCR. For amplification of the 5′ fragment, the primers Mii5 (5′-AGTTATCGATGCGCCTGTGGGAAAAACTAGA-3′; ClaI site underlined), and the primer Mii3 (5′-GGGAGTGGCCATCCGCATCTGGCTCACCGGTTTGGCAGATTT-3′; MscI-site underlined and an AgeI site in bold) were used. The 3′ fragment was amplified with the primers Mii4 (5′-TGCGGATGGCCACTCCCTTGCTGATGCGGCCGATGTCCATGGATAACATGC-3′; MscI site underlined and EagI site in bold) and Mii6 (5′-AGTTCCGCGGCTGCTGCTGTGCAGAGCTGGC-3′; SacII site underlined). The resulting PCR products were polished by Pfu DNA polymerase (Stratagene) and cloned into the EcoRV site of pBluescript KS+ (Stratagene). The 5′ fragment was cut with ClaI and MscI and the 3′ fragment with MscI and SacII, after which both fragments were ligated into pBluescript KS+ (resulting clone with correct sequence: 405–1). The additional internal SacI and AgeI sites were removed by using a PCR-based “megaprimer” method (10, 11). The first PCR was performed with the primers Mii5 (5′-AGTTATCGATGCGCCTGTGGGAAAAACTAGA-3′) and MIi1 (5′-GAGACTCCGGTGTACAGGGCGCCACGGCTGCACCTTTC-3′; mutagenesis sites underlined). The resulting PCR product was used as a megaprimer together with the primer MIi6 and 1 ng of KpnI-digested plasmid 405–1 as template for the second PCR. The second PCR product was then used as template for a PCR with the primers MIi5 and MIi6. The final PCR product was cut with ClaI and SacII and cloned into pBluescript KS+ (resulting clone with the correct sequence: 410–4). For bacterial protein expression of the mouse Ii, we ligated the Pfu-polished HindIII–SacII fragment of clone 410–4 (coding for the luminal part of mIi amino acid 80–219) into SmaI-linearized pQE 31 vector (Qiagen, Hilden, Germany) to generate pQE 31-mIi. All constructs were verified by DNA sequencing using the AmpliTaq FS Dye Deoxy Terminator Cycle Sequencing Kit (Applied Biosystems) and the PE-ABI PRISM 310 automated sequencer (Applied Biosystems). To exchange the wild-type CLIP segment for T cell epitopes, we used the CLIP-flanking AgeI and EagI sites. To generate Ii-MBP84–96, we mixed the oligonucleotides 5′-CCGGTGAGCCAGGTACACTTCTTCAAGAATATAGTGACGCCGAGGACCCCAC-3′ and 5′-GGCCGTGGGGTCCTCGGCGTCACTATATTCTTGAAGAAGTGTACCTGGCTCA-3′ (10 μM final concentration each) in 30 mM Tris⋅HCl, 10 mM MgCl2, 10 mM DTT, and 0.5 mM ATP (pH 7.8). The oligonucleotides were denaturated at 94°C for 2 min, annealed for 1 min at 70°C, and cooled down slowly to room temperature. The double-stranded fragment was ligated into the AgeI/EagI sites of pQE 31-mIi. Ii-PLP139–151 was created accordingly.
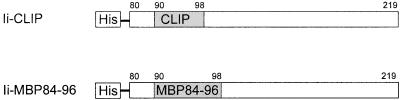
The final protein Ii-CLIP (Fig. 1) contains the N-terminal his tag and amino acids 80–219 of the murine Ii (MRGSHHHHHHTDPHASSVPQLPKSAKPV… . . VTL). The fusion protein Ii-MBP84–96 is thus Ii-CLIP with the CLIP core region (Ii90–98) replaced by the epitope MBP84–96 (MRGSHHHHHHTDPHASSVPQLPKSAKPVSQVHFFKNIVTPRTPRPMSMDN..VTL; MBP84–96 underlined). Both proteins were expressed in the bacterial strain M15[pREP4] (Qiagen) and purified under denaturing conditions using Ni-NTA Sepharose columns (Qiagen). The recombinant proteins were extensively dialyzed against PBS, and purity was confirmed by SDS/PAGE analysis. Proteins were then stored at −80°C.
Figure 1.
Design of the proteins. Depicted numbers indicate the amino acids of the murine Ii. The N-terminal hydrophobic region was replaced by a His6 tag. The CLIP core region at amino acids 90–98 was replaced by the epitope MBP84–96 to generate Ii-MBP84–96.
T Cell Proliferation Assays.
Mice were immunized in each hind footpad with 50 μl of antigen emulsified with complete Freund's adjuvant (CFA; Difco), and the popliteal draining lymph nodes were removed 10 days later. Lymph nodes of four mice per group were pooled in all experiments, and single cell suspensions were prepared. The cells were cultured in 96-well plates at 6 × 105 cells per well in 200 μl of HL-1 medium (BioWhittaker) containing 50 μM β-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin (all from Bio Whittaker). Titrated concentrations of the indicated antigen were tested in triplicate. As a positive control, purified protein derivative (PPD, Statens Serum Institut, Copenhagen), a constituent of CFA, was added at a concentration of 60 μg/ml, and at least 12 wells without antigen served as negative controls. After 96 h of culture, 1 μCi/well [3H]thymidine was added and the cells were harvested 12 h later. Incorporation of thymidine was measured by liquid scintillation counting. Proliferation data were presented as cpm.
Antigen Processing Assays.
Single cell suspensions prepared from spleen cells of naïve mice were irradiated at 5,000 rad. Antigen uptake by the spleenic antigen-presenting cells was blocked by fixing the cells with 1% paraformaldehyde (Sigma) for 15 min and subsequently washing them with 3.6% (wt/vol) dl-lysine (Sigma) for 30 min. The cells were seeded in 96-well microtiter plates at 2 × 104 cells per well and pulsed with the indicated amounts of antigen. A total of 5 × 104 cells per well of the T cell hybridoma 6F11 were then added. 6F11 is specific for MBP84–96 presented by I-As, the MHC II haplotype of SJL mice. After 36 h incubation, the IL-2 concentrations in the supernatants were determined by the proliferation of IL-2-dependent CTLL-2 cells. Five thousand CTLL-2 cells per well were incubated with 75 μl of cell culture supernatant. After 24 h, 1 μCi/well [3H]thymidine was added, and the cells were harvested 12 h later. Incorporation of thymidine was measured by liquid scintillation counting. Proliferation against titrated amounts of recombinant IL-2 (Cetus) was determined in each assay and used to calculate the IL-2 concentrations in the culture supernatants.
Induction and Assessment of EAE.
EAE was induced in mice by s.c. injection of 100 μl of an antigen/CFA emulsion spread over four sites in the flanks. The emulsion contained the indicated amounts of antigen and 200 μg Mycobacterium tuberculosis H37RA (Difco). For MBP84–96-induced EAE, mice were immunized on days 0 and 7 and in addition received 200 ng pertussis toxin (List Biological Laboratories, Campbell, CA) in PBS i.v. at days 0 and 2. PLP139–151-EAE was induced by a single s.c. injection of 50 nmol PLP139–151 in CFA and a single i.v. injection of 200 ng pertussis toxin at day 0. Clinical signs of EAE were assessed by using the following scores: 0, no apparent abnormalities; 1, tail or hind limb weakness; 2, limp tail and hind limb weakness; 3, severe hind limb paresis; 4, complete hind limb paralysis and front limb weakness; 5, dead (dead mice were scored 5 if they had previously shown signs of progressive disease).
Induction of Antigen-Specific Tolerance.
Mice were injected i.p. with 100 μl of an emulsion of IFA containing the indicated amount of antigen. In vitro tolerance was assessed by immunizing the mice in the hind footpads with antigen emulsified in CFA 10 days after the tolerizing regimen. After an additional 10 days, the popliteal lymph nodes were removed for in vitro proliferation assays as described above. In vivo tolerance, with regard to EAE induction, was determined by immunizing the mice for EAE induction 10 days after the tolerizing regimen. An alternative method of tolerance induction used was to inject mice i.v. with antigen in saline. This method was used according to Critchfield et al. (12) to determine whether disease could be suppressed after its commencement.
Results
Ii-MBP84–96 Activates MBP84–96-Primed T Cells in Vitro.
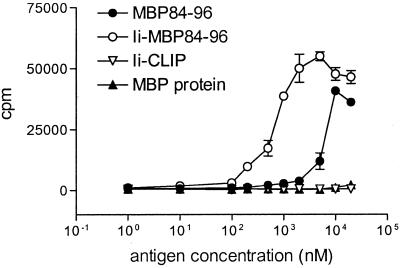
The peptide MBP84–96 can be presented by I-As MHC class II molecules and is an immunodominant MBP epitope in SJL mice (8). First, we assessed whether Ii-MBP84–96 is able to stimulate MBP84–96-specific T cells in vitro. T cells generated by MBP84–96 immunization proliferated when cultured with MBP84–96 or the fusion protein Ii-MBP84–96 but not with the control protein Ii-CLIP (Fig. 2). Relative to MBP84–96, ≈20-fold lower concentrations of Ii-MBP84–96 were sufficient for equivalent T cell activation. MBP did not induce any proliferation. Ii-MBP84–96 is thus more efficient than peptide in activating specific T cells in vitro. This enhanced antigenicity is Ii-specific, because MBP, which also contains the epitope MBP84–96, is not more immunogenic than the peptide.
Figure 2.
Lymph node cells of mice immunized with MBP84–96 proliferate in vitro when stimulated with Ii-MBP84–96 and MBP84–96 but not Ii-CLIP. Mice were immunized s.c. with 10 nmol MBP84–96 in CFA containing 200 μg M. tuberculosis. At day 10 postimmunization, single cell suspensions of draining lymph nodes were cultured along with the indicated concentrations of MBP84–96, Ii-MBP84–96, Ii-CLIP, or MBP. A total of 1 μCi/well [3H]thymidine was added after 96 h of culture, cells were harvested after an additional 12 h, and mean [3H]thymidine incorporation was measured by liquid scintillation counting. Background incorporation was less than 1,000 cpm and proliferation to the control antigen PPD, which is a component of the adjuvant, was >20,000 cpm (data not shown). Error bars represent SEM.
Ii-MBP84–96 Requires Endogenous Antigen Processing to Stimulate MBP84–96-Specific T Cells.
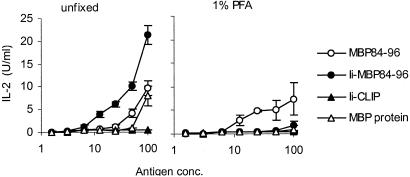
We next examined whether stimulation of MBP84–96-specific T cells by Ii-MBP84–96 requires endogenous processing by antigen-presenting cells or whether the fusion protein directly binds to class II molecules on the cell surface. Presentation of MBP84–96 by I-As molecules was detected with the specific T cell hybridoma 6F11. Blocking antigen uptake and endogenous antigen processing by antigen-presenting cells with paraformaldehyde (PFA) abolished completely the ability of Ii-MBP84–96 to stimulate 6F11 (Fig. 3). In contrast, the ability of MBP84–96 to activate 6F11 was not affected by PFA. Thus, the Ii protein, but not the peptide, requires uptake and endogenous antigen processing by antigen-presenting cells for efficient loading of MHC class II with the antigenic epitope. Consistent with the previous assay, Ii-MBP84–96, but not MBP, was more potent in stimulating 6F11 than the peptide.
Figure 3.
Ii-MBP84–96 requires endogenous antigen processing to stimulate MBP84–96-specific T cells. Spleen cells of naive mice were used unfixed or fixed with 1% paraformaldehyde and incubated along with titrated amounts of the indicated antigens. The MBP84–96-specific T cell hybridoma 6F11 was added for 36 h, and the IL-2 concentration in the supernatant was determined by proliferation of the IL-2-dependent cell line CTLL-2.
Ii-MBP84–96 Efficiently Primes MBP84–96-Specific T Cells in Vivo.
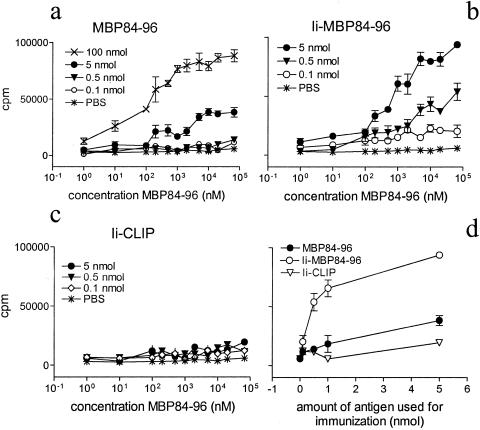
To determine whether Ii-MBP84–96 is capable of activating MBP84–96-specific T cells in vivo, mice were immunized with different amounts of either MBP84–96, Ii-MBP84–96, Ii-CLIP, or PBS emulsified with CFA. Primary T cell cultures from draining lymph nodes were then assessed for proliferation in response to titrated amounts of MBP84–96 (Fig. 4). The T cells from mice immunized with MBP84–96 and Ii-MBP84–96, but not Ii-CLIP or PBS, proliferated when cultured with MBP84–96. Amounts as low as 0.5 nmol Ii-MBP84–96 per mouse were sufficient for priming a substantial response in vivo, whereas 10 times as much peptide was required to induce comparable proliferation. In all groups, proliferation induced by the control antigen PPD was >40,000 cpm (data not shown), indicating that all immunizations had been effective. The Ii fusion protein is thus more potent than peptide in priming naïve T cells in vivo.
Figure 4.
MBP84–96 and Ii-MBP84–96, but not Ii-CLIP, can prime MBP84–96-specific T cells in vivo. Mice were immunized s.c. with the indicated amount of MBP84–96 (a), Ii-MBP84–96 (b), or Ii-CLIP (c) in CFA containing 200 μg M. tuberculosis. Ten days later, single cell suspensions of draining lymph nodes were cultured in vitro along with the indicated concentrations of MBP84–96. After 96 h of culture, 1 μCi/well [3H]thymidine was added, cells were harvested after an additional 12 h, and proliferation was measured by liquid scintillation counting. Proliferation induced by the control antigen PPD was >40,000 cpm (data not shown) and background incorporation was less than 2,500 cpm in all cases. (d) The proliferation at the highest in vitro dose of MBP84–96 was plotted against the amount of antigen used for immunization.
Ii-MBP84–96 Is More Efficient at Inducing Autoimmunity than MBP84–96.
Immunization with peptide MBP84–96 can break self-tolerance and induce autoimmunity in SJL mice (8). Based on the above data, we reasoned that Ii-MBP84–96 might be able to induce EAE. Mice immunized with either 10 or 5 nmol MBP84–96 developed weak disease symptoms, after which they recovered completely. They were free of symptoms 45 days after immunization (Fig. 5). A dose of 1 nmol MBP84–96 did not induce any signs of EAE. In contrast, the Ii protein at equivalent concentrations induced much more severe disease in all experimental groups. The course of the disease was a relapsing-remitting one, and moderate to severe symptoms were observed until the end of the observation period at day 60. These data document that the potency of the Ii protein to induce in vivo T cell effector functions (breaking of self-tolerance and induction of autoimmunity) is dramatically enhanced as compared with the peptide epitope.
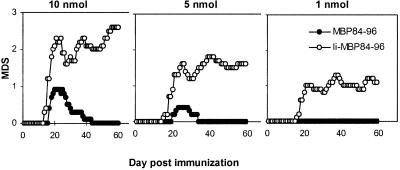
Figure 5.
Ii-MBP84–96 is more efficient in induction of EAE than MBP84–96. Groups of 10 mice were immunized on days 0 and 7 with 10, 5, or 1 nmol of either MBP84–96 or Ii-MBP84–96 in CFA containing 200 μg M. tuberculosis. On days 0 and 2, mice received in addition 200 ng pertussis toxin in PBS i.v. Clinical signs of EAE were assessed as described in Materials and Methods, and the mean disease score (MDS) was plotted against the day postimmunization. The incidence rates were determined as 10/10 in each of the groups immunized with 10, 5, and 1 nmol Ii-MBP84–96 and 8/10, 4/10, and 0/10 in the groups immunized with 10, 5, and 1 nmol MBP84–96, respectively.
Induction of Antigen-Specific Tolerance and Prevention of Autoimmunity with Ii-MBP84–96.
We next asked whether Ii-MBP84–96 is primarily more immunogenic than peptide or whether the Ii protein has in addition the capacity to induce antigen-specific tolerance in vivo. Antigenic peptides can induce tolerance in specific T cells when given i.p. in an emulsion with IFA (13). Mice therefore were treated with different amounts of either MBP84–96, Ii-MBP84–96, or PBS in IFA. Ten days later, they were immunized with MBP84–96 in CFA, and the T cell reactivity was determined by proliferation assays of draining lymph node cells. The i.p. antigen application suppressed the subsequent T cell proliferation in a dose-dependent manner (Fig. 6). About 20-fold lower amounts of Ii protein than peptide were required to achieve comparable reduction of MBP84–96-specific T cell responses. The induction of T cell unresponsiveness was specific for this epitope as the response to the control antigen PPD was unaltered and higher than 30,000 cpm in all experimental groups (data not shown).
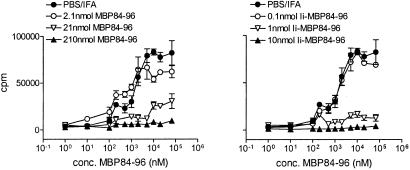
Figure 6.
Ii-MBP84–96 is more efficient than MBP84–96 in suppression of antigen-specific T cell responses. Mice received an i.p. injection of the indicated amount of MBP84–96, Ii-MBP84–96, or PBS emulsified in IFA at day 10 before s.c. immunization with 100 nmol MBP84–96 in CFA. Ten days postimmunization, proliferation of the draining lymph node cells cultured with MBP84–96 was determined as described in the legend to Fig. 4. Error bars indicate SEM. Background incorporation was between 1,450 and 5,400 cpm. The response to the control antigen PPD was >30,000 cpm in all cases.
The higher potency of the Ii protein in suppressing specific T cell reactivity was paralleled by its ability to prevent the development of autoimmunity. Intraperitoneal injections of different amounts of either MBP84–96, Ii-MBP84–96, or PBS in IFA and subsequent EAE induction (Fig. 7) showed that about 20-fold lower amounts of the fusion protein as compared with the peptide were sufficient to suppress EAE symptoms to an equivalent degree.
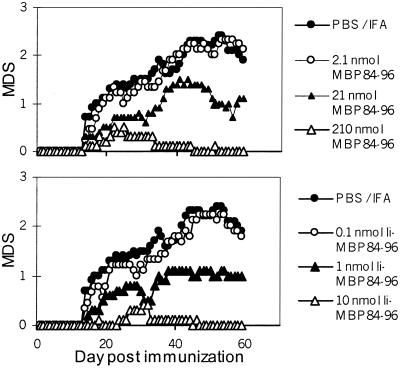
Figure 7.
Ii-MBP84–96 is more efficient than MBP84–96 in prevention of autoimmunity. Ten mice per group were injected i.p. with 100 μl of an emulsion containing the indicated amount of either MBP84–96, the fusion protein Ii-MBP84–96, or PBS in IFA. Ten days later, EAE was induced by s.c. injection of 130 nmol MBP84–96 emulsified in CFA at days 0 and 7 and i.v. injection of 200 ng pertussis toxin at days 0 and 2. EAE disease score was determined as described in Materials and Methods. The disease incidence for mice injected with PBS/IFA was 10/10, for mice injected with 2.1, 21, and 210 nmol MBP84–96, 9/9, 8/10, and 4/10 for mice that received 0.1, 1, and 10 nmol Ii-MBP84–96 8/8, 8/10, and 3/10, respectively.
Potent Treatment of EAE by i.v. Injection of Ii Proteins.
Intravenous injection of high antigen doses leads to T cell deletion and is able to abrogate ongoing EAE (12). To assess the possibility that Ii proteins could be used in the antigen-specific treatment of autoimmunity, mice were injected with either PBS alone, 50 nmol MBP84–96, or 50 nmol Ii-MBP84–96 in PBS on days 7 and 11 after EAE induction with MBP84–96. Ii-MBP84–96-treated mice were completely free of EAE symptoms, whereas the disease was only marginally suppressed by MBP84–96 (Fig. 8a). We then verified this finding in a second disease model, PLP139–151-induced EAE (9). Intravenous injection of 30 nmol Ii-PLP139–151 completely abolished PLP139–151-induced EAE, whereas similar injection of the peptide PLP139–151 did not significantly alter disease symptoms (Fig. 8b). PLP-induced EAE in SJL mice is more severe than MBP-induced EAE and the differences in disease severity between the peptide and the Ii protein-treated groups in this second model were even more pronounced than in EAE induced with the MBP epitope.
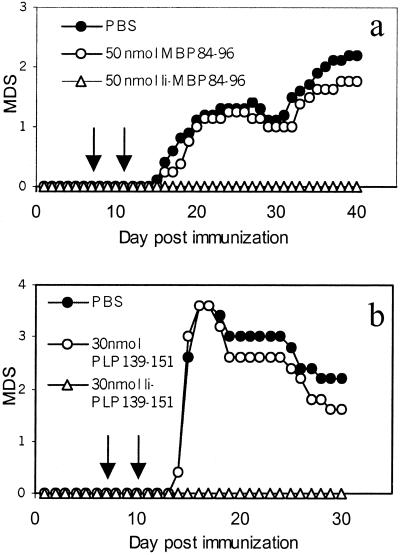
Figure 8.
Suppression of EAE by i.v. injection of recombinant Ii proteins. (a) EAE was induced by immunization with 130 nmol of MBP84–96 and i.v. injection of pertussis toxin as described in the legend to Fig. 7. At days 7 and 11 (arrows) mice received either 50 nmol MBP84–96, 50 nmol Ii-MBP84–96, or PBS i.v. Disease severity was scored as described in Materials and Methods. The incidence rates for the PBS- and MBP84–96-treated groups were 10/10 and for the Ii-MBP84–96-treated group 0/10. (b) EAE was induced by s.c. injection of 50 nmol PLP139–151 in CFA and i.v. injection of 200 ng pertussis toxin. Experimental groups received i.v. injections of PBS, 30 nmol PLP139–151, or 30 nmol Ii-PLP139–151, respectively. The incidence rates were 5/5 in the PBS- and peptide-treated groups and 0/5 in the Ii-PLP139–151- treated group. The results are representative of three independent experiments. MDS, mean disease score.
Discussion
Presentation of antigenic epitopes by MHC molecules to T cells is an essential step in the generation of an adaptive immune response. The chaperone molecule Ii has evolved to form complexes with MHC class II molecules, in which the CLIP region of Ii is targeted to the peptide binding groove. This study shows that Ii proteins with the CLIP region replaced by antigenic epitopes are superior to peptide epitopes in triggering specific T cells in vitro and in vivo and demonstrate the eligibility of Ii as a vehicle for improved antigen delivery in vivo.
We first established that placing the T helper epitope MBP84–96 in the CLIP region of Ii does not alter its antigenic specificity. We showed that the recombinant protein Ii-MBP84–96 activates MBP84–96-specific T cells both in vitro and in vivo, demonstrating that the epitopes generated from the peptide and the Ii protein are at least partially identical. Using polyclonal, primary T cell cultures and the specific T cell hybridoma 6F11, we assessed that the Ii protein was 10–20 times more efficient than peptide in activating antigen-specific T cells in vitro. The Ii protein was in addition superior to peptide in priming naive antigen-specific T cells in vivo and, importantly, in inducing in vivo T cell effector functions as documented by breaking of self-tolerance and the induction of autoimmunity.
Interestingly, the Ii-derived sequences themselves did not activate the T cells but they rather enhanced the T cell triggering capacity of the epitope, irrespective of whether this results in activation or inactivation. This was demonstrated by induction of antigen-specific tolerance in vivo. When delivered in a tolerogenic manner by i.p. injection in emulsion with IFA, Ii-MBP84–96 eliminated the proliferative capacity of antigen-specific T cells more potently than equivalent amounts of the peptide. Moreover, similar in vivo tolerance induction by the Ii protein efficiently prevented the subsequent evolvement of autoimmunity. This potency of the Ii proteins to suppress specific immune responses may be even more important than their immunostimulatory properties, and we therefore assessed whether the Ii proteins also would be feasible in suppressing the disease after its commencement.
Indeed, whereas i.v. injection of peptides only marginally suppressed disease symptoms, similar application of the Ii proteins completely abolished the disease. This finding points the way toward a possible therapeutic application of Ii proteins in the antigen-specific treatment of autoimmunity, and it was therefore assessed in two disease models, MBP84–96-induced EAE and PLP139–151-induced EAE. In both experimental systems, the Ii protein potently suppressed the disease, demonstrating that not only the epitope MBP84–96, but also PLP139–151 profits from Ii-derived sequences. This finding further indicates that this approach may be easily adaptable to other T helper epitopes. Taken together, these data consistently demonstrate the capacity of Ii proteins to suppress immune responses in vivo and suggest their potential use in the antigen-specific treatment of autoimmunity.
How do the Ii proteins improve the T cell-triggering capacity of antigenic epitopes? First, we showed that this effect was Ii-specific and not simply a consequence of the larger antigen size by directly comparing Ii-MBP84–96 with MBP. MBP, which also contains the epitope MBP84–96, was, in contrast to Ii-MBP84–96, not more immunogenic than the peptide. This finding is consistent with our previous observation that an Ii protein, in which the CLIP region is replaced by a T helper epitope from the human acetylcholine receptor α chain (αAChR), is 2 orders of magnitude more efficient in stimulating specific T cell clones than the recombinant αAChR (6). Moreover, the antigenicity of a T helper epitope is only enhanced when it is placed in the CLIP region and not when fused to the C terminus of Ii (6). This enhanced antigenicity is most likely accomplished by sequences on both sides of the CLIP region. These flanking residues bind to the MHC molecule in an allotype-independent manner (14, 15) and thereby increase the affinity of the epitope to the class II molecule (16). In wild-type Ii, the CLIP-flanking sequences enable the stable association to the class II molecule, whereas the CLIP region itself, while being able to interact with many different MHC alleles, usually binds with only moderate affinity (2). The CLIP region and the flanking residues (Ii72–110) form a functional domain that becomes structurally ordered upon binding to the MHC molecule and is then protected from proteolytic degradation (17). It is unlikely that protection from extracellular proteolysis contributes much to the improved antigenicity, because the epitope would be similarly protected in MBP, and MBP is not more immunogenic than the peptide. In addition, inhibition of extracellular proteolytic degradation would not be expected to enhance the T cell-stimulating capacity of the epitope to the same extend in vitro and in vivo, as observed in this study. In theory, the Ii proteins could act also by crosslinking T cell receptors via their C-terminal homotrimerization domain. This would imply that the Ii proteins remain relatively intact while the epitope is presented by the class II molecule on the surface of the antigen-presenting cell. However, the fact that the Ii proteins are taken up by the antigen-presenting cell and undergo endogenous processing (a process that usually involves sequential proteolytic degradation) before they are loaded onto the MHC molecules, makes this scenario unlikely.
The approach described here should be clearly distinguished from Ii-fusion proteins that use the N-terminal endosomal targeting sequence of Ii to drive endogenous antigens into the class II loading compartment (18). In contrast to such proteins, the Ii proteins used in this study take advantage of the ability of Ii to interact with the class II molecule in a way that targets the CLIP region into the peptide binding groove. They are applied as exogenous antigens and thus follow the endocytic pathway into the class II loading compartment. The endosomal targeting sequences of Ii are not required and are not present in these constructs.
In conclusion, Ii proteins with the CLIP region replaced by antigenic epitopes represent an efficient way to activate and down-regulate specific T cells in vivo, induce antigen-specific tolerance, and suppress autoimmunity. This approach may in addition be feasible for tolerance induction by oral antigen application (19) or DNA vaccination (20) or to induce immunity against infectious agents or tumors.
Ii fusion proteins may be particularly advantageous in activating and silencing self-specific T cells, as these cells are usually of low affinity and more difficult to trigger. Oligomerization of T helper epitopes has been proven to be very potent in enhancing the immunogenicity as well as the tolerance-inducing capacity of antigenic epitopes in vivo (21). How the Ii proteins described here compare in efficacy with oligomerized T cell epitopes remains to be established.
Acknowledgments
We thank Marjan Smook for performing some of the experiments resulting in Fig. 2, Marie Anne van Halem for help with preparation of the manuscript, and Kirsten Falk and Olaf Rötzschke for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (Bi 603/2 to F.B.), the American Multiple Sclerosis Society (RG-2940-A-1 to A.M.K.), and the European Commission (CT960069 to G.M.). A.M. is supported by the Deutsche Forschungsgemeinschaft Sonderforschungsbereich 510, the Federal Ministry of Education, Science, Research and Technology, Germany (Fö.01KS9602), and the Interdisciplinary Clinical Research Center (IZKF) Tübingen, Germany.
Abbreviations
- Ii
invariant chain
- MBP
myelin basic protein
- EAE
experimental allergic encephalomyelitis
- PLP
proteolipid protein
- IFA
incomplete Freund's adjuvant
- CFA
complete Freund's adjuvant
- PPD
purified protein derivative
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ghosh P, Amaya M, Mellins E, Wiley D C. Nature (London) 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 2.Malcherek G, Gnau V, Jung G, Rammensee H G, Melms A. J Exp Med. 1995;181:527–536. doi: 10.1084/jem.181.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avva R R, Cresswell P. Immunity. 1994;1:763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 4.Bijlmakers M J, Benaroch P, Ploegh H L. J Exp Med. 1994;180:623–629. doi: 10.1084/jem.180.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freisewinkel I M, Schenck K, Koch N. Proc Natl Acad Sci USA. 1993;90:9703–9706. doi: 10.1073/pnas.90.20.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcherek G, Wirblich C, Willcox N, Rammensee H G, Trowsdale J, Melms A. Eur J Immunol. 1998;28:1524–1533. doi: 10.1002/(SICI)1521-4141(199805)28:05<1524::AID-IMMU1524>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.van Bergen J, Schoenberger S P, Verreck F, Amons R, Offringa R, Koning F. Proc Natl Acad Sci USA. 1997;94:7499–7502. doi: 10.1073/pnas.94.14.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai K, Zamvil S S, Mitchell D J, Lim M, Rothbard J B, Steinman L. J Neuroimmunol. 1988;19:21–32. doi: 10.1016/0165-5728(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 9.Tuohy V K, Lu Z, Sobel R A, Laursen R A, Lees M B. J Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- 10.Barik S, Galinski M S. BioTechniques. 1991;10:489–490. [PubMed] [Google Scholar]
- 11.Sarkar G, Sommer S S. BioTechniques. 1990;8:404–407. [PubMed] [Google Scholar]
- 12.Critchfield J M, Racke M K, Zuniga-Pflucker J C, Cannella B, Raine C S, Goverman J, Lenardo M J. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 13.Gaur A, Wiers B, Liu A, Rothbard J, Fathman C G. Science. 1992;258:1491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- 14.Siebenkotten I M, Carstens C, Koch N. J Immunol. 1998;160:3355–3362. [PubMed] [Google Scholar]
- 15.Thayer W P, Ignatowicz L, Weber D A, Jensen P E. J Immunol. 1999;162:1502–1509. [PubMed] [Google Scholar]
- 16.Carstens C, Newman D K, Bohlen H, Konig A, Koch N. Int Immunol. 2000;12:1561–1568. doi: 10.1093/intimm/12.11.1561. [DOI] [PubMed] [Google Scholar]
- 17.Jasanoff A, Song S, Dinner A R, Wagner G, Wiley D C. Immunity. 1999;10:761–768. doi: 10.1016/s1074-7613(00)80075-8. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson S, Frauwirth K, Shastri N. Proc Natl Acad Sci USA. 1995;92:7217–7221. doi: 10.1073/pnas.92.16.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner H L, Mackin G A, Matsui M, Orav E J, Khoury S J, Dawson D M, Hafler D A. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 20.Lobell A, Weissert R, Storch M K, Svanholm C, de Graaf K L, Lassmann H, Andersson R, Olsson T, Wigzell H. J Exp Med. 1998;187:1543–1548. doi: 10.1084/jem.187.9.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falk K, Rotzschke O, Santambrogio L, Dorf M E, Brosnan C, Strominger J L. J Exp Med. 2000;191:717–730. doi: 10.1084/jem.191.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]