Abstract
Please cite this paper as: Ison. (2012) Clinical use of approved influenza antivirals: therapy and prophylaxis. Influenza and Other Respiratory Viruses 7(Suppl. 1), 7–13.
Currently, there are two commonly used classes of antiviral agents approved for the prevention of and treatment for influenza: the M2 Inhibitors (amantadine and rimantadine) and the neuraminidase inhibitors (oseltamivir, laninamivir, peramivir and zanamivir). These agents have been proven to be safe and effective alone or in combination for the treatment of uncomplicated influenza in otherwise healthy individuals. Although there are few prospective, randomized studies of these antivirals for the treatment of pregnant women, hospitalized patients, and immunocompromised patients infected with seasonal, pandemic, or avian H5N1 influenza, these agents are widely used for these indications. This article reviews the pharmacokinetics and clinical data available relative to the use of commercially available antiviral agents for the prevention of and treatment for influenza.
Keywords: Adamantanes, antiviral agents, influenza, M2 inhibitors, neuraminidase inhibitors
Introduction
Currently, there are two commonly used classes of antiviral agents approved for the prevention of and treatment for influenza: the M2 Inhibitors (amantadine and rimantadine) and the neuraminidase inhibitors (oseltamivir, laninamivir, peramivir and zanamivir). These agents have been proven to be safe and effective alone or in combination for the treatment of uncomplicated influenza in otherwise healthy individuals. Although there are few prospective, randomized studies of these antivirals for the treatment of pregnant women, hospitalized patients, and immunocompromised patients infected with seasonal, pandemic, or avian H5N1 influenza, these agents are widely used for these indications. The basic pharmacokinetics and data supporting the use of these agents are reviewed. Guidelines for the use of existing antivirals are updated regularly and should be consulted prior to use (see Table 1).
Table 1.
Existing guidelines for the treatment and prevention of influenza
| World Health Organization |
| WHO guidelines for pharmacological management of pandemic influenza A(H1N1) 2009 and other influenza viruses |
| http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf |
| US Advisory Committee on Immunization Practices (ACIP) |
| Fiore AE, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR. 2011; 60(RR01): 1–24 4 |
| http://www.cdc.gov/flu |
| UK National Institute for Health and Clinical Excellence |
| TA168: Amantadine, oseltamivir, and zanamivir for the treatment of influenza |
| http://egap.evidence.nhs.uk/amantadine-oseltamivir-and-zanamivir-for-the-treatment-of-influenza-ta168 |
M2 Inhibitors
Pharmacokinetics
The commercially available M2 inhibitors amantadine and rimantadine come as 100 mg tablets (and capsules in the case of amantadine) in addition to an oral solution and syrup (10 mg/ml) (See Table 2). 1 , 2 Both amantadine and rimantadine inhibit the M2 proton channel that allows for uncoating of the virus in the endosome, thereby inhibiting the replication of susceptible influenza A viruses at low concentrations (<1·0 μg/ml). 1 , 2 Although these agents are approved for prevention of and treatment for influenza A, widespread resistance among most currently circulating viruses precludes their use in most cases. 3 , 4 Both agents have excellent bioavailability and reach peak serum levels around 4 hours following ingestion. 1 , 2 Amantadine is predominately excreted unchanged in the urine by glomerular filtration and tubular secretion with a plasma elimination half‐life of approximately 11–15 hours in patients with normal renal function; elimination is prolonged in patients with renal insufficiency and in the elderly. 1 , 2 Rimantadine undergoes extensive metabolism in the liver before being excreted in the urine, resulting in a plasma half‐life of 24–36 hours and therefore only needs dose adjustment with serious renal (creatinine clearance ≤10 ml/min) or hepatic insufficiency. 1 , 2 Both agents are pregnancy class C drugs, which means that animal reproduction studies have shown an adverse effect on the fetus, and there are no adequate and well‐controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. Major side effects include central nervous system side effects (confusion, disorientation, mood alterations, memory disturbances, delusions, nightmares, ataxia, tremors, dizziness, anxiety, irritability, headache, slurred speech, visual disturbances, delirium, occulogyric episodes and hallucinations), gastrointestinal upset and anti‐muscarinic effects. 1 , 2 Compared to amantadine, rimantadine causes significantly fewer CNS side effects in both young and elderly adults. 1 , 2 , 5
Table 2.
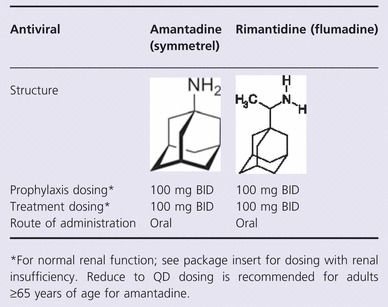
Prophylaxis
Both M2 inhibitors have been studied and approved for the prevention of influenza (34–85% effective). 5 , 6 Comparative effectiveness studies between the two agents suggest similar efficacy. 5 , 6 Post‐exposure prophylaxis also appears to be effective with these agents for susceptible strains, but not in households when the index case is also given treatment because of the transmission of M2 inhibitor‐resistant variants. 5 , 6
Therapy
Both M2 inhibitors have been studied and approved for the treatment of influenza. In placebo‐controlled studies, amantadine and rimantadine treatment is able to shorten the duration of fever by about 1 day. 5 , 6 Treatment is also associated with more rapid symptom resolution, functional recovery, and, in some studies, resolution of small airway functional abnormalities. 2 , 5 , 6 M2 inhibitors are associated with decreased progression to pneumonia and death in hematopoietic stem cell transplant patients and shorter duration of fever and hospitalization in hospitalized adults. 5 , 6 Available data suggest that the M2 inhibitors are safe and efficacious in reducing length of fever and illness in children older than 2 years of age, but data are far more limited in younger children. 7 , 8
Resistance
Resistance to the two agents occurs as a result of amino acid substitutions in the transmembrane portion of the M2 protein (position 26, 27, 30, 31, or 34) which result in reduced binding of the M2 inhibitors or in enlargement of the pore diameter; by either mechanism, the inherent function of the M2 pore is preserved in the presence of the inhibitor. 6 Resistance mutations emerging in vivo do not affect transmissibility or replication fitness as compared with wild‐type viruses; documented transmission from person to person has been well established. 6 Resistance affects both drugs in the class equally and appears to be persistent over time. Mutants may rapidly emerge within 2–4 days after the start of therapy in up to 30% of patients, more frequently in immunosuppressed individuals. 2 , 6 More recently, widespread resistance, as a result of the S31N mutation, among circulating influenza A(H3N2) and 2009 pandemic A(H1N1) viruses has rendered this class of antivirals ineffective. 3 , 4 , 6 , 9 The M2 inhibitors are also ineffective against all influenza B viruses. Resistance may be detected by plaque assays, which are not readily available, or by sequencing or pyrosequencing of the M2 gene. 6
Neuraminidase inhibitors
There are currently two neuraminidase inhibitors (NAIs) approved in most countries: oseltamivir (GS4104; Tamiflu®, Genentech, South San Francisco, CA, USA, and Chugai Pharmaceutical Co, Japan) and zanamivir (GG167; Relenza®, GlaxoSmithKline, Research Triangle Park, NC, USA) and two NAIs that are approved in more limited markets: laninamivir (CS08958; Inavir, Daiichi Sankyo, Japan, and Biota Holdings Ltd, Australia; approved in Japan only) and peramivir (BCX‐1812 and previously RWJ‐270201; Rapiacta® in Japan and Peramiflu in South Korea, BioCryst Pharmaceuticals, Birmingham, AL, USA) (See Table 3). 6 All 4 compounds inhibit the virus neuraminidase and thereby prevent destruction of sialic acid‐bearing receptors that are recognized by influenza A and B virus hemagglutinins. This prevents the virus from being released from infected cells and passing through respiratory secretions to initiate new cycles of replication, as the virions remain attached to the membrane of the infected cell and to each other; additionally, the NAIs may inhibit virus binding to cells. 10
Table 3.
Commercially available neuraminidase inhibitors 6
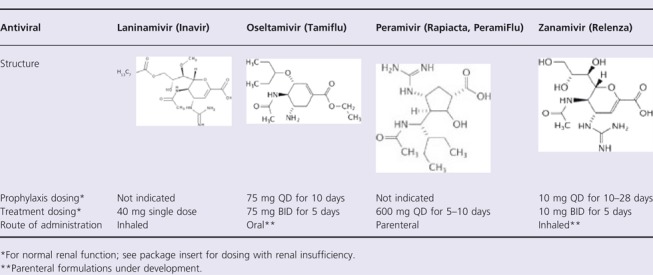
Laninamivir
Laninamivir octanoate (CS‐8958) is currently only licensed in Japan and is available as a 20‐mg dry powder inhaler. Laninamivir octanoate (CS‐8958) is a prodrug that is converted in the airway to laninamivir (R‐125489), the active neuraminidase inhibitor and is retained at concentrations that exceed the IC50 for most influenza neuraminidases for at least 240 hours (10 days) after a single inhalation of 40 mg. 11 Only 15% of the drug is orally bioavailable. Laninamivir has excellent in vitro activity, comparable or superior to other agents, against wild‐type influenza A and B viruses currently circulating, including those H1N1 viruses containing a H275Y mutation in the neuraminidase gene. Clinical studies in Asia found similar rates of nausea in laninamivir octanoate‐ and oseltamivir‐treated patients, lower rates of vomiting and similar to slightly higher rates of diarrhea in the laninamivir octanoate arm. 12 , 13 Dizziness was seen in 0·9–1·8% of laninamivir octanoate‐treated patients but not oseltamivir‐treated patients. 12 In studies in symptomatic children, laninamivir was associated with more rapid time to alleviation of influenza illness, while studies in adults demonstrated non‐inferiority versus oseltamivir. Of note, many of the patients in the adult study were infected with influenza viruses with a H275Y mutation, which confers resistance to oseltamivir but not laninamivir. 12 , 13
Oseltamivir
Oseltamivir is available in 30, 45, and 75 mg oral capsules and an oral suspension (6 mg/ml); not all formulations may be available in all countries. The ethyl ester prodrug (oseltamivir phosphate) is rapidly absorbed and converted by gastrointestinal tract, hepatic, and blood esterases to the active compound (oseltamivir carboxylate), achieving peak concentrations 3–4 hours following oral administration. 1 , 14 The carboxylate is renally cleared by both glomerular filtration and tubular secretion, and dose adjustment is required with renal dysfunction. 1 , 14 Protein binding is low and peak concentrations in the BAL, middle ear fluid and sinus approximate blood levels. Although the agent is active against circulating strains of influenza A and B, it is less active against influenza B, which has correlated with slower clinical responses. 15 , 16 Gastrointestinal side effects, mostly nausea and vomiting, occur in 10–15% of treated patients and are ameliorated with food; CNS adverse effects occur in ∼1% of treated patients. 1 , 14 In Japan, because of possible CNS side effects of oseltamivir, oseltamivir use is prohibited for treatment of patients with influenza who are 10–19 years old.
Oseltamivir has been shown to be 68–92% effective in preventing influenza‐proven illness after exposure of close contacts, such as household members, or as seasonal prophylaxis of otherwise healthy adults and children in the community. 14 In otherwise healthy, ambulatory adults and children, oseltamivir is associated with a significant reduction (0·5–4·1 days) in the length of illness as long as the medication is started within 48 hours after symptom onset, with greater impact on efficacy the earlier the onset of therapy. 14 Oseltamivir is also associated with a significant reduction in the use of antibiotic therapy for lower respiratory tract complications and/or otitis media. 17
Peramivir
Peramivir is available in 150 and 300 mg solutions for intravenous use as it has low oral bioavailability. Peramivir achieves exceptionally high maximum concentrations (∼45 000 ng/ml after a 600 mg IV dose) with excellent concentrations of drug in the nasal and pharyngeal secretions. 18 Peramivir is predominately eliminated unchanged by renal excretion with a plasma half‐life of 12–25 hours. 19 , 20 Peramivir has comparable or lower activity in vitro against influenza A and B viruses than oseltamivir carboxylate and zanamivir. 21 Recognized adverse events associated with the administration of peramivir are diarrhea, nausea, vomiting, and decreased neutrophil count; other less common adverse events observed in studies to date include dizziness, headache, somnolence, nervousness, insomnia, feeling agitated, depression, nightmares, hyperglycemia, hyperbilirubinemia, elevated blood pressure, cystitis, ECG abnormalities (prolonged QTc interval was observed in one patient in a phase 1 trial), anorexia, and proteinuria. 22
Peramivir has been studied in previously healthy adults and children infected with influenza. When compared to placebo, a single 300–600 mg infusion of peramivir was associated with a significantly shorter time to alleviation of symptoms, significantly shorter time to resumption of their usual activities, and more rapid clearance of virus. 23 Another study found that a single 300–600 mg infusion of peramivir was non‐inferior to 5 days of oral oseltamivir 75 mg BID in a season when many of the viruses were resistant to oseltamivir as the result of the H275Y mutation; these data question the efficacy of peramivir in the management of viruses with the H275Y mutation. 24 In a study comparing 5 days of 200 or 400 mg QD of peramivir with oral oseltamivir 75 mg BID in hospitalized adults, there was a trend toward more rapid resumption of usual activities in peramivir‐treated patients and greater reductions of influenza B virus titers in the nasopharynx than in oseltamivir‐treated patients over the first 48 hours.
Zanamivir
Zanamivir is available, pre‐packaged with the Diskhaler inhalation device, with four blisters, each containing 5 mg of zanamivir and 20 mg of lactose. Following inhalation of the dry powder, approximately 15% is deposited in the lower respiratory tract and the remainder in the oropharynx, where detectable levels remain for up to 24 hours. 1 , 14 The oral bioavailability of zanamivir is low, but can range from 4 to 17%. Zanamivir has comparable in vitro activity to oseltamivir for influenza A viruses with lower IC50 values against influenza B viruses. 1 , 14 Zanamivir is generally well tolerated, although it may cause cough, a reversible decrease in pulmonary function, or fatal bronchospasm in some patients, particularly those with underlying pulmonary disease. 1 , 14 The commercially available formulation should not be used for nebulization of intubated patients as it may cause ventilator failure and consequent death of the intubated patient. 25
Zanamivir has been shown to be 69–81% effective in preventing influenza‐proven illness after exposure for close contacts, such as household members, or as seasonal prophylaxis of otherwise healthy adults in the community. 14 In otherwise healthy ambulatory adults and children, zanamivir is associated with a significant reduction (1–3 days) in the length of illness as long as the medication is started within 48 hours after symptom onset, with a greater impact on efficacy the earlier the onset of therapy. 14 Zanamivir is also associated with a significant reduction in the use of antibiotic therapy for lower respiratory tract complications and/or sinusitis. 21
Resistance
Resistance to the neuraminidase inhibitors can develop as the result of mutations in the neuraminidase gene, the hemagglutinin gene, or both. 6 Each mutation results in different degrees of resistance dependent on the specific antiviral and the influenza type or subtype. The H275Y mutation has been most widely studied and is one of the most common resistance determinants among N1 subtypes of influenza A. 6 The mutation confers high‐level resistance to oseltamivir and 100‐fold reduction in peramivir susceptibility while laninamivir and zanamivir retain activity.
Combinations of approved antivirals
Combinations of multiple antivirals with different mechanisms of actions have been demonstrated to lead to improved clinical outcomes and protect against the emergence of resistance for numerous RNA virus infections. As such, combination therapy is considered an important potential option in the management of influenza. Two studies have provided significant insight into the role of antiviral combinations with approved agents. In the first study, hospitalized patients were randomized to receive rimantidine in combination with zanamivir or placebo in an era when most strains were susceptible to the M2 inhibitors. 26 Although the study was underpowered, the combination was associated with a statistically significant reduction in the severity of cough at day 3. 26 Furthermore, resistance to the M2 inhibitor emerged only in patients treated with rimantadine monotherapy, suggesting possible mitigation against resistance emergence by combination therapy. 26 In the second study, adults with seasonal influenza, mostly A(H3N2), were randomized to receive oseltamivir, zanamivir, or a combination of the two antivirals. 27 The oseltamivir–zanamivir combination appeared less effective, with fewer patients having an undetectable viral load at day 2 and longer time to resolution of illness, than oseltamivir monotherapy, and not significantly more effective than zanamivir monotherapy. 27
Special populations
Hospitalized adults
There are a number of factors that challenge clinical studies in patients hospitalized with influenza, and as a result few randomized studies have been performed and none have included a placebo arm. 28 Most data to support the use of antivirals in hospitalized adults and children are drawn from observational retrospective studies. 29 Given the limited data, the optimal dose, route, and duration of antiviral treatment for patients hospitalized with influenza have yet to be defined. 29 None‐the‐less, all of the studies to date have clearly documented that antivirals appear to be associated with reduced duration of virus detection, fever, and symptoms, reduced progression to pneumonia (OR 0·12, 95% CI 0·08–0·18), and reduction in the risk of death in severely ill, particularly those requiring admission to the ICU (OR, 24·2; 95% CI, 12–49); some studies have suggested that antivirals may be associated with reduced duration of hospitalization. 29 , 30 , 31 Likewise, although the greatest impact on morbidity and mortality appears to occur when antivirals are initiated within 48 hours after symptom onset, there is demonstrable benefit to therapy even begun up to 96 hours after symptom onset; there may be benefit for even later therapy, but the number of individuals studied, to date, has been small. 29 , 30 , 31 Lastly, because virus shedding, particularly in the lower airway, may be prolonged among hospitalized patients, particularly the elderly, those with underlying conditions, and those who receive steroids, may require courses of therapy longer than 5 days. 29 , 30 , 31
Immunocompromised adults
Stem cell and solid organ transplant patients, particularly lung transplant patients, have been clearly demonstrated to have higher morbidity and mortality following influenza infection. This is the result of higher rates of progression to viral pneumonia, higher frequencies of bacterial and fungal superinfections, prolonged virus replication with generally higher virus titers, and a resulting higher risk of resistance emergence. 32 , 33 , 34 Further, immune‐suppressed patients have a reduced response to influenza vaccination compared to healthy controls, particularly early post‐transplant and following rejection. 35 , 36 , 37 Although the optimal dose, route, and duration of antiviral treatment for influenza have yet to be defined, available data clearly demonstrate that early therapy is associated with a decreased risk of hospitalization, lower rates of ICU admission (8% versus 22%), and reduced mortality (1% versus 6%). 38 , 39 Further, antivirals may be associated with reduced development of chronic rejection among lung transplant recipients. 40 , 41
Pregnant women
There is a growing body of evidence from non‐randomized clinical experience, which clearly demonstrates a clinical benefit of the early use of antivirals in influenza‐infected individuals. Even though oseltamivir and zanamivir are classified as pregnancy category C drugs, transplacental transfer of metabolites appears to be low and the incidence of adverse maternal or fetal outcomes after exposure to oseltamivir has generally not been higher than background rates. 42 , 43 , 44 , 45 The available literature suggests a clear benefit of early antiviral use in terms of lower ICU admission (6% versus 31·5%) and mortality (0·5% versus 14·5%) among influenza‐infected pregnant women. 46 , 47 , 48 , 49 As a result, current treatment guidelines recommend early treatment of influenza‐infected individuals. 4 Limited PK studies suggest that there is no need for dosing adjustment in pregnancy, although levels may be slightly lower in the third trimester. 42 , 44 , 45 , 50
Avian H5N1 infections
The global experience of treatment of humans infected with highly pathogenic avian influenza A(H5N1) was recently reviewed and demonstrated significant morbidity and mortality (overall crude survival was 43·5%). 51 Survival was clearly improved among patients who received one dose of oseltamivir alone (60%) compared to those who received no therapy (24%; P < 0.001). 51 From the existing data, the survival benefit appears to persist with oseltamivir treatment initiation 6–8 days after symptom onset in all age groups. 51 Clinical failures, including those who had emergence of antiviral resistance during therapy, have been well described. 52 , 53 , 54 Early identification and initiation of therapy is, therefore, important to improve survival.
Conflicts of interest
The author has received research support, paid to Northwestern University Feinberg School of Medicine, from BioCryst, Cellex, Genentech/Roche and GlaxoSmithKlein. He is a paid member of a DSMB for NexBio and provided renumerated consultation to Abbott, Crucell, Genentech/Roche. Additionally, he has provided unremunerated consultation to Adamas, Alios, BioCryst, Biota, Cellex, Clarassance, GlaxoSmithKlein, MediVector/Toyama, TheraClone and Visterra.
Acknowledgements
This was initially presented at the first conference of the International Society for Influenza and Other Respiratory Virus Diseases Antiviral Group: Influenza Antivirals: Efficacy and Resistance held in Rio de Janeiro, Brazil, November 8–10, 2011.
References
- 1. Ison MG, Hayden FG. Therapeutic options for the management of influenza. Curr Opin Pharmacol 2001; 1:482–490. [DOI] [PubMed] [Google Scholar]
- 2. Hayden FG, Aoki FY. Amantadine, rimantadine, and related agents in Yu VL, Marigan TCJ, Barriere SL. (eds): Antimicrobial Therapy and Vaccines. Baltimore, MD: Williams & Wilkins, 1999; 1344–1365. [Google Scholar]
- 3. Bright RA, Medina MJ, Xu X et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 2005; 366:1175–1181. [DOI] [PubMed] [Google Scholar]
- 4. Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–24. [PubMed] [Google Scholar]
- 5. Jefferson T, Demicheli V, Di Pietrantonj C, Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database of Systematic Reviews 2006; Issue 2. Art. No.: CD001169. DOI: 10.1002/14651858.CD001169.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ison MG. Antivirals and resistance: influenza virus. Curr Opin Virol 2011; 1:563–573. [DOI] [PubMed] [Google Scholar]
- 7. Bryson YJ. The use of amantadine in children for prophylaxis and treatment of influenza A infections. Pediatr Infect Dis 1982; 1:44–46. [DOI] [PubMed] [Google Scholar]
- 8. Thompson J, Fleet W, Lawrence E, Pierce E, Morris L, Wright P. A comparison of acetaminophen and rimantadine in the treatment of influenza A infection in children. J Med Virol 1987; 21:249–255. [DOI] [PubMed] [Google Scholar]
- 9. Deyde VM, Nguyen T, Bright RA et al. Detection of molecular markers of antiviral resistance in influenza A (H5N1) viruses using a pyrosequencing method. Antimicrob Agents Chemother 2009; 53:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colman PM. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci 1994; 3:1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamashita M. Laninamivir and its prodrug, CS‐8958: long‐acting neuraminidase inhibitors for the treatment of influenza. Antiviral Chem Chemother 2010; 21:71–84. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y. Long‐acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double‐blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175. [DOI] [PubMed] [Google Scholar]
- 13. Sugaya N, Ohashi Y. Long‐acting neuraminidase inhibitor laninamivir octanoate (CS‐8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother 2010; 54:2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med 2005; 353:1363–1373. [DOI] [PubMed] [Google Scholar]
- 15. Bantia S, Parker CD, Ananth SL et al. Comparison of the anti‐influenza virus activity of RWJ‐270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother 2001; 45:1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugaya N, Mitamura K, Yamazaki M et al. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis 2007; 44:197–202. [DOI] [PubMed] [Google Scholar]
- 17. Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza‐related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003; 163:1667–1672. [DOI] [PubMed] [Google Scholar]
- 18. Boltz DA, Aldridge JR Jr, Webster RG, Govorkova EA. Drugs in development for influenza. Drugs 2010; 70:1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitley RJ, Hayden FG, Reisinger KS et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001; 20:127–133. [DOI] [PubMed] [Google Scholar]
- 20. Chairat K, Tarning J, White NJ, Lindegardh N. Pharmacokinetic properties of anti‐influenza neuraminidase inhibitors. J Clin Pharmacol 2012; Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 21. Kaiser L, Keene ON, Hammond JM, Elliott M, Hayden FG. Impact of zanamivir on antibiotic use for respiratory events following acute influenza in adolescents and adults. Arch Intern Med 2000; 160:3234–3240. [DOI] [PubMed] [Google Scholar]
- 22. Prevention CfDCa . Emergency Use Authorization of Peramivir IV: Fact Sheet for Health Care Providers In., 2009.
- 23. Kohno S, Kida H, Mizuguchi M, Shimada J. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother 2010; 54:4568–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohno S, Yen MY, Cheong HJ et al. Phase III randomized, double‐blind study comparing single‐dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother 2011; 55:5267–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiatboonsri S, Kiatboonsri C, Theerawit P. Fatal respiratory events caused by zanamivir nebulization. Clin Infect Dis 2010; 50:620. [DOI] [PubMed] [Google Scholar]
- 26. Ison MG, Gnann JW Jr, Nagy‐Agren S et al. Safety and efficacy of nebulized zanamivir in hospitalized patients with serious influenza. Antivir Ther 2003; 8:183–190. [PubMed] [Google Scholar]
- 27. Duval X, van der Werf S, Blanchon T et al. Efficacy of oseltamivir‐zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo‐controlled trial. PLoS Med 2010; 7:e1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ison MG, de Jong MD, Gilligan KJ et al. End points for testing influenza antiviral treatments for patients at high risk of severe and life‐threatening disease. J Infect Dis 2010; 201:1654–1662. [DOI] [PubMed] [Google Scholar]
- 29. Lee N, Ison MG. Diagnosis, management and outcomes of adults hospitalized with influenza. Antivir Ther 2012; 17 (1 Pt B):143–157. [DOI] [PubMed] [Google Scholar]
- 30. Bautista E, Chotpitayasunondh T, Gao Z et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708–1719. [DOI] [PubMed] [Google Scholar]
- 31. Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Clevel Clin J Med 2010; 77:812–820. [DOI] [PubMed] [Google Scholar]
- 32. Ison MG. Epidemiology, prevention, and management of influenza in patients with hematologic malignancy. Infect Disord Drug Targets 2011; 11:34–39. [DOI] [PubMed] [Google Scholar]
- 33. Khanna N, Steffen I, Studt JD et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2009; 11:100–105. [DOI] [PubMed] [Google Scholar]
- 34. Ison MG, Hirsch HH. Influenza: a recurrent challenge to transplantation. Transpl Infect Dis 2010; 12:95–97. [DOI] [PubMed] [Google Scholar]
- 35. Baluch A, Humar A, Egli A et al. Long term immune responses to pandemic influenza A/H1N1 infection in solid organ transplant recipients. PLoS ONE 2011; 6:e28627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar D, Blumberg EA, Danziger‐Isakov L et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant 2011; 11:2020–2030. [DOI] [PubMed] [Google Scholar]
- 37. Ljungman P, Cordonnier C, Einsele H et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant 2009; 44:521–526. [DOI] [PubMed] [Google Scholar]
- 38. Kumar D, Michaels MG, Morris MI et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid‐organ transplants: a multicentre cohort study. Lancet Infect Dis 2010; 10:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ljungman P, de la Camara R, Perez‐Bercoff L et al. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica 2011; 96:1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant 2008; 27:282–288. [DOI] [PubMed] [Google Scholar]
- 41. Ng BJ, Glanville AR, Snell G et al. The impact of pandemic influenza A H1N1 2009 on Australian lung transplant recipients. Am J Transplant 2011; 11:568–574. [DOI] [PubMed] [Google Scholar]
- 42. Donner B, Niranjan V, Hoffmann G. Safety of oseltamivir in pregnancy: a review of preclinical and clinical data. Drug Saf 2010; 33:631–642. [DOI] [PubMed] [Google Scholar]
- 43. Greer LG, Leff RD, Rogers VL et al. Pharmacokinetics of oseltamivir in breast milk and maternal plasma. Am J Obstet Gynecol 2011; 204:524–e521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Worley KC, Roberts SW, Bawdon RE. The metabolism and transplacental transfer of oseltamivir in the ex vivo human model. Infect Dis Obstet Gynecol 2008; 2008:927574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beigi RH, Han K, Venkataramanan R et al. Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol 2011; 204(6 Suppl 1):S84–S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cervantes‐Gonzalez M, Launay O. Pandemic influenza A (H1N1) in pregnant women: impact of early diagnosis and antiviral treatment. Expert Rev Anti Infect Ther 2010; 8:981–984. [DOI] [PubMed] [Google Scholar]
- 47. Greer LG, Sheffield JS, Rogers VL, Roberts SW, McIntire DD, Wendel GD Jr. Maternal and neonatal outcomes after antepartum treatment of influenza with antiviral medications. Obstet Gynecol 2010; 115:711–716. [DOI] [PubMed] [Google Scholar]
- 48. Siston AM, Rasmussen SA, Honein MA et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanaka T, Nakajima K, Murashima A, Garcia‐Bournissen F, Koren G, Ito S. Safety of neuraminidase inhibitors against novel influenza A (H1N1) in pregnant and breastfeeding women. CMAJ 2009; 181:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Greer LG, Leff RD, Rogers VL et al. Pharmacokinetics of oseltamivir according to trimester of pregnancy. Am J Obstet Gynecol 2011; 204(6 Suppl 1):S89–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adisasmito W, Chan PK, Lee N et al. Strengthening observational evidence for antiviral effectiveness in influenza A (H5N1). J Infect Dis 2011; 204:810–811. [DOI] [PubMed] [Google Scholar]
- 52. Beigel JH, Farrar J, Han AM et al. Avian influenza A (H5N1) infection in humans. N Engl J Med 2005; 353:1374–1385. [DOI] [PubMed] [Google Scholar]
- 53. de Jong MD, Bach VC, Phan TQ et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med 2005; 352:686–691. [DOI] [PubMed] [Google Scholar]
- 54. de Jong MD, Tran TT, Truong HK et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672. [DOI] [PubMed] [Google Scholar]


