Introduction
The global HIV response has been remarkably successful. More than 19 million persons living with HIV (PLHIV) have accessed life‐saving antiretroviral therapy (ART) 1 and the annual number of HIV‐related deaths and new HIV infections have both plummeted 1. As countries strive to reach the UNAIDS 90:90:90 targets (i.e. for 90% of PLHIV to be aware of their diagnosis, 90% of those who know their diagnosis to receive ART, and 90% of those on ART to have durable viral load suppression 2), new guidelines, tools and implementation strategies are vitally important.
Viral load measurement is a critical tool to assess the impact of HIV treatment efforts, and is now endorsed by the World Health Organization (WHO) as the primary methodology for monitoring response to ART 3. This recommendation is based on research demonstrating that viral suppression is associated with decreased HIV disease progression and mortality among PLHIV, and the prevention of HIV transmission to sexual partners 4, 5. Although stakeholders were initially slow to adopt this WHO recommendation, most funders and national programmes now strongly support scaling up access to routine viral load monitoring 6.
Because viral load measurement is a laboratory assay, it is unfortunately easy to misunderstand the challenge of viral load scale‐up as one for laboratorians only. However, experience with other laboratory assays in resource‐limited settings has shown that it takes far more than assuring availability for a test to realize its potential. For example, even with the availability of testing for early infant HIV diagnosis, multiple challenges have been described in relation to coverage, quality and utilization of results. These include ensuring correct sampling methodologies for dried blood spot samples from HIV‐exposed infants, obtaining specimens at the recommended timeframes, transporting specimens to the laboratory, ensuring tests are done in a timely fashion, enabling providers to promptly access test results and providing results to the infant's caregiver to enable appropriate clinical decision‐making 7. These impediments delay the diagnosis of HIV infection among infants, thus delaying ART initiation in this vulnerable population 8, 9, 10.
The experience of early infant diagnosis (EID) scale‐up highlights the importance of conceptualizing viral load testing as a continuum; a series of steps, each critical for achieving the ultimate goal – swift and appropriate clinical management to maximize the chance of sustained viral suppression (Figure 1). The first step in the viral load continuum is support for demand generation among both recipients of care and their health providers to motivate the former to request and the latter to order the test.
Figure 1.
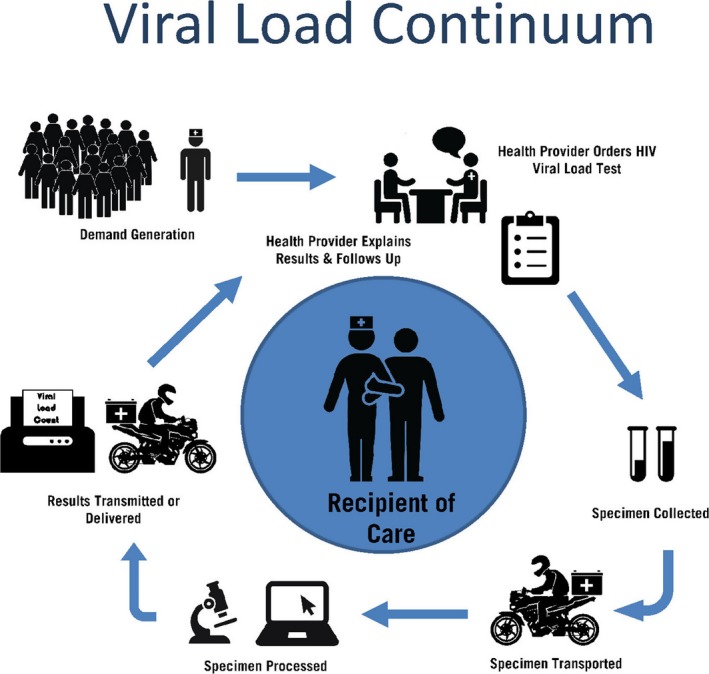
Viral load continuum.
Once the sample is obtained, transportation systems are required to convey specimens, whether plasma or dried blood spot samples, to the appropriate laboratories. Laboratories need staff, equipment, laboratory information systems and quality assurance/improvement capacity to ensure that specimens are logged, tracked, tested and documented – and that results are transmitted back to the health facility, either electronically or physically. Point‐of‐care assays for viral load measurement offer the potential for overcoming the challenges encountered in transport of specimens and results 11. Nonetheless, irrespective of laboratory method used, these results must then be readily available to the providers, whether in medical charts or via an electronic medical record.
Ultimately, the most critical step is for the providers to review the test results and to share them promptly with the recipients of services. Ensuring that clients are aware of the importance of viral load monitoring and viral suppression for both their own health as well as preventing onward transmission is critically important. Similarly, providers must be trained and supported to swiftly act upon viral load test results, reinforcing clients whose viral load is suppressed, and rapidly and accurately following national guidelines for patients with unsuppressed viral loads to increase adherence or adjust ART regimens if viral resistance is suspected.
In most countries, the first step after an unsuppressed viral load result is to provide enhanced adherence counselling (EAC) based on a careful assessment of all the factors that may be impeding clients’ ability to take ART regularly, followed by repeat viral load measurement 3. In the absence of viral resistance testing – which is unavailable in many of the countries most severely affected by the HIV epidemic – failure to achieve viral suppression after EAC is assumed to reflect viral resistance, motivating change to a second line regimen 12. With the recent expanding availability of integrase inhibitors, e.g. dolutegravir‐containing regimens, simplification of both first and second line treatment is on the immediate horizon with the potential to enhance adherence due to decreased risk of side effects 13. For children living with HIV, it is critically important to accelerate the development of similar regimens, particularly in formulations appropriate for young children 14.
Findings to date indicate that many clients who have unsuppressed viral load achieve viral suppression after EAC, demonstrating that in a significant proportion, the unsuppressed viral load is largely due to non‐adherence 15, 16, 17. This finding is in contrast to a recent report that raised concern regarding an increase in the prevalence of HIV resistance 18. Of note, results from the four recently conducted nationally representative population‐based HIV impact assessments (PHIAs) in Zimbabwe, Malawi, Zambia and Swaziland showed impressive viral load suppression (between 86.5% and 91.9%) among PLHIV who indicated that they were aware of their HIV‐positive status and were on ART, suggesting the robustness of the commonly used current first‐line regimen 19, 20.
This supplement aims to summarize a workshop focused on viral load scale‐up that took place from 27 to 30 June 2016 in Swaziland. The workshop, entitled “Reaching the Third 90: Implementing High Quality Viral Load Monitoring at Scale,” was attended by 150 participants from 16 sub‐Saharan African countries, including individuals from diverse backgrounds reflecting key elements of the viral load continuum 21, such as clinical providers, civil society representatives, laboratorians, programme managers, policy makers, researchers and funders. The workshop agenda was inspired by the concept of the viral load continuum and included cross‐disciplinary panels and small group discussions to encourage attendees to think broadly beyond their own disciplines and areas of interest. The articles included in this supplement reflect this premise.
In their article, Killingo et al. describe the efforts of the International Treatment Preparedness Coalition to mobilize communities of PLHIV to demand access to viral load testing and to empower them to advocate for such access in the countries where they live 22. Ensuring clients have immediate access to their results along with their health provider will increase client awareness of the importance of adherence. Peter et al. describe the lessons learned from scale‐up of other laboratory tests, such as EID and CD4+ cell count assays, which can inform scale‐up of viral load testing 23. Ellman et al. discuss the optimal viral load threshold to use when defining virological failure 24, while Saito et al. describe the unique experience of providing viral load results to individuals participating in the PHIA Project 25. Specific issues related to viral load testing among pregnant women, infants and children, adolescents and selected key populations are described in articles by Lesosky et al. 26, Arpadi et al. 27, Marcus et al. 28 and Schwartz et al. 29 respectively. Finally, the article by Barnabas et al. includes a systematic review of evidence related to the cost‐effectiveness of routine viral load monitoring in low‐ and middle‐income countries 30.
In summary, viral load measurement provides critical information for the management of individuals, as well as insight into the effectiveness of HIV programmes across the entire HIV care continuum. This one test serves as a unique measure of the coverage, quality and impact of HIV programmes. As access to viral load testing expands, it is critical to learn from the lessons of the past, and to take a systems approach to strengthening every step of the viral load continuum 31. All recipients of care deserve access to their viral load test results and all programmes need to move to utilize viral suppression as the indicator of programmatic effectiveness. Ultimately, viral load measurement is an asset that is too precious to waste.
Competing interests
The authors cite no conflicts of interest.
Authors’ contributions
All authors contributed equally to the writing and editing of this manuscript.
Acknowledgements
None.
Funding sources
The publication of this journal supplement was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under award # U1NHA28555. The contents of the supplement are the responsibility of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the US Government.
El‐Sadr, W. M. , Rabkin, M. , Nkengasong, J. and Birx, D. L. Realizing the potential of routine viral load testing in sub‐Saharan Africa. J Int AIDS Soc. 2017; 20 (S7):e25010
References
- 1. UNAIDS . Ending AIDS: Progress Towards the 90‐90‐90 Targets. Geneva: UNAIDS; 2017. [Google Scholar]
- 2. UNAIDS . 90‐90‐90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 3. WHO . What's New in Treatment Monitoring: Viral Load and CD4 Testing. Geneva: WHO; 2017. [Google Scholar]
- 4. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV‐1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease ‐inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 6. Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with scale‐up of HIV viral load monitoring ‐ seven Sub‐Saharan African Countries, January 2015‐June 2016. MMWR Morb Mortal Wkly Rep. 2016;65(47):1332–5. [DOI] [PubMed] [Google Scholar]
- 7. Essajee S, Bhairavabhotla R, Penazzato M, Kiragu K, Jani I, Carmona S, et al. Scale‐up of early infant HIV diagnosis and improving access to pediatric HIV care in global plan countries: past and future perspectives. J Acquir Immune Defic Syndr. 2017;75 Suppl 1:S51–8. [DOI] [PubMed] [Google Scholar]
- 8. Hsiao NY, Stinson K, Myer L. Linkage of HIV‐infected infants from diagnosis to antiretroviral therapy services across the Western Cape, South Africa. PLoS ONE. 2013;8(2):e55308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chatterjee A, Tripathi S, Gass R, Hamunime N, Panha S, Kiyaga C, et al. Implementing services for early infant diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nuwagaba‐Biribonwoha H, Werq‐Semo B, Abdallah A, Cunningham A, Gamaliel JG, Mtunga S, et al. Introducing a multi‐site program for early diagnosis of HIV infection among HIV‐exposed infants in Tanzania. BMC Pediatr. 2010;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drain PK, Rousseau C. Point‐of‐care diagnostics: extending the laboratory network to reach the last mile. Curr Opin HIV AIDS. 2017;12(2):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 13. Cahn P. Candidates for inclusion in a universal antiretroviral regimen: dolutegravir. Curr Opin HIV AIDS. 2017;12(4):318–23. [DOI] [PubMed] [Google Scholar]
- 14. Abrams EJ, Strasser S. 90‐90‐90‐‐Charting a steady course to end the paediatric HIV epidemic. J Int AIDS Soc. 2015;18 Suppl 6:20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS ONE. 2015;10(2):e0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bulage L, Ssewanyana I, Nankabirwa V, Nsubuga F, Kihembo C, Pande G, et al. Factors associated with virological non‐suppression among HIV‐positive patients on antiretroviral therapy in Uganda, August 2014–July 2015. BMC Infect Dis. 2017;17:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rangarajan S, Colby DJ, Giang LT, Bui DD, Hung Nguyen H, Tou PB, et al. Factors associated with HIV viral load suppression on antiretroviral therapy in Vietnam. J Virus Erad. 2016;2(2):94–101. [PMC free article] [PubMed] [Google Scholar]
- 18. WHO . HIV Drug Resistance Report 2017. Geneva: 2017. Licence: CC BY‐NC‐SA 3.0 IGO. [Google Scholar]
- 19. New Findings Show Significant Progress against HIV Epidemic in Africa, Bringing the 90‐90‐90 Goals within Reach[press release]. New York City, December 1, 2016. [Google Scholar]
- 20. Swaziland Survey Shows Impressive Progress in Confronting the HIV Epidemic[press release]. Paris, July 24, 2017. [Google Scholar]
- 21. Reaching the Third 90: Implementing High Quality Viral Load Monitoring [press release]. New York, August 1, 2016. [Google Scholar]
- 22. Killingo BC, Taro TB, Mosime WN. Community‐driven demand creation for the use of routine viral load testing: a model to scale up routine viral load testing. J Int AIDS Soc. 2017;20 Suppl 7:e25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peter T, Zeh C, Katz Z, Elbireer A, Alemayu B, Vojnov L, et al. Scaling up HIV viral load – lessons from the large‐scale implementation of HIV early infant diagnosis and CD4 testing. J Int AIDS Soc. 2017;20 Suppl 7:e25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellman TM, Alemayehu B, Abrams EJ, Arpadi S, Howard AA, El‐Sadr WM. Selecting a viral load threshold for routine monitoring in resource‐limited settings: optimizing individual health and population impact. J Int AIDS Soc. 2017;20 Suppl 7:e25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito S, Doung YT, Metz M, Lee K, Patel H, Sleeman K, et al. Returning HIV‐1 viral load results to participants’‐selected health facilities in national Population‐based HIV Impact Assessment (PHIA) household surveys in three sub‐Saharan African Countries, 2015‐2016. J Int AIDS Soc. 2017;20 Suppl 7:e25004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lesosky M, Glass T, Mukonda E, Hsiao NY, Abrams E, Myer L. Optimal timing of viral load monitoring during pregnancy to predict viremia at delivery in HIV‐infected women initiating ART in South Africa: a simulation study. J Int AIDS Soc. 2017;20 Suppl 7:e25000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arpadi SM, Shiau S, Pimentel De Gusmao E, Violari A. Routine viral load monitoring in HIV‐infected infants and children in low and middle‐income countries: challenges and opportunities. J Int AIDS Soc. 2017;20 Suppl 7:e25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marcus R, Ferrand RA, Kranzer K, Bekker LG. The case for viral load testing in adolescents in resource limited settings. J Int AIDS Soc. 2017;20 Suppl 7:e25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwartz SR, Kravanough MM, Sugarman J, Solomon SS, Njindam IM, Rebe K, et al. HIV viral load monitoring among key populations in low‐ and middle‐income countries: challenges and opportunities. J Int AIDS Soc. 2017;20 Suppl 7:e25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnabas RV, Revill P, Tan N, Phillips A. Cost‐effectiveness of routine viral load monitoring in low‐ and middle‐income countries: a systematic review. J Int AIDS Soc. 2017;20 Suppl 7:e25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McNairy ML, El‐Sadr WM. The HIV care continuum: no partial credit given. AIDS. 2012;26(14):1735–8. [DOI] [PubMed] [Google Scholar]


