Abstract
Vincristine (VCR) is a vinca alkaloid and common chemotherapeutic that is used to treat multiple pediatric and adult malignancies. Despite its common use, cases of anaphylaxis to VCR are rare and typically isolated to a single individual. We report a series of eight patients with adverse reactions to VCR over the course of 11 months at a single institution, four of which progressed to anaphylaxis and one of which resulted in cardiac arrest. Mass spectrometry analysis of medication lots was concerning for possible contaminant. Our findings highlight the risk of anaphylaxis during therapy with VCR.
Keywords: Vincristine, Allergy, Anaphylaxis, Mass spectrometry, Contaminant
Introduction
Vincristine (VCR) is a vinca alkaloid that was historically purified from the Madagascar periwinkle Catharanthus roseus, but is now synthesized.[1,2] VCR is a common chemotherapeutic that is used to treat multiple pediatric and adult malignancies. Cases of anaphylaxis to VCR have been reported but are rare, and typically isolated to a single individual.[3,4]
Between July of 2015 and May of 2016, eight patients had symptoms of adverse reactions immediately after receiving VCR at The Children’s Hospital of Philadelphia (CHOP), with similar reactions being colloquially reported at other institutions around the country. A subset of the patients treated at CHOP (4 of 8) progressed to anaphylaxis. We performed a manual chart review of the electronic medical records for these patients, as well as mass spectrometry analysis of medication lots, to determine the cause of the reactions. This study was reviewed by the CHOP Institutional Review Board and determined to be exempt from requiring approval.
Results
The demographic and clinical characteristics of the patients are shown in Table 1. There were five male and three female patients, with an average age of 5 years. Patients were treated with three lots of VCR, from two different manufacturers. Underlying oncologic diagnoses were acute lymphoblastic leukemia, vaginal sarcoma, oligodendroglioma, medulloblastoma, lymphoblastic lymphoma, and Wilm’s tumor. On review, we found no obvious change in the medication administration protocol, equipment associated with the medication infusion, or other similarity among the patients in question (other medications, underlying diagnosis, etc.) that would explain the reactions. Attention was given to other chemotherapeutic agents administered in proximity to VCR. In all cases, the reaction occurred during or immediately following VCR administration. In seven of eight cases, VCR was the first or only chemotherapeutic administered, excluding other chemotherapeutic agents as the etiology of the reactions. In the remaining case (patient #8), pegaspargase was administered six hours prior to vincristine without complication. As most medication reactions occur within one hour of administration, it is unlikely that pegaspargase was the cause of the reaction observed in this patient. Vincristine is administered in a saline mini-bag of 0.9% NaCl at our institution, offering an additional potential etiology of the reactions. There was no consistency of mini-bag lot numbers across patients that reacted, and no reactions were reported with other medications prepared with the same mini-bags, making it unlikely that the mini-bags were related to the reactions in question.
Table 1.
Demographic and clinical characteristics of patients and reactions. N, No; Y, Yes; N/A, Not applicable.
| Patient | Sex | Diagnosis | Age at reaction (years) | First exposure (Y/N) | Tolerated subsequently (Y/N or N/A) | Symptoms | Therapy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory | Vomiting | Flushing | Hypotension | Mouth Itch | Headache | Cardiac Arrest | Hives | Antihistamine | Intravenous fluids | Steroids | Epinephrine | Oxygen | Albuterol | ||||||
| 1 | Female | Vaginal Sarcoma | <1 | Y | Y | X | X | X | X | X | X | X | |||||||
| 2 | Male | Oligodendroglioma | 1 | N | Y | X | X | ||||||||||||
| 3 | Male | ALL | 6 | N | Y | X | X | X | X | X | X | X | X | ||||||
| 4 | Male | Medulloblastoma | 4 | N | N/A | X | X | X | X | X | X | X | |||||||
| 5 | Female | Lymphoblastic Lymphoma | 16 | N | Y | X | X | X | X | X | X | ||||||||
| 6 | Male | ALL | 4 | N | Y | X | X | X | X | X | |||||||||
| 7 | Male | Wilm’s Tumor | 2 | N | Y | X | X | X | X | ||||||||||
| 8 | Female | ALL | 7 | Y | Y | X | X | X | X | X | |||||||||
Symptoms experienced were immediate and included respiratory (100%), vomiting (50%), flushing (25%), hypotension (25%), mouth itch (13%), headache (13%), and cardiac arrest (13%) (Table 1). While two patients experienced flushing, no patients experienced hives. Patients required therapy with antihistamines (100%), intravenous fluids (75%), steroids (50%), epinephrine (50%), supplemental oxygen (25%), and albuterol (13%) (Table 1). Reactions occurred on first exposure to VCR in two of the eight patients. Seven of the patients were treated with VCR subsequently without reaction, and four of these received pre-medications (anti-histamines +/- steroids) prior to subsequent infusions. One patient required no further VCR due to completion of therapy (patient #4). There were no long-term, medically-related negative sequelae of these reactions.
Given the number of cases over a short period of time, and that most patients were tolerating VCR prior to their initial reaction and could tolerate VCR subsequently, we favored a contaminant as the etiology of the reactions. To identify potential contaminants, we performed mass spectrometry (MS) analysis. The experimental VCR sample was taken from a lot of medication to which patients clinically reacted. A control sample of VCR was taken from a lot from the same manufacturer to which patients did not react. For each sample, 20 μL of 1 mg/mL VCR was prepared for MS analysis by C18 reversed solid phase extraction (Zip Tip, Millipore Corp.). Briefly, columns were activated with 20 μL of 70% acetonitrile and VCR was bound via three aspiration/dispense cycles. Columns were washed seven times with 0.1 % formic acid to remove mannitol and residual salts. After eluting VCR in 20 μL 0.1% formic acid and 50% acetonitrile, the solution was analyzed on a Q Exactive HF mass spectrometer (Thermo Fisher Scientific) by nano electrospray ionization in positive ion mode, infused at a rate of 250 to 500 nL/min, and analyzed with a normalized collision energy of 30%, spray voltage of 1.8 kVolts, and ion transfer tube temperature of 275 C. Full scan MS and tandem MS spectra were recorded for both samples at a resolution of 240000.
The spectra and analysis of the control and experimental VCR samples are presented in Figure 1 and Supplemental Table S1. Most of the molecules in both the control and experimental preparations were VCR in either mono- or diprotonated forms (Figure 1 and Supplemental Table S1, peaks V1 & V2). Tandem mass spectrometry analysis confirmed the identity of these molecules as VCR (Supplemental Figure S1). We also identified three molecules present at up to 7% relative abundance in the experimental sample, and reduced or near absent in the control sample (Figure 1 and Supplemental Table S1, peaks α, β, γ). Tandem mass spectrometry analysis revealed common product ions between these peaks and VCR, indicating that these molecules were likely related to the vinca alkaloids (Supplemental Figure S1). Peaks α and γ, with m/z values of 399.209 and 797.411 respectively, represent di- or monoprotonated forms of a molecule with a combined relative abundance of 6.71% in the experimental sample compared with the predominant form of VCR.[5,6] This molecule is related to VCR, but predicted to be missing one carbon and one oxygen atom therefore likely representing N-dimethyl-vinblastine, a known decomposition product and proposed intermediate in the synthetic preparation of vincristine.[7,8] The third molecule had a m/z value of 584.264 and was present at 0.35% relative abundance compared with the predominant form of VCR (Figure 1 and Supplemental Table S1, peak β). The composition of peak β relative to VCR was not able to be predicted.
Figure 1.
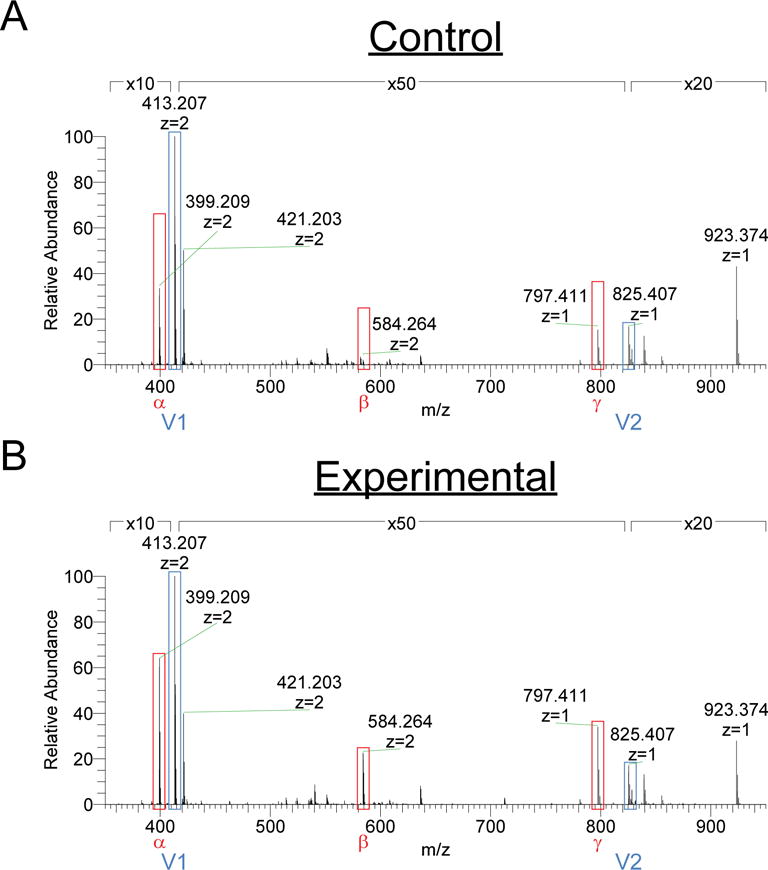
Mass spectra of vincristine samples. (A) Control sample to which no patients reacted. (B) Experimental sample to which patients reacted.
Discussion
Vincristine is a chemotherapeutic that is used to treat multiple pediatric and adult malignancies. Because of its common use, it is essential to understand the risks of adverse reactions with this medication. Though cases of anaphylaxis to VCR have been reported, they are rare and typically isolated to a single individual.[3,4] We report a series of eight adverse reactions to VCR, four of which progressed to anaphylaxis.
It is thought that most drugs are too small to directly illicit an IgE-dependent immune response. Rather, they act as haptens or prohaptens by binding endogenous proteins and lead to an IgE-mediated reaction. Alternatively, small molecules can lead to direct, IgE-independent mast cell activation, complement activation, and/or kallikrein activation. [9] It is therefore possible that seemingly subtle differences in molecular composition can result in dramatically different immune reactivity.
We cannot be certain that the identified molecules in our study were the etiology of the observed reactions, nor do not know the mechanism of the reactions in our population. While MS is a very sensitive technique for identification of compounds, it is not without limitations and can have difficulty identifying non-volatile and very rare substances, as well as compounds not currently available in spectra databases.[10,11] Furthermore, very small amounts of allergen can elicit clinically significant reactions making identification of the causal compound difficult.
Nevertheless, the frequency and characteristics of the reactions reviewed here are most consistent with a reaction to a contaminant. It is possible that the contaminants identified represent degradation products. However, VCR concentrate is stable for 24 months when stored appropriately, and seven days once diluted. All doses administered in this case series strictly adhered to these recommended time periods. As such, we favor a contaminant resulting from the manufacturing process as the etiology of the reactions. There is precedent for this hypothesis as a similar series of anaphylactic reactions occurred in the United States and Germany in 2007 as a result of heparin contaminated with over-sulfated chondroitin.[12] Our findings highlight the importance of recognizing the potential for complications related to anaphylaxis when treating patients with vincristine specifically, as well as the importance of recognizing the potential for allergic complications as a result of impure medication preparations in general.
Supplementary Material
Supplemental Figure S1 Tandem mass spectrometry analysis of molecules identified in the experimental vincristine sample. (A) Peak α, m/z value of 399.2, (B) Peak V1, m/z value of 413.2, (C) Peak β, m/z value of 584.3, (D) Peak γ, m/z value of 797.4, (E) Peak V2, m/z value of 825.4.
Supplemental Table S1 Analysis of molecules in control and experimental vincristine samples.
Acknowledgments
IRB, Funding:
We thank Lynn Spruce for her technical expertise. This study was reviewed by the CHOP Institutional Review Board. D.A.H. is supported by the CHOP NRSA Institutional Training in Pediatric Research grant (T32 HD043021). J.M.S. is supported by the Stuart Starr Endowed Chair of Pediatrics. D.A.H., J.M.S., and T.B.W. made contributions to conception and design of the work; D.A.H., S.H.S., and T.B.W. made contributions to the acquisition, analysis, and/or interpretation of the data;
Abbreviations
- MS
Mass spectrometry
- VCR
Vincristine
Footnotes
Author Contributions:
All authors made intellectual contributions during drafting and revision of the work, approved the final version to be published, and agree to be accountable for all aspects of the work including accuracy and integrity.
Disclosures:
The authors have no financial relationships or other conflicts of interest relevant to this article to disclose.
References
- 1.Noble RL. The discovery of the vinca alkaloids–chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68:1344–1351. [PubMed] [Google Scholar]
- 2.Kuboyama T, Yokoshima S, Tokuyama H, Fukuyama T. Stereocontrolled total synthesis of (+)-vincristine. Proc Natl Acad Sci USA. 2004;101:11966–11970. doi: 10.1073/pnas.0401323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernini JC, Timmons CF, Sandler ES. Acute basophilic leukemia in a child. Anaphylactoid reaction and coagulopathy secondary to vincristine-mediated degranulation. Cancer. 1995;75:110–114. doi: 10.1002/1097-0142(19950101)75:1<110::aid-cncr2820750118>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj B, Kalra SK, Gupta G. Fatal anaphylaxis following intravenous vincristine. Indian Pediatr. 1986;23:961. [PubMed] [Google Scholar]
- 5.Hada V, Dubrovay Z, Lako-Futo A, Galambos J, Gulyas Z, Aranyi A, Szantay C., Jr NMR and mass spectrometric characterization of vinblastine, vincristine and some new related impurities–part II. J Pharm Biomed Anal. 2013;84:309–322. doi: 10.1016/j.jpba.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Dubrovay Z, Hada V, Beni Z, Szantay C., Jr NMR and mass spectrometric characterization of vinblastine, vincristine and some new related impurities – part I. J Pharm Biomed Anal. 2013;84:293–308. doi: 10.1016/j.jpba.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Council of Europe. European pharmacopoeia. Strasbourg. 2013 [Google Scholar]
- 8.Marvin G. Nu-desmethylvinblastine. 3354163. US Patent. 1967
- 9.Kuruvilla M, Khan DA. Anaphylaxis to drugs. Immunol Allergy Clin North Am. 2015;35:303–319. doi: 10.1016/j.iac.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Meyer MR, Helfer AG, Maurer HH. Current position of high-resolution MS for drug quantification in clinical & forensic toxicology. Bioanalysis. 2014;6:2275–2284. doi: 10.4155/bio.14.164. [DOI] [PubMed] [Google Scholar]
- 11.Holzgrabe U, Diehl BW, Wawer I. NMR spectroscopy in pharmacy. J Pharm Biomed Anal. 1998;17:557–616. doi: 10.1016/s0731-7085(97)00276-8. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao G, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 Tandem mass spectrometry analysis of molecules identified in the experimental vincristine sample. (A) Peak α, m/z value of 399.2, (B) Peak V1, m/z value of 413.2, (C) Peak β, m/z value of 584.3, (D) Peak γ, m/z value of 797.4, (E) Peak V2, m/z value of 825.4.
Supplemental Table S1 Analysis of molecules in control and experimental vincristine samples.


