Abstract
Introduction
Adolescents and youth receiving antiretroviral treatment (ART) in sub‐Saharan Africa have high attrition and inadequate ART outcomes, and evaluations of interventions improving ART outcomes amongst adolescents are very limited. Sustainable Development Goal (SDG) target 3c is to substantially increase the health workforce in developing countries. We measured the effectiveness and cost‐effectiveness of community‐based support (CBS) provided by lay health workers for adolescents and youth receiving ART in South Africa.
Methods
A retrospective cohort study including adolescents and youth who initiated ART at 47 facilities. Previously unemployed CBS‐workers provided home‐based ART‐related education, psychosocial support, symptom screening for opportunistic infections and support to access government grants. Outcomes were compared between participants who received CBS plus standard clinic‐based care versus participants who received standard care only. Cumulative incidences of all‐cause mortality and loss to follow‐up (LTFU), adherence measured using medication possession ratios (MPRs), CD4 count slope, and virological suppression were analysed using multivariable Cox, competing‐risks regression, generalized estimating equations and mixed‐effects models over five years of ART. An expenditure approach was used to determine the incremental cost of CBS to usual care from a provider perspective. Incremental cost‐effectiveness ratios were calculated as annual cost per patient‐loss (through death or LTFU) averted.
Results
Amongst 6706 participants included, 2100 (31.3%) received CBS. Participants who received CBS had reduced mortality, adjusted hazard ratio (aHR) = 0.52 (95% CI: 0.37 to 0.73; p < 0.0001). Cumulative LTFU was 40% lower amongst participants receiving CBS (29.9%) compared to participants without CBS (38.9%), aHR = 0.60 (95% CI: 0.51 to 0.71); p < 0.0001). The effectiveness of CBS in reducing attrition ranged from 42.2% after one year to 35.9% after five years. Virological suppression was similar after three years, but after five years 18.8% CBS participants versus 37.2% non‐CBS participants failed to achieve viral suppression, adjusted odds ratio = 0.24 (95% CI: 0.06 to 1.03). There were no significant differences in MPR or CD4 slope. The cost of CBS was US$49.5/patient/year. The incremental cost per patient‐loss averted was US$600 and US$776 after one and two years, respectively.
Conclusions
CBS for adolescents and youth receiving ART was associated with substantially reduced patient attrition, and is a low‐cost intervention with reasonable cost‐effectiveness that can aid progress towards several health, economic and equality‐related SDG targets.
Keywords: HIV, antiretroviral treatment, adolescents, United Nations Sustainable Development Goals, community‐based support, cost‐effectiveness
1. Introduction
The UN Sustainable Development Goals (SDGs) are 17 universal, ambitious and interrelated goals established to guide the development policy and agenda of member states till 2030 1. UNAIDS has also set ambitious HIV treatment targets to help end the AIDS epidemic by 2030 (SDG 3.3) 2. For the SDGs to be achievable, evidence‐based interventions need to be implemented 3, and to reach the UNAIDS treatment goals, innovative and efficient healthcare service delivery models are required 4.
Amongst adolescents in sub‐Saharan Africa (SSA), progress towards the SDGs and HIV prevention and care goals are particularly lagging 5, 6. Adolescents in SSA have the highest HIV incidence globally 7, 8, and adolescents are the only demographic group in whom AIDS‐related mortality is increasing, having tripled since 2000 9, 10. Adolescents and youth receiving antiretroviral treatment (ART) have poorer patient retention and treatment outcomes than adults 11, 12, 13, 14, 15. Ensuring high retention is a crucial aspect of the ART programme to maximize treatment outcomes 16, as well as to reduce community viral load to prevent horizontal transmission 17, 18. ART programmes retention in SSA is poor, being only 56% after five years 19. The barriers to retention amongst adolescents and youth are numerous and diverse, and include the burden of multiple vulnerabilities, barriers to healthcare access, mental health needs, a lack of psychosocial support, a lack of trained healthcare workers focusing on adolescent‐specific care, and lack of support during the transition from paediatric to adult care 20, 21, 22, 23. Appropriate, individualized, holistic and durable interventions that support adolescent's clinical, psychosocial and nutritional care have been suggested 20, 21, 23.
In SSA, adolescents and youth form the greatest proportion of the population (over 33%), and SSA is the only region in which this group continues to grow substantially 24. The health of adolescents is crucial that they may meaningfully contribute to the economy 25, 26. Their economic potential will support progress towards SDGs 1, 2, 8 and 9 to reduce poverty and hunger, promote economic growth and build industry. As SSA has very high HIV prevalence amongst adolescents and youth 27, promoting the health of adolescents and youth living with HIV is essential for the region to make meaningful progress towards the SDGs over and beyond health‐related SDGs.
HIV‐infected adolescents are a neglected group 28. Recent systematic reviews indicate that the evidence base for adherence and retention‐enhancing interventions amongst HIV‐infected adolescents and youth is very sparse, and that most studies focussed on high‐income countries and had low participant numbers 23, 28, 29. These reviews conclude that identifying effective interventions that improve ART outcomes amongst adolescents is overdue. Evidence of the longer‐term effectiveness and cost‐effectiveness of adherence and retention‐enhancing interventions are particularly lacking 30. The limited evidence that exists suggests that interventions that include individualized psychosocial support, counselling and education, and the provision of specific adolescent‐tailored services are promising and require further investigation 23, 28, 29.
SSA also has critical shortages of professional healthcare workers–particularly aggravated due to the HIV/AIDS epidemic–and needs to substantially increase its health workforce to attain its development goals 31, 32. SDG target 3c is to substantially increase the recruitment, development and training of the health workforce in developing countries 1. Community‐based support (CBS) programmes are task‐shifting healthcare interventions involving lay healthcare workers that have been developed to increase the health workforce at limited cost in developing countries 33, 34. Amongst others, CBS programmes have aimed to support HIV‐infected adults receiving ART 35. The effectiveness of CBS for adolescents receiving ART requires evaluation, and cost‐effectiveness evaluations of CBS are lacking 36.
South Africa has the greatest number of people living with HIV globally, and is showing poor performance regarding its HIV‐related SDG target 3, 37. South Africa also has one of the most unequal societies worldwide 38. South Africa's unemployment rate (27%) is amongst the ten highest national unemployment rates globally, 39, 40 with youth unemployment being approximately 50% 41. Almost two‐thirds of young South African children live in poverty, and 20% of the population live in extreme poverty 38, 42.
This study aimed to evaluate the effectiveness and cost‐effectiveness of a large CBS programme for HIV‐infected adolescents and youth receiving ART (with five years of patient outcomes) in four South African provinces.
2. Methods
A retrospective cohort study was performed at 47 public ART facilities, using routinely collected clinical data. The facilities were located in KwaZulu‐Natal, Western Cape, Eastern Cape and Mpumalanga provinces, in both urban (33 facilities) and rural areas (14 facilities). Included facilities were all facilities supported by Kheth'Impilo, a non‐profit organization, which had a CBS programme for adolescents and youth. Kheth'Impilo supports the South African Department of Health with public health systems strengthening. The majority were primary healthcare facilities, and six were secondary‐level hospitals. Antenatal HIV prevalence in these provinces varied between 18.2% and 37.4% 43. Co‐infection with tuberculosis amongst adolescents and youth starting ART in South Africa is high (9% to 13%) 13.
Antiretroviral‐naive adolescents and youth aged 10 to 24 years who initiated ART between 01 January 2004 and 30 September 2010 were included. Follow‐up was until mortality, loss to follow‐up (LTFU), documented transfer‐out to other sites, 30 September 2011 (database closure) or five years on ART (whichever occurred first). To evaluate the effectiveness of CBS, ART outcomes were compared between adolescents and youth who received CBS plus standard clinic‐based care versus adolescents and youth who received standard care only. During the pre‐ART preparation period, patients initiating ART were evaluated by a facility‐based community co‐ordinator (named a “site‐facilitator”), who assigned patients in a non‐randomized manner to receive CBS in addition to usual care if the following criteria were fulfilled: CBS‐workers were active in the area of the patient's home, CBS‐worker capacity was available, and patient consent was obtained. As the development of the CBS programme was progressive, few patients initially received CBS but this increased as the programme expanded. Clinical and socioeconomic factors were not criteria in the allocation of patients to receive CBS. For analyses, patients were assigned to the CBS group if they were allocated to and received support from a named CBS‐worker from ART initiation.
2.1. CBS intervention
CBS‐workers are clinic‐linked, lay community health workers who provided ART patient support by undertaking home visits to ascertain and address household challenges impacting on clinic attendance and adherence. CBS‐workers resided in low socioeconomic, high HIV‐prevalence areas. Preference was given to employing previously unemployed people as CBS‐workers. They were trained regarding HIV and tuberculosis (TB) infection and treatment, including addressing psychosocial issues impacting adherence. Support started from the time of pre‐ART preparation and continued throughout long‐term care. Patient, family and household issues assessed by CBS‐workers included nutrition security, substance abuse, mental health including depression, domestic violence, non‐disclosure, and HIV‐related stigma and discrimination. Issues were discussed at clinic multidisciplinary team meetings and interventions agreed by the team were implemented by the CBS‐worker as appropriate. CBS included providing one‐on‐one counselling regarding adherence, and support and referral for psychosocial problems and nutrition security. Participants were provided with information and education regarding sexual and reproductive health and family planning. Adolescents' carers were offered educational sessions regarding HIV/TB information, medication adherence, and nutrition. Adolescents and youth who defaulted clinic visits were traced by CBS‐workers. Eligibility for government social assistance grants (for poverty relief) was assessed and support provided to obtain these where eligible.
Participants were scheduled for weekly visits during the first months following ART initiation, then monthly for at least six months. Once stable, home visits were performed at least quarterly, but if clinic visits were delayed, home visit frequency increased. Health promotion education and symptom screening for TB, opportunistic infections and sexually transmitted infections (STIs) were performed, with referral to clinics for further management if indicated.
CBS‐workers had a specific geographic area which they supported and were assigned 80 to 120 patients each. Career development of CBS‐workers was encouraged, with certain CBS‐workers subsequently employed as social auxiliary workers or home‐based care co‐ordinators 44.
2.2. Outcomes and definitions
The primary outcomes were as follows: time to all‐cause mortality after starting ART, and time till LTFU after starting ART. Attrition was defined as a combined endpoint due to patient losses due to either mortality or LTFU. The secondary outcomes were as follows: (i) Adherence to ART measured using Medication Possession Ratios (MPRs)–an adherence measure derived from pharmacy refill data (number of days of dispensed medication divided by the number of days between the first and last pharmacy refill during the study period) 45, 46; (ii) CD4 cell count increases between months 0 and 36 after starting ART; (iii) CD4 count slope (mean change in CD4 count per month) between months 0 to 6 and 6 to 60; and (iv) the proportion of patients not achieving virological suppression after three years and during the fifth year of ART. We were primarily interested in longer‐term immunological reconstitution and virological outcomes and not the initial rapid rise in CD4 count following ART initiation 47.
Deaths were recorded as reported by professional healthcare workers or family members. Patients were defined as LTFU if they were not known to have died or to have transferred out (as documented in site databases), and had no visit to the site for six months or more prior to database closure 48, 49. Patients who returned to care after treatment interruptions were considered remaining in care. The date of last contact was assigned for the outcome of mortality or LTFU in time‐to event analyses, with one day of follow‐up added for patients who did not return after initiating ART to include them in analyses. Patients documented as transferring to other facilities were censored on the last clinic visit date. Patients who did not receive CBS who missed appointments were traced by telephone or a district tracing team would visit the home where available. All patients visited the clinic at a frequency determined by clinic professional staff (generally monthly). Virological suppression was defined as viral load <400 copies/ml. Laboratory measurements were performed by the South African National Health Laboratory Service.
Individual‐level patient data were collected prospectively for programme monitoring purposes by designated site‐based data capturers at each visit using standardized custom‐designed databases, which were regularly pooled to a data warehouse, using standardized operating procedures. Site databases were designed in Microsoft Access®, and were used for clinical data collection and patient and clinic management. Regular data cleaning and quality control procedures were implemented.
Participant baseline characteristics were compared with medians, interquartile ranges and percentages, and binary variables were compared with risk ratios and 95% confidence intervals. Outcomes were by intention‐to‐treat ignoring changes in exposure status after ART initiation. Cumulative incidence functions were used to calculate time till mortality or LTFU, using a competing‐risks approach. Multivariable Cox regression and Fine and Grey competing‐risks regression were used to compare mortality and LTFU between patients who received and did not receive CBS, controlling for demographic, clinical and site‐related confounding. To account for clustering of observations within sites, stratified Cox regression was conducted allowing the baseline hazard for each site to vary 50, and for the competing‐risks models site was included as a fixed effect. Incidence rate ratios of attrition were calculated stratified by site, with the combined estimate calculated using Mantel–Haenszel weights.
Mean MPR was analysed using generalized estimating equations specifying for clustering within sites and using Huber–White (robust) variance estimates. MPR was also analysed as a binary variable with mixed‐effects logistic regression including site as a random intercept, using a threshold MPR of ≥95% to indicate high adherence. CD4 count increases were analysed with linear regression, and CD4 cell slopes were analysed with multilevel mixed‐effects linear regression including site and patient as random effects to account for the longitudinal nature of the data and clustering within sites. Models were adjusted for ART duration and baseline variables were included as fixed effects. Proportions of patients not achieving viral suppression were analysed using mixed‐effects logistic regression.
To impute missing baseline covariate data, multiple imputations by chained equations were conducted using 20 imputed datasets, under the assumption that missing data were likely missing at random. Multivariable analyses were run on each data set that included the imputed values and the results combined, using Rubin rules 51.
All available plausible demographic, clinical and site‐related variables were considered as potential confounders and were included in multivariable models when their inclusion altered the association between CBS and the outcomes or were significantly associated with the outcomes with p < 0.05. Modification of the effect of CBS on outcomes was assessed by stratifying effect measures by plausible modifiers. The number needed to treat (NNT) to prevent a case of death or LTFU were calculated as appropriate for time‐to‐event outcomes 52.
2.3. Cost‐effectiveness analyses
A top‐down expenditure approach was used to determine the incremental cost of CBS to usual ART care from a provider perspective. Expenditure of the CBS programme according to the financial records of the programme were collected, which included costs of human resources, training, management and administration, infrastructure and equipment, and monitoring and evaluation over a two‐year period between 01 April 2011 and 31 March 2013. The cost of usual ART patient care was not considered and was assumed to be equal between patients with and without CBS. The number of patient‐years of CBS during this period was calculated from programme monitoring data.
The cost outcomes were: (i) average cost of CBS per patient‐year of support, and (ii) cost‐effectiveness defined as cost per patient‐loss (through death or LTFU) averted. The effectiveness of CBS in preventing patient attrition at annual intervals after starting ART (compared to usual care) was calculated as the difference in patient attrition between patients who did and who did not receive CBS (estimated from a stratified Cox model) divided by attrition amongst patients who did not receive CBS 53. Incremental cost‐effectiveness ratios (ICERs) were calculated from one through five years of treatment. For cost calculations, patients lost to care were considered lost at the mid‐point of each year. Costs were converted to United States dollars at the average exchange rate of ZAR 1 = US$0.1219 in 2012 54. For ICERs, costs and patient losses averted were discounted at 3% per annum 55. Analyses were conducted with Stata® version 13.1 (College Station, TX, USA), and Microsoft Excel®. The University of Cape Town Human Research Ethics Committee provided the studies ethical approval, and the study conformed to the Declaration of Helsinki ethical principles.
3. Results
Database records of 85,997 patients who initiated ART were screened for inclusion, with the following excluded: 3756 patients aged <10 years when starting ART; 74,123 aged ≥25 years; and 1412 who started ART after the study enrolment period. Thus 6706 participants were included, of whom 2100 (31.3%) received CBS and 4606 (68.7%) who received standard care only. Most (82.4%) participants were female and 1810 (27.0%) were aged 10 to 19 years. At ART initiation, participants who received CBS had: a higher proportion with advanced clinical stage disease (World Health Organization (WHO) stages III/IV), a slightly higher median CD4 count, a higher proportion who received concomitant TB treatment, a higher proportion who were pregnant, a higher proportion who attended rural facilities and a higher proportion who attended primary healthcare clinics (Table 1). The proportion of patients who received CBS increased from 19.3% to 33.5% during the study period.
Table 1.
Characteristics of adolescents and youth at antiretroviral treatment initiation who received and did not receive CBS in South Africa
| Total (n = 6706) | Did not received CBS (n = 4606) | Received CBS (n = 2100) | Risk ratio (CBS vs. no CBS) (95% CI)a | |
|---|---|---|---|---|
| Female, n (%) (n = 6706) | 5523 (82.4) | 3752 (81.5) | 1771 (84.3) | 1.04 (1.01 to 1.06) |
| Median age, years, (IQR) (n = 6706) | 22.4 (19.6 to 23.9) | 22.4 (19.5 to 23.9) | 22.5 (19.9 to 23.9) | |
| Age categories, n (%) (n = 6706) | ||||
| Ages 10 to 19 years | 1810 (27.0) | 1268 (27.5) | 542 (25.8) | 0.93 (0.86 to 1.02) |
| Ages 20 to 24 years | 4896 (73.0) | 3338 (72.5) | 1558 (74.2) | |
| WHO clinical stage, n (%) (n = 4424) | ||||
| I/II | 1564 (35.4) | 1171 (37.5) | 393 (30.1) | |
| III/IV | 2860 (64.7) | 1949 (62.5) | 911 (69.9) | 1.12 (1.06 to 1.17) |
| CD4 cell count, cells/μl, median (IQR) (n = 5560) | 136 (70 to 187) | 131 (65 to 182) | 145 (82 to 195) | |
| Pregnancy amongst females, n (%) (n = 5166) | ||||
| Not pregnant | 4512 (87.3) | 3031 (88.4) | 1481 (85.3) | |
| Pregnant | 654 (12.7) | 399 (11.6) | 255 (14.7) | 1.26 (1.09 to 1.46) |
| Received tuberculosis treatment, n (%) (n = 6332) | ||||
| No | 5623 (88.8) | 3831 (89.6) | 1792 (87.1) | |
| Yes | 709 (11.2) | 443 (10.4) | 266 (12.9) | 1.25 (1.08 to 1.44) |
| Initial regimen, n (%) (n = 5657) | ||||
| d4T‐3TC‐EFV | 2792 (49.4) | 1961 (52.5) | 831 (43.2) | |
| d4T‐3TC‐NVP | 2006 (1342) | 1342 (36.0) | 664 (34.5) | |
| ZDV‐3TC‐EFV | 38 (0.7) | 19 (0.5) | 19 (1.0) | |
| ZDV‐3TC‐NVP | 106 (1.9) | 37 (1.0) | 69 (3.6) | |
| TDF‐3TC‐EFV | 339 (6.0) | 163 (4.4) | 176 (9.2) | |
| TDF‐3TC‐NVP | 322 (5.7) | 184 (4.9) | 138 (7.2) | |
| Other | 54 (1.0) | 27 (0.7) | 27 (1.4) | |
| Year of starting ART, n (%)(n = 6706) | ||||
| 2004 to 2005 | 218 (3.3) | 176 (3.8) | 42 (2.0) | |
| 2006 to 2007 | 1384 (20.6) | 1038 (22.5) | 346 (16.5) | |
| 2008 to 2010 | 5104 (76.1) | 3392 (73.6) | 1712 (81.5) | |
| Location of site attended, n (%) (n = 6706) | ||||
| Urban | 5238 (78.1) | 3784 (82.2) | 1454 (69.2) | |
| Rural | 1468 (21.9) | 822 (17.9) | 646 (30.8) | 1.72 (1.58 to 1.88) |
| Hospital‐based clinic/primary healthcare clinic attended, n (%) (n = 6706) | ||||
| Hospital | 1612 (24.0) | 1407 (30.6) | 205 (9.8) | |
| Primary healthcare clinic | 5094 (76.0) | 3199 (69.5) | 1895 (90.2) | 1.30 (1.27 to 1.33) |
| Province, n (%) (n = 6706) | ||||
| Western Cape | 803 (12.0) | 523 (11.4) | 280 (13.3) | |
| Eastern Cape | 1259 (18.8) | 587 (12.7) | 672 (32.0) | |
| KwaZulu‐Natal | 4035 (60.2) | 3243 (70.4) | 792 (37.7) | |
| Mpumalanga | 609 (9.1) | 253 (5.5) | 356 (17.0) | |
ART, antiretroviral treatment; CBS; community‐based support; WHO, World Health Organization; IQR, interquartile range; CI, confidence interval; d4T, stavudine; 3TC, lamivudine; EFV, efavirenz; NVP, nevirapine; ZDV, zidovudine; TDF, tenofovir.
For binary variables.
During 9215 person‐years of follow‐up, 87 (4.1%) and 256 (5.6%) of participants who received and did not receive CBS were reported as having died, respectively (p = 0.015). A further 286 (13.6%) and 885 (19.2%) became LTFU amongst those who received and did not receive CBS, respectively (p < 0.0001). 375 (8.5%) participants transferred out. After five years of ART, the cumulative incidence of mortality amongst adolescents and youth who received and did not receive CBS was 8.3% and 10.8%, respectively (p = 0.027), and the cumulative incidence of LTFU was 29.9% and 38.9%, respectively (p < 0.0001) (Figure 1).
Figure 1.
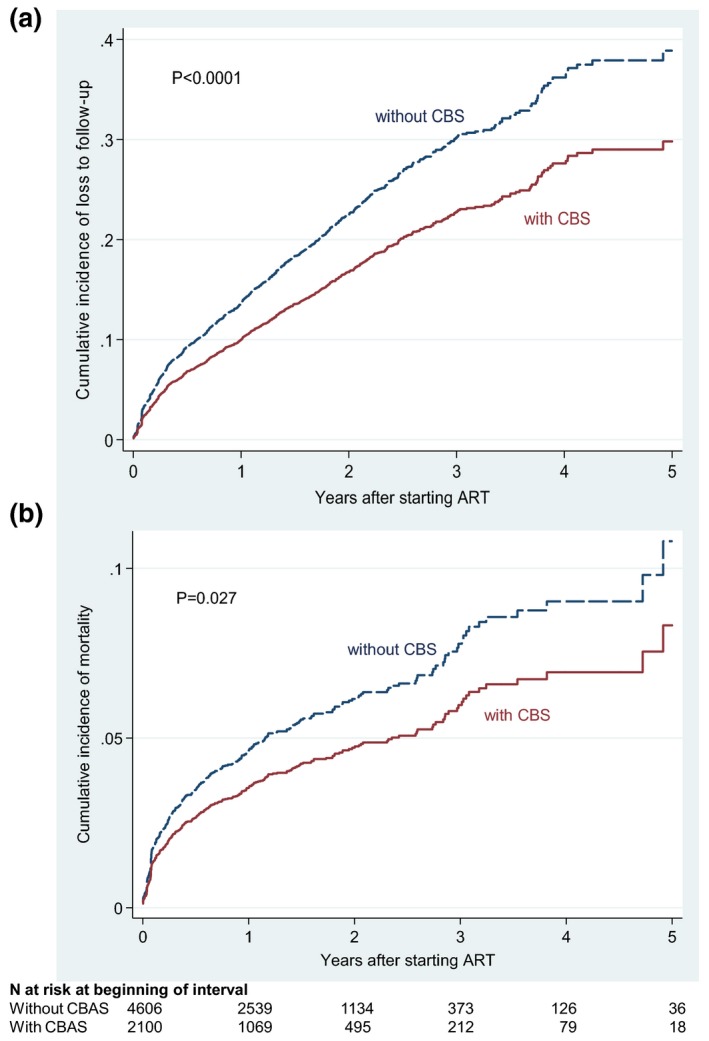
Cumulative incidence of (A) Loss to follow‐up and (B) mortality amongst adolescents and youth starting antiretroviral treatment in South Africa.
For multivariable analyses, the proportions of imputed baseline values were: TB treatment status‐5.6%; pregnancy status‐5.3%; CD4 count‐17.1%; initial regimen‐15.6%; WHO stage‐34.0%. After controlling for confounding using multivariable Cox regression, participants who received CBS had a significantly reduced probability of mortality, adjusted hazard ratio (aHR) = 0.52 (95% CI: 0.37 to 0.73; p < 0.0001) (Table 2). Estimates from the competing‐risks regression models were similar. Adolescents and youth who received CBS had a 40% reduced probability of becoming LTFU, aHR = 0.60 (95% CI: 0.51 to 0.71; p < 0.0001). The effect of CBS on LTFU was more pronounced at rural facilities, aHR = 0.43 (95% CI: 0.30 to 0.62) and slightly more pronounced amongst pregnant women, aHR = 0.53 (95% CI: 0.31 to 0.92).
Table 2.
Univariable and multivariable models of factors associated with loss to follow‐up and mortality amongst adolescents initiating ART in South Africa
| Predictor (baseline) | Loss to follow‐up | Mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Cox | Multivariable Cox | Multivariable competing risks | Univariable Cox | Multivariable Cox | Multivariable competing risks | |||||||
| HR (95% CI) | p‐value | aHR (95% CI) | p‐value | asHR (95% CI) | p‐value | HR (95% CI) | p‐value | aHR (95% CI) | p‐value | asHR (95% CI) | p‐value | |
| Received CBS | ||||||||||||
| Yes | 0.59 (0.50 to 0.70) | <0.0001 | 0.60 (0.51 to 0.71) | <0.0001 | 0.61 (0.52 to 0.73) | <0.0001 | 0.45 (0.32 to 0.63) | <0.0001 | 0.52 (0.37 to 0.73) | <0.0001 | 0.56 (0.41 to 0.76) | <0.0001 |
| No | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Age (years) | 1.03 (1.02 to 1.05) | <0.0001 | 1.03 (1.02 to 1.05) | <0.0001 | 1.04 (1.02 to 1.05) | <0.0001 | 1.00 (0.98 to 1.03) | 0.88 | 0.99 (0.96 to 1.01) | 0.29 | 0.98 (0.96 to 1.01) | 0.28 |
| Gender | ||||||||||||
| Female | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Male | 0.86 (0.73 to 1.00) | 0.048 | 0.97 (0.82 to 1.15) | 0.71 | 0.97 (0.82 to 1.15) | 0.70 | 1.02 (0.78 to 1.35) | 0.84 | 0.91 (0.67 to 1.22) | 0.52 | 0.90 (0.33 to 1.21) | 0.48 |
| WHO stage | ||||||||||||
| I/II | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| III | 1.10 (0.94 to 1.29) | 0.22 | 1.18 (1.00 to 1.39) | 0.049 | 1.18 (1.02 to 1.37) | 0.028 | 2.19 (1.54 to 3.11) | <0.0001 | 1.84 (1.29 to 2.64) | 0.001 | 1.86 (1.30 to 2.66) | 0.001 |
| IV | 1.12 (0.86 to 1.48) | 0.38 | 1.20 (0.91 to 1.60) | 0.19 | 1.21 (0.93 to 1.57) | 0.16 | 4.5 (2.79 to 7.27) | <0.0001 | 3.48 (2.15 to 5.62) | <0.0001 | 3.4 (2.12 to 5.51) | <0.0001 |
| CD4 count, cells/μl | ||||||||||||
| 0 to 99 | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| 100 to 199 | 1.06 (0.92 to 1.22) | 0.40 | 1.04 (0.90 to 1.20) | 0.63 | 1.09 (0.94 to 1.27) | 0.25 | 0.36 (0.28 to 0.47) | <0.0001 | 0.42 (0.31 to 0.54) | <0.0001 | 0.42 (0.32 to 0.55) | <0.0001 |
| 200 to 349 | 1.11 (0.91 to 1.36) | 0.30 | 1.11 (0.90 to 1.37) | 0.32 | 1.17 (0.94 to 1.45) | 0.17 | 0.27 (0.17 to 0.42) | <0.0001 | 0.36 (0.22 to 0.58) | <0.0001 | 0.35 (0.21 to 0.56) | <0.0001 |
| ≥350 | 1.03 (0.86 to 1.23) | 0.72 | 1.33 (0.93 to 1.92) | 0.12 | 1.47 (1.02 to 2.10) | 0.036 | 0.16 (0.05 to 0.51) | 0.002 | 0.18 (0.05 to 0.57) | 0.004 | 0.18 (0.05 to 0.60) | 0.005 |
| Pregnancy | ||||||||||||
| Yes | 1.42 (1.17 to 1.72) | <0.0001 | 1.43 (1.17 to 1.74) | <0.0001 | 1.45 (1.19 to 1.77) | <0.0001 | 0.25 (0.12 to 0.52) | <0.0001 | 0.38 (0.19 to 0.79) | 0.010 | 0.38 (0.19 to 0.79) | 0.009 |
| No | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | ||
| TB treatment | ||||||||||||
| Yes | 0.95 (0.79 to 1.15) | 0.61 | 0.98 (0.80 to 1.19) | 0.82 | 0.98 (0.81 to 1.19) | 0.87 | 1.10 (0.77 to 1.55) | 0.61 | 0.88 (0.61 to 1.29) | 0.48 | 0.90 (0.63 to 1.30) | 0.57 |
| No | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Year of starting ART (continuous) | 1.12 (1.05 to 1.19) | <0.0001 | 1.17 (1.10 to 1.25) | <0.0001 | 1.15 (1.08 to 1.22) | <0.0001 | 0.71 (0.64 to 0.79) | <0.0001 | 0.77 (0.69 to 0.86) | <0.0001 | 0.74 (0.67 to 0.82) | <0.0001 |
| Site location | ||||||||||||
| Urban | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Rural | 1.01 (0.27 to 3.75) | 0.98 | 1.15 (0.31 to 4.27) | 0.83 | 0.65 (0.17 to 2.50) | 0.54 | 1.46 (0.16 to 12.60) | 0.73 | 1.19 (0.13 to 11.03) | 0.88 | 1.31 (0.15 to 11.7) | 0.81 |
| PHC clinic /hospital | ||||||||||||
| Hospital | 0.68 (0.51 to 0.90) | 0.007 | 0.71 (0.53 to 0.96) | 0.025 | 0.57 (0.25 to 1.30) | 0.19 | 1.35 (0.77 to 2.37) | 0.30 | 0.88 (0.47 to 1.64) | 0.69 | 3.42 (0.40 to 28.9) | 0.26 |
| PHC clinic | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
Regression results using models with multiple imputation of missing covariate data, using 20 imputed datasets. To account for clustering within sites, Cox models were stratified by site, and a fixed‐effects approach was used for the competing risks models. Multivariable models were also adjusted for initial antiretroviral regimen. HR, hazard ratio; aHR, adjusted hazard ratio; asHR, adjusted subhazard ratio; CBS, community‐based support; ART, antiretroviral treatment; TB, tuberculosis; WHO, World Health Organization; PHC, primary healthcare; CI, confidence interval.
The NNT to prevent one case of mortality after one and three years was 6.4 (95% CI: 3.6 to 16.7) and 5.3 (3.2 to 13.0), respectively, and the NNT to prevent one case of LTFU after one and three years was 6.0 (95% CI: 4.4 to 9.4) and 5.4 (4.2 to 8.0), respectively.
Considering the combined endpoint of attrition, the incidence rate of attrition was 12.9 cases/100 person‐years (95% CI: 11.7 to 14.3) amongst adolescents and youth who received CBS, and 18.0 cases/100 person‐years (95% CI: 17.0 to 19.1) amongst adolescents and youth without CBS, incidence rate ratio (stratified by site) = 0.55 (95% CI: 0.48 to 0.64; p < 0.0001).
Mean MPR was similar between patients with and without CBS; 82.5% and 83.0%, respectively, adjusted mean difference = −1.0 % (95% CI: −2.6% to 0.5%), p = 0.20 (Table 3). There was no difference in the proportion of patients who achieved high adherence (MPR ≥95%), viz. 35.4% and 35.8% amongst patients with and without CBS, respectively, adjusted odds ratio (aOR) = 1.00 (95% CI: 0.86 to 1.19; p = 0.92).
Table 3.
Secondary outcomes of CBS for adolescents and youth receiving antiretroviral treatment in South Africa
| Outcome | Received CBS | Did not receive CBS | Crude effect measure (95% CI) (CBS vs. no CBS) | Crude p‐value | Adjusted effect measure (95% CI)a | Adjusted p‐value |
|---|---|---|---|---|---|---|
| Mean MPR, % (95% CI) | 82.5% (81.6% to 83.4%) | 83.0% (82.3% to 83.7%) | −0.6% (−1.7% to 0.6%)b | 0.33 | −1.0% (−2.6% to 0.5%)c | 0.20 |
| Proportion with MPR ≥95%, % (95% CI) | 35.4% (33.2% to 37.6%) | 35.8% (34.1% to 37.5%) | 0.99 (0.92 to 1.07)d | 0.79 | 1.00 (0.86 to 1.19)e | 0.92 |
| CD4 count increases after three years of ART, cells/μl (IQR) | 384.5 (152 to 521) | 366 (208 to 485) | 11.9 (−67.6 to 91.6)f | 0.76 | 21.8 (−60.2 to 103.9)f | 0.60 |
| CD4 cell slope between months 0 and 6 after ART initiation, cells/μl/month, median (IQR) | 27.0 (12.9 to 43.4) | 25.6 (11.9 to 42.0) | 1.31 (−1.92 to 4.55)g | 0.43 | 2.10 (−1.21 to 5.39)g | 0.22 |
| CD4 cell slope between months 6 and 60 after ART initiation, cells/μl/month, median (IQR) | 6.7 (−2.0 to 16.4) | 7.1 (−0.6 to 16.1) | 1.09 (−1.34 to 3.51)g | 0.38 | 1.28 (−1.12 to 3.68)g | 0.30 |
| Proportions not achieving viral suppression after three years of ART, % (95% CI) | 28.2% (19.7% to 37.9%) | 32.7% (26.1% to 39.7%) | 0.81 (0.48 to 1.36)e | 0.43 | 0.96 (0.41 to 2.28)e | 0.93 |
| Proportions not achieving viral suppression during fifth year of ART, % (95% CI) | 18.8% (7.2% to 36.4%) | 37.2% (24.1% to 51.9%) | 0.39 (0.14 to 1.11)e | 0.079 | 0.24 (0.06 to 1.03)e | 0.055 |
Adjusted for baseline confounding using 20 multiple imputed datasets.
Mean absolute difference.
Coefficient from generalized estimating equation specifying for clustering within sites.
Risk ratio.
Odds ratios using mixed‐effects logistic regression including site as a random intercept.
Coefficient from linear regression.
Coefficient from mixed‐effects linear regression (cells/μl/month) including site and individual as random effects, and adjusted for duration of ART.
CBS, community‐based support; MPR, medication possession ratios; IQR, interquartile range.
CD4 count increases were 384.5 cells/μl and 366 cells/μl amongst adolescents and youth with and without CBS, respectively, after 36 months. CD4 count slope between months 6 to 60 in adolescents and youth with and without CBS was 6.7 cells/μl/month and 7.1 cells/μl/month, respectively, with no difference in multivariable analyses; coefficient = 1.28 cells/μl/month (95% CI: −1.12 to 3.68; p = 0.30).
The proportions of adolescents with and without CBS who failed to achieve virological suppression after three years were similar, aOR = 0.96 (95% CI: 0.41 to 2.28), p = 0.93. During the fifth year of ART, the proportions with and without CBS who failed to achieve virological suppression were 18.8% and 37.2%, respectively, with the adjusted effect measure approaching a significant difference in favour of CBS, aOR = 0.24 (95% CI: 0.06 to 1.03), p = 0.055.
3.1. Cost‐effectiveness results
The average cost of CBS was US$49.5/patient/year, with 84% spent on human resources (Table 4). The entire programme employed 576 CBS‐workers. The effectiveness of CBS in reducing patient attrition ranged from 42.2% after one year to 35.9% after five years. The incremental cost of CBS per patient‐loss averted after one, two and five years was US$600, US$776 and US$1149, respectively (Table 5).
Table 4.
Costs of CBS for antiretroviral treatment patients in South Africa
| Total patient‐years supported | 126,485 |
| No. community workers employed | 576 |
| Item | Average costs per patient year supported, US$ (%) a |
| Human resources | 41.83 (84.4) |
| Training | 5.97 (12.1) |
| Infrastructure and equipment | 0.02 (0.05) |
| Clothing for CBS‐workers | 0.15 (0.3) |
| Management and administration | 0.48 (1.0) |
| Monitoring and evaluation | 0.10 (0.2) |
| Overhead costs | 0.99 (2.0) |
| Total cost per patient supported/year | 49.5 (100.0) |
Values in parentheses are percentages of the total cost.
Table 5.
Cost‐effectiveness of CBS for ART patients in South Africa
| Duration of ART (years) | Proportion of patients retained in care (%)a | Effectiveness of intervention in reducing patient attrition (%)b | No. patient losses averted due to CBS (per 100 patients initiating ART)c | Cumulative cost of CBS (per 100 patients initiating ART), US$c , d | Cost‐effectiveness ratio (US$/patient‐loss averted) | |
|---|---|---|---|---|---|---|
| With CBS | Without CBS | |||||
| 1 | 89.3 | 81.5 | 42.2 | 7.6 | 4549 | 600.7 |
| 2 | 82.7 | 71.0 | 40.3 | 11.0 | 8561 | 776.3 |
| 3 | 76.4 | 61.5 | 38.7 | 13.6 | 12,165 | 892.1 |
| 4 | 70.7 | 53.5 | 37 | 15.3 | 15,400 | 1007.7 |
| 5 | 66.9 | 48.4 | 35.9 | 16.0 | 18,337 | 1149.1 |
Estimated from the survivor function of a stratified Cox model.
The effectiveness of the CBS programme in preventing attrition (through death or loss to follow‐up) was calculated as the difference in patient attrition between patients who did and who did not receive CBS divided by attrition amongst patients who did not receive CBS.
Costs and no. of patient losses averted were discounted at 3% per annum.
Patients lost to the programme were considered lost at the mid‐point of each year.
CBS, community‐based support; ART, antiretroviral treatment.
4. Discussion
The SDGs are opportune to improve the health and wellbeing of disadvantaged groups globally. Government commitment to the SDGs needs to be translated into programmes that can deliver on the wide‐ranging goals and accompanying targets. The SDG targets are interrelated and overlap; notably 28 targets across 11 goals are health‐related 3, 26. To reach the SDGs for adolescents by 2030, the importance of innovations in adolescent health involving biomedical and behavioural interventions delivered together has recently been highlighted 56.
Adolescents are a key group for targeting of the UNAIDS 90‐90‐90 HIV treatment goals 57. In view of their poorer ART outcomes, there have previously been calls for adolescents and youth to receive specific additional support 11, 12, 13, 15. This study has found that CBS was associated with substantially improved retention in adolescents and youth receiving ART, and is a low‐cost intervention with reasonable cost‐effectiveness. Cost‐effectiveness of CBS was greatest during the first two years of treatment.
Improved programme retention increases the number of HIV‐infected adolescents and youth receiving ART, which would lead to greater numbers potentially being able to achieve viral suppression due to ART use. In turn, this can potentially decrease sexual transmission due to ART 58, 59 and aid progress towards SDG target 3.3 to reduce HIV incidence.
Community support has previously been found to reduce ART programme attrition amongst adults and children 35, 60. Mechanisms underlying these improvements include defaulter tracing, psychosocial support offered by CBS workers, improved patient links with clinics, decreased treatment fatigue, improved self‐management skills regarding HIV/AIDS, greater disclosure, greater social capital and a widened community safety net 35, 61, 62. The primary driver of decreased attrition associated with CBS in this study was reduced LTFU, with reduced mortality accounting for a small component only. Except for a trend towards improved viral suppression at five years amongst those who received CBS, significant differences in immunological restitution or the adherence measure utilized were not observed. In the absence of these, the reasons for the difference in mortality observed are unclear and require further research. It is plausible that CBS was associated with health aspects not measured in this study, such as earlier referral and treatment for incident opportunistic infections, improvements in nutritional status or mental health, or improved socioeconomic status through access to grants. Future research should also incorporate more accurate measures of adherence.
In adults, the cost‐effectiveness of strategies to reduce ART patient attrition have been evaluated in two previous studies. A hypothetical study found that interventions costing up to US$120/person/annum with effectiveness ≥40% in reducing attrition would be cost‐effective with high degrees of regional ART coverage 63. A Cote d'Ivoire study found that interventions preventing LTFU would result in a substantial saving of life‐years, and an intervention costing US$53 per person/annum would be cost‐effective by international criteria (<3 times gross domestic product per capita) if ≥28% effective 53. Although we did not model cost‐effectiveness based on disability‐adjusted life years averted, CBS was found to cost US$50/person/annum and have effectiveness between 42% to 36%, and would thus be expected to cost‐effectively reduce high attrition amongst SSA adolescents and youth.
The health workforce underpins every aspect of the health system, and is the rate‐limiting step in achieving universal health coverage by 2030 64. There is pronounced inequity in the distribution of health workers globally, with Africa carrying 25% of the world's disease burden but only 1.3% of the world's health workers, with little progress being evident in this regard 65, 66. To achieve health‐related SDGs, task‐shifting to maximize the use of available funds and health workers in the region will be essential. Efficiency and value for money will be important priorities. Amongst children, UNICEF is promoting task‐shifting from professional to community health workers to improve access to health interventions, in order to achieve SDG target 3.2 to prevent common causes of child mortality 67. The CBS programme evaluated in this study extends this model for the care of HIV‐infected adolescents and youth.
Community health workers can play a key role in attaining a number of SDGs, including health, ending poverty and hunger, equality, clean water and sanitation, and partnerships for global health (SDG 17), as highlighted in the recent Kampala statement 68, 69. Important actions to support the role of community health workers in this regard include financial and political support, partnerships with a range of healthcare providers, and disseminating cross‐country learnings. Rigorous research to expand the evidence base for policy and practice to maximize the contribution and potential of community health workers in progress towards these SDGs is vital 70. Research priorities include the roles of cross‐cutting enabling factors such as education and accreditation of community health workers, management, effective linkage with other professional staff cadres, remuneration, and motivation and performance 64, 68. Translating evidence to investment decisions will also be required to enable sustainable health solutions in pursuit of the SDGs. Including community engagement as an additional aspect of the SDG health targets has also been suggested 26.
Innovations in health worker training will be important in attaining the SDGs. CBS involves training previously unemployed persons living in impoverished areas and employing them as lay health workers, and assisting their further career development 44. As CBS is labour‐intensive, large CBS programmes will aid progress towards SDG targets 4.4, 8.5 and 8.6 (provision of skills to facilitate employment and job creation). Job‐creation further impacts other health‐related targets, as access to gainful employment improves the mental and physical well‐being of families and young people 26. Provision of jobs for CBS‐workers also increases income to the lowest 40% income group (SDG target 10.1) which can support the targets to reduce poverty and food insecurity amongst CBS‐workers and their families (SDG targets 1.1, 1.2 and 2.1).
HIV‐related interventions that have cross‐sectoral benefits produce development synergies and will accelerate progress across development goals 71. CBS‐workers provided counselling regarding mental health, sexual and reproductive health (particularly for adolescent girls), nutrition counselling, and support to access social grants. These interventions can aid progress towards SDG target 3.4 (promotion of mental health and wellbeing), SDG target 3.7 (universal access to sexual and reproductive healthcare services), as well as reduce poverty and hunger. As almost 85% of CBS‐supported participants were female, gender‐equality progress (SDG target 5.6) is also supported. The impact of these services was not assessed in this evaluation; however, future economic analyses may incorporate the potential cross‐sectoral benefits of CBS.
South Africa has recently introduced and is scaling‐up implementation of new national adherence guidelines 72. In line with this, CBS workers currently provide home and clinic‐based support for the initial 12 months after starting ART and for patients who are unstable. This study's results provide evidence of the effectiveness of an individualized approach to support adolescents and youth, and encourage scale‐up of implementation of these guidelines. Individual and group counselling and education for adolescents have shown promise in previous smaller studies conducted mostly in developed countries 28, 29. The role of CBS workers is currently expanding to include facilitation of community and clinic‐based adherence clubs for stable, virologically suppressed adults from 12 months of ART and beyond.
Challenges faced by the CBS programme include the rural context of many patients' homes with long travel distances and inadequate transport, and inconsistent availability of some adolescents for follow‐up counselling sessions. CBS is not a panacea, and other important facets of comprehensive care include youth‐friendly clinical management, peer‐support groups, and integrated management of the transition from child to adult care services 20, 21.
The strengths of this study include the large sample size drawn from many sites situated in low‐income, high HIV prevalence areas, with results thus likely being generalizable to other SSA areas. Prospectively collected individual‐level data were collected with up to five years of patient follow‐up. Additionally, clinical as well as cost outcomes were analysed.
The study limitations include the non‐random allocation of patients to groups, with the potential for selection bias and unmeasured or residual confounding. Effect measures were, however, adjusted for site‐related and individual‐level confounding using multiple imputation of missing covariate values. Differences in measured baseline characteristics were observed between CBS and non‐CBS patients; however, most confounders associated with increased attrition were more prevalent amongst CBS patients (advanced clinical stage disease 73, concurrent TB 74, pregnancy 75, more recent year of starting ART 14, 76, and attending rural facilities 77). Residual confounding is thus unlikely to have confounded effect measures in favour of CBS. The routine nature of the data may have produced information bias. Mortality was likely underestimated in both CBS and non‐CBS patients, as misclassification of patients who have died as being LTFU is common in SSA routine ART data 78. Patients who were classified as LTFU may have been undocumented transfers to other treatment sites outside the study facilities.
5. Conclusions
The SDG process reinforces the central importance of health in sustainable development. Greater attention to adolescent health, particular regarding HIV/AIDS, will be critical to achieve universal and sustainable development 56. This study found CBS to be a low‐cost intervention associated with substantially improved retention in adolescents and youth receiving ART, which had reasonable cost‐effectiveness. CBS for adolescents and youth can potentially aid progress towards twelve targets from eight health, economic, equality and education‐related SDGs. Future qualitative research may shed greater light on mechanisms that may improve outcomes and how community‐support may be further tailored specifically for adolescents.
Competing interests
The authors all declare that they have no conflicts of interests.
Authors' contributions
GF and AG conceived the study. GF designed the study. GF contributed to data collection and managed the data. GF analysed the data. GF drafted the manuscript. All authors interpreted the data and contributed to writing the manuscript. All authors have read and approved the final manuscript.
Acknowledgements and Funding
The authors gratefully acknowledge the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the Departments of Health of KwaZulu‐Natal, Eastern Cape, Western Cape and Mpumalanga. Funding for the study was provided by the US President's Emergency Plan for AIDS Relief, USAID. This research has been supported by the U.S. Presidents' Emergency Plan for AIDS Relief (PEPFAR, grant number U51HA02522) through Centers for Disease Control and Prevention under the terms of grant 5U2GPS001966.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the United States Agency for International Development or the President's Emergency Plan for AIDS Relief. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Fatti, G. , Jackson, D. , Goga, A. E. , Shaikh, N. , Eley, B. , Nachega, J. B. and Grimwood, A. The effectiveness and cost‐effectiveness of community‐based support for adolescents receiving antiretroviral treatment: an operational research study in South Africa. J Int AIDS Soc. 2018; 21(S1):e25041
References
- 1. United Nations . Transforming our world: the 2030 Agenda for Sustainable Development. United Nations; [Internet]. 2015. [cited 2017 Mar 31]. Available from: https://sustainabledevelopment.un.org/post2015/transformingourworld [Google Scholar]
- 2. UNAIDS . 90‐90‐90. An ambitious treatment target to help end the AIDS epidemic. [Internet]. 2014. [cited 2017 Mar 20]. Available from: http://www.unaids.org/en/resources/documents/2014/90-90-90
- 3. Lim SS, Allen K, Bhutta ZA, Dandona L, Forouzanfar MH, Fullman N, et al. Measuring the health‐related Sustainable Development Goals in 188 countries: a baseline analysis from the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1813–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Granich R, Williams B, Montaner J, Zuniga JM. 90‐90‐90 and ending AIDS: necessary and feasible. Lancet. 2017;390(10092):341–3. [DOI] [PubMed] [Google Scholar]
- 5. UNICEF . Annual Results Report 2016: HIV and AIDS [Internet]. 2017. [cited 2017 Oct 23]. Available from: https://www.unicef.org/publicpartnerships/files/2016arr_hivaids.pdf
- 6. UNICEF . Progress for children: a report card on adolescents (No. 10). [Internet]. 2012. [cited 2017 Oct 23]. Available from: https://www.unicef.org/publications/files/Progress_for_Children_-_No._10_EN_04272012.pdf
- 7. Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc. 2015;18 2 Suppl 1:19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdool Karim Q, Kharsany AB, Frohlich JA, Werner L, Mashego M, Mlotshwa M, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu‐Natal, South Africa. Int J Epidemiol. 2011;40:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. UNAIDS . Global AIDS update. [Internet]. 2016. [cited 2017 Mar 22]. Available from: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf
- 10. UNICEF . Statistical update on children, adolescents and AIDS. [Internet]. 2015. [cited 2017 Mar 22]. Available from: http://data.unicef.org/wp-content/uploads/2015/12/2015-Children-Adolescents-and-AIDS-Statistical-Update-Executive-Summary_244.pdf
- 11. Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, Munyaradzi D. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One. 2012;7(12):e52856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans D, Menezes C, Mahomed K, Macdonald P, Untiedt S, Levin L, et al. Treatment outcomes of HIV‐infected adolescents attending public‐sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses. 2013;29(6):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nglazi MD, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale‐up at a community‐based antiretroviral treatment service in South Africa. J Acquir Immune Def Syndr. 2011;56(1):e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koech E, Teasdale CA, Wang C, Fayorsey R, Alwar T, Mukui IN, et al. Characteristics and outcomes of HIV‐infected youth and young adolescents enrolled in HIV care in Kenya. AIDS. 2014;28(18):2729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bekker L, Venter F, Cohen K, Goemare E, Van Cutsem G, Boulle A, et al. Provision of antiretroviral therapy in South Africa: the nuts and bolts. Antivir Ther. 2014;19 Suppl 3:105–16. [DOI] [PubMed] [Google Scholar]
- 17. McNairy ML, El-Sadr WM. Antiretroviral therapy for the prevention of HIV transmission: what will it take? Clin Infect Dis. 2014;58(7):1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. Aids Patient Care STDS. 2010;24(10):607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low‐ and middle‐income countries: systematic review and meta‐analysis 2008‐2013. J Acquir Immune Defic Syndr. 2015;69(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dahourou DL, Gautier-Lafaye C, Teasdale CA, Renner L, Yotebieng M, Desmonde S, et al. Transition from paediatric to adult care of adolescents living with HIV in sub‐Saharan Africa: challenges, youth‐friendly models, and outcomes. J Int AIDS Soc. 2017;20:21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pettitt ED, Greifinger RC, Phelps BR, Bowsky SJ. Improving health services for adolescents living with HIV in sub‐Saharan Africa: a multi‐country assessment. Afr J Reprod Health. 2013;17(4):17–31. [PubMed] [Google Scholar]
- 22. Kung TH, Wallace ML, Snyder KL, Robson VK, Mabud TS, Kalombo CD, et al. South African healthcare provider perspectives on transitioning adolescents into adult HIV care. S Afr Med J. 2016;106:804–8. [DOI] [PubMed] [Google Scholar]
- 23. Lall P, Lim SH, Khairuddin N, Kamarulzaman A. Review: An urgent need for research on factors impacting adherence to and retention in care among HIV‐positive youth and adolescents from key populations. J Int AIDS Soc. 2015;18:19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. United Nations Population Fund and Population Reference Bureau . Status Report on Adolescents and Young People in Sub-Saharan Africa: Opportunities and Challenges. [Internet]. 2014. [cited 2017 Mar 28]. Available from: http://www.prb.org/Publications/Reports/2014/status-report-youth.aspx
- 25. Ashford LS. Africa's youthful population: risk or opportunity? Population Reference Bureau; [Internet]. 2007. [cited 2017 March 28]. Available from: http://www.prb.org/Publications/Reports/2007/AfricasYouthfulPopulation.aspx
- 26. International Council for Science, International Social Science Council . Review of Targets for the Sustainable Development Goals: The Science Perspective. International Council for Science ; [Internet]. 2015. [cited 2017 Oct 23]. Available from: https://www.icsu.org/cms/2017/05/SDG-Report.pdf
- 27. UNAIDS . The gap report. [Internet]. 2014. [cited 2017 Mar 23]. Available from: http://www.unaids.org/en/resources/documents/2014/20140716_UNAIDS_gap_report
- 28. MacPherson P, Munthali C, Ferguson J, Armstrong A, Kranzer K, Ferrand RA, et al. Service delivery interventions to improve adolescents' linkage, retention and adherence to antiretroviral therapy and HIV care. Trop Med Int Health. 2015;20(8):1015–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaw S, Amico KR. Antiretroviral therapy adherence enhancing interventions for adolescents and young adults 13–24 years of age: a review of the evidence base. J Aquir Immune Defic Syndr. 2016;72(4):387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Barnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS. 2014;28 Suppl 2:S187–204. [DOI] [PubMed] [Google Scholar]
- 31. Chen L, Evans T, Anand S, Boufford JI, Brown H, Chowdhury M, et al. Human resources for health: overcoming the crisis. Lancet. 2004;364(9449):1984–90. [DOI] [PubMed] [Google Scholar]
- 32. Lehmann U, Van Damme W, Barten F, Sanders D. Task shifting: the answer to the human resources crisis in Africa? Hum Resour Health. 2009;7(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rasschaert F, Philips M, Leemput LV, Assefa Y, Schoutenand E, Damme WV. Tackling health workforce shortages during antiretroviral treatment scale‐up—experiences from Ethiopia and Malawi. J Acquir Immune Def Syndr. 2011;57 Suppl 2:S109–12. [DOI] [PubMed] [Google Scholar]
- 34. Schneider H, Hlophe H, van Rensburg D. Community health workers and the response to HIV/AIDS in South Africa: tensions and prospects. Health Policy Plan. 2008;23(3):179–87. [DOI] [PubMed] [Google Scholar]
- 35. Wouters E, Van Damme W, van Rensburg D, Masquillier C, Meulemans H. Impact of community‐based support services on antiretroviral treatment programme delivery and outcomes in resource‐limited countries: a synthetic review. BMC Health Serv Res. 2012;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nachega JB, Adetokunboh O, Uthman OA, Knowlton AW, Altice FL, Schechter M, et al. Community‐based interventions to improve and sustain antiretroviral therapy adherence, retention in HIV care and clinical outcomes in low‐ and middle‐income countries for achieving the UNAIDS 90‐90‐90 targets. Curr HIV/AIDS Rep. 2016;13(5):241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980‐2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3(8):e361–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Statistics South Africa . Poverty Trends in South Africa: an examination of absolute poverty between 2006 and 2011. [Internet]. 2014. [cited 2017 Mar 20]. Available from: https://beta2.statssa.gov.za/publications/Report-03-10-06/Report-03-10-06March2014.pdf
- 39. International Labour Organization . World Employment and Social Outlook: Trends 2016. [Internet]. 2016. [cited 2017 Mar 20]. Available from: http://www.ilo.org/wcmsp5/groups/public/-dgreports/-dcomm/-publ/documents/publication/wcms_443480.pdf
- 40. Statistics South Africa . Quarterly Labour Force Survey, Quarter 3, 2016 [Internet]. 2016. [cited 2017 Mar 20]. Available from: http://www.statssa.gov.za/publications/P0211/P02113rdQuarter2016.pdf
- 41. Trading Economics [Internet]. 2017. [cited 2017 April 26]. Available from: http://www.tradingeconomics.com/south-africa/youth-unemployment-rate
- 42. Hall K, Sambu W, Berry L, Giese S, Almeleh C, Rosa S. South African Early Childhood Review. Children's Institute, University of Cape Town and Ilifa Labantwana; [Internet]. 2016. [cited 2017 Mar 20]. Available from: http://ilifalabantwana.co.za/wp-content/uploads/2016/05/SA-ECD-Review-2016-low-res-for-web.pdf
- 43. South African National Department of Health . The 2011 National Antenatal Sentinel HIV & Syphilis Prevalence Survey in South Africa. [Internet]. 2012. [cited 2017 Mar 10]. Available from: http://www.hst.org.za/publications/2011-national-antenatal-sentinel-hiv-syphilis-prevalence-survey-south-africa
- 44. Gittings L, Rundare A, Malahlela M, Jason A, Fatti G, Pududu B, et al. The Journey Project: an evaluation of the Impact of the Kheth'Impilo model on Patient Advocates. 5th South African AIDS Conference; 2011; Durban, South Africa. [Google Scholar]
- 45. Kozma CM, Dickson M, Phillips AL, Meletiche DM. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease‐modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013;7:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–8. [DOI] [PubMed] [Google Scholar]
- 47. Bosch RJ, Wang R, Vaida F, Lederman MM, Albrecht MA; Team ftACTGS . Changes in the slope of the CD4 cell count increase after initiation of potent antiretroviral treatment. J Acquir Immune Defic Syndr. 2006;43(4):433–5. [PubMed] [Google Scholar]
- 48. Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C, et al. Universal definition of loss to follow‐up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;8(10):e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shepherd BE, Blevins M, Vaz LM, Moon TD, Kipp AM, Jose E, et al. Impact of definitions of loss to follow‐up on estimates of retention, disease progression, and mortality: application to an HIV program in Mozambique. Am J Epidemiol. 2013;178(5):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Giganti MJ, Luz PM, Caro-Vega Y, Cesar C, Padgett D, Koenig S, et al. A comparison of seven Cox regression‐based models to account for heterogeneity across multiple HIV treatment cohorts in Latin America and the Caribbean. AIDS Res Hum Retroviruses. 2015;31(5):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubin D. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 52. Altman D, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Losina E, Toure H, Uhler LM, Anglaret X, Paltiel AD, Balestre E, et al. Cost‐effectiveness of preventing loss to follow‐up in HIV treatment programs: A Cote d'Ivoire appraisal. PLoS Med. 2009;6(10):e1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Average yearly exchange rates. [Internet]. [cited 2017 Mar 01]. Available from: http://www.oanda.com
- 55. Severens JL, Milne RJ. Discounting health outcomes in economic evaluation: the ongoing debate. Value Health. 2004;7(4):397–401. [DOI] [PubMed] [Google Scholar]
- 56. Sudfeld CR, Fawzi WW. Importance of innovations in neonatal and adolescent health in reaching the sustainable development goals by 2030. JAMA Pediatr. 2017;171(6):521–2. [DOI] [PubMed] [Google Scholar]
- 57. Davies M-A, Pinto J. Targeting 90–90–90 – don't leave children and adolescents behind. J Int AIDS Soc. 2015;18 7 Suppl 6:20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV‐1 transmission. N Engl J Med. 2016;375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Johnson LF, Chiu C, Myer L, Davies M-A, Dorrington RE, Bekker L-G, et al. Prospects for HIV control in South Africa: a model‐based analysis. Glob Health Action. 2016;9:30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grimwood A, Fatti G, Mothibi E, Malahlela M, Shea J, Eley B. Community adherence support improves programme retention in children on antiretroviral treatment: a multicentre cohort study in South Africa. J Int AIDS Soc. 2012;15(2):17381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Binagwaho A, Ratnayake N. The role of social capital in successful adherence to antiretroviral therapy in Africa. PLoS Med. 2009;6(1):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Foster G. Under the radar: community safety nets for AIDS‐affected households in sub‐Saharan Africa. AIDS Care. 2007;19 Suppl 1:54–63. [DOI] [PubMed] [Google Scholar]
- 63. Kessler J, Nucifora K, Li L, Uhler L, Braithwaite S. Impact and cost‐effectiveness of hypothetical strategies to enhance retention in care within HIV treatment programs in East Africa. Value Health. 2015;18(8):946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Freer J. Sustainable development goals and the human resources crisis. Int Health. 2017;9(1):1–2. [DOI] [PubMed] [Google Scholar]
- 65. Commission for Africa . Our common interest: report of the Commission for Africa. [Internet]. 2005. [cited 2017 Oct 31]. Available from: http://www.commissionforafrica.info/2005-report
- 66. Commission for Africa . Still our common interest: report of the Commission for Africa. [Internet]. 2010. [cited 2017 Oct 31]. Available from: http://www.commissionforafrica.info/2010-report
- 67. UNICEF . Annual Results Report 2016: Health. [Internet]. 2017. [cited 2017 October 23]. Available from: https://www.unicef.org/publicpartnerships/files/2016arr_health.pdf
- 68. Maher D. ‘Leaving no‐one behind’: how community health workers can contribute to achieving the Sustainable Development Goals. Public Health Action. 2017;7(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kampala Statement from the 1st International Symposium on Community Health Workers. Health Information for all (HIFA); [Internet]. 2017. [cited 2017 Nov 1]. Available from: http://www.hifa.org/sites/default/files/publications_pdf/Kampala_CHW_symposium_statement-FINAL.pdf
- 70. Maher D, Cometto G. Research on community‐based health workers is needed to achieve the sustainable development goals. Bull World Health Organ. 2016;94:786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Remme M, Vassall A, Lutz B, Luna J, Watts C. Financing structural interventions: going beyond HIV‐only value for money assessments. AIDS. 2014;28(3):425–34. [DOI] [PubMed] [Google Scholar]
- 72. South African National Department of Health . Adherence guidelines for HIV, TB and NCDs. Policy and service delivery guidelines for linkage to care, adherence to treatment and retention in care. [Internet]. 2016. [cited 2017 Oct 30]. Available from: https://www.nacosa.org.za/wp-content/uploads/2016/11/Integrated-Adherence-Guidelines-NDOH.pdf
- 73. Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub‐Saharan Africa. AIDS. 2008;22(15):1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bassett IV, Chetty S, Wang B, Mazibuko M, Giddy J, Lu Z, et al. Loss to follow‐up and mortality among HIV‐infected people co‐infected with TB at ART initiation in Durban, South Africa. J Acquir Immune Def Syndr. 2011;59(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow‐up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22(13):1679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cornell M, Grimsrud A, Fairall L, Fox M, van Cutsem G, Giddy J, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002‐2007. AIDS. 2010;24:2263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wandeler G, Keiser O, Pfeiffer K, Pestilli S, Fritz C, Labhardt ND, et al. Outcomes of antiretroviral treatment programs in rural Southern Africa. J Acquir Immune Defic Syndr. 2012;59(2):e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anderegg N, Johnson LF, Zaniewski E, Althoff KN, Balestre E, Law M, et al. All‐cause mortality in HIV‐positive adults starting combination antiretroviral therapy: correcting for loss to follow‐up. AIDS. 2017;31 Suppl 1:S31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


