Abstract
PURPOSE
Prognostic models for pleural mesothelioma (MPM) are needed to prevent potentially futile outcomes. We combined MPM plasma biomarkers with validated clinical prognostic indices to determine whether stratification of risk for death in 194 MPM patients improved.
PATIENTS AND METHODS
Individuals were recruited from three different centers: a discovery cohort (83 MPM) created by combining patients from two US centers and a separate, independent cohort from Canada (111 MPM). Univariable/multivariable analyses were performed on the initial discovery and independent cohorts separately. In multivariable analyses, prognostic factors were adjusted for the Mesothelioma EORTC Prognostic Index (PI). The prognostic significance of adding plasma biomarker data to the PI was determined using the likelihood ratio test, comparing models with and without the addition of biomarker to the clinical PI. The predictive ability of the biomarker was then assessed formally using Harrell’s c-index by applying the fitted model variables of the discovery cohort to the second, independent cohort, including and not including the biomarker with the PI.
RESULTS
Higher levels of osteopontin and mesothelin were individually associated with worse prognosis after adjusting for the PI. In the independent cohort, incorporating either plasma osteopontin or mesothelin into the baseline predictive PI model substantively and statistically sgnificantly improved Harrell’s c-statistic. In the final prognostic model, log-osteopontin, EORTC CPI, and hemoglobin remained as independently significant predictors, and the entire prognostic model improved the optimism-corrected Harrell’s c-index significantly from 0.718 (0.67–0.77) to 0.801 (0.77–0.84).
CONCLUSION
These data suggest a possible role for preoperative plasma biomarkers to improve prognostic capability of the MPM EORTC PI.
Despite growing reports describing improvement in median survival time for malignant pleural mesothelioma (MPM), current prognostic stratification methods remain suboptimal. Multiple single institution series have attempted to correlate clinical factors, standard laboratory parameters, and pathologic features in an attempt to better define MPM prognosis. The EORTC Prognostic Index(1) has been one standard for such prognostic quantification. Additionally, a surgery-based registry identified “best” clinical or pathologic stage, gender, age, histologic subtype, and curative intent surgery as associated with survival(2) and these factors were supplemented by white blood cell count (WBC), hemoglobin (Hgb) level, and platelet count(3). This registry serves as an excellent reference source for future studies; however, it does not have an embedded, prospective uniform biospecimen collection component.
Our laboratory has had a long interest in osteopontin (OPN) as a potential biomarker in MPM. However, we recognized the importance of using plasma (pOPN) instead of serum OPN, and that there were reproducibility issues depending upon the ELISA platform used(4). Reports in other malignancies of the prognostic value of OPN have been performed in chemotherapy treated patients(5) (6–8) where, in some cases, the ELISA assays were associated with poor coefficients of variation. Moreover, studies in the literature analyzing serum or plasma mesothelin or mesothelin related peptide (MRP)(9–11) have reported possible prognostic capabilities, but independent validations have been lacking. Fibulin-3 (FBLN3)(12, 13) is a new plasma marker of MPM with no prognostic evaluations published to date.
Using the highest quality ELISAs available, we therefore designed a trial investigating OPN, MRP, and FBLN3 as prognostic factors among other variables including EORTC Prognostic Index, stage and other reported laboratory biomarkers, such as the absolute neutrophil/absolute lymphocyte ratios (NLR)(14) in both cytoreduced and non-surgical patients with pleural MPM. We report that pre-therapy pOPN levels were significantly associated with overall survival in mixed populations of pleural MPM in an intial discovery set, and this finding was confirmed in a second, independent and blinded dataset. Moreover, plasma OPN significantly improved the concordance index (C index) when added to the EORTC Prognostic Index. Using these patient cohorts, a prognostic model for MPM combining plasma biomarker data with clinical variables is described for future validation.
Methods
Patient Populations
We retrospectively analyzed MPM patients who were prospectively recruited at the time of diagnosis from three different centers, all of whom had signed informed consent to obtain plasma for biomarker studies. An initial cohort (n=83) was created by combining patients from two centers: the NYU Langone Medical Center, New York (NYU; 44 MPMs treated between 2007 and 2012) and the Barbara Ann Karmanos Cancer Institute (KCI; 39 MPMs treated between 1998 and 2006). A separate, independent cohort came from the Princess Margaret Cancer Centre (PMCC; 111 MPMs treated between 2004 and 2012) whose levels of biomarkers were determined at NYU without advanced knowledge of their clinical and survival information. The sequencing of the component therapies for patients receiving multimodality therapy varied according to the individual institutions’ protocols (Supplementary Table I). When performed, surgery included maximal cytoreduction by pleurectomy decortication, extended pleurectomy, or extrapleural pneumonectomy along with nodal sampling/dissection(15). The EORTC clinical prognostic index (CPI) defined patients to be good (<1.27) or poor prognosis (>=1.27) using a weighting score of Eastern Cooperative Oncology Group (ECOG) performance status, histology, gender, and pre-treatment white blood cell (WBC) counts(1). The CALGB index used regression trees to examine prognostic variables in 337 patients treated in seven phase II clinical trials. Six prognostic groups were identified based on age, performance status, hemoglobin (Hb) level, WBC and the presence or absence of chest pain and weight loss(16).
Specimen Characteristics and Plasma Biomarker Analyses
EDTA plasma samples were collected prior to therapy, within a few weeks of initial histologic diagnosis of mesothelioma, at all three centers, and stored locally until use at −80°C. Enzyme-linked immunosorbent assays, in duplicate, were performed in the NYU Thoracic Surgical Laboratory for intial discovery and second, independent cohorts for osteopontin (R&D, Minneapolis, MN), mesothelin (R&D, Minneapolis, MN), and fibulin3 (USCN Life Science, Wuhan, Hubei, Peoples Republic China). All plasma biomarker analyses were performed blinded to patient information.
Statistical Analysis
Clinicopathologic, laboratory, treatment, and clinical prognostic index (CPI), and biomarker variables were compared across initial discovery and independent cohorts using Chi-squared and Kruskal-Wallis tests. Survival times were measured from start of first treatment (surgery, chemotherapy, or radiation) to death or last follow up time; for patients receiving solely supportive care, survival times were measured from time of diagnosis to death or last follow up. The primary analysis was to evaluate the role of the three plasma biomarkers as independent predictors of outcome using association analyses in the discovery dataset, and confirm the significant factors in the second, independent dataset. The discovery data was analyzed initially at NYU, while the PMCC data was analyzed at PMCC, independent and blinded to what was found at NYU. Final analyses for the manuscript were performed at PMCC. For this, the Kaplan–Meier method and log-rank tests were used to assess differences in overall survival curves. Cox proportional hazard models generated hazard ratios (HRs) and 95% confidence levels (CI) in univariable and multivariable analyses. The nonlinear effect of the clinical factors and biomarkers on overall survival was assessed using multiple fractional polynomials (MFP) models(17–20). The need of non-linear transformations was only observed for mesothelin and osteopontin: the logarithmic transformation, log(biomarker value/100) was used for all analyses, denoted by log-mesothelin and log-osteopontin, respectively.
Univariable and multivariable analyses were performed on the discovery and independent cohorts separately. In separate multivariable analyses, prognostic factors were either adjusted primarily for EORTC or CALGB CPIs. Because of the complexity of the CALGB CPI, limited clinical usage of this CPI, conventional reclassification of this index in subsequent use, and because the original derivation using a partitioning approach had some categories based on very small numbers of patients, this CPI was dichotomized into Groups 4–6 vs. 1–3; in contrast, EORTC CPI was treated as a continuous variable. For Cox proportional hazard models, the proportionality of hazards assumption was assessed for each of the models in two ways: graphically via Schoenfeld residuals and tested formally(17).
The prognostic significance of adding plasma biomarker data to different CPIs was determined using the likelihood ratio test, comparing models with and without the addition of biomarker to the EORTC or CALGB clinical prognostic indices. When significant association (P<0.05) was found between a plasma biomarker and survival in Cox models, the predictive ability of such a biomarker was then assessed formally using Harrell’s c-index(18, 21), by applying the fitted model variables of the initial cohort to the second, independent cohort. Furthermore, to measure the improvement of the discriminative power, Harrell’s c-indices were calculated for each of the model cohorts, including the biomarker and the model with the clinical prognostic index alone. A bootstrap resampling algorithm was applied to estimate the confidence intervals of the difference in the c-indices (CPI alone vs. CPI + biomarker) using 200 bootstrap replicates each time.
Lastly, a prognostic model was constructed using pooled data that included significant plasma biomarkers and clinical prognostic factors selected based on clinical rationale. Pooling was necessary to ensure that there were adequate events per predictor variable. However, using pooled data meant that no external validation was available. Thus, bias-corrected c-indices and confidence intervals for the difference in c-indices between final prognostic models with/without plasma biomarkers were calculated through a bootstrap algorithm to account for optimism(22). This difference in the c-indices reflects the improvement of the predictive ability attributed to the prognostic effect of log-osteopontin and log-mesothelin. Additionally, goodness of fit survival curves of the final models were assessed through visual inspection of observed versus predicted survival curves after grouping the risk scores derived from the prognostic model into tertiles.
All analyses were performed in R (v.3.0.2, R core development group). Parametric and Cox proportional hazards models were fitted using the survival package(23), c-indices were calculated using Hmisc package (24).
Results
Baseline information for the discovery (NYU/KCI) and second, independent (PMCC) cohorts is presented in Table 1. Compared to the NYU/KCI cohort, the PMCC cohort consisted of patients who had higher stage of disease at diagnosis, worse performance status, more significant weight loss, and worse poor prognosis by the EORTC CPI. However, patients in the validation cohort also had higher baseline hemoglobin levels, and were more likely to have received aggressive cytoreductive surgery combined with radiation (P<0.05, each comparison). Discovery and validation cohorts were also different in the therapies used (Supplemental Table 1). In contrast, within the discovery cohort, patients of the sub-cohorts from KCI and NYU had similar baseline characteristics (Supplementary Table 2).
Table 1.
Baseline Information for Initial Discovery (NYU/KCI) and Independent (PMCC) datasets
| Covariate | Units | NYU/KCI (N=83) | PMCC (N=111) | P-value1 |
|---|---|---|---|---|
| Clinico-pathologic Variables | ||||
|
| ||||
| Age | Median (Range); years | 63 (34–86) | 65 (36–83) | 0.54 |
| Gender | 0.04 | |||
| Female | N (%) | 21 (25%) | 14 (13%) | |
| Male | N (%) | 62 (75%) | 97 (87%) | |
| Performance Status (ECOG) | 0.007 | |||
| 0 | N (%) | 32 (39%) | 20 (18%) | |
| 1 | N (%) | 37 (45%) | 66 (59%) | |
| 2 or higher | N (%) | 14 (17%) | 25 (23%) | |
| Chest pain | 0.56 | |||
| No | N (%) | 37 (47%) | 57 (51%) | |
| Yes | N (%) | 42 (53%) | 54 (49%) | |
| Missing | N | 4 | 0 | |
| Weight loss | <0.001 | |||
| No | N (%) | 67 (85%) | 63 (57%) | |
| Yes | N (%) | 12 (15%) | 48 (43%) | |
| Missing | N | 4 | 0 | |
| Histology | 1.00 | |||
| Epithelial | N (%) | 58 (70%) | 77 (69%) | |
| Other | N (%) | 25 (30%) | 34 (31%) | |
| Stage | <0.001 | |||
| I/II | N (%) | 26 (31%) | 9 (8%) | |
| III/IV | N (%) | 57 (69%) | 102 (92%) | |
|
| ||||
| Laboratory Data | ||||
|
| ||||
| Hemoglobin | Median (Range); g/dL | 12.0 (8–15) | 13.3 (7–17) | <0.001 |
| Missing | N | 4 | 2 | |
| White blood cell | Median (Range); × 109/L | 8.0 (4–33) | 8.0 (4–23) | 0.54 |
| Missing | N | 4 | 1 | |
| NLR | Median (Range) | 3.2 (1.4–19) | 3.7 (1.3–15) | 0.31 |
| Missing | N | 39 | 1 | |
| Platelet number | Median (Range); × 109/L | 302 (41–895) | 326 (136–880) | 0.15 |
| Missing | N | 4 | 1 | |
|
| ||||
| Treatments | ||||
|
| ||||
| Cytoreductive surgery | 0.02 | |||
| No | N (%) | 32 (39%) | 62 (56%) | |
| Yes | N (%) | 51 (61%) | 49 (44%) | |
| Chemotherapy | 0.26 | |||
| No | N (%) | 19 (23%) | 34 (31%) | |
| Yes | N (%) | 64 (77%) | 77 (69%) | |
| Radiation | <0.001 | |||
| No | N (%) | 79 (95%) | 47 (42%) | |
| Yes | N (%) | 4 (5%) | 64 (58%) | |
|
| ||||
| Clinical Prognostic Indices | ||||
|
| ||||
| EORTC | 0.03 | |||
| Good Prognosis | N (%) | 26 (33%) | 20 (18%) | |
| Poor Prognosis | N (%) | 53 (67%) | 90 (82%) | |
| Missing | N | 4 | 1 | |
| CALGB2 | ||||
| Groups 1–3 | N (%) | 53 (67%) | 64 (58%) | 0.23 |
| Groups 4–6 | N (%) | 26 (33%) | 46 (42%) | |
| Missing | N | 4 | 1 | |
|
| ||||
| Plasma Biomarkers | ||||
|
| ||||
| Fibulin3 | Median (Range); ng/mL | 101 (40–316) | 54 (2–204) | <0.001 |
| Missing | N | 1 | 0 | |
| Mesothelin | Median (Range); ng/mL | 54 (4–272) | 43 (5–910) | 0.41 |
| Missing | N | 1 | 0 | |
| Osteopontin | Median (Range); ng/mL | 120 (23–849) | 108 (19–588) | 0.24 |
NYU – New York University; KCI – Karmanos Cancer Institute; PMCC – Princess Margaret Cancer Centre; ECOG – Eastern Cooperative Oncology Group; NLR – neutrophil-lymphocyte ratio; EORTC – European Organisation for Research and Treatment of Cancer; CALGB – Cancer and Leukemia Group B;
P value comparing NYU/KCI and PMCC datasets, using non-parametric Kruskal-Wallis tests.
EORTC clinical prognostic index was divided into good and poor prognosis based on a regression value cutpoint of 2.7. Weight loss was defined as greater or equal to 10% loss.
Although the distributions of plasma mesothelin and osteopontin levels were similar between KCI/NYU and PMCC cohorts, the validation cohort had lower baseline levels of fibulin3 (Table 1; Supplementary Figure 1). Higher stage of disease was associated with higher levels of plasma osteopontin and mesothelin (but not fibulin3) in the KCI/NYU cohort (Supplementary Table 3). In the PMCC cohort, higher stage disease was associated with higher levels of plasma mesothelin only, but only 8% of this cohort had early Stage I/II disease.
Despite having more aggressive disease, with more aggressive management, survival was better in the validation cohort than in the discovery cohort (P=0.04 by log-rank test; Supplementary Figure 2; Table 2). Survival was similar comparing the NYU and KCI sub-cohorts that comprised the discovery cohort (P=0.68). All deaths were attributable to MPM.
Table 2.
Univariable analysis of baseline characteristics on overall survival for initial discovery (NYU/KCI) dataset and for second, independent (PMCC) dataset.
| Survival Characteristic | NYU/KCI | PMCC | ||||
|---|---|---|---|---|---|---|
| Median follow-up time | 11 months | 16 months | ||||
| Median overall survival | 11 (95%CI: 8–14) months | 18 (95%CI: 14–22) months | ||||
| Percentage alive at 6 months | 70% | 86% | ||||
| Percentage alive at 12 months | 41% | 67% | ||||
| Percentage alive at 24 months | 23% | 26% | ||||
|
| ||||||
| Characteristic | HR (95%CI) | P value | Global P value1 | HR (95%CI) | P value | Global P value1 |
|
| ||||||
| Clinico-pathologic Variables | ||||||
|
| ||||||
| Age per 10 year increase | 1.18 (0.9–1.5) | 0.17 | 1.34 (1.02–1.8) | 0.04 | ||
| Gender | 0.24 | 0.24 | ||||
| Male vs. Female | 1.40 (0.8–2.5) | 1.52 (0.8–3.1) | ||||
| Performance Status (ECOG) | <0.001 | 0.011 | ||||
| 1 or higher vs. 0 | 2.99 (1.8–5.1) | 2.49 (1.2–5.0) | ||||
| Chest pain | 0.08 | 0.053 | ||||
| Yes vs. No | 1.58 (1.0–2.6) | 1.54 (0.99–2.4) | ||||
| Weight loss | <0.001 | <0.001 | ||||
| Yes vs. No | 3.32 (1.7–6.4) | 2.8 (1.8–4.4) | ||||
| Histology | 0.14 | <0.001 | ||||
| Other vs. Epithelial | 1.48 (0.0–2.5) | 2.65 (1.6–4.3) | ||||
| Stage | <0.001 | 0.92 | ||||
| III/IV vs. I/II | 3.93 (2.2–7.2) | 1.05 (0.5–2.4) | ||||
|
| ||||||
| Laboratory Data | ||||||
|
| ||||||
| Hemoglobin per 1g/dl increase | 0.83 (0.7,0.9) | 0.003 | 0.73 (0.6–0.9) | <0.001 | ||
| White blood cell per 109/L increase | 2.08 (1.2–3.6) | 0.01 | 2.69 (1.5–4.8) | 0.001 | ||
| NLR per unit increase | 1.13 (1.0–1.2) | 0.007 | 1.09 (1.0–1.2) | <0.05 | ||
| Platelets per 50 × 109/L increase | 1.15 (1.1–1.3) | <0.001 | 1.22 (1.1–1.3) | <0.001 | ||
|
| ||||||
| Treatments | ||||||
|
| ||||||
| Cytoreductive surgery | <0.001 | <0.001 | ||||
| Yes vs. No | 0.30 (0.2–0.5) | 0.37 (0.2–0.6) | ||||
| Chemotherapy | 0.001 | 0.30 | ||||
| Yes vs. No | 0.41 (0.2–0.7) | 0.78 (0.5–1.3) | ||||
| Radiation | 0.66 | <0.001 | ||||
| Yes vs. No | 1.26 (0.5–3.5) | 0.42 (0.3–0.7) | ||||
|
| ||||||
| Clinical Prognostic Indices | ||||||
|
| ||||||
| EORTC (per 1.0 unit increase in value) | 2.68 (1.8–4.1) | <0.001 | 3.07 (2.0–4.7) | <0.001 | ||
| CALGB (Groups 4–6 vs. Groups 1–3) | 4.07 (2.3–7.1) | <0.001 | 1.78 (1.1–2.8) | 0.01 | ||
|
| ||||||
| Plasma Biomarkers2 | ||||||
|
| ||||||
| Fibulin3 per 50 ng/ml increase | 0.87 (0.7–1.1) | 0.23 | 1.32 (1.0–1.7)3 | 0.06 | ||
| Log-mesothelin per log(ng/100mL) increase | 2.16 (1.5–3.1) | <0.001 | 1.28 (1.1–1.6) | 0.22 | ||
| Log-osteopontin per log(ng/100mL) increase | 3.31 (2.3–4.8) | <0.001 | 3.93 (2.9–5.4) | <0.001 | ||
NYU – New York University; KCI – Karmanos Cancer Institute; PMCC – Princess Margaret Cancer Centre; HR – hazard ratio; CI – confidence interval; ECOG – Eastern Cooperative Oncology Group; NLR – neutrophil-lymphocyte ratio; EORTC – European Organisation for Research and Treatment of Cancer; CALGB – Cancer and Leukemia Group B; Weight loss was defined as greater or equal to 10% loss.
Global P values compare across all levels of a single variable. Although discovery and validation analyses are provided in the same table, the analyses were performed sequentially.
Each plasma biomarker was analyzed as a continuous variable. For the plasma biomarkers, the primary key findings are in the discovery cohort; univariable analysis of plasma biomarkers for the validation cohort is presented for completeness.
This value is being presented for completeness; as there was no significance in the discovery cohort, fibulin3 was not formally validated.
In all survival analyses, no violations of the assumption of proportionality were observed. Non-linear effects of mesothelin and osteopontin (but not fibulin3) on OS were observed (Supplementary Figure 3); log-transformation was found to best address this issue, and log-mesothelin and log-osteopontin were used for all prognostic analyses. The relationships between individual clinicopathologic, laboratory, and treatment variables with survival in the discovery and validation datasets are presented in Table 2. Many of the non-treatment variables have been incorporated into the EORTC(1) and CALGB(16) CPIs, which were both significantly prognostic in both initial discovery and second, independent cohorts (Table 2). Of the plasma biomarkers evaluated in the KCI/NYU cohort, higher levels of log-osteopontin and log-mesothelin (but not fibulin3) were each individually associated with worse prognosis after adjusting for CPIs (Table 3), and were therefore further evaluated for their predictive ability in the independent PMCC cohort. In th PMCC cohort, incorporating either plasma log-osteopontin or log-mesothelin into the baseline predictive CPI models substantively and significantly improved Harrell’s c-statistic as the confidence intervals of the difference in c-statistics did not cross zero (Table 3).
Table 3.
Predictive models of overall survival for log-osteopontin and log-mesothelin
| Cohort | Variable | Adjusted for EORTC CPI | Adjusted for CALGB CPI | ||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| NYU/KCI | log-osteopontin | 2.70 (1.8–4.0) | <0.001 | 2.71 (1.8–4.1) | <0.001 |
| log-mesothelin | 1.94 (1.4–2.8) | <0.001 | 1.63 (1.1–2.4) | 0.009 | |
| PMCC | log-osteopontin | 3.53 (2.6–4.9) | <0.001 | 4.05 (2.9–5.6) | <0.001 |
| log-mesothelin | 1.27 (1.1–1.5) | <0.001 | 1.40 (1.2–1.7) | <0.001 | |
| Discovery (NYU/KCI) Cohort
| ||
|---|---|---|
| Prognostic variables | EORTC CPI | CALGB CPI |
| CPI alone (for log-osteopontin analysis)1, c-index (95%CI) | 0.649 (0.59–0.70) | 0.641 (0.59–0.69) |
| CPI alone (for log-mesothelin analysis)1, c-index (95% CI) | 0.645 (0.59–0.70) | 0.640 (0.59–0.69) |
| CPI + log-osteopontin, c-index (95%CI) | 0.767 (0.71–0.82) | 0.763 (0.71–0.81) |
| CPI + log-mesothelin, c-index (95%CI) | 0.692 (0.63–0.76) | 0.724 (0.66–0.79) |
| Improvement in Harrell’s c-indices when adding log-osteopontin2 | 0.118 (0.10–0.18) | 0.122 (0.11–0.18) |
| Improvement in Harrell’s c-indices when adding log-mesothelin2 | 0.045 (0.03–0.11) | 0.084 (0.06–0.13) |
| Validation (PMCC) Cohort
| ||
|---|---|---|
| Prognostic variables | EORTC CPI | CALGB CPI |
| CPI alone, c-index (95%CI) | 0.596 (0.55–0.64) | 0.602 (0.54–0.66) |
| CPI + log-osteopontin, c-index (95%CI) | 0.811 (0.76–0.86) | 0.781 (0.73–0.83) |
| CPI + log-mesothelin, c-index (95%CI) | 0.650 (0.58–0.72) | 0.649 (0.58–0.71) |
| Improvement in Harrell’s c-indices when adding log-osteopontin2 | 0.216 (0.20–0.26) | 0.179 (0.16–0.23) |
| Improvement in Harrell’s c-indices when adding log-mesothelin2 | 0.054 (0.03–0.12) | 0.047 (0.03–0.10) |
Top panel: Cox proportional hazard models of association; p-values are derived from likelihood ratios comparing the models with/without the biomarker of interest. Middle and Bottom panels: Prognostic model evaluation comparing Harrell’s c-indices with/without biomarker of interest in the model.
The baseline CPI-only c-indices reflect the patient samples available for the associated biomarker analyses; there was one missing log-mesothelin value (and no missing los-osteopontin values) in the discovery dataset that results in minor differences in the CPI-only indices.
Confidence intervals (CIs) for the difference in Harrell’s c-indices were calculated using 200 bootstrap replicates. Intervals that did not cross zero are interpreted as having the Harrell’s c-index demonstrate significant improvement with the addition of the biomarker.
NYU – New York University; KCI – Karmanos Cancer Institute; PMCC – Princess Margaret Cancer Centre; CPI – clinical prognostic index; HR – hazard ratio; CI – confidence interval; EORTC – European Organisation for Research and Treatment of Cancer; CALGB – Cancer and Leukemia Group B. Log-mesothelin and log-osteopontin are in log(ng/100mL) units.
In developing a prognostic model that includes log-osteopontin and log-mesothelin, we utilized the entire dataset of individuals who had complete data on all key variables (n=154); missing individuals had clinico-demographic and outcomes data similar to the discovery cohort. Predictors included in this model were chosen on the basis of clinical reasons: in addition to log-osteopontin and log-mesothelin, we included CPI, clinical stage, and three laboratory values associated with outcome in our datasets (hemoglobin, NLR, and platelet counts). The EORTC CPI was included in the model over the CALGB CPI because it had better performance in our population (Table 3), is the more commonly used CPI in clinical practice, and was generated from a regression model as a continuous variable. Within this prognostic model, log-osteopontin, EORTC CPI, and hemoglobin continued to remain as independently significant predictors, and the entire prognostic model improved the optimism-corrected Harrell’s c-index significantly from 0.718 (0.67–0.77) to 0.801 (0.77–0.84)(Table 4). Model calibration curves (Figure 1) visually confirm good fit between predicted and observed survival curves, using these prognostic scores.
Table 4.
Prognostic Model Development: Pooled analysis
| Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|
| Log-osteopontin | 3.57 (2.5–5.1) | <0.001 |
| Log-mesothelin | 0.97 (0.8–1.2) | 0.80 |
| EORTC CPI | 1.99 (1.4–2.9) | <0.001 |
| Stage III/IV vs I/II | 1.36 (0.8–2.4) | 0.30 |
| Hemoglobin | 0.10 (0.0–0.3) | <0.001 |
| Platelets | 2.56 (0.6–10) | 0.19 |
| Neutrophil-Lymphocyte Ratio | 0.96 (0.5–2.0) | 0.91 |
| Prognostic Model | c-index original | c-index optimism-corrected | Difference in c-index |
|---|---|---|---|
| Without log-osteopontin, log-mesothelin | 0.726 (0.68–0.78) | 0.718 (0.67–0.77) | |
| With log-osteopontin, log-mesothelin | 0.813 (0.78–0.85) | 0.801 (0.77–0.84) | 0.087 (0.08–0.12) |
CPI – clinical prognostic index; EORTC – European Organisation for Research and Treatment of Cancer.
Because of missing data, only 154 pooled cases were analyzed. Selection of variables for the prognostic model was based on clinical rationale. EORTC CPI was included in the model as it was the more commonly used CPI, and because its general performance was better than with the CALGB CPI (see Table 3); as this CPI did not include stage, we included that variable separately. Finally, three laboratory variables not found in the EORTC CPI were also included. The regression equation generating the risk score is RS = 1.273 * log(osteopontin(ng/mL)/100) − 0.025 * log(Mesothelin(ng/mL)/100) + 0.686 * EORTC CPI − 2.349 * Hemoglobin(g/dL) − 0.0402 * NLR/10 + 0.941 * Platelets(×109/L)/1000 + 0.307 (if Stage III/IV; if I/II omit this last term). Median (range) of risk score values is 0.046 (−2.88 to 3.28).
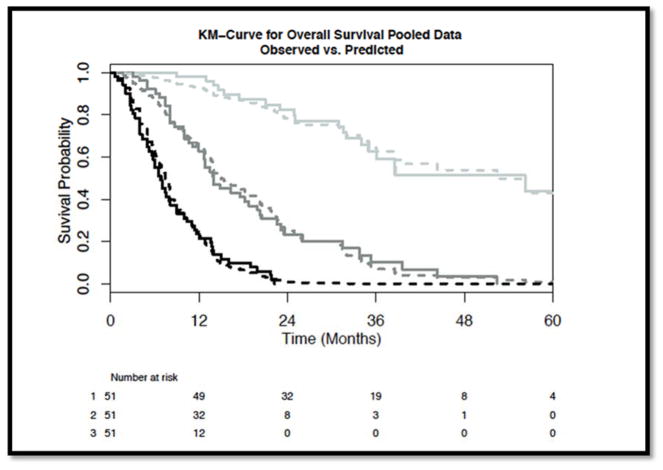
Figure 1. Visual inspection of model fit curves evaluating tertiles of the risk score generated from the pooled prognostic model.
The observed (solid) and predicted (dashed) survival curves are presented. Darkest color represent the worse prognostic risk scores, with progressively lighter lines representing the higher tertiles of risk score. The tertile risk score ranges are: worst prognosis category (risk scores −2.88 to −0.619), intermediate prognosis category (risk scores −0.619 to 0.710), and best prognosis (risk scores 0.71 to 3.28).
Discussion
Prognostic biomarkers, including clinico-demographic information, standard laboratory parameters, and novel plasma or tissue based tests have tremendous potential to improve care by assisting clinicians in appropriately tailoring therapies to individual patient needs. The low disease incidence and diverse treatment strategies have hindered the discovery of prognostic biomarkers in MPM. Nonetheless, studies attempting to define novel prognostic markers in MPM have been reported, chiefly in patients having supportive care or chemotherapy only. Both the EORTC prognostic score and the CALGB index, which includes a combination of clinical and laboratory variables, have been validated prognostically in studies of non-cytoreduced MPMs, and recently were again validated by Meniawy et al.(25) in 274 MPM patients; of the two, the EORTC prognostic index has been more commonly used.
Novel MPM prognostic biomarkers include tissue based assays including a four-gene expression testing (26), expression arrays (27), P16/CDKN2A homozygous deletion (28), high nuclear grade (29), and mir-29c* (30), all of which have been reported to have prognostic significance, but few have been blindly validated. Published candidate blood-based prognostic biomarkers include soluble MRP(10, 31) (32–34), osteopontin, NLR, and fibrinogen(35). At least three single reports point to OPN as a possible prognostic factor in MPM in non-surgical MPM cohorts (34, 36, 37). Nevertheless, biomarker studies of the prognostic implications of pOPN have been largely ignored due to the inability to reproduce its value as a specific diagnostic marker of MPM, and because of the aforementioned performance differences across available ELISA platforms. Another recently described biomarker, FBLN3(38) was reported to have diagnostic discrimination in plasma for diagnostic purposes but not prognostic in plasma. Two studies have reported that FBLN3 may be prognostic when measured in the pleural effusions of MPM patients(38,41). The NLR could not be validated as a reliable prognostic biomarker in a recent report from Meniawy et al. (25).
The present study, therefore, was designed to examine whether plasma biomarkers have added value to known clinical prognostic indices in order to chose which MPM patients have a higher chance of early death from the disease. We attempted to examine the majority of the reported biomarkers in MPM, both in cytoreduced (56%) and non-cytoreduced patients (44%), and an independent cohort was used for validation. The number of patients in the analysis (n=188) is small compared to the recently published IASLC database (n=1494) but compares favorably to the number of patients analyzed by Curran(1) (n=204) and most recently by Meniawy (25). To maintain maximum stringency, we required all plasma biomarker levels to be measured on samples obtained prior to the initiation of therapy, and employed a discovery set followed by a blinded validation cohort. Unlike MPM, other tumor types can allow for “matching” of cohorts due to a large number of available patients. Our patients were recruited prospectively from institutions with considerable expertise in the treatment of MPM, but the components and timing of the multimodality treatment approaches were not uniform across the cohorts. In fact, the PMCC investigators are unique in their reports of promising reports for preoperative radiation therapy and EPP in MPM(39). The test set from KCI was collected prior to the discovery and validation sets, which were collected simultaneously from NYU and PMCC.
Studying mesothelioma is a special case because of its rarity compared to lung cancer. Methodologies which can readily be applied to common cancers are difficult to apply to mesothelioma. In univariable analyses, some of the variables that were significantly associated with reduced survival across all cohorts have been previously reported to be similarly prognostic, including poor performance status, weight loss, low hemoglobin, high white count, increased NLR, a high platelet count and the inability to cytoreduce. The EORTC prognostic index was significant in both the discovery and validation cohorts, and it was used as a surrogate for all non-lab-related clinical variables.
As stated earlier, what has been lacking in previous MPM biomarker reports which we have addressed is whether any of the reported plasma biomarkers provide added value to the clinical CPI by c-statistic comparison. Both log-osteopontin and log mesothelin when added to EORTC CPI were predictive of survival, and both increased the c-index for the discovery and validation sets. Only after we demonstrated that there was added value to both a discovery and validation set did we use a pooled analysis model which should be validated in future trials. By using a bootstrap internal validation modeling approach, we attempted to compensate for overfit or “optimism”, and this approach has greater power in demonstrating improvement in prognostication. In our analysis, using separate development and validation datasets may actually be inferior to pooling all the data and using bootstrap internal validation. As endorsed by Harrell (22) and by Steyerberg (40) the pooled effect estimates in the model are more likely to be accurate compared to the estimates in either group alone.
As the MPM staging system undergoes revision, supplementary prognostic factors in addition to the usual clinic pathologic demographics could add value in the selection of patients for radical and potentially morbid procedures. These supplementary factors, including plasma biomarkers, could alert clinicians that certain MPM patients are not candidates for cytoreductive surgery since their chance of survival is limited by a more aggressive phenotype. In order to potentially validate the CPI for the pooled data described in this study, an international effort to collect blood elements prior to treatment must be addressed. Such efforts should not only be limited to potential cytoreducible patients but also to those enrolled in ongoing novel therapeutic trials.
Supplementary Material
Acknowledgments
Funding: NCI Supported by funding from the Early Detection Research Network, National Institutes of Health, to Dr. Pass (U01 CA-111295), the Princess Margaret Hospital Foundation and the Princess Margaret Hospital Mesothelioma Research Program (funded by the Masters Insulators Association of Ontario, International Association of Heat and Frost Insulators and Asbestos Workers, Local 793, and other unions, and the Imperial Oil Charitable Foundation) for plasma banking; by the M. Qasim Choksi Chair in Lung Cancer Translational Research (held by Dr. Tsao), the Alan B. Brown Chair in Molecular Genetics and CCO Chair in Experimental Therapeutics and Population Studies (both held by Dr. Liu), and the Ontario Ministry of Health and Long-Term Care; and by donations from Belluck and Fox, the Simmons Foundation, Levi Phillips and Konigsberg, the Stephen E. Banner Fund for Lung Cancer Research, the Rosenwald Family, and the Anderson Family
Footnotes
This study has not been presented elsewhere.
Disclaimer: Dr. Pass and Wayne State University have filed a patent application for the use of osteopontin in the diagnosis and prognosis of pleural mesothelioma (US 20090311721 A1).
References
- 1.Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol. 1998;16:145–152. doi: 10.1200/JCO.1998.16.1.145. [DOI] [PubMed] [Google Scholar]
- 2.Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol. 2012;7:1631–1639. doi: 10.1097/JTO.0b013e31826915f1. [DOI] [PubMed] [Google Scholar]
- 3.Pass HI, Giroux D, Kennedy C, et al. Supplementary prognostic variables for pleural mesothelioma: a report from the IASLC staging committee. J Thorac Oncol. 2014;9:856–864. doi: 10.1097/JTO.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anborgh PH, Wilson SM, Tuck AB, et al. New dual monoclonal ELISA for measuring plasma osteopontin as a biomarker associated with survival in prostate cancer: clinical validation and comparison of multiple ELISAs 1. Clin Chem. 2009;55:895–903. doi: 10.1373/clinchem.2008.117465. [DOI] [PubMed] [Google Scholar]
- 5.Thoms JW, Dal PA, Anborgh PH, et al. Plasma osteopontin as a biomarker of prostate cancer aggression: relationship to risk category and treatment response. Br J Cancer. 2012;107:840–846. doi: 10.1038/bjc.2012.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CY, Wu MS, Chiang EP, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782–789. doi: 10.1136/gut.2006.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrik D, Lavori PW, Cao H, et al. Plasma osteopontin is an independent prognostic marker for head and neck cancers. J Clin Oncol. 2006;24:5291–5297. doi: 10.1200/JCO.2006.06.8627. [DOI] [PubMed] [Google Scholar]
- 8.Bramwell VH, Doig GS, Tuck AB, et al. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res. 2006;12:3337–3343. doi: 10.1158/1078-0432.CCR-05-2354. [DOI] [PubMed] [Google Scholar]
- 9.Hollevoet K, Reitsma JB, Creaney J, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J Clin Oncol. 2012;30:1541–1549. doi: 10.1200/JCO.2011.39.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dipalma N, Luisi V, Di SF, et al. Biomarkers in malignant mesothelioma: diagnostic and prognostic role of soluble mesothelin-related peptide. Int J Biol Markers. 2011;26:160–165. doi: 10.5301/JBM.2011.8614. [DOI] [PubMed] [Google Scholar]
- 11.Creaney J, Francis RJ, Dick IM, et al. Serum soluble mesothelin concentrations in malignant pleural mesothelioma: relationship to tumor volume, clinical stage and changes in tumor burden. Clin Cancer Res. 2011;17:1181–1189. doi: 10.1158/1078-0432.CCR-10-1929. [DOI] [PubMed] [Google Scholar]
- 12.Creaney J, Dick IM, Yeoman D, et al. Auto-antibodies to beta-F1-ATPase and vimentin in malignant mesothelioma. PLoS One. 2011;6:e26515. doi: 10.1371/journal.pone.0026515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pass HI, Goparaju C. Fibulin-3 as a biomarker for pleural mesothelioma. N Engl J Med. 2013;368:190. doi: 10.1056/NEJMc1213514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao SC, Vardy J, Chatfield M, et al. Validation of prognostic factors in malignant pleural mesothelioma: a retrospective analysis of data from patients seeking compensation from the New South Wales Dust Diseases Board. Clin Lung Cancer. 2013;14:70–77. doi: 10.1016/j.cllc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol. 2011;6:1304–1312. doi: 10.1097/JTO.0b013e3182208e3f. [DOI] [PubMed] [Google Scholar]
- 16.Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113:723–731. doi: 10.1378/chest.113.3.723. [DOI] [PubMed] [Google Scholar]
- 17.Therneau TMN, Granbsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 18.Harrell FE, Califf DB, Pryor KL, et al. Evaluating the yideld of medical tests. JAMA. 1982;247:2543–2436. [PubMed] [Google Scholar]
- 19.Royston D, Altman D. Regression using fractional polynomials of continous covariatges parsimonious parametric modelling. J Royal Stat Soc. 2015;43:429–467. [Google Scholar]
- 20.Sauerbrei W, Royston D. Building multivariable prognostic and diagnostic models: transformation of the predictors by using fractional polynomials. J Royal Stat Soc. 1999;162:71–94. [Google Scholar]
- 21.Harrell FE, Lee K, Mark DB. Multivariable prognostic models: Issues in developing models, evaluation assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE. Regression Modeling Strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 23.A Package for Survival Analysis in S. 2013. [Google Scholar]
- 24.Harrell Micscellaneous. 2013. Hmisc. [Google Scholar]
- 25.Meniawy TM, Creaney J, Lake RA, et al. Existing models, but not neutrophil-to-lymphocyte ratio, are prognostic in malignant mesothelioma. Br J Cancer. 2013;109:1813–1820. doi: 10.1038/bjc.2013.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De RA, Richards WG, Yeap BY, et al. Sequential binary gene ratio tests define a novel molecular diagnostic strategy for malignant pleural mesothelioma. Clin Cancer Res. 2013;19:2493–2502. doi: 10.1158/1078-0432.CCR-12-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pass HI, Liu Z, Wali A, et al. Gene expression profiles predict survival and progression of pleural mesothelioma. Clin Cancer Res. 2004;10:849–859. doi: 10.1158/1078-0432.ccr-0607-3. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Rios F, Chuai S, Flores R, et al. Global gene expression profiling of pleural mesotheliomas: overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray-based prognostic prediction. Cancer Res. 2006;66:2970–2979. doi: 10.1158/0008-5472.CAN-05-3907. [DOI] [PubMed] [Google Scholar]
- 29.Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol. 2012;25:260–271. doi: 10.1038/modpathol.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pass HI, Goparaju C, Ivanov S, et al. hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res. 2010;70:1916–1924. doi: 10.1158/0008-5472.CAN-09-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollevoet K, Nackaerts K, Thas O, et al. The effect of clinical covariates on the diagnostic and prognostic value of soluble mesothelin and megakaryocyte potentiating factor. Chest. 2012;141:477–484. doi: 10.1378/chest.11-0129. [DOI] [PubMed] [Google Scholar]
- 32.Cristaudo A, Bonotti A, Simonini S, et al. Combined serum mesothelin and plasma osteopontin measurements in malignant pleural mesothelioma. J Thorac Oncol. 2011;6:1587–1593. doi: 10.1097/JTO.0b013e31821e1c08. [DOI] [PubMed] [Google Scholar]
- 33.Schneider J, Hoffmann H, Dienemann H, et al. Diagnostic and prognostic value of soluble mesothelin-related proteins in patients with malignant pleural mesothelioma in comparison with benign asbestosis and lung cancer. J Thorac Oncol. 2008;3:1317–1324. doi: 10.1097/JTO.0b013e318187491c. [DOI] [PubMed] [Google Scholar]
- 34.Grigoriu BD, Scherpereel A, Devos P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res. 2007;13:2928–2935. doi: 10.1158/1078-0432.CCR-06-2144. [DOI] [PubMed] [Google Scholar]
- 35.Ghanim B, Klikovits T, Hoda MA, et al. Ki67 index is an independent prognostic factor in epithelioid but not in non-epithelioid malignant pleural mesothelioma: a multicenter study. Br J Cancer. 2015;112:783–792. doi: 10.1038/bjc.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappia S, Righi L, Mirabelli D, et al. Prognostic role of osteopontin expression in malignant pleural mesothelioma. Am J Clin Pathol. 2008;130:58–64. doi: 10.1309/TWCQV536WWRNEU51. [DOI] [PubMed] [Google Scholar]
- 37.Hollevoet K, Nackaerts K, Gosselin R, et al. Soluble mesothelin, megakaryocyte potentiating factor, and osteopontin as markers of patient response and outcome in mesothelioma. J Thorac Oncol. 2011;6:1930–1937. doi: 10.1097/JTO.0b013e3182272294. [DOI] [PubMed] [Google Scholar]
- 38.Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med. 2012;367:1417–1427. doi: 10.1056/NEJMoa1115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the “SMART” approach for resectable malignant pleural mesothelioma. J Thorac Oncol. 2014;9:397–402. doi: 10.1097/JTO.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 40.Steyerberg EW, Lingsma HF. Predicting citations: Validating prediction models 10. BMJ. 2008;336:789. doi: 10.1136/bmj.39542.610000.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirschner MB, Pulford E, Hoda MA, et al. Fibulin-3 levels in malignant pleural mesothelioma are associated with prognosis but not diagnosis. Br J Cancer. 2015 Sep 15;113(6):963–9. doi: 10.1038/bjc.2015.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.