Abstract
Problem
Autism-spectrum disorder (ASD) is one of the most commonly diagnosed neurodevelopmental disorders in the USA. While ASD can be significantly influenced by genetics, prenatal exposure to maternal infections has also been implicated in conferring risk. Despite this, the effects of several important maternal pathogens, such as cytomegalovirus (CMV) and herpes simplex virus-2 (HSV2), remain unknown.
Methods of Study
We tested whether maternal CMV and/or HSV2 seropositivity was associated with ASD symptoms in children. ELISA was used to assay for CMV IgG and HSV2 IgG in serum from the mothers of 82 children whose ASD symptoms were assessed at 3-6 years of age using the Social Responsiveness Scale-2 (SRS-2).
Results
Associations between maternal viral serostatus and SRS-2 scores were estimated using linear regression with covariate adjustments. The children of mothers seropositive for CMV, but not for HSV2, had SRS-2 scores 3.6 – 4.2 points higher, depending on the adjustment model, than seronegative women, a significant finding, robust to several statistical adjustments.
Conclusions
Our results suggest that maternal CMV infections may influence ASD symptoms. These findings are being further evaluated in ongoing prospective studies with larger population samples.
Keywords: Cytomegalovirus, herpes simplex virus, autism spectrum disorders, prenatal exposure
Introduction
Autism spectrum disorder (ASD) is one of the most common neurodevelopmental disorders diagnosed in the United States, with the most recent estimates of prevalence at 1 in 68 children1. While the core symptoms range in severity, all individuals with ASD experience clinically significant impairment in functioning resulting from persistent deficits in social interactions and/or communication as well as repetitive patterns of behaviors or actions2. The development of ASD can be significantly influenced by genetic factors, although differences in study methodology (e.g., sample selection, diagnostic strategy, data analytic methods) have resulted in substantial variation in estimates of genetic contributions2-5. Importantly, several perinatal exposures, including infections and environmental toxins, have also been implicated in conferring risk for ASD, and the contribution of these and related environmental factors to the etiology of the disorder have received increasing attention in the past decade6-11.
Maternal inflammation during pregnancy is one prenatal environmental factor that has been associated with ASD risk in children9,12. High levels of maternal prenatal circulating pro-inflammatory cytokines, including interleukin-6 (IL-6), interferon-gamma (IFNg), and IL-1alpha, have been associated with ASD with co-morbid intellectual disability in offspring13, while high levels of maternal circulating IFNg, IL-4, and IL-5 have been associated with ASD regardless of comorbid intellectual disability status14. The most obvious cause of maternal inflammation is infection, and indeed a study by Atladottir and associates (2010) determined that pregnant women admitted to the hospital for treatment of infections had greater risk of offspring being diagnosed with ASD15. Interestingly, this study also found that mothers hospitalized for viral infections, specifically in the first trimester, had three times greater risk of having a child diagnosed with ASD15. Despite this, the effects of maternal viral infections during pregnancy have remained understudied10.
The only maternal viral infection to receive significant consideration in the context of its indirect effects on fetal development is influenza. Seasonal and pandemic influenza strains rarely infect the fetus16, yet they have been associated with numerous neurodevelopmental outcomes, including ASD and schizophrenia10,17-20. But unlike influenza, many common viral infections, such as cytomegalovirus (CMV) and herpes simplex virus-2 (HSV2) infections, are asymptomatic and do not result in hospitalization. But despite being sub-clinical, these viruses still induce an immune response21-28. In addition, they establish latency and can be persistently reactivated throughout the lifetime of the host29. This means that women seropositive for CMV or HSV2 harbor the latent virus, which can become reactivated at any time. Unfortunately, little is known of how reactivation of these viruses affect the maternal immune system and what consequences they might have on fetal neurodevelopment and ASD.
Therefore, we designed the current study with the goal of determining if mothers who were CMV or HSV2 seropositive had children who displayed higher levels of ASD symptoms. We used a nested sample (n=82) within the ARCH cohort, a longitudinal pregnancy cohort recruited from two clinics in Lansing, Michigan. Maternal viral serostatus was determined by detection of CMV and/or HSV2 IgG in maternal serum collected at the first prenatal visit. The symptoms of ASD were assessed in children using the parent-reported Social Responsiveness Scale-2 (SRS-2) questionnaire, a pre-clinical ASD assessment tool30. Linear regression models were used to assess the relationship between maternal viral serostatus and ASD symptoms in children.
Methods
Study cohort
The Archive for Research in Child Health (ARCH) is a population-based pregnancy cohort developed to study the effect of prenatal exposures on postnatal childhood outcomes. Between 2008-2016, 854 women were enrolled at first prenatal visit (mean gestational age, 13.1 weeks) from three clinics in Lansing, Michigan. Exclusion criteria included age <18 years and inability to be interviewed in English. At enrollment, women provided information on diet, physical activity, depression, spousal abuse and socio-economic status. Permission was granted to access state-archived data on pregnancy and birth, including birth certificates and archived neonatal blood spots. Biological specimens archived at −80 C included maternal blood (1st/2nd trimester), urine (1st/2nd/3rd trimester) and a placental specimen when possible. Maternal blood was derived from extra tubes set-aside during routine blood draws. The present study utilized the ARCH Child Development cohort (N=132), a nested sample that was recruited from among ARCH participants from whom comprehensive child behavioral data was obtained. All procedures were approved by the Michigan State University Institutional Review Board.
Behavioral assessments
The Social Responsiveness Scale, Second Edition (SRS-2) was used to assess social communication deficits and repetitive/stereotypic behavior consistent with ASD symptoms in children. Mothers responded to 65 questions that assessed their child’s social awareness, social cognition, social communication, social motivation, restricted interests, and repetitive behaviors31. The population mean of the SRS-2 is expected to be 50, with a standard deviation of 10. Items were summed to compute a total score that can be used as an index of ASD symptomatology, such that scores <60 are indicative of behaviors that are within normal limits, 60-65 are indicative of mild deficits in social interactions, and >65 are indicative of more severe behavioral disturbances with substantial interference in everyday social interactions. The SRS-2 is normed by child age and sex, has evidence of good inter-rater reliability, high internal consistency, and convergent validity with the Autism Diagnostic Interview-Revised (ADI-R)30. The SRS-2 scores were used as a continuous outcome variable, as opposed to using ASD diagnosis as a categorical outcome, due to limited sample size, and also in order to identify exposures that influence the entire range of autism-related symptoms.
While the SRS-2 has been widely used to assess ASD symptoms, scores can be inflated due to unrelated behavioral problems. For that reason, the Child Behavior Checklist (CBCL) was used to assess more general maladaptive functioning and behavior problems32,33. Scores in the broadband domains of internalizing (CBCL IB; i.e., depression, anxiety, social withdrawal, and somatic complaints) and externalizing (CBCL EB; i.e. aggression and rule-breaking) were used to adjust SRS-2 score for behavioral problems in these other domains.
The Broad Autism Phenotype Questionnaire (BAPQ) was used to assess aloofness, rigid personality, and pragmatic language deficits that are characteristic of the broader autism phenotype (BAP) in mothers34. BAP traits are qualitatively similar to autistic symptoms, but are subclinical. These characteristics have been previously identified in mothers of autistic children and maternal BAP is considered an indication of genetic liability for autism. A normative BAPQ cutoff score of 3.1 was previously established for mothers35.
Qualitative analysis of maternal antibodies using ELISA
Maternal serum was thawed on ice, diluted 1:100 and assayed using colorimetric ELISA developed for qualitative analysis of CMV IgG (ab108724, Abcam, Cambridge, MA) and HSV2 IgG (ab108739, Abcam) according to the manufacturer’s protocol. Briefly, samples were diluted and incubated in microplates that were pre-coated with viral antigen, which served to “capture” virus-specific antibodies present in the serum. Following incubation, plates were washed and then incubated with horseradish peroxidase (HRP)-conjugated antibodies specific to CMV or HSV2 IgG. Finally, plates were washed, HRP substrate was added for 15 minutes, and the reaction was quenched. The sample absorbance was measured at 450nm and a cut-off control provided by the assay manufacturer was used to determine if women were seropositive for CMV or HSV2.
Data analysis
The characteristics of the analytic sample (e.g., race/ethnicity, maternal age, parity, behavior scores, etc.) were described as frequencies or means and standard deviations. The frequencies of the sample characteristic were also calculated according to CMV or HSV2 serostatus and compared using Chi-squared or Fisher’s Exact Test. Separate general linear models were used to evaluate the association between CMV or HSV2 seroprevalence and ASD symptoms in children. For antibodies to each virus, we first performed unadjusted analyses, and then used two adjusted models. Adjusted Model 1 included maternal age, BAP, education, income, race/ethnicity, parity, and child sex as covariates. Maternal age and BAP were analyzed as continuous variables. Our Adjusted Model 2 included the previous covariates plus additional adjustments for parent-reported CBCL IB and CBCL EB (continuous variables). All linear regression analyses are reported as β-coefficient, 95% confidence intervals (CI), and p-values <.05 were considered significant (Statistical Analysis System, NC).
Results
Study protocol and sample characteristics
We tested the hypothesis that mothers seropositive for CMV and/or HSV2 (IgG+) would have children with higher levels of ASD symptoms. The study protocol included all singleton, term pregnancies with maternal serum available and associated childhood neurodevelopment assessments (final analytic sample, n=82). Maternal viral serostatus was determined by detection of CMV and/or HSV2 IgG in maternal serum collected at the first prenatal visit. The symptoms of ASD and other behavioral symptoms were assessed in children between 3-6 years of age, using the SRS-2 and CBCL questionnaires. Linear regression models were used to assess the relationship between maternal viral serostatus and ASD symptoms in children with and without adjustments for maternal, pregnancy and behavioral characteristics (Figure 1: schematic representation of the study protocol).
Figure 1. Schematic representation of study protocol.
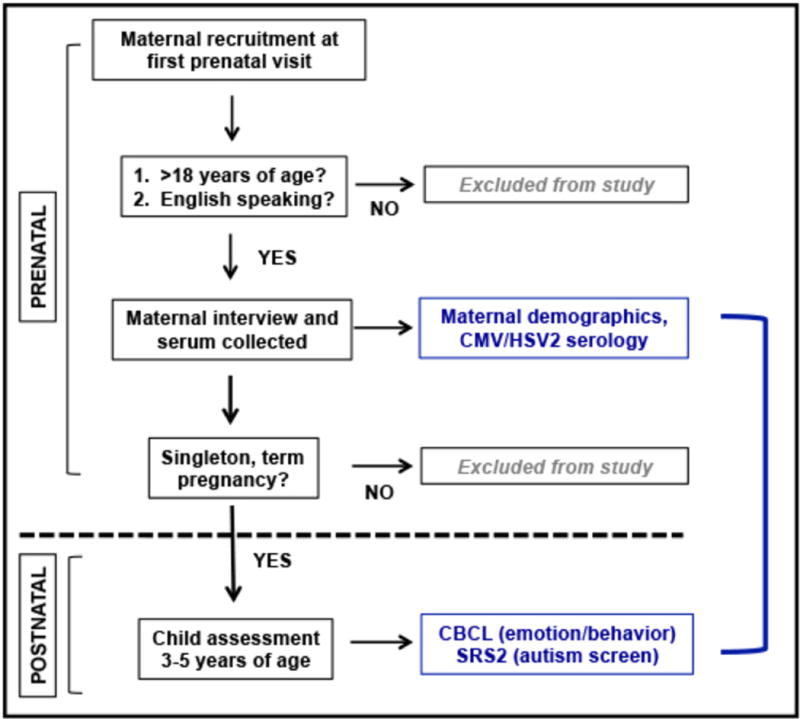
Pregnant women who were 18 years of age or older, and English-speaking, were recruited during their first prenatal visit at two clinics in Lansing, MI. Maternal demographic and pregnancy information was gathered and serum was collected following patient consent. Maternal viral serostatus was evaluated using these first trimester serum specimens. Child behavior assessments were performed between 3-6 years of age, using parent-reported SRS-2 and CBCL scores. The relationship between maternal serostatus and child behavior scores was evaluated using unadjusted and adjusted linear regression.
The characteristics of our analytic sample are reported in Table 1. In total, 53% of our sample was CMV seropositive (IgG) and 21% were HSV2 (IgG) seropositive and the mean SRS-2 score was 53.1 (SD 8.7). Maternal and pregnancy characteristics were further evaluated according to CMV and HSV2 seroprevalence and are reported in Table 2. HSV2 frequency was significantly higher in African American and Hispanic women than in non-Hispanic White women (Table 2)(Chi-squared test, p= .02), and was greatest in older women (Table 2)(Chi-squared test, p< .001). CMV frequency did not significantly differ across maternal and pregnancy characteristics.
Table 1. Maternal, pregnancy and child sample characteristics.
Characteristics of analytic sample (n=82) derived from the ARCH Child Development Cohort (N=132), after excluding premature labor (<37w), twin samples, and samples missing maternal serum or child SRS-2/CBCL behavioral assessments (n=82). BAPQ, Broad Autism Phenotype Questionnaire; CMV, cytomegalovirus; HSV2, herpes simplex virus-2; SRS-2, social responsiveness score version 2; CBCL IB/EB, child behavior checklist internalizing behaviors/externalizing behaviors.
| Maternal characteristics | N | (%) | Maternal viral serostatus | N | (%) |
|---|---|---|---|---|---|
|
|
|
||||
| Race/Ethnicity | CMV IgG | ||||
| Non-Hispanic White/Other | 61 | (74) | Seropositive | 44 | (54) |
| Non-Hispanic Black | 10 | (12) | Seronegative | 38 | (46) |
| Hispanic | 11 | (14) | HSV2 | ||
| Education | Seropositive | 15 | (18) | ||
| < High school | 9 | (11) | Seronegative | 67 | (82) |
| High school/some college | 47 | (58) | Child characteristics | N | (%) |
| College graduate | 25 | (31) | Sex | ||
| Income | Male | 41 | (50) | ||
| < $25,000 | 45 | (57) | Female | 41 | (50) |
| $25,000-$49,000 | 20 | (25) | Mean | (SD) | |
| ≥$50,000 | 14 | (18) | Behavior score | ||
| Parity | SRS-2 | 53.1 | (8.7) | ||
| Primiparous | 39 | (48) | CBCL IB | 48.7 | (10.9) |
| Multiparous | 43 | (52) | CBCL EB | 47.7 | (10.7) |
| Mean | (SD) | ||||
| Maternal age at delivery | 25.5 | (5.1) | |||
| Maternal BAPQ score | 2.7 | (0.6) |
Table 2. Maternal and pregnancy characteristics and viral serostatus.
Frequency analyses performed using Fisher’s Exact or Chi-square test. BAPQ, Broad Autism Phenotype Questionnaire, score ≥3.1 indicative of BAP; CMV, cytomegalovirus; HSV2, herpes simplex virus-2.
| CMV Negative |
CMV Positive |
HSV2 Negative |
HSV2 Positive |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Maternal characteristics
|
N (%) | N (%) | p | N (%) | N (%) | p |
| Race/Ethnicity | ||||||
| Non-Hispanic White/Other | 31 (82) | 30 (68) | 0.19 | 54 (80) | 7 (47) | 0.02 |
| Non-Hispanic Black | 2 (5) | 8 (18) | 6 (9) | 4 (27) | ||
| Hispanic | 5 (13) | 6 (14) | 7 (10) | 4 (27) | ||
| Education | ||||||
| < High school | 4 (11) | 6 (14) | 0.54 | 8 (12) | 2 (13) | 0.93 |
| =High school | 8 (21) | 14 (33) | 17 (26) | 5 (33) | ||
| Some college | 13 (34) | 13 (30) | 22 (33) | 4 (27) | ||
| College graduate | 13 (34) | 10 (23) | 19 (29) | 4 (27) | ||
| Income | ||||||
| < $25,000 | 19 (51) | 26 (62) | 0.59 | 35 (55) | 10 (67) | 0.15 |
| $25,000-$49,000 | 10 (27) | 10 (24) | 19 (30) | 1 (7) | ||
| ≥$50,000 | 8 (22) | 6 (14) | 10 (16) | 4 (27) | ||
| Maternal BAPQ | ||||||
| <3.1 | 31 (91) | 31 (78) | 0.13 | 52 (84) | 11 (85) | 0.9 |
| ≥3.1 | 3 (9) | 9 (22) | 10 (16) | 2 (15) | ||
| Maternal age (years) | ||||||
| 18-25 | 5 (82) | 10 (68) | 0.2 | 14 (22) | 3 (21) | <0.001 |
| 26-35 | 22 (5) | 29 (18) | 41 (64) | 1 (7) | ||
| >35 | 8 (13) | 4 (14) | 9 (14) | 10 (72) | ||
| Parity | ||||||
| Primiparous | 20 (53) | 19 (43) | 0.39 | 34 (51) | 5 (33) | 0.28 |
| Multiparous | 18 (47) | 25 (57) | 33 (49) | 10 (67) | ||
Maternal CMV IgG is associated with ASD symptoms in children
Linear regression was used to evaluate the relationship between maternal viral serostatus and ASD symptoms in children. Maternal CMV IgG was associated with higher levels of ASD symptoms in children (Unadjusted model, CMV; Table 3), and this effect was not driven by SRS-2 score outliers (n=2, >1.5* IQR). However, there was no significant relationship between maternal HSV2 serostatus and ASD symptoms (Unadjusted Model, HSV2; Table 3). Next, the above models were adjusted for sample characteristics for which there were differences in viral prevalence and for those implicated in prior reports. Specifically, we adjusted for maternal age, race/ethnicity, education level36, maternal BAP37, parity38, and child sex39. The association between CMV seropositivity and ASD symptoms persisted following these adjustments (Adjusted Model 1, CMV; Table 3) and the association between HSV2 and ASD symptoms remained non-significant (Adjusted Model 1, HSV2; Table 3). We further adjusted for CBCL IB and CBCL EB scores since these behaviors may inflate SRS-2 scores in children with behavioral problems unrelated to ASD. The significant association between CMV seropositivity and ASD symptoms persisted following these adjustments, and the outcome effect was greater (Adjusted Model 2, β= 4.3 verses Adjusted Model 1, β= 3.6). The association between HSV2 and ASD symptoms remained non-significant (Adjusted Model 2, HSV2; Table 3).
Table 3. Associations between maternal serostatus and child SRS-2.
Unadjusted and adjusted linear regression models were utilized to characterize the relationship between maternal viral serostatus and SRS-2 scores in children. Adjusted model 1: Adjustments include maternal age, race/ethnicity, education level, maternal BAP, parity, and child sex. Adjusted model 2: Adjustments include all in Adjusted model 1, plus adjustment for CBCL IB and CBCL EB. Negative, seronegative; Positive, seropositive; CMV, cytomegalovirus; HSV2, herpes simplex virus-2; SRS-2, social responsiveness score version 2; CBCL IB, child behavior checklist internalizing behaviors; CBCL EB, child behavior checklist externalizing behaviors.
| CMV IgG | Negative (n= 38) |
Positive (n= 44) |
||
|---|---|---|---|---|
|
| ||||
| SRS2 Score | Mean (SE) | Mean (SE) | β (95% CI) | p |
| Unadjusted model | 51.1 (1.4) | 54.8 (1.3) | 3.8 (0.0, 7.5) | 0.05 |
| Adjusted model 1 | 50.5 (1.5) | 54.1 (1.3) | 3.6 (0.5, 6.7) | 0.03 |
| Adjusted model 2 | 50.5 (1.4) | 54.1 (1.3) | 4.3 (1.2, 7.4) | 0.01 |
| HSV2 IgG | Negative (n= 67) |
Positive (n= 15) |
||
|---|---|---|---|---|
|
| ||||
| SRS2 Score | Mean (SE) | Mean (SE) | β (95% CI) | p |
| Unadjusted model | 52.9 (1.1) | 54.0 (2.3) | 1.1 (−3.9, 6.1) | 0.66 |
| Adjusted model 1 | 51.8 (1.4) | 54.2 (2.1) | 2.4 (−2.7, 7.5) | 0.35 |
| Adjusted model 2 | 51.8 (1.4) | 54.2 (2.1) | 1.9 (−2.8, 6.7) | 0.42 |
Finally, because behavior scores were analyzed as continuous variables, and not dichotomized into referent/clinical categories, we removed children with clinically elevated SRS-2 scores (i.e. ≥ 60; n=17) and repeated the analyses. We did this to confirm that associations between viral serostatus and SRS-2 scores were largely driven by children with clinically-significant ASD symptoms. Indeed this was confirmed; removing those cases with elevated SRS-2 scores resulted in attenuation of the unadjusted and fully adjusted findings by 63% and 53%, respectively, and there was no longer an association between maternal CMV seropositivity and SRS-2 scores.
Discussion
The effects of maternal viral infections during pregnancy on fetal brain development have been understudied. While seasonal and pandemic influenza infections during pregnancy have been associated with neurodevelopmental outcomes such as ASD and schizophrenia10,17-20, the effect of other common viral pathogens, such as CMV, remain unstudied. Extant research has primarily investigated the effects of congenital CMV infections, which occur as a result of direct viral transmission to the fetus and lead to significant neurodevelopmental impairments10,40-42. But congenital CMV infection is rare, estimated to occur in 0.5-2.0% of births despite the fact that 60-90% of women are CMV seropositive43. Therefore, the majority of maternal CMV infections do not result in viral transmission to the fetus. However, the virus can still indirectly affect the fetus by affecting maternal immunity and/or placental function. It is the indirect effects of these infections on neurodevelopmental outcomes such as ASD that remain unknown.
Viruses from the family Herpesviradae include many common human pathogens such as CMV and HSV2. One important characteristic of these viruses is that, after primary infection, they establish latency and can then be reactivated throughout the lifetime of the host29. Furthermore, they can undergo persistent reactivation in response to stress, growth factors, hormonal changes, or co-infections29. Importantly, while these viral infections, or reactivations, tend to be subclinical, they do elicit an immune response. Herpesviruses can inhibit the host’s anti-viral defense by blocking interferon, which can induce inflammation21,25,44. Cytomegalovirus infection affects CD8 T-cell function22,23, and there is evidence that CD8 cells play a role in perinatal brain injury45. At the maternal-fetal interface, CMV can affect natural killer cell function28, induce local macrophage and T-cell infiltration46, and disrupt trophoblast invasion47, proliferation47 and immune function24,26,27.
These observations formed the rationale for the present study. If pregnancy-related stress can increase the risk for persistent viral reactivations, leading to chronic immune activation in the mother, this could have negative consequences on fetal brain development. If this were the case, as we hypothesize, then children from mothers harboring these latent viruses would exhibit higher levels of ASD symptoms compared to children from seronegative mothers. Here we tested that hypothesis and determined that, indeed, maternal CMV seropositivity was associated with more severe ASD symptoms in children. Since we assessed maternal viral serostatus, which indicates past infection and not necessarily active infection, this study doesn’t link active infection to ASD symptoms. But because CMV established latency, these findings suggest that harboring the latent virus could be associated with ASD symptoms. A direct role for the virus, and maternal immune activation, in these outcomes has yet to be determined and is the focus of ongoing work.
Few other studies have investigated the effect of maternal viral infections, rather than congenital viral infections, on neurodevelopmental outcomes. Indeed, only one recent study has investigated the association between maternal CMV and HSV2 serostatus during pregnancy and ASD diagnosis in children48. In that study, HSV2 was determined to be associated with ASD, but only when HSV2 IgG was analyzed as a continuous variable. Unfortunately, these results are difficult to interpret because the assay used to determine HSV2 serostatus is strictly qualitative and it is inappropriate to analyze HSV2 IgG on a continuous scale. That study also did not find an association between maternal CMV serostatus and ASD in children. The disparate results between that study and ours are likely due to significant differences in outcome reporting and analysis. In the previous study, ASD was analyzed as a categorical variable and children with ASD were identified through multiple mechanisms including screening via parent questionnaires at 3, 5 and 7 years of age, professional and parent referrals of participants suspected of having ASD, linkages through the Norwegian Patient Register, and a subset of children that were clinically diagnosed at a clinic48. These represent a broad range of diagnostic protocols, some of which depend on treatment-seeking behaviors. These factors can contribute a considerable amount of variation to ASD diagnoses, making it increasingly difficult to study associations, especially in higher functioning individuals.
In conclusion, we determined that maternal CMV seropositivity was associated with ASD symptoms in children. An important limitation of this study was the small sample size, therefore future studies with larger samples will be required to confirm and further evaluate these findings. Further evaluation of CMV activation, and the associated maternal immune response, will be also be important next steps for understanding the mechanisms by which maternal viral exposures influence the development of ASD.
Acknowledgments
Environmental Influences on Child Health Outcomes (ECHO), Office of the Director, NIH UG3OD023285; Michigan State University RAIND Institute; and the Jean and Robert Schultz Biomedical Research Fund. No conflicts of interest to declare.
Footnotes
DR ARIANNA SMITH (Orcid ID : 0000-0003-0025-3932)
DR KAREN RACICOT (Orcid ID : 0000-0002-2887-2543)
References
- 1.Frieden T, Jaffe H, Cono J, Richards C, Iademarco M. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. CDC MMWR. 2014;63(2) [PubMed] [Google Scholar]
- 2.Constantino JN, Marrus N. The Early Origins of Autism. Child Adolesc Psychiatr Clin N Am. 2017;26(3):555–570. doi: 10.1016/j.chc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Colvert E, Tick B, McEwen F, et al. Heritability of Autism Spectrum Disorder in a UK Population-Based Twin Sample. JAMA Psychiatry. 2015;72(5):415–423. doi: 10.1001/jamapsychiatry.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of general psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. The American journal of psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 6.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. International journal of epidemiology. 2014;43(2):443–464. doi: 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matelski L, Van de Water J. Risk factors in autism: Thinking outside the brain. Journal of autoimmunity. 2016;67:1–7. doi: 10.1016/j.jaut.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. doi: 10.1186/s13229-017-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racicot K, Mor G. Risks associated with viral infections during pregnancy. The Journal of clinical investigation. 2017;127(5):1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Tang S, Xu S, Weng S, Liu Z. Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Scientific reports. 2016;6:34248. doi: 10.1038/srep34248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447–1454. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones KL, Croen LA, Yoshida CK, et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Molecular psychiatry. 2017;22(2):273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goines PE, Croen LA, Braunschweig D, et al. Increased midgestational IFN-g, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular Autism. 2011;2(13):1–11. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atladottir HO, Thorsen P, Ostergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2005;23(2–3):299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophrenia bulletin. 2006;32(2):200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of general psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein JM, Cherkerzian S, Seidman LJ, et al. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychological medicine. 2014:1–13. doi: 10.1017/S0033291714000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abate DA, Watanabe S, Mocarski ES. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. Journal of virology. 2004;78(20):10995–11006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agatsuma Y, Fitzpatrick P, Lele A, Kaul A, Ogra PL. Cell-mediated immunity to cytomegalovirus in pregnant women. American journal of reproductive immunology. 1981;1(4):174–179. doi: 10.1111/j.1600-0897.1981.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 23.Cicin-Sain L, Brien JD, Uhrlaub JL, Drabig A, Marandu TF, Nikolich-Zugich J. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS pathogens. 2012;8(8):e1002849. doi: 10.1371/journal.ppat.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jun Y, Kim E, Jin M, et al. Human Cytomegalovirus Gene Products US3 and US6 Down-Regulate Trophoblast Class I MHC Molecules. The Journal of Immunology. 2000;164(2):805–811. doi: 10.4049/jimmunol.164.2.805. [DOI] [PubMed] [Google Scholar]
- 25.Miller D, Zhang Y, Rahill B, Waldman J, Sedmak D. Human Cytomegalovirus inhibits IFNa stimulated antiviral and immunoregulatory responses by blocking multiple levels of IFNa signal transduction. Journal of immunology. 1999;162:6107–6113. [PubMed] [Google Scholar]
- 26.Pizzato N, Garmy-Susini B, Le Bouteiller P, Lenfant F. Down-regulation of HLA-G1 cell surface expression in human cytomegalovirus infected cells. American journal of reproductive immunology. 2003;50(4):328–333. doi: 10.1034/j.1600-0897.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 27.Chou D, Ma Y, Zhang J, McGrath C, Parry S. Cytomegalovirus infection of trophoblast cells elicits an inflammatory response: a possible mechanism of placental dysfunction. American journal of obstetrics and gynecology. 2006;194(2):535–541. doi: 10.1016/j.ajog.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 28.Siewiera J, El Costa H, Tabiasco J, et al. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS pathogens. 2013;9(4):e1003257. doi: 10.1371/journal.ppat.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traylen CM, Patel HR, Fondaw W, et al. Virus reactivation: a panoramic view in human infections. Future Virol. 2011;6(4):451–463. doi: 10.2217/fvl.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingersoll B, Hopwood C, Wainer A, Donnellan B. A comparison of three self-reported measures of the broader autism phenotype in a non-clinical sample. J of Autism and Developmental Disorders. 2011;41(12):1646–1657. doi: 10.1007/s10803-011-1192-2. [DOI] [PubMed] [Google Scholar]
- 31.Constantino J, Gruber CJ. Social Responsiveness Scale. Los Angeles, CA; Western Psychological Services; 2005. [Google Scholar]
- 32.Ivanova MY, Achenbach TM, Rescorla LA, et al. Preschool psychopathology reported by parents in 23 societies: testing the seven-syndrome model of the child behavior checklist for ages 1.5-5. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1215–1224. doi: 10.1016/j.jaac.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- 34.Hurley R, Losh M, Parlier M, Reznick S, Piven J. The broad autism phenotype questionnaire. J of Autism and Developmental Disorders. 2007;37(9):1679–1690. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- 35.Sasson NJ, Lam KS, Childress D, Parlier M, Daniels JL, Piven J. The broad autism phenotype questionnaire: prevalence and diagnostic classification. Autism Res. 2013;6(2):134–143. doi: 10.1002/aur.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osborne LA, McHugh L, Saunders J, Reed P. Parenting stress reduces the effectiveness of early teaching interventions for autistic spectrum disorders. J Autism Dev Disord. 2008;38(6):1092–1103. doi: 10.1007/s10803-007-0497-7. [DOI] [PubMed] [Google Scholar]
- 37.Maxwell C, Parish-Morris J, Hsin O, Bush J, Schultz R. The broad autism phenotype predicts child functioning in autism spectrum disorders. J Neurodevelopmental Disorders. 2013;5(25):1–7. doi: 10.1186/1866-1955-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheslack-Postava K, Jokiranta E, Suominen A, Lehti V, Sourander A, Brown AS. Variation by diagnostic subtype in risk for autism spectrum disorders associated with maternal parity among Finnish births. Paediatr Perinat Epidemiol. 2014;28(1):58–66. doi: 10.1111/ppe.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen D, Baio J, Braun K, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morb Mortal Wkly Rep. 2016;65(3) doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andriesse G, Weersink A, de Boer J. Visual impairment and deafness in young children: consider the diagnosisi of congenital infection with cytomegalovirus, even years after birth. Arch Opththalmol. 2006;124:743. doi: 10.1001/archopht.124.5.743. [DOI] [PubMed] [Google Scholar]
- 41.Kylat RI, Kelly EN, Ford-Jones EL. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur J Pediatr. 2006;165(11):773–778. doi: 10.1007/s00431-006-0172-6. [DOI] [PubMed] [Google Scholar]
- 42.Smithers-Sheedy H, Raynes-Greenow C, Badawi N, McIntyre S, Jones CA, Australian Cerebral Palsy Register G Congenital cytomegalovirus is associated with severe forms of cerebral palsy and female sex in a retrospective population-based study. Dev Med Child Neurol. 2014;56(9):846–852. doi: 10.1111/dmcn.12467. [DOI] [PubMed] [Google Scholar]
- 43.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clinical microbiology reviews. 2009;22(1):99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Racicot K, Kwon JY, Aldo P, et al. Type I Interferon Regulates the Placental Inflammatory Response to Bacteria and is Targeted by Virus: Mechanism of Polymicrobial Infection-Induced Preterm Birth. American journal of reproductive immunology. 2016;75(4):451–460. doi: 10.1111/aji.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei J, Xie L, Zhao H, et al. Maternal CD8(+) T-cell depletion alleviates intrauterine inflammation-induced perinatal brain injury. American journal of reproductive immunology. 2017 doi: 10.1111/aji.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aronoff DM, Correa H, Rogers LM, Arav-Boger R, Alcendor DJ. Placental pericytes and cytomegalovirus infectivity: Implications for HCMV placental pathology and congenital disease. American journal of reproductive immunology. 2017;78(3) doi: 10.1111/aji.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Zheng X, Li Q, et al. Role of human cytomegalovirus in the proliferation and invasion of extravillous cytotrophoblsts isolated from early placentae. Int J Clin Exp Med. 2015;8(10):17248–17260. [PMC free article] [PubMed] [Google Scholar]
- 48.Mahic M, Mjaaland S, Bovelstad HM, et al. Maternal Immunoreactivity to Herpes Simplex Virus 2 and Risk of Autism Spectrum Disorder in Male Offspring. mSphere. 2017;2(1) doi: 10.1128/mSphere.00016-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


