Atherosclerosis is an arterial disease that progresses with age and affects many people in the world1. In the United States and Western Europe, cardiovascular disease (CVD) caused by atherosclerosis is the leading cause of death. In advanced atherosclerosis, there is narrowing of the arterial lumen to the extent that tissue ischemia can occur when metabolic demands increase (e.g. coronary ischemia with angina). In severe cases, acute rupture or erosion of atherosclerotic plaques lead to the rapid formation of intravascular thrombi, a process referred to atherothrombosis. Clinical manifestations of atherothrombosis occur in the heart (coronary artery disease, CAD, and myocardial infarction, MI), brain (ischemic stroke) and in peripheral arteries (peripheral artery disease, PAD). CAD can be divided into stable disease and unstable disease that includes acute coronary syndrome (ACS) with ST-elevated MI (STEMI) or non ST-elevated MI (NSTEMI).
Arterial thrombi are primarily composed of platelets (so-called “white clots”)2. However, cross-linked fibrin strands stabilize the clot. Plaques contain many platelet activators, including collagen (Figure). In addition, plaques contain high levels of tissue factor that activates the coagulation cascade (Figure). Importantly, platelets and the coagulation cascade are activated in parallel and there is crosstalk between the two pathways. For instance, thrombin is a potent activator of human platelets through cleavage of protease-activated receptors (PARs) (PAR1 and PAR4 (reviewed in3,4) and fibrinogen is used to bridge activated platelets. Conversely, activated platelets enhance coagulation by providing coagulation factors and by presenting a negatively-charged phospholipid surface that facilitates the assembly of cofactor/coagulation protease complexes and thrombin generation.
Figure 1. Role of platelets and the coagulation cascade in atherothrombosis and vascular inflammation.
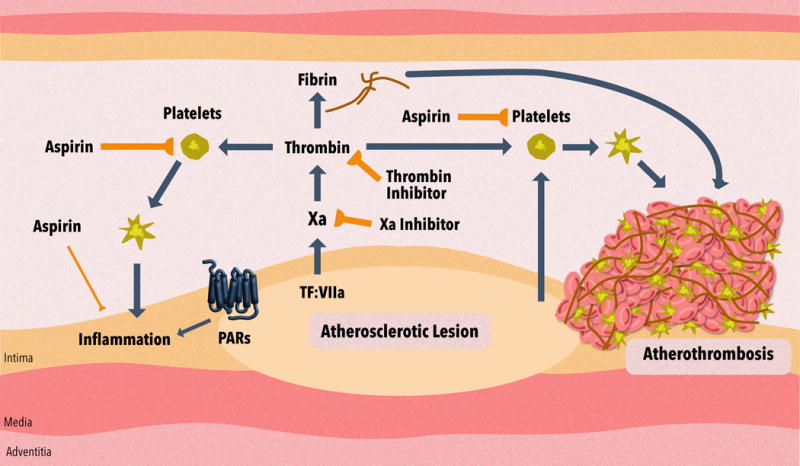
Atherothrombosis involves atherosclerotic plaque rupture or erosion and the formation of an intravascular thrombus. The atherosclerotic plaque contains agents, such as collagen, that activate platelets, and tissue factor (TF) that activates the coagulation cascade. Aspirin reduces the thrombus by inhibiting platelet activation. Anticoagulants reduce the thrombus by inhibiting fibrin generation and also by reducing thrombin activation of platelets. These inhibitors include the thrombin inhibitor dabigatran etexilate and the factor Xa inhibitors rivaroxaban, apixaban, edoxaban or betrixaban. Activated platelets and activation of protease-activated receptors (PARs) by coagulation proteases may also increase inflammation in the vessel wall. Aspirin and anticoagulants may also reduce vascular inflammation and limit the progression of atherosclerosis.
Platelet inhibitors are the primary therapy used to prevent arterial thrombosis in CVD patients5,6. The standard medical treatment for ACS patients and patients receiving percutaneous intervention (PCI) with an intracoronary stent is dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor5. Aspirin irreversibly inhibits COX-1 and prevents platelet-dependent thromboxane formation. P2Y12 inhibitors include thienopyridines, such as clopidogrel and prasugrel, and cyclopentyltriazolopyrimidine-type inhibitors, such as ticagrelor7. Clopidogrel and prasugrel are prodrugs that require activation by cytochrome P450 enzymes in the liver. Ticagrelor is a direct acting non-competitive inhibitor of P2Y12.
DAPT is recommended for a minimum of 6 months in patients with stable CAD receiving a drug eluting stent and for 12 months in patients with ACS (the majority of whom undergo PCI with stenting)8. After this time, therapy can be changed to a single antiplatelet agent depending on the balance between risk of recurrent ischemic event versus risk of bleeding with DAPT. Despite continued antiplatelet therapy, patients remain at risk for recurrent cardiovascular events, particularly in those at highest risk, such as patients with diabetes, PAD and complex CAD. The current recommended treatment for patients with stable CAD is low-dose aspirin or clopidogrel if they do not tolerate aspirin9. The recommended treatment for patients with PAD is single antiplatelet therapy10. However, despite antiplatelet therapy, ~2-5% of CAD and PAD patients annually have major adverse cardiovascular events (MACE)11-15.
Clinical trials have compared the efficacy and safety of different antiplatelet agents and combinations of antiplatelet agents in CVD patients, attempting to find more effective and safer strategies to prevent recurrent atherothrombotic events (Table). A recent review discusses these different trials in detail16. In this editorial, we selected a few trials to illustrate differences between trials and the relationship between efficacy and safety. Comparison of different trials is difficult because they are performed at different times and include different groups of patients.
Table.
Effect of different single and combined antithrombotic drugs on thrombotic events and bleeding in patients with cardiovascular disease. The table shows a select group of the different clinical trials. Details of each study are provided in the original references.
Abbreviations. ACS: acute coronary syndrome, ASA: aspirin, CAD: coronary arterial disease, CV: cardiovascular, CVD: cardiovascular disease, DAPT: dual antiplatelet therapy, ICH: Intracerebral hemorrhage, MI: myocardial infarction, PAD: peripheral arterial disease.
| Name of Trial | Patient Group | # Patients | Therapeutic Strategy | Efficacy | Safety | Ref |
|---|---|---|---|---|---|---|
| CHARISMA | CVD | 15,603 | ASA vs ASA+Clopidogrel | No effect on CV death | Similar bleeding | 22 |
| TRITON-TIMI 38 | ACS with PCI | 13608 | Prasugrel vs Clopidogrel | Decrease in CV death | Increased bleeding | 18 |
| PLATO | ACS with or without ST-elevation | 18624 | Tricagrelor vs Clopidogrel | Decrease in CV death | Similar bleeding | 17 |
| TRILOGY ACS | Unstable angina or MI with or without ST-elevation | 7243 | Prasugrel vs Clopidogrel | No effect on CV death | Similar bleeding | 19 |
| PEGASUS-TIMI 54 | MI within 3 yrs | 21,162 | ASA vs ASA+Ticagrelor | Reduced CV death | Increased bleeding | 23 |
| EUCLID | Symptomatic PAD | 13885 | Tricagrelor vs Clopidogrel | No effect on CV death | Similar bleeding | 13 |
| TRA 2P-TIMI 50 | History of MI, ischemic stroke or PAD | 26,449 | ASA vs ASA/DAPT+Vorapaxar | Reduced CV death in patients with stable atherosclerosis | Increased bleeding, ICH | 26 |
| TRACER | ACS without ST-elevation | 12,944 | ASA vs ASA/DAPT+Vorapaxar | No effect on CV death | Increased bleeding, ICH | 25 |
| APPRAISE-2 | Recent ACS and 2 risk factors | 7,392 | DAPT vs DAPT+Apixaban | No effect on CV death | Increased bleeding | 30 |
| ATLAS ACS 2-TIMI 51 | ACS | 15,526 | DAPT vs DAPT+Rivaroxaban | Reduced CV death* | Increased bleeding | 32 |
| COMPASS | Stable atherosclerotic disease, CAD and PAD | 27,395 | ASA vs ASA+Rivaroxaban | Reduced CV death* | Increased bleeding | 35 |
Reduced the composite end point of death from cardiovascular causes, myocardial infarction, or stroke.
The TRITON-TIMI 38 trial enrolled >13000 patients with ACS (either STEMI or NSTEMI) of which 99% underwent PCI. The more potent P2Y12 blocker prasugrel was superior to clopidogrel in reducing the combined endpoint of vascular death, MI or stroke in patients with ACS but there was a higher rate of bleeding with prasugrel (Table)17,18. In contrast, prasugrel was not superior to clopidgrel in reducing cardiovascular (CV) death in patients with NSTEMI ACS who did not undergo revascularization in the TRILOGY ACS trial (Table)19. In the PLATO trial, the direct acting P2Y12 blocker ticagrelor was superior to clopidogrel in >18000 patients with ACS (either STEMI or NSTEMI) undergoing PCI who were treated with aspirin, but not in the EUCLID trial in which patients with symptomatic PAD were treated with P2Y12 inhibitor monotherapy (Table)13,17. However, the beneficial effect of ticagrelor in PLATO was driven by a reduction in MI and vascular death whereas there was no difference in stroke17. In addition to differences in receptor binding kinetics, part of the difference in efficacy between clopidogrel and prasugrel/ticagrelor in ACS patients treated with PCI may be due to a lack of response to clopidogrel. Clopidogrel must be metabolized to an active form via a pathway that involves the CYP2C19 pathway, resulting in a broad variation in concentrations of the active metabolite. Recently, the IGNITE study showed a higher risk for MACE in patients with a CYP2C19 loss-of-function allele if clopidogrel versus alternative therapy was prescribed20.
Because of the lack of optimal protection of single antiplatelet therapy in patients with stable CAD and/or risk factors, the use of DAPT was studied in the CHARISMA trial. This study of >15000 patients showed that adding clopidogrel to aspirin did not reduce the risk of cardiovascular death, MI or stroke compared to aspirin alone21. While there was no benefit in patients with CAD risk factors, a subgroup analysis of patients with prior MI, stroke or PAD (9,478 patients) found a significant reduction in death without an increase in major bleeding in the patients treated with DAPT compared with aspirin alone22. The PEGASUS-TIMI 54 trial investigated the effect of DAPT beyond 1 year in patients with a prior MI and found that addition of ticagrelor to aspirin reduced CV death in patients but significantly increased major bleeding23.
Vorapaxar is a PAR1 inhibitor that represents a new class of antiplatelet therapy24. Two studies determined the effect of triple antiplatelet therapy on CVD patients by adding Vorapaxar on top of standard antiplatelet therapy. The TRACER trial found that vorapaxar did not reduce CV death in ACS patients but increased intracranial hemorrhage25. The TRA 2P-TIMI 50 observed a reduced risk of cardiovascular death in patients with stable atherosclerosis but was stopped due to the risk of intracranial bleeding (Table)25,26. Interestingly, in the TRA 2P-TIMI 50 trial 14% of the patients had PAD and while vorapaxar had no effect on the composite endpoint of CV death, MI or stroke, vorapaxar significantly reduced acute limb ischemic (ALI) events and peripheral revascularization in PAD patients compared to placebo15,26. These studies indicate that, in general, an intensification of antiplatelet therapy is associated with increased efficacy but often with increased bleeding.
An alternative strategy to prevent thromboembolic events in CVD patients is to add an anticoagulant agent to standard antiplatelet therapy. Early studies evaluated warfarin in ACS patients. Warfarin is a vitamin K antagonist that reduces blood coagulation by inhibiting the formation of gamma-carboxyglutamic domains on the coagulation factors prothrombin, factor VII, factor IX and factor X. A meta-analysis of 10 trials found that ACS patients receiving aspirin and warfarin had a decrease in MI and ischemic stroke but at the cost of an increase in major bleeding compared with patients receiving aspirin alone27. These studies were performed prior to the widespread usage of PCI and DAPT in patients with ACS and thus do not reflect current practice.
Recently, a new family of anticoagulants has been developed that are called direct oral anticoagulants (DOACs) because they directly bind and inhibit either thrombin or factor28. DOACs consist of the thrombin inhibitor dabigatran etexilate and the factor Xa inhibitors apixaban, rivaroxaban, edoxaban and betrixaban28. Importantly, DOACs have lower rates of intracerebral bleeding compared to vitamin K antagonists29. However, the major side-effect of all antithrombotic agents is bleeding because both platelets and the coagulation cascade are required for hemostasis.
The concept of combined antiplatelet and anticoagulant agents for the secondary prevention of atherothrombotic events has been revisited since the approval of DOACs because they have a lower risk of intracerebral bleeding compared with vitamin K antagonists28. Two trials have analyzed the effect of adding two different factor Xa (FXa) inhibitors to standard antiplatelet therapy in ACS patients (Table). In APPRAISE-2, the addition of apixaban to DAPT did not significantly affect the primary outcome of cardiovascular death (7.5% versus 7.9%) but significantly increased major bleeding which led to the termination of the study30. The dose of apixaban used in this trial was 5 mg bid, which is the same dose used to treat patients with venous thromboembolism or atrial fibrillation. In ATLAS ACS 2-TIMI 51, ACS patients received low doses of rivaroxaban (2.5 or 5 mg bid)31 which are lower than the therapeutic doses used to treat patients with venous thromboembolism or prevent thromboembolic stroke in atrial fibrillation (20 mg qd). Both low doses of rivaroxaban reduced the primary endpoint of cardiovascular death, MI or stroke when compared to placebo. Importantly, the 2.5 mg bid dose of rivaroxaban significantly reduced cardiovascular death (2.7% versus 4.1%) compared with standard antiplatelet therapy alone, whereas no survival benefit was observed with the 5 mg bid dose. Compared to placebo, rivaroxaban was associated with increased rates of major bleeding and intracranial hemorrhage but not fatal bleeding. This study indicates that addition of rivaroxaban at a dose of 2.5 mg bid to standard antiplatelet therapy reduced death in ACS patients but at the cost of increased bleeding. Rivaroxaban is approved together with antiplatelet therapy for ACS in Europe but not the US.
It is intriguing that the lower dose of rivaroxaban improved survival whereas the higher dose did not have this effect. This result has to be interpreted very cautiously as the study was not designed nor powered to look at differences in death between the two doses. It is interesting however to speculate on possible etiologies. One potential explanation is that this effect is because of the higher rates of bleeding with rivaroxaban 5 mg bid compared with 2.5 mg bid. Another explanation is that thrombin can act as both a procoagulant and an anticoagulant. When thrombin binds its receptor thrombomodulin on endothelial cells it changes its substrate specificity and is able to activate the anticoagulant protein C. Indeed, infusion of a low dose of thrombin into baboons increases levels of activated protein C (APC)32. In addition to its anticoagulant activity, APC has anti-inflammatory and cytoprotective activities33,34. Therefore, it is possible that thrombin-dependent generation of the APC and its vascular protective functions is preserved with a low dose of rivaroxaban. It would be interesting to test this hypothesis by measuring levels of plasma APC in patients treated with 2.5 mg bid and 5 mg bid doses of rivaroxaban.
The standard therapy for patients with stable CAD and/or PAD is treatment with a single antiplatelet agent, such as aspirin or clopidogrel. To determine if outcomes would be improved by adding an anticoagulant, the recent COMPASS trial compared the effect of aspirin alone (100 mg qd), rivaroxaban alone (5 mg bid) or aspirin (100 mg qd) plus rivaroxaban (2.5 mg bid) in stable CAD and PAD patients35. Importantly, the composite of CV death, stroke, or MI was significantly decreased in the rivaroxaban-plus-aspirin group compared to the aspirin group (4.1% versus 5.4%). However, there was a significant increase in major bleeding (3.1% versus 1.9%)35. Interestingly, rivaroxaban alone also reduced the primary outcome of CV death, MI or stroke compared with aspirin but this reduction was not statistically significant (p = 0.12). Similar results were observed when the stable CAD patients (24,824 patients) and the PAD patients (7470 patients) were analyzed separately, although the net clinical benefit was stronger in PAD compared to CAD patients36,37.
The combination of rivaroxaban-plus-aspirin was associated with fewer CV deaths and death from any cause than aspirin alone. What is the mechanism by which rivaroxaban-plus-aspirin reduced death in stable CAD and PAD patients compared with aspirin alone? The simplest explanation is that the combination of an anticoagulant agent and an antiplatelet agent has superior antithrombotic activity compared to aspirin alone in preventing major thrombotic events. Anticoagulants, such as rivaroxaban, not only reduce fibrin generation but also indirectly inhibit platelet activation by reducing the amount of thrombin (Figure). This hypothesis is supported by the reduction in ischemic endpoints in the COMPASS trial.
Interestingly, the combination of rivaroxaban and aspirin significantly reduced major adverse limb events in the PAD patients compared with aspirin alone37. Similarly, PAD patients treated with the PAR1 inhibitor had a significant reduction in ALI events and revascularization compared to placebo15. At present, the relative contribution to vascular pathogenesis in PAD patients of thrombotic events and vascular inflammation are not well understood.
It is intriguing to speculate that dual aspirin and rivaroxaban therapy in the COMPASS trial has an effect beyond simply reducing thrombosis. Platelets and activation of PARs by coagulation proteases can enhance inflammation and atherosclerosis. Aspirin has anti-inflammatory activity and blocking FXa would reduce PAR signaling (Figure). In mouse studies, a deficiency of P-selectin, P2Y12 or CalDAG-GEFI, which is required for calcium-dependent platelet activation, is associated with reduced atherosclerosis38-40 Mouse studies have also shown that thrombin and FXa inhibitors stabilize plaques by reducing plaque inflammation and increasing the fibrous cap41. An early study found that the thrombin inhibitor melagatran reduced atherosclerosis in ApoE−/− mice and this was associated with reduced MMP9 expression and an increase in the fibrous cap42. Similar results were observed with the thrombin inhibitor dabigatran43-45. Importantly, rivaroxaban also reduced atherosclerosis in ApoE−/− mice and this was associated with reduced levels of MMP9 and macrophages46. A further study found that rivaroxaban did not affect established lesions in ApoE−/− mice but decreased inflammatory mediators and increased plaque stability47. However, thrombin and FXa inhibitors may not be equal since FXa is upstream of thrombin and is a more potent activator of PAR2 than thrombin. For instance, in a mouse model of sickle cell disease we found that rivaroxaban but not dabigatran reduced IL-6 expression48. Furthermore, we found that PAR2−/− mice but not PAR1−/− mice have reduced atherosclerosis in Apoe-/- and Ldlr-/- models49(Owens, Mackman, in revision). Despite these interesting mouse studies, we need clinical data on the plaques themselves and/or levels of circulating biomarkers before we can conclude that the protection by dual aspirin-plus-rivaroxaban therapy observed in COMPASS is due to effects beyond simply antithrombotic activity.
DOACs target coagulation proteins in the common pathway of the coagulation protease cascade. This pathway is essential for hemostasis since in mice deficiencies in either prothrombin, factor V or factor X leads to fatal bleeding50-53. The use of DOACs are associated with an increased the risk of bleeding and this has led to the search for new, safer target for anticoagulant therapy. One such target is factor XI, which is near the top of the intrinsic pathway of the coagulation protease cascade. One recent small phase II proof of concept study found that reducing levels of factor XI using an antisense oligonucleotide led to a reduction in VTE in patients undergoing total knee arthroplasty with less bleeding than patients receiving the low-molecular-weight heparin enoxaparin54. This suggests that factor XIa might have a larger therapeutic window (efficacy versus safety) than factor Xa (Weitz J, ATVB in press). However, these studies need to be confirmed in larger studies. An intriguing possibility is that addition of a factor XIa inhibitor to aspirin in patients with CAD or PAD will reduce cardiovascular death without increasing bleeding.
Antiplatelet agents have been the cornerstone of therapy for preventing ischemic events and death in cardiovascular disease patients. Intensification of antiplatelet therapy with more potent antiplatelet agents or combinations of antiplatelet agents reduces thrombotic events and CV death in CVD patients. However, this is often associated with an increase in bleeding, such as seen in studies with triple antiplatelet therapy, because platelets are essential for hemostasis. The ATLAS ACS 2-TMI 51 and COMPASS trials show that addition of low doses of the FXa inhibitor rivaroxaban to antiplatelet agents can have a beneficial effect on ischemic events in CVD patients, although there is an increase in bleeding. Future challenges include 1/finding patients that will benefit most from combined antiplatelet and anticoagulant therapy, 2/what are the optimal doses of each agent, 3/what are the best anticoagulant and antiplatelet drugs, 4/understanding of the mechanisms by which this dual therapy protects CVD patients.
Supplementary Material
Acknowledgments
NM, HS, GS and HtC wrote the manuscript.
Sources of Funding NM was supported by grants from the National Institutes of Health and the John C. Parker Professorship. HtC and HMHS are supported by grants from the CardioVascularResearchNetwork (“CVON”) grants RACE-5 and CONTRAST from the Netherlands Heart Foundation.
Disclosure NM is a consultant for Bayer and Janssen Pharmaceuticals. HS is a consultant for Bayer and has a research grant from Bayer. HtC is a consultant for Bayer and Stago and has a research grant from Bayer.
Non-standard abbreviation and acronyms
- ACS
acute coronary syndrome
- APC
activated protein C
- APT
antiplatelet therapy
- CAD
coronary artery disease
- CVD
cardiovascular disease
- DAPT
dual antiplatelet therapy
- DOAC
direct oral anticoagulant
- FXa
factor Xa
- MACE
major adverse cardiovascular event
- MI
myocardial infarction
- NSEMI
non ST-elevated myocardial infarction
- PAD
peripheral artery disease
- PAR
protease-activated receptor
- PCI
percutaneous coronary intervention
- STEMI
ST-elevated myocardial infarction
References
- 1.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 4.Posma JJN, Posthuma JJ, Spronk HMH. Coagulation and non-coagulation effects of thrombin. J Thromb Haemost. 2016;14(10):1908–1916. doi: 10.1111/jth.13441. [DOI] [PubMed] [Google Scholar]
- 5.Patrono C, Morais J, Baigent C, Collet J-P, Fitzgerald D, Halvorsen S, Rocca B, Siegbahn A, Storey RF, Vilahur G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J Am Coll Cardiol. 2017;70(14):1760–1776. doi: 10.1016/j.jacc.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Chan NC, Eikelboom JW, Weitz JI. Evolving Treatments for Arterial and Venous Thrombosis: Role of the Direct Oral Anticoagulants. Circ Res. 2016;118(9):1409–1424. doi: 10.1161/CIRCRESAHA.116.306925. [DOI] [PubMed] [Google Scholar]
- 7.Gresele P, Page CP, Fuster V, Vermylen J. Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics. 2002 [Google Scholar]
- 8.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Vol. 133. American Heart Association, Inc.; 2016. pp. 1135–1147. [DOI] [PubMed] [Google Scholar]
- 9.Task Force Members. Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 10.European Stroke Organisation. Tendera M, Aboyans V, Bartelink M-L, et al. SC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(22):2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL. Identification of and management approaches for the high-risk patient. Am J Cardiol. 2006;98(12A):22Q–29Q. doi: 10.1016/j.amjcard.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Bonaca MP, Bhatt DL, Steg PG, et al. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients with prior myocardial infarction: insights from PEGASUS-TIMI 54. Eur Heart J. 2016;37(14):1133–1142. doi: 10.1093/eurheartj/ehv531. [DOI] [PubMed] [Google Scholar]
- 13.Hiatt WR, Fowkes FGR, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS, Blomster J, Millegård M, Reist C, Patel MR, EUCLID Trial Steering Committee and Investigators Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N Engl J Med. 2017;376(1):32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 14.Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, Mehrhof F, Merlini PA, Murphy SA, Sabatine MS, Tendera M, Van de Werf F, Wilcox R, Morrow DA, TRA 2°P-TIMI 50 Steering Committee Investigators Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet. 2012;380(9850):1317–1324. doi: 10.1016/S0140-6736(12)61269-0. [DOI] [PubMed] [Google Scholar]
- 15.Bonaca MP, Scirica BM, Creager MA, Olin J, Bounameaux H, Dellborg M, Lamp JM, Murphy SA, Braunwald E, Morrow DA. Vorapaxar in patients with peripheral artery disease: results from TRA2{degrees}P-TIMI 50. Circulation. 2013;127(14):1522-9–1529e1-6. doi: 10.1161/CIRCULATIONAHA.112.000679. [DOI] [PubMed] [Google Scholar]
- 16.Olie RH, Cate ten H, van der Meijden PEJ. The coagulation system in atherothrombosis; implications for new therapeutic strategies. ResPractThrombHemost. 2018 doi: 10.1002/rth2.12080. InPress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 18.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM, TRITON-TIMI 38 Investigators Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 19.Roe MT, Armstrong PW, Fox KAA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297–1309. doi: 10.1056/NEJMoa1205512. [DOI] [PubMed] [Google Scholar]
- 20.Cavallari LH, Lee CR, Beitelshees AL, et al. Multisite Investigation of Outcomes With Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2017 Oct; doi: 10.1016/j.jcin.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982–1988. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 24.Chackalamannil S, Wang Y, Greenlee WJ, Hu Z, Xia Y, Ahn H-S, Boykow G, Hsieh Y, Palamanda J, Agans-Fantuzzi J, Kurowski S, Graziano M, Chintala M. Discovery of a novel, orally active himbacine-based thrombin receptor antagonist (SCH 530348) with potent antiplatelet activity. J Med Chem. 2008;51(11):3061–3064. doi: 10.1021/jm800180e. [DOI] [PubMed] [Google Scholar]
- 25.Tricoci P, Huang Z, Held C, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366(1):20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- 26.Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366(15):1404–1413. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 27.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143(4):241–250. doi: 10.7326/0003-4819-143-4-200508160-00005. [DOI] [PubMed] [Google Scholar]
- 28.Weitz JI, Harenberg J. New developments in anticoagulants: Past, present and future. Thromb Haemost. 2017;117(7):1283–1288. doi: 10.1160/TH16-10-0807. [DOI] [PubMed] [Google Scholar]
- 29.Weitz JI, Jaffer IH, Fredenburgh JC. Recent advances in the treatment of venous thromboembolism in the era of the direct oral anticoagulants. F1000Res. 2017;6:985. doi: 10.12688/f1000research.11174.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365(8):699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 31.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 32.Hanson SR, Griffin JH, Harker LA, Kelly AB, Esmon CT, Gruber A. Antithrombotic effects of thrombin-induced activation of endogenous protein C in primates. J Clin Invest. 1993;92(4):2003–2012. doi: 10.1172/JCI116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esmon CT. Protein C anticoagulant system–anti-inflammatory effects. Semin Immunopathol. 2012;34(1):127–132. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin JH, Zlokovic BV, Mosnier LO. Protein C anticoagulant and cytoprotective pathways. Int J Hematol. 2012;95(4):333–345. doi: 10.1007/s12185-012-1059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017 Aug; doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 36.Connolly SJ, Eikelboom JW, Bosch J, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017 Nov 10; doi: 10.1016/S0140-6736(17)32458-3. [DOI] [PubMed] [Google Scholar]
- 37.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017 Nov 10; doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 38.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101(7):2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 39.Li D, Wang Y, Zhang L, Luo X, Li J, Chen X, Niu H, Wang K, Sun Y, Wang X, Yan Y, Chai W, Gartner TK, Liu J. Roles of purinergic receptor P2Y, G protein-coupled 12 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(8):e81–e89. doi: 10.1161/ATVBAHA.111.239095. [DOI] [PubMed] [Google Scholar]
- 40.Boulaftali Y, Owens AP, Beale A, Piatt R, Casari C, Lee RH, Conley PB, Paul DS, Mackman N, Bergmeier W. CalDAG-GEFI Deficiency Reduces Atherosclerotic Lesion Development in Mice. Arterioscler Thromb Vasc Biol. 2016;36(5):792–799. doi: 10.1161/ATVBAHA.115.306347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loeffen R, Spronk HMH, Cate ten H. The impact of blood coagulability on atherosclerosis and cardiovascular disease. J Thromb Haemost. 2012;10(7):1207–1216. doi: 10.1111/j.1538-7836.2012.04782.x. [DOI] [PubMed] [Google Scholar]
- 42.Bea F, Kreuzer J, Preusch M, Schaab S, Isermann B, Rosenfeld ME, Katus H, Blessing E. Melagatran reduces advanced atherosclerotic lesion size and may promote plaque stability in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26(12):2787–2792. doi: 10.1161/01.ATV.0000246797.05781.ad. [DOI] [PubMed] [Google Scholar]
- 43.Lee I-O, Kratz MT, Schirmer SH, Baumhäkel M, Böhm M. The effects of direct thrombin inhibition with dabigatran on plaque formation and endothelial function in apolipoprotein E-deficient mice. J Pharmacol Exp Ther. 2012;343(2):253–257. doi: 10.1124/jpet.112.194837. [DOI] [PubMed] [Google Scholar]
- 44.Pingel S, Tiyerili V, Mueller J, Werner N, Nickenig G, Mueller C. Thrombin inhibition by dabigatran attenuates atherosclerosis in ApoE deficient mice. Arch Med Sci. 2014;10(1):154–160. doi: 10.5114/aoms.2014.40742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borissoff JI, Otten JJT, Heeneman S, et al. Genetic and pharmacological modifications of thrombin formation in apolipoprotein E-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner. PLoS ONE. 2013;8(2):e55784. doi: 10.1371/journal.pone.0055784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, Yagi S, Yamada H, Soeki T, Wakatsuki T, Shimabukuro M, Sata M. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis. 2015;242(2):639–646. doi: 10.1016/j.atherosclerosis.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Q, Bea F, Preusch M, Wang H, Isermann B, Shahzad K, Katus HA, Blessing E. Evaluation of plaque stability of advanced atherosclerotic lesions in apo E-deficient mice after treatment with the oral factor Xa inhibitor rivaroxaban. Mediators Inflamm. 2011;2011:432080. doi: 10.1155/2011/432080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sparkenbaugh EM, Chantrathammachart P, Mickelson J, van Ryn J, Hebbel RP, Monroe DM, Mackman N, Key NS, Pawlinski R. Differential contribution of FXa and thrombin to vascular inflammation in a mouse model of sickle cell disease. Blood. 2014;123(11):1747–1756. doi: 10.1182/blood-2013-08-523936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuo P, Zuo Z, Zheng Y, Wang X, Zhou Q, Chen L, Ma G. Protease-Activated Receptor-2 Deficiency Attenuates Atherosclerotic Lesion Progression and Instability in Apolipoprotein E-Deficient Mice. Front Pharmacol. 2017;8:647. doi: 10.3389/fphar.2017.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui J, O’Shea KS, Purkayastha A, Saunders TL, Ginsburg D. Fatal haemorrhage and incomplete block to embryogenesis in mice lacking coagulation factor V. Nature. 1996;384(6604):66–68. doi: 10.1038/384066a0. [DOI] [PubMed] [Google Scholar]
- 51.Dewerchin M, Liang Z, Moons L, Carmeliet P, Castellino FJ, Collen D, Rosen ED. Blood coagulation factor X deficiency causes partial embryonic lethality and fatal neonatal bleeding in mice. Thromb Haemost. 2000;83(2):185–190. [PubMed] [Google Scholar]
- 52.Sun WY, Witte DP, Degen JL, Colbert MC, Burkart MC, Holmbäck K, Xiao Q, Bugge TH, Degen SJ. Prothrombin deficiency results in embryonic and neonatal lethality in mice. Proc Natl Acad Sci USA. 1998;95(13):7597–7602. doi: 10.1073/pnas.95.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue J, Wu Q, Westfield LA, Tuley EA, Lu D, Zhang Q, Shim K, Zheng X, Sadler JE. Incomplete embryonic lethality and fatal neonatal hemorrhage caused by prothrombin deficiency in mice. Proceedings of the National Academy of Sciences. 1998;95(13):7603–7607. doi: 10.1073/pnas.95.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI, FXI-ASO TKA Investigators Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232–240. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


