Abstract
Introduction
Low income populations are especially likely to smoke and have difficulty quitting. This study evaluated a monetary incentive intended to increase smoking treatment engagement and abstinence amongst Medicaid recipients who smoke.
Study Design
2-group randomized clinical trial of Incentive (n=948) and Control interventions (n=952) for smoking.
Setting/Participants
Medicaid recipients recruited from primary care patients (n=920) and callers to the Wisconsin Tobacco Quit Line (WTQL; n=980).
Intervention
Participants were offered five quitline cessation calls and were encouraged to obtain cessation medication (covered by Medicaid). Only Incentive condition participants received compensation for taking counseling calls ($30 per call) and for biochemically-verified abstinence at the 6-month visit ($40). All participants received additional payment for completing a baseline assessment and a 6-month smoking test.
Main Outcome Measures
7-day point-prevalence smoking abstinence 6-months post study entry and cost/quit.
Results
Incentive condition participants had significantly higher biochemically determined 7-day point-prevalence smoking abstinence rates 6 months after study induction than did Controls (21.6% vs. 13.8%, respectively: p<.0001). A positive treatment effect of incentives was present across other abstinence indices, but the size of effects and levels of abstinence varied considerably across indices. Incentive condition participants were also significantly more likely than non-incentivized Control participants to accept WTQL treatment calls and their acceptance of calls mediated their attainment of higher abstinence rates at 6-month follow-up. The cost/quit/participant averaged $4,268.26 for the Control participants and $3,601.37 for the Incentive participants.
Conclusions
This study shows that fairly moderate levels of incentive payments for treatment engagement and abstinence (a total possible payment of = $190) increased very low income smokers’ engagement and success in smoking cessation treatment.
Smoking and its resultant harms are becoming increasingly concentrated in smokers who are low-income.1–5 Such smokers are less successful than other smokers in their stop smoking attempts1–4,6,7 and tend not to use evidence based treatment.5,8,9 To date it has been difficult to increase low income smokers’ use of evidence based treatments and their quitting success.5,10–12
A wealth of evidence from laboratory studies shows that incentives for abstinence can decrease addictive drug use.13–18 Also, relatively large incentives for smoking abstinence ($750-800) have approximately tripled cessation rates amongst employee groups;19,20 also see.21–23
However, the effectiveness of large scale incentive programs has not been convincingly demonstrated with low income populations. Hand et al.24 note that incentive interventions used by State Medicaid programs have yielded disappointing results, perhaps due to the population involved, the size of the incentives used, or the fact that incentives were often not delivered in a timely manner.14,24,25
The present research explored the effectiveness of an incentive intervention for State of Wisconsin Medicaid recipients who smoked. In contrast to much prior work,19,20,22,23,26,27 moderate magnitude incentives were used, which should encourage dissemination, and incentives were focused more on treatment engagement than on treatment outcome (abstinence). Treatment engagement is easier to assess than abstinence, and, there is substantial evidence that greater treatment exposure increases smoking cessation success.26,27
The study design compared two groups. Both the Incentive and Control conditions had access to the same Wisconsin Tobacco Quitline (WTQL) smoking treatment program, and both could receive $40 for attending each of two assessment visits (total = $80). The Incentive condition could also receive compensation for taking WTQL calls and for being abstinent at 6-months follow-up (total = $190). We hypothesized that reinforcing treatment engagement for Incentive participants would increase their treatment exposure, which in turn would lead to increased abstinence.26,28,29
Methods
Setting
This research was conducted by the Center for Tobacco Research and Intervention (UW-CTRI) at the University of Wisconsin-Madison School of Medicine and Public Health, in collaboration with the State of Wisconsin Department of Health Services (DHS), and the Wisconsin Tobacco Quitline (WTQL) vendor (Alere Wellbeing, now Optum), based in Seattle, WA.
Study design
All participants were provided access to a standard 5-call WTQL counseling treatment. Participants were randomized by WTQL immediately following screening and administration of a free and informed consent using language provided by the research team, to either an Incentive or Control condition without racial or gender bias. Randomization occurred via computer generated lists (see supplemental information), with order stratified by county and race. The WTQL counseling protocol is for pre-quit and quit day (usually 2 weeks later) calls, and three more calls at 2 week intervals. All participants were incentivized for participating in baseline and 6-month follow-up biochemical assessment visits. Incentive condition participants were additionally incentivized for participating in WTQL calls and for biochemically determined abstinence at the 6-month follow-up visit. Counselors at the WTQL were not blinded; the WTQL staff mentioned the incentive payments to Incentive participants consistent with real world delivery.
Participant recruitment
Recruitment spanned May 2013 – June 2015, occurring via three routes: clinic-based, quitline-based, and community-based referral (See supplemental Figure 1 and Supplementary text). In clinic-based referral, clinic staff performed the following with regard to patients making normal healthcare visits: identified smokers on Medicaid, read a brief script to outline the study, and arranged for cotinine/nicotine testing (done via clinic laboratory). The clinic then faxed relevant information to the WTQL, which performed additional screening, consented cleared persons, gave the baseline survey, and performed randomization. For quitline-based referrals, WTQL staff screened all potentially eligible new callers to the WTQL for their interest in, and eligibility for, participation. Racial and/or gender bias was not part of the selection process. The WTQL then contacted the UW-CTRI research staff to determine if those passing screening were registered Wisconsin Medicaid members, and then sent a letter referring registered members to a nearby testing site, which performed a carbon monoxide (CO) test and transmitted the results to WTQL. WTQL then consented cleared individuals, gave them a baseline survey, randomized them, and began proactive treatment calls. For community-based referrals individuals presented themselves directly to community biochemical the testing sites, which then performed a CO test and confirmed their Medicaid membership. Contact information and CO results were then transmitted to WTQL, which performed further screening, assessment and randomization as appropriate.
Paid media advertisements and outreach to community groups were used to boost recruitment. The study catchment area comprised 16 Wisconsin counties, which each had a research testing site for biochemical verification for participants not in the clinic-based recruitment arm (see Supplemental material for further details).
Screening Assessments
All participants, regardless of referral route, had their Medicaid registration verified via DHS records. WTQL staff asked all participants general screening questions: e.g., age ≥18 years, English or Spanish speaking, resident of a participating county (not required for clinic fax referrals), and willingness to set a quit date in the next 30 days (see Figure 1 and Supplemental Figure 1). Biochemical confirmation of initial smoking status was required: carbon monoxide (for quitline-community based referrals) or cotinine or nicotine tests (for most clinic-based referrals). DHS allowed participating clinics to select the form of the biochemical test used and the cut-score for smoking. Of a total of 64 clinics, all but two used laboratory tests of urine cotinine; the remaining two used NicCheck test strips. The CO cut-score for smoking was CO ≥ 7ppm. Clinics chose different cut-scores for the urine cotinine test; the great majority of clinics chose to define smoking as a value that exceeded either 50 ng/ml, 100 ng/mil, or 200 ng/ml, depending on the clinic. Four clinics used 300 ng/ml as the smoking cut-score. The testing method and cut-score was the same for initial screening and follow-up for all but 16 participants.
Figure 1.
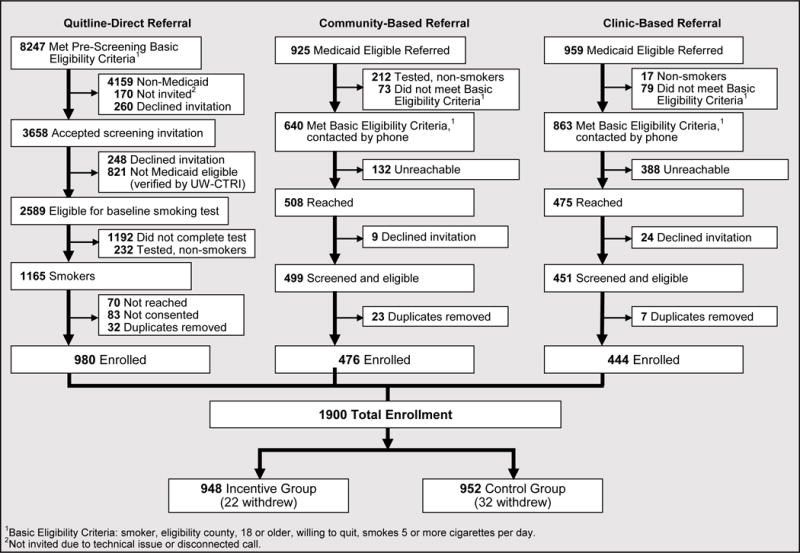
STQ Quitline (QL) CONSORT Diagram
Study treatments
Quitline coaching included a prequit call that typically occurred at study enrollment and 4 additional proactive calls (see Supplemental Figure 2). Participants could also initiate calls to the WTQL for additional assistance. WTQL quit coaches made three attempts (per protocol) on different days to reach a participant for each proactive call, leaving messages at least twice if possible. Those callers not reached on the first two proactive calls were sent a letter urging them to call. Study participants also received a mailed quit guide, access to recorded medication information (via phone), and access to Web Coach®, an online cessation program maintained by the quitline. WTQL quit coaches routinely recommended that participants obtain a prescription for a Medicaid-approved smoking cessation medication from their primary care provider (at minimal or no co-pay).
Incentives
Participants in the Incentive condition could receive a total payment of $270: $30/call for up to five WTQL calls, $40/visit for attending the baseline and 6-month follow-up assessment visit, and $40 for producing biochemical evidence of abstinence at the 6-month follow-up visit. Participants in the control condition could receive a total incentive of $80: $40 each for attendance at the baseline and 6-month follow-up biochemical assessment visits. Compensation was in the form of prepaid Visa gift cards and took 2-4 weeks from the point of contact.
Data Collection and Measures
WTQL staff collected WTQL registration data via a baseline questionnaire, which addressed sociodemographic status, current and past tobacco use, dependence (the Fagerstrom Test of Cigarette Dependence,30,31 pregnancy, nonsmoking tobacco product use, smoking environment, quitting motivation and confidence, chronic disease, past quit attempts and relapses, and basic health information (see The Minimum Data Set for Evaluating Quitlines [NAQC]).32 The WTQL made 5-month reminder calls reminding participants of the 6-month follow-up visit, testing site, and compensation. This call also assessed past 7-day smoking status and the use of any cessation aids. The WTQL also sent all participants a letter conveying information about their 6-month follow-up test.
The 6-month in-person follow up visit was used to collect biological samples for determination of smoking status from all study participants. The results of the CO and the urine cotinine tests were recorded dichotomously by testers as abstinent vs. smoking. No further data (e.g., self-report of smoking status) were collected at this visit because the IRB would have deemed all involved clinic staff to be researchers.
Outcome Measures
The primary outcome was biochemical evidence of smoking status at the 6-month follow-up visit. Secondary outcomes included; treatment engagement (number of proactive treatment calls taken: range = 0-5); use of cessation medications via healthcare system pharmacy records; and self-reported smoking status (via a 6-month follow-up call, separate from the 6-month visit).
Analyses
Treatment groups were compared on demographic and smoking history characteristics via χ2 tests (for categorical variables) and independent-groups t-tests (for continuous variables). Treatment group differences in binary abstinence outcomes were tested via logistic regression models which yielded odds ratios (ORs) and 95% confidence intervals (CIs). Risk differences (RDs; i.e., the difference between the Control and Incentive abstinence rates) and 95% CIs for RDs were calculated using Proc Freq (SAS Institute Inc) via the RISKDIFF option and are reported for abstinence outcomes. Independent-groups t-tests were used to test treatment group differences in treatment engagement (number of proactive calls, minutes of quitline counseling, number of participant-initiated ad hoc calls). For comparisons based on type of referral route, the quitline-based and community-based routes were combined and contrasted with the clinic-based route. Both quitline-direct and community-based referrals originate with quitline contact and analyses revealed that recruits produced by these routes were similar to one another. Mediation analyses were computed via the SAS PROCESS macro.33
The original grant proposal estimated power based on a total sample size of 4000. However, sample size estimates changed due to the pace of recruitment (see Supplementary material) so that the ultimate sample size was N=1900. Recalculation of power based on N=1900 for the predicted effect size (25% vs. 35%) yielded power= .99.
The primary analyses of costs for this project focused on first identifying the costs of project activities that would be required to implement the incentive program on an ongoing basis. Costs of planning the project, grant administration, and research within the project are not included in the analysis. Project costs were allocated to three categories: 1) Service costs, including billed staff time for counseling and testing, as well as all incidentals connected with services; 2) Incentives and distribution costs; and 3) Service-related administrative costs, including promotion/marketing and staff time for administering the intervention. Costs were calculated on a per-participant basis for the 980 participants recruited via the quitline recruitment method. This was done because this method was the one that yielded the greatest number of participants, and also was viewed as most representative of recruitment that would occur in real world implementation. All costs were adjusted to reflect actual expense of the project in the field; no budgeted costs were used. Supplemental Table 3 breaks down the costs for the Incentive and Control Groups and for the 3 types of cost categories listed above.
Results
Demographics and smoking history characteristics
Supplemental Table 1 displays demographic and smoking history characteristics of participants in the two experimental conditions. This table reveals that participants in the two conditions were about 60% female, about 45 years of age, 51% and 41 % Black and White respectively, smoked about 17 cigarettes/day, and about 70% had smoked for 20 years or more. Incentive and Control condition participants differed on two measures: FTCD Item 1 (dichotomized as smoking within 30 minutes of awakening vs. later) and Motivation to Quit Smoking (analyzed as a continuous variable on a 1-10 scale). Participants in the Incentive Condition had lower scores on both measures. Supplemental Table 1 shows the characteristics of those who were recruited via quitline direct/community-based referral vs. clinic-based referral. Compared with those recruited via clinics, quitline direct/community recruits were more likely to be older, nonwhite, less educated, and heavier smokers, and less likely to have tried to quit on their own, or used prescribed cessation medications (p’s <.05).
Participants Recruited into Treatment
Participants (N=1900) were smokers recruited over the course of the study recruitment period (May 2013-May 2015), including 980 (51.6%) recruited via quitline-direct referral, 476 (25%) via community-based referral, and 444 (23%) via clinic-based referral (from 48 clinics). The quitline-direct referrals constituted about 12% of all WTQL callers (most callers were not Medicaid registered). Community-based and clinic-based referral caused 51 & 46%, respectively, of Medicaid registered individuals to enter the study.
Biochemical Evidence of Abstinence at 6-Month Follow-up (Primary Outcome)
Results indicated that the mean and median number of days post-enrollment to the occurrence of the biochemical test were 189 and 180, respectively. About 80% of participants had their test within +/- 40 days of the 6-month mark.
Table 1 depicts the abstinence rates for the two conditions at 6-months post study induction adhering to the intent-to-treat (ITT) principle (N = 1900), where those with missing data were counted as smoking. Biochemically assessed abstinence for the Incentive and Control conditions was 21.6% and 13.8%, respectively (Table 1), Risk Difference (RD)=−7.9, p<.001.
Table 1.
Abstinence and Treatment Engagement Outcomes by Treatment Group
| Primary Abstinence Outcome and Key Treatment Engagement Outcomes | ||||
|---|---|---|---|---|
|
Primary or Key Outcome
|
||||
|
Primary Abstinence Outcome: Post-Enrollment Abstinence at the 6-Month Follow-up Based on Biochemical Testa Intention-to-Treat (ITT) Sample (N=1900) |
Abstinence Rates, N Abstinent/Total (%) |
Abstinence Risk Difference (95% CI), P-Valuec |
Unadjusted Odds Ratio (95% Cl)d |
|
| Control | Incentive | Control vs. Incentive | Control vs. Incentive | |
|
| ||||
| 131/952 (13.76%) |
206/948 (21.62%) |
−7.86 (−11.28 to −4.5) P<.0001 |
0.58 (0.46 to 0.74) |
|
|
| ||||
| Key Treatment Engagement Outcomes: | Mean (SD) | t-test (df) | P-Value | |
|
| ||||
| Control | Incentive | |||
|
|
||||
| Number of Proactive Treatment Calls Taken | 2.9 (1.5) | 3.8 (1.4) | t(1898)= −14.6 | <.0001 |
| Total Number of Minutes of Counseling | 46.1 (26.5) | 65.2 (27.1) | t(1898)= −15.6 | <.0001 |
|
| ||||
| Secondary Abstinence Outcomes | ||||
|
| ||||
|
Secondary Post-Enrollment Abstinence Measure At 6-Months Follow-up |
Abstinence Rates, N Abstinent/Total (%) |
Abstinence Risk Difference (95% CI), P-Valuec |
Unadjusted Odds Ratio (95% Cl)d |
|
| Control | Incentive | Control vs. Incentive | Control vs. Incentive | |
|
| ||||
| Abstinence Based on Biochemical Test, Responder Only Sample (N=1114) |
131/562 (23.31%) |
205/552 (37.14%) |
−13.83 (−19.16 to −8.49) P<.0001 |
0.52 (0.40 to 0.67) |
| Abstinence Based on Biochemical Test, ITT Sample Removing Participants Disenrolled from Medicaid (N=1710) |
127/848 (14.98%) |
196/862 (22.74%) |
−7.76 (−11.45 to −4.07) P<.0001 |
0.60 (0.47 to 0.77) |
| Abstinence Based on Biochemical Test, ITT Sample Participants Tested with Carbon Monoxide Breath Test (N=1458) |
118/736 (16.03%) |
173/722 (23.96%) |
−7.93 (−12.02 to −3.84) P=.0002 |
0.61 (0.47 to 0.79) |
| Abstinence Based on Biochemical Test, ITT Sample Participants Tested with Urine Cotinine Test (N=384) |
10/188 (5.32% |
26/170 13.27% |
−7.95 (−13.68 to −2.22) P=.0076 |
0.37 (0.17 to 0.78) |
| Abstinence Based on Biochemical Test, ITT Sample Participants from the Quitline-Community-Based Referral (N=1456 |
119/732 (16.26%) |
174/724 (24.03%) |
−7.78 (−11.9 to −3.67) P=.0002 |
0.61 (0.47 to 0.80) |
| Abstinence Based on Biochemical Test, ITT Sample Participants from the Clinic-Based Referral (N=444) |
12/220 (5.45%) |
31/224 (13.84%) |
−8.38 (−13.81 to −2.96) P=.0029 |
0.36 (0.18 to 0.72) |
| Abstinence Based on Self-Report,b ITT Sample (N=1900) |
98/952 (10.29%) |
136/948 (14.35%) |
−4.05 (−7.00 to −1.10) P=.0072 |
0.69 (0.52 to 0.90) |
| Abstinence Based on Self-Report, Responder Only Sample (N=862) |
98/411 (23.84%) |
136/451 (30.16%) |
−6.31 (−12.22 to −0.40) P=.0374 |
0.73 (0.54 to 0.98) |
| Abstinence Based on Combined Biochemical Test and Self-Report, ITT Sample (N=1900) |
39/952 (4.10%) |
72/948 (7.59%) |
−3.50 (−5.60 to −1.39) P=.0012 |
0.52 (0.35 to 0.78) |
| Abstinence Based on Combined Biochemical Test and Self-Report, Responder Only Sample (N=651) |
39/326 (11.96%) |
72/325 (22.15%) |
−10.19 (−15.92 to −4.46) P=.0005 |
0.48 (0.31 to 0.73) |
Biochemical Test of Abstinence at the 6-month follow-up visit based on breath carbon monoxide test (n=1458; 77%), urine cotinine testing (n=384; 20%), or urine test strip (n=58; 3%).
Abstinence self-report was assessed during the 6-month follow-up call.
Pairwise comparisons of Abstinence Risk Differences were tested via Proc Freq (SAS Institute) by specifying the RISKDIFF option which provides standard Wald asymptotic confidence limits for the risks.
Unadjusted odds ratios based on logistic regression analysis.
Note: Table 1 provides absolute and relative effect sizes. Absolute effect sizes are presented as group-specific abstinence rates along with odds ratios (and 95% confidence intervals) as the effect size for the group comparison. Relative effect sizes are presented as abstinence Risk Differences with 95% confidence intervals
Treatment Engagement
Quitline calls. Table 2 shows the number of participants in the two experimental conditions taking 0-5 proactive quitline calls. While 46% of Incentive participants took 5 proactive calls, only about 21% of Control participants did so. The association between the number of calls taken and the two experimental conditions was statistically significant, (χ2 = 196.1, p<.001: Table 2), with Incentive participants taking a mean of 3.8 (SD=1.4) proactive calls and Control participants a mean of 2.9 (SD=1.5; t(1898)= −14.6, p<.001). The mean number of minutes of counseling received across such calls was about 65.2 minutes (SD=27.1) for Incentive participants and about 46.1 minutes (SD=26.5; t(1898)= −15.6, p<.001) for Control participants. 503 of the 1900 participants initiated calls to the quitline with the mean number of calls differing significantly: Incentive condition = 0.5 calls (SD=1.2) and Control condition = 0.3 (SD=0.8); t(1898)= −4.45, p<.001. (NOTE: No significant harms were reported by any participant in this counseling-only study.)
Table 2.
Quitline Call Acceptance and Medication Pick-up Rates for Participants in the Control and Incentive Conditions
| Treatment Group
|
|||
|---|---|---|---|
| Control (N=952) |
Incentive (N=948) |
||
|
| |||
| N (%) | N (%) | ||
| Proactive Quitline Calls Completed: | Zero Calls | 6 (0.6%) | 3 (0.3%) |
| 1 Call | 245 (25.7%) | 76 (8.0%) | |
| 2 Calls | 179 (18.8%) | 113 (11.9%) | |
| 3 Calls | 154 (16.2%) | 122 (12.9%) | |
| 4 Calls | 165 (17.3%) | 199 (21.0%) | |
| 5 Calls | 203 (21.3%) | 435 (45.9%) | |
| (χ2= 196.1, p<.0001) | |||
|
| |||
| Medication Pick-up: | No medications picked up | 497 (52.2%) | 430 (45.4%) |
| 1+ Nicotine Replacement Medications | 255 (26.8%) | 283 (29.9%) | |
| Varenicline Only | 78 (8.2%) | 83 (8.8%) | |
| Bupropion Only | 38 (4.0%) | 59 (6.2%) | |
| Multiple Medications | 84 (8.8%) | 93 (9.8%) | |
| (χ2= 11.5, p=.022) | |||
Medication use
Healthcare system pharmacy records revealed that 55% and 48% of the Incentive and Control condition participants, respectively, received some form of cessation medication. Table 2 displays the number and percentages of participants in the two experimental conditions who used the different forms of medication. The distribution across these categories differed significantly between experimental conditions (χ2 =11.5, p=.022). Bupropion data may reflect some use for depression.
Secondary Biochemical Abstinence Outcomes
A variety of sensitivity analyses were conducted to ascertain the robustness of the obtained findings (see Table 1 and the supplemental material). Such sensitivity analyses addressed outcomes in: just those actually tested at the 6-month mark, those for whom different forms of biochemical assessment were used, and those recruited via different routes (clinic-based vs. quitline-community based). Significant condition effects were obtained in all comparisons (p’s<.01 Table 1).
Self-Reported Smoking Abstinence
Table 1 also displays abstinence rates based on self-report in the phone follow-up time point that occurred closest to the 6-month mark. The mean and median number of days post study induction until the relevant follow-up call were 160 and 152, respectively, and 87.6% of participants had their call within +/- 40 days of the 6-month mark. Table 1 shows that the 6-month self-report abstinence rates for the Incentive and Control conditions for the ITT sample (N=1900) were 14.4% and 10.3% respectively, RD=−4.1, p=.01. Relatively few participants (n=651) supplied both self-report and biochemical evidence of abstinence at 6-months follow-up. Data from these participants showed discordance between the biochemical and self-report measures in 26.2% of the sample (see Supplemental Table 2). However, when biochemically confirmed self-report of 7-day point prevalence abstinence was used as the outcome, significant condition effects were present. In the ITT (N=1900) analysis, the abstinence rates for the Incentive and Control conditions were 7.6% and 4.1%, respectively, RD=−3.5, p=.0012.
The Mediation of 6-Month Abstinence by Treatment Engagement
Mediation analyses used biochemically determined abstinence at 6-months (ITT sample; N=1900) as the outcome and number of proactive quitline calls as the mediator. Analyses focused on whether the increase in calls taken by Incentive versus Control participants could account statistically for the former group’s higher biochemically determined abstinence rate (21.6 vs. 13.8%, respectively). A simple logistic regression (non-mediational) model testing only the relation between treatment condition and the 6-month outcome revealed a significant effect of treatment condition, c = −0.55, p < .0001 (see Figure 2). When number of calls was entered in the full mediational model, the path from treatment condition to number of proactive calls (a’) was significant (a’ = 0.96, p < .0001), as was the path from the number of proactive calls to 6-month abstinence (b’= .40, p < .0001). However, the direct path from treatment condition to outcome (c’) was no longer significant in the full model (c’ = 0.21, p = .10). The indirect, mediated effect of number of calls (the product of paths a’ and b’) was significant (a’b’ = 0.35, p < .0001).
Figure 2.
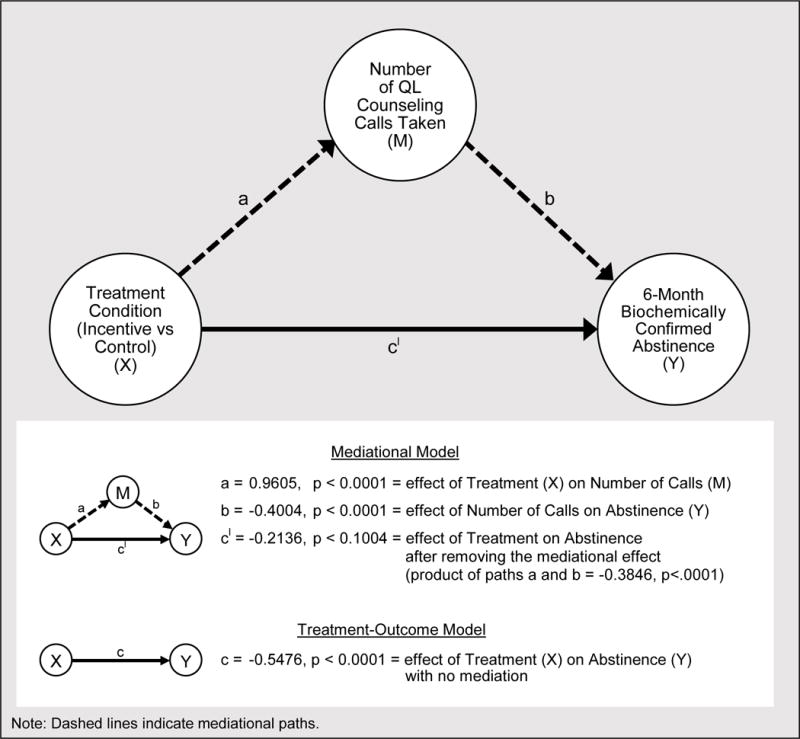
Mediation Model of Incentive Effects on Abstinence by Quitline Call Acceptance
Cost-Effectiveness
When estimated amongst those participants recruited via the quitline recruitment method, the cost of implementing the program with the full set of incentives in this protocol was $920.43 for the Incentive condition, versus $744.97 for the Control condition. The cost/quit/participant for the two different study groups is depicted in Supplemental Table 3. The analysis of cost/quit/group found that Control Group participants had an average cost per quit of $4,268.26 and Incentive Group participants averaged $3,601.37 per quit (see Supplemental Table 3). Thus, the clinical impact of the enhanced incentives used in the Incentive condition outweighed its higher cost; providing such incentives yielded a $667 lower cost/quit.
DISCUSSION
Participants receiving financial incentives for treatment engagement and abstinence were significantly more likely than non-incentivized participants to accept quitline treatment calls and be abstinent from smoking 6 months after study induction. The clinical impact of the Incentive intervention outweighed its greater costs; costs/quit were about $670 lower in the Incentive intervention than in the Control condition, which received access to the same cessation treatment but without enhanced incentives. Moreover, a mediation analysis showed that the effect of the Incentive intervention on abstinence could be accounted for statistically by its effects on the number of quitline calls taken.
Low income individuals are notable for their high prevalence of smoking and smoking related disease,34–38 their infrequent use of evidence based smoking treatment, and their low rate of smoking cessation success.5,8,9 The present research suggests that financial incentives for engaging in quitline smoking cessation counseling increases low income smokers’ use of evidence based treatment and their quitting success.
This research used several methods for participant recruitment: i.e., recruitment from quitline callers, the community, and primary care. Quitline recruitment was the most feasible and productive recruitment route; clinic recruitment entailed considerable staff training and incentives to clinics. One obstacle to translating the quitline recruitment method is the need for community based testing sites to obtain biochemical determination of smoking status.
The treatment engagement data showed that the Incentive condition produced significantly higher rates of treatment engagement than did the Control condition: e.g., in the number of proactive calls taken and receipt of cessation medication. Moreover, the mediation analysis showed that the effect of incentives on quitline calls taken, accounts statistically for the greater abstinence rates of Incentive participants.
Variability in methods and data creates challenges to drawing inferences from this real-world study. For instance, participating clinics chose their own methods and cut-scores for biochemical evaluation of smoking abstinence and there was a time gap between the self-report of abstinence and biochemical ascertainment. Also, the rate of completion of follow-up phone calls was modest, leading to considerable missing data (participants were incentivized to make biochemical follow-up assessment visits not to take follow-up calls). These factors might have affected the results by increasing error.
Despite these limitations, significant treatment effects were consistently found amongst participants differing in recruitment route, type of biochemical test, and self-reported vs. biochemical determination of abstinence. The results did reveal considerable variability in abstinence rates and effect sizes across the various analyses, though (see Table 1 and supplemental materials). Of those in the incentive condition who tested as abstinent via a biochemical test, almost half (37/76) reported during their 6-month follow-up phone call that they had smoked (Supplemental Table 2). This suggests either that the biochemical test was insensitive, or that the smoker’s status had changed between the call and the biochemical test (the call was generally first). The latter suggests that the abstinence detected at the 6-month biochemical assessment visit was often short-lived.
Another limitation of this research is that Control participants did not receive the same amount of total payment as Incentive participants (an alternative design might have given Controls greater noncontingent payments so that their total payment matched that of the Incentive participants). Therefore, some of the treatment effect may have been due to the amount of payment per se.
Another limitation of this research is that because engagement and abstinence incentives were bundled, we do not know how either would have worked by itself. For instance, dropping the modest abstinence incentive might have improved estimated cost-effectiveness. Or, if the same total amount of incentive had been used to incentivize abstinence per se, even higher abstinence rates might have been obtained. On the other hand, this might have produced a greater demand effect to become temporarily abstinent for the follow-up test(s). This concern could be addressed by checking abstinence repeatedly via biochemical testing, but this can be more challenging and expensive than documenting treatment receipt. Last, these results show that incentivizing treatment engagement appeared to boost the effects of quitline smoking counseling; it is unknown whether incentivizing treatment engagement would work with other type of treatment or with other behavioral problems.
This research joins a growing list of studies suggesting that incentives can exert beneficial effects on health related behaviors and outcomes (e.g.,15,20,39). It may be the first study to demonstrate that moderate levels of incentive payment (treatment incentives maximally totaling $190) increased low income smokers’ engagement and success in smoking cessation treatment. Thus, the methods used in this research appear to have successfully addressed important obstacles to the effective large scale application of incentive programs (see 40) including needs to: (1) increase program awareness among targeted participants, (2) identify an effective, but scalable, incentive magnitude, (3) clearly communicate the contingencies for incentive receipt, and (4) engage relevant recruitment and delivery systems (e.g., the quitline).
Supplementary Material
Acknowledgments
People who have made substantive contributions to the study: David L. Fraser, MS, Michael C Fiore, MD, MPH, MBA, Kate Kobinsky, MPH, Robert Adsit, MEd, Stevens S. Smith, PhD, Mimi L. Johnson, and Timothy B. Baker, PhD
Disclaimers. The manuscripts is solely the responsibility of the authors and does not necessarily represent the views of the United States Department of Health and Human Services (HHS) or any of its agencies nor have the results been reviewed and verified by any HHS evaluation contract.
Sources of support in the form of grants, equipment, or drugs, and describe the role of the study sponsor(s), if any, in study design. IRB numbers should be included here, when applicable.
- Disclosure of which tasks each author completed.
-
○Mr. Fraser oversaw all aspects of the study design and implementation as well as the production of this manuscript
-
○Dr. Fiore is the Director of the Center for Tobacco Research and Intervention. He supervised those involved in the study and was sdirectly involved in authoring the final paper. He is the corresponding author.
-
○Ms. Kobinsky was the lead researcher, overseeing study data collection and operational issues including the contract with the WTQL and management of the IRB submissions and was involved in writing the study design portion of the manuscript
-
○Mr. Adsit managed the contract with the State of Wisconsin and oversaw the research staff who performed data collection and biochemical testing. He reviewed the paper for accuracy in those areas.
-
○Dr. Smith was the lead data analyst and oversaw all aspects of data cleaning as well as performed all of the final analyses.
-
○Ms. Johnson was the lead contract representative from the State of Wisconsin and was involved in all aspects of project design, including reviewing that aspect of this paper.
-
○Dr. Baker was PI of the study and has played a major role in reviewing study design, recruitment and outcome data in order to produce this manuscript. He is the senior author.
-
○
Clinical trial registration number: www.clinicaltrials.gov NCT02713594
The contents of this article have not been previously presented elsewhere
Declaration of Interest: This research was supported by Funding Opportunity Number 1B1CMS330876 from the Centers for Medicare & Medicaid Services. The manuscripts is solely the responsibility of the authors and does not necessarily represent the views of the United States Department of Health and Human Services (HHS) or any of its agencies nor have the results been reviewed and verified by any HHS evaluation contract. No financial disclosures were reported by the authors of this paper.
IRB and Clinical trial information: The study was approved by the UW-Health Sciences Institutional Review Board as Exempt Research for Public Benefit on 2/26/2013. The trial was previously registered in clinical trials.gov in May 2012 as NCT01569477. The exempt research study required re-registration at www.clinicaltrials.gov as NCT02713594 on 2/16/2016 (see supplemental material for detailed timeline of study IRB/registry history)
Footnotes
This study is registered at www.clinicaltrials.gov: NCT02713594
Disclosure of financial conflicts of interest: No financial disclosures were reported by the authors of this paper.
References
- 1.Hiscock R, Judge K, Bauld L. Social inequalities in quitting smoking: what factors mediate the relationship between socioeconomic position and smoking cessation? J Public Health (Oxf) 2011;33(1):39–47. doi: 10.1093/pubmed/fdq09. [DOI] [PubMed] [Google Scholar]
- 2.Kotz D, West R. Explaining the social gradient in smoking cessation: it’s not in the trying, but in the succeeding. Tob Control. 2009;18(1):43–46. doi: 10.1136/tc.2008.025981. [DOI] [PubMed] [Google Scholar]
- 3.Levy DT, Romano E, Mumford E. The relationship of smoking cessation to sociodemographic characteristics, smoking intensity, and tobacco control policies. Nicotine Tob Res. 2005;7(3):387–396. doi: 10.1080/14622200500125443. [DOI] [PubMed] [Google Scholar]
- 4.Neumann T, Rasmussen M, Ghith N, Heitmann BL, Tonnesen H. The Gold Standard Programme: smoking cessation interventions for disadvantaged smokers are effective in a real-life setting. Tob Control. 2013;22(6):e9. doi: 10.1136/tobaccocontrol-2011-050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock BC, Papandonatos GD, de Dios MA, et al. Tobacco cessation among low-income smokers: motivational enhancement and nicotine patch treatment. Nicotine Tob Res. 2014;16(4):413–422. doi: 10.1093/ntr/ntt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid JL, Hammond D, Boudreau C, Fong GT, Siahpush M, Collaboration ITC Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: findings from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2010;12(Suppl):S20–33. doi: 10.1093/ntr/ntq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caleyachetty A, Lewis S, McNeill A, Leonardi-Bee J. Struggling to make ends meet: exploring pathways to understand why smokers in financial difficulties are less likely to quit successfully. Eur J Public Health. 2012;22(Suppl 1):41–48. doi: 10.1093/eurpub/ckr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings KM, Hyland A. Impact of nicotine replacement therapy on smoking behavior. Annu Rev Public Health. 2005;26:583–599. doi: 10.1146/annurev.publhealth.26.021304.144501. [DOI] [PubMed] [Google Scholar]
- 9.Murphy JM, Mahoney MC, Hyland AJ, Higbee C, Cummings KM. Disparity in the use of smoking cessation pharmacotherapy among Medicaid and general population smokers. J Public Health Manag Pract. 2005;11(4):341–345. doi: 10.1097/00124784-200507000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Okuyemi KS, James AS, Mayo MS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav. 2007;34(1):43–54. doi: 10.1177/1090198106288046. [DOI] [PubMed] [Google Scholar]
- 11.Okuyemi KS, Goldade K, Whembolua GL, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction. 2013;108(6):1136–1144. doi: 10.1111/add.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant J, Bonevski B, Paul C, McElduff P, Attia J. A systematic review and meta-analysis of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups. Addiction. 2011;106(9):1568–1585. doi: 10.1111/j.1360-0443.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 13.Higgins ST, Washio Y, Heil SH, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55(Suppl):S33–40. doi: 10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 15.Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Prev Med. 2012;55(Suppl):S24–32. doi: 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigmon SC, Lamb RJ, Dallery J. Tobacco. In: Higgins ST, Silverman K, Heil SH, editors. Contingency management in substance abuse treatment. New York: The Guilford Press; 2008. pp. 99–119. [Google Scholar]
- 17.Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend. 2000;58(1-2):103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 18.Roll JM. Assessing the feasibility of using contingency management to modify cigarette smoking by adolescents. J Appl Behav Anal. 2005;38(4):463–467. doi: 10.1901/jaba.2005.114-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halpern SD, French B, Small DS, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372(22):2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 21.Kaper J, Wagena EJ, Willemsen MC, van Schayck CP. Reimbursement for smoking cessation treatment may double the abstinence rate: results of a randomized trial. Addiction. 2005;100(7):1012–1020. doi: 10.1111/j.1360-0443.2005.01097.x. [DOI] [PubMed] [Google Scholar]
- 22.Tappin D, Bauld L, Purves D, et al. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ. 2015;350:h134. doi: 10.1136/bmj.h134. [DOI] [PubMed] [Google Scholar]
- 23.Volpp KG, Gurmankin Levy A, Asch DA, et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev. 2006;15(1):12–18. doi: 10.1158/1055-9965.EPI-05-0314. [DOI] [PubMed] [Google Scholar]
- 24.Hand DJ, Heil SH, Sigmon SC, Higgins ST. Improving medicaid health incentives programs: lessons from substance abuse treatment research. Prev Med. 2014;63:87–89. doi: 10.1016/j.ypmed.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendzor DE, Businelle MS, Poonawalla IB, et al. Financial incentives for abstinence among socioeconomically disadvantaged individuals in smoking cessation treatment. Am J Public Health. 2015;105(6):1198–1205. doi: 10.2105/AJPH.2014.302102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. 2008 http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. Accessed 6 July 2016.
- 27.Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2012;12:CD009670. doi: 10.1002/14651858.CD009670.pub2. [DOI] [PubMed] [Google Scholar]
- 28.McAfee TA. Quitlines: a tool for research and dissemination of evidence-based cessation practices. Am J Prev Med. 2007;33(6 Suppl):S357–367. doi: 10.1016/j.amepre.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347(14):1087–1093. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 30.Fagerstrom KO. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 31.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 32.North American Quitline Consortium (NAQC) The minimal data set for evaluating quitlines. Phoenix, AZ: NAQC; 2012. [Google Scholar]
- 33.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: The Guilford Press; 2013. [Google Scholar]
- 34.Centers for Disease Control, National Center for Health Statistics, National Health Interview Survey. Prevalence of current cigarette smoking among adults aged 18 and over: United States 1997-March 2014. 2015 Early release data. http://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201409_08.pdf.
- 35.Baggett TP, Rigotti NA. Cigarette smoking and advice to quit in a national sample of homeless adults. Am J Prev Med. 2010;39(2):164–172. doi: 10.1016/j.amepre.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Baggett TP, Tobey ML, Rigotti NA. Tobacco use among homeless people–addressing the neglected addiction. N Engl J Med. 2013;369(3):201–204. doi: 10.1056/NEJMp1301935. [DOI] [PubMed] [Google Scholar]
- 37.Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, Lopez AD. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet. 2006;368(9533):367–370. doi: 10.1016/S0140-6736(06)68975-7. [DOI] [PubMed] [Google Scholar]
- 38.Giesinger I, Goldblatt P, Howden-Chapman P, Marmot M, Kuh D, Brunner E. Association of socioeconomic position with smoking and mortality: the contribution of early life circumstances in the 1946 birth cohort. J Epidemiol Community Health. 2014;68(3):275–279. doi: 10.1136/jech-2013-203159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins ST, Silverman K, Heil SH, editors. Contingency management in the treatment of substance use disorders: a science-based treatment innovation. New York, NY: Guilford Press; 2008. [Google Scholar]
- 40.Blumenthal KJ, Saulsgiver KA, Norton L, et al. Medicaid incentive programs to encourage healthy behavior show mixed results to date and should be studied and improved. Health Aff (Millwood) 2013;32(3):497–507. doi: 10.1377/hlthaff.2012.0431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


