Abstract
Purpose of review
Tissue injury stimulates an inflammatory response that is mediated in part by alarmins. Alarmins are a group of endogenous molecules that trigger inflammation in response to damage. This class of molecules is becoming increasingly recognized for their ability to influence wound healing. This article will provide an overview of alarmins and outline the latest findings on these mediators in cutaneous wound healing.
Recent findings
In addition to stimulating inflammatory cells, recent evidence suggests that alarmins can act on other cells in the skin to affect wound closure and the extent of scar tissue production. This review will focus on HMGB-1 and IL-33, two alarmins that have received recent attention in the wound healing field.
Summary
Because a properly regulated inflammatory response is critical for optimal healing, further research must be done to fully understand the role of alarmins in the wound repair process.
Keywords: HMGB-1, Inflammation, IL-33, Repair, Skin, Wound
Introduction
The repair of cutaneous wounds requires a complex set of events that include inflammation, proliferation, and scar formation. Despite being induced immediately after injury and contributing to the earliest stages of healing, the inflammatory response can also help regulate later phases of the repair process [1]. A carefully balanced inflammatory response is critical for proper healing – too little inflammation can increase the risk of wound infection which can impair healing, while too much inflammation can delay healing and result in excessive scarring.
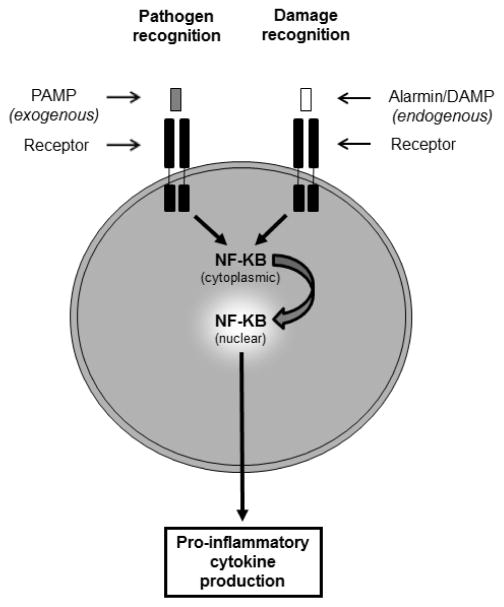
Many different signals can induce inflammation in a wound, and inflammatory cells are capable of recognizing both exogenous and endogenous danger signals [2, 3] (Figure 1). One of the primary functions of inflammation is to prevent infection, and inflammatory cells are equipped with multiple pattern recognition receptors that recognize potential pathogens. These receptors, which include toll-like receptors (TLRs), bind to pathogen-associated molecular patterns (PAMPs) associated with microbes. This binding triggers signaling events that activate antimicrobial defense systems and stimulate pro-inflammatory cytokine production by the inflammatory cells, usually through activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Inflammation can also be induced by tissue injury in even in the absence of microbes through recognition of alarmins (also known as damage-associated molecular patterns or DAMPs). Alarmins are endogenous molecules released by damaged host cells [2, 3]. Many alarmins are proteins that are located in the nucleus or another intracellular compartment where they are not normally exposed to inflammatory cells. However, when cell damage occurs these molecules are released into the extracellular space where they are recognized by receptors on the surface of inflammatory cells (and other cell types harboring the receptor). In addition to being released by damaged cells, alarmins can also be secreted by activated immune cells, enhancing inflammation [2]. Many different molecules have been identified as alarmins, such as HMGB-1 (high-mobility group box-1), S100 proteins, heat shock proteins, uric acid, adenosine triphosphate (ATP), and members of the interleukin-1 (IL-1) family, such as IL-1α and IL-33 [2–4]. Two alarmins that have gained recent attention for their involvement in wound healing are HMGB-1 and IL-33. In the sections below, publications examining these two alarmins in wound healing will be discussed.
Figure 1. Basic recognition of PAMPs and alarmins.
The presence of microbes is recognized by the binding of exogenous PAMPs to pattern recognition receptors on inflammatory cells (left). In the setting of tissue injury, endogenous alarmins/DAMPs released by damaged cells are recognized by pattern recognition receptors or other specific receptors (right). Typically, binding of PAMPs or alarmins to their respective receptors will induce signaling pathways that result in translocation of the transcription factor NF-κB from the cytoplasm to the nucleus. Activation of NF-κB leads to the transcription and secretion of pro-inflammatory cytokines.
High mobility group box-1 (HMGB-1)
HMGB-1 is a non-histone DNA binding protein that facilitates interactions between transcription factors and chromatin [2, 3]. HMGB-1 normally resides in the nucleus, but in damaged cells it moves to the cytoplasm and is released into the extracellular space. Outside of the cell, HMGB-1 stimulates inflammation by binding to toll-like receptors (TLR2 and TLR4) and receptor of advanced glycation end products (RAGE) on the surface of inflammatory cells [3]. Stimulation of both receptor signaling pathways by HMGB-1 causes activation of NF-κB and the production of pro-inflammatory cytokines.
Many different cell types contribute to the repair process, including keratinocytes in the epidermis as well as fibroblasts and endothelial cells in the dermis. There is evidence that HMGB-1 affects all three of these cell types. HMGB-1 was shown to promote viability, proliferation and migration in HaCaT keratinocytes via RAGE signaling [5]. Similarly, Straino et al showed that HMGB-1 acts as a chemoattractant for primary human keratinocytes [6]. Studies have also shown that estrogen, which is known to accelerate wound closure [7–9], increases HMGB-1 in keratinocytes and that HMGB-1 mediates estrogen-induced keratinocyte migration [10]. HMGB-1 has also been shown to act as a chemoattractant in primary human fibroblasts [6]. Subsequent studies using 3T3 fibroblasts showed that HMGB-1 promotes fibroblast proliferation and migration and that RAGE-mediated mitogen-activated protein kinase (MAPK) activation is required for this activity [11]. RAGE activation by HMGB-1 also controls endothelial cell behavior, causing an increase in endothelial cell proliferation, chemotaxis and vascular sprouting in vitro [12], suggesting a role for HMGB-1 in neovascularization.
In addition to studies on the in vitro effects of HMGB-1 on cell types relevant to wound healing, the in vivo functions of HMGB-1 during wound healing have been assessed in multiple types of experimental wounds, including burn injuries, diabetic wounds, fetal wounds and subcutaneous sponge implants. In burn wound models, HMGB-1 has been shown to be a useful marker for necrosis [13–16]. The pattern of HMGB-1 staining is altered after burn injury, with strong nuclear staining in normal cells changing to cytoplasmic staining in necrotic cells. HMGB-1 staining used in conjunction with active caspase-3 staining can be used to distinguish between necrotic cells (cytoplasmic HMGB-1 staining) and apoptotic cells (active caspase-3 staining) in burn wounds [14]. Nuclear HMGB-1 levels decrease with burn contact time [15], indicating a correlation between nuclear HMGB-1 localization and the severity of the burn injury. In addition, HMGB-1 staining may be useful for visualizing burn progression, as the initially healthy area of tissue surrounding the burn eventually becomes non-viable due to expansion of the necrotic zone [14, 16]. A recent study has also suggested a link between HMGB-1 and cholinergic activation, as alterations in cholinergic mediators are associated with the induction of HMGB-1 after burn injury [17].
HMGB-1 also plays a role in diabetic wounds. Straino and colleagues performed a detailed study of HMGB-1 in normal and diabetic wound healing [6]. HMGB-1 was present in the nucleus of epidermal keratinocytes in normal skin but accumulated in the cytoplasm in wounded skin, which is suggestive of extracellular release. Diabetic human and mouse skin, which are characterized by delayed healing, showed reduced HMGB-1 levels supporting a beneficial role for HMGB-1 in wound repair. Topical treatment of diabetic wounds with HMGB-1 accelerated wound closure and increased vessel density and granulation tissue deposition. Interestingly, HMGB-1 treatment did not augment would closure rates in non-diabetic animals, but inhibition of HMGB-1 function with a truncated form of the protein (Box A) impaired wound healing in normal mice [6]. The results suggest that adequate levels of HMGB-1 are present under non-diabetic conditions, but that healing is impaired when HMGB-1 function is inhibited or when levels are reduced as in the case of diabetic wounds.
Several other studies have suggested a link between diabetes and HMGB-1 function. HMGB-1 has been shown to be a substrate for cleavage by dipeptidyl peptidase-4 (DPP-4), a protease that is associated with diabetes [18]. The cleaved form of HMGB-1 was found at high levels in the serum of diabetic patients. This could affect wound closure, as the cleaved form of HMGB-1 has reduced capacity to stimulate endothelial cells and induce angiogenesis [18] and deficiencies in neovascularization are believed to contribute to the delayed healing observed in diabetic wounds [19]. Furthermore, studies have shown that treatment with a DPP-4 inhibitor increases the migration of NTCC2544 human keratinocytes by increasing HMGB-1 levels [20]. Treatment with DPP-4 inhibitors restores serum levels of full-length HMGB-1 [18], which could partially explain the benefit of DPP-4 inhibitors in diabetic wounds that has been reported in the literature [21, 22]. Taken together, these studies show that sufficient levels of functional full-length HMGB-1 is important for efficient wound repair.
HMGB-1 function has also been examined in fetal wounds. Fetal skin displays different healing properties depending on the developmental age of the skin [23, 24]. At early stages of development, mammalian skin heals without significant inflammation and undergoes regenerative healing which leads to a scarless healing outcome. As the skin becomes more developed, fetal wounds begin to heal with a strong inflammatory response and the formation of a well-defined scar. HMGB-1 was examined in a fetal wound healing model in an attempt to understand its role in inflammation and scar formation [25]. Developmental differences in HMGB-1 expression were observed in epidermal keratinocytes, with an increase in nuclear HMGB-1 staining in the keratinocytes of more developed skin. Spatial and temporal HMGB-1 staining patterns also differed in scarless and scar-forming wounds. Late-gestation fetal wounds, which heal with inflammation and subsequent scarring, displayed a stronger reduction of nuclear HMGB-1 staining and enhanced cytoplasmic staining in keratinocytes, implying more extensive extracellular release of HMGB-1. In addition, the staining patterns suggested that a larger number of keratinocytes were releasing HMGB-1 at the wound edge and that HMGB-1 was released for longer periods of time in scar-forming fetal wounds. The fibrogenic potential of HMGB-1 was also analyzed in fetal wounds. When HMGB-1 was injected into early-gestation wounds that normally heal scarlessly, the wounds healed with a prominent collagenous scar. In addition, wounds that received HMGB-1 had an increase in macrophages, more fibroblasts, and an elevated blood vessel density [25]. The results suggested that HMGB-1 promotes inflammation, collagen production, and scar formation in fetal wounds. In contrast to the results observed in fetal wounds, another study suggested that HMGB-1 may inhibit collagen production. In this experiment ethyl pyruvate, which inhibits the release of HMGB-1, reduced wound breaking strength in incisional wounds and increased collagen content in subcutaneously implanted polyvinyl alcohol sponges [26]. However, it is possible that off-target effects were contributing to the reduction in collagen in the sponges, as ethyl pyruvate has other activities and is not a specific inhibitor of HMGB-1. The authors also found that HMGB-1 treatment of cultured fibroblasts caused a reduction in collagen synthesis [26]. The effective concentration of HMGB-1 used in this study (1000 ng/ml) was quite high compared to what has been used previously in fibroblast studies [6, 11], so it is possible that the effects of HMGB-1 on fibroblast function vary depending on the concentration used. More studies will have to be done to clarify the effects of HMGB-1 on collagen production under different conditions and in different models.
Overall, studies suggest a role for HMGB-1 in promoting inflammation, angiogenesis, and wound healing in vivo. The effects of HMGB-1 on collagen synthesis appear to be model- and concentration-dependent, as HMGB-1 promotes granulation tissue deposition in diabetic wounds and scar formation in fetal wounds, but appears to reduce collagen production in cultured fibroblasts (at least with high concentrations).
Interleukin-33 (IL-33)
IL-33 is a relatively newly described member of the IL-1 cytokine family that has been described as a dual function protein [27]. In the nucleus, IL-33 can bind to chromatin and act as a transcriptional repressor [28]. One reported property of full-length nuclear IL-33 that could impact inflammation and wound healing is the ability to bind NF-κB and reduce its transcriptional activity, which effectively suppresses pro-inflammatory cytokine production [27]. Importantly, IL-33 has also been shown to function as an alarmin, acting as a pro-inflammatory cytokine when released extracellularly. In this role, IL-33 stimulates inflammation by binding to a heterodimeric receptor complex on the surface of inflammatory cells [29]. Suppression of tumorigenicity 2 (ST2) is the ligand binding component, which interacts with the co-receptor IL-1 receptor accessory protein (IL-1RAcP) required for signaling [30]. Dimerization of ST2 and IL-1RAcP triggers a signaling cascade which leads to the activation of NF-κB and the MAPK pathway [31], thereby stimulating inflammation. IL-33 is highly expressed in epithelial barrier tissues, including the skin [32, 33]. Multiple recent studies have now shown that IL-33 regulates the activity of several cell types involved in cutaneous wound healing, and a functional role for IL-33 in wound healing has been demonstrated using several different genetically modified mouse strains in various wound healing models.
One of the first hints that IL-33 may play a role in wound healing came from studies on endothelial cells. In quiescent cutaneous blood vessels, endothelial cells showed strong nuclear expression of IL-33 [34], an observation that has also been reported by other groups [32]. IL-33 staining was lost in endothelial cells after cutaneous injury [34]. In in vitro studies, downregulation of IL-33 occurred during endothelial cell migration and after stimulation with tumor necrosis factor-α or vascular endothelial growth factor [34]. This suggests that nuclear IL-33 may maintain blood vessel quiescence under normal circumstances and that loss of nuclear IL-33 may help facilitate angiogenesis in healing wounds.
In addition to functioning in the cutaneous vasculature, several studies have shown a role for IL-33 in regulating keratinocyte function and wound closure rates. IL-33 expression levels are upregulated in the skin after injury [35, 36], particularly in keratinocytes [37]. This increase in IL-33 seems to be functionally significant, as multiple in vivo approaches have shown that IL-33 is beneficial for healing. Studies by Yin et al showed that intraperitoneal administration of IL-33 increased wound closure and also resulted in an increase in the mRNA expression of the extracellular matrix proteins fibronectin and type III collagen [35]. IL-33 treatment also increased the number of alternatively activated macrophages (or M2 macrophages) in the wounds [35], which are believed to have pro-healing properties [38, 39].
Studies have also shown that wound healing is delayed in mice lacking either IL-33 or its receptor, ST2. In ST2 knockout mice, no changes were observed in macroscopic wound closure, but reduced reepithelialization was seen microscopically in wounds from ST2 knockout mice 5 days after injury [40]. Reduced CD-31 staining and an increase in mature versus immature collagen was observed with picrosirius red staining, suggesting that IL-33 signaling may affect angiogenesis and extracellular matrix composition in the dermis. Changes in inflammation were also observed in ST2 knockout mice, with an increase in the proportion of pro-inflammatory macrophages despite no change in overall macrophage numbers, as well as an increase in neutrophils compared to wild-type mice [40]. Rak et al examined wound healing in IL-33 knockout mice using a splinted wound model [36]. They were specifically interested in examining the regulation of group 2 innate lymphoid cell (ILC2) responses by IL-33 during wound healing. They found that elevated IL-33 expression levels after wounding correlate with an increase in the presence of ILC2 cells in the wound. In IL-33 knockout mice, wound closure and reepithelialization were delayed, which was likely due in part to reduced ILC2 numbers. In addition, intraperitoneal injection of IL-33 increased the number of IL-13+ ILC2 cells and accelerated wound closure [36]. Therefore, the ability of IL-33 to promote repair seems to be based in part on its ability to recruit ILC2 cells to the wound.
While the studies described above all seem to support a role for extracellular IL-33 in wound healing, a recent study by Oshio et al suggested that nuclear IL-33 in keratinocytes plays a role in wound healing [37]. In these studies, wound healing was examined in two different strains of mice. Transgenic mice expressing the soluble form of the IL-33 receptor, sST2, which normally acts to neutralize extracellular IL-33 activity, showed comparable wound healing rates to wild-type mice. This would seem to indicate that the extracellular alarmin function of IL-33 does not play an important role in wound healing. However, IL-33 knockout mice, which are deficient in both nuclear and extracellular IL-33, displayed a delay in wound closure along with a prolonged neutrophil presence in the wounds. Keratinocytes from IL-33 knockout mice also showed delayed closure in an in vitro scratch assay. Interestingly, IL-33 expression was prominent in keratinocytes at the wound margins in acute human wounds but not in chronic wounds [37], which supports the idea that sufficient IL-33 levels are needed for rapid wound healing.
The role of IL-33 in wound infection has also been examined. Yin and colleagues [41] showed that IL-33 is increased in wounds challenged with Staphylococcus aureus (S. aureus), a common wound pathogen. Intraperitoneal injection of IL-33 inhibited S. aureus colonization and accelerated repair. IL-33 treatment also resulted in increased neutrophil numbers, which was likely due to enhanced expression of CXCR2 [41], a chemokine receptor involved in neutrophil trafficking. Additional studies by Li et al in a skin infection model showed that IL-33 increases the antimicrobial activity of macrophages against S. aureus by elevating inducible nitric oxide synthase expression and nitric oxide production [42]. It is possible that the ability of IL-33 to stimulate antimicrobial responses in macrophages is also contributing to the results described by Yin et al in infected wounds where IL-33 reduced bacterial colonization and accelerated healing.
Taken together, the studies described above provide strong support that IL-33 is involved in the regulation of cutaneous wound healing. However, more studies will have to be done to dissect the contributions of nuclear and extracellular IL-33 in wound repair and to define the role of IL-33 in other aspects of the wound healing process.
Conclusions
Inflammation is an important event during the wound repair process that is regulated in part by alarmins. These molecules alert the body to the injury and trigger an inflammatory response in an attempt to aid in the repair process. Recent studies support a role for alarmins, including HMGB-1 (Table 1) and IL-33 (Table 2), in cutaneous wound healing. Published results demonstrating the importance of alarmins in wound healing suggest that this group of molecules may have the potential to become useful therapeutically. Elevating HMGB-1 levels in wounds, particularly diabetic wounds, may help accelerate wound closure [6]. On the other hand, if cosmesis is a priority, neutralizing HMGB-1 activity may be advantageous [25]. With regard to IL-33, supplementing wounds with this molecule may be beneficial by boosting the immune response and in turn enhancing the antimicrobial response [41] and accelerating wound closure [36]. Overall, the current state of research in this area suggests that further studies are needed to fully understand the role of HMGB-1 and IL-33 as well as other alarmins in all phases of the repair process and to determine whether manipulating alarmin levels could be beneficial for clinical wound management.
Table 1.
Summary of documented HMGB-1 functions in cutaneous wound healing.
| Target cell/tissue | Stimulated activity | Reference(s) |
|---|---|---|
|
| ||
| Keratinocytes/epithelium | Proliferation/Migration (in vitro) | 5–6, 10 |
| Wound closure (in vivo) | 6 | |
|
| ||
| Endothelial cells/vasculature | Proliferation/Migration (in vitro) | 12 |
| Vascular sprouting (in vitro) | 12 | |
| Vascular density (in vivo) | 6, 25 | |
|
| ||
| Fibroblasts | Proliferation/Migration (in vitro) | 6, 11 |
| Granulation tissue (in vivo) | 6 | |
| Fibroblast density (in vivo) | 25 | |
| Scar formation (in vivo) | 25 | |
|
| ||
| Immune cells | Macrophage density (in vivo) | 25 |
Table 2.
Summary of documented IL-33 functions in cutaneous wound healing.
| Target cell/tissue | Stimulated activity | Reference(s) |
|---|---|---|
|
| ||
| Keratinocytes/epithelium | Scratch closure (in vitro) | 37 |
| Wound closure (in vivo) | 35–37, 40 | |
|
| ||
| Endothelial cells/vasculature | Migration (in vitro) | 34 |
| Vascular density (in vivo) | 40 | |
|
| ||
| Fibroblasts | ECM deposition (in vivo) | 35, 40 |
|
| ||
| Immune cells | Neutrophil number (in vivo) | 41 |
| M2 macrophage number (in vivo) | 35 | |
| ILC2 number (in vivo) | 36 | |
References
Recently published papers of particular interest have been highlighted as:
• Of importance
- 1.Wilgus TA. Immune cells in the healing skin wound: influential players at each stage of repair. Pharmacol Res. 2008;58(2):112–6. doi: 10.1016/j.phrs.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 3.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14(7–8):476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Millar NL, O’Donnell C, McInnes IB, Brint E. Wounds that heal and wounds that don’t - The role of the IL-33/ST2 pathway in tissue repair and tumorigenesis. Semin Cell Dev Biol. 2017;61:41–50. doi: 10.1016/j.semcdb.2016.08.007. This is an informative review article on IL-33 signaling and the role of this pathway in the repair of multiple tissue types. [DOI] [PubMed] [Google Scholar]
- 5.Ranzato E, Patrone M, Pedrazzi M, Burlando B. HMGb1 promotes scratch wound closure of HaCaT keratinocytes via ERK1/2 activation. Mol Cell Biochem. 2009;332(1–2):199–205. doi: 10.1007/s11010-009-0192-4. [DOI] [PubMed] [Google Scholar]
- 6.Straino S, Di Carlo A, Mangoni A, De Mori R, Guerra L, Maurelli R, et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol. 2008;128(6):1545–53. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3(11):1209–15. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 8.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155(4):1137–46. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111(9):1309–18. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin JU, Noh JY, Lee JH, Lee WJ, Yoo JS, Kim JY, et al. In vivo relative quantitative proteomics reveals HMGB1 as a downstream mediator of oestrogen-stimulated keratinocyte migration. Exp Dermatol. 2015;24(6):478–80. doi: 10.1111/exd.12713. [DOI] [PubMed] [Google Scholar]
- 11.Ranzato E, Patrone M, Pedrazzi M, Burlando B. Hmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys. 2010;57(1):9–17. doi: 10.1007/s12013-010-9077-0. [DOI] [PubMed] [Google Scholar]
- 12.Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176(1):12–5. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Hirth DA, Singer AJ, Clark RA, McClain SA. Histopathologic staining of low temperature cutaneous burns: comparing biomarkers of epithelial and vascular injury reveals utility of HMGB1 and hematoxylin phloxine saffron. Wound Repair Regen. 2012;20(6):918–27. doi: 10.1111/j.1524-475X.2012.00847.x. [DOI] [PubMed] [Google Scholar]
- 14.Lanier ST, McClain SA, Lin F, Singer AJ, Clark RA. Spatiotemporal progression of cell death in the zone of ischemia surrounding burns. Wound Repair Regen. 2011;19(5):622–32. doi: 10.1111/j.1524-475X.2011.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNamara AR, Zamba KD, Sokolich JC, Jaskille AD, Light TD, Griffin MA, et al. Apoptosis is differentially regulated by burn severity and dermal location. J Surg Res. 2010;162(2):258–63. doi: 10.1016/j.jss.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Singer AJ, McClain SA, Taira BR, Guerriero JL, Zong W. Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Acad Emerg Med. 2008;15(6):549–54. doi: 10.1111/j.1553-2712.2008.00115.x. [DOI] [PubMed] [Google Scholar]
- 17.Holmes CJ, Plichta JK, Gamelli RL, Radek KA. Burn Injury Alters Epidermal Cholinergic Mediators and Increases HMGB1 and Caspase 3 in Autologous Donor Skin and Burn Margin. Shock. 2017;47(2):175–83. doi: 10.1097/SHK.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchetti C, Di Carlo A, Facchiano F, Senatore C, De Cristofaro R, Luzi A, et al. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia. 2012;55(1):236–44. doi: 10.1007/s00125-011-2213-6. [DOI] [PubMed] [Google Scholar]
- 19.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 20.Sinagra T, Merlo S, Spampinato SF, Pasquale RD, Sortino MA. High mobility group box 1 contributes to wound healing induced by inhibition of dipeptidylpeptidase 4 in cultured keratinocytes. Front Pharmacol. 2015;6:126. doi: 10.3389/fphar.2015.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marfella R, Sasso FC, Rizzo MR, Paolisso P, Barbieri M, Padovano V, et al. Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp Diabetes Res. 2012;2012:892706. doi: 10.1155/2012/892706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schurmann C, Linke A, Engelmann-Pilger K, Steinmetz C, Mark M, Pfeilschifter J, et al. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J Pharmacol Exp Ther. 2012;342(1):71–80. doi: 10.1124/jpet.111.191098. [DOI] [PubMed] [Google Scholar]
- 23.Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy Wound Manage. 2007;53(6):16–31. [PubMed] [Google Scholar]
- 24.Walmsley GG, Maan ZN, Wong VW, Duscher D, Hu MS, Zielins ER, et al. Scarless wound healing: chasing the holy grail. Plast Reconstr Surg. 2015;135(3):907–17. doi: 10.1097/PRS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 25.Dardenne AD, Wulff BC, Wilgus TA. The alarmin HMGB-1 influences healing outcomes in fetal skin wounds. Wound Repair Regen. 2013;21(2):282–91. doi: 10.1111/wrr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, O’Hearn S, Kavalukas SL, Barbul A. Role of High Mobility Group Box 1 (HMGB1) in Wound Healing. J Surg Res. 2011 doi: 10.1016/j.jss.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 27.Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, et al. The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription. J Immunol. 2011;187(4):1609–16. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- 28.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104(1):282–7. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179(4):2551–5. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 30.Palmer G, Lipsky BP, Smithgall MD, Meininger D, Siu S, Talabot-Ayer D, et al. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine. 2008;42(3):358–64. doi: 10.1016/j.cyto.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7(10):827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3(10):e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488–95. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 34.Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173(4):1229–42. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin H, Li X, Hu S, Liu T, Yuan B, Gu H, et al. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol Immunol. 2013;56(4):347–53. doi: 10.1016/j.molimm.2013.05.225. [DOI] [PubMed] [Google Scholar]
- 36.Rak GD, Osborne LC, Siracusa MC, Kim BS, Wang K, Bayat A, et al. IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J Invest Dermatol. 2016;136(2):487–96. doi: 10.1038/JID.2015.406. This paper demonstrates an important role for IL-33 in the recruitment of ILC2 cells during skin repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Oshio T, Komine M, Tsuda H, Tominaga SI, Saito H, Nakae S, et al. Nuclear expression of IL-33 in epidermal keratinocytes promotes wound healing in mice. J Dermatol Sci. 2017;85(2):106–14. doi: 10.1016/j.jdermsci.2016.10.008. This study suggests opposing nuclear and extracellular activities of IL-33 in wound healing. [DOI] [PubMed] [Google Scholar]
- 38.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5(3):e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579–87. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JS, Seppanen E, Patel J, Rodero MP, Khosrotehrani K. ST2 receptor invalidation maintains wound inflammation, delays healing and increases fibrosis. Exp Dermatol. 2016;25(1):71–4. doi: 10.1111/exd.12833. [DOI] [PubMed] [Google Scholar]
- 41.Yin H, Li X, Hu S, Liu T, Yuan B, Ni Q, et al. IL-33 promotes Staphylococcus aureus-infected wound healing in mice. Int Immunopharmacol. 2013;17(2):432–8. doi: 10.1016/j.intimp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Li H, Jiang Z, Zhang T, Wang Y, Li Z, et al. Interleukin-33 increases antibacterial defense by activation of inducible nitric oxide synthase in skin. PLoS Pathog. 2014;10(2):e1003918. doi: 10.1371/journal.ppat.1003918. [DOI] [PMC free article] [PubMed] [Google Scholar]