Abstract
Objective
Identify factors that may predispose to excess gestational weight gain (GWG).
Methods
Seventy-two healthy women with obesity (30 Class I, 24 Class II, 18 Class III) expecting a singleton pregnancy were studied at 13-16 weeks gestation. Energy expenditure (EE) was measured during sleep (SleepEE, average EE from 0200-0500h) in a whole-room calorimeter, and total daily EE (TDEE) over seven days using doubly-labeled water. Glucose, insulin, thyroid hormones and catecholamines were measured.
Results
Body composition explained 70% variability in SleepEE, and SleepEE accounted for 67-73% of TDEE. While there was no evidence of consistent low metabolism, there was considerable variability. Low SleepEE was associated with insulin resistance and low T3 concentrations (both, p=0.01). Physical activity level was 1.47±0.02. For women with SleepEE within 100kcal/d of their predicted EE, TDEE was significantly less than the estimate (2530±91 vs. 2939kcal/d, p<0.001) provided from the most recent gestational energy requirement model.
Conclusions
Pregnant women with obesity are inactive, possibly predisposing them to excess GWG. Current energy requirement models overestimate activity and may promote excess GWG in women with obesity. Further, we speculate that the observed large inter-individual variability in basal metabolism may be important to consider when assessing the risk for excess GWG.
Keywords: Pregnancy, Obesity, Energy expenditure, Doubly labeled water, Physical activity
Introduction
According to the 2011-2014 NHANES, 34.4% of reproductive aged women (20-39 years) are obese, which translates to approximately 1.4 million births every year from women with obesity in the United States (1). Alarmingly, the prevalence of obesity in women entering pregnancy is further increasing. Obese pregnancies are likely to be complicated by excess gestational weight gain (GWG, >60% prevalence) (2) and have increased incidence of gestational diabetes, hypertensive disorders, nonelective cesarean section, and macrosomic infants (3).
Lifestyle modification interventions are now specifically targeting pregnant women with obesity with the goal to tilt energy balance towards better control of weight (and fat) gain and improvement of maternal and infant outcomes. The efficacy of randomized controlled trials designed at reducing GWG is however inconsistent with modest mean effect sizes for weight gain attenuation, reduction of pregnancy complications, and detrimental infant health outcomes including growth restriction, catch-up growth and childhood adiposity (4, 5). So far, the focus of such interventions has been based mostly on modification of diet and/or physical activity similarly to what has been done for obesity treatment in non-pregnant adults (6, 7, 8).
Understanding energy balance in early pregnancy may better guide the design of interventions which are appropriately tailored to pregnant women with obesity. The aim of this study was to combine stable isotopes and indirect calorimetry to characterize the components and determinants of energy expenditure and to assess energy requirements in early pregnancy in women with obesity. Measuring and understanding the components of energy expenditure in early pregnancy could lead to more effective lifestyle interventions to attenuate GWG and thus improve maternal and infant outcomes.
Methods
Study design
This analysis is part of a larger prospective observational study to assess the determinants of GWG in pregnant women with obesity (clinicaltrials.gov: NCT01954342). Energy metabolism including energy expenditure in sedentary and free-living conditions, substrate oxidation, physical activity and endocrine mediators of energy metabolism were measured between 13 and 16 weeks of gestation in 72 pregnant women at Pennington Biomedical Research Center (Baton Rouge, LA, USA). The study was approved by the Pennington Biomedical Research Center Institutional Review Board, and all participants provided written informed consent prior to participation.
Participants and Recruitment: Seventy-two women aged 18 to 40 years, with obesity (BMI ≥30 kg/m2) at screening (<15 weeks of gestation), and a confirmed singleton, viable pregnancy were enrolled. Women were excluded for recent history of smoking, alcohol or drug use, pre-existing hypertension (i.e. systolic blood pressure>160mmHg and diastolic blood pressure>110mmHg), diabetes (HbA1c≥6.5%), HIV or AIDS, severe anemia (hemoglobin<8g/dL and/or hematocrit<24%), contraindications to MRI (implanted metal objects, claustrophobia), prior or planned (within 1 year of expected delivery) bariatric surgery, psychological or eating disorders. Furthermore, women using contraindicated medications or supplements that influence energy intake or expenditure, planned to move out of the study area within the next 2 years or planned to be out of the study area for more than 4 weeks in the next 12 months, planned termination, or were unwilling to avoid pregnancy for 12 months following delivery were excluded. Study participants were recruited from January 2015 to January 2017 through community and social media advertisements and referrals by local obstetricians as previously described (9).
Anthropometrics and Body Composition
At the screening visit (11 weeks ± 3 days), body height and weight were measured in a clinic gown to assess eligibility and classify obesity as class I (30≤BMI<35), class II (35≤BMI<40) or class III (BMI≥40). At the study visit (14.7±0.1 weeks), body weight was measured in the morning following an overnight fast with participants wearing a gown (gown weight was subtracted), and body composition was measured by air displacement plethysmography using BodPod® (COSMED USA, Inc., Concord, CA, USA) with women wearing provided spandex clothing. Thoracic gas volume was estimated by the software and corrected for a pregnancy-related decline of 100mL (10). Body fat percentage was calculated per Siri (11), in which density of fat mass (FM) was 0.9kg/L (12) and density of fat-free mass (FFM) was calculated based on gestational age using an exponential regression (13).
Total Daily Energy Expenditure
Total daily energy expenditure (TDEE) was measured using doubly labeled water over seven days (14). Briefly, participants provided two urine samples before being dosed (1.25g of 10% enriched H218O and 0.10g of 99.9% enriched 2H2O per kg) and subsequent urine samples at 4.5h, 12h and day 6 and 7 after dosing. 18O and 2H abundance was measured by isotope ratio mass spectrometry (15). CO2 production rate (rCO2) was calculated using the equation (A5) of Schoeller (14). TBW was calculated as the average of TBW-estimates by the dilution spaces of 18O and 2H (NO and ND) using the 0-intercepts; NO/1.007 and ND/(1.007*(ND/NO)), in which ND/NO is the dilution space ratio, calculated as the average of the group mean (1.0315±0.0023) and each individual value (if ND/NO≥1, or if ND/NO≤1.07; n=1 with ND/NO≤1) as previously described (16, 17). TDEE was calculated by multiplying rCO2 by the energy equivalent of CO2 for a respiratory quotient of 0.866 (18), reflecting the mean 12h-RQ of the cohort in pregnancy as measured in the metabolic chamber and a diet that provides approximately 50%, 30%, and 20% of energy from carbohydrate, fat, and protein, respectively. TDEE was not available for two participants due to missed urine collections or suspected misreporting of urine collection times.
Energy Metabolism by Metabolic Chamber
Sleeping and resting energy expenditure (SleepEE and REE, respectively) were measured during an overnight stay in a metabolic chamber (19). Participants entered the chamber at 1830 after refraining from exercise, caffeine and alcohol for the previous 36 hours. At 1900, a standard dinner was served providing 30% of the estimated daily energy requirements (20) as 30% fat, 55% carbohydrate, and 15% protein. Lights were turned off between 2230 and 0600 the next morning. Beginning at approximately 0615, after emptying of the bladder, REE was measured with the participant awake and lying supine on the bed for 30 minutes before exiting the chamber at 0700. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured continuously (For O2: Siemens Oxymat 6e, software v4.8.3, Bartlesville, OK, US; for CO2: ABB Uras AO2020 26, software v3.4.0, Houston, TX). Energy expenditure (EE) was calculated using the Weir-equation adjusted for urinary nitrogen excretion rate which was measured during the chamber stay and extrapolated to 24 hours (21). The respiratory quotient (RQ) was calculated as VCO2/VO2. Infrared sensors detected activity in the chambers (% of minutes during which activity was detected). SleepEE is the mean EE between 0200 and 0500 (minutes when activity was <1%), extrapolated to 24 hours. REE is the mean of the last 20 min of the REE measurement, extrapolated to 24 hours. Metabolic flexibility, or the increase in postprandial RQ over fasting RQ, was calculated as the difference of the 4h post-dinner RQ minus sleeping RQ. SleepEE and REE were not available for 2 and 3 participants, respectively, due to technical failure of the instrumentation.
Prediction of Individual Variability of Energy Expenditure
Linear regression was used to develop prediction equations for energy expenditure during sleep (SleepEE) and free-living conditions over seven days (TDEE), using fat-free mass and fat mass as independent variables. Age was not a significant predictor of EE and was therefore not included in the models. If EE is proportional to the metabolic mass, the EE predicted from the regression equation will be equal to the EE measured. The difference between the measured and predicted EE (called Residual EE) allows categorization of participants into three groups; those with an EE (kcal/d) that is significantly higher than the regression line which reflects high metabolism, those with an EE that is on the regression line which reflects average metabolism, or those with an EE (kcal/d) that is significantly lower than the regression line which reflects low metabolism. For the present analysis, we used a previously published threshold of ±100kcal/d (22), which was a more conservative estimate as compared to using tertiles (23).
Physical activity
Physical activity was estimated from TDEE and REE using three different calculations. First, activity-related EE (AREE) was calculated in absolute terms as the remaining EE from 0.9TDEE-REE, which assumes diet-induced thermogenesis as 0.1*TDEE. Second, physical activity level (PAL) was calculated as PAL=TDEE/REE (24). Third, because of the inherent problem of using ratios when the two variables have an intercept not equal to zero, we expressed physical activity as residual AREE. Residual AREE is calculated as measured TDEE minus TDEE predicted using regression with TDEE as dependent and SleepEE as independent variable (25). This value is positive for subjects with higher physical activity than average and is negative for subjects with lower physical activity than average independent of metabolic body size. Because residual AREE is adjusted for metabolic body size (SleepEE), this value is directly proportional to the amount of physical activity. In addition, physical activity was assessed over 24 hours including the chamber stay using a SenseWear Armband™ accelerometer (SWA, Model MF-SW, BodyMedia Inc. USA).
Clinical Chemistry
Urinary nitrogen, creatinine, norepinephrine and epinephrine were measured by ELISA in an overnight pooled urine sample collected during the chamber stay (Bio Rad Microplate reader, DLD Diagnostika, Hamburg, Germany). A fasting blood sample was collected after exiting the chamber for measurement of insulin, triiodothyronine, thyroxine, thyroid stimulating hormone (Immulite 2000, Siemens, Broussard, LA) and glucose (DXC600, Beckman Coulter Inc., Brea, CA).
Fetus
Medical record data pertaining to the pregnancy was obtained before study enrollment and at the end of pregnancy to confirm gestational age and fetal sex. Fetal weight in early pregnancy was estimated using 2D ultrasound assessments of head circumference, biparietal diameter, abdominal circumference, femoral length (26) obtained by the same sonographer.
Statistics
All analyses were carried out using SAS/STAT software, Version 9.4 of the SAS System for Windows© (SAS Institute Inc. Cary, NC, USA). All tests were evaluated using significance level α=0.05. To test for differences between obesity classes, linear models were produced to obtain estimates used in f-tests and two-sample t-tests for continuous variables and Chi-Square-tests were performed for categorical variables. Post-hoc testing was used for pair-wise comparisons. Data is expressed as means±SEM derived from the linear models.
Results
Participant Characteristics
Enrolled women (n=72, Table 1) were 27±0.6 years old and 53% had at least one previous live birth. By design, the women all were classified as obese, but were healthy as confirmed by normal glucose toositively correlated with weilerance and blood pressure. First trimester body weight measured at approximately 11 weeks, 3 days classified 42% of participants with class I obesity (30/72), 33% with class II (24/72) and 25% with class III (18/72). Across obesity classes, participants were similar with respect to age, race, fasting glucose, parity and infant sex. As expected, FM was proportional to obesity (p<.001) and FFM was higher with class III compared to class II and class I, but not different between class I and class II. Diastolic BP and insulin were the only metabolic parameters that were significantly different between obesity classes.
Table 1. Demographics, body composition and metabolic characteristics of healthy women with obesity in early pregnancy.
| Variable | Overall N=72 |
Class I N=30 |
Class II N=24 |
Class III N=18 |
P For Obesity Class |
|---|---|---|---|---|---|
| GA at Visit, week/day | 14 4/7 ± 1/7 | 14 4/7 ± 1/7 | 14 5/7 ± 1/7 | 14 4/7 ± 1/7 | 0.88 |
| GWG, 1st Trimester, g/wk | 118 ± 21 | 125 ± 34 | 99 ± 36 | 129 ± 43 | 0.83 |
| Age, y | 27.7 ± 0.6 | 27.3 ± 1.0 | 27.0 ± 0.9 | 29.4 ± 1.2 | 0.24 |
| Race (W/B/O), N | 32, 34, 6 | 16, 13, 1 | 11, 11, 2 | 5, 10, 3 | 0.35 |
| BMI, kg/m2 | 37.1 ± 0.7 | 32.3 ± 0.3 | 37.0 ± 0.3 | 45.2 ± 1.1 | |
| FFM, kg | 54.2 ± 1.0 | 50.4 ± 1.1 | 54.0 ± 1.5 | 60.7 ± 2.1 | <0.001 |
| FM, kg | 45.9 ± 1.5 | 36.8 ± 1.0 | 44.8 ± 1.1 | 62.7 ± 2.9 | <0.001 |
| FM, % | 45.4 ± 0.6 | 42.2 ± 0.7 | 45.4 ± 0.8 | 50.6 ± 1.2 | <0.001 |
| HbA1c, % | 5.4 ± 0.04 | 5.4 ± 0.1 | 5.3 ± 0.1 | 5.6 ± 0.1 | 0.12 |
| Glucose, mg/dL | 89 ± 1 | 88 ± 1 | 87 ± 2 | 92 ± 2 | 0.09 |
| Insulin, IU/mL | 15.6 ± 1.0 | 12.3 ± 1.0 | 13.8 ± 1.6 | 23.6 ± 1.8 | <0.001 |
| Systolic BP, mmHg | 108 ± 1 | 106 ± 2 | 107 ± 2 | 112 ± 2 | 0.07 |
| Diastolic BP, mmHg | 67 ± 1 | 65 ± 1 | 66 ± 1 | 71 ± 2 | 0.01 |
| Parity (0, 1, 2+), N | 34, 22, 16 | 12, 8, 10 | 14, 8, 2 | 8, 6, 4 | 0.29 |
| Infant Sex (M/F), N | 33, 36 | 15, 14 | 11, 12 | 7, 10 | 0.79 |
GA, gestational age; GWG, gestational weight gain; W/B/O, white, black or African American, other; BMI, body mass index; FFM, fat-free mass; FM, fat mass; BP, blood pressure; M, male; F, female.
Data is expressed as means ± SEM. Differences in participant characteristics were tested with linear models.
Total Daily Energy Expenditure
In early pregnancy, TDEE was 2639±46kcal/d (Table 2, Figure 1A). TDEE positively correlated with weight (r=0.46, p <0.001), FFM (r=0.57, p <0.001, Figure 1A) and FM (r=0.32, p<0.01). The prediction equation for relating TDEE to FFM and FM, and to weight were:
Table 2. Energy Expenditure in Obese Pregnancy.
| Variable | Overall N=72 |
Class I N=30 |
Class II N=24 |
Class III N=18 |
P For Obesity Class |
|---|---|---|---|---|---|
| TDEE, kcal/d | 2639 ± 46 | 2540 ± 57 | 2623 ± 86 | 2847 ± 99 | 0.03 |
| SleepEE, kcal/d | 1768 ± 33 | 1621 ± 29 | 1724 ± 47 | 2053 ± 62 | <0.001 |
| REE, kcal/d | 1825 ± 34 | 1686 ± 39 | 1775 ± 50 | 2102 ± 58 | <0.001 |
| AREE, kcal/d | 569 ± 30 | 596 ± 47 | 585 ± 56 | 499 ± 50 | 0.43 |
| PAL | 1.47 ± 0.02 | 1.51 ± 0.04 | 1.48 ± 0.04 | 1.38 ± 0.02 | 0.04 |
Energy expenditure was measured between 13-16 weeks of gestation. Abbreviations: TDEE, total daily EE; EE, energy expenditure; SleepEE, sleeping EE; REE, resting EE; PAL, physical activity level; AREE, activity-related EE. Data is expressed as means ± SEM. Differences in energy by obesity class were tested with linear models.
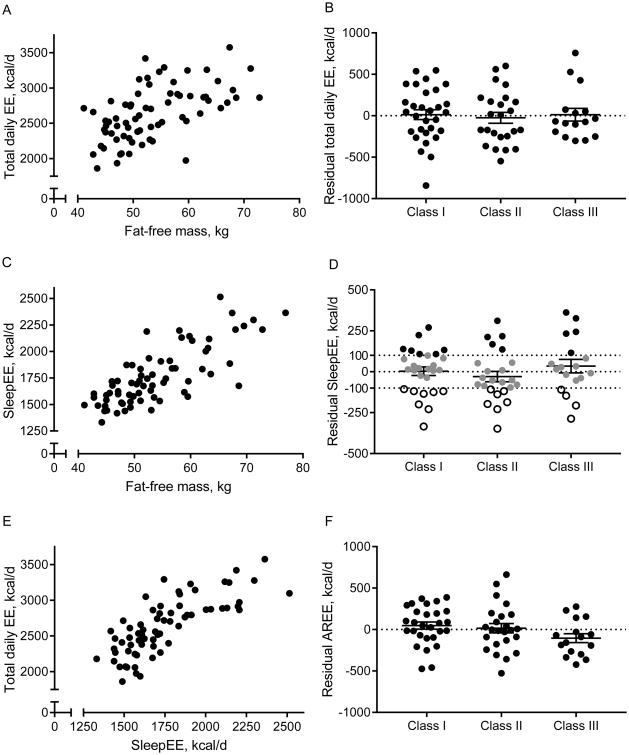
Figure 1.
Correlations between fat-free mass, sleeping and total daily energy expenditure (A, C, E) and residuals for total daily energy expenditure (TDEE, B), sleeping EE (SleepEE, D), and activity-related EE (AREE, F), by obesity class. Each data point represents one participant. For residual SleepEE, -100 kcal/d and 100 kcal/d are used to identify low and high metabolism.
TDEEpredicted[kcal/d]=1081+[26.5*FFM, kg]+[3.1*FM, kg], R2=0.33, p<0.001;
TDEEpredicted[kcal/d]=1507+[11.5*weight, kg], R2=0.21, p<0.001.
Sedentary Energy Expenditures (SleepEE and REE)
In early pregnancy, SleepEE was 1768±33kcal/d, with a high degree of variability among pregnant women (n=70, Figure 1C). As expected, SleepEE in early pregnancy correlated with weight (r=0.79, p<0.001), FFM (r=0.77, p<0.001, Figure 1C) and FM (r=0.68, p<0.01).
The prediction equation for relating SleepEE to FFM and FM and to weight were:
SleepEEpredicted[kcal/d]=388+[19.0*FFM, kg]+[7.6*FM, kg], R2=0.69, p<0.001;
SleepEEpredicted[kcal/d]=588+[11.8*weight, kg], R2=0.62, p<0.001. Findings were similar for REE (N=69; Table 2). The prediction equation for relating REE to FFM and FM, and to weight were:
REEpredicted[kcal/d]=368+[20.7*FFM, kg]+[7.2*FM, kg], R2=0.68, p<0.001;
REEpredicted[kcal/d]=604+[12.1*weight, kg], R2=0.61, p<0.001.
Physical Activity
The energy expended during activity (AREE) was not proportional to body mass and not different between obesity classes (Table 2). According to the physical activity guidelines (27), 88% (59/67) of the participants were sedentary (PAL <1.7), 12% (8/67) were moderately active (1.7≤PAL<2.0) and no participant was highly active (PAL≥2.0). The average PAL was 1.47±0.02 and PAL was lowest in women with obesity class III as compared to women in class I and II (p=0.01 and p=0.06, respectively, Table 2). Physical activity expressed as residual AREE (TDEE adjusted for SleepEE, TDEE[kcal/d]=618+[1.154*SleepEE, kcal/d], R2=0.59, p<0.001) was not statistically different between obesity classes (p=0.14, Figure 1E-F). Reduced physical activity with higher obesity class was confirmed by the accelerometer and the time spent in various intensities of physical activity. Women with obesity class III spent significantly less time in moderate or vigorous activities than women with obesity class I and II (I: 243±62 min/week, II: 102±15, and III: 86±1, p=0.02), and consequently average metabolic equivalents (METS) per day was also significantly lower (p<0.001).
Components of Energy Expenditure
The primary constituent of TDEE in early pregnancy was SleepEE accounting for 67±1%. Estimating diet-induced thermogenesis as 10% of TDEE for all subjects, 3.0±0.4% of TDEE was attributed to the energy cost of arousal (N.S. between obesity classes). The remaining component of TDEE was AREE (22±1%) which is the energy cost of structured exercise and activities of daily living or non-exercise activity thermogenesis.
Endocrine Mediators of Energy Expenditure
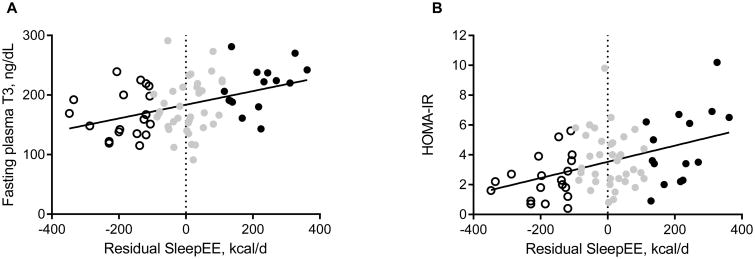
Fasting concentrations of triiodothyronine (T3) were significantly higher in women with obesity class III as compared to class I and II (214±11ng/dL vs 173±7ng/dL, and 174±8ng/dL, respectively, p=0.002 and p=0.003). T4 and TSH were not different between obesity classes. Energy expenditure (TDEE, SleepEE and REE) correlated with T3 (Figure 2A) but not with urinary catecholamine excretion. Further, fasting glucose, insulin and HOMA-IR were also positively correlated with energy expenditure (Figure 2B). Women expecting male infants tended to have a higher SleepEE compared to women expecting female infants (p=0.06), but there was no relationship between maternal EE and infant size measured by fetal ultrasound at the same study visit (r=-0.03, p=N.S.).
Figure 2.
Correlations between residual SleepEE, thyroid hormone T3 and insulin resistance. Each data point represents one participant. Subjects classified as having low, average and high metabolic rates are presented as open, grey and black circles, respectively. Regression lines are significant (T3: R2=0.15, and HOMA-IR: R2=0.17, both p<0.001).
Metabolic Status
Using the linear regression models TDEE and SleepEE derived from the whole cohort, we predicted EE for each participant based on their FFM and FM in early pregnancy. There was a large degree of variability observed among the participants for the residual energy expenditure of TDEE (Figure 1B) and SleepEE (Figure 1D). Residual EE was not significantly different between obesity classes (p=0.41). When applying a published threshold of ±100kcal/d to SleepEE (22), approximately 50% of women had metabolic rate commensurate with their respective metabolic mass, whereas 27% (19/70) had a residual EE≤-100kcal/d which could be considered low metabolic rate and 24% (17/70) had a residual EE≥100kcal/d which could be considered high metabolic rate.
Irrespective of obesity class, the sub-group with high SleepEE had higher fasting glucose (94±3 vs 85±1mg/dL, p=0.003), insulin (18.9±2.2 vs 11.5±1.5IU/mL, p=0.007) and T3 concentrations (214±9 vs 168±9ng/dL, p=0.001) compared to the sub-group with low SleepEE. There was no difference in the distribution of infant sex between the women with low metabolic rate (8 girls, 9 boys) and high metabolic rate (5 girls, 12 boys, p=0.29). These results are robust against the use of other thresholds for high vs low EE (eg. tertiles, >48kcal/d and <-65kcal/d: both n=23, or 10% of SleepEE: >212kcal/d and <-186kcal/d, both n=9).
Substrate Oxidation: The average RQ during the total overnight stay and during sleep (0200-0500am) were 0.875±0.004 and 0.854±0.005, respectively, and were not different between obesity classes. Thus, there was no difference in the overnight oxidation rates for fat and carbohydrate. Moreover, metabolic flexibility in response to the standardized dinner was also comparable between obesity classes (overall, 0.042±0.003).
Energy Requirements
In the 49% of women with energy metabolism within 100kcal/d of the predicted EE (n=13, 12, and 9 in class I, II and III, respectively), the mean TDEE was 2530±91kcal/d (PAL=1.46±0.09). To sustain weight gain within the IOM guidelines (170-270 grams/week) from week 13 until delivery (28), between 2754 and 2835kcal/d is required. Using the most recent model to estimate maternal TDEE (20), TDEE was calculated to be 2939kcal/d (P<0.001 vs measured TDEE) with 3163-3245 kcal/d necessary to promote appropriate GWG.
Discussion
Gestational weight gain above the recommendations of 2009 by the IOM is most prevalent among women with obesity. In this study we successfully measured the different components of daily energy expenditure during early pregnancy (∼15 weeks) and assessed the physiological determinants of energy expenditure during pregnancy in women with obesity. Our major goal was to determine energy requirements during early pregnancy and to identify potential risk factors for excess GWG that could be targeted for intervention strategies. Studies have seldom measured EE in early gestation and none have phenotyped metabolism in this manner in women with obesity (29, 30, 31, 32, 33, 34). In 72 pregnant women with obesity, we found that total daily energy expenditure in early pregnancy is highly variable but is positively correlated with metabolic mass, systemic thyroid hormone concentration and insulin resistance. Importantly, the calculated AREE comprised only 20 % of TEE, which is low compared to other reports (29, 30, 31, 32, 33, 35, 36). Further, while there was no evidence for low EE in this cohort of women with obesity, at the individual level, approximately one-third of women had evidence of low metabolic rate (measured EE<-100kcal/d than predicted EE) which may predispose them to excess GWG. In the women with energy metabolism proportional to their metabolic mass, this study estimates that the energy requirement needed to maintain weight gain within the IOM guideline is between ∼2760 and ∼2840kcal/d. Alarmingly, this estimate is significantly less than the individual estimate that would be derived with a currently applied model (3160-3240kcal/d) (20).
Possible causes for the high prevalence of excess GWG among women with obesity are likely multifactorial. First, low levels of physical activity reduce overall TDEE and therefore favor positive energy balance. Second, a low energy expenditure for a given metabolic mass has been shown to increase risk for weight gain in non-pregnant individuals (23, 37) and in pregnant women (38, 39). Third, weight gain could be the result of a particular metabolic phenotype that includes impaired fat oxidation, low thyroid function and low sympathetic nervous system activity (40). Lastly, GWG could simply be the result of energy intake exceeding energy requirements. Thus, both behavior and physiology are important considerations when identifying risk factors for excess GWG in pregnancy.
In this cohort of pregnant women with obesity, TDEE was primarily comprised of sedentary energy expenditures (70%), i.e. resting and sleep. Assuming diet-induced thermogenesis to account for 10% of TDEE, physical activity contributed to TDEE only in very small quantities. Leaner women seemed to spend a smaller portion (∼60%) of their TDEE in sedentary conditions in early pregnancy and therefore were more active as reported in other studies (29, 30, 31, 32, 33). In line with these findings, physical activity levels were low among our cohort of women with obesity, and more so in those with obesity class III as compared to women without obesity (29, 30, 31, 32, 33, 35, 36). Using accelerometers, we confirmed that pregnant women with obesity maintain a very sedentary lifestyle in early pregnancy. Strikingly, women in obesity class II and III failed to engage in moderate physical activity for the recommended 150min/week almost exclusively (83%, 94% in class II and III, respectively) (27, 41, 42). The benefits of physical activity during pregnancy are well-described (42). Physical activity during pregnancy may reduce the likelihood for pregnancy complications and may improve infant health outcomes such as infant birth weight and abdominal circumference (5). Therefore, in line with the American College of Obstetricians and Gynecologists (52), our data suggests that there is an opportunity to position public health messages on maintaining physical activity throughout pregnancy with an emphasis toward women with obesity.
After correction for body mass, we found no difference in resting energy expenditure between obesity classes, nor when this cohort was compared to a non-obese group of women in early pregnancy (29, 43). Although resting energy expenditure may not be physiologically high or low in women with obesity overall, we found evidence for both low and high metabolic rates of up to ±17% of SleepEE (and REE) among our cohort (±350kcal/d). This variability is comparable to studies in both non-pregnant and pregnant individuals and thus not specific to obesity (23, 29). Given the close relationship between metabolism and body weight regulation (40), the observation of low EE in some women with obesity in early pregnancy may have clinical relevance to GWG. Having low EE is believed to be a homeostatic mechanism activated when energy balance is manipulated to defend body mass and favor weight gain (22). While GWG data is not yet available, the low metabolic rate was supported by lower concentrations of thyroid hormone and insulin. Indeed, in non-pregnant individuals, both low thyroid hormone level and reduced insulin sensitivity are considered to be metabolic mediators of weight gain (40). Interestingly, other metabolic mediators such as low sympathetic nervous system activity and low rates of fat oxidation that also predict weight gain, were not observed in the sub-group with low metabolic rate. Future studies should further investigate a role of endocrine mediators of energy balance throughout pregnancy and in relation to GWG in obesity.
To guide pregnant women toward healthy GWG, studies have been conducted to determine trimester-specific energy requirements. In 2009, data from the classic study by Butte et al. was used to develop a maternal energy requirement model (20). This model, validated against two independent cohorts of pregnant women (31, 32) uses individual age, height and body weight, and GWG to make assumptions regarding individual body composition and physical activity and to estimate trimester-specific energy requirements (20). Using this model, energy expenditure is significantly higher than measured TDEE for women in our cohort in the second and third trimester when weight gain is thought to be linear. The data used in the development and validation of this published energy requirement model were comprised primarily of non-obese women and the reference cohort included only 3 women with pre-gravid obesity (20). According to a secondary analysis we performed on this data, all 3 women with obesity gained in excess of the 2009 IOM guidelines (43). Our study is the largest to metabolically phenotype a cohort of pregnant women with obesity and raises the question of whether the assumptions for maternal energy expenditure, specifically physical activity may significantly overestimate the energy requirement for women with obesity. Specifically, the PAL of pregnant women in the model (i.e. 1.7) is higher than what we observed in pregnant women with obesity overall (1.5) and substantially higher than for women with obesity class III (1.4). Thus, although this energy requirement model has been validated, albeit in non-obese cohorts, it does not appear to be valid for women with obesity. Moreover, caution should be used when applying estimates from this model to pregnant women with obesity to guide weight gain treatment.
In conclusion, this is the first cross-sectional study to precisely measure the different components of energy expenditure in pregnant women with obesity. Given that maternal obesity is a risk factor for excess GWG and adverse pregnancy outcomes, characterization of energy balance with an overarching aim to define energy requirements and to identify components and determinants of energy expenditure could inform the development of future targeted intervention approaches. Our study, combining stable isotopes, indirect calorimetry, accelerometry and body composition assessments suggests that an increased risk for excess GWG among women with obesity may be driven by low physical activity present in early pregnancy. In addition, our data also suggests that metabolic impairments may be implicated in about 30% of cases (conservatively defined as residual SleepEE < -100kcal/d). Our findings point to the need for interventions to promote healthy weight gain and to ameliorate obesity-induced pregnancy risk factors by targeting low physical activity which is evident from the start of pregnancy. Physical activity likely benefits the mother and fetus and also positively contributes to healthy maintenance of energy balance throughout gestation. Finally, this work identified that currently utilized energy intake recommendations are likely overestimating the energy requirements of pregnant women with obesity, and therefore it is imperative that current energy intake recommendations are expanded to include women with maternal obesity. Future studies investigating the mechanisms of gestational weight gain in women with obesity and the relevance of metabolism early in pregnancy are needed.
What is already known about this subject?
Pregnant women with obesity are at increased risk for excessive gestational weight gain
Both behavioral and metabolic factors have been proposed to increase the risk of excessive gestational weight gain in women with obesity
What does your study add?
This is the first detailed characterization of energy metabolism in pregnant women with obesity
Energy requirement models overestimate energy expenditure in pregnant women with obesity as a result of low physical activity
Sleeping metabolic rate is quite variable during pregnancy and may pre-dispose to weight gain in some women
Acknowledgments
We would like to acknowledge technical assistance of Dr. Jennifer Rood, Loren E. Cain, Kimberly Landry and Brian Gilmore, administrative support from Elizabeth F. Sutton, Kelsey Olson, Alexandra Beyer, Alexis O'Connell and Natalie Comardelle and the recruitment and retention support from Drs. Ralph Dauterive, Evelyn Griffin and Evelyn Hayes. Above all, we thank the participants for allowing us to follow their pregnancies.
Funding: This study was funded by the National Institutes of Health (R01DK099175; Redman) and Core support via U54 GM104940; Ryan, P30DK072476; Ravussin.
Footnotes
Trial registered: at Clinicaltrials.gov: NCT01954342
Disclosures: The authors declared no conflict of interest.
Author contribution: JM, LAG, MSA, DSH, ER and LMR conceived the experiments and designed the study. JM, PMV, LAG, MSA, and ADA carried out experiments. JM, LAG, MSA, RAB, ER and LMR analyzed data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993-2003. Obesity (Silver Spring) 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 2.Gavard JA, Artal R. The association of gestational weight gain with birth weight in obese pregnant women by obesity class and diabetic status: a population-based historical cohort study. Matern Child Health J. 2014;18:1038–1047. doi: 10.1007/s10995-013-1356-0. [DOI] [PubMed] [Google Scholar]
- 3.Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health. 2012;9:1263–1307. doi: 10.3390/ijerph9041263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Weight Management in Pregnancy Collaborative G. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119. doi: 10.1136/bmj.j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015:CD007145. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd JM, Turnbull D, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348:g1285. doi: 10.1136/bmj.g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767–777. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 8.Clifton RG, Evans M, Cahill AG, et al. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring) 2016;24:305–313. doi: 10.1002/oby.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton EF, Cain LE, Vallo PM, Redman LM. Strategies for Successful Recruitment of Pregnant Patients Into Clinical Trials. Obstetrics and gynecology. 2017;129:554–559. doi: 10.1097/AOG.0000000000001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen D, Webb KA, Davies GA, O'Donnell DE. Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: implications for respiratory sensation. The Journal of physiology. 2008;586:4735–4750. doi: 10.1113/jphysiol.2008.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961 Nutrition. 1993;9:480–491. discussion 480, 492. [PubMed] [Google Scholar]
- 12.Keys A, Brozek J. Body fat in adult man. Physiological reviews. 1953;33:245–325. doi: 10.1152/physrev.1953.33.3.245. [DOI] [PubMed] [Google Scholar]
- 13.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr. 1988;48:24–29. doi: 10.1093/ajcn/48.1.24. [DOI] [PubMed] [Google Scholar]
- 14.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. The American journal of physiology. 1986;250:R823–830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 15.Martin CK, Heilbronn LK, de Jonge L, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15:2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt MJ, Going SB, Williams DP, Lohman TG. Hydration of the fat-free body mass in children and adults: implications for body composition assessment. The American journal of physiology. 1993;265:E88–95. doi: 10.1152/ajpendo.1993.265.1.E88. [DOI] [PubMed] [Google Scholar]
- 17.Sagayama H, Yamada Y, Racine NM, Shriver TC, Schoeller DA. Dilution space ratio of 2H and 18O of doubly labeled water method in humans. Journal of applied physiology. 2016;120:1349–1354. doi: 10.1152/japplphysiol.01037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Medical & biological engineering & computing. 2003;41:572–578. doi: 10.1007/BF02345320. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DM, Navarro-Barrientos JE, Rivera DE, et al. Dynamic energy-balance model predicting gestational weight gain. Am J Clin Nutr. 2012;95:115–122. doi: 10.3945/ajcn.111.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annual review of nutrition. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Technical Report Series No 724. Geneva: 1985. Energy and protein requirements: Report of a joint FAO/WHO/UNU expert consultation. [PubMed] [Google Scholar]
- 25.Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–540. doi: 10.1148/radiology.150.2.6691115. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services, editor. U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Office of Disease Prevention and Health Promotion; Washington (DC): 2008. [Google Scholar]
- 28.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Determining Optimal Weight Gain. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): 2009. [PubMed] [Google Scholar]
- 29.Butte NF, Wong WW, Treuth MS, Ellis KJ, O'Brian Smith E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr. 2004;79:1078–1087. doi: 10.1093/ajcn/79.6.1078. [DOI] [PubMed] [Google Scholar]
- 30.Forsum E, Kabir N, Sadurskis A, Westerterp K. Total energy expenditure of healthy Swedish women during pregnancy and lactation. Am J Clin Nutr. 1992;56:334–342. doi: 10.1093/ajcn/56.2.334. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg GR, Prentice AM, Coward WA, et al. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am J Clin Nutr. 1993;57:494–505. doi: 10.1093/ajcn/57.4.494. [DOI] [PubMed] [Google Scholar]
- 32.Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Longitudinal assessment of energy balance in well-nourished, pregnant women. Am J Clin Nutr. 1999;69:697–704. doi: 10.1093/ajcn/69.4.697. [DOI] [PubMed] [Google Scholar]
- 33.Lof M, Forsum E. Activity pattern and energy expenditure due to physical activity before and during pregnancy in healthy Swedish women. Br J Nutr. 2006;95:296–302. doi: 10.1079/bjn20051497. [DOI] [PubMed] [Google Scholar]
- 34.Heini A, Schutz Y, Diaz E, Prentice AM, Whitehead RG, Jequier E. Free-living energy expenditure measured by two independent techniques in pregnant and nonpregnant Gambian women. The American journal of physiology. 1991;261:E9–17. doi: 10.1152/ajpendo.1991.261.1.E9. [DOI] [PubMed] [Google Scholar]
- 35.Melzer K, Schutz Y, Boulvain M, Kayser B. Pregnancy-related changes in activity energy expenditure and resting metabolic rate in Switzerland. Eur J Clin Nutr. 2009;63:1185–1191. doi: 10.1038/ejcn.2009.49. [DOI] [PubMed] [Google Scholar]
- 36.Singh J, Prentice AM, Diaz E, et al. Energy expenditure of Gambian women during peak agricultural activity measured by the doubly-labelled water method. Br J Nutr. 1989;62:315–329. doi: 10.1079/bjn19890033. [DOI] [PubMed] [Google Scholar]
- 37.Tataranni PA, Harper IT, Snitker S, et al. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Metab Disord. 2003;27:1578–1583. doi: 10.1038/sj.ijo.0802469. [DOI] [PubMed] [Google Scholar]
- 38.Berggren EK, O'Tierney-Ginn P, Lewis S, Presley L, De-Mouzon SH, Catalano PM. Variations in resting energy expenditure: impact on gestational weight gain. Am J Obstet Gynecol. 2017;217:445 e441–445 e446. doi: 10.1016/j.ajog.2017.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng Y, Groth SW, Stewart P, Smith JAAn. Exploration of the Determinants of Gestational Weight Gain in African American Women: Genetic Factors and Energy Expenditure. Biol Res Nurs. 2017:1099800417743326. doi: 10.1177/1099800417743326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravussin E, Gautier JF. Metabolic predictors of weight gain. Int J Obes Relat Metab Disord. 1999;23 Suppl 1:37–41. doi: 10.1038/sj.ijo.0800793. [DOI] [PubMed] [Google Scholar]
- 41.Currie S, Sinclair M, Murphy MH, Madden E, Dunwoody L, Liddle D. Reducing the decline in physical activity during pregnancy: a systematic review of behaviour change interventions. PLoS One. 2013;8:e66385. doi: 10.1371/journal.pone.0066385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126:e135–142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 43.Gilmore LA, Butte NF, Ravussin E, Han H, Burton JH, Redman LM. Energy Intake and Energy Expenditure for Determining Excess Weight Gain in Pregnant Women. Obstet Gynecol. 2016;127:884–892. doi: 10.1097/AOG.0000000000001372. [DOI] [PMC free article] [PubMed] [Google Scholar]