Abstract
Anabolic-androgenic steroid (AAS) abuse is implicated in maladaptive decision making such as increased risk taking and problem gambling. Endogenous testosterone correlates with economic risk taking in both the stock market (Coates and Herbert, 2008) and in the laboratory, as measured by the Iowa Gambling Task (Stanton et al. 2011). Additionally, AAS use has been associated with problem gambling behavior in adolescents (Proimos, 1998). Thus, AAS may impair economic decision making. However, studies of human AAS users cannot control for pre-existing risky behavior or normalize androgen levels. Accordingly, the present study investigated AAS effects on decision making in rats using a novel, balanced rodent model of the IGT. Adolescent male Long-Evans rats were treated chronically with high-dose testosterone (7.5 mg/kg) or vehicle (13% cyclodextrin in water) sc, and trained to work for sugar pellets in an operant chamber equipped with 4 levers, each associated with a different schedule of reward magnitude (number of pellets), probability, and punishment (time-out) duration. By RM-ANOVA, there was a main effect of lever (F3,78=25.33, p<0.05), such that all rats preferred lever L4 offering a large reward (4 pellets), but with low probability (45%) and a long (35 sec) time-out. There was also a significant interaction of testosterone x lever (F3,78=2.78, p<0.05), with testosterone increasing preference for L4 and decreasing preference for the other levers, relative to vehicle-treated controls. These data extend our previous findings of altered decision making in AAS-treated rats, and suggest that AAS may alter economic decision making in human users.
Keywords: anabolic agents, cognition, operant behavior, food reward, rats
INTRODUCTION
Anabolic-androgenic steroids (AAS) are drugs of abuse associated with maladaptive behavioral effects such as impaired decision making and increased risk taking (Pope et al, 2014). Once limited to elite athletes in professional sports, AAS abuse has spread to college and high-school athletics. It is estimated that 4 million Americans have used AAS, including 4–6% of high-school boys (Johnston et al., 2013; Pope et al, 2014). High-school students who report AAS use also exhibit higher rates of risk taking: drinking and driving, carrying a weapon, and not wearing a helmet or seat belt (Middleman et al. 1995), activities that can result in physical harm. AAS use has also been associated with problematic gambling in adolescents (Proimos, 1998), suggesting AAS may contribute to poor economic decision making, as well. Problem gambling is a costly disorder, with the magnitude of monetary losses increasing rapidly in recent decades. Americans lost $92 billion through gambling in 2007, nearly 10 times the amount lost in 1982 (Skolnik, 2011). Furthermore, adolescents and young adults demonstrate a high prevalence of problem gambling, which is frequently associated with substance abuse (Barnes et al, 2009). Adverse effects on decision making are particularly problematic for young AAS users, as the neural circuitry underlying cognition is still under development in the adolescent brain (Blakemore and Choudhury, 2006). Therefore, it is important to determine if AAS use may cause increased economic risk taking.
Studies of AAS in humans are complicated by users’ motivation to increase muscle-mass and enhance appearance, and by preexisting potential for risky behavior (Pope et al, 2014). Animal studies eliminate this confound, and also control for AAS type and dose. In particular, behavioral paradigms adapted from human tasks allow us to study AAS effects on executive function. We have previously shown that AAS alter decision-making behavior in discounting tasks where rats choose between two levers (Wood et al, 2013; Cooper et al. 2014; Wallin et al. 2015). One delivers a small “safe” reward (1 sugar pellet), while the other lever gives a large reward (3 or 4 pellets) that is “discounted” or made less desirable by pairing with a cost such as delay, effort, uncertainty, or punishment. In effort, delay and punishment discounting, AAS-treated rats were willing to work harder, wait longer and accept discomfort in order to earn bigger reward (Wood et al, 2013; Cooper et al. 2014; Wallin et al. 2015). These findings are perhaps not surprising, as athletes and body builders exert tremendous effort and endure physical pain in pursuit of their aesthetic and athletic goals. However, we were surprised that AAS-treated rats were more sensitive to reward uncertainty in probability discounting (PD). Compared with vehicle controls, they were less likely to choose a large uncertain reward, and instead preferred a small reward delivered with 100% probability (Wallin et al., 2015). These studies demonstrate that AAS do not cause a “win-at-all-costs” mentality. Rather, AAS sensitize subjects to different costs in discounting tasks.
Thus far, we have tested the effects of AAS on discounting tasks that require consideration of only 1 variable. In real life, decisions are seldom 1-dimensional. Instead, we integrate multiple cost and reward variables to make choices that maximize reward. To study decision making in humans, participants in the Iowa Gambling Task (IGT) choose among 4 card decks, which offer monetary gains and losses of varying magnitude and probability (Bechara et al. 1994). By sampling the decks, subjects aim to earn as much money as possible throughout the session. To investigate AAS effects on decision making in rats, we developed a balanced rat model of the Iowa Gambling Task (brIGT), offering 4 lever choices to obtain food reward. Previous studies with a rat IGT task (Zeeb et al. 2009) showed that rats develop a stable preference for the reward option that maximizes pellets earned over the 30-minute session. The brIGT task expands on these previous rat IGT models, by holding each decision variable (reward magnitude, win probability, and time-out duration) constant between two lever choices. This allowed a direct comparison of the relative importance of each variable to vehicle- and testosterone-treated rats. With this model, we can evaluate how AAS alter the salience of and sensitivity to different reward variables.
We hypothesized that AAS would increase risk taking and decrease performance on the brIGT, similar to IGT studies in humans. In the IGT, men with high testosterone are more likely to choose cards from decks offering large monetary gains paired with larger, infrequent losses (Stanton et al. 2011). As a result, they earned less money throughout the session, relative to men with lower levels of testosterone. High levels of endogenous testosterone also correlate with economic risk taking outside the lab. In a study of London stock traders, morning testosterone levels predicted risk taking throughout the day (Coates and Herbert, 2008). Conversely, when the sexes are compared, men generally perform better than women on the IGT (Bolla et al, 2004), even though men have higher testosterone than women. Together, these findings suggest that the relationship between testosterone and decision making in the IGT is not linear. Rather, a moderate level of testosterone may reduce loss aversion to facilitate optimal decision making, while high levels can impair performance.
METHODS
Animals
28 male Long-Evans rats (5 weeks old at the start, Charles River Laboratories, MA) were treated chronically with either vehicle or testosterone (n=12–16/group) and tested for decision making in the brIGT. Rats were pair-housed with ad libitum access to water under a reversed 14L:10D photoperiod, and were tested daily (5 days/week) during the dark phase. Rats remained gonad-intact to approximate human AAS use. To facilitate operant responding, rats were food restricted to maintain a slow rate of growth (3–4 g BW/day), as in our previous studies (Wood et al, 2013; Cooper et al. 2014; Wallin et al. 2015). Vehicle- and testosterone-treated groups did not differ in body weight at the start of the study or throughout testing. Experimental procedures were approved by USC’s Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Ed (National Research Council, National Academies Press, Washington DC; 2011).
AAS treatments
As a model for AAS abuse, the present study exposed rats to chronic high-dose testosterone beginning in adolescence (5 weeks of age; Spear, 2000), at least 2 weeks prior to behavioral testing. Rats received daily injections of testosterone (7.5 mg/kg; Steraloids, RI) or vehicle [3% ethanol and 13% cyclodextrin (RBI, MA)] sc immediately before behavioral training or testing for the duration of the experiment (5 days/week for 8 weeks). Testosterone was used because it is the prototypical AAS, and is the most common performance-enhancing substance (55.5%) detected in urine tests by World Anti-Doping Agency-accredited laboratories (World Anti-Doping Agency, 2012). Among human users, testosterone is a popular choice due to its low price, easy availability, and the difficulty differentiating exogenous testosterone from endogenous production in drug testing. The 7.5 mg/kg dose approximates heavy doses of AAS used by humans, and has been previously used to test effects of AAS in discounting tasks (Wood et al, 2013; Cooper et al. 2014; Wallin et al. 2015).
Operant Chambers
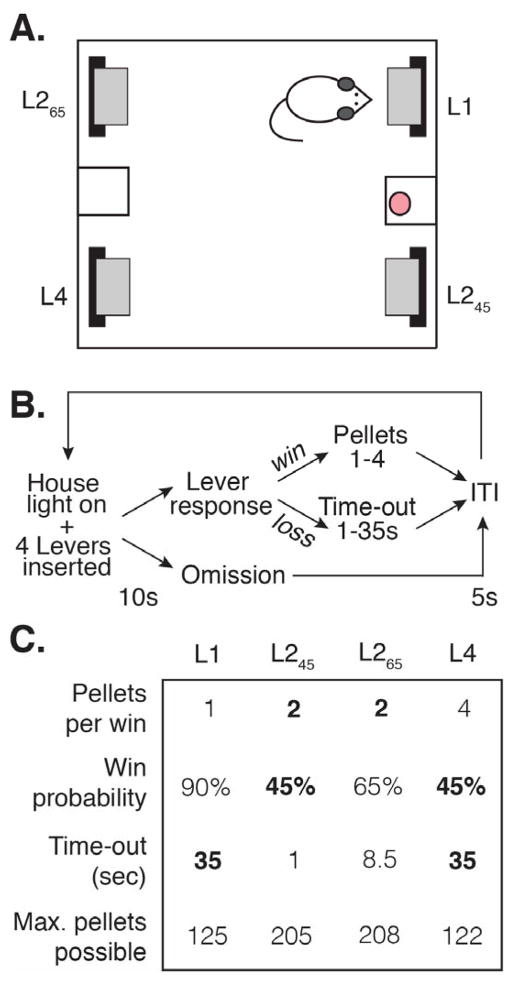
Studies were conducted in operant chambers (Med Associates, VT) with a houselight for illumination and two pellet dispensers connected to food cups on opposite sides of the chamber. Each food cup was flanked by two retractable levers (Figure 1A). Chambers were enclosed in sound-attenuating boxes with fans for ventilation.
Figure 1.
Experimental design in brIGT. A: Location of levers and pellet cups in the operant chamber. B: Flow chart for each trial. See Methods for details. C: Reward profile for each lever. Pairs of identical values in each row are in bold.
Lever Training
Initially, rats were trained to respond on each lever to receive 45mg sucrose pellets (Bio-Serv Inc., Frenchtown, NJ) as in Wallin et al (2015). Next, rats were habituated to lever insertion in 100-trial daily sessions (25 trials/lever). Each trial began in darkness with all levers retracted in the inter-trial interval (ITI) state. Three seconds later, the house-light was illuminated and 1 lever was inserted into the chamber in pseudorandom order. If the rat responded on the extended lever within 30 sec, 1 pellet was delivered to the adjacent food cup, and the house-light stayed on for 4 seconds before returning to ITI. If the rat failed to respond, the chamber reverted to ITI and the trial counted as an omission. The criterion for success in lever training was omission of <10% of trials. After reaching criterion, lever extension was decreased to 20 seconds, and subsequently to 10 seconds. Once criterion was met on the 10-second task, rats began brIGT testing.
Balanced Rat Iowa Gambling Task (brIGT)
Operant procedure
In 30-minute daily sessions, trials began in darkness with all levers retracted. On each trial, all 4 levers were inserted, and rats had 10 seconds to respond. Once a lever was selected, the 3 other levers retracted while the chosen lever remained extended for the duration of the trial. If the lever choice was rewarded (win), the house light remained illuminated for 5 seconds while pellets were delivered. If the lever choice was not rewarded (loss), no pellets were delivered, and the house light blinked at 0.5 Hz for the duration of the time-out period (1, 8.5 or 35 seconds, Figure 1B). Additional responses on the chosen lever were recorded but had no effect. A 5-second ITI followed each reward or time-out period. If no lever was selected in 10 seconds, the trial was counted as an omission, and the chamber reverted to ITI. Rats were tested for 22 days until behavior was stable over 5 days of testing, as defined by no effect of day on choice behavior by repeated measures ANOVA (RM-ANOVA) with day as the repeated measure, as in St. Onge and Floresco (2009); Cooper et al. (2014); and Wallin et al. (2015).
Lever reward schedules
Each lever offered a distinct reward profile which varied the reward magnitude (number of pellets per win), probability (percent chance of winning) and time-out duration (seconds of time-out after a loss; Figure 1C). Because rats were not able to earn any pellets during time-outs, losses not only resulted in no reward in the current trial, but also decreased the time available to earn future pellets. Thus, levers varied in their objective value—number of available pellets per unit time. As in the human IGT, there were two advantageous and two disadvantageous choices. The two advantageous levers (L265 and L245) delivered 2 pellets with each win, for a maximum of 208 and 205 pellets/session, respectively. L265 and L245 differed in reward probability, with L245 offering a less certain reward (45%) compared with L265 (65%). The two disadvantageous levers (L1 and L4) delivered 1 and 4 pellets per win, respectively, with a maximum of only 125 and 122 pellets/session, respectively (Figure 1C). Lever position did not vary between rats, but was arranged so that each side of the chamber had one advantageous lever and one disadvantageous lever (Figure 1A).
Data analysis
Lever choice
Data for each session included the total number of trials, number of trials completed, trials omitted, responses on each lever, wins and losses on each lever, total pellets won, and responses on the selected lever after wins or losses. Lever choice was calculated as the proportion of completed trials in which each lever was chosen. Lever choice was analyzed via RM-ANOVA, with day of testing and lever as repeated measures and drug (vehicle vs testosterone) as the between-subjects factor. An arcsine transformation was performed before analysis of variables expressed as a proportion to limit the effect of an artificially imposed ceiling, as in Zeeb et al. (2009). Responses per second on inactive levers after wins and losses were averaged for vehicle- and testosterone-treated rats and compared by RM-ANOVA, with wins versus losses as the within-subjects factor, and drug as the between-subjects factor. Trials completed, omissions and pellets won were averaged for vehicle- and testosterone-treated rats, and compared by Student’s t-test.
Decision variable salience
By holding each variable constant between two of the lever choices, the brIGT permits comparison of sensitivity to different aspects of choice (i.e. reward magnitude, win probability, and time-out duration) for vehicle and testosterone-treated rats. As shown in Fig. 1C, L265 and L245 offered the same reward magnitude, L245 and L4 offered the same win probability (45%), and L1 and L4 gave the same time-out duration (35 sec) following a loss. Six planned comparisons were used to detect differences in preference between levers in a pair, and vehicle- and testosterone-treated rats’ lever preference within each of the 2 lever pairs. For instance, a preference for L4 over L1, suggests that rats value reward magnitude over win probability, whereas a preference for L265 over L245 indicates that win probability is more salient than time-out duration. Comparisons were made by Student’s t-test with Bonferroni’s correction for multiple comparisons (α=0.008).
Win-stay/Lose-shift behavior
To measure sensitivity to reward delivery and omission, win-stay (WS) and lose-shift (LS) ratios were calculated as in Wallin et al. (2015) and Stopper and Floresco (2011). A WS occurred when the rat received a reward (win), and responded on the same lever again in the following trial (stay). A LS occurred when the rat received no pellets on a trial (loss), and selected a different lever on the following trial (shift). Overall WS and LS ratios were calculated by collapsing the number of wins, losses, stays and shifts across all levers, and then calculating win-stay and lose-shift ratios based on these numbers. This method is analogous to collapsing win-stay and lose-shift data over blocks, as in St. Onge et al. (2011) and Stopper and Floresco, (2011). Individual ratios for each individual lever were calculated as “win-stays/total wins” and “lose-shifts/total losses” on each individual lever. The overall ratio is not a sum or a simple average of the individual lever ratios. Rather, relative to the overall ratio, the individual lever ratios have different weights depending on how often each lever was selected. Overall WS and LS ratios were compared by Student’s t-test. WS and LS ratios for each lever were compared by RM-ANOVA with lever as the repeated measure and drug (vehicle vs. testosterone) as the between-subjects factor. All statistical analyses were conducted in SPSS for Windows, with p<0.05 considered significant.
RESULTS
Choice behavior
Choice behavior was stable for vehicle- and testosterone-treated rats over days 18–22 of testing, and there was no significant effect of day on behavior (F4,104=0.74, p>0.05). With continued testing beyond 22 days, there was no change in choice behavior (data not shown). Figure 2 compares lever choice by vehicle- and testosterone-treated rats in the brIGT. By RM-ANOVA, there was a significant main effect of lever (F3,78=25.33, p<0.05), such that all rats significantly preferred L4 to the other choices. Ranking of available choices by both vehicle- and testosterone-treated rats followed the order: L4>L265>L245>L1. Although vehicle- and testosterone-treated rats ranked levers in the same order, testosterone altered choice behavior, indicated by a significant testosterone x lever interaction (F3,78=2.78, p<0.05). In particular, testosterone-treated rats exhibited a significantly greater preference for L4 (57.1±7.1% of trials) than vehicle-treated controls (38.8±5.0% of trials; p<0.05 by post-hoc comparison with Student’s t-test).
Figure 2.
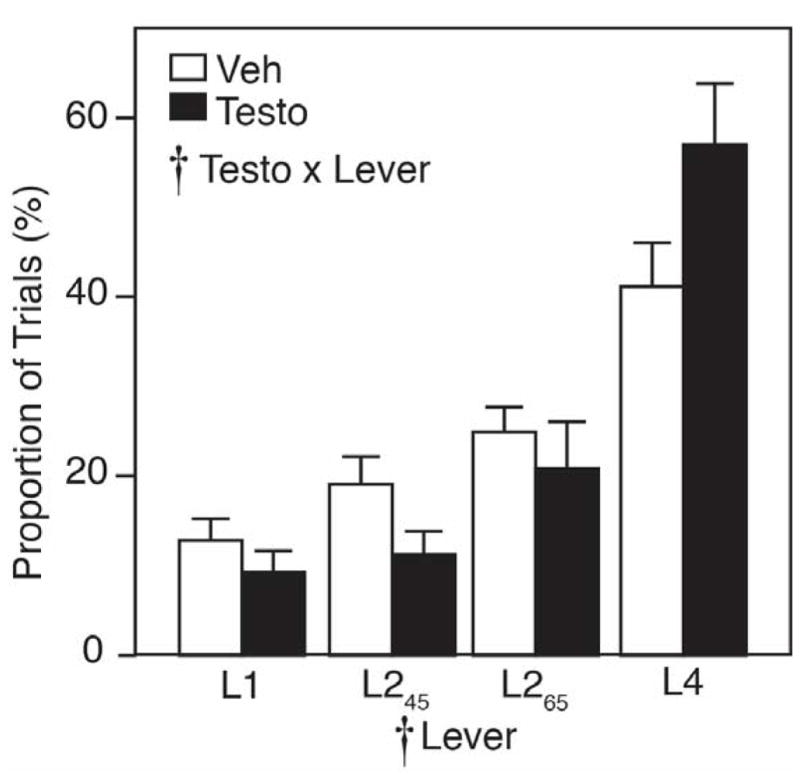
Lever choice behavior in rats playing brIGT as a proportion of total trials. Vehicle controls (Veh) are in open bars; testosterone-treated rats (Testo) are in closed bars. Cross indicates significant effect by RM-ANOVA.
Decision Variable Salience
Reward magnitude vs probability (L1 vs L4)
L1 and L4 offered the same time-out duration (35 sec) and comparable numbers of possible pellets per session (125 or 122), but with 4x more pellets per win (L4) or 2x probability of winning (L1). Vehicle-treated rats significantly preferred L4 over L1 (41.6±4.7% vs 13.9±2.6%; p<0.008). Testosterone-treated rats also preferred L4 over L1 (57.1±7.1% vs 9.2±2.4%; p<0.008), indicating that both groups of rats valued the large reward magnitude over a high win probability.
Reward magnitude vs time-out (L245 vs L4)
L245 and L4 offered the same probability of winning (45%), but with 2x more pellets per win (L4) or a substantially shorter time-out duration after a loss (L245). As a result, the maximum possible pellets from responses on L4 was only 60% of those from L245. Nonetheless, both groups preferred L4 over L245 (vehicle: 17.5±2.6%; p<0.008, testosterone: 11.7±2.5%; p<0.008) suggesting that the large reward magnitude was more important to rats than a short time-out duration following a loss.
Time-out vs probability (L245 vs L265)
A response on L245 or L265 delivered 2 pellets, with similar potential maximum pellets per session (205 or 208). With L245, wins occurred less frequently (45% vs 65% for L265), but time-outs were shorter (1 sec vs 8.5 sec for L265). Vehicle-treated rats displayed no statistically significant preference for L245 (17.5±2.6%) vs L265 (24.5±2.9%; p>0.008) reflecting that win probability was equally salient to time-out duration in our model. However, testosterone-treated rats did exhibit a trending preference for L265 (21.3±5.5%) over L245 (11.7±2.5%; p=0.03), suggesting that testosterone increases sensitivity to win probability, as in our previous study of PD (Wallin et al, 2015).
Win-Stay and Lose-Shift Behavior
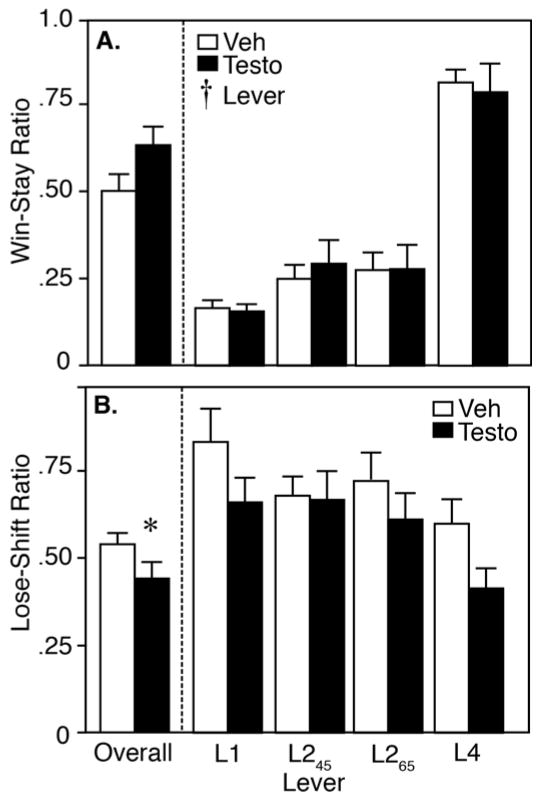
WS and LS ratios, measuring sensitivity to reward delivery and omission, respectively, are shown in Figure 3. For the overall WS ratio, there was no effect of drug, suggesting that testosterone does not affect sensitivity to reward delivery (p>0.05). By RM-ANOVA, there was a main effect of lever (F3,54=31.21, p<0.05), such that all rats were more likely to “stay” after a win on L4 than on the other levers, suggesting that rats are highly sensitive to the delivery of a large magnitude reward. Additionally, there was no effect of drug (F1,18=0.20, p>0.05), and no drug x lever interaction (F4,104=0.11, p>0.05; Figure 3A).
Figure 3.
A: Win-Stay and B: Lose-Shift ratios in rats playing brIGT, including overall session ratios (left) and response ratios for individual levers (right). Vehicle controls (Veh) are in open bars; testosterone-treated rats (Testo) are in closed bars. Asterisk indicates significant difference by Student’s t-test. Cross indicates significant effect by RM-ANOVA.
However, there was a main effect of testosterone on the overall LS ratio, where testosterone-treated rats were less likely to shift after a loss (0.43±0.07) compared to vehicle-treated controls (0.59±0.04; p<0.05; Figure 3B). By RM-ANOVA, there was no effect of lever on the LS ratio (F1,18=1.96, p>0.05), and no drug x lever interaction (F1,18=0.87, p>0.05). There was a trend toward a main effect of testosterone, with testosterone decreasing LS ratio across all levers (F1,18=3.93, p=0.063).
Trials Completed, Omissions, Pellets Won, and Responses on Inactive Levers
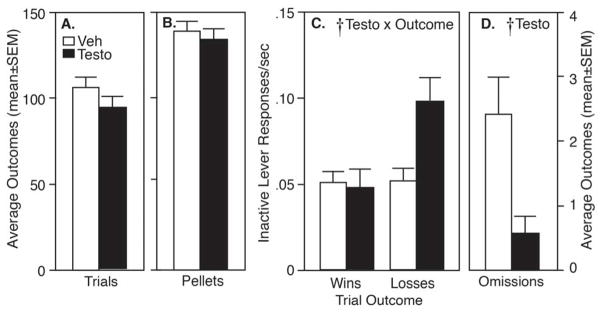
Figure 4A shows the average number of completed trials for vehicle- and testosterone-treated rats over the last 5 days of brIGT testing. By Student’s t-test, there was no effect of drug on number of trials completed (p>0.05). However, there was a significant effect of drug on trials omitted, with vehicle-treated rats omitting more trials per session (2.4±0.6) than testosterone-treated rats (0.6±0.2; p<0.05). Somewhat surprisingly, although vehicle- and testosterone-treated rats exhibited significantly different lever choices, the groups did not differ in average number of pellets earned per session (p>0.05; Figure 4B). Rats in both groups averaged 98.7±4.3 trials in each 30-min session, earning 137.6±4.4 pellets. In AAS-treated rats, the increase in selection of L4 was paired with a decreased selection of L1 (also disadvantageous) and a decrease in omissions, so that the total pellets earned was not significantly different from that in vehicle-treated rats. Figure 4C shows responses per second on the inactive lever during pellet delivery (after wins) and during time-outs (after losses). While there was no main effect of testosterone (F1,26=1.19, p<0.05), there was a testosterone x trial outcome interaction (F1,26=6.18, p<0.05) with testosterone-treated rats making significantly more responses per second on the inactive lever during the time-out period following a loss.
Figure 4.
A: Total trials and B: pellets earned in rats playing brIGT. C: Responses on inactive levers after wins and losses. D: Trial omissions. Vehicle controls (Veh) are in open bars; testosterone-treated rats (Testo) are in closed bars. Cross indicates significant effect by RM-ANOVA.
DISCUSSION
The present study investigated effects of testosterone treatment on decision-making in a balanced rodent version of the IGT. As hypothesized, testosterone altered decision making on the brIGT, significantly increasing selection of the most disadvantageous lever (L4), compared to vehicle-treated controls. This altered choice behavior was due to diminished loss sensitivity, as testosterone significantly decreased the LS ratio. Testosterone-treated rats also made significantly more inactive lever responses after losses than vehicle-treated rats, perhaps indicating frustration when rewards were not delivered. These results show that testosterone modifies decision making in a task incorporating multiple variables including reward magnitude, win probability, and punishment duration.
Results from the brIGT show that reward magnitude is the most salient decision variable in this paradigm. Although L1 and L4 offered equal time-out duration (35 sec) and similar objective value (125 and 122 total possible pellets, respectively), all rats exhibited a drastic preference for L4 over L1. Thus, it appears that a large reward magnitude with low win probability (L4, 45%) is more desirable than a small reward magnitude with a high win probability (L1, 90%). Likewise, when win probability was held constant, all rats preferred L4 over L245. This was surprising, as L4 is objectively worse than L245, offering only 122 vs 208 total possible pellets, respectively. However, rats again preferred the lever offering a large magnitude reward, even when it resulted in fewer pellets earned overall. Finally, when reward magnitude was held constant (L245 and L265), testosterone-treated rats tended to favor the lever offering a higher win probability, despite the longer time-out following a loss.
The strong preference for L4, an objectively disadvantageous lever, suggests the rats may have not correctly integrated the probability of reward delivery and time-out, plus time out length, with reward magnitude in order to choose optimally. We know that rats can do this. In the rodent IGT model of Zeeb et al. (2009), rats were able to determine the optimal strategy, exhibiting a significant preference for the most advantageous nose-poke choice (2 pellets delivered with 80% probability). So why can’t they do it in our brIGT? It is important to note that the layout of our brIGT task (2 pairs of levers on opposite sides of the chamber) differed from that of the rodent IGT (4 nose-pokes on 1 wall, with the pellet dispenser on the opposite wall). Nonetheless, the increased L4 responses in the brIGT cannot be explained by a consistent preference for levers near the door (L4 and L245) or for the levers to the left of the pellet trough (L4 and L1). Although lever position was not counter-balanced in our study, changing lever positions within the chamber during pilot testing of the brIGT did not affect lever choice. Likewise, Zeeb et al. (2009) varied the arrangement of nose-pokes in their model, and found no effect on choice behavior.
Instead, it seems likely that the animals are simply responding based on which option delivers the largest amount of sugar per trial win, and hence prefer L4. The different results with the brIGT and the rodent IGT of Zeeb et al. (2009) may be due to differences in the relative value of available choices. In the brIGT, L4 yielded a maximum of 122 total pellets, while L265 offered 208 pellets (a 170% increase). In Zeeb et al. (2009) the disadvantageous 4-pellet option gave 99 total pellets, while the advantageous 2-pellet option yielded 411 total pellets (a 415% increase). This large difference in objective value likely allowed rats to overcome preference for a large magnitude reward and discern the optimal strategy. However, when the difference is smaller, as in brIGT, it appears that rats are swayed to an ultimately disadvantageous choice by the temptation of a large magnitude reward. These findings have important implications for the design of decision making tasks. From the comparison of these IGT tasks, it appears that choice behavior is not transitive, but is influenced by the context of the choices available (i.e. choice architecture, discussed below). Thus decision-making behavior in one task cannot be predicted by behavior previously observed on similar paradigms.
Comparing responses of vehicle- and testosterone-treated rats in the brIGT, the two groups show the same rank order of lever preferences (L4>L265>L245>L1), but testosterone increased preference for L4. This, combined with the drop in omissions, could indicate that testosterone-treated rats are more sensitive to reward magnitude, or more motivated. To address these possibilities, it is useful to consider the current findings in the context of our previous work on chronic high-dose testosterone and decision making in discounting paradigms. Compared to control rats, testosterone-treated rats were significantly more likely to choose the large reward despite an effort cost, delivery delay, or pairing with a footshock (Wood et al. 2013; Cooper et al. 2014; Wallin et al. 2015). Thus, testosterone decreases sensitivity to these costs, which is consistent with increased sensitivity to reward magnitude. On the other hand, with PD, testosterone-treated rats chose the large/uncertain reward significantly less than vehicle-treated controls (Wallin et al. 2015), indicating increased sensitivity to reward uncertainty. This argues that testosterone-treated rats are not more motivated towards the large reward in all circumstances, compared with vehicle-treated rats. While the effects of testosterone on lever choice in the brIGT and PD may seem contradictory, there are some important differences between the two tasks. For instance, PD offers a small reward delivered with 100% certainty, while no option in brIGT is a guaranteed win. Furthermore, in PD, the win probability associated with the large reward decreases throughout the session. An unstable win probability may be more aversive to testosterone-treated rats than a stable one, especially since testosterone impairs cognitive flexibility (Wallin and Wood, 2015).
Like rats in the present study, humans value short-term rewards and may not choose outcomes that maximize their long-term interests (reviewed in Kalenscher and van Wingerden, 2011). However, only animal studies question the potential cognitive limitations of their subjects. Instead, comparing choice behavior on PD, brIGT, and Winstanley’s IGT (Zeeb et al. 2009) suggests that rats are prone to some of the same cognitive biases and irrational behavior exhibited by humans. For instance, rats appear to exhibit the phenomenon of probability distortion—meaning that the subjective values assigned to probabilities from 0–100% are not linear. Humans tend to overestimate the likelihood of small probabilities and over-weight values close to 0 or 100%. For example, the difference between a 100% and 90% chance of winning seems much more meaningful than a difference between a 45% and 55% chance of winning, even though the objective difference in value between the two pairs of chances are the same (Kahneman and Tversky, 1979; Baron, 2000; Ariely, 2008). Likewise, testosterone-treated rats show a substantial difference in subjective value assigned to a 1 pellet reward delivered at 100% probability (preferred in Wallin et al. 2015) and a 1 pellet reward delivered at 90% probability (strongly disfavored in current study). Additionally, it is well known that choice architecture can influence decision behavior in humans. Both the relative value of available options (the reference effect) and increasing the number of options (choice overload) can alter behavior and result in suboptimal decision making (Ariely, 2008). Thus, the availability of 4 vs 2 choices may influence the contrasting preferences exhibited in PD and brIGT, and the difference in relative values between choices likely causes behavioral differences in brIGT compared to Zeeb et al. (2009). These phenomena are well-documented in human behavioral economic studies, and the current study suggests that rats are subject to some of the same cognitive biases as well.
In human patients, deficits on the IGT are seen with neurological damage to brain regions including the OFC, mPFC, and amygdala, suggesting these areas are important for optimal economic decision making (Bechara et al. 1999; Fellows and Farah, 2005). Animal studies have confirmed and clarified these associations, showing that mPFC inactivation or BLA lesion impair decision making on a rodent version of the IGT, increasing selection of the disadvantageous options (Zeeb et al. 2011 and 2015). Additionally, OFC lesions impair initial acquisition of the optimal strategy on the rodent IGT, suggesting that this brain region is required for the learning phase of a decision making task (Zeeb et al. 2011). In addition to supporting economic decision making behavior in the IGT, PFC is required for other types of decision making and executive function. For instance, both the mPFC and OFC are involved in cognitive flexibility (Floresco et al. 2008; Ghods-Sharifi et al. 2008). We have previously shown that testosterone impairs both set-shifting (dependent on mPFC) and reversal learning (supported by OFC; Wallin and Wood; 2015). Thus, it is not surprising that testosterone also impairs decision making on the brIGT, which likely depends on similar mesocorticolimbic brain circuitry.
Human imaging studies have also shown sex differences in OFC function during decision making. In an fMRI study, females exhibited more dynamic OFC activity than males during performance of the Risky Gains decision making task (Lee et al. 2009). In an fMRI study of the Ultimatum Game, a social economic decision making task, high testosterone levels correlated with both reduced OFC activity and with “aggressive” responses to unfair offers—rejection of the offers, resulting in no monetary gain for either player (Mehta and Beer, 2010). This suggests that decreased OFC activity mediated by testosterone is a likely candidate for impaired decision-making behavior in testosterone-treated rats. Altered OFC function may also underlie the striking increase in inactive lever responses by testosterone-treated rats. The tendency to continue lever pressing after a loss (when no pellets can be earned) resembles perseverative behavior, which is associated with OFC dysfunction (Bechara et al, 2000) and high testosterone in human and animal studies (Broverman et al, 1964; Andrew and Rogers, 1972). Continued responding after a loss does not reflect motor impulsivity, and we have previously found no effect of testosterone in a “go/no-go” task of impulsive behavior (Cooper et al. 2013). Furthermore, there is no effect of testosterone to increase general locomotor activity (Wallin-Miller et al, in press). It is worth noting that our model begins testosterone treatment in late adolescence to model the human condition of AAS use, as most AAS abusers begin use as teenagers or young adults (Pope et al, 2013). Testosterone treatment may have different effects on decision making if not initiated until adulthood, when the underlying prefrontal cortical circuitry is mature (Spear, 2000). It is possible that adult rats may be more resilient to the effects of exogenous testosterone on decision making.
Our body of work investigating testosterone effects on decision making shows that AAS often increase the drive to go for a big payout despite the cost, above and beyond what is normal. In particular, testosterone-treated rats in the current study exhibited increased selection of a disadvantageous choice associated with large reward magnitude and low win probability, with the caveat that neither group of rats preferred the advantageous levers in this task. Likewise, problematic gambling in humans involves compulsive pursuit of large, tempting payouts despite the unlikely odds of winning. This study suggests a causal relationship between AAS use and problematic gambling, behaviors previously shown to be associated in human subjects (Proimos, 1998). The finding that testosterone increases risk-taking behavior in a gambling paradigm also corresponds with trends observed in human populations. Among humans, males are twice as likely as females to be problem gamblers (Welte et al. 2015). Thus, it is possible that even normal levels of testosterone are conducive to maladaptive gambling behavior, perhaps explaining the preference for a large, risky reward exhibited by even our control rats (intact males). Furthermore, adolescents exhibit higher rates of problematic gambling than any other age group (Welte et al. 2015). Therefore, the increase in circulating gonadal hormones during adolescence may both facilitate problem gambling and render adolescents especially vulnerable to AAS effects on decision making and risk taking. This study reveals previously unknown side effects of AAS use, and highlights the association between drug abuse and behavioral addiction—common comorbidities. Both drug and gambling addiction are costly to individuals and society, and rodent models of substance abuse and decision making will be crucial to understanding the neurobiology underlying these disorders.
Acknowledgments
We thank Jordyn Chesley for assistance with animal handling. This work was supported by the National Institutes of Health (NIH R01-DA029613 to RIW).
Footnotes
A preliminary report of this work was presented at the 2014 Annual Meeting of the Society for Neuroscience. Washington, DC (Abstract #8287).
References
- Andrew RJ, Rogers LJ. Testosterone, search behaviour and persistence. Nature. 1972;237:343–346. doi: 10.1038/237343a0. [DOI] [PubMed] [Google Scholar]
- Ariely D. Predictably irrational. New York: HarperCollins; 2008. [Google Scholar]
- Barnes GM, Welte JW, Hoffman JH, Tidwell MC. Gambling, alcohol, and other substance use among youth in the United States. J Stud Alcohol Drugs. 2009;70(1):134–142. doi: 10.15288/jsad.2009.70.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J. Thinking and deciding. New York: Cambridge University Press; 2000. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47(3–4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14(11):1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Broverman DM, Broverman IK, Vogel W, Palmer RD, Klaiber EL. The automatization cognitive style and physical development. Child Dev. 1964;35:1343–1359. doi: 10.1111/j.1467-8624.1964.tb05272.x. [DOI] [PubMed] [Google Scholar]
- Coates JM, Herbert J. Endogenous Steroids and Financial Risk Taking on a London Trading Floor. Proc Natl Acad Sci USA. 2008;105:6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Goings SP, Kim JY, Wood RI. Testosterone enhances risk tolerance without altering motor impulsivity in male rats. Psychoneuroendocrinology. 2014;40:201–12. doi: 10.1016/j.psyneuen.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Maric TL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89(4):567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 2012 Overview, Key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47(2):263–291. [Google Scholar]
- Kalenscher T, van Wingerden M. Why we should use animals to study economic decision making- a perspective. Front Neurosci. 2011;5:82. doi: 10.3389/fnins.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Chan CC, Leung AW, Fox PT, Gao JH. Sex-related differences in neural activity during risk taking: an fMRI study. Cereb Cortex. 2008;19(6):1303–1312. doi: 10.1093/cercor/bhn172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PH, Beer J. Neural mechanisms of the testosterone–aggression relation: the role of orbitofrontal cortex. J Cog Neurosci. 2010;22(10):2357–2368. doi: 10.1162/jocn.2009.21389. [DOI] [PubMed] [Google Scholar]
- Middleman AB, Faulkner AH, Woods ER, Emans SJ, Durant RH. High-risk behaviors among high school students in Massachusetts who use anabolic steroids. Pediatrics. 1995;96(2 Pt 1):268–272. [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- Pope HG, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am J Addict. 2013;23(4):371–377. doi: 10.1111/j.1521-0391.2013.12118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Homicide and near-homicide by anabolic steroid users. J Clin Psychiatry. 1990;51:28–31. [PubMed] [Google Scholar]
- Proimos J, DuRant RH, Pierce JD, Goodman E. Gambling and other risk behaviors among 8th-to 12th-grade students. Pediatrics. 1998;102(2):e23. doi: 10.1542/peds.102.2.e23. [DOI] [PubMed] [Google Scholar]
- Skolnik S. High stakes: The rising cost of America’s gambling addiction. Boston: Beacon Press; 2011. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34(3):681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa Gambling Task. Horm Behav. 2011;59(2):252–256. doi: 10.1016/j.yhbeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB. Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cogn Affect Behav Neurosci. 2011;11(1):97–112. doi: 10.3758/s13415-010-0015-9. [DOI] [PubMed] [Google Scholar]
- Wallin KG, Alves JM, Wood RI. Anabolic–androgenic steroids and decision making: Probability and effort discounting in male rats. Psychoneuroendocrinology. 2015;57:84–92. doi: 10.1016/j.psyneuen.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin KG, Wood RI. Anabolic–androgenic steroids impair set-shifting and reversal learning in male rats. Eur Neuropsychopharmacol. 2015;25(4):583–90. doi: 10.1016/j.euroneuro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin-Miller KG, Kreutz F, Li G, Wood RI. Anabolic-androgenic steroids (AAS) increase sensitivity to uncertainty by inhibition of dopamine D1 and D2 receptors. Psychopharmacology. doi: 10.1007/s00213-017-4810-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte JW, Barnes GM, Tidwell MC, Hoffman JH, Wieczorek WF. Gambling and problem gambling in the United States: Changes between 1999 and 2013. J Gambl Stud. 2015;31(3):695–715. doi: 10.1007/s10899-014-9471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Armstrong A, Fridkin V, Shah V, Najafi A, Jakowec M. Roid rage in rats? Testosterone effects on aggressive motivation, impulsivity and tyrosine hydroxylase. Physiol Behav. 2013;110:6–12. doi: 10.1016/j.physbeh.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Anti-doping Agency. [Accessed 05/02/13];2012 http://www.wada-ama.org/Documents/Resources/Testing-Figures/WADA-2011-Laboratory-Testing-Figures.pdf.
- Zeeb FD, Baarendse PJ, Vanderschuren LJ, Winstanley CA. Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology. 2015;232(24):4481–4491. doi: 10.1007/s00213-015-4075-y. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34(10):2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci. 2011;31(6):2197–2204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]