Abstract
Background
Congenital heart surgery has improved the survival of patients with even the most complex defects, but the long-term survival after these procedures has not been fully described.
Objectives
Evaluate the long-term survival of patients (<21 years) operated for congenital heart defects.
Methods
This study used the Pediatric Cardiac Care Consortium (PCCC) data, a U.S.-based, multicenter registry of pediatric cardiac surgery. Survival analysis included 35,998 patients who survived their first congenital heart surgery at less than 21 years of age and had adequate identifiers for linkage with the National Death Index through 2014. Survival was compared to that in the general population using standardized mortality ratios (SMRs).
Results
After a median follow-up of 18 years (645,806 person-years), 3,191 deaths occurred with an overall SMR of 8.3 (95% CI: 8.0-8.7). The 15-year SMR decreased from 12.7 (95% CI: 11.9-13.6) in the early era (1982-1992) to 10.0 (95% CI: 9.3-10.8) in the late era (1998-2003). The SMR remained elevated even for mild forms of CHD such as patent ductus arteriosus (4.5) and atrial septal defects (4.9). The largest decreases in SMR occurred for patients with transposition of great arteries (early: 11.0 vs late: 3.8, p<0.05), complete atrioventricular canal (31.3 vs 15.3, p<0.05), and single ventricle (53.7; vs 31.3, p<0.05).
Conclusions
In this large U.S. cohort, long-term mortality after congenital heart surgery was elevated across all forms of CHD. Survival has improved over time, particularly for severe defects with significant changes in their management strategy, but still lags the general population.
Keywords: congenital heart defects, outcomes research, mortality, children’s health
Introduction
Congenital heart defects (CHD) affect nearly 1% of children born in the United States (1) who, without interventions, experience significant morbidity and mortality as described by the first and second natural history studies (2,3). Surgery for CHD dramatically changed the outcomes for patients with even complex defects, and surgical mortality has significantly decreased over time (4–8). As a result, there is a continuously growing population of CHD survivors whose long-term health must be monitored (9–12). However, robust long-term outcomes after congenital heart surgery (CHS) in the United States are lacking (13).
Most data on long-term outcomes after CHS have been reported from countries with national health systems (14–19). Other sources include data from individual states (20), reports on single lesions and operative strategies collected over a short time span as part of a major center’s experience (9,21–36), or population-based data lacking surgery-related information (37–39). Although valuable, these data do not adequately capture the experience across the United States for all CHD, and, often, do not possess sufficient patient numbers or follow-up time for meaningful long-term outcome evaluation. In the absence of longitudinal registries for CHD in the United States, assessment of long-term survival in this population remains incomplete. To fill this gap, the National Center on Birth Defects at the Centers for Disease Control and Prevention (CDC) outlined a public health research agenda for CHD that includes database linkage (40,41).
In this study, we used the Pediatric Cardiac Care Consortium (PCCC), a large U.S.-based registry of interventions for CHD that has collected patient-level data since 1982 (42,43). Recently, we linked the PCCC to the National Death Index (NDI) creating a cohort describing the long-term mortality of patients with repaired CHD more thoroughly than prior studies (44). Using this linkage, we now report on the mortality in a large cohort of patients who survived their first operation for CHD (1982-2003) with NDI follow-up through 2014.
Methods
This is a retrospective cohort study with prospectively collected data through linkage with the NDI. The study was approved by the Institutional Review Boards of the University of Minnesota and Emory University, the NDI, and the state birth registries of Minnesota, Arkansas, Ohio, South Carolina, and Missouri for patients enrolled in the PCCC up to April 15, 2003, the date stricter Health Insurance Portability and Accountability Act rules took effect.
PCCC Registry
We queried the PCCC registry for patients who were US residents and underwent congenital heart surgery (CHS) in a US center between January 1, 1982 and April 15, 2003. All patients less than 21 years of age were included except isolated ductal ligation in preterm infants weighing less than 2.5 kg because of the significant morbidity associated with prematurity rather than the CHD itself. To avoid introducing immortal person-time bias, we included only patients with their first cardiac surgery in the PCCC (45). Details regarding the PCCC are available in the Online Appendix (42).
Data Collection
Variables for analysis included sex, age at first surgery (0-27 days, 28-365 days, 1-5 years, and 6-20 years), era of first surgery, type of surgery, CHD diagnosis, and presence of chromosomal anomalies. Surgical era was classified into approximate tertiles as early (1982- 1992), middle (1993-1997), or late (1998-2003). Information about ancestry was obtained from the PCCC forms when available or by linkage to state birth records when possible. Race and ethnicity were considered separately for consistency with data from the general US population. The subset with ancestry information was classified as Black, White, and other race, and as Hispanic or non-Hispanic ethnicity.
Cardiac diagnosis and classification of defects
Each patient was assigned one primary diagnosis using a severity-based list of CHD and the operative strategy for the first CHS (Online Table 1) (14,46). To compare the impact of the underlying condition we grouped patients as mild, moderate, or severe according to the classification introduced in the Canadian Conference on the care of adults with CHD in 1996 (12); this classification scheme is suitable for studies on long-term outcomes and has been adapted by other reports on the same topic (47). The severe category was subdivided into single- and two-ventricular lesions. Further subgroupings were used for lesions sharing common anatomo-pathophysiologic characteristics (for example left-to-right shunt), whenever possible. If more than one CHD is present, patients are classified by the hierarchically most severe diagnosis, except when lesions with different pathophysiology coexist, in which case the lesion is listed separately within the complex group to distinguish them from the plain forms of the primary CHD lesion. Details about this classification of CHD are available in Online Tables 2 and 3.
Death ascertainment
Death was ascertained from the PCCC and by matching to NDI records through December 31, 2014. Records submitted to the NDI included first name, middle initial (when available), surname, date of birth, sex, state of last known residence, and state of birth (imputed from state of residence for those <1 year of age at first surgery). Patients without these identifiers could not be linked and were excluded from the analysis. The matching process and our custom algorithm for assigning death events have been previously described and are summarized in the Online Appendix (44).
U.S. Mortality Data
Mortality data for the US population were downloaded from the CDC Wonder website and comprise age-, sex-, year-, race-, and ethnicity-specific death rates per 100,000 people (48).
Statistical Analysis
For the primary analysis, we computed standardized mortality ratios (SMRs) by dividing the observed by the expected number of deaths based on the US mortality rates matched to the PCCC cohort on age, sex, and calendar year (Online Methods).
A one-sample log-rank test was used to compare survival in the PCCC cohort to the US population (49). Confidence intervals (CI) (95%) and P values are reported for the overall comparison and in subgroups of interest. Comparisons among SMRs were considered significant when there was no overlap between CIs. For other comparisons, two-sided P values <0.05 were considered statistically significant.
Kaplan-Meier survival curves were generated for each comparison, as well as for the US population (49). Survival time was calculated from the date of first CHS in PCCC to date of death or the end of 2014 for patients who were not identified as deceased in either the PCCC or NDI. We used a multivariable Cox model, to compare survival between eras adjusting for sex, age, disease severity, and presence of chromosomal abnormalities. The proportional hazards assumption was assessed graphically and, when violated, addressed by including the interaction between the natural log of time and era in the model. Hazard ratios (HR) were estimated at 1, 5, 10, and 15 years post-surgery. Similar models were used to compare survival between males and females and between blacks and whites.
A sensitivity analysis was performed excluding patients with chromosomal defects from the analysis. To address the possibility of differential short-term survival of patients with significant residual morbidity due to more aggressive support of the sickest patients in more recent era, we also calculated estimates excluding deaths within the first 90 days after surgery or 1 year after surgery (early mortality). We selected the 90-day time point based on the inflection point of the observed mortality after surgery. Secondary analyses matched on race/ethnicity were conducted for the subgroup with ancestry information available. To estimate potential selection bias due to the inability to link patients with inadequate identifiers, available characteristics were compared between those with and without identifiers and then used to calculate inverse probability weights (IPW) of outcome linkage. Application of these weights to the study cohort allowed for approximation of survival estimates had all patients been linked. All analyses were performed in SAS version 9.4 (SAS Institute Inc, NC) and R Studio version 1.0.136.
Results
Study Population
Among the 47,039 patients who met inclusion criteria, 8,524 had inadequate identifiers for linkage with the NDI, and 28 matched to a death record prior to their last admission into PCCC and were excluded (Online Figure 1). Those with inadequate identifiers were more likely to have their first CHS as neonates, die in-hospital after the surgery, and have more severe forms of CHD. They were also more likely to have ancestry information available, reflecting differential information provided by specific centers (Online Table 4).
Of the final sample of 38,487 patients, 2,489 (6.5%) died in hospital after CHS and 35,998 were discharged alive for long-term mortality assessment including patients from 47 states. The median age at first CHS was 0.8 years (IQR: 0.2-3.8). Race information was available for 34.0% of the cohort (6,180 from the PCCC records and 7,911 from birth certificates). Among those with race/ethnicity available, most (81.3%) were White, 16.1% were Black and 2.6% other races. A chromosomal defect occurred in 13.5% of the cohort with a higher percentage in the late era possibly due to increased genetic testing and changing societal and medical attitudes towards offering CHS in this population. Distribution of disease severity was 34.7%, 37.8%, 12.9%, and 6.8% for mild, moderate, severe two-ventricle, and single ventricle (SV) disease, respectively. The most frequent individual CHD diagnoses were atrial septal defects (ASDs) (17.2%) and plain ventricular septal defects (VSDs) (12.6%), while the least common were cor-triatriatum (0.2%) and congenital mitral stenosis (0.2%) (Table 1).
Table 1.
Characteristics of eligible patients in PCCC with adequate identifiers for linkage with NDI
| In-hospital death at 1st surgery | Discharged alive after 1st surgery | Deaths post discharge | ||
|---|---|---|---|---|
|
| ||||
| Demographics | N = 2,489 | N = 35,998 | N = 3,191 | |
| Age at first surgery | 0 - 27 days | 1,804 (72.5) | 6,624 (18.4) | 1,158 |
| 28 - 365 days | 622 (25.0) | 12,674 (35.2) | 1,251 | |
| 1- 4 years | 40 (1.6) | 9,778 (27.2) | 414 | |
| 5 - 20 years | 23 (0.9) | 6,922 (19.2) | 368 | |
| Median (IQR) yrs | 0.0 (0.0-0.1) | 0.8 (0.2-3.8) | 0.2 (0.0-0.9) | |
| Sex | Male | 1,353 (54.4) | 18,713 (52.0) | 1,826 |
| Female | 1,136 (45.6) | 17,285 (48.0) | 1,365 | |
| Race | White | 1,465 (80.5) | 9,952 (81.3) | 1,168 |
| Black | 307 (16.9) | 1,967 (16.1) | 295 | |
| Other | 48 (2.6) | 317 (2.6) | 35 | |
| Missing | 669 | 23,762 | 1,693 | |
| Ethnicity | Not Hispanic | 1,739 (93.9) | 13,860 (93.2) | 1,575 |
| Hispanic | 113 (6.1) | 1,013 (6.8) | 99 | |
| Missing | 637 | 21,125 | 1,517 | |
|
Type of Defect | ||||
| Two ventricle lesions | ||||
| Left-to-right shunt | Total | 270 (10.9) | 15,906 (44.2) | 827 |
| PDA | 20 (0.8) | 2,976 (8.3) | 98 | |
| ASD | 27 (1.1) | 6,198 (17.2) | 184 | |
| VSD (simple) | 64 (2.6) | 4,522 (12.6) | 204 | |
| CAVC (simple) | 159 (6.4) | 2,210 (6.1) | 341 | |
| RVOTO | Total | 207 (8.3) | 3,927 (10.9) | 298 |
| PS/Sub-PS | 22 (0.9) | 724 (2.0) | 34 | |
| PA/IVS | 66 (2.7) | 199 (0.6) | 23 | |
| TOF | 119 (4.8) | 3,004 (8.3) | 241 | |
| LHOL | Total | 291 (11.7) | 5,642 (15.7) | 375 |
| Cor-Tri | 6 (0.2) | 77 (0.2) | 5 | |
| MS | 9 (0.4) | 70 (0.2) | 14 | |
| AS/Sub-AS | 59 (2.4) | 1,284 (3.6) | 92 | |
| CoA | 124 (5.0) | 3,899 (10.8) | 215 | |
| IAA | 93 (3.7) | 312 (0.9) | 49 | |
| APVR | Total | 118 (4.7) | 1,461 (4.1) | 69 |
| TAPVR | 114 (4.6) | 684 (1.9) | 51 | |
| PAPVR | 4 (0.2) | 777 (2.2) | 18 | |
| TGA physiology | d-TGA (simple) | 192 (7.7) | 1,554 (4.3) | 113 |
| Complete mixing | TAC | 129 (5.2) | 268 (0.7) | 59 |
| Complex Lesions | Total | 220 (8.8) | 2,792 (7.8) | 423 |
| Complex CAVC | 29 (1.2) | 60 (0.2) | 31 | |
| Complex d-TGA | 57 (2.3) | 232 (0.6) | 75 | |
| Complex VSD | 32 (1.3) | 1,729 (4.8) | 90 | |
| Complex TOF | 102 (4.1) | 771 (2.1) | 227 | |
| Miscellaneous physiology | Total | 73 (2.9) | 2,003 (5.6) | 189 |
| l-TGA (2V) | 18 (0.7) | 200 (0.6) | 48 | |
| MR/AI | 6 (0.2) | 407 (1.1) | 44 | |
| TVA | 13 (0.5) | 160 (0.4) | 28 | |
| Other | 36 (1.5) | 1,236 (3.4) | 69 | |
| SV | Total | 989 (39.7) | 2,445 (6.8) | 838 |
| RV | 668 (26.8) | 833 (2.3) | 370 | |
| LV | 195 (7.8) | 1,027 (2.9) | 265 | |
| Other | 126 (5.1) | 585 (1.6) | 203 | |
| Severity | Mild | 97 (3.9) | 12,497 (34.7) | 429 |
| Moderate | 430 (17.3) | 13,590 (37.8) | 957 | |
| Severe 2V | 752 (30.2) | 4,658 (12.9) | 694 | |
| Not classifiable | 221 (8.9) | 2,808 (7.8) | 273 | |
| Critical | No | 464 (18.6) | 22,435 (62.3) | 1,249 |
| Yes | 2,025 (81.4) | 13,563 (37.7) | 1,942 | |
| Chromosomal abnormality | No | 2,177 (87.5) | 31,132 (86.5) | 2,527 |
| Yes | 312 (12.5) | 4,866 (13.5) | 664 | |
Abbreviations: 2V, two ventricle APVR, abnormal pulmonary venous return; AS/Sub-AS, aortic and sub-aortic stenosis; ASD, atrial septal defect; CAVC, common atrio-ventricular canal; CoA, coarctation of the aorta; Cor-Tri, cor-triatriatum; d-TGA, dextro-transposition of the great arteries; IAA, interrupted aortic arch; l-TGA, levo-transposition of the great arteries; LHOL, left heart obstructive lesions; LV, left ventricle; MR/AI, mitral regurgitation or aortic insufficiency; MS, mitral stenosis; PA/IVS, pulmonary atresia with intact ventricular septum; PAPVR, partial anomalous pulmonary venous return; PDA, patent ductus arteriosus; PS/Sub PS, pulmonary and sub-pulmonary stenosis; RV, right ventricle; RVOTO, right ventricular outflow tract obstruction; SV, single ventricle; TAC, truncus arteriosus communis; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of Fallot; TVA, tricuspid valve anomalies; VSD, ventricular septal defect
Survival Analysis
Over 645,806 person-years of observation, 3,191 deaths occurred following discharge after the first CHS. Almost half of the deaths (47.2%) occurred within the first year after surgery (median time of death: 1.2 years after surgery; IQR: 0.3-7.9). The median age at the end of follow-up for those last indicated alive was 21.2 years (IQR: 16.9-26.4) (Online Figure 2). Survival at 1, 10, 15, 20, and 25 years post-surgery is presented by era for the overall cohort and individual lesions in Tables 2 and 3.
Table 2.
Comparison of survival estimates across eras by CHD patient characteristics
| % Surviving hospital discharge | % Survival after initial surgery | Total % survival after initial surgery | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Early | Middle | Late | Early | Middle | Late | |||
| Overall*† | 91.9 | 93.5 | 94.9 | 1-year | 94.6 | 96.0 | 96.6 | 89.6 |
| 5-year | 92.0 | 94.2 | 94.9 | 87.8 | ||||
| 10-year | 91.0 | 93.4 | 94.2 | 87.0 | ||||
| 15-year | 90.1 | 92.7 | 93.3 | 86.2 | ||||
| 20-year | 88.7 | 91.8 | – | 85.1 | ||||
| 25-year | 87.5 | – | – | 84.0 | ||||
| 0 - 27 days*† | 71.6 | 77.6 | 83.7 | 1-year | 85.7 | 90.5 | 91.9 | 70.7 |
| 5-year | 78.7 | 85.8 | 88.4 | 67.0 | ||||
| 10-year | 77.0 | 84.9 | 87.9 | 66.3 | ||||
| 15-year | 76.2 | 83.9 | 86.9 | 65.6 | ||||
| 20-year | 74.9 | 83.3 | – | 64.8 | ||||
| 25-year | 73.4 | – | – | 63.5 | ||||
| 28 – 365 days*† | 92.7 | 95.5 | 96.9 | 1-year | 92.5 | 94.6 | 96.3 | 90.3 |
| 5-year | 89.5 | 92.6 | 94.6 | 88.3 | ||||
| 10-year | 88.4 | 91.8 | 93.7 | 87.4 | ||||
| 15-year | 87.9 | 91.2 | 93.0 | 86.8 | ||||
| 20-year | 86.6 | 90.6 | – | 85.8 | ||||
| 25-year | 85.2 | – | – | 84.4 | ||||
| 1 - 4 years*† | 99.2 | 99.7 | 99.9 | 1-year | 98.0 | 99.1 | 99.1 | 98.3 |
| 5-year | 96.9 | 98.4 | 98.5 | 97.5 | ||||
| 10-year | 96.4 | 97.9 | 98.2 | 97.1 | ||||
| 15-year | 95.5 | 97.5 | 97.6 | 96.5 | ||||
| 20-year | 94.1 | 96.4 | – | 95.3 | ||||
| 25-year | 93.2 | – | – | 94.3 | ||||
| 5 - 20 years* | 99.4 | 99.7 | 99.9 | 1-year | 99.2 | 99.3 | 99.3 | 98.9 |
| 5-year | 98.2 | 98.7 | 98.7 | 98.2 | ||||
| 10-year | 97.0 | 97.8 | 97.4 | 97.1 | ||||
| 15-year | 95.3 | 96.5 | 96.0 | 95.6 | ||||
| 20-year | 93.8 | 95.3 | – | 94.3 | ||||
| 25-year | 92.7 | – | – | 93.1 | ||||
| Sex | ||||||||
| Male*† | 91.6 | 93.1 | 94.7 | 1-year | 94.5 | 95.5 | 96.4 | 89.1 |
| 5-year | 91.8 | 93.5 | 94.6 | 87.2 | ||||
| 10-year | 90.7 | 92.8 | 93.9 | 86.4 | ||||
| 15-year | 89.5 | 91.9 | 92.9 | 85.4 | ||||
| 20-year | 87.8 | 90.8 | – | 84.1 | ||||
| 25-year | 86.2 | – | – | 82.5 | ||||
| Female*† | 92.2 | 93.9 | 95.1 | 1-year | 94.8 | 96.6 | 96.7 | 90.2 |
| 5-year | 92.3 | 94.9 | 95.2 | 88.5 | ||||
| 10-year | 91.4 | 94.1 | 94.5 | 87.7 | ||||
| 15-year | 90.7 | 93.6 | 93.8 | 87.1 | ||||
| 20-year | 89.7 | 92.9 | – | 86.3 | ||||
| 25-year | 88.9 | – | – | 85.6 | ||||
| Severity | ||||||||
| Mild* | 98.6 | 99.5 | 99.5 | 1-year | 98.9 | 99.1 | 98.9 | 98.2 |
| 5-year | 98.1 | 98.5 | 98.2 | 97.5 | ||||
| 10-year | 97.6 | 97.8 | 97.8 | 97.0 | ||||
| 15-year | 97.0 | 97.4 | 97.3 | 96.5 | ||||
| 20-year | 96.1 | 96.7 | – | 95.7 | ||||
| 25-year | 95.3 | – | – | 94.9 | ||||
| Moderate*† | 95.8 | 96.9 | 97.8 | 1-year | 95.1 | 96.7 | 97.8 | 93.7 |
| 5-year | 93.5 | 95.4 | 96.6 | 92.4 | ||||
| 10-year | 92.7 | 94.8 | 95.9 | 91.7 | ||||
| 15-year | 92.0 | 94.3 | 95.3 | 91.1 | ||||
| 20-year | 90.8 | 93.3 | – | 90.1 | ||||
| 25-year | 89.4 | – | – | 88.7 | ||||
| Severe 2V*† | 82.7 | 85.3 | 89.4 | 1-year | 89.1 | 91.5 | 93.1 | 78.7 |
| 5-year | 84.1 | 88.9 | 91.0 | 76.1 | ||||
| 10-year | 82.7 | 88.0 | 90.2 | 75.2 | ||||
| 15-year | 81.7 | 86.9 | 89.3 | 74.4 | ||||
| 20-year | 80.0 | 86.3 | – | 73.3 | ||||
| 25-year | 78.4 | – | – | 71.8 | ||||
| SV*† | 66.3 | 69.4 | 76.0 | 1-year | 76.6 | 82.5 | 85.6 | 58.7 |
| 5-year | 61.6 | 72.0 | 78.1 | 51.3 | ||||
| 10-year | 57.2 | 70.3 | 76.8 | 49.7 | ||||
| 15-year | 54.4 | 68.6 | 74.9 | 48.2 | ||||
| 20-year | 51.2 | 66.9 | – | 46.3 | ||||
| 25-year | 48.9 | – | – | 44.5 | ||||
| Chromosomal abnormality | ||||||||
| No*† | 91.7 | 93.5 | 94.9 | 1-year | 95.1 | 96.3 | 97.0 | 89.9 |
| 5-year | 92.7 | 94.6 | 95.6 | 88.2 | ||||
| 10-year | 91.8 | 93.9 | 95.0 | 87.5 | ||||
| 15-year | 90.9 | 93.2 | 94.2 | 86.8 | ||||
| 20-year | 89.6 | 92.4 | – | 85.8 | ||||
| 25-year | 88.5 | – | – | 84.8 | ||||
| Yes† | 93.1 | 93.7 | 94.8 | 1-year | 91.5 | 94.5 | 93.7 | 87.8 |
| 5-year | 87.8 | 91.7 | 90.9 | 84.9 | ||||
| 10-year | 85.9 | 90.6 | 89.4 | 83.5 | ||||
| 15-year | 84.6 | 89.7 | 88.3 | 82.5 | ||||
| 20-year | 82.5 | 88.1 | – | 80.8 | ||||
| 25-year | 80.4 | – | – | 78.8 | ||||
Abbreviations: 2V, two ventricle; SV, single ventricle
Chi-squared p-value < 0.05 comparing in-hospital death between eras
Log-rank p-value < 0.05 comparing survival after hospital discharge between era
Table 3.
Comparison of survival estimates across eras by CHD patient characteristics
| % Surviving hospital discharge | % Survival after initial surgery | Total % survival after initial surgery | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Early | Middle | Late | Early | Middle | Late | % | ||
| Left-to-Right shunt*† | 97.6 | 98.7 | 98.6 | 1-year | 96.9 | 98.1 | 98.2 | 96.2 |
| 5-year | 95.7 | 97.3 | 97.2 | 95.2 | ||||
| 10-year | 95.0 | 96.6 | 96.7 | 94.6 | ||||
| 15-year | 94.4 | 96.2 | 96.0 | 94.0 | ||||
| 20-year | 93.2 | 95.2 | – | 93.1 | ||||
| 25-year | 92.3 | – | – | 92.1 | ||||
| PDA* | 98.7 | 99.9 | 99.6 | 1-year | 99.1 | 99.4 | 98.6 | 98.4 |
| 5-year | 98.7 | 98.5 | 98.0 | 97.8 | ||||
| 10-year | 97.9 | 97.7 | 98.0 | 97.2 | ||||
| 15-year | 97.6 | 97.3 | 97.8 | 96.9 | ||||
| 20-year | 96.4 | 96.9 | – | 96.0 | ||||
| 25-year | 95.9 | – | – | 95.5 | ||||
| ASD | 99.3 | 99.7 | 99.6 | 1-year | 99.2 | 99.2 | 99.3 | 98.8 |
| 5-year | 98.8 | 99.0 | 98.9 | 98.5 | ||||
| 10-year | 98.4 | 98.4 | 98.3 | 97.9 | ||||
| 15-year | 97.7 | 98.1 | 97.5 | 97.4 | ||||
| 20-year | 96.9 | 97.0 | – | 96.5 | ||||
| 25-year | 95.9 | – | – | 95.5 | ||||
| VSD (simple)* | 97.4 | 98.8 | 99.2 | 1-year | 97.8 | 98.2 | 98.4 | 96.8 |
| 5-year | 96.1 | 97.4 | 97.3 | 95.7 | ||||
| 10-year | 95.5 | 96.9 | 97.0 | 95.3 | ||||
| 15-year | 95.0 | 96.4 | 96.6 | 94.7 | ||||
| 20-year | 94.1 | 95.7 | – | 94.1 | ||||
| 25-year | 93.1 | – | – | 93.1 | ||||
| CAVC (simple)† | 91.6 | 93.2 | 94.6 | 1-year | 85.2 | 92.3 | 94.8 | 85.2 |
| 5-year | 80.8 | 89.5 | 92.2 | 82.2 | ||||
| 10-year | 79.4 | 88.5 | 91.1 | 81.1 | ||||
| 15-year | 78.3 | 87.9 | 90.2 | 80.3 | ||||
| 20-year | 75.9 | 86 | – | 78.4 | ||||
| 25-year | 74.4 | – | – | 76.7 | ||||
| RVOTO*† | 92.2 | 95.0 | 97.5 | 1-year | 94.9 | 96.5 | 97.5 | 91.5 |
| 5-year | 93.1 | 94.7 | 95.9 | 89.9 | ||||
| 10-year | 92.4 | 94.3 | 95.2 | 89.3 | ||||
| 15-year | 91.7 | 93.8 | 94.4 | 88.7 | ||||
| 20-year | 90.5 | 93.3 | – | 87.8 | ||||
| 25-year | 89.2 | – | – | 86.6 | ||||
| PS/Sub-PS | 96.6 | 96.7 | 98.1 | 1-year | 97.7 | 98.1 | 99.5 | 95.4 |
| 5-year | 97.3 | 97.4 | 98.5 | 94.8 | ||||
| 10-year | 96.9 | 97.0 | 98.0 | 94.4 | ||||
| 15-year | 96.1 | 96.6 | 97.5 | 93.8 | ||||
| 20-year | 95.3 | 96.2 | – | 93.2 | ||||
| 25-year | 93.4 | – | – | 91.4 | ||||
| PA/IVS*† | 63.8 | 80.7 | 85.1 | 1-year | 83.6 | 94.7 | 98.3 | 69.1 |
| 5-year | 80.6 | 92.0 | 98.3 | 67.6 | ||||
| 10-year | 79.1 | 92.0 | 98.3 | 67.2 | ||||
| 15-year | 79.1 | 90.7 | 98.3 | 66.8 | ||||
| 20-year | 77.6 | 90.7 | – | 66.2 | ||||
| 25-year | 77.6 | – | – | 66.2 | ||||
| TOF* | 94.1 | 95.9 | 98.0 | 1-year | 94.9 | 96.2 | 97.1 | 92.5 |
| 5-year | 92.9 | 94.2 | 95.3 | 90.7 | ||||
| 10-year | 92.2 | 93.7 | 94.5 | 90.0 | ||||
| 15-year | 91.4 | 93.2 | 93.7 | 89.3 | ||||
| 20-year | 90.1 | 92.7 | – | 88.3 | ||||
| 25-year | 88.9 | – | – | 87.2 | ||||
| LHOL*† | 93.2 | 94.6 | 97.1 | 1-year | 96.0 | 96.9 | 97.6 | 92.2 |
| 5-year | 94.5 | 96.0 | 96.7 | 91.1 | ||||
| 10-year | 93.9 | 95.2 | 96.1 | 90.5 | ||||
| 15-year | 92.6 | 94.4 | 95.3 | 89.6 | ||||
| 20-year | 91.7 | 93.8 | – | 88.8 | ||||
| 25-year | 90.2 | – | – | 87.5 | ||||
| Cor-Tri | 84.0 | 96.9 | 96.2 | 1-year | 90.5 | 96.8 | 96.0 | 88.0 |
| 5-year | 90.5 | 96.8 | 96.0 | 88.0 | ||||
| 10-year | 90.5 | 96.8 | 96.0 | 88.0 | ||||
| 15-year | 85.7 | 96.8 | 96.0 | 86.6 | ||||
| 20-year | 85.7 | 96.8 | – | 86.6 | ||||
| 25-year | 85.7 | – | – | 86.6 | ||||
| MS | 77.8 | 86.7 | 96.8 | 1-year | 100 | 96.2 | 93.3 | 86.1 |
| 5-year | 100 | 96.2 | 86.7 | 82.3 | ||||
| 10-year | 100 | 92.3 | 80.0 | 78.5 | ||||
| 15-year | 100 | 88.5 | 76.2 | 75.9 | ||||
| 20-year | 92.9 | 84.6 | – | 71.8 | ||||
| 25-year | 74.3 | – | – | 57.4 | ||||
| AS/Sub-AS* | 91.8 | 96.4 | 98.4 | 1-year | 98.3 | 98.0 | 98.1 | 93.8 |
| 5-year | 96.5 | 96.7 | 97.9 | 92.9 | ||||
| 10-year | 95.5 | 96.0 | 96.5 | 91.8 | ||||
| 15-year | 92.8 | 94.0 | 94.8 | 89.8 | ||||
| 20-year | 91.1 | 92.5 | – | 88.3 | ||||
| 25-year | 90.3 | – | – | 87.6 | ||||
| CoA† | 96.6 | 96.2 | 97.7 | 1-year | 96.0 | 97.0 | 97.9 | 94.1 |
| 5-year | 94.8 | 96.3 | 97.0 | 93.2 | ||||
| 10-year | 94.3 | 95.7 | 96.8 | 92.8 | ||||
| 15-year | 93.7 | 95.4 | 96.3 | 92.3 | ||||
| 20-year | 93.1 | 95.1 | – | 91.8 | ||||
| 25-year | 91.5 | – | – | 90.4 | ||||
| IAA* | 70.9 | 74.0 | 85.4 | 1-year | 86.3 | 91.5 | 93.5 | 69.9 |
| 5-year | 81.1 | 88.3 | 91.1 | 67.2 | ||||
| 10-year | 81.1 | 86.2 | 91.1 | 66.7 | ||||
| 15-year | 77.9 | 84.0 | 90.1 | 65.0 | ||||
| 20-year | 77.9 | 84.0 | – | 65.0 | ||||
| 25-year | 76.6 | – | – | 63.9 | ||||
| APVR* | 88.6 | 91.9 | 96.7 | 1-year | 96.8 | 96.6 | 97.9 | 89.9 |
| 5-year | 96.5 | 96.0 | 97.2 | 89.4 | ||||
| 10-year | 96.0 | 96.0 | 96.8 | 89.1 | ||||
| 15-year | 95.7 | 95.6 | 96.5 | 88.8 | ||||
| 20-year | 94.6 | 95.1 | – | 88.1 | ||||
| 25-year | 93.7 | – | – | 87.2 | ||||
| TAPVR* | 81.8 | 85.1 | 90.0 | 1-year | 95.3 | 93.5 | 96.7 | 81.6 |
| 5-year | 94.8 | 92.6 | 95.1 | 80.7 | ||||
| 10-year | 93.8 | 92.6 | 94.7 | 80.3 | ||||
| 15-year | 93.8 | 92.6 | 94.2 | 80.2 | ||||
| 20-year | 92.4 | 92.1 | – | 79.3 | ||||
| 25-year | 91.9 | – | – | 78.9 | ||||
| PAPVR | 99.4 | 99.2 | 99.7 | 1-year | 98.8 | 99.6 | 98.7 | 98.5 |
| 5-year | 98.8 | 99.2 | 98.7 | 98.3 | ||||
| 10-year | 98.8 | 99.2 | 98.1 | 98.1 | ||||
| 15-year | 98.1 | 98.4 | 98.1 | 97.7 | ||||
| 20-year | 97.5 | 97.9 | – | 97.1 | ||||
| 25-year | 96.0 | – | – | 95.6 | ||||
| Transposition physiology | ||||||||
| d-TGA (simple)† | 87.9 | 87.5 | 91.1 | 1-year | 91.8 | 97.0 | 98.4 | 85.5 |
| 5-year | 89.5 | 96.6 | 97.6 | 84.5 | ||||
| 10-year | 89.5 | 96.4 | 97.1 | 84.3 | ||||
| 15-year | 88.6 | 95.4 | 96.1 | 83.5 | ||||
| 20-year | 87.0 | 95.2 | – | 82.6 | ||||
| 25-year | 85.3 | – | – | 80.8 | ||||
| Complete mixing | ||||||||
| TAC*† | 54.6 | 69.4 | 75.3 | 1-year | 76.9 | 86.7 | 89.1 | 57.7 |
| 5-year | 73.8 | 84.0 | 87.5 | 56.2 | ||||
| 10-year | 70.8 | 81.3 | 85.2 | 54.4 | ||||
| 15-year | 67.7 | 81.3 | 83.6 | 53.3 | ||||
| 20-year | 66.2 | 80.0 | – | 52.0 | ||||
| 25-year | 66.2 | – | – | 51.1 | ||||
| Complex lesions | ||||||||
| Complex CAVC† | 74.1 | 68.8 | 60.0 | 1-year | 90.0 | 54.6 | 77.8 | 49.4 |
| 5-year | 75.0 | 36.4 | 72.2 | 40.5 | ||||
| 10-year | 60.0 | 36.4 | 72.2 | 37.1 | ||||
| 15-year | 60.0 | 36.4 | 72.2 | 37.1 | ||||
| 20-year | 50.0 | 36.4 | – | 33.4 | ||||
| 25-year | 45.0 | – | – | 30.1 | ||||
| Complex d-TGA*† | 69.5 | 85.3 | 84.1 | 1-year | 64.9 | 82.8 | 85.6 | 64.0 |
| 5-year | 56.1 | 78.1 | 82.0 | 59.9 | ||||
| 10-year | 54.4 | 73.4 | 80.2 | 57.8 | ||||
| 15-year | 54.4 | 67.2 | 78.4 | 55.5 | ||||
| 20-year | 52.6 | 62.2 | – | 52.7 | ||||
| 25-year | 49.3 | – | – | 49.4 | ||||
| Complex VSD | 97.3 | 98.3 | 98.7 | 1-year | 97.3 | 97.4 | 99.0 | 96.4 |
| 5-year | 96.6 | 97.4 | 98.4 | 95.8 | ||||
| 10-year | 95.8 | 96.5 | 97.4 | 94.9 | ||||
| 15-year | 95.3 | 95.6 | 96.6 | 94.2 | ||||
| 20-year | 94.5 | 94.6 | – | 93.0 | ||||
| 25-year | 94.1 | – | – | 92.4 | ||||
| Complex TOF | 88.1 | 85.8 | 90.5 | 1-year | 88.3 | 84.7 | 83.8 | 75.4 |
| 5-year | 73.4 | 77.3 | 79.7 | 68.2 | ||||
| 10-year | 69.6 | 75.2 | 78.1 | 66.1 | ||||
| 15-year | 67.8 | 73.1 | 76.9 | 64.7 | ||||
| 20-year | 65.4 | 71.9 | – | 62.8 | ||||
| 25-year | 62.8 | – | – | 60.2 | ||||
| Miscellaneous | ||||||||
| l-TGA (2V) | 91.4 | 88.6 | 95.1 | 1-year | 88.7 | 92.9 | 92.2 | 83.9 |
| 5-year | 81.1 | 88.6 | 81.8 | 77.1 | ||||
| 10-year | 79.2 | 87.1 | 80.5 | 75.7 | ||||
| 15-year | 75.5 | 87.1 | 76.8 | 73.6 | ||||
| 20-year | 64.2 | 85.7 | – | 68.7 | ||||
| 25-year | 64.2 | – | – | 68.7 | ||||
| MR/AI | 99.0 | 99.2 | 97.8 | 1-year | 98.0 | 96.9 | 99.4 | 96.9 |
| 5-year | 94.0 | 93.9 | 98.3 | 94.4 | ||||
| 10-year | 91.0 | 93.1 | 94.9 | 92.0 | ||||
| 15-year | 89.0 | 91.5 | 93.2 | 90.1 | ||||
| 20-year | 86.0 | 90.6 | – | 86.7 | ||||
| 25-year | 83.5 | – | – | 84.2 | ||||
| TVA | 90.7 | 96.4 | 90.7 | 1-year | 89.7 | 94.3 | 95.6 | 84.3 |
| 5-year | 87.2 | 88.7 | 91.2 | 82.7 | ||||
| 10-year | 82.1 | 86.8 | 89.7 | 80.4 | ||||
| 15-year | 76.9 | 84.9 | 89.7 | 78.3 | ||||
| 20-year | 71.8 | 83.0 | – | 75.4 | ||||
| 25-year | 68.4 | – | – | 71.8 | ||||
| Other* | 94.3 | 97.8 | 98.4 | 1-year | 98.0 | 97.2 | 98.5 | 95.2 |
| 5-year | 96.6 | 96.2 | 97.6 | 94.2 | ||||
| 10-year | 96.3 | 95.0 | 97.0 | 93.5 | ||||
| 15-year | 96.0 | 94.0 | 95.8 | 92.6 | ||||
| 20-year | 94.6 | 92.6 | – | 91.3 | ||||
| 25-year | 94.3 | – | – | 90.7 | ||||
| SV | ||||||||
| RV*† | 47.8 | 52.0 | 62.8 | 1-year | 66.3 | 76.6 | 77.3 | 41.6 |
| 5-year | 49.4 | 64.5 | 67.2 | 34.8 | ||||
| 10-year | 44.2 | 63.0 | 65.4 | 33.4 | ||||
| 15-year | 41.3 | 60.8 | 62.8 | 32.1 | ||||
| 20-year | 37.8 | 58.1 | – | 30.2 | ||||
| 25-year | 35.1 | – | – | 28.3 | ||||
| LV† | 82.5 | 82.2 | 86.8 | 1-year | 82.7 | 86.1 | 91.1 | 73.2 |
| 5-year | 68.9 | 79.6 | 85.7 | 66.5 | ||||
| 10-year | 66.4 | 77.8 | 84.7 | 65.1 | ||||
| 15-year | 63.3 | 77.2 | 84.5 | 64.0 | ||||
| 20-year | 59.7 | 76.3 | – | 61.9 | ||||
| 25-year | 57.7 | – | – | 60.1 | ||||
| Other*† | 71.4 | 85.5 | 88.1 | 1-year | 77.0 | 84.1 | 89.8 | 69.6 |
| 5-year | 61.8 | 68.8 | 83.2 | 60.1 | ||||
| 10-year | 54.6 | 67.2 | 82.0 | 57.7 | ||||
| 15-year | 52.6 | 64.0 | 78.0 | 55.0 | ||||
| 20-year | 50.7 | 62.2 | – | 53.3 | ||||
| 25-year | 48.0 | – | – | 50.8 | ||||
Abbreviations: 2V, two ventricle APVR, abnormal pulmonary venous return; AS/Sub-AS, aortic and sub-aortic stenosis; ASD, atrial septal defect; CAVC, common atrio-ventricular canal; CoA, coarctation of the aorta; Cor-Tri, cor-triatriatum; d-TGA, dextro-transposition of the great arteries; IAA, interrupted aortic arch; l-TGA, levo-transposition of the great arteries; LHOL, left heart obstructive lesions; LV, left ventricle; MR/AI, mitral regurgitation or aortic insufficiency; MS, mitral stenosis; PA/IVS, pulmonary atresia with intact ventricular septum; PAPVR, partial anomalous pulmonary venous return; PDA; PS/Sub PS, pulmonary and sub-pulmonary stenosis; RV, right ventricle; RVOTO, right ventricular outflow tract obstruction; SV, single ventricle; TAC, truncus arteriosus communis; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of Fallot; TVA, tricuspid valve anomalies; VSD, ventricular septal defect
Chi-squared p-value < 0.05 comparing in-hospital death between eras
Log-rank p-value < 0.05 comparing survival after hospital discharge between era
In the PCCC cohort that survived their first CHS mortality was 8.3 times greater than in the age- and sex-standardized US population (95% CI: 8.0-8.7). The increased SMR persisted after exclusion of patients with chromosomal defects (7.5, 95% CI: 7.2-7.8) (Figure 1). Kaplan- Meier survival plots for the overall cohort and by sex, era and disease severity are displayed in Figure 2, while plots by major groups and individual lesions are presented in Online Figure 3. SMRs increased substantially with disease severity, in particular for SV physiology (35.0, 95% CI: 33.0-38.0), but were still significantly elevated for mild forms of CHD (3.6, 95% CI: 3.2-4.0). Within patients with plain forms of two-ventricle physiology, including over 15,000 person- years of follow up, patients with ASD (3.1; 95% CI: 2.6-3.6) and patent ductus arteriosus (PDA) (3.4; 95% CI: 2.7-4.2) had the lowest SMRs, while complete atrioventricular canal (CAVC) had the highest (17.0, 95% CI: 15.0-19.0). Despite the high number of patients with chromosomal anomalies in the CAVC group (77%), the SMR did not meaningfully change after excluding these patients. Tetralogy of Fallot, total anomalous pulmonary venous return, and dextro- transposition of the great arteries (d-TGA), also had SMRs greater than five. Complex forms of disease had variably elevated SMRs compared to the corresponding plain form.
Figure 1. Standardized mortality ratios (SMRs) of patients discharged alive after their initial CHD surgery compared with the age-, sex-, and year-matched US general population.
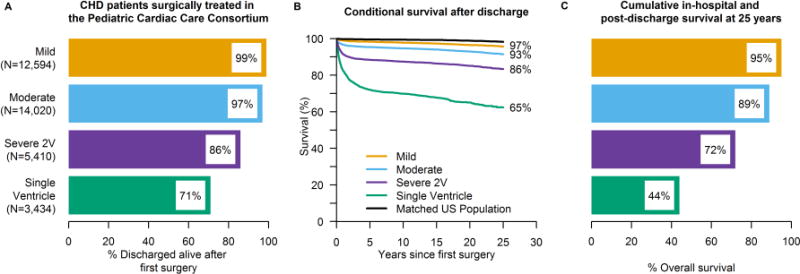
A) SMRs of the cumulative PCCC cohort and separately for the subgroup without chromosomal defects, for males/females and for each severity group. B) SMRs by individual lesions.
Figure 2. Long-term survival of patients after discharge from initial congenital heart surgery.
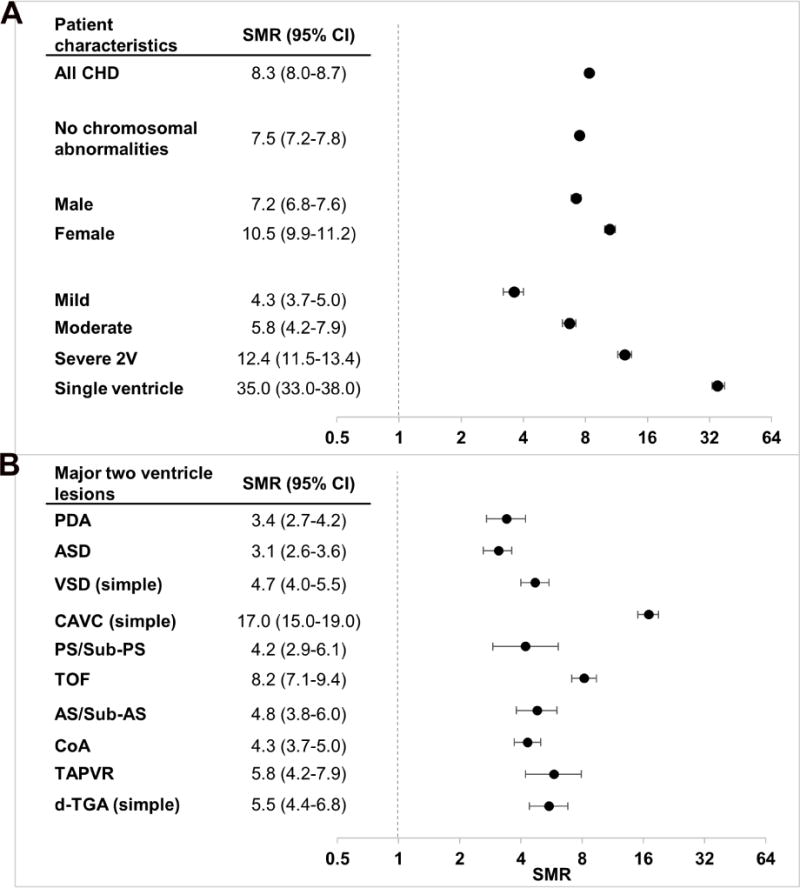
A) with/without a chromosomal (chr) abnormality, B) by sex, C) by era of initial surgery and D) by severity.
Females had a higher SMR (10.5; 95% CI: 9.9-11.2) than males (7.2; 95% CI: 6.8-7.6). However, males had higher absolute and adjusted mortality than females beyond the first year after surgery [15-year post-surgery adjusted HR: 1.3; 95% CI: 1.2-1.4] (Online Table 5). For those with race information, the SMR matched on race in addition to age, sex, and year was slightly higher in whites (11.0; 95% CI: 10.3-11.7) than blacks; (9.1; 95% CI: 8.0-10.3). Nevertheless, blacks had higher absolute 15-year mortality than whites (adjusted HR: 2.1; 95% CI: 1.7-2.5). Survival curves by race are presented in Online Figure 4 and HRs in Online Table 6.
When examining the effect of era, there was a trend towards operations at younger age (Table 1, Online Figure 5) and in-hospital survival increased across almost all forms of CHD in the recent era. Significant improvement in the long-term survival after discharge was observed for most lesions when comparing them for an equal follow up period. Specifically, the 15-year SMR decreased from 12.7 (95% CI: 11.9-13.6) in the early to 10.0 (95% CI: 9.3-10.8) in the more recent era. In the adjusted time-varying model, the rate of death was reduced in the late era for all cardiac defects and at all time points tested [15 years post-surgery adjusted HR = 0.7 (95% CI: 0.6-0.8)] (Online Table 7).
The decrease in 15-year SMR was noted for all lesions that were operated in neonatal age and infancy, and generally persisted after removal of early mortality from the analysis. Comparison of trends in SMRs by era for all subgroups and individual lesions are presented in Online Table 8. The lesions with the most dramatic decrease in SMR were d-TGA (late 3.8 [2.4-6.1] vs early 11.0 [8.0-15.0]), CAVC (late 15.3 [12.0-19.5] vs early 31.3 [25.9-37.9]), and SV (late 31.3 [27.3-36.0] vs early 53.7 [46.9-61.4]).
Discussion
In this study, the largest to date on long-term mortality after CHS, we found that the overall risk of premature death is elevated compared to the general population (Central Illustration). The excess mortality persisted after exclusion of patients with chromosomal abnormalities. We noted elevated SMRs in all CHD types, and, as expected, SMRs were higher in the most severe conditions. Higher SMRs were also observed in patients operated at younger age, reflecting the severity of lesions requiring earlier age of surgery. The higher risk of premature death up to thirty years after surgery was more notable in moderate and severe lesions, but mild lesions were not spared. Nevertheless, for many CHDs, most patients survive into adulthood with an overall 25-year survival of about 90% after CHS.
Central Illustration. Survival after initial operation for a congenital heart defect by severity of the condition.
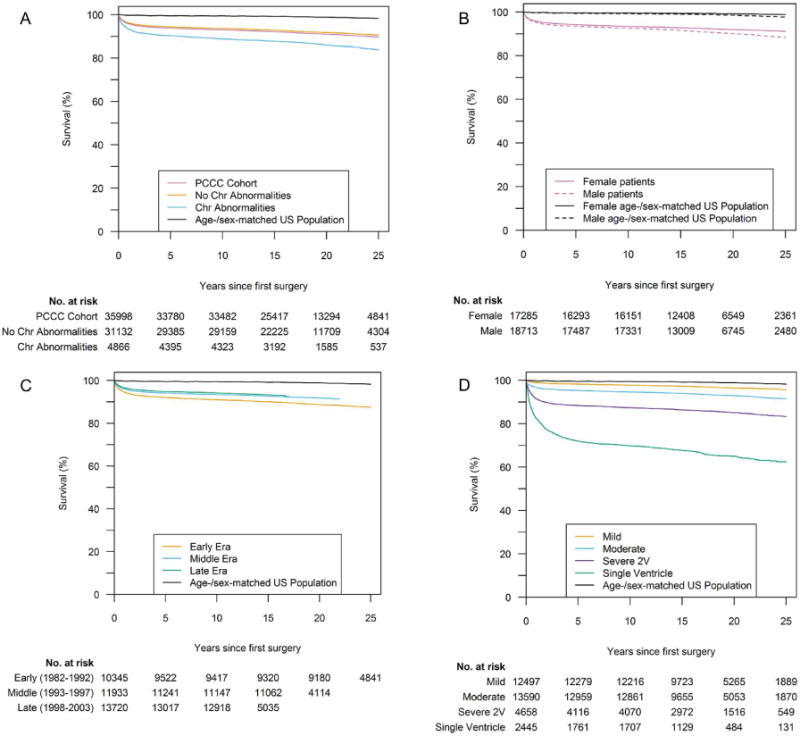
A) Survival to hospital discharge, B) conditional survival post- discharge from initial surgery and C) cumulative long-term survival including in-hospital and post-discharge survival. Patients whose CHD could not be classified into a severity category are not included here (N=3,029).
Survival trends
In the more recent era, we observed a remarkable improvement in surgical mortality and long-term survival for almost all CHD. The lesions that demonstrated the most significant increase in survival were d-TGA, CAVC, and SV with two-ventricle lesions benefiting mostly from reduction in early mortality, while the single ventricle lesions demonstrated more widespread improvement in survival over time. This improvement may reflect the impact of improved diagnostic modalities, surgical techniques, and general supportive measures on the most vulnerable patients. Survival for simple lesions such as PDA, ASDs, VSDs, and pulmonic stenosis has reached a plateau, and even in the most recent era still lags the survival of the general population. Survival for all other lesions continues to show incremental increase.
Disparities in outcomes
We found that in patients with operated CHD, as is true in the general population, males experienced higher mortality than females. However, a history of operated CHD attenuated the survival advantage of female sex as evidenced by the higher SMRs for females when compared to the sex-matched general population. In the subgroup with available ancestry, whites undergoing CHS had higher survival than blacks, but their survival advantage also dissipated when compared to the race-matched general population. This finding contrasts with previous reports, although not directly comparable since these reports did not estimate SMR by race (41,53,54). This paradox does not seem to be due to differences in underlying conditions and may reflect better access to healthcare for blacks who underwent CHS in PCCC than those in the general population. (38,50,51).
Comparison with other studies
Direct comparison to the population-based Finnish, Norwegian, and Danish studies is difficult because of differences in methodology, years covered, and inclusion criteria for each CHD diagnosis, lack of information on chromosomal defects, and varying introduction of diagnostic and therapeutic advancements in each country (14,19,39,52). For example, detailed diagnostic and surgical information in PCCC allow classification of the primary CHD into plain and complex forms, whereas, the European studies categorize these together. Nevertheless, we generally observed similar trends in mortality over an equivalent era and follow up time despite some small differences in individual lesions. For example, each European registry reported higher survival for patients with single ventricle lesions than that in PCCC (Online Tables 9 and 10). This likely reflects the low numbers of patients with hypoplastic left heart syndrome in the European cohorts, particularly in the earlier years of these registries.
Strengths and limitations
Although some European registries have longer follow up than the PCCC registry, these include administrative rather than clinical codes for CHD diagnoses and procedures. As a result, the PCCC is the oldest clinical registry containing outcomes for interventions for CHD. After linkage with the NDI, the dataset has the largest number of patient-years of follow-up reported, which is a major strength of this study. The size and depth of the dataset offers an unprecedented opportunity to compare not only grouped lesions but also individual defects and eras of surgical treatment. In addition, the design of the PCCC allowed us to avoid important limitations of prior studies, such as lack of information on chromosomal abnormalities and less rigorous characterization of the diagnoses and surgical interventions. Additionally, the case-mix and surgical results reported in PCCC have been shown to reflect a representative sample of patients receiving CHS in the US through its comparison to large clinical and administrative datasets for CHD in the US for the years covered by this report (42). About 18% of the PCCC cohort had insufficient data available for linkage to the NDI. These patients were younger and had more severe forms of CHD; it is thus likely that were they able to be included, that our estimates of SMRs would be somewhat higher. The results of the sensitivity analysis using inverse probability weighting were congruent with this hypothesis (Online Table 11).
Linkage to the NDI, considered the “gold standard” for death ascertainment in this country, is estimated to ascertain 83-97% of deaths in the US depending on the quality and number of identifiers used (53,54). For those with the complete set of identifiers we achieved a sensitivity of nearly 90% for deaths known to have occurred in hospital (44). Importantly, we found no difference in ascertainment of in-hospital deaths by lesion, suggesting that comparison of mortality between lesions would be unbiased. However, we cannot be certain that the sensitivity for deaths after discharge is the same, which is a limitation. It is also possible that some deaths were missed because of errors in matching variables or occurrence outside the US, but these are expected to be rare and unlikely to be related to clinical factors.
Despite inclusion of centers enrolling patients from 47 states, the PCCC cohort lacks the demographic diversity of the whole country. Also, because the PCCC did not systematically collect race/ethnicity, ancestry information was only available for a subset of patients. While the availability of race/ethnicity may be related to factors affecting long-term mortality, we found small differences in terms of clinical characteristics between patients with and without such information (44).
Finally, by the nature of this study, the reported survival reflects surgical strategies and management of previous eras, while therapies for CHD continuously evolve. Nevertheless, the available cohort includes well-established management strategies that have undergone minimal or no modifications and their outcome will be applicable for many years and numerous patients that had CHS between 1982 and 2003.
Conclusions
In summary, our data from a large, multicenter US cohort provide physicians, families and patients with operated CHD nuanced information about expected long-term prognosis. The persistently elevated risk of death after CHS emphasizes the need for close clinical monitoring of patients with even the mildest lesions to address residual morbidities (11) and to understand the reasons for increased mortality in these patients who often have no residual hemodynamic abnormality. Since management differs between centers and evolves over time, continued analysis of lesion- and surgical strategy-specific outcomes may reveal differences in mortality that will inform current practice.
Supplementary Material
Condensed abstract.
Long-term survival after congenital heart surgery has not been fully described. This study evaluates this outcome in patients (<21 years of age) operated for congenital heart defects. Compared with the general population, the 15-year SMR decreased from 12.7 (95% CI: 11.9-13.6) in the early era (1982-1992) to 10.0 (95% CI: 9.3-10.8) in the late era (1998-2003). The largest decreases in SMR occurred for patients with transposition of great arteries, complete atrioventricular canal, and single ventricle. These results suggest survival has improved over time, particularly for severe defects with significant changes in their management strategy, but still lags the general population.
Clinical Perspectives.
Competency in Medical Knowledge
Although surgical outcomes for patients with congenital heart defects have improved over the last several decades, long-term survival remains lower than the general population for even those with the mildest forms of this condition. This should be considered when communicating prognosis to families and planning follow-up care.
Translational Outlook 1
Continued monitoring of patients with operated congenital heart defects is necessary to determine their long-term adverse events and comorbidities. In addition, such an approach may identify vulnerable time-periods and high-risk subgroups for which future interventions could be directed in hopes of preventing or minimizing premature mortality.
Acknowledgments
We thank the program directors and data collection coordinators from the participating PCCC centers; without their effort and dedication, this work could not have been completed. Susan Anderson and Brian Harvey were especially instrumental in the management of PCCC and initial linkage of this cohort with mortality information. We also thank Alvin Chin, MD for critically reviewing the manuscript.
Funding: This study was supported by National Heart, Lung, and Blood Institute R01 HL122392 and NIH CTSA Award UL1TR000114.
Abbreviations
- CHD
Congenital heart defects
- PCCC
Pediatric Cardiac Care Consortium
- NDI
National Death Index
- CHS
Congenital Heart Surgery
- CDC
Centers for Disease Control and Prevention
- SMRs
Standardized Mortality Ratios
- CAVC
Complete Atrioventricular Canal
- d-TGA
dextro-Transposition of the Great Arteries
- PDA
Patent Ductus Arteriosus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No relevant disclosures
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Nadas AS. Report from the Joint Study on the Natural History of Congenital Heart Defects. I. General introduction. Circulation. 1977;56:I3–4. 36–8. [PubMed] [Google Scholar]
- 3.O’Fallon WM, Crowson CS, Rings LJ, et al. Second natural history study of congenital heart defects. Materials and methods. Circulation. 1993;87:I4–15. [PubMed] [Google Scholar]
- 4.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in outcomes for risk-stratified pediatric cardiac surgical operations: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94:564–71. doi: 10.1016/j.athoracsur.2012.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in outcomes for benchmark operations: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2011;92:2184–91. doi: 10.1016/j.athoracsur.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs JP, O’Brien SM, Pasquali SK, et al. The importance of patient-specific preoperative factors: an analysis of the society of thoracic surgeons congenital heart surgery database. Ann Thorac Surg. 2014;98:1653–8. doi: 10.1016/j.athoracsur.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinocur JM, Menk JS, Connett J, Moller JH, Kochilas LK. Surgical volume and center effects on early mortality after pediatric cardiac surgery: 25-year North American experience from a multi-institutional registry. Pediatr Cardiol. 2013;34:1226–36. doi: 10.1007/s00246-013-0633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempny A, Dimopoulos K, Uebing A, et al. Outcome of cardiac surgery in patients with congenital heart disease in England between 1997 and 2015. PLoS One. 2017;12:e0178963. doi: 10.1371/journal.pone.0178963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122:2264–72. doi: 10.1161/CIRCULATIONAHA.110.946343. [DOI] [PubMed] [Google Scholar]
- 10.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–72. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 11.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) J Am Coll Cardiol. 2008;52(23):e143–263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Connelly MS, Webb GD, Somerville J, et al. Canadian Consensus Conference on Adult Congenital Heart Disease 1996. Can J Cardiol. 1998;14:395–452. [PubMed] [Google Scholar]
- 13.Gurvitz M, Burns KM, Brindis R, et al. Emerging Research Directions in Adult Congenital Heart Disease: A Report From an NHLBI/ACHA Working Group. J Am Coll Cardiol. 2016;67:1956–64. doi: 10.1016/j.jacc.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erikssen G, Liestol K, Seem E, et al. Achievements in congenital heart defect surgery: a prospective, 40-year study of 7038 patients. Circulation. 2015;131:337–46. doi: 10.1161/CIRCULATIONAHA.114.012033. [DOI] [PubMed] [Google Scholar]
- 15.Nieminen HP, Jokinen EV, Sairanen HI. Late results of pediatric cardiac surgery in Finland: a population-based study with 96% follow-up. Circulation. 2001;104:570–5. doi: 10.1161/hc3101.093968. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population-based study. J Am Coll Cardiol. 2007;50:1263–71. doi: 10.1016/j.jacc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 17.Raissadati A, Nieminen H, Haukka J, Sairanen H, Jokinen E. Late Causes of Death After Pediatric Cardiac Surgery: A 60-Year Population-Based Study. J Am Coll Cardiol. 2016;68:487–98. doi: 10.1016/j.jacc.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Raissadati A, Nieminen H, Jokinen E, Sairanen H. Progress in late results among pediatric cardiac surgery patients: a population-based 6-decade study with 98% follow- up. Circulation. 2015;131:347–53. doi: 10.1161/CIRCULATIONAHA.114.011190. [DOI] [PubMed] [Google Scholar]
- 19.Larsen SH, Olsen M, Emmertsen K, Hjortdal VE. Interventional Treatment of Patients With Congenital Heart Disease: Nationwide Danish Experience Over 39 Years. J Am Coll Cardiol. 2017;69:2725–2732. doi: 10.1016/j.jacc.2017.03.587. [DOI] [PubMed] [Google Scholar]
- 20.Morris CD, Menashe VD. 25-year mortality after surgical repair of congenital heart defect in childhood. A population-based cohort study. JAMA. 1991;266:3447–52. [PubMed] [Google Scholar]
- 21.Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–57. doi: 10.1016/j.jacc.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 22.Fricke TA, Brizard C, d’Udekem Y, Konstantinov IE. Arterial switch operation in children with interrupted aortic arch: long-term outcomes. J Thorac Cardiovasc Surg. 2010;141:1547–8. doi: 10.1016/j.jtcvs.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Fricke TA, d’Udekem Y, Richardson M, et al. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg. 2012;94:139–45. doi: 10.1016/j.athoracsur.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Chiu SN, Wang JK, Chen HC, et al. Long-term survival and unnatural deaths of patients with repaired tetralogy of Fallot in an Asian cohort. Circ Cardiovasc Qual Outcomes. 2012;5:120–5. doi: 10.1161/CIRCOUTCOMES.111.963603. [DOI] [PubMed] [Google Scholar]
- 25.Buratto E, McCrossan B, Galati JC, et al. Repair of partial atrioventricular septal defect: a 37-year experience. Eur J Cardiothorac Surg. 2015;47:796–802. doi: 10.1093/ejcts/ezu286. [DOI] [PubMed] [Google Scholar]
- 26.Buratto E, Ye XT, King G, et al. Long-term outcomes of single-ventricle palliation for unbalanced atrioventricular septal defects: Fontan survivors do better than previously thought. J Thorac Cardiovasc Surg. 2017;153:430–438. doi: 10.1016/j.jtcvs.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 27.Wong DJ, Iyengar AJ, Wheaton GR, et al. Long-term outcomes after atrioventricular valve operations in patients undergoing single-ventricle palliation. Ann Thorac Surg. 2012;94:606–13. doi: 10.1016/j.athoracsur.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 28.Hoashi T, Kagisaki K, Miyazaki A, et al. Anatomic repair for corrected transposition with left ventricular outflow tract obstruction. Ann Thorac Surg. 2013;96:611–20. doi: 10.1016/j.athoracsur.2013.03.095. [DOI] [PubMed] [Google Scholar]
- 29.Shuhaiber JH, Robinson B, Gauvreau K, et al. Outcome after repair of atrioventricular septal defect with tetralogy of Fallot. J Thorac Cardiovasc Surg. 2012;143:338–43. doi: 10.1016/j.jtcvs.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Pundi KN, Johnson JN, Dearani JA, et al. 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol. 2015;66:1700–10. doi: 10.1016/j.jacc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 31.Yong MS, Yim D, Brizard CP, et al. Long-term outcomes of patients with absent pulmonary valve syndrome: 38 years of experience. Ann Thorac Surg. 2014;97:1671–7. doi: 10.1016/j.athoracsur.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Brizard CP, Lee A, Zannino D, et al. Long-term results of anatomic correction for congenitally corrected transposition of the great arteries: A 19-year experience. J Thorac Cardiovasc Surg. 2017;154:256–265 e4. doi: 10.1016/j.jtcvs.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 33.d’Udekem Y, Iyengar AJ, Galati JC, et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation. 2014;130:S32–8. doi: 10.1161/CIRCULATIONAHA.113.007764. [DOI] [PubMed] [Google Scholar]
- 34.Poh CL, Zannino D, Weintraub RG, et al. Three decades later: The fate of the population of patients who underwent the Atriopulmonary Fontan procedure. Int J Cardiol. 2017;231:99–104. doi: 10.1016/j.ijcard.2017.01.057. [DOI] [PubMed] [Google Scholar]
- 35.Shi G, Zhu Z, Chen J, et al. Total Anomalous Pulmonary Venous Connection: The Current Management Strategies in a Pediatric Cohort of 768 Patients. Circulation. 2017;135:48–58. doi: 10.1161/CIRCULATIONAHA.116.023889. [DOI] [PubMed] [Google Scholar]
- 36.Khairy P, Clair M, Fernandes SM, et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013;127:331–9. doi: 10.1161/CIRCULATIONAHA.112.135046. [DOI] [PubMed] [Google Scholar]
- 37.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131:e1502–8. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Liu G, Druschel CM, Kirby RS. Maternal race/ethnicity and survival experience of children with congenital heart disease. J Pediatr. 2013;163:1437–42 e1. 2. doi: 10.1016/j.jpeds.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 39.Videbaek J, Laursen HB, Olsen M, Hofsten DE, Johnsen SP. Long-Term Nationwide Follow-Up Study of Simple Congenital Heart Disease Diagnosed in Otherwise Healthy Children. Circulation. 2016;133:474–83. doi: 10.1161/CIRCULATIONAHA.115.017226. [DOI] [PubMed] [Google Scholar]
- 40.Oster ME, Riehle-Colarusso T, Simeone RM, et al. Public health science agenda for congenital heart defects: report from a Centers for Disease Control and Prevention experts meeting. J Am Heart Assoc. 2013;2:e000256. doi: 10.1161/JAHA.113.000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasquali SK, Jacobs JP, Farber GK, et al. Report of the National Heart, Lung, and Blood Institute Working Group: An Integrated Network for Congenital Heart Disease Research. Circulation. 2016;133:1410–8. doi: 10.1161/CIRCULATIONAHA.115.019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinocur JM, Moller JH, Kochilas LK. Putting the Pediatric Cardiac Care Consortium in context: evaluation of scope and case mix compared with other reported surgical datasets. Circ Cardiovasc Qual Outcomes. 2012;5:577–9. doi: 10.1161/CIRCOUTCOMES.111.964841. [DOI] [PubMed] [Google Scholar]
- 43.Moller JH, Hills CB, Pyles LA. A multi-center cardiac registry. A method to assess outcome of catheterization intervention or surgery. Progress in Pediatric Cardiology. 2005;20:7–12. [Google Scholar]
- 44.Spector LG, Menk JS, Vinocur JM, et al. In-Hospital Vital Status and Heart Transplants After Intervention for Congenital Heart Disease in the Pediatric Cardiac Care Consortium: Completeness of Ascertainment Using the National Death Index and United Network for Organ Sharing Datasets. J Am Heart Assoc. 2016;5:e003783. doi: 10.1161/JAHA.116.003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lash TL, Cole SR. Immortal person-time in studies of cancer outcomes. J Clin Oncol. 2009;27:e55–6. doi: 10.1200/JCO.2009.24.1877. [DOI] [PubMed] [Google Scholar]
- 46.Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:375–461. [PubMed] [Google Scholar]
- 47.Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–5. doi: 10.1016/s0735-1097(01)01272-4. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention, National Center for Health Statistics. Compressed Mortality File 1999-2015 on CDC WONDER Online Database, released December 2016. Data are from the Compressed Mortality File 1999-2015 Series 20 No. 2U, 2016, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/cmf-icd10.html on Jun 5, 2017.
- 49.Finkelstein DM, Muzikansky A, Schoenfeld DA. Comparing survival of a sample to that of a standard population. J Natl Cancer Inst. 2003;95:1434–9. doi: 10.1093/jnci/djg052. [DOI] [PubMed] [Google Scholar]
- 50.Nembhard WN, Xu P, Ethen MK, Fixler DE, Salemi JL, Canfield MA. Racial/ethnic disparities in timing of death during childhood among children with congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2013;97:628–40. doi: 10.1002/bdra.23169. [DOI] [PubMed] [Google Scholar]
- 51.Nembhard WN, Salemi JL, Ethen MK, Fixler DE, Dimaggio A, Canfield MA. Racial/Ethnic disparities in risk of early childhood mortality among children with congenital heart defects. Pediatrics. 2011;127:e1128–38. doi: 10.1542/peds.2010-2702. [DOI] [PubMed] [Google Scholar]
- 52.Raissadati A, Nieminen H, Jokinen E, Sairanen H. Progress in late results among pediatric cardiac surgery patients: a population-based 6-decade study with 98% follow- up. Circulation. 2015;131:347–53. doi: 10.1161/CIRCULATIONAHA.114.011190. [DOI] [PubMed] [Google Scholar]
- 53.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 54.Skopp NA, Smolenski DJ, Schwesinger DA, Johnson CJ, Metzger-Abamukong MJ, Reger MA. Evaluation of a methodology to validate National Death Index retrieval results among a cohort of U.S. service members. Ann Epidemiol. 2017;27:397–400. doi: 10.1016/j.annepidem.2017.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


