Abstract
Background
To improve stroke care, the Brain Attack Coalition recommended establishing primary stroke center (PSC) and comprehensive stroke center (CSC) certification. This study aimed to compare ischemic stroke care and in-hospital outcomes between CSCs and PSCs.
Methods and Results
We analyzed patients with acute ischemic stroke who were hospitalized at stroke centers participating in Get With The Guidelines (GWTG)-Stroke from 2013 to 2015. Multivariable logistic regression models were generated to examine the association between stroke center certification (CSC vs PSC) and performances and outcomes. This study included 722,941 patients who were admitted to 134 CSCs and 1047 PSCs. Both CSCs and PSCs had good conformity to seven performance measures and the summary defect-free care measure. Among Emergency Department (ED) admissions, CSCs had higher intravenous tissue plasminogen activator (IV tPA) and Endovascular Thrombectomy (EVT) rates than PSCs (14.3% vs 10.3%, 4.1% vs 1.0 %, respectively). Door to IV tPA time was shorter at CSCs [Median 52 vs 61 minutes, adjusted risk ratio (aRR) 0.92; 95% CI 0.89-0.95]. More patients at CSCs had door to IV tPA time ≤60 minutes [79.7 vs 65.1%, aOR 1.48; 95% CI 1.25-1.75]. For transferred patients, CSCs and PSCs had comparable overall performance in defect-free care, except higher EVT therapy rates. The overall in-hospital mortality was higher at CSCs in both ED admissions [4.6% vs 3.8%, aOR 1.14; 95% CI 1.01-1.29] and transferred patients [7.7% vs 6.8% aOR 1.17; 95% CI 1.05-1.32]. In-hospital outcomes were comparable between CSCs and PSCs in patients who received IV tPA or EVT.
Conclusions
CSCs and PSCs achieved similar overall care quality for acute ischemic stroke patients. CSCs exceeded PSCs in timely acute reperfusion therapy for ED admissions, while PSCs had lower risk-adjusted in-hospital mortality. This information may be important for acute stroke triage and targeted quality improvement.
Keywords: Primary Stroke Center, Comprehensive Stroke Center, Ischemic Stroke, Quality of Care, Outcome
Stroke is the fifth leading cause of death in the United States and the leading cause of long-term disability.1 To improve the delivery of evidence-based stroke care, the Brain Attack Coalition suggested that two levels of stroke centers should be established: primary stroke center (PSC) and comprehensive stroke center (CSC).2 A PSC has the necessary staffing, infrastructure, and programs to stabilize and treat most acute stroke patients.2 A CSC should provide complete care to patients experiencing the most complex strokes that require specialized testing and interventions.2 CSCs are also expected to act as resource centers for other facilities in their region.2 Similarly to the US, European Stroke Organization recommended to establish two levels of stroke care certification: Stroke Unit and Stroke Center, in participating European countries.3 Interhospital transfer is a resource intensive pattern of care.4 Transferred patients had higher in-hospital mortality compared with patients who were directly admitted from the emergency room and were more likely to suffer complications.5 It remains unclear whether CSCs have better performances and outcomes than PSCs for transferred patients and ED admissions in the US.
The majority of stroke centers participate in the Get With The Guidelines (GWTG)-Stroke program, which collects prospective data for evidence-based and guideline directed performance measures, and in-hospital outcomes.6,7 This study aimed to compare the performances of CSCs and PSCs for quality of care and in-hospital outcomes in treating ischemic stroke patients. Given the presumed different disease severity and triage algorithm, patients who were admitted directly from the emergency department (ED admissions) and those who were transferred in (transfer-in patients) were examined separately.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results. Study data are confidential and cannot be shared according to the terms of the contracts signed between participating hospitals and the American Heart Association.
GWTG-Stroke is an ongoing voluntary web-based registry and performance improvement initiative that collects patient level data on patient characteristics, diagnostic testing, treatments, adherence to quality measures, and in-hospital outcomes in patients hospitalized with stroke. Trained hospital personnel are instructed to collect data of consecutive patients treated for acute ischemic stroke by either prospective clinical identification, retrospective identification using International Classification of Diseases 9th revision codes, or a combination.6,7 Additional descriptions of the case ascertainment, data collection, and quality auditing methods have been previously published.6,7 Although large, urban and teaching hospitals are overrepresented, the patients included in GWTG-Stroke have similar characteristics to the overall US Medicare stroke population.8
Each participating hospital received either human research approval to enroll cases without individual patient consent under the common rule, or a waiver of authorization and exemption from subsequent review by their institutional review board. The Duke Clinical Research Institute serves as the data analysis center and has an agreement to analyze the aggregate deidentified data for research purposes.
Patient Population
This study included patients who were admitted to CSCs and PSCs that participated in the GWTG-Stroke program between January 1, 2013 and December 31, 2015 with a final diagnosis of acute ischemic stroke. During the study period, 1626 hospitals in the US were certified as stroke centers. Among them, 165 hospitals were CSCs and 1461 hospitals were PSCs. Seventy-three percent of the certified stroke centers participated in the GWTG-Stroke, including 134 CSCs and 1047 PSCs. The final study population consisted of 605,136 ED admission and 117,805 transfer-in patients. Patients without the ischemic stroke diagnosis (n=1,302,419), discharge status missing or those who left against medical advice (n=6,874), and those whose transfer information was missing (n=3,906) were excluded. We excluded in-hospital stroke (n=18,360) because they represent a unique patient population compared to community-onset stroke.9
Stroke Center Certification and Hospital Data
The hospitals that were listed as having maintained or obtained CSC or PSC certification by the Joint Commission, Healthcare Facilities Accreditation Program, and Det Norske Veritas during the study period were publically available on-line at www.qualitycheck.org, www.hfap.org, and http://dnvglhealthcare.com. The lists of CSCs and PSCs certified by the state agencies were obtained from the state health department websites. Data on hospital characteristics (i.e., bed size, academic status, and geographical region) were obtained from the American Hospital Association annual survey database.
Stroke Measure Definitions
The American Heart Association/American Stroke Association came to an agreement with the Joint Commission and the Centers for Disease Control and Prevention to jointly release a set of standardized stroke performance measures.10 The seven GWTG-Stroke performance measures and eight quality measures were previously published.6,7 An all-or-none measure of care, termed as defect-free care, was used to summarize the overall conformity with the 7 achievement measures for each hospital.6 These measures and additional in-hospital outcomes were specified in Supplemental Table 1.
Statistical Analysis
The patient demographics, comorbidities, and hospital characteristics were compared between CSCs and PSCs. Due to the large sample size, statistical significance was detected in nearly all the measures despite often very small differences. To avoid the influence of the sample size, standardized differences were calculated, using the difference in the mean of a variable between two groups divided by the standard deviation of that variable. Standardized difference greater than 10% was considered significant imbalance. Hospital performances in ED admissions, transfer-in patients, and overall ischemic stroke patients were compared between CSCs and PSCs. Pearson chi-square test was used for categorical variables and Wilcoxon Rank-Sum test was used for continuous variables.
Multivariable logistic regression models were generated to examine the association between the performance and stroke center certification (CSC vs PSC). The generalized estimating equation (GEE) method with exchangeable working correlation matrix was applied to provide valid inference to account for in-hospital clustering. The adjusted models were controlled for potential confounders as previously described: patient age, sex, race, medical history of atrial fibrillation/flutter, prior stroke/transient ischemic attack, coronary artery disease/prior myocardial infarction, carotid stenosis, diabetes, peripheral vascular disease, hypertension, dyslipidemia, smoking, heart failure, arrival during off hours, hospital annual ischemic stroke admission, annual intravenous tissue plasminogen activator (IV tPA) volume, number of beds, region, academic status, and urban/rural location.11 The collinearity between all covariates in the model was assessed using variance inflation factors (VIFs). Large VIFs (VIF > 5) may be indicative of collinearity. The correlation between variables with VIF > 5 and the other covariates was examined. When there was evidence of strong correlation between two covariates, one covariate may need to be dropped from the candidate list to achieve a stable model fit. The mode of arrival (arrival by emergency medical service) was included in the modeling for ED admissions, not transfer-in patients. Due to skewed distribution of door to IV tPA time and door to EVT therapy time, Poisson regression model with log link was used and risk ratios were reported. The quality measure door to IV tPA time ≤ 60 minutes had very few eligible patients in the transfer-in cohort so it was not included in the modeling analysis.
National Institutes of Health Stroke Score (NIHSS), a surrogate of stroke severity, was not included in the primary analysis due to high missing rate in GWTG-Stroke (up to 15%). As previously reported, the missing of NIHSS in GWTG-Stroke may not be completely random early on when the report rate was low.12 NIHSS was missing more often in patients with less severe stroke.13 Sensitivity analyses were further performed where the NIHSS score was added to the models. As secondary analyses, we compared the performance and outcomes between CSCs vs PSCs among patients who received acute reperfusion therapy, IV tPA and EVT, separately. NIHSS report rate was 100% in patients who received IV tPA and EVT treatment thus it was included in the modeling.
All statistical analyses were performed using SAS Version 9.4 software (SAS Institute). All hypothesis tests were 2-sided, with P<0.05 considered statistically significant.
Results
The patients' demographics and comorbidities are shown in Table 1. There were few differences between CSCs and PSCs in patient characteristics. Overall, patients at CSCs were younger, more likely arrived by EMS, and had more severe stroke represented by higher NIHSS score. Patients who were transferred to CSCs had higher NIHSS than those transferred to PSCs (standardized difference -10.87).
Table 1. Patient Characteristics of the Study Subjects.
| ED admission | Transfer-in | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CSCs | PSCs | StdDiff (%) | CSCs | PSCs | StdDiff (%) | CSC | PSC | StdDiff (%) | |
| Number of Patients | 110624 | 494512 | 48376 | 69429 | 159000 | 563941 | |||
| Age (years) (Mean±SD) | 70±15 | 71±14 | 8.1 | 66±15 | 68±14 | 11.8 | 69±15 | 70±15 | 13.5 |
| Female (%) | 50.4 | 51.4 | 2.0 | 46.4 | 47.2 | 1.7 | 49.2 | 50.9 | 3.4 |
| Race (%) | |||||||||
| White | 66.0 | 68.7 | 5.8 | 77.4 | 73.8 | -8.4 | 69.5 | 69.3 | -0.2 |
| Black | 21.7 | 17.4 | -11.1 | 11.9 | 12.5 | 1.9 | 18.7 | 16.8 | -5.1 |
| Asian | 2.8 | 3.2 | 2.3 | 1.4 | 1.7 | 2.5 | 2.4 | 3.0 | 3.9 |
| Hispanic | 5.7 | 7.0 | 5.3 | 3.4 | 6.3 | 13.6 | 5.0 | 6.9 | 8.0 |
| Arrival by EMS (%) | 59.0 | 55.5 | -7.2 | - | - | - | 41.3 | 48.9 | 15.5 |
| Arrival during off Hours (%) | 45.1 | 43.7 | -2.9 | 60.3 | 58.0 | -4.8 | 49.7 | 45.4 | -8.7 |
| Onset to Arrival Time, Median (IQR) (minute) | 195 (71,612) | 192 (66,603) | 0.5 | 371 (236,780) | 384 (245,775) | -0.9 | 270 (100,660) | 225 (73,621) | -4.5 |
| Atrial fibrillation (%) | 19.1 | 18.8 | -0.8 | 19.3 | 18.9 | -1 | 19.1 | 18.8 | -0.9 |
| Prior Stroke/TIA (%) | 32.2 | 31.6 | -1.2 | 26.7 | 27.8 | 2.6 | 30.5 | 31.2 | 1.4 |
| Myocardial Infarction (%) | 23.5 | 24.4 | 2.1 | 24.3 | 24.7 | 0.9 | 23.7 | 24.4 | 1.6 |
| HF (%) | 9.5 | 9.3 | -0.5 | 9.4 | 9.0 | -1.2 | 9.5 | 9.3 | -0.6 |
| Carotid Stenosis (%) | 3.5 | 3.7 | 1.6 | 4.0 | 4.4 | 2 | 3.6 | 3.8 | 1.1 |
| Diabetes (%) | 33.3 | 34.8 | 3.1 | 32.2 | 33.7 | 3.2 | 33.0 | 34.7 | 3.6 |
| PAD (%) | 4.1 | 4.8 | 3.2 | 4.0 | 4.5 | 2.7 | 4.1 | 4.8 | 3.3 |
| Hypertension (%) | 76.6 | 76.9 | 0.7 | 73.0 | 74.2 | 2.6 | 75.5 | 76.6 | 2.5 |
| Smoker (%) | 19.2 | 17.3 | -4.9 | 24.0 | 21.1 | -7.0 | 20.6 | 17.7 | -7.3 |
| Dyslipidemia (%) | 45.1 | 46.3 | 2.5 | 42.4 | 45.1 | 5.5 | 44.3 | 46.2 | 3.8 |
| NIHSS | 4 (1,10) | 3 (1,8) | -8.03 | 6 (2,14) | 5 (2,12) | -10.9 | 4 (2,11) | 4 (1,9) | -13.1 |
Abbreviations: StdDiff, Standardized Difference; EMS, emergency medical services; IQR, interquartile range; TIA, transient ischemic attack; HF, heart failure; PAD, peripheral vascular disease; NIHSS, National Institute of Health stroke scale.
StdDiff was calculated using the difference in the mean between two groups divided by the pooled standard deviation of a variable. StdDiff greater than 10% was considered significant imbalance. The overall population included ED admissions and those patients who presented from non-acute care hospitals.
The hospital characteristics at patient level and hospital level are shown in Table 2. CSCs were larger than PSCs in terms of total number of beds and annual ischemic stroke volume. CSCs had higher annual IV tPA volume than PSCs. More CSCs were teaching hospitals.
Table 2. Hospital Characteristics at Patient Level.
| CSC | PSC | Standardized difference (%) | |
|---|---|---|---|
| Number of Hospitals | 134 | 1047 | |
|
| |||
| Total Number of Beds | 591±273 | 398±256 | -72.81 |
|
| |||
| Annual Stroke Volume | 431±187 | 247±136 | -112.52 |
|
| |||
| Annual IV tPA Volume | 40±20 | 22±15 | -102.82 |
| Teaching Hospital (%) | 88.0 | 56.1 | -76.09 |
| Regional Distribution | (%) | ||
| West | 10.4 | 20.7 | 28.50 |
| South | 36.6 | 35.8 | 0.48 |
| Midwest | 29.5 | 18.3 | -26.44 |
| Northeast | 24.5 | 25.2 | 1.68 |
|
| |||
| Rural (%) | 0 | 4.7 | 31.25 |
Abbreviations: IV tPA, intravenous tissue plasminogen activator.
Standardized difference greater than 10% or less than -10% was considered imbalance.
The performances and in-hospital outcomes are shown in Table 3. Both CSCs and PSCs had overall good conformity to the seven performance measures and the summary defect-free care measure. However, CSCs outperformed PSCs in several key measures in treating ED admissions, especially for IV tPA and EVT therapy. Compared to PSCs, more patients at CSCs who arrived by 2 hours of stroke onset received IV tPA by 3 hours, and more patients who arrived by 3.5 hours received IV tPA by 4.5 hours. The proportion of door to IV tPA time less than 60 minutes, 45 minutes and 30 minutes were all much higher at CSCs than PSCs. Among all ischemic stroke patients, CSCs had higher IV tPA and EVT therapy rates than PSCs (14.3% vs 10.3%, 4.1% vs 1.0 %, respectively). The median door to IV tPA time at CSCs was 9 minutes shorter than that at PSCs. The median door to EVT therapy time at CSCs was 7 minutes shorter than that at PSCs. Interestingly, CSCs had higher mortality than PSCs (4.6% vs 3.8%) (Table 3).
Table 3. The Achievement and Quality Measures and In-hospital Outcomes.
| ED admission | Transfer-in | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| CSCs | PSCs | StdDiff (%) | p | CSCs | PSCs | StdDiff (%) | p | CSC | PSC | StdDiff (%) | p | |
| Defect-free Care Measure (%) | 94.2 | 93.8 | -1.62 | <.0001 | 95.5 | 95.2 | -1.83 | .0026 | 94.6 | 94.0 | -2.66 | <.0001 |
|
| ||||||||||||
| Early Antithrombotics (%) | 97.1 | 97.7 | 3.38 | <.0001 | 96.4 | 97.1 | 3.75 | <.0001 | 97.0 | 97.6 | 4.03 | <.0001 |
|
| ||||||||||||
| Antithrombotics (%) | 98.9 | 99.1 | 1.68 | <.0001 | 99.3 | 99.2 | -0.32 | .6174 | 99.0 | 99.1 | 0.87 | .0041 |
|
| ||||||||||||
| Anticoagulation for Atrial Fibrillation (%) | 97.0 | 96.2 | -4.71 | <.0001 | 97.3 | 97.0 | -2.12 | .1844 | 97.1 | 96.3 | -4.75 | <.0001 |
|
| ||||||||||||
| DVT Prophylaxis (%) | 98.9 | 98.8 | -1.28 | .0007 | 99.2 | 99.1 | -0.59 | .3598 | 99.0 | 98.8 | -1.67 | <.0001 |
|
| ||||||||||||
| LDL 100 or Statin (%) | 98.1 | 97.7 | -2.29 | <.0001 | 98.6 | 98.2 | -3.15 | <.0001 | 98.2 | 97.8 | -3.03 | <.0001 |
|
| ||||||||||||
| Smoking Cessation (%) | 98.0 | 97.5 | -3.33 | <.0001 | 98.6 | 97.4 | -8.41 | <.0001 | 98.2 | 97.5 | -4.88 | <.0001 |
|
| ||||||||||||
| Dysphagia Screen (%) | 86.0 | 86.5 | 1.45 | <.0001 | 90.3 | 87.1 | -9.92 | <.0001 | 87.2 | 86.6 | -1.97 | <.0001 |
|
| ||||||||||||
| Stroke Education (%) | 94.8 | 95.7 | 3.97 | <.0001 | 96.6 | 95.8 | -4.05 | <.0001 | 95.3 | 95.7 | 1.83 | <.0001 |
|
| ||||||||||||
| Rehabilitation Considered (%) | 98.6 | 98.6 | 0.44 | .2119 | 99.1 | 99.0 | -0.48 | .4579 | 98.7 | 98.7 | -0.43 | .1568 |
|
| ||||||||||||
| LDL Measured (%) | 93.2 | 92.7 | -2.19 | <.0001 | 94.5 | 92.6 | -7.75 | <.0001 | 93.6 | 92.7 | -3.73 | <.0001 |
|
| ||||||||||||
| Intensive Statin Therapy (%) | 58.9 | 44.7 | -28.80 | <.0001 | 69.2 | 53.5 | -32.66 | <.0001 | 62.0 | 45.8 | -33.05 | <.0001 |
|
| ||||||||||||
| NIHSS Reported (%) | 88.2 | 84.2 | -11.47 | <.0001 | 90.5 | 80.3 | -29.28 | <.0001 | 88.9 | 83.7 | -15.03 | <.0001 |
|
| ||||||||||||
| IV tPA Rate (%) | 14.3 | 10.3 | -12.38 | <.0001 | 1.4 | 1.6 | 1.32 | .0227 | 10.4 | 9.2 | -4.05 | <.0001 |
| IV tPA 3 hr/Arrive 2 hr (%) | 93.6 | 89.4 | -14.94 | <.0001 | 86.2 | 84.0 | -6.06 | .4630 | 93.4 | 89.4 | -14.61 | <.0001 |
| IV tPA 4.5 hr /Arrive 3.5 hr (%) | 84.9 | 73.7 | -27.85 | <.0001 | 62.1 | 57.9 | -8.59 | .0497 | 83.8 | 73.4 | -25.77 | <.0001 |
|
| ||||||||||||
| Door to IV tPA ≤60 minutes (%) | 79.7 | 65.1 | -33.12 | <.0001 | 78.1 | 58.6 | -42.56 | .0470 | 79.7 | 65.0 | -33.14 | <.0001 |
| Door to IV tPA ≤45 minutes (%) | 45.9 | 30.2 | -32.81 | <.0001 | 37.5 | 29.3 | -17.28 | 0.386 | 45.9 | 30.2 | -32.77 | <.0001 |
| Door to IV tPA ≤30 minutes (%) | 14.5 | 7.5 | -22.33 | <.0001 | 9.4 | 5.0 | -16.57 | 0.376 | 14.5 | 7.5 | -22.32 | <.0001 |
| Door to IV tPA Time (minutes), Median (IQR) | 52 (39, 70) | 61 (47, 83) | 26.56 | <.0001 | 44 (31, 61) | 53 (36, 73) | 10.95 | <.0001 | 52 (39, 70) | 60 (47,83) | 25.72 | <.0001 |
|
| ||||||||||||
| Thrombolytic Complications (%) | 4.4 | 4.7 | 1.57 | .0708 | 7.3 | 6.7 | -2.39 | .2701 | 5.0 | 4.8 | -0.63 | .4169 |
|
| ||||||||||||
| EVT Rate (%) | 4.1 | 1.0 | -18.06 | <.0001 | 8.5 | 4.3 | -16.12 | <.0001 | 5.5 | 1.4 | -20.55 | <.0001 |
| Door to EVT Time (minutes), Median (IQR) | 145 (105,192) | 152 (112,215) | 11.29 | <.0001 | 85 (49,138) | 85 (53,141) | 5.67 | .0806 | 119 (75,174) | 132 (85,195) | 12.32 | <.0001 |
|
| ||||||||||||
| In-hospital Mortality (%) | 4.6 | 3.8 | -3.56 | <.0001 | 7.7 | 6.8 | -3.61 | <.0001 | 5.5 | 4.2 | -6.13 | <.0001 |
|
| ||||||||||||
| Length of Stay>4 Days (%) | 40.8 | 37.6 | -7.39 | <.0001 | -- | -- | -- | 40.8 | 37.6 | -8.24 | <.0001 | |
|
| ||||||||||||
| Ambulatory at Discharge (%) | 45.3 | 43.5 | -3.72 | <.0001 | 40.1 | 38.9 | -2.33 | <.0001 | 43.7 | 42.9 | -1.63 | <.0001 |
|
| ||||||||||||
| Discharge Home (%) | 51.1 | 51.3 | 1.15 | .2906 | 45.4 | 47.4 | 4.86 | <.0001 | 49.4 | 50.8 | 4.12 | <.0001 |
Abbreviations: StdDiff, Standardized Difference; DVT, deep vein thrombosis; LDL, low density lipoprotein; NIHSS, National Institute of Health stroke scale; IV tPA; intravenous tissue plasminogen activator; IQR, interquartile range; EVT, endovascular thrombectomy.
Standardized difference greater than 10% or less than -10% was considered imbalance
CSCs and PSCs had similar conformity to the performance measures in treating transfer-in patients except that CSCs had higher EVT therapy rates than PSCs (8.5% vs 4.3%). Transferred patients had much higher mortality and lower discharge home rates than ED admissions in both CSCs and PSCs (Table 3).
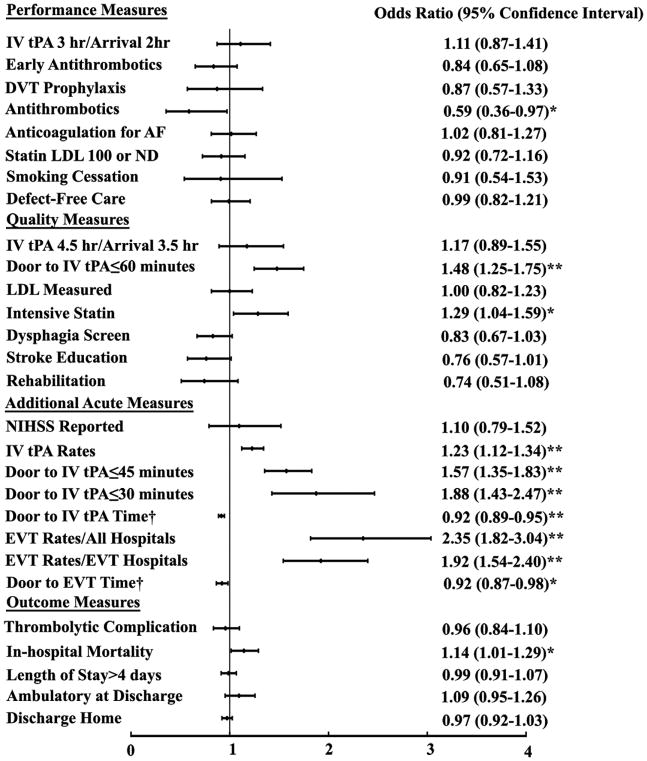
We further examined the association of performance with stroke center certification status (CSC vs PSC) using multivariable logistic regression analysis. The forest plots of the adjusted odds ratios are shown in Figures 1 and 2. Detail results including the unadjusted odds ratios are shown in supplemental Tables 2 and 3. CSCs exceeded PSCs in many key quality measures in treating ED admissions, particularly for the acute reperfusion therapies (Figure 1 and Supplemental Table 2). Compared to PSCs, the door to IV tPA time at CSCs was 15% shorter in the unadjusted model and 8% shorter in the adjusted model. Patients at CSCs were more likely to receive IV tPA with door to needle time less than 60, 45, and 30 minutes in both the unadjusted and adjusted models. Patients at CSCs were more likely to receive IV tPA within 3 hours or 4.5 hours if they arrived by 2 hours or 3.5 hours respectively in the unadjusted model, but not after risk adjustment. The risk-adjusted door to EVT therapy time for patients at CSCs was 7.8% shorter than that at PSCs. The IV tPA and EVT rates among all the ED admissions were higher at CSCs than PSCs. For in-hospital outcomes, CSCs had higher in-hospital mortality than PSCs, although the odds were smaller after risk-adjustment. More patients at CSCs had length of stay >4 days in the unadjusted model but not the adjusted model.
Figure 1.
The association of the performances and outcomes with stroke center certification (CSCs vs PSCs) in ED admissions.
The multivariable logistic regression analysis were adjusted for potential confounders. †Due to skewed distribution, Poisson regression models with log link was used for these two variables and the results shown were risk ratios. Higher odds ratio or risk ratio indicated that the performance or outcome occurred more frequently in CSCs. *Represented p<0.05. **Represented p<0.001.
Abbreviations: hr: hour; DVT, deep vein thrombosis; AF: Atrial fibrillation; LDL, low density lipoprotein; NIHSS, National Institute of Health Stroke Scale; IV tPA; intravenous tissue plasminogen activator; EVT, endovascular thrombectomy; EVT hospitals, hospitals that performed endovascular thrombectomy.
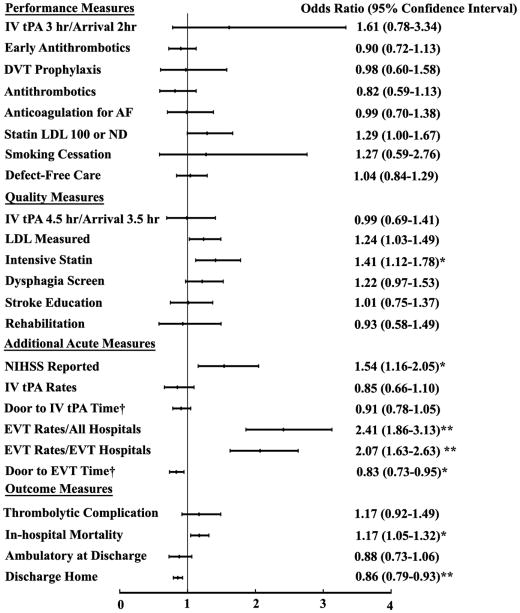
Figure 2.
The association of the performances and outcomes with stroke center certification (CSCs vs PSCs) in Transfer-in Patients.
The multivariable logistic regression analysis were adjusted for potential confounders. † Due to skewed distribution, Poisson regression models with log link was used for these two variables and the results shown were risk ratios. Higher odds ratio or risk ratio indicated that the performance or outcome occurred more frequently in CSCs. *Represented p<0.05. **Represented p<0.001.
Abbreviations: hr: hour; DVT, deep vein thrombosis; AF: Atrial fibrillation; LDL, low density lipoprotein; NIHSS, National Institute of Health Stroke Scale; IV tPA; intravenous tissue plasminogen activator; EVT, endovascular thrombectomy; EVT hospitals, hospitals that performed endovascular thrombectomy.
CSCs and PSCs showed similar overall performance in treating transferred patients except for EVT therapy and in-hospital mortality (Figure 2 and Supplemental Table 2). Patients who were transferred to CSCs were more likely to undergo EVT therapy than those who were transferred to PSCs. CSCs had shorter risk-adjusted door to EVT therapy time than PSCs. The risk-adjusted discharge home rate was lower at CSCs, and in-hospital mortality was higher. The above findings did not significantly change in the sensitivity analyses where the NIHSS score was added to the models (Supplemental Table 3).
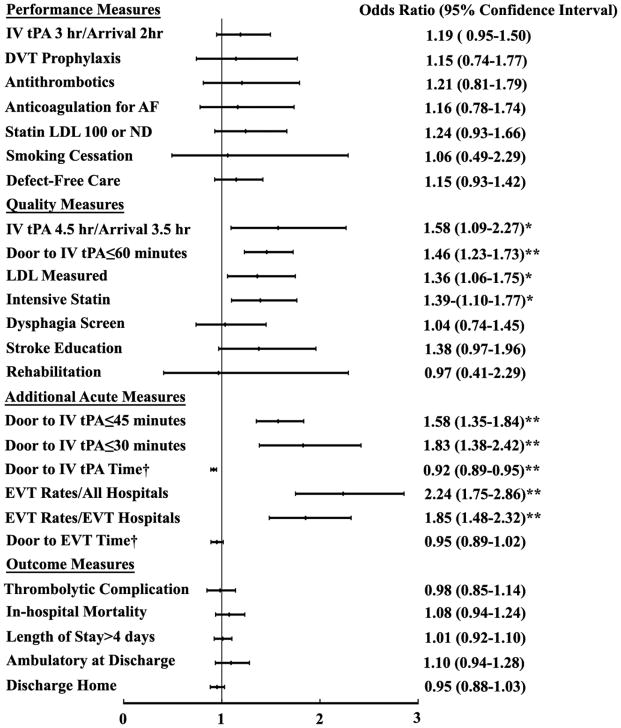
In the secondary analysis, we compared the performances and outcomes between CSCs and PSCs for ED admissions that received IV tPA and EVT therapy. The performances and in-hospital outcomes are shown in Supplemental Table 4. The adjusted odds ratios are shown in Figures 3 and 4. Detail results of the multivariable analysis including the unadjusted odds ratios are shown in Supplemental Table 5. Similar to the primary analysis, patients at CSCs had shorter door to IV tPA time, more likely to receive IV tPA within 60, 45, and 30 minutes than those at PSCs. The patients who received IV tPA at CSCs were more likely to receive EVT therapy. CSCs had shorter door to EVT time in the unadjusted model but not the adjusted model. The risk-adjusted in-hospital outcomes including mortality, discharge home rate, discharge mRS and ambulatory status did not differ between CSCs and PSCs in this population.
Figure 3.
The Association of performances and outcomes with stroke center certification (CSCs vs PSCs) in ED Admissions that received intravenous thrombolytic treatment.
The multivariable logistic regression analysis were adjusted for potential confounders. † Due to skewed distribution, Poisson regression models with log link was used for these two variables and the results shown were risk ratios. Higher odds ratio or risk ratio indicated that the performance or outcome occurred more frequently in CSCs. *Represented p<0.05. **Represented p<0.001.
Abbreviations: hr: hour; DVT, deep vein thrombosis; AF: Atrial fibrillation; LDL, low density lipoprotein; IV tPA; intravenous tissue plasminogen activator; EVT, endovascular thrombectomy; EVT hospitals, hospitals that performed endovascular thrombectomy.
Figure 4.
The Association of performances and outcomes with stroke center certification (CSCs vs PSCs) in ED Admissions that underwent endovascular thrombectomy.
The multivariable logistic regression analysis were adjusted for potential confounders. † Due to skewed distribution, Poisson regression models with log link was used for these two variables and the results shown were risk ratios. *Represented p<0.05.
Abbreviations: IV tPA; intravenous tissue plasminogen activator; EVT, endovascular thrombectomy; mRS: modified Rankin Scale.
Discussion
This study compared the performance of CSCs and PSCs that participated in the GWTG-Stroke program in treating ischemic stroke patients. For many components of care for acute ischemic stroke patients, including the defect-free care measure, performance was comparable at CSCs and PCSs. These findings suggest for these domains of care both CSCs and PSCs are capable of achieving similar care quality for acute ischemic stroke patients. CSCs did exceed PSCs in performance on several key measures of acute reperfusion therapies for ED admissions. CSCs had shorter door to IV tPA times and door to EVT therapy time than PSCs. At CSCs, significantly more patients had door to IV tPA time less than 60 minutes, 45 minutes, and 30 minutes. CSCs had higher EVT rates in treating ED admissions than PSCs. In-hospital mortality was found to be modestly lower in PSCs compared with CSCs. This study identifies opportunities for PSCs as well as CSCs to improve the acute stroke care.
Improved treatment rates and more timely treatment with IV tPA therapy by hospitals are essential components of stroke care quality. Intravenous tPA and endovascular thrombectomy have been proven to improve the outcome of acute ischemic stroke in randomized trials.14-16 Earlier thrombolytic treatment was associated with reduced mortality and symptomatic intracranial hemorrhage, higher rates of independent ambulation at discharge and discharge to home following acute ischemic stroke.17 Meta-analysis of clinical trials of patients with large-vessel ischemic stroke showed that earlier treatment with endovascular thrombectomy was associated with lower degrees of disability at 3 months.18 The advantages of CSCs over PSCs in providing acute reperfusion therapy did not translate into better in-hospital outcomes in our study. Whether such benefits would be apparent in post-discharge follow-up requires further study.
This study showed modest differences in in-hospital mortality favoring PCSs. These differences, although persisted, were attenuated when adjusting for stroke severity. More patients at CSCs had length of stay >4 days in the unadjusted model. Although the result could have been influenced by residual confounding, it may indicate an opportunity for further quality improvement in the CSCs. Further study of other care quality measures such as post-discharge mortality, functional outcomes, and readmissions will be helpful to understand the differences between CSCs and PSCs.
Our results confirmed that PSCs remained equivalent resources of CSCs for interhospital transfer of acute ischemic stroke when EVT therapies were not needed. Among transfer-in patients, NIHSS was modestly higher in CSCs than PSCs, indicating more patients with severe stroke were transferred to CSCs. We suspect that CSCs received more transferred patients who needed EVT therapy resulting in higher EVT rates. Many PSCs may not provide EVT and transfer patients to CSCs. A less likely explanation would be that PSCs were reluctant to provide EVT therapy. The time period over which the data was collected was prior to the most recent EVT trials that provided sufficient evidence to make it routine practice.14 It is possible that the low rates of EVT particularly in PSCs, and potentially low numbers transferred to CSCs for intervention reflected the evidence-base and presumed risk associated with the procedure. Study using GWTG-Stroke has shown an increase in EVT after the publication of MR Clean and the pivotal trials.19
It is notable that the onset to arrival time in transferred patients was nearly three hours longer than that in the ED admissions. If this delay happened in patients who were EVT therapy candidates, it might have disqualified many patients from EVT therapy. A prior study had shown that one out of three patients became ineligible for EVT because of unfavorable deterioration on neuroimaging following interhospital transfer.20 Timely transport of patients to an endovascular-capable hospital is crucial because delay to reperfusion was associated with less favorable degree of disability and less functional independence.18 The current triage algorithm needs to be modified to ensure the fastest possible reperfusion therapy.
This study has important limitations. Participation in GWTG-Stroke is voluntary and data on patient characteristics and quality measures were self-reported by participating hospitals. However, prior quality audits of GWTG-Stroke data showed high concordance rates with source documentation.7 Stroke centers that did not participate in the GWTG-Stroke program were not included in this study, though the number of such centers is small. We cannot determine whether a greater proportion of patients who were triaged to CSCs were eligible for IV tPA or EVT therapy. The mRS at discharge was not available in a significant proportion of patients so it was not used in the primary analysis. There might be unmeasured confounding factors which may influence the results of the multivariable analyses. Although in-hospital mortality is an important in-hospital outcome measure, and we adjusted for comorbid conditions and other risk factors, it can be influenced by stroke severity, transfer policies, length of stay, and other unmeasured confounding factors. Other measures to assess care quality were not collected in this study, including procedure complications, health status, patient satisfaction, and post discharge outcomes such as mortality, functional status, and preventable readmissions.
Conclusions
This study showed that CSCs and PSCs achieved similar overall care quality for acute ischemic stroke patients. CSCs exceeded PSCs for timely acute reperfusion therapy in treating ED admissions. CSCs and PSCs had comparable performance in treating transferred patients, except that patients who were transferred to CSCs were more likely to undergo EVT. The risk adjusted in-hospital mortality was modestly higher at CSCs than PSCs in both ED admissions and transfer-in patients. The risk-adjusted in-hospital mortality and functional outcomes were similar in CSCs and PSCs for patients undergoing reperfusion therapy. These findings suggest that both CSCs and PSCs are capable of and achieving similar care quality for acute ischemic stroke patients, with some exceptions. PSCs have further opportunities to improve the rate and timing for reperfusion therapies including IV tPA and EVT therapy. The findings from this study may have important implications for stroke triage to PSCs versus CSCs, targeted quality improvement at CSCs and PSCs, and for further improving stroke systems of care.
Supplementary Material
What is Known
Two levels of stroke centers, primary stroke centers (PSCs) and comprehensive stroke centers (CSCs), were established to provide evidence-based stroke treatment and improve the quality of stroke care.
What the Study Adds
CSCs and PSCs achieved similar overall care quality for acute ischemic stroke patients with some exceptions.
CSCs exceeded PSCs for timely acute reperfusion therapy, including intravenous thrombolytic therapy and endovascular thrombectomy.
The risk adjusted in-hospital mortality was modestly higher at CSCs than PSCs, but similar between CSCs and PSCs in patients received acute reperfusion therapy.
These findings may have important implications for stroke triage and targeted quality improvement at CSCs and PSCs.
Acknowledgments
Sources of Funding: This study was funded by American Heart Association/ American Stroke Association to S. Man. Funding number: 15CRP23100013. The American Heart Association receives a portion of the fees paid by hospitals to the Joint Commission for Primary Stroke Certification. The GWTG-Stroke program is currently supported in part by a charitable contribution from Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership and the American Heart Association Pharmaceutical Roundtable. GWTG-Stroke has been funded in the past through support from Boehringer-Ingelheim and Merck. These funding agencies did not participate in design or analysis, manuscript preparation, or approval of this study.
Footnotes
Disclosures: S Man: Research support from the American Heart Association, Award Number 15CRP23100013.
X Zhao: None.
K Uchino: None.
M.S. Hussain: None
E.E. Smith: None.
D. L. Bhatt: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; clinical trial steering committee), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda.
L.H. Schwamm: Serving as the chair of the American Heart Association/American Stroke Association GWTG stroke clinical work group and Healthcare Accreditation Science Committee; stroke systems consultant to the Massachusetts Department of Public Health; scientific consultant regarding trial design and conduct to Penumbra; and scientific consultant regarding trial design and conduct to Medtronic.
S. Shah: None.
Y. Khan: Employed at American Heart Association
G.C. Fonarow: Research support from the Patient Centered Outcome Research Institute and the National Institutes of Health, and an employee of University of California which holds a patent on an endovascular device for stroke.
References
- 1.Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Alberts MJ, Hademenos G, Latchaw RE, Jagoda A, Marler JR, Mayberg MR, Starke RD, Todd HW, Viste KM, Girgus M, Shephard T, Emr M, Shwayder P, Walker MD. Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA. 2000;283:3102–3109. doi: 10.1001/jama.283.23.3102. [DOI] [PubMed] [Google Scholar]
- 3.Ringelstein EB, Chamorro A, Kaste M, Langhorne P, Leys D, Lyrer P, Thijs V, Thomassen L, Toni D ESO Stroke Unit Certification Committee. European Stroke Organisation recommendations to establish a stroke unit and stroke center. Stroke. 2013;44:828–840. doi: 10.1161/STROKEAHA.112.670430. [DOI] [PubMed] [Google Scholar]
- 4.Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11:245–250. doi: 10.1002/jhm.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickles AV, Roberts S, Shell E, Mitchell M, Hussain S, Lyon-Callo S, Reeves MJ. Characteristics and Outcomes of Stroke Patients Transferred to Hospitals Participating in the Michigan Coverdell Acute Stroke Registry. Circ Cardiovasc Qual Outcomes. 2016;9:265–274. doi: 10.1161/CIRCOUTCOMES.115.002388. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, Hernandez AF, Peterson ED, Schwamm LH GWTG-Stroke Steering Committee and Investigators. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes. 2010;3:291–302. doi: 10.1161/CIRCOUTCOMES.109.921858. [DOI] [PubMed] [Google Scholar]
- 7.Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, Schwamm LH, Smith EE. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J. 2012;163:392–8. 398.e1. doi: 10.1016/j.ahj.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Reeves MJ, Fonarow GC, Smith EE, Pan W, Olson D, Hernandez AF, Peterson ED, Schwamm LH. Representativeness of the Get With The Guidelines-Stroke Registry: comparison of patient and hospital characteristics among Medicare beneficiaries hospitalized with ischemic stroke. Stroke. 2012;43:44–49. doi: 10.1161/STROKEAHA.111.626978. [DOI] [PubMed] [Google Scholar]
- 9.Cumbler E. In-Hospital Ischemic Stroke. Neurohospitalist. 2015;5:173–181. doi: 10.1177/1941874415588319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41:1573–1578. doi: 10.1161/STROKEAHA.109.577171. [DOI] [PubMed] [Google Scholar]
- 11.Man S, Cox M, Patel P, Smith EE, Reeves MJ, Saver JL, Bhatt DL, Xian Y, Schwamm LH, Fonarow GC. Differences in Acute Ischemic Stroke Quality of Care and Outcomes by Primary Stroke Center Certification Organization. Stroke. 2017;48:412–419. doi: 10.1161/STROKEAHA.116.014426. [DOI] [PubMed] [Google Scholar]
- 12.Reeves MJ, Smith EE, Fonarow GC, Zhao X, Thompson M, Peterson ED, Schwamm LH, Olson D. Variation and Trends in the Documentation of National Institutes of Health Stroke Scale in GWTG-Stroke Hospitals. Circ Cardiovasc Qual Outcomes. 2015;8:S90–8. doi: 10.1161/CIRCOUTCOMES.115.001775. [DOI] [PubMed] [Google Scholar]
- 13.Thompson MP, Luo Z, Gardiner J, Burke JF, Nickles A, Reeves MJ. Quantifying Selection Bias in National Institute of Health Stroke Scale Data Documented in an Acute Stroke Registry. Circ Cardiovasc Qual Outcomes. 2016;9:286–293. doi: 10.1161/CIRCOUTCOMES.115.002352. [DOI] [PubMed] [Google Scholar]
- 14.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama a Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 15.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, Cardona P, Devlin TG, Frei DF, du Mesnil de Rochemont R, Berkhemer OA, Jovin TG, Siddiqui AH, van Zwam WH, Davis SM, Castano C, Sapkota BL, Fransen PS, Molina C, van Oostenbrugge RJ, Chamorro A, Lingsma H, Silver FL, Donnan GA, Shuaib A, Brown S, Stouch B, Mitchell PJ, Davalos A, Roos YB, Hill MD HERMES Collaborators. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316:1279–1288. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Saver JL, Cox M, Liang L, Matsouaka R, Xian Y, Bhatt DL, Fonarow GC, Schwamm LH. Increase in Endovascular Therapy in Get With The Guidelines-Stroke After the Publication of Pivotal Trials. Circulation. 2017;136:2303–2310. doi: 10.1161/CIRCULATIONAHA.117.031097. [DOI] [PubMed] [Google Scholar]
- 20.Mokin M, Gupta R, Guerrero WR, Rose DZ, Burgin WS, Sivakanthan S. ASPECTS decay during inter-facility transfer in patients with large vessel occlusion strokes. J Neurointerv Surg. 2017;9:442–444. doi: 10.1136/neurintsurg-2016-012331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.