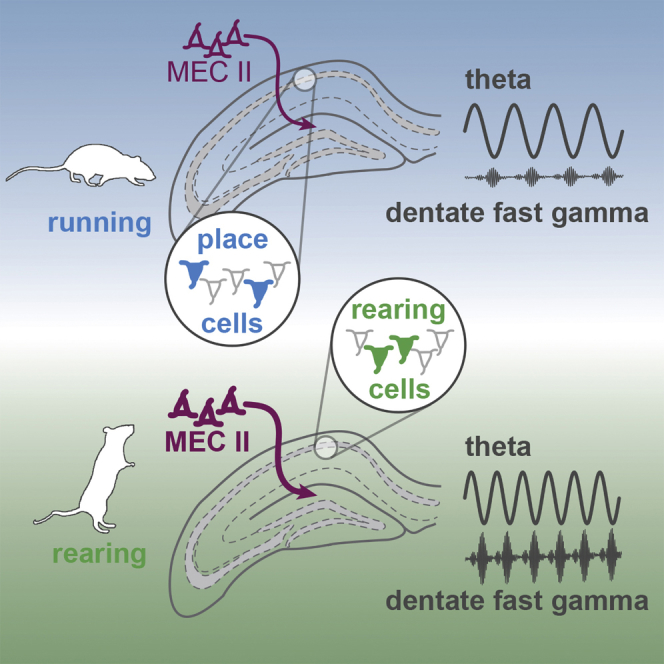
Summary
Animals build a model of their surroundings on the basis of information gathered during exploration. Rearing on the hindlimbs changes the vantage point of the animal, increasing the sampled area of the environment. This environmental knowledge is suggested to be integrated into a cognitive map stored by the hippocampus. Previous studies have found that damage to the hippocampus impairs rearing. Here, we characterize the operational state of the hippocampus during rearing episodes. We observe an increase of theta frequency paralleled by a sink in the dentate gyrus and a prominent theta-modulated fast gamma transient in the middle molecular layer. On the descending phase of rearing, a decrease of theta power is detected. Place cells stop firing during rearing, while a different subset of putative pyramidal cells is activated. Our results suggest that the hippocampus switches to a different operational state during rearing, possibly to update spatial representation with information from distant sources.
Keywords: theta rhythm, gamma oscillation, cross-frequency coupling, place cell, motion capture, silicone probe, independent component analysis, CA1, dentate gyrus, navigation
Graphical Abstract
Highlights
-
•
Theta frequency increased during rearing coupled with an elevated dentate theta sink
-
•
Robust theta-fast gamma phase coupling in the dentate gyrus accompanied rearing
-
•
Rearing-specific firing rate increase of putative pyramidal cells was detected
-
•
Conversely, if rearing occurred in a neuron’s place field, its firing rate decreased
By combining high speed tracking in three dimensions and multichannel recording, Barth et al. characterize the network dynamics in the hippocampus during rearing episodes. They observe augmented impulse flow through the medial perforant pathway accompanied by the activation of certain principal cells and decrease of place cell activity.
Introduction
When animals are exposed to novelty or experience alterations of a familiar environment, a complex set of behaviors unfolds. All of these behavioral actions aim to map the environment, and as such they are indispensable for the survival of the animal (O’Keefe and Nadel, 1979). A key component of this complex behavioral pattern is termed rearing and manifests as standing on the hindlimbs and scanning the environment from an elevated perspective (Lever et al., 2006). Changing the vantage point of the animal during exploration allows access to information from sources not immediately detectable during horizontal ambulation. Indeed, the occurrence of rearing is correlated with novelty, and can be used as a sensitive measure of exploratory behavior (Lever et al., 2006, Mun et al., 2015, Wells et al., 2013).
In addition to the spatial memory deficits, hippocampal damage impairs novelty detection and disrupts the normal pattern of rearing (Deacon et al., 2002, Harley and Martin, 1999). Rearing deficits may occur because of failures in spatial memory, where the novelty detection is impaired, thus failing to elicit the relevant behaviors normally triggered in a new space. Spatial memory is thought to rely upon comparing sensory inputs with the hippocampal-dependent representation of familiar places. Such spatial representations are supported by hippocampal “place cells” that are spatially selective and fire during movement throughout an animal’s environment (O’Keefe and Nadel, 1979, Wilson and McNaughton, 1993).
Place cell activity during exploration is organized by the 5–12 Hz theta rhythm as well as higher frequency gamma oscillations (Bragin et al., 1995, Buzsáki et al., 1983) that reflect local computations and dynamic changes of the information flow via hippocampal afferents (Colgin et al., 2009, Schomburg et al., 2014). Therefore, novelty-related behaviors may be triggered by, or in turn influence, place cell activity and the rhythms that coordinate the hippocampal system.
To address this question, we recorded hippocampal activity during rearing. We hypothesized that the information flow through the hippocampus would change when the animal changes its vantage point to switch from a focus on local cues to a focus on the more distant landmarks within an environment (Knierim and Hamilton, 2011). In the few studies that have examined the hippocampal electrophysiological correlates of rearing, robust theta was detected (Vanderwolf, 1969, Vanderwolf, 1975, Young and McNaughton, 2009). However, lack of appropriate tracking of the animal’s position precluded sufficiently detailed analysis of rearing-specific hippocampal activity. We combined a high-speed, three-dimensional (3D) behavioral tracking system with electrophysiological recordings from multiple hippocampal subregions to uncover rearing-associated network and single unit dynamics.
Results
Rearing Is Accompanied by Increased Hippocampal Theta Frequency
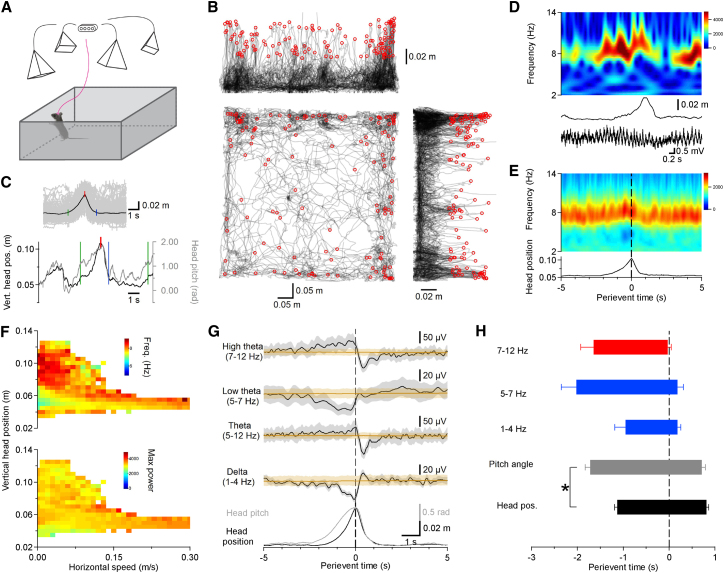
To investigate rearing behavior, mice (n = 12, both transgenic and wild-type; see Experimental Procedures; Table S1) were recorded during open-field exploration. Local field potentials (LFPs) and single units were registered across hippocampal subregions with multisite silicon probes, and the position of the animal was captured with simultaneous high-speed 3D behavioral tracking (Figure 1A). We defined rearing events on the basis of the distribution of vertical head positions (see Experimental Procedures; Figures S1A, 1B, and 1C). As expected (Lever et al., 2003, Mun et al., 2015), we found an initial surge in rearing during the first few minutes of an open-field test, followed by a gradual decrease (Figures S1B and S1C). Previous studies demonstrated a positive correlation between the frequency and power of theta oscillation and the animal’s horizontal running speed (McFarland et al., 1975). Therefore, we investigated the dependence of theta frequency and power on horizontal speed as well as vertical head position. The frequency of theta recorded in the dorsal CA1 pyramidal layer significantly increased during rearing episodes (Figures 1D–1G; −10 to −5 s before rearing peak 7.9 ± 0.1 Hz; −0.5 s to rearing peak 8.2 ± 0.1 Hz; t[11] = −4.73; p = 0.0006, paired t test; n = 12 mice). In addition to the known correlation between horizontal speed and theta frequency (Figure S1D; Pearson’s r = 0.45, p = 0.0003, n = 12 mice) and power (Pearson’s r = 0.51, p = 0.00003, n = 12 mice), our analysis also revealed high-frequency theta oscillations at elevated head positions, which typically occur during slow horizontal velocities (Figure 1F). The peak frequency during rearing episodes, which occur almost exclusively in the absence of horizontal motion, was similar to the highest value detected during running (Figure S1D; 8.2 ± 0.1 Hz at 0.12 m/s running speed versus 8.2 ± 0.1 Hz during rearing; t[11] = −0.371; p = 0.72, paired t test; n = 12 mice). We then asked if the rearing-coupled increase of theta frequency was correlated with vertical head position and the speed of movement along the vertical dimension. Cross-correlogram of vertical head position and high theta power indicated that increase of high theta power leads the elevation of vertical head position (Figure S1E; lag −0.43 ± 0.11 s; p = 0.0026, t test; n = 12 mice). Theta oscillation frequency and vertical head position showed a weak correlation, and the correlation at higher head positions had disappeared (Figure S1G; Pearson’s r = 0.32, p = 0.002; above 0.03 m from baseline, Pearson’s r = −0.04, p = 0.77; n = 12 animals). Because the horizontal and vertical components of 3D speed can be associated with different theta frequencies (Figure S1F), we used only its vertical component. We found that theta oscillation frequency correlated with vertical speed during both upward and downward movement (Figure S1H; Pearson’s r for upward movement = 0.51, p = 1.1 × 10−7; Pearson’s r for downward movement = −0.28, p = 0.005; comparison of correlations by Fisher r-to-z transformation, z = 1.84, p = 0.07; n = 12 animals). We suppose that the decreasing vertical head speed and the correlated theta frequency reduction close to peak positions (Figure S1H, right) can explain the flattening of theta frequency and vertical head position correlation. Theta frequency had a tendency to increase coincident with the change in head pitch angle before the elevation of vertical head position and continued accelerating during the lift-up of the body (Figures 1G and 1H). Although low-frequency (5–7 Hz) theta is typically observed in the absence of horizontal movement (Vanderwolf, 1969), during rearing we found an increase in high-frequency (7−12 Hz) theta (Figure 1G). These results suggest that rearing may be associated with a distinct brain state compared with that observed during running or immobility.
Figure 1.
Rearing Events Are Accompanied by Theta Oscillation Alterations
(A) Arrangement of the 3D behavioral observation system. Four cameras recorded the position of markers on the mouse’s head implant. The motion capture software reconstructed the exact position and orientation of the animal’s head in real time.
(B) Sample trajectory from an animal in the open arena during one trial (top right, side views, bottom left, top view). Red circles indicate rearing events.
(C) Example rearing event: black, vertical head position; gray, head pitch angle; green vertical line, start; red vertical line, peak; blue vertical line, stop of rearing event. Inset shows the average of n = 132 rearing events of one animal in black; the overlaid individual events are in gray.
(D) Example LFP recording in the CA1 pyramidal cell layer (bottom trace) during one rearing episode (vertical head positions, middle trace) and corresponding wavelet spectrogram of the LFP (top). Note the elevated frequency of theta oscillation during the rearing event.
(E) Rearing peak-triggered average vertical head position (bottom) and the corresponding average wavelet spectrogram (top) of all rearing events of an animal.
(F) Theta oscillation frequency (top diagram) and power (bottom) as a function of running speed and vertical head position. Note the high theta frequency values at low running speed and high head position.
(G) Mean power in different frequency bands around rearing events calculated for all mice. A prominent increment of high theta power is paralleled by the reduction of low theta power. Brown traces indicate the shuffled control (n = 12 animals; shaded gray area corresponds to SEM; beige area represents the 99% band of the shuffled control).
(H) Timing of changes in the delta and theta bands during rearing. Horizontal bars indicate the duration of the LFP and behavioral events; error bars represent SEM. Red, power increment; blue, power reduction. Note that power alterations of high and low theta tended to precede the start of head lift-up but nearly coincided with change of pitch angle (∗p < 0.05, paired t test; n = 12 animals).
See also Figure S1.
Increased Entorhinal-Driven Theta Currents during Rearing
Theta oscillation power and phase exhibit a characteristic depth profile across the layers of the hippocampal formation explained by the presence of different layer-specific theta current generators (Kocsis et al., 1999, Montgomery et al., 2009). Temporal shifts in the dominance of one theta generator over another can offer indirect insight into which afferent region is driving downstream activity on a moment-to-moment basis. Therefore, we investigated which theta current generators are involved in the observed theta oscillation alterations during rearing and hypothesized that rearing would be associated with an increased feedforward entorhinal drive due to the availability of new sensory inputs.
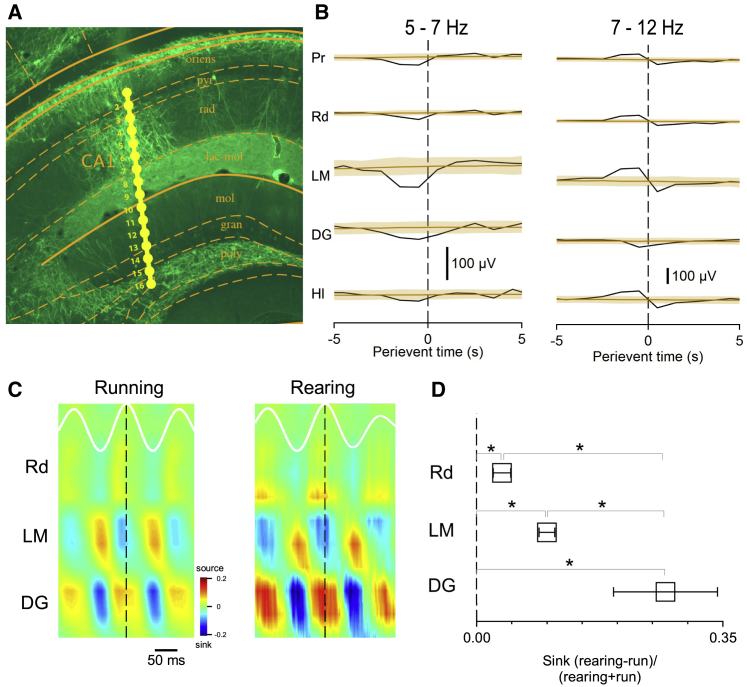
We used linear electrode arrays that spanned all CA1 layers and extended into the dentate gyrus to map layer-specific alterations during rearing episodes (Figure 2A). Similar to what we observed in the CA1 pyramidal cell layer, low-frequency theta was decreased and high-frequency theta was increased throughout all of the sampled hippocampal layers with the exception of the dentate molecular layer (Figure 2B). To uncover the contribution of the major hippocampal theta generators during rearing, we performed current source density (CSD) analysis (Buzsáki et al., 1986) on LFPs recorded during rearing and running (horizontal speed above 4 cm/s) epochs. During running episodes, as reported earlier (Buzsáki et al., 1986, Brankačk et al., 1993), a large-magnitude sink at the peak of pyramidal layer theta oscillation was found in the stratum lacunosum-moleculare (the target domain of entorhinal layer III axons), accompanied by a phase-shifted sink in the dentate gyrus molecular layer (the target domain of entorhinal layer II terminals) and a weaker sink in CA1 stratum radiatum (the target domain of CA3 axons) (Figure 2C). Rearing episodes were characterized by moderate elevations of CA1 radiatum and lacunosum-moleculare sinks and a prominent increase of the dentate gyrus sink (Figures S2, 2C, and 2D; rearing-coupled change of sink values compared with running: radiatum sink 3.6% ± 1.3%, lacunosum-moleculare sink 10.0% ± 1.1%, dentate gyrus sink 26.8% ± 7.4%; dentate versus lacunosum-moleculare sink, p = 0.02; dentate versus radiatum sink, p = 0.004; n = 4 mice; ANOVA F[2,9] = 7.53, p = 0.012 with post hoc Dunnett’s test). This laminar pattern of current sinks implies that rearing is associated with increased entorhinal inputs through the dentate gyrus via the perforant pathway as well as a weaker entorhinal layer III input to CA1 through the temporoammonic pathway.
Figure 2.
Enhancement of the DG Sink during Rearing
(A) Histological image shows the linear silicone probe track through all layers of the hippocampus. Oriens, stratum oriens; pyr, pyramidal layer; rad, radiatum; lac-mol, lacunosum-moleculare; mol, molecular layer; gran, granule cell layer; poly, polymorphic layer.
(B) Perievent binned (bin size = 1 s) average power in the low and high theta bands in the main layers of the hippocampal formation. Brown traces indicate the shuffled control (n = 4 animals; shaded area represents the 99% band of the shuffled control). Note the high theta power elevations in the lacunosum-moleculare and in the hilus of the DG.
(C) CSD maps from an animal of averaged LFP profile during running (left) and rearing (right). White traces at the top indicate theta oscillation in the pyramidal layer.
(D) Comparison of current sink magnitude during running and rearing (∗p < 0.05, ANOVA with post hoc Dunnett’s test; n = 4 animals; values are mean ± SEM). Note the prominent increase of dentate gyrus sink during rearing compared to running. Pr, pyramidal layer; Rd, radiatum; LM, lacunosum-moleculare; DG, dentate gyrus; Hl, hilus.
See also Figure S2.
Theta-Gamma Coupling in the Dentate Gyrus during Rearing Episodes
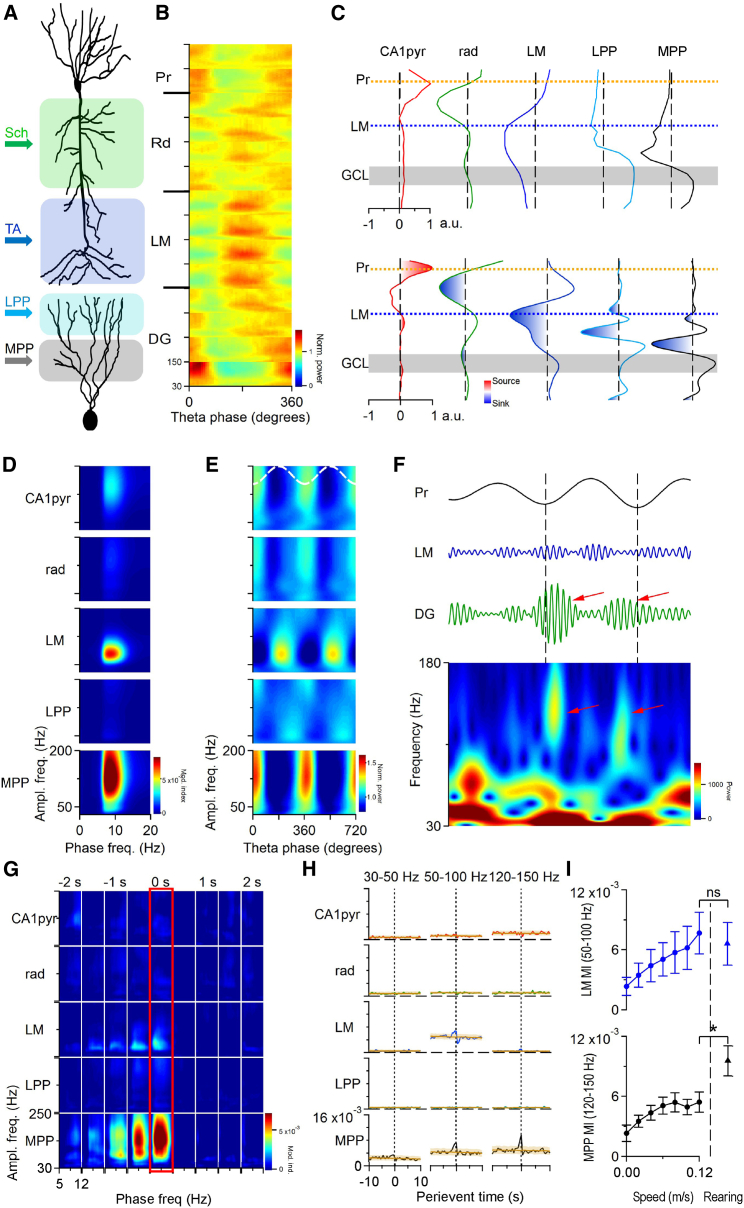
Theta and gamma oscillations are tightly coupled throughout the hippocampal formation (Bragin et al., 1995, Colgin et al., 2009, Csicsvari et al., 2003). Recently, the different theta phase-coupled gamma oscillations have been linked to specific pathways in the hippocampal formation (Figure 3A) (Colgin et al., 2009, Fernández-Ruiz et al., 2017, Lasztóczi and Klausberger, 2017, Schomburg et al., 2014). Previous studies have separated three different gamma frequency bands in CA1 (slow 30–50 Hz, mid 50–100 Hz, fast 120–150 Hz; Belluscio et al., 2012, Schomburg et al., 2014) that are linked to different current generators (i.e., synaptic inputs) and coupled to different phases of ongoing theta oscillation (Figures 3A and 3B). Because LFPs recorded at any location in the hippocampus are a mixture of local and distant volume-conducted fields (Buzsáki et al., 2012), we sought to disentangle the distinct gamma generators by applying independent-component analysis (ICA) to wide gamma band LFPs (30–200 Hz) in CA1 and the dentate (Fernández-Ruiz and Herreras, 2013). In each animal, five main independent components (ICs) were separated and identified as previously described (Fernández-Ruiz and Herreras, 2013, Fernández-Ruiz et al., 2012, Schomburg et al., 2014). Each component showed characteristic voltage and CSD depth profiles corresponding to the anatomical location of the underlying current sources and sinks (Figure 3C). Three ICs had activity restricted to the CA1 region, in the pyramidal (CA1pyr), radiatum (rad), and lacunosum-moleculare (LM) layers, respectively (Figure 3C, columns 1–3). Previous work identified the synaptic origin of these current generators: CA1 local circuits (CA1pyr), CA3 input to CA1 through Schaffer collaterals (rad), and entorhinal layer III input to CA1 through the temporoammonic pathway (LM) (Benito et al., 2014, Fernández-Ruiz et al., 2012, Fernández-Ruiz et al., 2017, Schomburg et al., 2014). Two additional ICs were restricted to the dentate gyrus, with main currents in the outer third (LPP) and middle third (MPP) of the molecular layer (Fernández-Ruiz et al., 2013), the target dendritic regions of perforant pathway axons from lateral and medial entorhinal cortex respectively (Figure 3C, columns 4 and 5).
Figure 3.
Robust Enhancement of Theta-Gamma Coupling in the Dentate Gyrus during Rearing Events
(A) Anatomical sketch that depicts the main inputs of CA1 pyramidal and dentate granule cells. Sch, Schaffer; TA, temporoammonic; LPP, lateral perforant; MPP, medial perforant pathways.
(B) CSD wavelet spectrograms indicate the characteristic gamma bands and their theta phase along the hippocampal formation. Pr, pyramidal layer; Rd, radiatum; LM, lacunosum-moleculare; DG, dentate gyrus.
(C) Independent-component analysis (ICA) of hippocampal LFPs resulted in five main components (ICs), three restricted to the CA1 area and two to the dentate gyrus. Top row: voltage loadings of the five identified LFP generators in the hippocampal formation (from left to right: CA1pyr, CA1 pyramidal layer; rad, radiatum; LM, lacunosum-moleculare; LPP, lateral perforant path; MPP, medial performant path generators; from top to bottom: Pr, pyramidal layer; LM, lacunosum-moleculare; GCL, granule cell layer). Bottom row: CSD loadings of the same generators. In the dentate gyrus one component had maximal negative amplitude in the outer molecular layer (LPP) and the other (MPP) in the middle of it and both had positive polarity through the granular layer and the hilus.
(D) Average cross-frequency-amplitude comodulograms for the five ICs during running (averages of n = 5 animals).
(E) Average theta phase-gamma amplitude coupling matrices for the five ICs during running (averages of n = 5 animals). Two theta cycles are shown for visualization purposes. Top dashed trace indicates theta oscillation in the CA1 pyramidal layer. Note the opposite theta-phase preference of LM and MPP gamma oscillations, matching the phase distribution of entorhinal layer II and III spiking and local gamma oscillations (Fernández-Ruiz et al., 2017). (D) and (E) were calculated from IC segments during which the animal movement speed was above 0.04 m/s for at least 2 s.
(F) Fast gamma in the dentate gyrus. First row: filtered (5–12 Hz) CA1 pyramidal layer LFP; second row: 120–150 Hz filtered lacunosum-moleculare LFP; third row: 120–150 Hz filtered dentate gyrus LFP; bottom row: wavelet spectrogram of the dentate gyrus LFP. Red arrows highlight fast gamma transients on the dentate gyrus recordings and corresponding wavelet spectrogram. Vertical dashed lines indicate the pyramidal layer theta troughs. Fast gamma components were not detected in the lacunosum-moleculare LFP.
(G) Average comodulograms of the five ICs for different time intervals around rearing peak (averages of n = 5 animals). Plots were constructed by averaging individual comodulograms from five animals followed by the subtraction of baseline (−10 to −5 s to rearing peak) comodulograms. Red rectangle highlights comodulograms at rearing peak (0 s). Note the robust enhancement of the theta coupling of the medial perforant path generator (MPP).
(H) Average perievent modulation index values of the five ICs (rows) in three different gamma frequency bands (columns; averages of n = 5 animals, brown traces indicate the shuffled control, shaded area represents the 99% band of the shuffled control). Vertical dashed lines at zero indicates rearing peak. The dentate fast gamma (MPP generator) has broad frequency range appearing in both 50–100 Hz and 120–150 Hz frequency bands.
(I) The coupling of LM mid gamma and MPP dentate fast gamma to theta oscillation increases with running speed. However, rearing-coupled MPP theta-gamma coupling is about two times larger than that is observed during running (∗p < 0.05, paired t test; n = 5 animals; values are mean ± SEM).
See also Figure S3.
Having identified dissociable gamma generators, we next sought to link that pathway-specific activity with theta oscillations and rearing episodes. Previous studies have demonstrated that gamma oscillatory activity in the hippocampus is tightly modulated by theta phase, a phenomenon called cross-frequency phase-amplitude coupling (CFC) (Bragin et al., 1995, Colgin et al., 2009). Therefore, we calculated the theta phase-gamma amplitude comodulograms of the five ICs for running episodes by using CA1 pyramidal layer theta as a reference. Phase-amplitude comodulograms of the five ICs calculated for running epochs showed modulation in specific gamma sub-bands and preferred theta phases, corresponding to isolated layer-specific gamma oscillations (Figures 3D and 3E), in agreement with previous reports in both rats and mice (Fernández-Ruiz et al., 2017, Lasztóczi and Klausberger, 2016, Lasztóczi and Klausberger, 2017, Schomburg et al., 2014).
We hypothesized that alteration of the animal’s vantage point and the resulting change in multimodal stimuli would lead to the redistribution of inputs and thus to the reorganization of theta-modulated gamma oscillations. Consistent with this view, theta-fast gamma coupling in the middle molecular layer of the dentate gyrus (MPP component) increased prominently during rearing (modulation index [MI] values of baseline 5–10 s before rearing peak versus rearing peak 0.0047 ± 0.00067 versus 0.0098 ± 0.0018; t[4] = −3.081; p = 0.037, paired t test; n = 5 animals) and showed maximal theta phase coupling during peak vertical head positioning (Figures 3G, 3H, and S3). Importantly, this fast gamma was likely not due to contamination from multiunit activity, as the cross-frequency coupling between fast gamma and high-frequency oscillations above 300 Hz was insignificant (Figure S3H). No other components exhibited significant rearing-coupled changes (CA1pyr fast gamma 0.0021 ± 0.00049 versus 0.0024 ± 0.00072, t[4] = −1.027, p = 0.36; rad slow gamma 0.00087 ± 0.00031 versus 0.0011 ± 0.0005, t[4] = −0.729, p = 0.51; LM mid gamma 0.005 ± 0.0019 versus 0.0066 ± 0.0026, t[4] = −2.038, p = 0.11; LPP slow gamma 0.0005 ± 0.00009 versus 0.0007 ± 0.0003, t[4] = −0.855, p = 0.44; paired t test; n = 5 animals). Note that the two strongest gamma components, LM and MPP, have opposite theta phase preferences (Figure 3E). Their preferred theta phase matches with the reported firing phase of entorhinal cortex layer III and layer II principal cells, respectively (Fernández-Ruiz et al., 2017, Mizuseki et al., 2009) and with the phase-shifted theta sinks in stratum lacunosum-moleculare and dentate (Figure 2C). These results suggest that during rearing episodes, medial entorhinal cortex (MEC) inputs to the hippocampus are enhanced, especially those originating in layer II (van Groen et al., 2003).
Running speed has been shown to modulate gamma amplitude in CA1 (Ahmed and Mehta, 2012, Zheng et al., 2015). However, the decoupling of theta frequency and speed of horizontal movement during rearing events raises the possibility of dissociation between horizontal movement and theta-modulated gamma patterns. Therefore, we determined the relationship between running and rearing-coupled dynamics of theta phase-gamma amplitude CFC. First, we found that theta-lacunosum-moleculare mid gamma CFC increased linearly with running speed (LM; Figures 3I, S3E, and S3G; for speed > 0.04 m/s: Pearson’s r > 0.6, p < 0.005; n = 5 animals), in agreement with previous reports (Chen et al., 2011). In contrast, theta-dentate fast gamma (MPP) coupling and speed were not correlated (Figure 3I; for speed > 0.04 m/s: Pearson’s r < 0.3, p > 0.1; n = 5 animals). The theta-lacunosum-moleculare mid gamma CFC during rearing did not surpass values that characterized high running speeds, whereas an almost 2-fold elevation was uncovered for the theta-dentate fast gamma coupling at the peak of rearing versus running (Figure 3I; LM IC 50–100 Hz CFC at rearing versus running at 0.12 m/s 81.15% ± 11.96%, t[4] = −1.577, p = 0.19; MPP IC 120–150 Hz CFC at rearing versus running at 0.12 m/s 190.28% ± 32.18%, t[4] = 2.805, p < 0.05; n = 5 animals). The above results regarding theta-gamma CFC during rearing compared with running were verified by both LFP and CSD analyses (Figures S3E and S3G). Taken together, the reorganization of theta-gamma patterns point to the redistribution of network activity both in hippocampal circuits and in input pathways dominated by an enhanced theta-gamma input from MEC to dentate gyrus (DG).
A Subpopulation of Hippocampal Units Shows Rearing-Coupled Facilitation
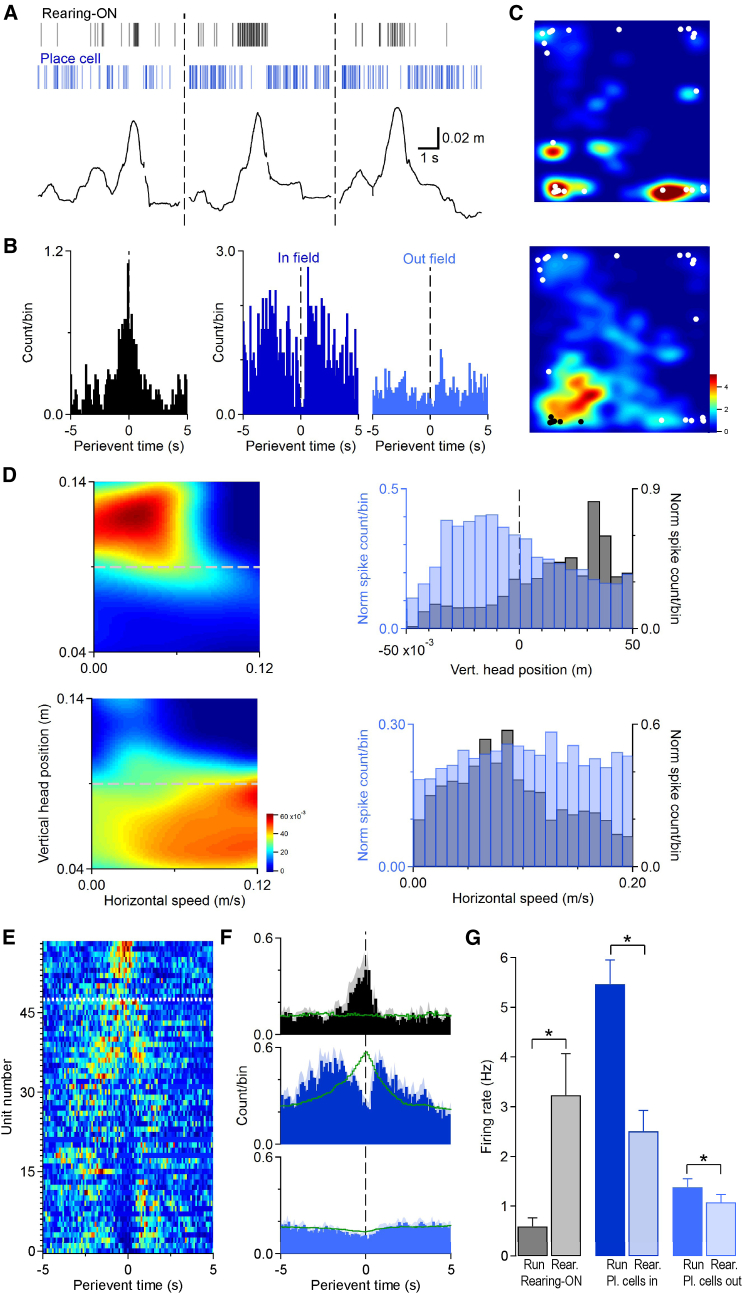
The analysis on network oscillations suggests a redistribution of inputs during rearing. Such redistribution may also affect neuronal firing dynamics, resulting in rearing-specific activity patterns of hippocampal units. To test this hypothesis, we next analyzed how single neurons respond during rearing epochs. On the basis of waveform features and firing rates, pyramidal layer single units were separated into putative interneurons and principal cells (Figures S4A and S4B; n = 124 putative pyramidal cells and n = 25 putative interneurons from n = 5 mice). Rearing peak-triggered firing histograms uncovered hippocampal principal units that showed robust firing rate increases around the rearing peak (rearing-on units, n = 11 from five animals; Figure 4A). The analysis of individual rearing events revealed that rearing-on units were activated in 27.5% ± 4.1% of rearing epochs (on average there were 20 ± 7 “active” rearing events for each rearing-on unit). We compared the spatial distribution of these “active” rearing events with randomly selected rearing events. For each rearing-on unit, the mean difference of horizontal positions of the “active” rearing events was calculated (see Experimental Procedures). We found no significant difference when we compared these values with randomized ones (average mean difference values for rearing-on units 0.20 ± 0.022 m, randomized 0.23 ± 0.007 m; n = 11; W = 20; p = 0.28, Wilcoxon signed-rank test). This calculation indicates that the “active” rearing events did not occur in a spatially restricted area. The comparison of the durations of the active and non-active rearing events did not reveal significant differences (duration of active versus non-active rearing episodes 2.08 ± 0.51 versus 2.06 ± 0.42 s; t[10] = 0.097; n = 11; p = 0.92, paired t test). We also compared rearing events occurring at the periphery (within 5 cm of the walls) or in the center (beyond 5 cm from the wall). On average 84.3% ± 4% of the total rearing numbers occurred within 5 cm of a wall. Active rearing events were also observed at larger percentage at the periphery (periphery versus center 30.0% ± 4.6% versus 17.3% ± 5.5%; n = 9 rearing-on units; W = 3; p = 0.02, Wilcoxon signed-rank test). The higher incidence of rearing-on spiking near the walls indicated an apparent border preference of these units. However, this might be explained by the larger number of wall-proximal compared with central rearing events (Figures S4D and S4E). The acquisition of visual information is a key function of rearing, and visually guided directional tuning of hippocampal neuronal activity has recently been described (Acharya et al., 2016), so we tested if rearing-on units exhibit head directional preference. Because “behavioral constrains” (i.e., close to the wall, the mice preferentially head toward the wall) can result in apparent directional preference, we weighted the head direction distributions in each location bin by the firing rate of rearing-on units in that bin and calculated the correlation between the observed and reconstructed (weighted) head direction curves (Muller et al., 1994, Rubin et al., 2014). The reconstructed and observed head directions of rearing-on units were highly correlated, suggesting that instead of real head directional preference, behavioral constraints (i.e., proximity to the wall) could explain their apparent head direction tuning (Figure S4F). Finally, we found that rearing-on units showed a significant decrease in firing during movement (Figure 4G; firing rate during rearing 3.2 ± 0.8 Hz, during movement 0.6 ± 0.2 Hz; n = 11 rearing-on units in five mice; W = 5; p = 0.01, Wilcoxon signed-rank test).
Figure 4.
Single-Unit Activity during Rearing Behavior
(A) Rearing-on units and place cells show contrasting rearing-dependent activity. Bottom row: vertical head position during rearing events. Middle row: blue vertical lines, sample place cell spikes. Top row: black vertical lines, sample rearing-on unit spikes. The rearing-on unit increased and the place cell decreased activity during rearing epochs.
(B) Rearing peak-triggered perievent firing histograms corresponding to the units in A. Left: rearing-on unit; middle: place cell infield (within place field); right: place cell outfield (out of place field).
(C) Spatial firing maps of a rearing-on (top) and place cell (bottom, black dots indicate the infield, white dots the outfield rearing events).
(D) Rearing-on units and place cells have different firing preference for vertical head position and horizontal speed. Two-dimensional histograms show the horizontal speed and vertical head position dependence of a rearing-on unit (left top) and a place cell (left bottom). Units are the same as in (C). Horizontal gray dashed lines mark the threshold of rearing events. Average vertical head position (right top, overlaid histograms) and horizontal speed distributions (right bottom, overlaid histograms) highlight the differences between rearing-on units (gray) and place cells (blue). Vertical head positions are measured form the rearing threshold.
(E) Rearing peak-triggered perievent firing histograms of rearing-on units (above horizontal dashed line) and infield perievent histograms for place cells (below horizontal dashed line). The Z-scored perievent histograms were ordered by their average value between −0.25 and +0.25 s around rearing peaks.
(F) Average rearing peak-triggered perievent histograms of rearing-on units and place cells: rearing-on units (top; n = 11 units), place cells infield (middle; n = 48 units), place cells outfield (bottom; n = 48 units). Green traces indicate the shuffled data. Shaded area around the histogram bars corresponds to SEM.
(G) Summarized data showing the firing rate during running and rearing for rearing-on units and place cells. In the case of place cells, infield and outfield firing data are shown separately (∗p < 0.05, Wilcoxon signed-rank test; n = 11 rearing-on units, n = 48 place cells; error bars represent SEM).
See also Figure S4.
In order to determine if rearing-on units correspond to a separate functional subgroup among hippocampal cells, we compared their activity with that of place cells. We identified place cells (see Experimental Procedures) and separated “infield” and “outfield” rearing events on the basis of whether the rearing occurred inside or outside the unit’s place field (Figures 4B and 4C). Surprisingly, the activity of place cells was significantly decreased during rearing epochs, especially when the animal reared within the place cell’s place field (Figures 4E–4G; infield firing rate during rearing 2.5 ± 0.4 Hz, during running 5.5 ± 0.5 Hz W = 120, p < 0.001; outfield firing rate during rearing 1.1 ± 0.2 Hz, during running 1.4 ± 0.2 Hz, n = 48 place cells in five mice, W = 220, p < 0.001 [Wilcoxon signed-rank test]). The decrease in the place cells’ firing rate in a 0.5 s time window around rearing peaks was significantly higher when the rearing occurred infield versus outfield (percentage decrease of firing rate infield 46.3% ± 10.5% versus outfield 25.9% ± 5.7%; n = 48 place cells; p < 0.001, Wilcoxon signed-rank test). To further differentiate between rearing-on units and place cells, we calculated the distributions of vertical head positions and horizontal speed values corresponding to each spike. The average distributions show strikingly different patterns: place cells preferentially fire at “low” head positions (below rearing threshold), whereas rearing-on units fire at elevated head positions (Figure 4D; average head position from rearing threshold for rearing-on units 0.014 ± 0.003 m, n = 11; place cells −0.005 ± 0.001 m, n = 57; U = 47; p < 0.001, Wilcoxon-Mann-Whitney test). On average, rearing-on units also show lower horizontal speed preference (Figure 4D; average horizontal speed values for rearing-on units 0.098 ± 0.006 m/s, n = 11 cells; place cells 0.117 ± 0.002 m/s, n = 57 cells; U = 142; p = 0.003, Wilcoxon-Mann-Whitney test). However, on the basis of conventional place cell metrics (spatial information for rearing-on units 2.06 ± 0.35 bits/spike, n = 11 units; for place cells 2.02 ± 0.16 bits/spike, n = 57 units; U = 307; p = 0.92, Wilcoxon-Mann-Whitney test; sparsity for rearing-on units 0.21 ± 0.03, n = 11 units; for place cells 0.26 ± 0.02, n = 57 units; U = 305; p = 0.89, Wilcoxon-Mann-Whitney test), rearing-on units and place cells formed overlapping groups (n = 8 of 11 rearing-on units satisfied the criteria for place cells).
It has recently been reported that pyramidal cells in the CA2 area of the hippocampus show increased firing during immobility or low running speed, and these cells either decrease their activity during sharp wave ripples or increase their firing right before them (Kay et al., 2016, Oliva et al., 2016). However, the robust elevation of rearing-on units firing rate at sharp wave ripple peaks (Figure S4C) distinguishes them from the immobility-related units described previously. Additionally, our histological reconstruction of electrode tracks verified that recordings were never performed close to the CA2 region. However, we cannot exclude a possible partial overlap between the recently characterized immobility-associated place cells in the CA1 and rearing-on units, though the former cells were mostly active near reward zones (Yu et al., 2017), whereas studying the reward-related activity of rearing-on units was beyond the scope of our study. In our experiments, we recorded free exploration without delivering reward, so comparison of data from the cited report with ours is not possible.
Discussion
Using a combination of high-speed motion tracking in three dimensions and the recording of activity in multiple layers and subregions, we identified and characterized hippocampal network dynamics while mice were rearing on hindlimbs. During rearing, we found an increase in power of high-frequency theta across multiple hippocampal subregions. We also found an increase in theta phase-fast gamma amplitude coupling in the DG middle molecular layer, as well as an insignificant increase in theta-mid gamma coupling in the stratum lacunosum-moleculare. This theta-phase-modulated gamma amplitude is consistent with an increased flow of information from the entorhinal cortex (Fernández-Ruiz et al., 2017, Lasztóczi and Klausberger, 2017, Schomburg et al., 2014), with strong MEC layer II signaling to the DG and relatively moderate MEC layer III signaling to CA1. Finally, we observed different populations of pyramidal cells that were spatially tuned during running and decreased their activity during rearing (“place cells”) and a different population that increased firing during rearing and showed reduced activity while the animal was running (“rearing-on units”). Such behavioral modulation of single cell activity suggests that the switch in afferent drive during rearing is sufficient to impose different processing modes on the hippocampus.
A prime function of rearing is to make alternative sources of multimodal sensory information (i.e., context) accessible for the exploring animal. In turn, the acquired information enables the recognition of novelty, the discrimination of different environments and ultimately, the refinement of the forming spatial map. DG is important for novelty-induced rearing and for learning new spatial information (Saab et al., 2009). In our study, the DG was found to be the main locus of rearing-coupled change of input patterns, suggesting that contextual information sampled during rearing events is conveyed mainly by the medial perforant pathway to the hippocampal formation through the DG (Kitamura et al., 2015). Our results may also support the possibility that rearing can contribute to the correction of position estimation. Navigation is known to be controlled by self-motion cues and distal landmark information (Etienne and Jeffery, 2004). Depriving the navigating animal from external cues leads to the accumulation of errors in the estimation of position (Séguinot et al., 1993). Visual input is thus crucial for correcting the positional signal (Maaswinkel and Whishaw, 1999) and rearing may be a critical moment when such information is used to calibrate the cognitive map. Notably, without visually detectable external cues, the characteristic hexagonal grid pattern of activity in the MEC is also disrupted (Chen et al., 2016), possibly contributing to the deterioration of hippocampal position estimation. Therefore, increased input from the MEC to the DG in the rearing state implies that the information acquired during rearing can be pivotal for realigning the spatial map in the MEC as well as for updating the hippocampal spatial representation (Chen et al., 2016, Pérez-Escobar et al., 2016). In support of this role for rearing in reorganization of the cognitive map, new place fields often emerge in the locations in which rats stop and scan their environment (Monaco et al., 2014).
Hippocampal pyramidal cells can be tuned for multiple types of information (Wood et al., 2000). Rearing-on units were only activated in about a third of rearing events, suggesting that their activity is not representing the behavioral act of rearing but rather the specific information afforded by the change in vantage point. It is possible that the rearing-on units formed an unidentified subgroup of pyramidal cells, perhaps defined by the pattern of afferent connectivity. Alternatively, rearing-on cells could be activated by the altered neuromodulatory tone or by unreliable feedback from the motor system. We observed a slight preference of rearing-on unit activation for rearing events close to the walls of the arena, possibly reflecting a modulatory influence by boundary vector cells (Lever et al., 2009). Importantly, the lack of firing during ambulation of rearing-on units clearly differentiate them from other spatially modulated neurons of the hippocampal formation e.g., border cells (Solstad et al., 2008), grid cells (Hafting et al., 2005), or place cells (O’Keefe and Dostrovsky, 1971). However, the partial overlap of the rearing-on and place cell groups raises the possibility that the former neurons can be a subgroup of place cells, but it also highlights the limitations of determining the spatial correlates of activity without tracking the behavior in all three dimensions.
In contrast to rearing-on units, place cells exhibited decreased activity when rearing events occurred within their place fields. Reduction of firing can be caused by an active inhibitory process or can be due to the tuning of place cells’ activity for representing location on the horizontal plane only during movement. Because of the firing rate reduction, the coding of the rearing-coupled location may be temporarily segregated from the code carried by typical “place cells.” We hypothesize that rearing events may be triggered by moments of uncertainty about allocentric positioning, times when the cognitive map would contain the highest error and could most benefit from sensory driven re-alignment. Intra-place field reduction of place cell firing temporarily decouples the activity of place cells from that of rearing-related inputs. Thus, rearing can anchor the forming map to external cues and prevent the formation of erroneous associations between the cognitive map that has drifted and incoming perceptual inputs. Taken together, the rearing state may aid in the formation of a reliable map of the environment by allowing the repeated updating and correction of the spatial code during exploration.
Experimental Procedures
Further details on the methods can be found in Supplemental Experimental Procedures.
Mice
Twelve male mice (two Vglut3-ires-Cre, seven Som-ires-Cre, one Vgat-ires-Cre, and two wild-type C57BL/6J, 2–5 months old) were used in this study (Table S1). All mice were used in other projects as well. Mice were kept in the vivarium on a 12 hr light/dark cycle and provided with food and water ad libitum. The animals were housed two or three per cage before surgery and individually after it. All experiments were approved by the Ethical Committee for Animal Research at the Institute of Experimental Medicine, Hungarian Academy of Sciences, and conformed to Hungarian (1998/XXVIII Law on Animal Welfare) and European Communities Council Directive recommendations for the care and use of laboratory animals (2010/63/EU) (license number PE/EA/2552-6/2016).
Summary of Methods
Details on the methods can be found in Supplemental Experimental Procedures. Briefly, mice were implanted with silicone probes targeting the dorsal hippocampus. Hippocampal activity was recorded while the animals were freely exploring a rectangular arena. Simultaneously with electrophysiological recording, the animals’ movement was tracked in three dimensions using a multi-camera system. At the end of the experiments, mice were transcardially perfused, the brains were removed and sectioned, and the probe tracks were reconstructed. Rearing episodes were detected on the basis of the distribution of vertical head positions. Hippocampal LFP oscillations (delta, theta, and gamma) were separated using the time-frequency decomposition of the wide band LFP signal by continuous wavelet transformation or on the basis of the Hilbert magnitude of the band-pass-filtered signal (ripple). The relationship of hippocampal oscillations to rearing events was analyzed by peri-rearing distribution of power. The underlying current generators of theta and gamma oscillations were separated and analyzed by CSD and independent-component analyses. Theta-gamma cross-frequency coupling was calculated by using the phase of theta extracted from time-frequency decomposition and power, CSD, or separated ICs of gamma. Multiple single units were separated by principal-component analysis combined with clustering or by a template matching algorithm. Final spike clusters were generated by manual curation. The correlation of putative single units and rearing events was analyzed by perievent distribution of spikes. Various parameters (state-dependent firing rates, spatial information, sparsity, head direction tuning) of units that exhibited rearing-related and/or spatially modulated firing were calculated.
Quantification and Statistical Analysis
All statistical analyses were performed with standard IgorPro7 and MATLAB functions. For two-sample comparisons, the parametric Student’s t test, non-parametric Wilcoxon signed-rank test, or Wilcoxon-Mann-Whitney test was used. For multiple comparisons, ANOVA with Tukey’s honest significant difference or Dunnett’s post hoc test was used. In the case of parametric statistics, normality was tested using the Shapiro-Wilk test, and equality of variances was tested using the F test or Levene’s test. All graphs indicate mean ± SEM; n represents animal number or unit number as indicated. For significance, p = 0.05 was used.
Acknowledgments
We wish to thank Gabrielle Girardeau, Marta Jelitai, Gyorgy Buzsaki, John Long, Peter Petersen, Yuta Senzai, and especially Sam McKenzie for reading, commenting, and correcting various versions of the manuscript. We would also like to acknowledge the contribution of our expert reviewers to improving our manuscript. We thank Emoke Simon, Katalin Ivanyi, Katalin Lengyel, and Gyozo Goda for technical assistance as well as Laszlo Barna and the Nikon Microscopy Centre at the Institute of Experimental Medicine, Nikon Austria GmbH, and Auro-Science Consulting Ltd. for help with histological imaging. This study was supported by European Research Council grant ERC-2011-ADG-294313-SERRACO to T.F.F., National Research, Development and Innovation Office grant K109970 to V.V. and grant PD121248 to A.M.B., a Sir Henry Wellcome Postdoctoral Fellowship to A.F.-R., and by the National Brain Program of Hungary.
Author Contributions
A.D., A.M.B., and V.V. designed the experiments. A.D. and A.M.B. developed techniques. A.D. and A.M.B. conducted the experiments. A.M.B. and A.F.-R. analyzed data. A.D., A.M.B., and V.V. conceptualized the study. V.V. supervised the project. A.M.B., A.F.-R., T.F.F., and V.V. wrote the paper. T.F.F. and V.V. acquired funding.
Declaration of Interests
The authors declare no competing interests.
Published: May 8, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.04.021.
Supplemental Information
References
- Acharya L., Aghajan Z.M., Vuong C., Moore J.J., Mehta M.R. Causal influence of visual cues on hippocampal directional selectivity. Cell. 2016;164:197–207. doi: 10.1016/j.cell.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Ahmed O.J., Mehta M.R. Running speed alters the frequency of hippocampal gamma oscillations. J. Neurosci. 2012;32:7373–7383. doi: 10.1523/JNEUROSCI.5110-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio M.A., Mizuseki K., Schmidt R., Kempter R., Buzsáki G. Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J. Neurosci. 2012;32:423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito N., Fernández-Ruiz A., Makarov V.A., Makarova J., Korovaichuk A., Herreras O. Spatial modules of coherent activity in pathway-specific LFPs in the hippocampus reflect topology and different modes of presynaptic synchronization. Cereb. Cortex. 2014;24:1738–1752. doi: 10.1093/cercor/bht022. [DOI] [PubMed] [Google Scholar]
- Bragin A., Jandó G., Nádasdy Z., Hetke J., Wise K., Buzsáki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankačk J., Stewart M., Fox S.E. Current source density analysis of the hippocampal theta rhythm: associated sustained potentials and candidate synaptic generators. Brain Res. 1993;615:310–327. doi: 10.1016/0006-8993(93)90043-m. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Leung L.W., Vanderwolf C.H. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Czopf J., Kondákor I., Kellényi L. Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res. 1986;365:125–137. doi: 10.1016/0006-8993(86)90729-8. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Anastassiou C.A., Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Resnik E., McFarland J.M., Sakmann B., Mehta M.R. Speed controls the amplitude and timing of the hippocampal gamma rhythm. PLoS ONE. 2011;6:e21408. doi: 10.1371/journal.pone.0021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Manson D., Cacucci F., Wills T.J. Absence of visual input results in the disruption of grid cell firing in the mouse. Curr. Biol. 2016;26:2335–2342. doi: 10.1016/j.cub.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin L.L., Denninger T., Fyhn M., Hafting T., Bonnevie T., Jensen O., Moser M.-B., Moser E.I. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Csicsvari J., Jamieson B., Wise K.D., Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Deacon R.M.J., Croucher A., Rawlins J.N.P. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav. Brain Res. 2002;132:203–213. doi: 10.1016/s0166-4328(01)00401-6. [DOI] [PubMed] [Google Scholar]
- Etienne A.S., Jeffery K.J. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz A., Herreras O. Identifying the synaptic origin of ongoing neuronal oscillations through spatial discrimination of electric fields. Front. Comput. Neurosci. 2013;7:5. doi: 10.3389/fncom.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A., Makarov V.A., Herreras O. Sustained increase of spontaneous input and spike transfer in the CA3-CA1 pathway following long-term potentiation in vivo. Front. Neural Circuits. 2012;6:71. doi: 10.3389/fncir.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A., Muñoz S., Sancho M., Makarova J., Makarov V.A., Herreras O. Cytoarchitectonic and dynamic origins of giant positive local field potentials in the dentate gyrus. J. Neurosci. 2013;33:15518–15532. doi: 10.1523/JNEUROSCI.0338-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A., Oliva A., Nagy G.A., Maurer A.P., Berényi A., Buzsáki G. Entorhinal-CA3 dual-input control of spike timing in the hippocampus by theta-gamma coupling. Neuron. 2017;93:1213–1226.e5. doi: 10.1016/j.neuron.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T., Fyhn M., Molden S., Moser M.B., Moser E.I. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Harley C.W., Martin G.M. Open field motor patterns and object marking, but not object sniffing, are altered by ibotenate lesions of the hippocampus. Neurobiol. Learn. Mem. 1999;72:202–214. doi: 10.1006/nlme.1998.3902. [DOI] [PubMed] [Google Scholar]
- Kay K., Sosa M., Chung J.E., Karlsson M.P., Larkin M.C., Frank L.M. A hippocampal network for spatial coding during immobility and sleep. Nature. 2016;531:185–190. doi: 10.1038/nature17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Sun C., Martin J., Kitch L.J., Schnitzer M.J., Tonegawa S. Entorhinal cortical ocean cells encode specific contexts and drive context-specific fear memory. Neuron. 2015;87:1317–1331. doi: 10.1016/j.neuron.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim J.J., Hamilton D.A. Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation. Physiol. Rev. 2011;91:1245–1279. doi: 10.1152/physrev.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B., Bragin A., Buzsáki G. Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis. J. Neurosci. 1999;19:6200–6212. doi: 10.1523/JNEUROSCI.19-14-06200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasztóczi B., Klausberger T. Hippocampal place cells couple to three different gamma oscillations during place field traversal. Neuron. 2016;91:34–40. doi: 10.1016/j.neuron.2016.05.036. [DOI] [PubMed] [Google Scholar]
- Lasztóczi B., Klausberger T. Distinct gamma oscillations in the distal dendritic fields of the dentate gyrus and the CA1 area of mouse hippocampus. Brain Struct. Funct. 2017;222:3355–3365. doi: 10.1007/s00429-017-1421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C., Cacucci F., Wills T., Burton S., McClelland J., Burgess N., O’Keefe J. Spatial coding in the hippocampal formation: input, information type, plasticity, and behaviour. In: Jeffery K.J., editor. The Neurobiology of Spatial Behavior. Oxford University Press; 2003. [Google Scholar]
- Lever C., Burton S., O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 2006;17:111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Lever C., Burton S., Jeewajee A., O’Keefe J., Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H., Whishaw I.Q. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav. Brain Res. 1999;99:143–152. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- McFarland W.L., Teitelbaum H., Hedges E.K. Relationship between hippocampal theta activity and running speed in the rat. J. Comp. Physiol. Psychol. 1975;88:324–328. doi: 10.1037/h0076177. [DOI] [PubMed] [Google Scholar]
- Mizuseki K., Sirota A., Pastalkova E., Buzsáki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J.D., Rao G., Roth E.D., Knierim J.J. Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat. Neurosci. 2014;17:725–731. doi: 10.1038/nn.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.M., Betancur M.I., Buzsáki G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J. Neurosci. 2009;29:1381–1394. doi: 10.1523/JNEUROSCI.4339-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R.U., Bostock E., Taube J.S., Kubie J.L. On the directional firing properties of hippocampal place cells. J. Neurosci. 1994;14:7235–7251. doi: 10.1523/JNEUROSCI.14-12-07235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun H.S., Saab B.J., Ng E., McGirr A., Lipina T.V., Gondo Y., Georgiou J., Roder J.C. Self-directed exploration provides a Ncs1-dependent learning bonus. Sci. Rep. 2015;5:17697. doi: 10.1038/srep17697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J., Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J., Nadel L. Précis of O’Keefe & Nadel’s The hippocampus as a cognitive map. Behav. Brain Sci. 1979;2:487–494. [Google Scholar]
- Oliva A., Fernández-Ruiz A., Buzsáki G., Berényi A. Role of hippocampal CA2 region in triggering sharp-wave ripples. Neuron. 2016;91:1342–1355. doi: 10.1016/j.neuron.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Escobar J.A., Kornienko O., Latuske P., Kohler L., Allen K. Visual landmarks sharpen grid cell metric and confer context specificity to neurons of the medial entorhinal cortex. eLife. 2016;5:5. doi: 10.7554/eLife.16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A., Yartsev M.M., Ulanovsky N. Encoding of head direction by hippocampal place cells in bats. J. Neurosci. 2014;34:1067–1080. doi: 10.1523/JNEUROSCI.5393-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab B.J., Georgiou J., Nath A., Lee F.J.S., Wang M., Michalon A., Liu F., Mansuy I.M., Roder J.C. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron. 2009;63:643–656. doi: 10.1016/j.neuron.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Schomburg E.W., Fernández-Ruiz A., Mizuseki K., Berényi A., Anastassiou C.A., Koch C., Buzsáki G. Theta phase segregation of input-specific gamma patterns in entorhinal-hippocampal networks. Neuron. 2014;84:470–485. doi: 10.1016/j.neuron.2014.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguinot V., Maurer R., Etienne A.S. Dead reckoning in a small mammal: the evaluation of distance. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1993;173:103–113. doi: 10.1007/BF00209622. [DOI] [PubMed] [Google Scholar]
- Solstad T., Solstad T., Boccara C.N., Boccara C.N., Kropff E., Kropff E., Moser M.-B., Moser M.-B., Moser E.I., Moser E.I. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- van Groen T., Miettinen P., Kadish I. The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus. 2003;13:133–149. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- Vanderwolf C.H. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vanderwolf C.H. Neocortical and hippocampal activation relation to behavior: effects of atropine, eserine, phenothiazines, and amphetamine. J. Comp. Physiol. Psychol. 1975;88:300–323. doi: 10.1037/h0076211. [DOI] [PubMed] [Google Scholar]
- Wells C.E., Amos D.P., Jeewajee A., Douchamps V., Rodgers J., O’Keefe J., Burgess N., Lever C. Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. J. Neurosci. 2013;33:8650–8667. doi: 10.1523/JNEUROSCI.5040-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.A., McNaughton B.L. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wood E.R., Dudchenko P.A., Robitsek R.J., Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Young C.K., McNaughton N. Coupling of theta oscillations between anterior and posterior midline cortex and with the hippocampus in freely behaving rats. Cereb. Cortex. 2009;19:24–40. doi: 10.1093/cercor/bhn055. [DOI] [PubMed] [Google Scholar]
- Yu J.Y., Kay K., Liu D.F., Grossrubatscher I., Loback A., Sosa M., Chung J.E., Karlsson M.P., Larkin M.C., Frank L.M. Distinct hippocampal-cortical memory representations for experiences associated with movement versus immobility. eLife. 2017;6:6. doi: 10.7554/eLife.27621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Bieri K.W., Trettel S.G., Colgin L.L. The relationship between gamma frequency and running speed differs for slow and fast gamma rhythms in freely behaving rats. Hippocampus. 2015;25:924–938. doi: 10.1002/hipo.22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.