Abstract
Objective We aimed to compare major complication rates in patients undergoing open versus endoscopic resection of olfactory neuroblastoma (ONB) and to determine the prognostic utility of the Kadish staging and Hyams grading systems with respect to progression-free survival (PFS) and overall survival (OS).
Methods It is a retrospective review of experience in treating ONB at a single tertiary care hospital from 1987 through 2015. Major complications were defined as cerebrospinal fluid (CSF) leak, meningitis, osteomyelitis, tracheostomy, and severe neurologic injury.
Results Forty-one patients were included. An open approach was used in 34 (83%), endoscopic in 6 (15%), and combined in 1 (2%) case. Rates of major complications by surgical approach were 17% after endoscopic versus 31% after open ( p = 0.65). There was no significant difference in PFS or OS based on Kadish B versus C (PFS, p = 0.28; OS, p = 0.11) or Hyams grade 1 and 2 versus Hyams grade 3 and 4 (PFS, p = 0.53; OS, p = 0.38).
Conclusions There was no significant difference in major complications between open and endoscopic approaches for the treatment of ONB. Patient stratification using the Kadish staging and Hyams grading systems did not show significant differences in PFS or OS. Further research is needed to determine if a different staging system would better predict patient outcomes.
Keywords: olfactory neuroblastoma, endoscopic, complication, recurrence, survival, Kadish, Hyams
Introduction
Olfactory neuroblastoma (ONB) is a rare neuroectodermal tumor usually originating from the olfactory mucosa of the cribriform plate, upper third of the nasal septum, or superior turbinate. 1 Since it was first described in 1924 by Berger and Richard, 2 our understanding and treatment of this tumor has evolved. It has been shown that recurrence rates are reduced with multimodality treatment. 3 4 5 6 7 8 9 Staging systems have been proposed based on tumor extent, including the Kadish system 10 (and modifications of this) and the TNM system developed by Dulguerov. 11 Additionally, Hyams developed a grading system based on histologic features. 12
In the recent era of endoscopic cranial base surgery, there has been a trend to perform surgical resection endoscopically rather than via a craniofacial or subcranial approach. 13 14 Komotar et al performed a review of the literature comparing endoscopic, craniofacial and combined cranionasal approaches. They reviewed 47 studies, 19 of which reported endoscopic results and found increased overall and disease-free survival with endoscopic compared with craniofacial resection. These results were confounded by the fact that endoscopically resected tumors tended to be a lower Kadish stage. 13 They found similar rates of cerebrospinal fluid (CSF) leak between the two surgical approaches. In 2015, Feng et al added their institutional experience to the body of research, concluding that their complication rate was lower when using an endoscopic approach. 14
The main objectives of this study were to compare major complication rates for endoscopic versus open surgical approaches and to describe the progression-free survival (PFS) and overall survival (OS) of our cohort. Our secondary aim was to determine the prognostic value of the Kadish staging and Hyams grading systems for our cohort of patients.
Methods
Retrospective review of cases of ONB at a single tertiary care hospital was performed after obtaining institutional review board approval. Forty-six patients diagnosed with ONB from 1987 to 2015 were initially identified. Four patients with ONB were excluded from data analysis due to prior treatment at another institution. One patient was excluded because pathologic review revealed melanoma, not ONB.
Prior to May 2010, the open subcranial approach was the only approach performed at our institution. Since May 2011, an endoscopic approach has been selected for all patients whose disease is assessed as resectable via an endoscopic approach on preoperative imaging. Our standard open technique used was a subcranial approach for resection and reconstruction using a pericranial flap. The endoscopic technique varied based on tumor location, but always included image guidance. Turbinate and septal resection extent were limited to that required for access, tumor resection, and achievement of negative surgical margins. Reconstruction was determined by the size and quality of the defect. Commonly used reconstructive tools were the vascularized pedicled nasoseptal flap alone or multilayered closure with fat and mucosa (free or pedicled).
Kadish stage was determined by reviewing clinical notes, imaging, and operative reports. Hyams grade was determined by slide review by our senior head and neck pathologist. Major complications were defined as CSF leak, meningitis, osteomyelitis, tracheostomy, and severe neurologic injury. Severe neurologic injury was defined as permanent neurologic deficit that impacts the ability to perform activities of daily life. It could be due to any cause and did not need to be due to stroke. Major complications were identified by reviewing the postoperative documentation for each patient. Follow-up time was calculated from surgical date to confirmed recurrence or to the last clinical evaluation.
Statistical analysis was performed using SPSS software. All p values were calculated using the Fisher's exact test or log rank survival analysis for PFS and OS. Since some surgical procedures did not achieve gross total resection (due to extensive multisite involvement that would lead to significant morbidity), we chose to use PFS rather than recurrence-free survival. A p value of 0.05 was considered statistically significant.
Results
Forty-one patients were included in the study. Twenty-nine (71%) were male, and 12 (29%) were female. Median age was 48 years (range: 20–76 years). A clear bimodal distribution of age was not seen ( Fig. 1 ). Median follow-up was 6.5 years (range: 0.2–21 years). The most common presenting symptoms were nasal obstruction and epistaxis. Three patients had visual symptoms, including epiphora, pain, and decreased visual acuity. An open subcranial approach 9 was used in 34 (83%) cases, an endoscopic approach was used in 6 (15%), and a combined approach was used in 1 (2%) case. Fig. 2 depicts the number of cases as per 5-year period and surgical approach.
Fig. 1.
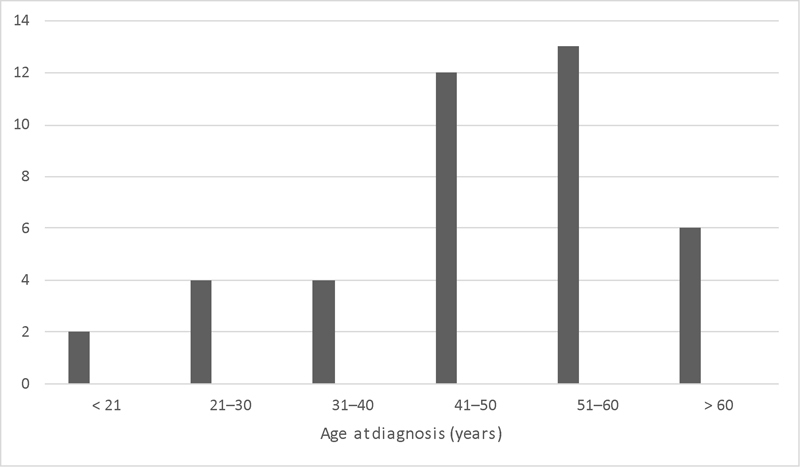
Age at diagnosis. All patients included in study. Unlike most reports, we did not observe a bimodal age distribution.
Fig. 2.
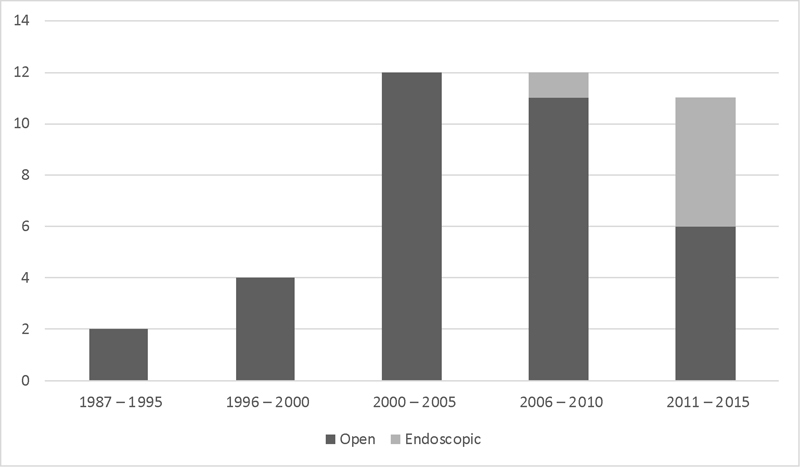
Year of treatment and surgical approach. Open indicates subcranial surgical approach. Endoscopic indicates completely endoscopic surgical approach. There was an increase in patients treated since 2000.
One (2.5%) patient was Kadish stage A, 20 (49%) were Kadish stage B, 19 (46%) were Kadish stage C, and 1 (2.5%) was Kadish stage D. No endoscopic case was converted to an open approach due to unresectable disease. The combined case was a planned endoscopic debulking followed by staged definitive subcranial approach for resection and, therefore, is included in the open approach cohort for statistical analyses. Table 1 shows comparison of endoscopic and open group characteristics including stage at presentation, surgical margin status, recurrence, and length of follow-up.
Table 1. Tumor stage, adjuvant treatment administration, surgical margin status, and recurrence broken down by surgical approach. Median follow-up is shown in years (range).
| Open n (%) |
Endoscopic n (%) |
p Value | |
|---|---|---|---|
| Kadish B | 16 (46) | 4 (67) | 0.41 |
| Kadish C | 17 (49) | 2 (33) | 0.67 |
| Adjuvant treatment | 28 (80) | 5 (83) | 1 |
| Negative margin resection | 25 (71) | 5 (83) | 1 |
| Gross total resection | 4 (11) | 1 (17) | 0.57 |
| Residual disease | 6 (17) | 0 (0) | 0.57 |
| Recurrence | 17 (49) | 0 (0) | 0.03 |
| Death | 3 (9) | 0 (0) | 1 |
| Median follow-up | 7 (0.2–21) | 1.8 (0.3–4) |
The major complication rate was not statistically significant between the open and endoscopic approaches ( p = 0.65). Differences in complication rates did not differ significantly when stratified by Kadish stage (Chi-square: p = 0.74). Table 2 shows a list of complications by surgical approach. The CSF leak after endoscopic surgery was repaired endoscopically. Four of the five CSF leaks after open surgery were treated with lumbar drains. The fifth required reconstruction with a free tissue microvascular flap.
Table 2. Major complications after endoscopic and open resection of esthesioneuroblastoma. Percent was calculated using the total number of patients undergoing that surgical approach.
| CSF leak n (%) |
Meningitis n (%) |
Osteomyelitis n (%) |
Severe neurologic n (%) |
Tracheostomy n (%) |
Total n (%) |
|
|---|---|---|---|---|---|---|
| Open | 5 (14) | 0 | 4 (11) | 1 (3) | 1 (3) | 11 (31) |
| Endoscopic | 1 (17) | 0 | 0 | 0 | 0 | 1 (17) |
Abbreviation: CSF, cerebrospinal fluid.
p = 0.65 for total number of complications.
Seventeen (41%) patients developed recurrence. All 17 recurrences were after open resection compared with zero recurrences after endoscopic resection ( p = 0.03). This finding is confounded by a shorter follow-up in the endoscopic resection group. PFS at 5, 10, and 15 years was 69%, 48%, and 27%, respectively. There were three deaths, which were all after open resection. OS at 5, 10, and 15 years was 97%, 92%, and 83%, respectively. There was no significant difference in PFS or OS stratified by Kadish B versus C or Hyams grade. Fig. 3 shows PFS and Fig. 4 shows OS at 5, 10, and 15 years, stratified by Kadish stage. Fig. 5 shows PFS and Fig. 6 shows OS at 5, 10, and 15 years, stratified by Hyams grade.
Fig. 3.
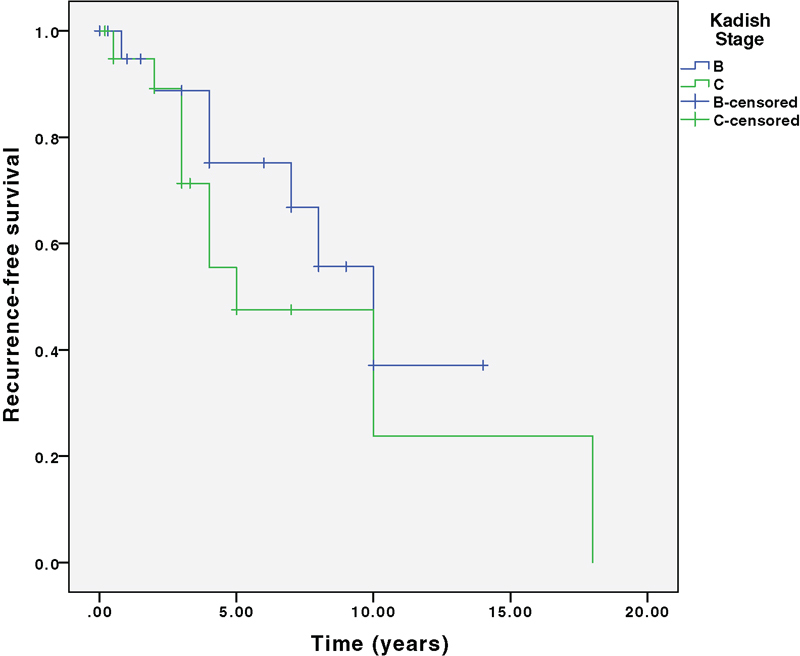
Progression-free survival stratified by Kadish stage. Log rank survival analysis comparing Kadish B and C. p = 0.28.
Fig. 4.
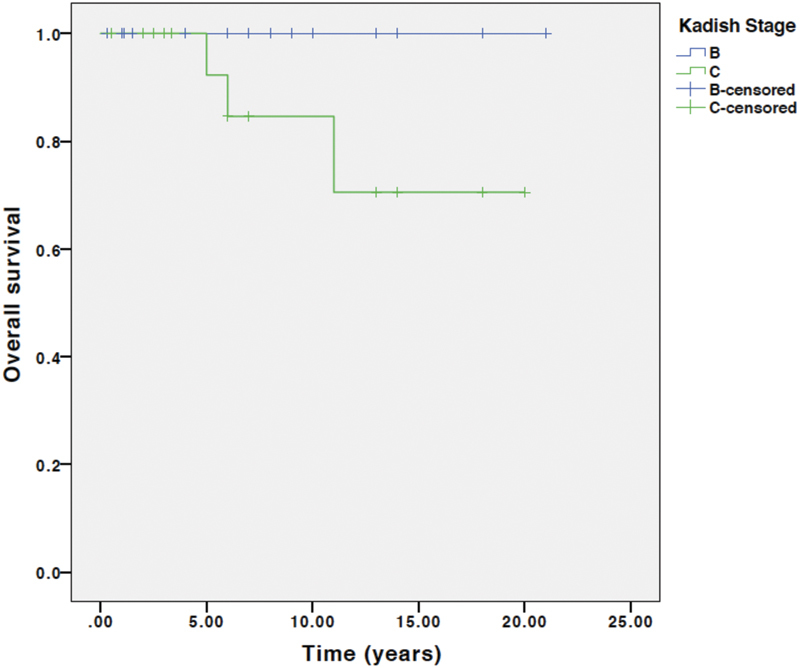
Overall survival stratified by Kadish stage. Log rank survival analysis comparing Kadish B and C. p = 0.11.
Fig. 5.
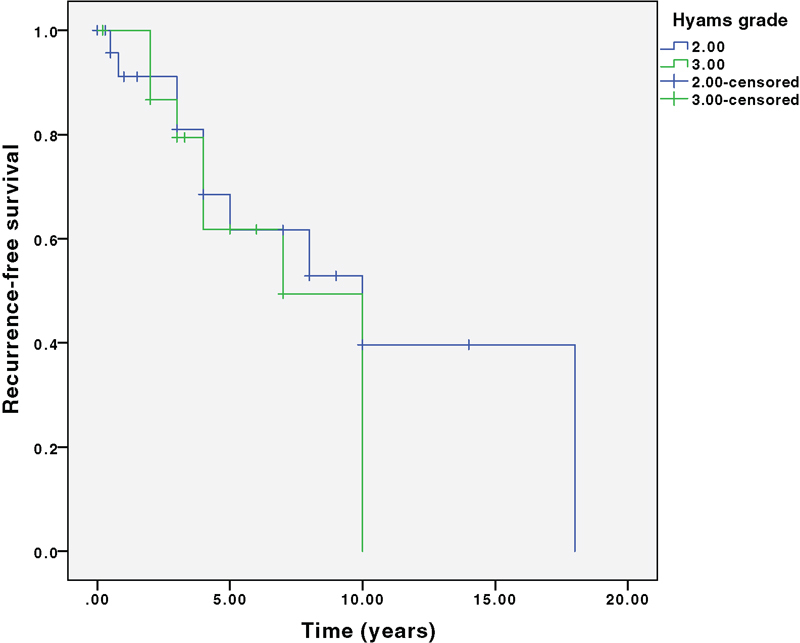
Progression-free survival stratified by Hyams grade. Log rank survival analysis comparing Hyams grade 1 and 2 (labeled 2 above) with 3 and 4 (labeled 3 above). p = 0.53.
Fig. 6.
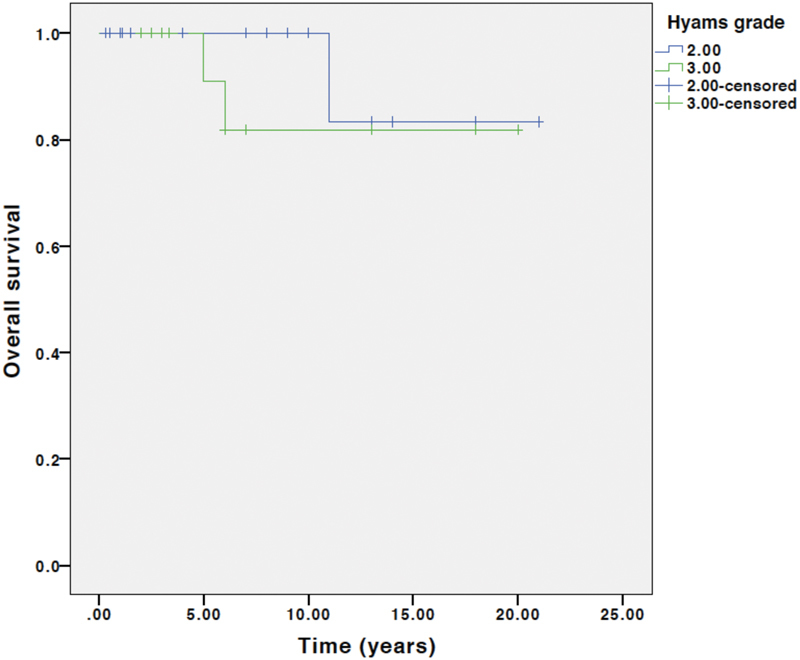
Overall survival stratified by Hyams grade. Log rank survival analysis comparing Hyams grade 1 and 2 (labeled 2 above) with 3 and 4 (labeled 3 above). p = 0.38.
Discussion
This study reviews the University of Michigan experience treating ONB over the past 28 years. It includes patients whose outcomes we have previously published. 9 However, this study has a longer median follow-up time and over double the number of patients as our previous publication had. The large increase in patients since our 2008 publication was due to an increase in cases treated by our team. Whether this is indicative of increasing incidence or change in referral patterns is unknown. Interestingly, PFS is higher in this series, while OS has decreased from 100% to 97% at 5 years. The increase in PFS may be due to a greater proportion of patients undergoing adjuvant radiation therapy or may reflect random variation given the relatively small sample sizes of both studies. While at face value, this study shows a statistically significant difference in recurrence after endoscopic versus open surgery, the endoscopic cohort consists of just six patients with far reduced length of follow-up compared with the open cohort. The decreased OS is likely due to longer follow-up time, as all deaths were from the initial cohort and were after the previous 2008 publication.
Twenty-nine percent of recurrences occurred >5 years after the initial treatment. This again highlights the importance of long-term follow-up and recurrence surveillance. In addition to regular physical examinations and yearly chest X-rays, we obtain magnetic resonance imaging (MRI) every 3 to 4 months for the first 3 years, followed by an MRI every 6 months for 3 years, and then yearly MRI thereafter.
No statistically significant difference in major complication rates was appreciated. This may be due to the small sample size and even smaller percentage of patients who underwent endoscopic tumor resection or may reflect true equivalence in complication rates. Other studies have shown similar to improved complication rates after endoscopic versus open surgical resection. 13 14 It is worth noting that CSF leak was the only major complication after endoscopic resection as compared with several cases of severe neurologic injury, tracheostomy, and osteomyelitis after open resection.
A non-statistically significant difference in OS stratified by Kadish stage was identified with improved OS for Kadish B stage compared with Kadish C. However, Kadish staging was not statistically significant for predicting OS or PFS. In contrast, Feng et al found the Kadish staging system to be predictive of PFS, but not OS. 14 Hyams histologic grading did not stratify PFS or OS in a statistically significant way. Some studies have found that the Hyams grading provides good prognostic information, particularly for grades I and IV disease, while other studies have concluded that the Hyams grading did not provide useful prognosis stratification, 15 16 17 18 19 20 similar to the current study's findings. We hypothesize that a staging system that stratifies patients by intradural and intraparenchymal extent would provide greater prognostic stratification, and we are currently studying this.
This study is limited by its retrospective nature and sample size. Because this study is retrospective, data are imperfect as documented for the objectives of this study. Additionally, a sample size of 41 patients, while a large series for a single institution, limits our ability to detect significant differences in complications rates, PFS and OS. It is possible if the entire population of ONB were studied, PFS and OS would be stratified well by Kadish stage and Hyams grade.
Conclusion
There was no statistically significant difference in major complications between open and endoscopic approaches for the surgical treatment of ONB at our institution. Patient stratification using the Kadish staging and Hyams grading systems did not show statistically significant differences in PFS or OS. This may be due to a lack of power to detect a difference or may indicate a need for a staging system that better stratifies PSF and OS.
Footnotes
Conflict of Interest None.
References
- 1.Bragg T M, Scianna J, Kassam Aet al. Clinicopathological review: esthesioneuroblastoma Neurosurgery 20096404764–770., discussion 770 [DOI] [PubMed] [Google Scholar]
- 2.Berger L, Luc G, Richard D. The olfactory esthesioneur- oepithelioma. Bull Assoc Fr Etud Cancer. 1924;13:410–421. [Google Scholar]
- 3.Loy A H, Reibel J F, Read P W et al. Esthesioneuroblastoma: continued follow-up of a single institution's experience. Arch Otolaryngol Head Neck Surg. 2006;132(02):134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 4.Resto V A, Eisele D W, Forastiere A, Zahurak M, Lee D J, Westra W H. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck. 2000;22(06):550–558. doi: 10.1002/1097-0347(200009)22:6<550::aid-hed2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Dulguerov P, Allal A S, Calcaterra T C. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2(11):683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 6.Simon J H, Zhen W, McCulloch T M et al. Esthesioneuroblastoma: the University of Iowa experience 1978-1998. Laryngoscope. 2001;111(03):488–493. doi: 10.1097/00005537-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Argiris A, Dutra J, Tseke P, Haines K. Esthesioneuroblastoma: the Northwestern University experience. Laryngoscope. 2003;113(01):155–160. doi: 10.1097/00005537-200301000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Chao K S, Kaplan C, Simpson J R et al. Esthesioneuroblastoma: the impact of treatment modality. Head Neck. 2001;23(09):749–757. doi: 10.1002/hed.1107. [DOI] [PubMed] [Google Scholar]
- 9.Ward P D, Heth J A, Thompson B G, Marentette L J. Esthesioneuroblastoma: results and outcomes of a single institution's experience. Skull Base. 2009;19(02):133–140. doi: 10.1055/s-0028-1096195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadish S, Goodman M, Wang C C. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37(03):1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970-1990. Laryngoscope. 1992;102(08):843–849. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Hyams V. Chicago, IL: American Society of Clinical Pathologists; 1983. Olfactory neuroblastoma (case 6) pp. 24–29. [Google Scholar]
- 13.Komotar R J, Starke R M, Raper D MS, Anand V K, Schwartz T H.Endoscopic endonasal compared with anterior craniofacial and combined cranionasal resection of esthesioneuroblastomas World Neurosurg 201380(1-2):148–159. [DOI] [PubMed] [Google Scholar]
- 14.Feng L, Fang J, Zhang L et al. Endoscopic endonasal resection of esthesioneuroblastoma: a single center experience of 24 patients. Clin Neurol Neurosurg. 2015;138:94–98. doi: 10.1016/j.clineuro.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Morita A, Ebersold M J, Olsen K D, Foote R L, Lewis J E, Quast L M.Esthesioneuroblastoma: prognosis and management Neurosurgery 19933205706–714., discussion 714–715 [DOI] [PubMed] [Google Scholar]
- 16.Kane A J, Sughrue M E, Rutkowski M J et al. Posttreatment prognosis of patients with esthesioneuroblastoma. J Neurosurg. 2010;113(02):340–351. doi: 10.3171/2010.2.JNS091897. [DOI] [PubMed] [Google Scholar]
- 17.Constantinidis J, Steinhart H, Koch M et al. Olfactory neuroblastoma: the University of Erlangen-Nuremberg experience 1975-2000. Otolaryngol Head Neck Surg. 2004;130(05):567–574. doi: 10.1016/j.otohns.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Dias F L, Sa G M, Lima R A et al. Patterns of failure and outcome in esthesioneuroblastoma. Arch Otolaryngol Head Neck Surg. 2003;129(11):1186–1192. doi: 10.1001/archotol.129.11.1186. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto R C, Gleich L L, Biddinger P W, Gluckman J L. Esthesioneuroblastoma and sinonasal undifferentiated carcinoma: impact of histological grading and clinical staging on survival and prognosis. Laryngoscope. 2000;110(08):1262–1265. doi: 10.1097/00005537-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Polin R S, Sheehan J P, Chenelle A G et al. The role of preoperative adjuvant treatment in the management of esthesioneuroblastoma: the University of Virginia experience. Neurosurgery. 1998;42(05):1029–1037. doi: 10.1097/00006123-199805000-00045. [DOI] [PubMed] [Google Scholar]


