Abstract
Objectives/Hypotheses The endoscopic endonasal approach (EEA) is the workhorse endoscopic procedure for sellar and parasellar pathology. Various reconstruction techniques have been reported following EEA surgery, ranging from no reconstruction to vascularized flaps. We review our institution's experience with sellar reconstruction following EEA and propose an evidence-based algorithm.
Design Retrospective review.
Setting Tertiary academic medical center.
Participants Patients who underwent endoscopic EEA surgery for sellar or parasellar pathology between March 1, 2013 and August 31, 2016.
Main Outcome Measures Patient demographic and clinicopathologic data were collected. Outcome measures included intraoperative and postoperative cerebrospinal fluid (CSF) leak rates and extent of resection (gross or subtotal).
Results Three hundred consecutive patients were included. Depending on the presence and grade of intraoperative CSF leak, cases were reconstructed using either a free mucosal graft (FMG) or nasoseptal flap (NSF). Intraoperative and postoperative CSF leak rates were 30.7% and 2.3%, respectively. Multivariable logistic regression found that intraoperative CSF leak was associated with recurrent disease (odds ratio [OR] 2.47, p = 0.004), with no apparent predictors of postoperative CSF leak.
Conclusions Based on this large series, we propose the following algorithm for sellar reconstruction: FMG for no CSF leak; fat graft + FMG ± rigid fixation for low-grade leaks; and fat graft + NSF ± rigid fixation for high-grade leaks.
Keywords: pituitary surgery, cerebrospinal fluid leak, transsphenoidal, sellar reconstruction, nasoseptal flap, mucosal graft
Introduction
In the last decade, the endoscopic endonasal approach (EEA) to the sella and parasellar region has become the workhorse to address pituitary pathology. Utilizing the EEA approach allows access to the sella and parasellar regions without extensive sinonasal dissection. Depending on the pathologic and surgical defect characteristics and the presence of a cerebrospinal fluid (CSF) leak, a myriad of sellar reconstructive techniques have been proposed, ranging from no reconstruction to vascularized pedicled flaps and from autologous tissue to synthetic materials. Due to this variety, no consensus has been reached regarding the best means to reconstruct the sella.
One of the most prominent and commonly cited algorithms examined 114 EEA cases, and it was determined that the presence of an intraoperative CSF leak signified the need for fat obliteration and use of a nasoseptal flap (NSF) for closure of the defect; 1 otherwise, no additional reconstruction was generally indicated. They noted a 3% postoperative CSF leak rate, suggesting that this approach is robust and time tested. 1
At our institution, sellar reconstruction is performed differently based on the presence of an intraoperative CSF leak, and options for reconstruction include using a free mucosal graft (FMG), NSF, abdominal fat, bone graft, or rigid plate or a combination of the above. In this study, we review our institution's experience on sellar reconstruction based on a large series of 300 consecutive patients, with attention to intraoperative and postoperative CSF leak rates, and we also aim to identify clinicopathologic factors associated with CSF leak. Based on the findings, we then propose an alternative, evidence-based algorithm for sellar reconstruction.
Methods
Institutional review board approval was obtained for this study. We performed a retrospective chart review of all patients who underwent EEA at a tertiary academic medical center for any sellar or parasellar pathology between March 1, 2013 and August 31, 2016 by a single skull base surgery team comprised of one otolaryngologist (Marilene B. Wang) and one neurosurgeon (Marvin Bergsneider). Patient demographics (age, gender, height, and weight), lesion characteristics (pathology, size, primary versus recurrent disease, history of prior radiation, presence of suprasellar extension, or cavernous sinus invasion), extent of resection (gross versus subtotal), use of specific reconstruction techniques and options (abdominal fat, rigid fixation using either septal bone or KLS Martin Resorb-X 1.0 mm double-Y extra plate [Tuttlingen, Germany], FMG, NSF, and Foley catheter), rate and grade of intraoperative CSF leak, and rate of postoperative CSF leak were collected. Intraoperative CSF leak was graded based on a previously described method, where grade 0 represented no leak observed, grade 1 small leak without obvious diaphragmatic defect, grade 2 moderate leak, and grade 3 large diaphragmatic or dural defects. 2
Statistical analysis for determining relationships between continuous variables was performed using the two-tailed Student's t -tests, while those involving categorical data were compared using the Fisher's exact tests. Binary logistic regression was performed to identify statistically significant covariates between independent variables and outcome variables, which in this study included subtotal resection, intraoperative CSF leak, and postoperative CSF leak. Multivariate logistic regression was then performed using these covariates to determine independent predictors of a given event. A significance level of p < 0.006 (= 0.05/9, Bonferroni correction for nine covariates) was used in all cases.
Reconstruction Principles
After resection of the sellar or parasellar pathology, the sellar defect is copiously irrigated, and careful inspection is performed to determine the presence of a CSF leak, including a Valsalva maneuver to 30 mm Hg. If abdominal fat is to be used, it is harvested sterilely from the left lower quadrant of the abdomen and used to obliterate the dead space of the sellar defect in an intradural manner. For certain defects with high tension on the dura or thinning of the dura based the senior neurosurgeon's (Marvin Bergsneider) intraoperative visual assessment, this is followed by placement of an intracranial, extradural rigid “plate,” which is either septal bone or the Resorb-X plate. The bone or plate is trimmed to size so that it can be tucked under the bony edges of the central aspect of the defect. There was no encroachment of the plate or bone graft laterally into the cavernous sinus region. Sometimes, the fat graft is sutured to the bone or plate to prevent migration into the suprasellar space. Fat grafts are placed for all but grade 0 leaks, and generally, no rigid fixation is placed for grade 0 leaks. After placement of the underlay materials, a Valsalva maneuver to 30 mm Hg is performed to check for persistent leak. After confirmation of no further leak, the FMG and NSF (described later) are placed extracranially and, depending on sellar anatomy and flap contour, may be held in place by a 14-French Coude tip Foley catheter containing 5 cc of water. A Foley balloon was utilized if the surgeons believed that placement of the anterior edge of the NSF was not secure. This was usually due to angulation of the skull base that resulted in the anterior edge of the NSF being prone to slipping inferiorly. The Foley balloon offered additional support in maintaining the flap in an optimal position. We generally deflate and remove this catheter on postoperative day 3. No lumbar drains are placed preoperatively.
Free Mucosal Grafting Technique
For all cases with a grade 0 and some cases with a grade 1 CSF leak, a FMG taken from the posterior nasal septum is used to reconstruct the sella. As we routinely harvest a NSF from the right side, the graft is harvested from the contralateral (left) side. Bovie electrocautery is used to incise the mucosa down to septal bone superiorly, anteriorly, and inferiorly. The mucosa is elevated with a Freer and grasped posteriorly with a Blakesley forceps, then saved for later use. At the end of the resection, the FMG is placed over the denuded bone (or, in the case of RCC, within the inferior portion of the marsupialized tract 3 ) in an effort to promote remucosalization. 4
Nasoseptal Flap
For grade 2 and 3 CSF leaks, we utilize the NSF as previously described by Hadad et al, 5 with the “rescue flap” modification. 6 7 The pedicle of the posterior septal branch of the sphenopalatine artery is elevated off of the anterior face of the sphenoid sinus, and a superior incision is made in the posterior septal mucosa below the olfactory fibers. No further elevation and incisions are made while the resection takes place. If an intraoperative CSF leak is encountered, Bovie electrocautery is used to make the inferior, lateral, and anterior incisions, with flap elevation commencing from an anterior to posterior direction. The flap is then inset into the sphenoid sinus to provide coverage of the sella.
Results
Three hundred consecutive patients were identified and included in the analysis. There were 166 (55.3%) females and 134 (44.7%) males. The mean age at diagnosis was 49 ± 17 years (range, 7–85). The mean lesion size was 19.6 ± 10.7 mm (maximum 67 mm). The types and frequencies of sellar and parasellar pathologic diagnoses are presented in Table 1 . Fifty-four (18%) of the cases were recurrent lesions requiring revision surgery. Only nine (3%) patients underwent prior radiation therapy. A total of 130 (43.3%) lesions had suprasellar extension, while 60 (20%) had cavernous sinus invasion. Gross total resection was achieved in 80% of cases, with 60 (20%) having subtotal resection.
Table 1. Pathologic diagnoses of the current series ( n = 300) .
| Diagnosis | n (%) |
|---|---|
| Pituitary adenoma | 235 (78.3%) |
| Rathke's cleft cyst | 28 (9.3%) |
| Craniopharyngioma | 12 (4.0%) |
| Other benign lesions | 12 (4.0%) |
| Meningioma | 8 (2.7%) |
| Malignant lesions | 5 (1.7%) |
Intraoperative CSF leak was encountered in 92 (30.7%) cases, of which there were 45 (15%) grade 1, 32 (10.7%) grade 2, and 15 (5%) grade 3 leaks. This was further analyzed by grade and method of repair (FMG versus NSF), which is summarized in Table 2 . Of note, 24 (53%) and 21 (47%) grade 1 CSF leaks were repaired using an FMG and NSF, respectively. Only one grade 2 or 3 leak was repaired with an FMG, with the remainder being repaired with NSF. Sixty-three (21%; 1.4% [3/208] grade 0, 58% [26/45] grade 1, 75% [24/32] grade 2, and 67% [10/15] grade 3) of the repairs employed rigid fixation, while 24 (8%; 1.0% [2/208] grade 0, 13% [6/45] grade 1, 25% [8/32] grade 2, and 53% [8/15] grade 3) had the repair held in place postoperatively with a Foley balloon catheter. Of those cases with rigid fixation, 7.9% (5/63) developed postoperative CSF leaks, whereas only 0.8% (2/237) developed leaks in the non-fixated group ( p = 0.005); there was no difference in postoperative CSF leak rates with Foley balloon bolstering ( p = 0.101). The decision to use a Foley balloon catheter for additional support of the flap was made based on factors such as anterior extent of the defect, ability to employ rigid fixation, and grade of leak. In general, a Foley balloon was used for high-grade leaks where rigid fixation was difficult to achieve due to lack of adequate bony “shelf” in the skull base defect, and the bone or plate was not able to be placed securely although both may be used together ( n = 5, 1.7%). In these cases, abdominal fat was placed in the dural defect, followed by placement of the NSF, and finally the balloon.
Table 2. Method of reconstruction using free mucosal graft versus nasoseptal flap as distributed by grade of intraoperative CSF leak.
| Free mucosal graft | Total | |||
|---|---|---|---|---|
| No | Yes | |||
| CSF leak grade | 0 | 27 | 181 | 208 |
| 1 | 21 | 24 | 45 | |
| 2 | 31 | 1 | 32 | |
| 3 | 14 | 0 | 15 | |
| Total | 94 | 206 | 300 | |
| Nasoseptal flap | Total | |||
| No | Yes | |||
| CSF leak grade | 0 | 187 | 21 | 208 |
| 1 | 24 | 21 | 45 | |
| 2 | 1 | 31 | 32 | |
| 3 | 0 | 15 | 15 | |
| Total | 212 | 88 | 300 | |
| 7 | 3 | Fat + NSF + rigid + Foley | 3 | Lumbar drain |
Abbreviations: CSF, cerebrospinal fluid; NSF, nasoseptal flap.
There were seven (2.3%) postoperative CSF leaks, detailed in Table 3 . All cases of CSF leak resolved after salvage management. For cases 1 and 2, there was initially no CSF leak noted intraoperatively; thus, they likely both represent subtle and, therefore, missed grade 1 leaks. In comparing FMG versus NSF reconstruction for low-grade leaks, there was no difference in postoperative CSF leak rates ( p > 0.05), signifying that FMG repair may be adequate for grade 1 intraoperative CSF leaks. Results from univariate regression analysis are reported in Table 4 . On multivariate analysis, subtotal resection was independently associated with non-adenoma pathology (hazard ratio [HR] 1.60, p = 0.001) and cavernous sinus invasion (OR 3.43, p = 0.001). The risk of intraoperative CSF leak was increased with recurrent disease (OR 2.47, p = 0.004), and there were no independent predictors of postoperative CSF leak ( Table 5 ).
Table 3. Postoperative CSF leaks and clinical characteristics.
| Case | Intraoperative CSF leak grade | Initial reconstruction | POD of leak | Management |
|---|---|---|---|---|
| 1 | 0 | FMG | 8 | Bilateral MT flaps (due to tumor destruction of anterior wall of sphenoid sinus) |
| 2 | 0 | FMG | 1 | Fat + NSF + Foley |
| 3 | 1 | Fat + FMG + rigid | 7 | NSF |
| 4 | 2 | Fat + NSF + rigid | 12 | Fat + NSF, lumbar drain |
| 5 | 2 | Fat + NSF + rigid | 2 | Lumbar drain |
| 6 | 2 | Fat + NSF + rigid | 7 | Lumbar drain |
| 7 | 3 | Fat + NSF + rigid + Foley | 3 | Lumbar drain |
Abbreviations: CSF, cerebrospinal fluid; FMG, free mucosal graft; MT, middle turbinate; NSF, nasoseptal flap; POD, postoperative day.
Table 4. Univariate analysis of predictors of subtotal resection and intraoperative and postoperative CSF leak.
| Factor | Subtotal resection ( p ) | Intraoperative CSF leak ( p ) | Postoperative CSF leak ( p ) |
|---|---|---|---|
| Age | 0.124 | 0.488 | 0.889 |
| Sex | 0.002* | 0.464 | 0.922 |
| BMI | 0.989 | 0.237 | 0.229 |
| Non-adenoma pathology | <0.001* | 0.038 | 0.408 |
| Lesion size | <0.001* | 0.031 | 0.014 |
| Recurrent lesion | <0.001* | 0.001* | 0.796 |
| Prior radiation therapy | 0.015 | 0.030 | 0.999 |
| Suprasellar extension | <0.001* | 0.002* | 0.151 |
| Cavernous sinus invasion | <0.001* | 0.900 | 0.145 |
Abbreviation: BMI, body mass index; CSF, cerebrospinal fluid.
*Statistical significance at the 0.006 level.
Table 5. Multivariate regression analysis of independent predictors of subtotal resection and intraoperative CSF leak.
| Factor | Subtotal resection (95% CI) | Intraoperative CSF leak (95% CI) |
|---|---|---|
| Sex | NS | |
| Non-adenoma pathology | 1.596 (1.204–2.116) | |
| Lesion size | NS | |
| Recurrent lesion | NS | 2.467 (1.332–4.569) |
| Prior radiation therapy | ||
| Suprasellar extension | NS | NS |
| Cavernous sinus invasion | 3.426 (1.601–7.335) |
Abbreviation: CSF, cerebrospinal fluid; CI, confidence intervals; NS, not significant.
Only those covariates that are statistically significant at the 0.006 level have 95% CI reported.
Discussion
With the advent and popularization of endoscopic approaches, skull base surgery for the sellar and parasellar region has evolved and is currently considered both safe and minimally invasive, while not sacrificing treatment outcomes. Concurrent to surgical approaches is developments in reconstruction of the surgical defect. Perhaps the most important innovation in sellar reconstruction is the Hadad et al pedicled NSF, which in essence provides reliable vascular tissue supplied by a named vessel. 5 Prevention of postoperative CSF leak outcomes with the NSF are outstanding, with the incidence of leak to be ∼3%. 1 8 9 However, donor site morbidity following NSF harvest is potentially very bothersome for patients, with certain reports of prolonged nasal crusting lasting for weeks to months. 10 11 Though several donor site reconstructive options have been reported, such as the use of fascia lata, 12 free septal 4 or middle turbinate 13 mucosal graft, or “reverse” flap, 14 careful selection of reconstructive options prior to flap harvest remains the best way to minimize morbidity. Initially, several grades 0 and 1 intraoperative CSF leaks were primarily reconstructed using NSF. As expected, this produced outstanding reconstructive outcomes, but also consequently led to increased donor site morbidity (e.g., crusting), prompting us to consider a change in reconstructive strategy. 15 Thus, the aim of the current study is to review our institution's technique for sellar reconstruction in a large, consecutive series, with special attention to postoperative CSF leak rates.
Numerous techniques have been previously reported for intracranial reconstruction of the sella following pituitary surgery. In most cases, autologous fat taken from the abdomen serves as a helpful buttress for obliteration of dead space. However, the role of rigid fixation, as in some of our cases, is unclear. Surgeons have reported success with acellular dermal matrix, 16 synthetic dural substitutes, 17 or collagen sponge, 18 though fascia lata has always remained a readily accessible and robust tissue for reconstruction. In their series of four patients, Tabaee et al demonstrated success in repair of high-flow CSF leaks with an absorbable miniplate, similar to the one used in the current series. 19 Although no prospective and large-sample series are currently available, we tend to utilize rigid fixation for lesions with low-grade CSF leak where the diaphragma sella is thinned significantly or in cases with high-grade leaks. In this study, we found that there was a significantly higher rate of postoperative CSF leak in cases with rigid fixation, but this is likely due to bias toward higher rates of rigid fixation in cases with higher grade intraoperative CSF leaks.
In our series, a FMG harvested from the nasal septum was largely sufficient for extracranial reconstruction of the sella for low-grade CSF leaks. Specifically, only 3 (1.5%) of 205 grades 0 and 1 intraoperative leaks developed postoperative leaks. These findings mirror those of Roxbury et al, where synthetic or autologous-free grafts successfully prevented postoperative CSF leaks in cases of low-grade intraoperative CSF leak. 20 A systematic review by Soudry et al comparing methods of reconstruction for low- and high-flow leaks found that there was no significant difference between NSF and FMG for low-flow leaks. 21 Although an argument can certainly be made for using no extradural reconstruction following intracranial repair of low-grade leaks, at our institution, we advocate placement of an FMG for additional coverage of the defect and for promotion of mucosalization of the posterior wall of the sphenoid sinus. In following these patients postoperatively in the outpatient setting, there is anecdotal evidence suggesting improved wound healing, without significantly added morbidity from the graft donor site. 4 Nevertheless, mucosal grafting outcomes on sinonasal symptoms are unknown and are needed to determine the true value of this technique.
For high-grade leaks, we usually resort to the NSF, which has become the workhorse flap for anterior skull base reconstruction. A systematic review by Harvey et al noted that FMG reconstruction of large dural defects (equivalent to a grade 3 CSF leak) was relatively unreliable, with 15.6% developing postoperative leaks, while NSF reconstruction had improved outcomes with only 6.7% leaks. 22 Another study by Zanation et al found that the NSF was robust (94% initial success rate) for preventing postoperative CSF leaks following high-flow intraoperative leaks, with pediatric patients and revision cases being at high risk. 23 In the current series, 4 (8.5%) of 47 high-grade intraoperative CSF leaks developed postoperative leaks. We did not find an association between postoperative CSF leak and revision surgery, though an association was established between intraoperative CSF leak and revision surgery for recurrence, significantly increasing the risk of by two fold.
Through multivariate analysis, we identified that the risk of subtotal resection was increased with cavernous sinus invasion and non-adenoma pathology. There is often a balance between the extent of resection and the risk of CSF leak, as several studies have also noted cavernous sinus invasion being a risk factor for subtotal resection. 24 25 26 Lesions demonstrating cavernous sinus invasion require more extensive lateral and posterior dissection, which increases manipulation of the surrounding dura and, thus, the risk of a surgically created dural defect. Similarly, for the purpose of this study, non-adenoma pathology includes several benign and malignant neoplasms of the central nervous system (e.g., craniopharyngiomas and meningiomas), of which many also require extensive lateral and posterior dissection. As such defects tend to be larger, the skull base surgeon, when reviewing imaging preoperatively, should anticipate the higher probability of encountering a high-grade intraoperative CSF leak and consider a more extensive reconstruction technique (e.g., NSF instead of FMG even if no leak or a low-grade leak is encountered).
There were two postoperative CSF leaks, which occurred after grade 0 intraoperative leaks. These likely represent missed intraoperative CSF leaks or perhaps dural tearing in the postoperative period, and possibly a combination of both factors, and were successfully repaired secondarily. Sanders-Taylor et al cited two possible explanations for postoperative CSF leaks following no intraoperative leaks: unrecognized/missed intraoperative leaks and postoperative changes (e.g., unanticipated Valsalva, shifting of repair). 27 They further advocate universal reconstruction regardless encountering intraoperative CSF leak to provide preemptive bolstering of the sellar defect in case of postoperative changes. 27 We agree with this assessment and support full extradural coverage of the defect regardless of CSF leak grade, even in the absence of any intraoperative leak, as this may potentially seal any small, difficult-to-recognize leaks. One patient required reconstruction using bilateral middle turbinate flaps. This patient had a macroadenoma filling the sphenoid sinus, which destroyed part of the anterior wall of the sphenoid sinus, thus disrupting the vascular supply of the NSFs. Furthermore, complete tumor resection necessitated removal of the remaining anterior face of the sphenoid sinus. As such, bilateral middle turbinate flaps were necessary for reconstruction. This underscores the importance of reserving additional vascularized tissue as a precaution. 28
The current study has several limitations. First and foremost, the decision of whether to use rigid fixation and/or Foley balloon support for reconstruction is largely subjective and based on the surgeon's intraoperative assessment of the defect (e.g., thinness and tenseness of the dura and contour of the flap). As noted earlier, use of rigid fixation did not appear to prevent postoperative CSF leak rates, likely due to bias toward use of rigid fixation in higher grade leaks. Second, the proposed algorithm is a capitulation of 300 consecutive cases based on a single institution's (and single skull base team) experience, and, though generalizable due to larger sample size, may benefit from further external validation. Third, for the purpose of focusing on reconstructive outcomes in consecutive cases, we included all pathologies within a given timeframe of interest and, thus, combined all non-adenoma pathologies into a single category. Needless to say, surgical decision-making related to a Rathke's cleft cyst may differ significantly from that for intracranial tumors. We recognize this as potentially biasing the results though the sample size for each of these individual pathologies is much smaller (<30 per pathology) compared with pituitary adenomas, making statistical analysis of each distinct pathology challenging.
Based on our series, we propose an alternative algorithm for sellar reconstruction ( Fig. 1 ). The algorithm is similar to previously introduced pathways such as that described by Patel et al 1 or Jalessi et al, 29 but we believe that it lends additional support to the importance of preoperative planning and intraoperative decision making.
Fig. 1.
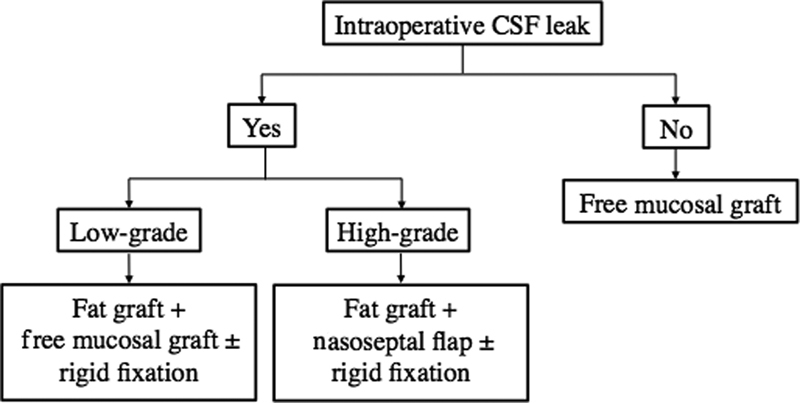
Proposed evidence-based algorithm for sellar reconstruction based on our institution's experience of 300 cases.
Conclusion
Based on this large series of 300 consecutive cases spanning all sellar and parasellar pathologies, we propose the following evidence-based algorithm for sellar reconstruction: FMG for no CSF leak; fat graft with FMG overlay ± rigid fixation for low-grade leaks; and fat graft with NSF ± rigid fixation for high-grade leaks. In our experience, this algorithm is associated with a low rate of postoperative CSF leak and appears to be versatile for different diagnoses and tumor characteristics. The absence of intraoperative CSF leak or a low-grade leak in the context of adverse tumor features, such as cavernous sinus invasion or recurrent disease, may prompt consideration of more aggressive reconstructive options.
Conflicts of Interest None.
Note
This study was presented at the North American Skull Base Society 26th Annual Meeting, March 4, 2017, in New Orleans, Louisiana.
Financial Disclosures
None.
References
- 1.Patel M R, Stadler M E, Snyderman C H et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(06):397–404. doi: 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito F, Dusick J R, Fatemi N, Kelly D F.Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery Neurosurgery 2007600402295–303., discussion 303–304 [DOI] [PubMed] [Google Scholar]
- 3.Kuan E C, Yoo F, Chyu J, Bergsneider M, Wang M B. Treatment outcomes of Rathke's cleft cysts managed with marsupialization. J Neurol Surg B Skull Base. 2017;78(02):112–115. doi: 10.1055/s-0036-1585088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo F, Kuan E C, Bergsneider M, Wang M B. Free mucosal graft reconstruction of the septum after nasoseptal flap harvest: a novel technique using a posterior septal free mucosal graft. J Neurol Surg B Skull Base. 2017;78(02):201–206. doi: 10.1055/s-0036-1597086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadad G, Bassagasteguy L, Carrau R L et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 6.Rivera-Serrano C M, Snyderman C H, Gardner P et al. Nasoseptal “rescue” flap: a novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope. 2011;121(05):990–993. doi: 10.1002/lary.21419. [DOI] [PubMed] [Google Scholar]
- 7.Kim B Y, Shin J H, Kang S G et al. Bilateral modified nasoseptal “rescue” flaps in the endoscopic endonasal transsphenoidal approach. Laryngoscope. 2013;123(11):2605–2609. doi: 10.1002/lary.24098. [DOI] [PubMed] [Google Scholar]
- 8.Horridge M, Jesurasa A, Olubajo F, Mirza S, Sinha S. The use of the nasoseptal flap to reduce the rate of post-operative cerebrospinal fluid leaks following endoscopic trans-sphenoidal surgery for pituitary disease. Br J Neurosurg. 2013;27(06):739–741. doi: 10.3109/02688697.2013.795525. [DOI] [PubMed] [Google Scholar]
- 9.Thorp B D, Sreenath S B, Ebert C S, Zanation A M. Endoscopic skull base reconstruction: a review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak. Neurosurg Focus. 2014;37(04):E4. doi: 10.3171/2014.7.FOCUS14350. [DOI] [PubMed] [Google Scholar]
- 10.Kassam A B, Thomas A, Carrau R Let al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 2008630101ONS44–ONS52., discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]
- 11.Soudry E, Psaltis A J, Lee K H, Vaezafshar R, Nayak J V, Hwang P H. Complications associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015;125(01):80–85. doi: 10.1002/lary.24863. [DOI] [PubMed] [Google Scholar]
- 12.Zeinalizadeh M, Sadrehosseini S M, Barkhoudarian G, Carrau R L. Reconstruction of the denuded nasoseptal flap donor site with a free fascia lata graft: technical note. Eur Arch Otorhinolaryngol. 2016;273(10):3179–3182. doi: 10.1007/s00405-016-3962-0. [DOI] [PubMed] [Google Scholar]
- 13.Kimple A J, Leight W D, Wheless S A, Zanation A M. Reducing nasal morbidity after skull base reconstruction with the nasoseptal flap: free middle turbinate mucosal grafts. Laryngoscope. 2012;122(09):1920–1924. doi: 10.1002/lary.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasemsiri P, Carrau R L, Otto B A et al. Reconstruction of the pedicled nasoseptal flap donor site with a contralateral reverse rotation flap: technical modifications and outcomes. Laryngoscope. 2013;123(11):2601–2604. doi: 10.1002/lary.24088. [DOI] [PubMed] [Google Scholar]
- 15.Thompson C F, Suh J D, Liu Y, Bergsneider M, Wang M B. Modifications to the endoscopic approach for anterior skull base lesions improve postoperative sinonasal symptoms. J Neurol Surg B Skull Base. 2014;75(01):65–72. doi: 10.1055/s-0033-1356492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citardi M J, Cox A J, III, Bucholz R D. Acellular dermal allograft for sellar reconstruction after transsphenoidal hypophysectomy. Am J Rhinol. 2000;14(01):69–73. doi: 10.2500/105065800781602920. [DOI] [PubMed] [Google Scholar]
- 17.Cappabianca P, Cavallo L M, Mariniello G, de Divitiis O, Romero A D, de Divitiis E.Easy sellar reconstruction in endoscopic endonasal transsphenoidal surgery with polyester-silicone dural substitute and fibrin glue: technical note Neurosurgery 20014902473–475., discussion 475–476 [DOI] [PubMed] [Google Scholar]
- 18.Kelly D F, Oskouian R J, Fineman I.Collagen sponge repair of small cerebrospinal fluid leaks obviates tissue grafts and cerebrospinal fluid diversion after pituitary surgery Neurosurgery 20014904885–889., discussion 889–890 [DOI] [PubMed] [Google Scholar]
- 19.Tabaee A, Kamat A, Shrivastava R. Complex reconstruction of the sella using absorbable mini-plate in revision endoscopic pituitary surgery: technical note. J Neurol Surg A Cent Eur Neurosurg. 2013;74(05):313–317. doi: 10.1055/s-0032-1333129. [DOI] [PubMed] [Google Scholar]
- 20.Roxbury C R, Saavedra T, Ramanathan M, Jr et al. Layered sellar reconstruction with avascular free grafts: acceptable alternative to the nasoseptal flap for repair of low-volume intraoperative cerebrospinal fluid leak. Am J Rhinol Allergy. 2016;30(05):367–371. doi: 10.2500/ajra.2016.30.4356. [DOI] [PubMed] [Google Scholar]
- 21.Soudry E, Turner J H, Nayak J V, Hwang P H. Endoscopic reconstruction of surgically created skull base defects: a systematic review. Otolaryngol Head Neck Surg. 2014;150(05):730–738. doi: 10.1177/0194599814520685. [DOI] [PubMed] [Google Scholar]
- 22.Harvey R J, Parmar P, Sacks R, Zanation A M. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122(02):452–459. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]
- 23.Zanation A M, Carrau R L, Snyderman C H et al. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy. 2009;23(05):518–521. doi: 10.2500/ajra.2009.23.3378. [DOI] [PubMed] [Google Scholar]
- 24.Juraschka K, Khan O H, Godoy B L et al. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J Neurosurg. 2014;121(01):75–83. doi: 10.3171/2014.3.JNS131679. [DOI] [PubMed] [Google Scholar]
- 25.Woodworth G F, Patel K S, Shin B et al. Surgical outcomes using a medial-to-lateral endonasal endoscopic approach to pituitary adenomas invading the cavernous sinus. J Neurosurg. 2014;120(05):1086–1094. doi: 10.3171/2014.1.JNS131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tajudeen B A, Mundi J, Suh J D, Bergsneider M, Wang M B. Endoscopic endonasal surgery for recurrent pituitary tumors: technical challenges to the surgical approach. J Neurol Surg B Skull Base. 2015;76(01):50–56. doi: 10.1055/s-0034-1383856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders-Taylor C, Anaizi A, Kosty J, Zimmer L A, Theodosopoulos P V. Sellar reconstruction and rates of delayed cerebrospinal fluid leak after endoscopic pituitary surgery. J Neurol Surg B Skull Base. 2015;76(04):281–285. doi: 10.1055/s-0034-1544118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel M R, Taylor R J, Hackman T G et al. Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014;124(04):846–852. doi: 10.1002/lary.24319. [DOI] [PubMed] [Google Scholar]
- 29.Jalessi M, Sharifi G, Mirfallah Layalestani M R et al. Sellar reconstruction algorithm in endoscopic transsphenoidal pituitary surgery: experience with 240 cases. Med J Islam Repub Iran. 2013;27(04):186–194. [PMC free article] [PubMed] [Google Scholar]


