Abstract
Purpose
There is a lack of prognostic biomarkers in idiopathic pulmonary fibrosis (IPF) patients. The objective of this study is to investigate the potential of 18F-FDG-PET/ CT to predict mortality in IPF.
Methods
A total of 113 IPF patients (93 males, 20 females, mean age ± SD: 70 ± 9 years) were prospectively recruited for 18F-FDG-PET/CT. The overall maximum pulmonary uptake of 18F-FDG (SUVmax), the minimum pulmonary uptake or background lung activity (SUVmin), and target-to-background (SUVmax/ SUVmin) ratio (TBR) were quantified using routine region-of-interest analysis. Kaplan–Meier analysis was used to identify associations of PET measurements with mortality. We also compared PET associations with IPF mortality with the established GAP (gender age and physiology) scoring system. Cox analysis assessed the independence of the significant PET measurement(s) from GAP score. We investigated synergisms between pulmonary 18F-FDG-PET measurements and GAP score for risk stratification in IPF patients.
Results
During a mean follow-up of 29 months, there were 54 deaths. The mean TBR ± SD was 5.6 ± 2.7. Mortality was associated with high pulmonary TBR (p = 0.009), low forced vital capacity (FVC; p = 0.001), low transfer factor (TLCO; p < 0.001), high GAP index (p = 0.003), and high GAP stage (p = 0.003). Stepwise forward-Wald–Cox analysis revealed that the pulmonary TBR was independent of GAP classification (p = 0.010). The median survival in IPF patients with a TBR < 4.9 was 71 months, whilst in those with TBR > 4.9 was 24 months. Combining PET data with GAP data (“PET modified GAP score”) refined the ability to predict mortality.
Conclusions
A high pulmonary TBR is independently associated with increased risk of mortality in IPF patients.
Electronic supplementary material
The online version of this article (10.1007/s00259-017-3917-8) contains supplementary material, which is available to authorized users.
Keywords: Interstitial lung disease, Positron-emission tomography and computed tomography, Fluorine-18 FDG
Introduction
Interstitial lung disease (ILD) has an incidence of ~57/100,000 per year and is associated with significant morbidity [1]. The ILDs consist of a heterogeneous group of diseases with varying amounts of interstitial inflammation and fibrosis [2]. However, there is heterogeneity in outcome, with survival in idiopathic pulmonary fibrosis (IPF) particularly poor. Some patients gradually deteriorate, some undergo stepwise progression, whilst others decline rapidly. Moreover, much of the prognostic data heralds from an era when the criteria for diagnosing IPF were less well and differently defined than at present [2–4].
Positron emission tomography (PET) offers the ability to non-invasively investigate cellular metabolism in vivo. PET studies in animals have yielded valuable insights into the biology of IPF and ILD [5, 6], and there is encouraging evidence that PET may aid the development of therapeutic interventions to treat these debilitating conditions [7]. It has recently been demonstrated that18F-fluorodeoxyglucose (18F-FDG) PET signal is consistently raised and can be objectively measured in patients with IPF [8]. Moreover, these PET signals are shown to be stable and reproducible [9].
The prevailing theory of IPF is that alveolar epithelial damage results in a chronic wound-healing response that leads to self-propagating scar formation and end-stage fibrosis [10]. 18F-FDG uptake by tissues is a marker of glucose utilization, correlating with tissue metabolism. Our hypothesis was that although the cellular basis of the FDG-PET signal is unclear, on-going metabolic activity, in areas of scarred lung, would indicate a process that could be manipulated therapeutically. In addition, a significant correlation between FDG-PET signal and disease progression would strengthen the validity and usefulness of this signal as a novel biomarker in IPF [11].
With the use of current treatment (e.g., anti-fibrinolytic therapy) in IPF patients [12, 13] and given the rationale for recommending these drugs as set out in some guidelines [14, 15], it has been recently highlighted that there is an urgent need for biomarkers and end points in order to develop therapy and treatment regimens for patients with IPF [3].
In this study, we investigate the potential for baseline measures of pulmonary 18F-FDG PET signal to predict survival in patients with IPF compared to the more established GAP (gender age and physiology) prognostic score [16].
Methods
Patients
From 2008 to 2017, there were a total of 113 (93 male, 20 female, mean age 70 ± 9 years) prospectively and consecutively consented patients with IPF who underwent 18F-FDG PET/CT from a single institution. All patients underwent full clinical assessment including multidisciplinary team (MDT) review, baseline pulmonary function tests (PFTs), and high-resolution computed tomography (HRCT) evaluation. Infection and neoplasia were excluded on clinical and radiological grounds. The diagnosis of IPF was made on clinico-radiological grounds following MDT review. The study had ethics board approval and all patients gave written informed consent.
Patient follow-up
The patient follow-up period was defined from date of scan to death (all causes, as previously adopted in prognostic studies [16]) or 9 years. Patient survival was confirmed by the use of patient charts, electronic database, primary health care physician records, or telephone interview.
PET/CT acquisition
PET/CT imaging was performed after the diagnosis was made following MDT review described above and all images were acquired on the same PET CT instrument (VCT PET/64-detector CT instrument, GE Healthcare Technology, Waukesha, WI, USA). Three imaging sequences of the thorax were performed whilst the patient remained supine on the table throughout. A CT was performed for attenuation correction. Maintaining the patient position, a whole-body 18F-FDG PET emission scan (8 min per bed position) was performed 1 h after injecting 200 MBq of 18F-FDG and covered an area identical to that covered by CT. Next, maintaining the patient position, a deep inspiratory breath-hold diagnostic high-resolution CT (HRCT) was performed using the following parameters: 64 × 1.25-mm detectors, a pitch of 0.53, and 1.25-mm collimation (120 kVp and 100 mAs).
Image analysis
Observers
PET images were analyzed by a PET radiologist and senior PET technologist with > 5 years’ experience in quantifying pulmonary 18F-FDG PET uptake in IPF. CT images were reviewed by a dedicated thoracic radiologist, independent of the PET CT analysis.
Image display and processing
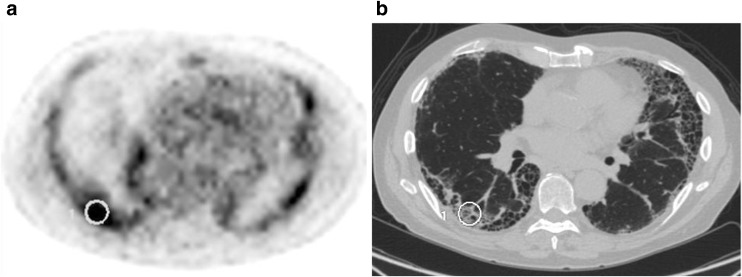
All images were loaded onto an ADW (GE Healthcare Technology, Waukesha, WI, USA) workstation. All datasets underwent image processing as previously described in detail [7]. Using a region of interest, the area of most intense pulmonary 18F-FDG uptake was identified and the highest image value (SUVmax) measured (see Fig. 1).
Fig. 1.
Co-registered PET (a) and CT (b) of a patient with IPF showing region of interest placement as part of measuring maximal pulmonary 18F-FDG uptake [7]. The dark grey/black regions are regions of high18F-FDG metabolism on the PET image (a)
In addition, the region of pulmonary parenchyma with the lowest SUV was identified as previously described and was shown to have high inter- and intra-observer reproducibility [7]. In all cases, this region was confirmed by the dedicated thoracic radiologist to conform to morphologically normal lung parenchyma on co-registered CT. The lowest image value (i.e., SUVmin) in this region was recorded as a measure of the background lung uptake and in turn to calculate the lung target-to-background ratio (TBR = SUVmax/SUVmin) [7]. TBR can be interpreted as a measure of variation of FDG uptake in the lung where a TBR close to 1 indicates relatively uniform uptake. In addition, tissue-to-blood ratios have been reported in several studies to be highly correlated with Ki [17–19], therefore we also measured lung-to-blood background levels by drawing an ROI as described by the European Association of Nuclear Medicine position paper [20], and thus “TBR blood” was calculated as lung SUVmax divided by average value of the blood SUVmean.
GAP calculation
GAP calculation based on the four variables (gender (G), age (A), and 2 lung physiology variables (P) forced vital capacity (FVC) and transfer factor (TLCO) as previously described was computed [16]. This comprised a model using continuous predictors (GAP calculator) and a simple point-scoring system (GAP index), which varies from 0, potentially indicating a good outcome, to 8, potentially indicating a worse outcome.
Based on the GAP index, the three stages (stages I, II, III) identified are: GAP stage I included GAP index 0, 1, 2, 3; GAP stage II included GAP index 4, 5; and GAP stage III included GAP index 6, 7, 8.
Statistical analysis
Statistical analyses were performed using SPSS for Windows version 19.0 (IBM, Armonk, NY, USA).
Univariate survival analysis
The relationships of SUVmax, SUVmin, TBR, and TBR blood with patient survival were assessed using univariate Kaplan–Meier (KM) survival analysis. Median values for these parameters were used as thresholds (cut-offs) to separate the survival plots (poor and good prognostic groups) and the difference in the survival plots was further evaluated using non-parametric log-rank test. KM curves for patients above and below the median cut-off for each PET parameter were constructed to display the proportion of patients surviving at a given time.
Cross-validation
Although the use of median value as a cut-off is unbiased, to further reduce bias, cross-validation was undertaken for each of the significant univariate PET markers of survival within the respective patient sub-group [21]. Because of the sample size, cross-validation could not be carried out on the basis of a sample split (training and validation). Instead, the statistically recognized k-fold (e.g., k = 4 in our study) cross-validation procedure was carried out [21]. See Supplemental Material for further detail.
Multivariate Cox regression analysis
Multivariate step-wise forward Wald–Cox regression was used to determine which significant cross-validated PET parameters (SUVmax, SUVmin, TBR, and TBR blood) were independent of the GAP (GAP index and GAP stage) parameters in predicting patient survival.
Modeling PET data with GAP analysis
The GAP scores were reclassified and modified based on the PET parameters to ascertain if the combinations were synergistic and improved the ability to predict survival.
For the PET-modified GAP calculation, we proposed adding a fourth PET variable (SUVmax or SUVmin or TBR or TBR blood that demonstrated the best prognostic ability in our study, based on the above univariate Kaplan–Meier survival analysis, cross-validation, and multivariate Cox regression analysis) to the existing GAP index calculation. For each patient, the best PET marker was binarized, based on the median cut-off, as an adverse (coded as 1) or favorable (coded as 0) PET signal similar to the coding employed in GAP calculation. This was further added to the existing GAP index calculation where the modified GAP index ranged from 0 to 9. For example, if a patient with original GAP index “0” had an adverse PET marker i.e., “1”, the modified GAP index would be “0 + 1” = “1”. Conversely, if the patient with original GAP index “0” had a favorable PET marker i.e., “0”, the modified GAP index would be “0 + 0” = “0”. So the “new” modified GAP index (mGAP) ranged from 0 to 9 in comparison to the original GAP index, which ranged from 0 to 8. Based on the mGAP index, we redefined the mGAP stages (stages I, II, III) as follows: mGAP stage I includes mGAP index 0, 1, 2, 3; mGAP stage II includes mGAP index 4, 5, 6; and mGAP stage III includes mGAP index 7, 8, 9.
A second PET altered (risk stratified) GAP model was also formulated where GAP stage was risk stratified (S-GAP) is presented in the Supplemental Section.
C-index (goodness of fit)
To further assess how good the modified GAP stage was at predicting a binary outcome (mortality) in comparison to GAP stage, the concordance statistic (C-index or C-statistic) was measured as the area under the receiver operating characteristic (ROC) curve along with the 95% confidence interval. In all analyses, p values < 0.05 were considered significant.
Results
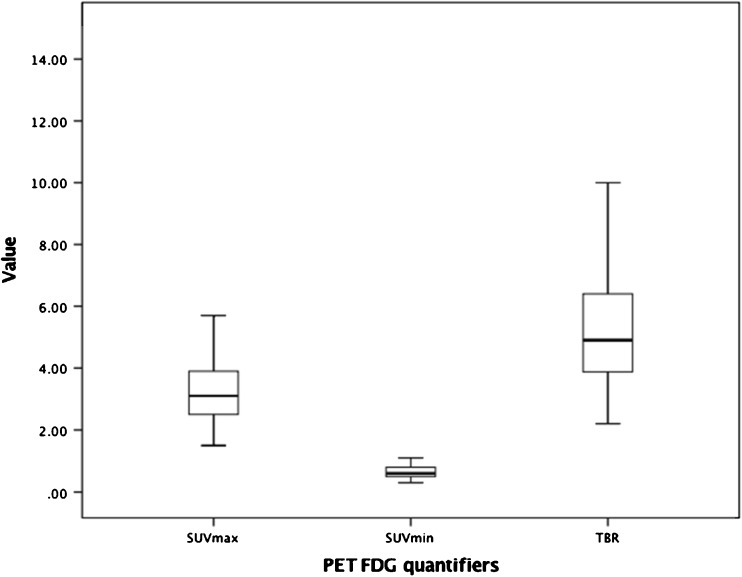
There were 113 IPF patients in total. Eight percent of patients were known to have a co-morbidity of cancer, 18% cardiovascular disease 6% diabetes and 41% other diseases. Twenty patients were known to have a history of anti-fibrotics, a further 44% had a trial of steroids, and 17% immunosuppressants, but not at the time of scanning. The mean ± 1 SD follow-up period was 29 ± 25.2 months. There were 54 deaths during the follow-up. The mean ± SD for SUVmax was 3.4 ± 1.4, SUVmin (background lung activity) was 0.7 ± 0.2 and the mean TBR 5.6 ± 2.7 (Fig. 2).
Fig. 2.
Box plots highlights the distribution of the individual PET markers (SUVmax, SUVmin, TBR) for the entire patient population
The mean ± SD for FVC (percentage predicted) was 74.0% ± 18.2. The mean ± SD for TLCO (percentage predicted) was 45.3 ± 14.4. GAP analysis was performed on 112 patients as FVC was unobtainable in one patient. KM analysis showed that patients that demonstrated a high pulmonary FDG uptake value had poorer survival scores (Table 1). Of the 14 patents who were treated with pirfenidone and six who were treated with nintedanib, no statistically significant survival difference was detected in these groups.
Table 1.
Summary of the cohort associations with survival incorporating the components of GAP analysis. PET (SUVmax, SUVmin, TBR and TBR blood), age, gender, FVC, TLCO, and GAP (index and stage) parameters, median cut-off (direction indicates poor prognosis), hazard ratio (HR), 95% confidence interval (CI) and their association with mortality (as assessed by log rank test from Kaplan–Meier analysis). Statistically significant results are shown in bold.
| Parameter | IPF | ||
|---|---|---|---|
| Median | HR (95% CI) | p value | |
| SUVmax | > 3.1 | 1.3 (0.8–2.3) | 0.317 |
| SUVmin | > 0.6 | 1.2 (0.7–2.3) | 0.490 |
| TBR | > 4.9 | 2.1 (1.2–3.6) | 0.009 |
| TBR blood | > 2.1 | 1.5 (0.9–2.6) | 0.147 |
| Age | > 71.0 | 1.5 (0.9–2.7) | 0.138 |
| Gender | Male | 1.6 (0.7–3.7) | 0.275 |
| FVC | < 72.5% | 2.5 (1.4–4.5) | 0.001 |
| TLCO | < 45.0% | 3.4 (1.8–6.8) | <0.001 |
| GAP index | > 4 | 2.9 (1.4–5.9) | 0.003 |
| GAP stage | > II | 2.9 (1.4–5.9) | 0.003 |
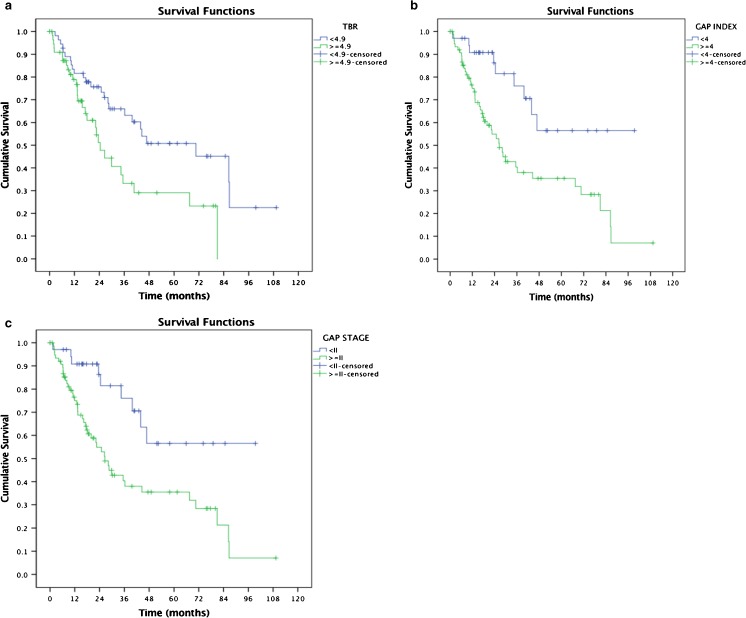
Of the 113 IPF patients, there was a significant association (Fig. 3a) between uptake of pulmonary 18F-FDG and survival (TBR, p = 0.009, before cross-validation), where patients below the median cut-off (< 4.9) had a better prognosis than patients with a TBR > 4.9 (hazard ratio: 2.1, 95% CI: 1.2–3.6). After the fourfold cross-validation, the median cut-off for each of the individual (four) folds was found to be the same as the entire population, whereby patients below the median cut-off (< 4.9) had a better prognosis than patients with a TBR > 4.9 had worse prognosis (hazard ratio: 2.1, 95% CI: 1.2–3.6).
Fig. 3.
a Kaplan–Meier survival curve analysis demonstrating a relationship between TBR (cut-off value of 4.9 = median) and survival in IPF patients after cross-validation. b Kaplan–Meier survival curve analysis demonstrating a relationship between GAP index (cut-off value of 4) and survival in IPF patients. c Kaplan–Meier survival curve analysis demonstrating a relationship between GAP stage (cut-off value of II) and survival in IPF patients
Patients with a TBR below a threshold of 4.9 had a 3-year survival of > 65% compared to a 3-year survival of < 35% for patients with a TBR above the threshold. Below the threshold, ~ 75% survived at 2 years and ~ 50% at 5 years, whilst survival was ~ 50 and 30%, respectively, above the threshold. The 50% mortality (median survival) below the threshold was ~ 6 years, whilst above the threshold it was ~ 2 years.
There was a significant association (Fig. 3b) between GAP “index” and survival (GAP index, p = 0.003), where patients below the median cut-off (< 4) had good prognosis and patients > 4 had poor prognosis with a hazard ratio of 2.9 (95% CI: 1.4–5.9).
In patients with a GAP index below a threshold of 4, ~ 80% survived at 3 years, and ~ 45% at 3 years above the threshold. In patients with a GAP index below a threshold of 4, ~ 86% survived at 2 years and ~ 56% at 5 years, whilst survival was ~ 55 and 35%, respectively, above the threshold. The 50% mortality below the threshold was greater than 8 years whilst above the threshold it was less than 2.5 years.
There was a significant association (Fig. 3c) between GAP stage and survival (GAP stage, p = 0.003), where patients below the median cut-off (< II) had good prognosis and patients > II had poor prognosis with a hazard ratio of 2.9 (95% CI: 1.4–5.9).
In patients with a GAP “stage” below a threshold of II, ~ 75% survived at 3 years whilst survival was ~ 40% above the threshold. In patients with a GAP stage below a threshold of II ~ 86% survived at 2 years and ~ 56% at 5 years, whilst survival was ~ 55 and 35%, respectively, above the threshold. The 50% mortality below the threshold was greater than 8 years whilst above the threshold it was less than 2.5 years.
The effect of the individual components of GAP (FVC and TLCO) on PET survival stratification are shown in the Supplementary Section.
Cox analysis to assess the independence of PET (TBR) and GAP
PET parameter (TBR) was independent of GAP analysis. By including TBR and GAP index in a Cox regression model, TBR (median threshold > 4.9, HR: 2.092, 95% CI: 1.192–3.674, p = 0.010) and GAP Index (median threshold > 4, HR: 2.894, 95% CI: 1.409–5.945, p = 0.004) were both independent predictors of survival.
By including TBR and GAP stage in a Cox regression model, TBR (median threshold > 4.9, HR: 2.092, 95% CI: 1.192–3.674, p = 0.010) and GAP stage (median threshold: > II, HR: 2.894, 95% CI: 1.409–5.945, p = 0.004) were both independent predictors of survival.
Modeling PET-derived TBR by combining with GAP analysis
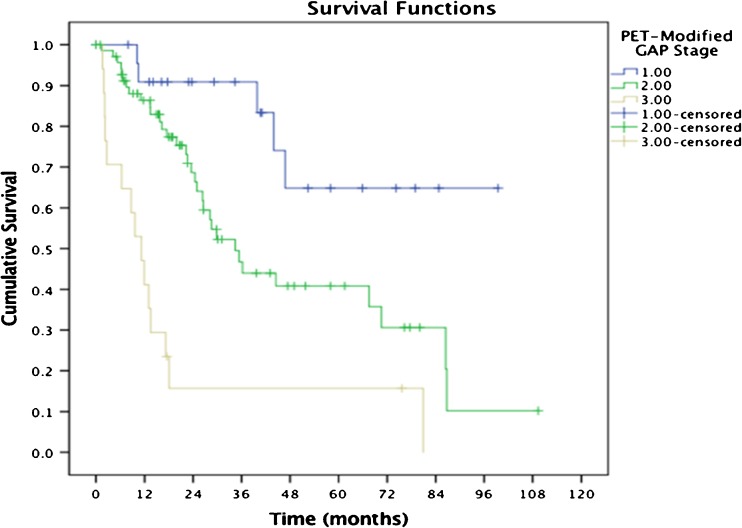
There was synergy in survival associations (Tables 2 and 3, Fig. 4, and Supplementary Table 1 see below).
Table 2.
Three-year mortality for the univariate PET, PFT, and GAP indices as stratified based on above/below the threshold identified in Table 1
| 3-year mortality | |
|---|---|
| TBR < median, (n = 56) | 35% |
| TBR > median, (n = 57) | 70% |
| GAP I (n = 35) | 24% |
| GAP II (n = 50) | 56% |
| GAP III (n = 27) | 65% |
| FVC > median (n = 56) | 29% |
| FVC < median (n = 56) | 68% |
| TLCO > median (n = 45) | 19% |
| TLCO < median (n = 47) | 73% |
Table 3.
Comparison between original GAP 3-year mortality PET versus PET modified GAP Stage (mGAP). The PET modified GAP stage classification shows improved risk stratification compared to GAP on its own especially in the stage I and III groups
| 3-year mortality | |
|---|---|
| GAP I (n = 35) | 24% |
| mGAP I (n = 24) | 9% |
| GAP II (n = 50) | 56% |
| mGAP II (n = 71) | 53% |
| GAP III (n = 27) | 65% |
| mGAP III (n = 17) | 84% |
Fig. 4.
KM-derived survival curves from the modified GAP groups showing that PET can refine the mortality prediction (see also Fig. 3b and c, and Table 3 above). *The difference in the survival curves based on the PET modified GAP stage yielded a p < 0.001 (log-rank test)
PET-modified GAP calculation
The findings from the modified GAP using the TBR are presented in Table 3. It shows improved risk stratification over the original GAP analysis and this additional benefit is further shown in the ROC analysis in the next section below.
C-index
Area under the ROC curve (AUC) along with the 95% confidence interval and p value for GAP stage and m-GAP stage are as follows: 0.667 (0.567–0.767, p = 0.002) and 0.694 (0.598–0.791, p < 0.001) demonstrates increased ability of m-GAP to predict patient mortality.
Discussion
In a population of 113 IPF patients, we have shown that baseline objective measures of 18F-FDG uptake on PET are related to patient survival. High pulmonary 18F-FDG uptake (TBR) was associated with poor survival and was independent of GAP stage. The GAP classification in our population was also a strong survival predictor, but as is recognized, many patients were unable to perform TLCO and PFTs can be skewed in IPF patients with emphysema. Moreover, the GAP classification measurements were synergistic with the prognostic potential of lung pulmonary 18F-FDG uptake. As shown in Table 3 above, PET signal reclassifies a subset of patients at GAP stage 1, as modified GAP stage 2 despite their relatively preserved lung function. Thus, even in groups of patients with good PFTs, the pulmonary 18F-FDG uptake could identify sub-populations of patients that would perform poorly. This may have clinical implication as treatment recommendations can depend on PFT performance [14, 15]. As such, pulmonary 18F-FDG uptake measured with PET is a potential prognostic biomarker in IPF.
The synergies between PET and GAP (Table 3 and Supplemental Table 1) are interesting. In GAP stage III patients, we identified IPF patients that have a better outcome (similar to GAP II) than the other GAP III patients, whose 3-year mortality was nearly 90%. Likewise, there were patients in GAP stage I that had high 18F-FDG uptake, whose outcome was so poor that they may have benefited from treatment. Currently, IPF patients with FVC of 80% predicted and above are considered to have mild disease and treatment with pirfenidone or nintedanib is not recommended in the UK by the National Institute of Clinical Excellence [14, 15]. However, there was evidence of benefit of anti-fibrotics even in patients with mild disease (FVC > 80% predicted) in the original studies [16, 17]. Our data show (see supplement Fig. 1) that a high pulmonary TBR may identify a sub-group of patients that have a poor survival despite only mildly impaired lung function (FVC > 80% predicted); compared to a group of patients with moderately impaired lung function (FVC < 80% predicted) that progress slowly. Thus, the use of 18F-FDG PET in this context raises the possibility of selecting patients for pirfenidone or nintedanib in a wider patient population.
Given the heterogeneous outcome of IPF patients, predictors of mortality in these patients are important for many reasons. Patients may need to understand their prognosis to plan for the future and assist them in their decision-making before taking experimental therapeutics. The patients’ physicians need information to determine the required intensity of follow-up, to enable them to balance the risks of entering their patients into drug trials versus the possible benefits, and also guide them in the timing of referral for lung transplant assessment and the listing of patients on an active transplant waiting list. As such, we show that those IPF patients with a pulmonary FDG uptake with a TBR > 4.9 have twice the risk of death compared to those with low measurements (see Cox models in the Results section above).
Given the inherent problems in using one modality for prognosis there has been much interest in clinical models of risk prediction that take into account age, sex, lung function, and other parameters, such as imaging in some cases, to build a risk score. These have been aimed mainly at IPF, although the original GAP (gender, age, physiology) model has been modified for use across all ILDs and shown to perform well [10, 22, 23]. However, even using these models, there currently remains a lack of survival endpoints. This has prompted the drive for serum biomarkers that can prospectively predict patients at most risk of disease progression. For example, in the PROFILE study, concentrations of 11 novel epitopes derived from matrix metalloprotease (MMP)-degraded ECM proteins were related to subsequent progression of IPF [24]. Future studies may be aimed at seeing if combinations of clinical models, serum biomarkers, and functional imaging can further refine the theranostic approach in IPF.
Since the prognosis is generally poor in IPF, effective treatments are required and the development of new therapeutic agents is urgently needed. However, at present, there is a lack of useful biomarkers to detect or monitor disease activity in IPF or predict survival. Using the currently available clinical predictors, such as GAP and survival data, trials of novel agents will be long in duration and require costly clinical studies [3, 4, 25]. Therefore, if the pulmonary uptake of 18F-FDG on PET is associated with survival in IPF patients, it would be interesting to investigate whether this could be a potential cost-effective response biomarker for drug development.
HRCT remains the main diagnostic imaging investigation in IPF. However, there are more limited data showing the prognostic use of HRCT. Previous investigations have provided associations between survival data and visual scoring/grading systems of HRCT findings in acute exacerbation, a specific situation in which the overall prognosis is generally dire [26, 27]. In the more general prognostic bio-marker role of baseline HRCT in ILD, one single-center study showed that baseline HRCT could be used to predict survival [28] and a large study of mild-to-moderate IPF showed that the extent of honey combing and reticulation was inversely associated with survival [29]. The latter study was a post hoc analysis to a phase III intervention study and thus potentially complicated using pharmacological agents. Another HRCT study has been used to directly assess response to therapy as well as survival [30]. More recently, quantitated CT has been explored with some interesting findings. In one such retrospective IPF study, textural CT patterns derived from HRCT were shown to correlate with lung function [31]. In another, a computer algorithm was used to quantify CT changes in IPF patients, and these were shown to be associated with mortality and add further information to the combined physiological index (COI) or GAP scores [32]. Similarly, another group used textural analysis using dual-energy contrast CT in patients with ILD, showing mortality associations [33]. In contrast, our imaging approach is novel and has the potential advantage of exploiting the functional data and sensitivity of PET [34]. Nonetheless, it is recognized that the underlying CT changes may affect the PET CT signal per se, as has been previously recognized [35]. Differences observed in SUV as used in this paper could be due to a combination of differences in tracer uptake and tissue density. However, it is worth noting that the SUV measures presented in this paper can be computed with standard software.
Although HRCT is essential for diagnosis, the PET signal was important for prognosis when measured from regions where HRCT lung parenchyma findings were normal. In a retrospective analysis, it was shown that increased 18F-FDG in normal lung was associated with poor survival in ILD patients [36]. This is in keeping with another study that showed that in IPF patients normal lung parenchyma as demonstrated on HRCT showed higher 18F-FDG uptake than control patients [37]. This may suggest that PET can detect disease signals before anatomical changes on HRCT are revealed, and as such, PET, in addition to detecting severe disease, may be detecting early or mild disease.
The precise cellular mechanisms underlying the observed FDG-PET signal in IPF are as of yet unknown. We and others have considered the possibility that the FDG signal is generated by inflammatory cells in the alveolar space that may not be seen on lung biopsy, and act as sentinels of lung damage. However, there is accumulating evidence that glucose uptake plays a fundamental role in the fibrotic process. The facilitative glucose transporter 1 GLUT-1 is up-regulated on fibroblasts in the fibrotic regions of both patients with IPF and mice that are subjected to a fibrosis-inducing bleomycin treatment [38]. GLUT1 is induced by transforming growth factor (TGF)-β in fibroblast lines and primary cells and is required for the profibrotic effects of T GF-β. Further evidence that metabolically active cells, taking up increased amounts of glucose, are present in the lungs of IPF patients come from the increased lactic acid levels found in the lungs of IPF patients compared to healthy and COPD controls [39]. This lactic acid may contribute to the pathogenesis of the disease by activating TGF-β and driving collagen production from fibroblasts [39]. Regardless of the underlying mechanism, the utility of FDG-PET as a prognostic marker in IPF remains valid and reflects the presence of metabolically active cells in the lungs of these patients.
There are many technical factors that need to be appreciated in this type of imaging study, such as density-induced artefacts, a varying blood fraction in the lung, and respiratory motion due to free breathing during the acquisition. The methodological considerations involved in tackling these challenges have been dealt with elsewhere [7, 8, 40–42]. Furthermore, respiratory gating would allow reducing the effect of the breathing, albeit at the expense of increased noise. Despite these challenges, we were able to make significant survival observations using routine PET measurements. There are a number of standard clinical 18F-FDG measurements that can be acquired. The three measurements we have performed are easily implemented and are used to quantify the glucose uptake in routine clinical practice. The advantage of assessing glucose uptake in this way is that use of a background 18F-FDG region allows standardization of measurements. We show that TBR in IPF patients is the most prognostic of our three measurements. Our results are in keeping with the previous findings from a dual time point imaging PET study (imaging at 1 and 3 h post-injection of 18F-FDG), where a single SUV measurement at 1 h was not found to have an association with survival. However, with the addition of increased SUV information, (the additonal data from the 2nd time point in the dual time point study, and the ratio of SUVs in our study) it did allow for an association with survival [43]. In another interesting study [44], there was a lack of association between SUVmax (and mean) with IPF survival, in keeping with our findings. Only more complex measurements of 18F-FDG (e.g., tumor lesion glycolysis and metabolic lesion volume), which are more arbitrary, showed any significant survival associations.
Other study limitations include the fact that the timing of the PET study was not always at the time of diagnosis and thus patients would have varying stages of disease. Our data imply that prognosis may be determined at various stages of disease. What would be of particular interest would be an investigation of the use of serial PET scanning in the disease setting. Similarly, serial measurements in PFT (e.g., FVC) and other indicators (e.g., respiratory, hospitalization, preceding change in physiology, disease trajectory some of which are not validated biomarkers) could be assessed in the future. However, it is recognized that there is inherent variability in PFT and PET measurements that serial imaging cannot entirely correct. Another challenge in the IPF patient population is the difficulty in differentiating the contribution of IPF to death from co-morbidities, further compounded by the regular palliative setting of IPF patients at the end of life. For these reasons, we adopted a recognized approach in IPF, and used the all-cause mortality as also in the original GAP analyses [16]. Finally, minimizing bias is challenging in this type of study. The use of test and validation populations can be helpful, but instead we used a k-fold cross-validation technique, which is appropriate in a population size such as in our study [25]. We further minimized bias using median value as cut-offs.
Conclusions
We have shown that high pulmonary uptake of 18F-FDG (TBR) is associated with mortality in our population of IPF patients. These PET findings were statistically independent of GAP survival associations, but were also shown to act synergistically with the GAP classification to help further risk stratify IPF patients.
Electronic supplementary material
(PDF 221 kb)
Acknowledgements
This work was undertaken at the University College London Hospital/University College London (UCLH/UCL), which received a proportion of the funding from the UK’s Department of Health’s National Institute of Health Research, Biomedical Research Centre’s funding scheme. We acknowledge the advice from GSK’s Respiratory DPU and their Craft Consortium.
We would also like to acknowledge the contribution of Vesna Cuplov, Gabrielle Azzopardi, John Hoath, and Irfan Kayani.
Funding
This work was undertaken at UCLH/UCL, which received a proportion of the funding from the UK’s Department of Health’s NIHR Biomedical Research Center’s funding scheme. We acknowledge the advice from GSK’s Respiratory Discovery Performance Units and their Craft Consortium.
Compliance with ethical standards
Disclosure
Although not directly related to this manuscript, our institution receives funding for idiopathic pulmonary fibrosis research from GSK (CRT115549) Research and Development in Stevenage UK.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00259-017-3917-8) contains supplementary material, which is available to authorized users.
References
- 1.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Resp Crit Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 2.Joint Statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Spagnolo P, Maher TM. Clinical trial research in focus: why do so many clinical trials fail in IPF? Lancet Respiratory. 2017;5:372–374. doi: 10.1016/S2213-2600(17)30122-4. [DOI] [PubMed] [Google Scholar]
- 4.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 5.Jones HA, Cadwallader KA, White JF, Uddin M, Peters AM, Chilvers ER. Dissociation between respiratory burst activity and deoxyglucose uptake in human neutrophil granulocytes: implications for interpretation of 18F-FDG PET images. J Nucl Med. 2002;43:652–657. [PubMed] [Google Scholar]
- 6.Wallace WE, Gupta NC, Hubbs AF, Mazza SM, Bishop HA, Keane MJ, et al. Cis-4-[18F]fluoro-l-proline PET imaging of pulmonary fibrosis in a rabbit model. J Nucl Med. 2002;43:413–420. [PubMed] [Google Scholar]
- 7.Chen DL, Schuster DP. Imaging pulmonary inflammation with positron emission tomography: a biomarker for drug development. Mol Pharm. 2006;3:488–495. doi: 10.1021/mp060050w. [DOI] [PubMed] [Google Scholar]
- 8.Groves AM, Win T, Screaton NJ, Berovic M, Endozo R, Booth H, et al. Idiopathic pulmonary fibrosis and diffuse Parenchymal lung disease: implications from initial experience with 18F-FDG-PET/CT. J Nucl Med. 2009;50:538–545. doi: 10.2967/jnumed.108.057901. [DOI] [PubMed] [Google Scholar]
- 9.Win T, Lambrou T, Hutton BF, Kayani I, Screaton NJ, Porter JC, et al. 18F-Fluorodeoxyglucose positron emission tomography pulmonary imaging in idiopathic pulmonary fibrosis is reproducible: implications for future clinical trials. Eur J Nucl Med Mol Imaging. 2012;39:521–528. doi: 10.1007/s00259-011-1986-7. [DOI] [PubMed] [Google Scholar]
- 10.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers RC, Mercer PF. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc. 2015;12(Suppl 1):S16–S20. doi: 10.1513/AnnalsATS.201410-448MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence: NICE technology appraisal guidance [TA282]. Pirfenidone for treating idiopathic pulmonary fibrosis, https://www.nice.org.uk/guidance/ta282 (2013). Accessed 11 Jul 2017.
- 13.National Institute for Health and Care Excellence: NICE technology appraisal guidance [TA379]. Nintedanib for treating idiopathic pulmonary fibrosis. Published date: 27 January 2016. https://www.nice.org.uk/guidance/ta379 (2016). Accessed 11 Jul 2017.
- 14.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 15.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 16.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;15(156):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hunter GJ, Hamberg LM, Alpert NM, Choi NC, Fischman AJ. Simplified measurement of deoxyglucose utilization rate. J Nucl Med. 1996;37(6):950–955. [PubMed] [Google Scholar]
- 18.Krak NC, van der Hoeven JJ, Hoekstra OS, Twisk JW, van der Wall E, Lammertsma AA. Measuring [(18)F]FDG uptake in breast cancer during chemotherapy: comparison of analytical methods. Eur J Nucl Med Mol Imaging. 2003;30(5):674–681. doi: 10.1007/s00259-003-1127-z. [DOI] [PubMed] [Google Scholar]
- 19.Chen DL, Mintun MA, Schuster DP. Comparison of methods to quantitate 18F-FDG uptake with PET during experimental acute lung injury. J Nucl Med. 2004;45(9):1583–1590. [PubMed] [Google Scholar]
- 20.Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, et al. Cardiovascular Committee of the European Association of Nuclear Medicine (EANM). Position paper of the cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43(4):780–789. doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med. 1996;15:2203–2213. doi: 10.1002/(SICI)1097-0258(19961030)15:20<2203::AID-SIM357>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, Collard HR, Anstrom KJ, Flaherty KR, Fleming TR, King TE, et al. Idiopathic pulmonary fibrosis: clinically meaningful primary endpoints in phase 3 clinical trials. Am J Respir Crit Care Med. 2012;185:1044–1048. doi: 10.1164/rccm.201201-0006PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3:462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 25.Wells AU, Behr J, Costabel U, Cottin V, Poletti V, Richeldi L. Hot of the breath: mortality as a primary end-point in IPF treatment trials: the best is the enemy of the good. Thorax. 2012;67:938–940. doi: 10.1136/thoraxjnl-2012-202580. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto K, Taniguchi H, Johkoh T, Kondoh Y, Ichikado K, Sumikawa H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol. 2012;22:83–92. doi: 10.1007/s00330-011-2211-6. [DOI] [PubMed] [Google Scholar]
- 27.Akira M, Kozuka T, Yamamoto S, Sakatani M. Computed tomographic findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:372–378. doi: 10.1164/rccm.200709-1365OC. [DOI] [PubMed] [Google Scholar]
- 28.Mogulkoc N, Brutsche MH, Bishop PW, Greaves SM, Horrocks AW, Egan JJ, Greater Manchester Pulmonary Fibrosis Consortium Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med. 2001;164:103–108. doi: 10.1164/ajrccm.164.1.2007077. [DOI] [PubMed] [Google Scholar]
- 29.Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, et al. Idiopathic pulmonary fibrosis study group. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172:488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 30.Gay SE, Kazerooni EA, Toews GB, Lynch JP, III, Gross BH, Cascade PH, et al. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med. 1998;157:1063–1072. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- 31.Humphries SM, Yagihashi K, Huckleberry J, Rho BH, Schroeder JD, Strand M, Schwarz MI, Flaherty KR, Kazerooni EA, van Beek EJR, Lynch DA. Idiopathic pulmonary fibrosis: data-driven textural analysis of extent of fibrosis at baseline and 15-month follow-up. Radiology. 2017;10:16117. doi: 10.1148/radiol.2017161177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, et al. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. 2017. 49(1). [DOI] [PubMed]
- 33.Moon JW, Bae JP, Lee HY, Kim N, Chung MP, Park HY, et al. Perfusion- and pattern-based quantitative CT indexes using contrast-enhanced dual-energy computed tomography in diffuse interstitial lung disease: relationships with physiologic impairment and prediction of prognosis. Eur Radiol. 2016;26:1368–1377. doi: 10.1007/s00330-015-3946-2. [DOI] [PubMed] [Google Scholar]
- 34.Groves AM, Win T, Haim SB, Ell PJ. Non-[18F] FDG PET in clinical oncology. Lancet Oncol. 2007;8:822–830. doi: 10.1016/S1470-2045(07)70274-7. [DOI] [PubMed] [Google Scholar]
- 35.Lambrou T, Groves AM, Erlandsson K, Screaton N, Endozo R, Win T, et al. The importance of correction for tissue fraction effects in lung PET: preliminary findings. Eur J Nucl Med Mol Imaging. 2011;3812:2238–2246. doi: 10.1007/s00259-011-1906-x. [DOI] [PubMed] [Google Scholar]
- 36.Nobashi T, Kubo T, Nakamoto Y, Handa T, Koyasu S, Ishimori T, et al. FDG uptake in less affected lung field provides prognostic stratification in patients with interstitial lung disease. J Nucl Med. 2016;57:1899–1904. doi: 10.2967/jnumed.116.174946. [DOI] [PubMed] [Google Scholar]
- 37.Win T, Thomas BA, Lambrou T, Hutton BF, Screaton NJ, Porter JC, et al. Areas of normal pulmonary parenchyma on HRCT exhibit increased FDG PET signal in IPF patients. Eur J Nucl Med Mol Imaging. 2014;41:337–342. doi: 10.1007/s00259-013-2514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrianifahanana M, Hernandez DM, Yin X, Kang JH, Jung MY, Wang Y, Yi ES, Roden AC, Limper AH, Leof EB. Profibrotic up-regulation of glucose transporter 1 by TGF-β involves activation of MEK and mammalian target of rapamycin complex 2 pathways. FASEB J. 2016;30(11):3733–3744. doi: 10.1096/fj.201600428R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med. 2012;186:740–751. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holman BF, Cuplov V, Hutton BF, Groves AM, Thielemans K. The effect of respiratory induced density variations on non-TOF PET quantitation in the lung. Phys Med Biol. 2016;61:3148–3163. doi: 10.1088/0031-9155/61/8/3148. [DOI] [PubMed] [Google Scholar]
- 41.Chen DL, Cheriyan J, Chilvers ER, Choudhury G, Coello C, Connell M, et al. Quantification of lung PET images: challenges and opportunities. J Nucl Med. 2017;58:201–207. doi: 10.2967/jnumed.116.184796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holman BF, Cuplov V, Millner L, Hutton BF, Maher TM, Groves AM, et al. Improved correction for the tissue fraction effect in lung PET/CT imaging. Phys Med Biol. 2015;60:7387–7402. doi: 10.1088/0031-9155/60/18/7387. [DOI] [PubMed] [Google Scholar]
- 43.Umeda Y, Demura Y, Morikawa M, Anzai M, Kadowaki M, Ameshima S, et al. Prognostic value of dual-time-point 18F-FDG PET for idiopathic pulmonary fibrosis. J Nucl Med. 2015;56:1869–1875. doi: 10.2967/jnumed.115.163360. [DOI] [PubMed] [Google Scholar]
- 44.Justet A, Laurent-Bellue A, Thabut G, Dieudonne A, Debray MP, Borie R, et al. [18F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. 2017. Respir Res. 2017;18:74. doi: 10.1186/s12931-017-0556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 221 kb)