Abstract
A defining feature of dentitions in modern sharks and rays is the regulated pattern order that generates multiple replacement teeth. These are arranged in labio‐lingual files of replacement teeth that form in sequential time order both along the jaw and within successively initiated teeth in a deep dental lamina. Two distinct adult dentitions have been described: alternate, in which timing of new teeth alternates between two adjacent files, each erupting separately, and the other arranged as single files, where teeth of each file are timed to erupt together, in some taxa facilitating similarly timed teeth to join to form a cutting blade. Both are dependent on spatiotemporally regulated formation of new teeth. The adult Angel shark Squatina (Squalomorphii) exemplifies a single file dentition, but we obtained new data on the developmental order of teeth in the files of Squatina embryos, showing alternate timing of tooth initiation. This was based on micro‐CT scans revealing that the earliest mineralised teeth at the jaw margin and their replacements in file pairs (odd and even jaw positions) alternate in their initiation timing. Along with Squatina, new observations from other squalomorphs such as Hexanchus and Chlamydoselachus, together with representatives of the sister group Galeomorphii, have established that the alternate tooth pattern (initiation time and replacement order) characterises the embryonic dentition of extant sharks; however, this can change in adults. These character states were plotted onto a recent phylogeny, demonstrating that the Squalomorphii show considerable plasticity of dental development. We propose a developmental‐evolutionary model to allow change from the alternate to a single file alignment of replacement teeth. This establishes new dental morphologies in adult sharks from inherited alternate order.
Keywords: chondrichthyan, dentitions, evolution, replacement, teeth
Introduction
In modern‐grade elasmobranch fishes, Neoselachii (including all living sharks, rays and skates), replacement tooth positions along the jaw have been described as two distinct arrangements, single file or alternate file (e.g. Reif, 1980; Smith et al. 2013; Underwood et al. 2015; Fig. 1A,C), or modifications of these basic patterns, producing, for example, a single cutting blade of imbricated teeth across the jaw (Strasburg, 1963; Underwood et al. 2015). During development, an asynchronous timed series of tooth germs is initiated along the jaw (Smith et al. 2009), at alternate positions, as labio‐lingual files of replacement teeth (Figs 1D and 2). In this arrangement of replacement teeth, two adjacent files form (Smith 2003; Smith et al. 2013: figs 1, 2), from the first rudimentary teeth, as sequentially added teeth (SAT unit), proposed as a clonal unit of differentially timed teeth [SAT unit tf 6 + 7; Fig. 2]. In these, timing and position alternate within each pair of tooth files (tf 6 + 7) to provide closely spaced teeth in alternately timed labio‐lingual addition (Fig. 1B). Each pair of files is added disto‐proximally along the jaw (Fig. 2, 1–12), developmentally linked as iteratively timed ‘SAT units’ (Fig. 2, stages 1–9; Smith, 2003; Smith et al. 2013: figs 1A, 2), so that in the adult, the teeth erupt at different times. By comparison, the single file arrangement in the adult is composed of teeth in adjacent files at the same developmental phase, recognised by the youngest teeth in adjacent files being at the same stage of development and the oldest at the same position relative to the jaw margin, erupting together (Fig. 1A). In this arrangement, timing of eruption at the jaw margin could be as single teeth or simultaneously for all teeth, forming a blade (Fig. 1A; Underwood et al. 2016), and as alternate teeth (Fig. 1B). Dentitions may also have disto‐proximally staggered times (Fig. 2, 1–12) and many rows of erupted (functional) teeth together (Fig. 1C). Until recently, it was unclear how these different adult morphologies developed and if neoselachian dentitions showed developmental plasticity, through transformation of tooth order from embryo to adult, and whether alternate or single file addition is the primitive state for skates and rays
Figure 1.
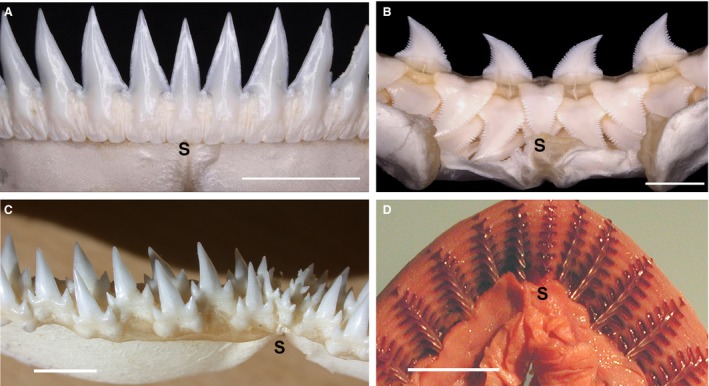
Adult and embryonic jaws with tooth arrangement at the jaw margin. Single file (A) vs. alternate dentitions (B,C), with (D) unknown timed order in embryo. (A) Scymnodon ringens (Knifetooth dogfish), lower jaw in labial view with single, symmetrical tooth across the jaw symphysis (S). (B) Prionace glauca (Blue shark), upper jaw in lingual view with replacement row teeth including symmetrical symphyseal tooth, other teeth are polarised left–right (modified from Smith et al. 2013; figs 1B, 5C; photos Tom Diekwisch). (C) Triaenodon obesus (White tip reef shark) adult dentition with alternate dentition, reduced symphyseal teeth. (D) Carcharhinus leucas (Blacktip shark) embryonic lower jaw (lingual epithelium removed, tissues stained) with tooth files central cusp‐aligned but all successor teeth appear in single file arrangement from rudimentary cusp of first tooth (volumetric data not available) with space for attachment bases to increase in size (from Smith, 2003: fig. 9A; Smith et al. 2013: fig. 4D). Scale bars: 1.0 cm.
Figure 2.
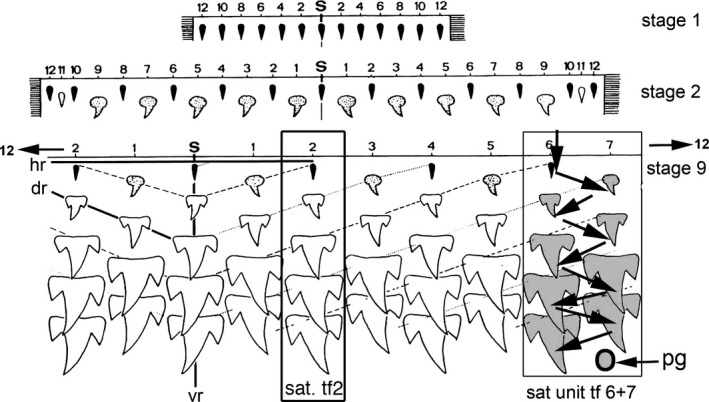
Developmental model of dentition in alternate file order in Grey reef sharks. Carcharhinidae, single cusp teeth are first initiated along the jaw, formed as mineralised tissue in embryos with one tooth row (stage 1), then two rows (stage 2) and, later in development, nine tooth rows (stage 9). Jaw positions (distal to proximal) numbered 1–12 from the symphyseal tooth (S), first as even number positions, then odd in the second row. Smallest teeth (black, stage 1) then larger alternate teeth with polarised shape (grey, stage 2); later, larger teeth with lateral cusps form by row 3 (Smith et al. 2013: fig. 2). Sequential tooth initiation in a clonal set (arrows, direction of timing for teeth 1–9) shows the alternate timing of tooth initiation order in adjacent tooth files 6 and 7 (SAT unit tf 6 + 7), with the next putative tooth germ (pg) to form in odd number row position. An example as if it was a single file, a sequential addition model is superimposed on this alternate model at file position 2 (SAT tf 2; Smith et al. 2013: fig. 2; modified from Reif, 1978).
Within the Batoidea (skates and rays), the sister group to modern sharks (Squalomorphii + Galeomorphii, Fig. 3), both embryos and adults possess the alternate pattern for arrangement of successor teeth. Within many batoids, teeth in alternating order are close‐packed, forming continuous surfaces, for crushing dentitions (Underwood et al. 2015). In sharks, all members of the Galeomorphii so far studied (Carcharhiniformes, Orectolobiformes and Heterodontiformes; see Smith et al. 2013; Fig. 1B,D) have alternate tooth replacement in adults, from alternate developmental order in embryos. The status of the Lamniformes has been considered uncertain (Smith et al. 2013). In Squalomorphii, many taxa have distinctive single‐file successor teeth demonstrated to be the result of developmental modification of an embryonic alternate pattern (Underwood et al. 2016; Fig. 1A). The alternate pattern can also be present in the upper jaw, or both jaws, as an example of developmental independence (plasticity) of upper and lower jaws, as in Hexanchidae (lower dentition single file, but an alternate pattern in the upper jaw and teeth that lie closest to the jaw hinges).
Figure 3.
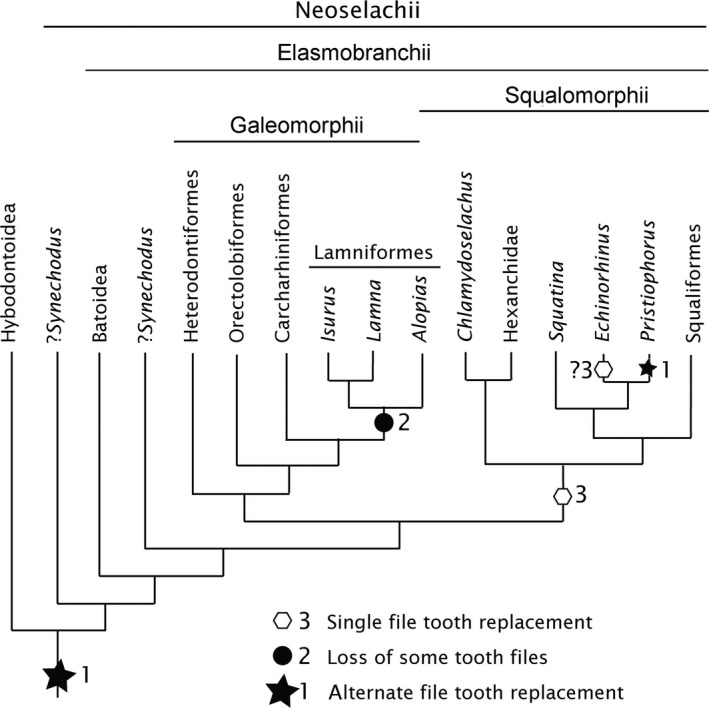
Neoselachian phylogeny with character state distribution. Alternate and single tooth file replacement in embryos and adults, with these character states plotted on a recent phylogeny (Naylor et al. 2012). The basal position and monophyly of the Synechodontiformes, including Synechodus, follows Klug (2010). All neoselachians, as well as the Hybodontoidea, show alternate tooth replacement in some part of their dentition in the embryo, even if this is not retained in the adult (state 1). Within the Lamniformes (Galeomorphii) some tooth files are lost to produce the appearance of single file tooth addition, found in only certain parts of the jaw, reflecting irregular tooth file loss (state 2). Within the Squalomorphii, the single file tooth replacement pattern is developed from secondary modification of an alternate pattern (state 3). Within Chlamydoselachus an alternate pattern may be present in the embryo but not in the adult (except proximally); the majority of the dentition shows a single file arrangement. In Hexanchus (Hexanchidae), single file addition is present in early development and is retained in the adult, whereas in other adult hexanchids (e.g. Notorhychus) the alternate pattern is only retained in proximal rudimentary teeth. In Squatina an alternate pattern is present in the embryo, whereas the adult dentition possesses a single file arrangement but has retained alternate addition in proximal tooth files. In these three taxa, state 3 (single file) may be related to a fixed number of tooth files and independent jaw growth, allowing in Squatina interdigitation of upper with lower jaw teeth.
Several other clades of squalomorph sharks appear to have a single file tooth replacement order in adults, with well‐spaced tooth files, including Squatina (angel shark), the Hexanchidae (six‐ and seven‐gilled sharks) and Chlamydoselachus (frilled shark) as well as the Lamniformes within the Galeomorphii (Mako, Thresher and White sharks and relatives), but in each case their developmental order is unknown. Our study of the embryonic and adult dentitions of Squatina, along with examination of dentitions of Hexanchus, Chlamydoselachus and Isurus (Galeomorphii), allows the early stages of tooth development to be compared and contrasted with the embryonic alternate patterning in Heterodontus (Heterodontiformes), as detailed by Reif (1976). These data are used to explore the hypothesis that the alternate pattern can be transformed into a single file during development through both alteration of tooth germ timing and loss of individual tooth files, with these being two different potential mechanisms for evolutionary transformation. We propose that the alternate addition of teeth is plesiomorphic for the Neoselachii, retained in embryonic and adult rays, and present in fossil relatives, but can be modified to single file from the embryonic condition in some adult sharks (Galeomorphii and Squalomorphii). Embryonic Squatina is an excellent model to test this hypothesis in squalomorphs along with Chlamydoselachus and Hexanchus as basal members of the group (Fig. 3), all with putatively single file dentitions in the adult.
To determine the plesiomorphic state, fossils of taxa closely related to extant sharks and rays were studied, including Acrodus (Hybodontoidea) and Synechodus. The phylogenetic relationships of Synechodus are uncertain (Maisey, 1985; Klug, 2010; Fig. 3, where ‘Neoselachii’ includes Synechodus + Elasmobranchii), although they shares many characters with extant sharks and rays, including that of a complex tooth histology (e.g. Enault et al. 2015). These two taxa were investigated with the aim of testing whether the alternate arrangement of teeth in adults is the basal state for modern sharks as well as rays (Fig. 3) and to determine where clades with the derived state (single file replacement) occurred on the phylogeny.
Materials and methods
Materials
Wet specimens
Squatina californica, embryo (Natural History Museum, Life Sciences collection, BMNH 91.5.19.240)
Isurus oxyrhinchus, embryo (BMNH 1961.11.2.3)
Chlamydoselachus anguineus, adult jaw (BMNH 2016.4.11.1)
Lamna nasus, adult head, jaw sections (BMNH 2015.3.13 1–3)
Hexanchus sp. (?H. nakamurai), embryo (BMNH 1973.7.12.4–6)
Fossil specimens
Synechodus dubrisiensis, jaw (Booth Museum, Brighton, BMB 008523)
Acrodus anningae, jaw (Natural History Museum, Earth Sciences collection, NHMUK PV P2732)
Dried specimens (Birkbeck reference collection)
Squatina spp. (Squatina guggenheim, Squatina argentina, Squatina tergocellata, Squatina ?caillaiti)
Echinorhinus brucus
Hexanchidae (four species; Hexanchus griseus, Hexanchus nakamurai, Heptranchias perlo, Notorynchus cepidianus)
Lamniformes (eight species; Lamna nasus, Isurus oxyrinchus, Pseudocarcharias kamoharai, Carcharias taurus, Odontaspic ferox, Alopias pelagicus, Alopias superciliosus, Alopias vulpinus)
Cleared and stained specimens
Chlamydoselachus anguineus embryos and juvenile [Nos. 1984/5/6/6, 1984/9/2/3 and 1985/5/3C: total length (TL) 28.6 cm; 40.1 cm]. These were stained with Alizarin red, Alcian blue, from the Tokai University Museum, Shimizu, Japan (TMFE), courtesy of Sho Tanaka. Also studied were Nos. 1984/5/6/6, 1984/9/2/3, and 1985/5/3C: TL 28.6 cm; 40.1 cm).
Methods
Imaging
We used X‐ray computed tomography to examine the head region of whole embryos [micro‐CT, Nikon Metrology HMX ST 225, Image and Analysis Centre, Natural History Museum, London (NHM)] to visualise the teeth present within the jaws of specimens, especially the earliest teeth (mineralised cusps) from the 3D volume‐rendered models. Photomacrographs were taken with a Nikon Coolpix camera in natural light; drawings made with the software inkscape and the x11 window System.
Terminology
For use of directional terms such as distal and proximal, see Underwood et al. (2016). The systematic terminology follows Compagno (1973, 1977) and Nelson (2006).
Measurements
In embryonic Squatina, measurements were taken when the first initiated teeth were set iteratively along the jaw (Fig. 4, 1–8, distal to proximal) with three to four labio‐lingual successive teeth and none shed from the jaw margin (Figs 4 and 5, stages 1–3). We compared these data with embryos of Chlamydoselachus and Isurus (taxa also with apparent single file tooth replacement). We also investigated various adult galeomorph and squalomorph taxa, along with fossil dentitions, selected from key positions on a neoselachian phylogeny (Fig. 3).
Figure 4.
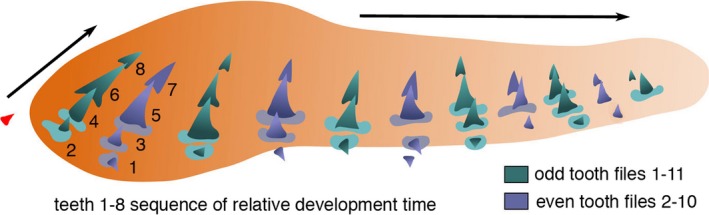
Schematic drawing of timed developmental order of first dentition teeth. Left lower jaw of Squatina californica embryo (BMNH91.5.19.240), teeth in apparent single file arrangement (from xCT render, Fig. 5) with odd (green) and even number (purple) tooth files and symphyseal tooth (red); timing order of clonal units shows successional tooth initiation (1–8) between first two adjacent files (based on size and morphology of files t1–7 and t2–8 in Fig. 5A–E). Teeth at the labial jaw margins are rudimentary, first to form on the jaw cartilage at the even numbered jaw positions and closer to eruption and shedding. The newest lingual tooth (8) is smallest in odd numbers, representing mineralised central cusp tip. Arrows indicate labio‐lingual tooth addition and disto‐proximal tooth file addition.
Figure 5.
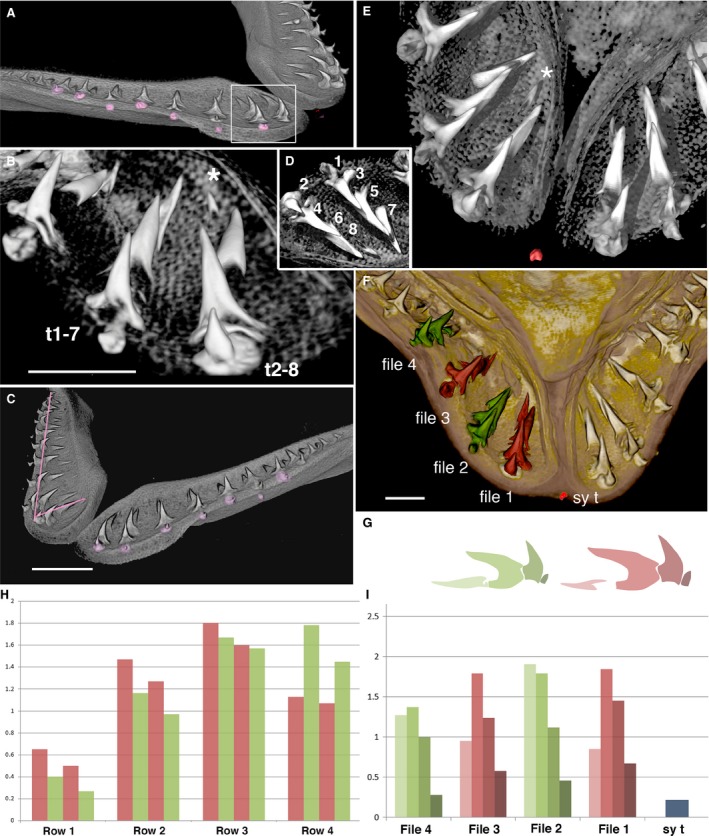
Spaced tooth files in lower jaw of Squatina californica embryo. BMNH 91.5.19.240, comparison of micro‐CT 3D‐renders using vgstudio max (B,D,E) and avizo (A,C,F). (A) Labial view of right jaw, alternate position of rudimentary teeth at the jaw margin (pink). The nearest are even number positions.White box indicates field in (B,D). Labial view from symphysis (B,E) and lingual view (D) indicate developmental series of replacement teeth in time series from 1–8 (D). (B) The first file (t2–8) and second file (t1–7), at even tooth positions (t1–7) is first rudimentary tooth; at odd position (t2–8) are youngest (asterisk, 8). (C) Oblique anterior view shows left jaw with alternate positions of shedding teeth (pink), right jaw central cusp‐aligned file (short pink line), alternate cusp‐aligned file (long pink line). (D) Tooth files 1 and 2 as in (B,E) with sequence of initiation time order t1–8, with tooth tip 8 the latest to form (see Fig. 4). (E) Symphyseal region, labial view of tooth files in (B,D), symphyseal tooth at jaw margin (red), the newest tooth in replacement series of file 1 (asterisk, 8 in D). (F) First four files segmented as alternate odd (red) and even positions (green) from symphyseal tooth (sy t), volume of central cusps measured. (G) Colour profiles of first four teeth shown in each file in histogram, the darkest is first in the file (I). (H) Histogram showing relative sizes of individual teeth in rows (labio‐lingual) first row smallest. (I) Histogram shows relative heights of each tooth in each alternate file; the first is the smallest in even numbers (colours as in G). Scale bars: 5 mm (B,C,F).
Tooth size was measured in two different ways, depending on the overall morphology of the tooth. The first focused on cusp height, as the distance between the tip of the central cusp to the crown/base boundary. Distances between the selected points were calculated using avizo 9.2 software measurement tools. A 3D‐rendered image was used for Squatina (embryo and adult) and Chlamydoselachus (adult), with the distance between the two points being recorded for each tooth in the file, starting at the first file next to the symphysis. Thus each tooth file includes separate measurements of tooth cusp height from the oldest tooth (labial) to the youngest developing mineralised tooth (lingual).
The second method, where tooth file size differed along the jaw, as in the Isurus embryo, and where the central cusp was asymmetrical relative to the base, used a different approach. Here, tooth volume was estimated to overcome problems such as the wide range of asymmetrical variation of each tooth file (Supporting Information Fig. S4). Measuring volume required individually segmented tooth elements (avizo 9.2), performed slice by slice, selecting mineralised regions of each tooth. A label analysis tool was then used to acquire an individual tooth volume metric (× mm3).
The histograms produced (Figs 5G–I and 6H, Supporting Information Fig. S4, Video S2) used a colour code indicating the developmental order for each tooth; darker colours represent the oldest tooth, grading to the lightest colours for the developmentally youngest teeth. Also, within each tooth file, red and green represent odd and even tooth files, respectively, beginning at the symphyseal region.
Figure 6.
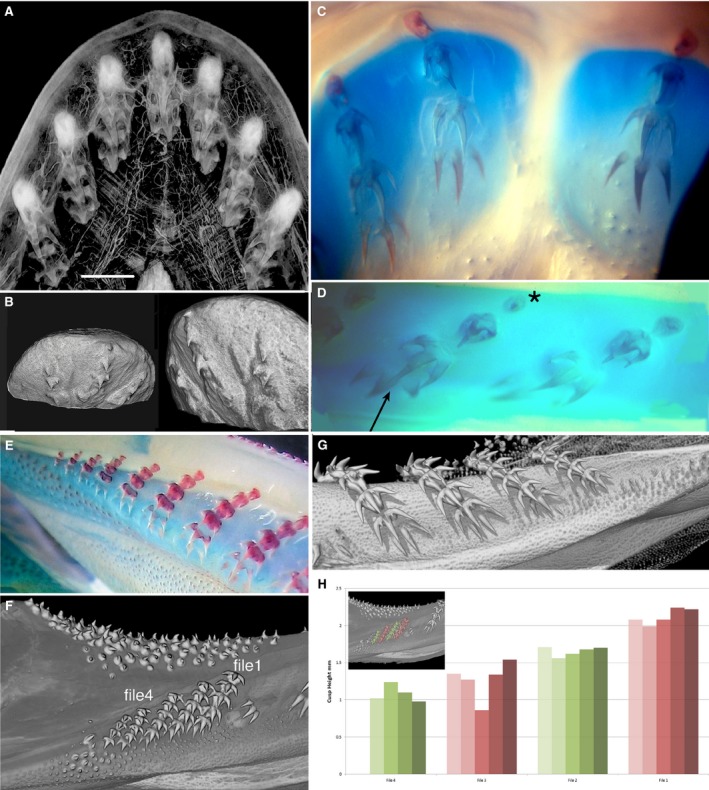
Single file dentition of embryo and adult of Chlamydoselachus angineus. (A) Micro‐CT scan through dissected symphyseal segment of the embryo lower jaw dentition, tissue contrast‐enhanced with phosphotungstic acid. Symphyseal file with three tooth files either side, each with five tooth germs, separately encapsulated in connective tissue; the last has three developing cusps, as in adult teeth (G). (B) Two views, lower jaw region across the symphysis of alcohol‐dried, younger embryo, isosurface render of three tooth files, symphyseal with the smallest, single cusp, nearest of the three files to the labial margin as first tooth formed; tooth files left and right also have single cusp first, but larger than the first symphyseal. The second teeth (in all three) are larger single cusps and two small lateral cusps. (C,D,E) Photomicrographs of cleared and stained embryos (Alcian Blue, Alizarin Red). (C) Upper jaw symphysis lacking symphyseal tooth file, first file (left) has small rudimentary first tooth of one cusp, a second with two cusps, a third with three cusps, with base outlined, a fourth with three large cusps not joined at base. (D) Files 2 and 3 of lower jaw may show the smallest first tooth in even files (asterisk), and in same file the fourth tooth has developing central cusps (arrow), larger than in adjacent file. (E) Juvenile, proximal eight files, reducing tooth numbers proximally, first teeth in all files rudimentary, increasing overall size distally. (F, G) Rendered and segmented adult lower jaw (BMNH2016.4.11.1) (F) Lingual view, smallest, but most proximal ordered tooth files (1–4 used for volume meacurements, (H) Contrast seen with small cluster of oro‐pharyngeal denticles lacking organisation and demal denticles, top, (see (G), and Fig. S3B). (G) Lingual view, four more distal files in which all teeth are above the jaw cartilage (no separate bullae), only loosely held in connective tissue (see Fig. S3A). (H) histograms of most proximal tooth files (coloured inset; files 1–4 in F); labial tooth is darkest colour in each set), insignificant size differences seen between first tooth in green files relative to red. Scale bars: 3 mm (A).
Results
Developmental interpretation of tooth replacement
In chondrichthyans, the developmental dental lamina restricts tooth induction (odontogenic potential) to the tissues in the lamina (Reif, 1982; Smith et al. 2009; and references therein; Martin et al. 2016; Rasch et al. 2016, 2016), which is present along the lingual face of the jaw cartilage. Here, tooth files are established in the embryo from the initiator teeth, ordered sequentially along the jaw margins, where morphogenesis gradually assumes the adult shape, and new teeth are continually initiated in the adult (Figs 1B,D and 2, pg). Because of this, developmental interpretations can be made from observations of static morphology, with teeth effectively suspended in a time sequence (Reif, 1980, 1982). Hence the smallest, oldest teeth are at the jaw margin, whereas the newest teeth are in various stages of development deep in the lingual furrow (Figs 1D and 2). The newest or youngest teeth are visible only as the mineralised central cusp tip, but in each tooth file they form in a sequentially timed developmental order with increasing morphological differentiation (e.g. cusps joined to base) to become the largest in the tooth file. These observations from tooth germs in the dental lamina can be used to assign a relative timed order to sequential stages of the developing teeth within two adjacent files (Figs 1D and 2, arrows).
Squalomorphii
Squatina embryonic dentition
In embryos, as in adults, teeth are arranged in well‐spaced single files, with 10 files in the upper jaw and 11 in the lower, in each half (Figs 4 and 5, Supporting Information Fig. S2C). In the embryonic lower jaw, the youngest developing teeth occur lingually as small cusps (no. 8, Figs 4 and 5B,D,E, asterisks), with the oldest teeth labially, towards the jaw symphysis. These have a single central cusp joined to a small tooth base (nos 1 and 2 in Figs 4 and 5D). A single rudimentary symphyseal tooth is present in one specimen (red, Figs 4and 5E,F) but is not observed in others. This is only present in the first tooth row, with subsequent rows lacking symphyseal teeth. When all tooth files along the jaw are established (disto‐proximally, Fig. 4) it was apparent that the oldest (first) teeth are the smallest and the nearest to the labial jaw margin, and are located in even positions relative to the symphyseal tooth [Fig. 5 (sy t, red t)1,t2, B,D]. These teeth are about to erupt, or be shed (purple, Fig. 4; Fig. 5A,C, alternate pink). Thus, teeth in even positions indicate the first initiation time in each pair of alternate files and of first loss from the jaw margin. Similarly, those at odd positions (Fig. 4, green) are the second teeth to be initiated; then alternate sequential tooth initiation within the pairs (odd and even files, Fig. 2) occurs with the latest to form, the newest tooth germ, visible as a cusp tip (Figs 4 5D, no. 8 and 5B,E, asterisk).
The first four teeth of the four files were compared, with the fourth still a developing tooth (Fig. 5G,I). When represented as graphs comparing cusp volumes in rows parallel to the jaw margin, even first teeth are smaller up to row 3 (green, Fig. 5H). In row 1, as noted, the first teeth are smallest in even files (2 and 4) representing the first to form. In row 4, all developing teeth have only the central cusp, and in even files (green) cusps are larger than odd files because these are the first file teeth to develop and are now further advanced morphologically at this stage of development (row 4, Fig. 5H).
All teeth are central cusp‐aligned (Fig. 5C, short pink line), in progressive states of morphological development of central cusp and the tooth base in file numbers t1–7, t2–8 (Fig. 4). Each file contains four teeth, with two in the most proximal files (presumed to be the newest files added proximally to the jaw, Fig. 4).
Interpretation of developmental timing in embryonic tooth files
As described above, teeth in files 1 and 2 on the lower right jaw illustrate relative sizes and morphologies, and therefore developmental order of timing for sequential tooth addition, alternating between these files (Fig. 5B,D–F). The first tooth of the series is the most labial and the smallest formed tooth with an attachment base; sequential addition starts from file 2 (Fig. 5B,t1–7), tooth number 1 being formed before the morphogenetic program was perfected (Fig. 5B,D,E). The sequential time series ends with the newly initiated tooth tip of tooth number 8, as a mineralised but incomplete central cusp (Fig. 5B,E, asterisk). This alternate file developmental set represents the SAT unit (Fig. 2, SAT tf 6 + 7). A single file is equivalent to one even set (Fig. 2, SAT tf2). Order of initiation (t1–8, Figs 4 and 5D) may also determine timing of shedding, as the labial positions of first teeth in each alternate file indicated an alternate shedding order (pink, Fig. 5A,C). In each separate file, central cusps are aligned (Fig. 5C, short pink line); in addition, only alternate tooth cusps are aligned with a straight disto‐proximal line along the jaw, showing that adjacent files are offset (Fig. 5C, long pink line).
Measurements of central cusp heights and alignment
Morphological evidence of progressive, developmental tooth order as clonal SAT units (paired odd and even files) was tested with measurements of tooth size, both along the jaw in rows parallel to the jaw margin and within files (Fig. 5C, pink lines). These are represented as histograms (Fig. 5H,I) taken from tooth files 1–4 (alternate red and green, Fig. 5F,G vignettes of two alternate files). The comparative central cusp heights, represented by the graphs, confirm that the smallest, complete first teeth are in the even files (green, Fig. 5G,I), whereas the first tooth in odd files is larger (red, Fig. 5H, row 1). When represented as graphs comparing cusp heights in rows parallel to the jaw margin, again even first teeth are smaller up to row 3 (green, Fig. 5H); in row 4 teeth the central cusp is just developing and even numbers are larger than odd because, as these are the first of the teeth to develop, they are further advanced in morphology at this stage of development (row 4, Fig. 5H).
Adult jaws of S. guggenheim
In the early adult dentition, occlusion of upper jaws with lower jaws show teeth fitting between lower jaws, with teeth organised in widely spaced files along the jaw (Fig. S2B–D) so that tooth file spacing allows upper teeth to fit between lower ones. From the relative cusp heights it appears that the alternate initiation of teeth seen in an embryo does not continue in the adult (Fig. S2F); measurements in the adult dentition were taken, as for the embryonic teeth, from the first teeth of each of four files; these showed little difference between alternate positions [the fifth and sixth teeth are smaller (partially developed) and so cannot be compared with the four fully formed main cusp volumes]. However, in proximal files closer to the jaw articulation (Fig. S2C, white box, D, file numbers 6–10), the position of the oldest teeth located at the jaw margins seems to alternate and suggests they still show different times of origination, as in the embryonic alternate developmental pattern.
Other Squalomorphii
Chlamydoselachus anguineus embryo and adult dentitions
In the embryo, at the four‐tooth stage (Fig. 6A–D) teeth are superficial and encapsulated in individual sheaths of connective tissue, separate for each file (Fig. 6A, contrast‐enhanced density). A symphyseal file is fully expressed in the lower jaw (Fig. 6A,B) but absent in the upper jaw (Fig. 6C). In the dried specimen (Fig. 6B), the symphyseal file has a rudimentary single cusp tooth that is the nearest of all files to the jaw margin, and as such is the initiator tooth of the lower jaw dentition; to either side is also a single cusp first tooth, and each file then acquires morphological competence as three‐cusped teeth. This morphology (Fig. 6C–G) is demonstrated with calcium‐positive staining (red, Fig. 6C–E), first as separate cusps (Fig. 6D, arrow, 6C) and later joined to the base. In the lower jaw, files 3 and 4 (Fig. 6D), the smallest first tooth (oldest, asterisk) is positioned in the even number file 4, whereas the file 3 first tooth is slightly larger (i.e. began developing later). The third and fourth teeth of file 4 have developing, mineralised cusps (fourth tooth largest, as latest and most morphogenetically competent). These size differences in adjacent files suggest the presence of alternate tooth initiation timing. In the upper jaw at the four‐tooth stage in each file, teeth are absent from the symphysis, the first tooth of each has only one cusp and the base has formed. Measurements of tooth sizes were not possible in these embryos, as they were only soft tissue preparations or were long‐term fixed specimens and had lost mineralisation.
The lower jaw of a more mature embryo has tooth files proximally that have not completed morphogenesis (Fig. 6E), as all files have a rudimentary first labial tooth, so the embryo has not shed the first teeth in these proximal files and had not reached maturity (see Fig. 6F). These gradually reduce in total tooth number in files to four or three, all diminishing in size (Fig. 6E). In all files, tooth number 6 is the youngest, with only cusp tips mineralised, but teeth in older positions have all cusps joined and the first four teeth also have cusps joined to mineralised bases. In the adult lower jaw these proximal files still have minute teeth but the first tooth is three‐cusped (Fig. 6E), is on the labial side of the jaw and is not rudimentary. We segmented and measured the teeth of these last four files (Fig. 6F) to test whether we could show size differences that represented ordered, alternate files created by alternate timing, as in the embryo. The general tooth sizes of the four measured files (1–4 in Fig. 6F,H) decreases proximally; nevertheless, the first teeth are smaller in green files than in red files (Fig. 6F,H). We interpret this as resulting from the alternate developmental program seen in the embryo, still present in adult proximal teeth; however, this needs to be tested on more suitable material.
Considering the fully formed teeth of the adult lower jaw, distal to these proximal files (Fig. 6G, Supporting Information Fig. S3), each file has similarly sized teeth, four teeth fully erupted and locked together as a functional unit, a fifth tooth lingually with an incomplete base and a sixth tooth forming as three separate cusps lingual to the completed teeth (Fig. 6G). These developing successional teeth are located on a lingual shelf on Meckel's cartilage deep to the oral surface (not in a separate bulla); older teeth are held in the connective tissue of the skin, as in the virtual section next to one of the tooth files (Fig. S3A). In the symphyseal file and three files either side, tooth size differences between odd and even files were inconspicuous (Fig. S3C, histogram). It would seem that any evidence for timing difference between tooth files seen in the embryos was not present in the adult dentition but might be present in the smallest, most proximal tooth files.
Hexanchidae: Hexanchus spp. embryo and adult dentitions; N. cepedianus, adult dentitions
A single embryo of Hexanchus was studied, in which the teeth are well developed, but with those in the first row of the lower jaw only starting to rotate into a pre‐functional position (Fig. 7A,C). Thus, most teeth have not been lost, as bulk‐shedding only occurs after the replacement teeth are in their functional positions (Underwood et al. 2016). The lower teeth are large and distinct, but no obvious alternation or overlapping of tooth bases could be observed there or in the upper jaw (Fig. 7B).
Figure 7.
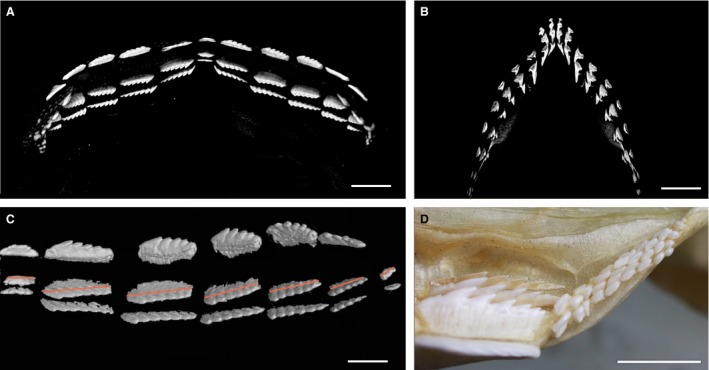
Single file dentition of embryo of Hexanchus. (A–C) Hexanchus ?nakamurai (BMNH1973.7.12.4–6), micro‐CT renders of late stage embryo, upper and lower jaws. (A) Lower jaw dentition labial view, compared with (B) upper jaw dentition. (C) Lower right, lingual view (of A), three teeth in each file (file 7 has two), teeth are aligned in a single file replacement pattern, but within each file they are arranged at an oblique angle relative to the jaw margin (red line). (D) Proximal teeth of adult Notorynchus cepedianus, adult lower dentition, adjacent to last tooth of typical bladelike morphology, tiny rudimentary teeth showing and alternate arrangement. Scale bars: 5 mm (A,B), 2.5 mm (C), 10 mm (D).
In the embryo, individual replacement teeth in seven adjacent single files, including the symphyseal file, are obliquely arranged within disto‐proximal rows (Fig. 7C, red lines). Although tooth size decreased to the most proximal, 7th tooth file, equivalent individual teeth in files were of approximately equal size. This oblique, developmental alignment of teeth in a disto‐proximal row has also been recognised in the Squalomorphii, demonstrated to be as a result of realignment of teeth to form a single file arrangement, altered from the embryonic alternate arrangement of the first teeth (Underwood et al. 2016). By comparison, in Hexanchus, embryonic teeth are aligned as single file and no detectable alternation of replacement teeth is apparent. This arrangement is retained in the adult, where teeth are also single file, aligned labio‐lingually (e.g. Smith et al. 2013: fig. 4G).
An abrupt change in tooth form and arrangement occurs in the lower dentition of adult hexanchid genera, where the most proximal teeth are very reduced in size, and appear to show an irregular but alternating pattern (Notorhynchus, Fig. 7D). These reduced teeth in the adult are suggested to be a ‘remnant’ of the ancestral developmental alternate pattern (as described above for Chlamydoselachus) but are not observed in Hexanchus, where alternate tooth addition was absent even in the embryo. This suggests that dental arrangement in the hexanchids is highly plastic, which appears to be a general feature of Squalomorphii, as discussed below (Fig. 3).
Galeomorphii
As the sister group to the Squalomorphii, we also considered the distribution of alternate file vs. single file tooth addition for Galeomorphii, focusing on the Lamniformes (Fig. 3) and one embryo of Heterodontiformes but excluding Orectolobiformes and Carcharhiniformes, both of which have alternate tooth replacement, as previously described (see Smith et al. 2013).
Isurus oxyrinchus, embryo (Lamniformes)
The dentition in the embryo of Isurus comprises teeth of adult‐like morphology (Fig. 8A–E) in the oldest and smallest fully mineralized teeth; however, none has rotated into a functional position (except two teeth, see below). Tooth files 1–3 are held within a small, distal bulla next to the jaw symphysis, and more proximal files within a longer bulla, the two being separated by a space, or diastema (between files 3 and 4, Fig. 8C,E). In the adult of Lamna (Fig. 8F) the disto‐proximal number of tooth files (1–13) is the same as in the embryo (Fig. 8C–E, 1–13), indicating that the latter is a fully formed, unerupted dentition.
Figure 8.
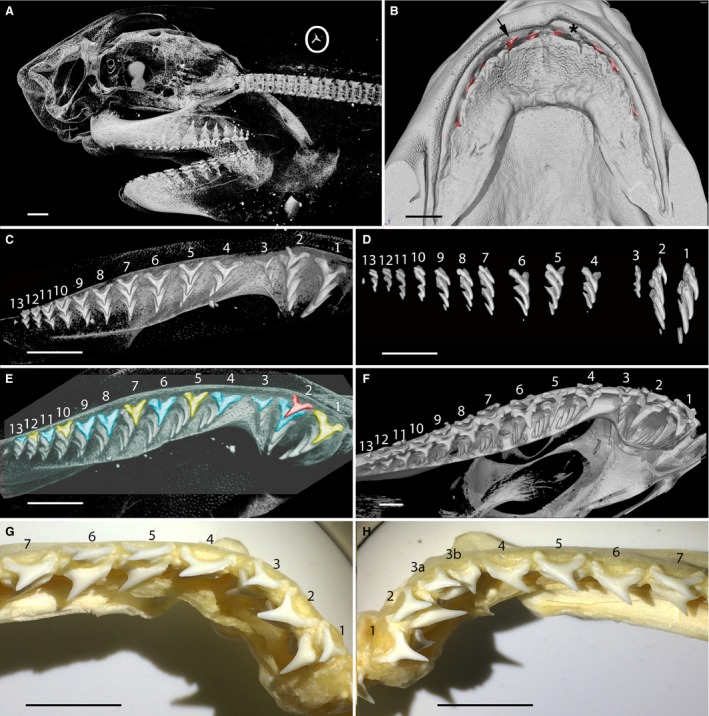
Single file embryonic dentitions of Isurus, adult Lamna and Alopias (Lamniformes). (A–E) Isurus oxyrinchus late stage embryo (BMNH1961.11.2.3), 3D‐rendered micro‐CT images. (A) Braincase, jaws, anterior vertebral column, lateral view. Developing teeth are visible in single file organisation and a loose tooth (upper right, white circle) in the gill region demonstrates that teeth are being shed at this stage. (B) Upper jaw region, internal view, partially erupted teeth, red. Tooth loss is confirmed by comparison between the fully erupted second tooth (black arrow), with a gap in the corresponding position on the right (black asterisk). (C) Left upper dentition, oldest teeth in adjacent files are at different positions relative to the jaw margin (see colour scheme, E). (D) Upper jaw, tooth rows in oblique lateral view, note the youngest teeth are in alternate evened files. (E) Upper left dentition with the oldest teeth colour coded to show their relative timing of development (see above). The tooth in file 2 is the most developed (red) and probably the oldest (relates to adjacent odd number files being the younger of each alternate pair, as in alternate model). Yellow teeth represent the next oldest with blue teeth being younger. Note the lack of alternation between files 3–4 (diastema) and 8–9 as presumed missing files. (F) Lamna nasus adult, upper dentition, diastema between files 3–4. Teeth in most adjacent files alternate, but this is not seen in files 8–9. (G,H) Alopias pelagicus, macrophotos of adult, upper dentition. (G) Right upper dentition showing the typical lamniform arrangement of three teeth within an anterior bulla. (H) Part of the left upper jaw of the same individual; extra tooth file present in position 3. Scale bars: 10 mm.
Despite tooth size varying dramatically along the jaw, size measurements of the first five files on either side of the jaw symphysis (taken as in Squatina) did not show differences between teeth in odd vs. even files (Fig. S4). However, this analysis did indicate the presence of a developmentally missing file (number 3 on each side). As mentioned above, alternate timing of tooth development in adjacent files can also be assessed via the relative position of the oldest teeth in each file relative to the jaw margin, and the overlap of root base lobes. This was far less problematic in the upper jaws than in the lower, in part due to the more oval cross‐section of the Meckel's cartilage, making assessment of tooth proximity to the jaw margin less certain; assessment was therefore done on the upper dentition.
In the upper dentition, the tooth in file 2 is the oldest (Fig. 8E, red), as the only representative of the most labial disto‐proximal tooth row, but without other teeth; evidence of shedding is shown by a tooth of identical morphology that has become lodged in the branchial region (Fig. 8A, white circle). The second row of alternating teeth includes teeth in files 1, 5, 7, 10 and 12 (Fig. 8C–E, yellow). The third tooth row includes teeth in files 2, 3, 4, 6, 8, 9, 11 and 13. This pattern is irregular (i.e. teeth in files 3 and 4 and in files 8 and 9 do not alternate in position) and as such forms a partial single file dentition because both alternating tooth replacement and some regions of single file tooth replacement occur at this stage of development. The most likely explanation for the highly specific regional lack of alternating files (corroborating the graphic data, Fig. S4A,C) is that files have been developmentally lost. For example, the diastema between distal and more proximal teeth could mark the position of one of the missing tooth files.
Lamna nasus, adult (Lamniformes)
In the upper dentition in adults of Lamna, tooth files are also held in proximal and distal bullae, with an intervening diastema (between 3 and 4) even more prominent than in the Isurus embryo. In the upper jaw, the oldest teeth in files 3 and 4 are at the same position relative to the jaw margin (Fig. 8F). Although the relative positions of the distal tooth files are not clear, teeth in files 8 and 9 do not appear to alternate and likewise have their oldest teeth at the same position relative to the jaw margin and their youngest teeth at the same stage of development. The teeth in the upper jaw of an adult Lamna thus resemble the Isurus embryo, with an alternate file differential in timing but with small portions of the dentition showing single file replacement due to probable regional loss of intervening tooth files (e.g. at the diastema).
Alopias pelagicus, adult (Lamniformes)
The upper dentition of adult A. pelagicus has the lamniform arrangement of three tooth files positioned within an anterior bulla, although this bulla is smaller and less distinct than in Isurus and Lamna. In Alopias, tooth files show an alternate pattern of eruption times, or tooth bases, relative to the jaw margin (Fig. 8G,H, files 1 and 3, and 2 and 4). Also, the oldest teeth in files 5 and 7 (Figs 8H, 4 and 6, and 5 and 7) are in the same position relative to the jaw margin, evidence of alternate timing events.
Heterodontus sp. (Heterodontiformes)
In a late‐stage embryo (labial view, Fig. 9A), teeth have rolled over the jaw margin, with the symphyseal tooth and one tooth present from file 2 in the initial row, these being the earliest to form. Here arrangement is alternate (Fig. 9A), but file 2 on the right jaw may have shed a tooth, consistent with tooth shedding in the earliest embryos, as described previously (Reif, 1976: fig. 8F). Teeth with cusped morphology in distal files have a bilateral symmetry (as in symphyseal teeth) and alternate in their position with respect to the jaw margin, as do the more proximal molariform teeth with broad bases and low cusps (tooth positions 7–9) in the less curved part of the jaw (Fig. 9B–D). New teeth are added proximally to molariform teeth at jaw position 10, as a large, open base to the developing but narrow crown (Fig. 9E, arrows). In the adult (Smith et al. 2013: fig. 3A,B), the close packing of both tooth morphologies seems to present as ‘single file’, but in the embryo the molariform teeth are slightly staggered in each file to accommodate wide teeth formed in an alternate pattern.
Figure 9.
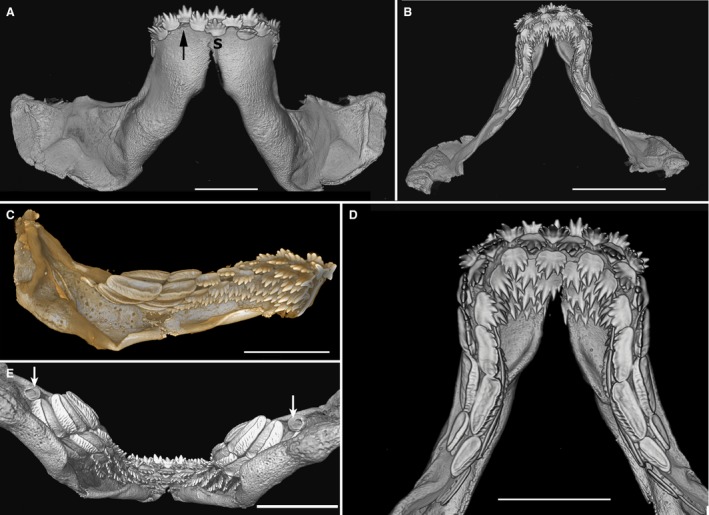
Heterodontus sp. late stage embryo, 3D‐rendered micro‐CT images. (A) Lower dentition, labial view, file 2 of the right jaw shows a lost tooth (black arrow) from same row as symphyseal tooth (S). (B) Lingual view. (C) Left jaw, lingual view showing high‐cusped distal teeth (jaw positions 1–6) and molariform teeth (jaw positions 1–9), all in alternate arrangement. (D) Close‐up of lower jaw, lingual view, tooth files showing alternate pattern, both sides of symphyseal tooth file. (E) Lower jaw, oblique lingual view, latest tooth position (10) with tooth germs added proximally (white arrows). Scale bars: 5 mm (A,C–E), 1 cm (B).
Fossil taxa
Synechodus (Synechodontiformes)
The fossil shark genus Synechodus is generally considered to be part of a monophyletic clade of neoselachians (Synechodontiformes; Klug, 2010), although this is not universally accepted (e.g. Maisey, 1985). Although most specimens of Synechodus and other Synechodontiformes comprise isolated teeth, several skulls and well‐preserved skeletons are known (Maisey, 1985). Within these, however, dentitions are commonly poorly exposed or partly disarticulated. In contrast, a dentition from the Late Cretaceous of SE England (Fig. 10A–I) comprises an articulated suite of teeth with no jaw cartilages preserved. This shows a high degree of heterodonty (examples of isolated teeth, Fig. 10J–L) with erect and cuspate teeth distally (Fig. 10D,G–I) and wide, low cusped teeth in more proximal positions (Fig. 10A–C,E,F,L), but all are arranged with bases and crowns in close‐packed, alternate arrangement. At the symphysis (Fig. 10D,H) two files of relatively small, close‐packed parasymphyseal teeth clearly alternate in their positions labio‐lingually. This alternate packing of in situ teeth in all positions of this fossil specimen indicates that the dentition derives from an alternating tooth addition pattern at their initiation.
Figure 10.
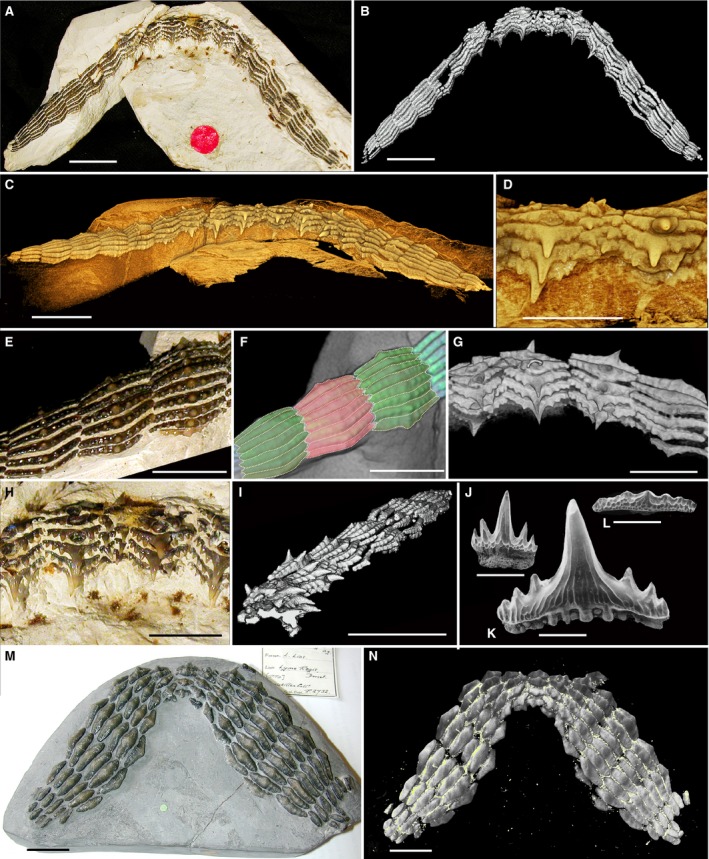
Articulated dentition of Synechodus dubrisiensis (Cretaceous, Chalk). (A–I, BSB008523, Booth Museum, Brighton). (A,E,H) Macrophotos (B–D,F,G,I) 3D‐rendered micro‐CT images. (A) Occlusal view, teeth are articulated as in situ but no cartilages are preserved. (B) Rock surrounding tooth files removed virtually. (C) Oblique lingual view showing the general arrangement of the teeth. (D) Detail of the symphyseal region showing alternating small teeth. (E) Lingual view, wide, low crown shape and alternating arrangement. (F) Same region, with pseudocoloured crowns to show close‐packed alternating pattern of adjacent files. (G) Teeth with high cusps (as in H–K) close to the symphysis show an alternating pattern of adjacent files in contrast to those more proximally. (H) Symphyseal region with alternation of parasymphyseal teeth and large teeth on adjacent files. (I) Oblique view of the dentition, showing that several tooth rows would have been simultaneously functional. Scale bars: 10 mm (A–C,I), 5 mm (D–H). (J–L) Synechodus dubrisiensis isolated teeth (Early Cretaceous, Underwood et al. 1999: pl. 1.1, 1.4, 1.5), SEM images, demonstrating the high degree of heterodonty. (J) Symphyseal tooth. (K) High cusped anterior tooth. (L) Low crowned posterior tooth. Scale bars: 1 mm. (M,N) Acrodus anningae (NHMUK PV P2732), articulated dentition, as prepared (M) compared with segmented micro‐CT image with rock removed virtually showing new teeth lingually (N). Pyrite is indicated in yellow. Note the alternating pattern of the tooth bases.
Acrodus (Hybodontoidea)
The extinct Hybodontoidea are a clade forming a sister group to Neoselachii (Maisey, 1987; Lane, 2010; Fig. 3). Observations were made on an exceptionally well‐preserved dentition of Acrodus from the Early Jurassic of southern England (Fig. 10M,N). This specimen preserves an entire dentition but, as with the Synechodus specimen, no jaw cartilages are preserved. CT‐renders revealed some additional teeth (unprepared from the fossil matrix) on the labial margin of the dentition and in the process of shedding. They also showed the presence of pyrite, an iron sulphide that forms early in the fossilisation process, between the teeth, suggesting that teeth have not shifted relative to each other postmortem. The exposed biting surface of the dentition reveals wide, low teeth on either side of a file of smaller teeth at the jaw symphysis. The extended tooth bases in adjacent files overlap, demonstrating an alternate pattern of replacement teeth. Observations of other hybodont sharks such as Asteracanthus (Rees & Underwood, 2006) show that a similar alternating pattern was present across the clade.
Discussion
The aims of our study were focused on the spatio‐temporal order of initiation of replacement teeth in the Neoselachii. The relative timing of successive teeth in the files was determined from observations and measurements of relative size differences of teeth, compared both within and between adjacent tooth files, in adults and embryos wherever possible.
Focusing on embryos of the Angel shark Squatina (Supporting Information Figs S1 and S2), we have proposed a model for all neoselachians (i.e. iterative sequence of tooth addition), from a structural pattern of the dentition that we interpret as timed developmental differences based on a clonal generative unit (SAT tf unit) that comprises two adjacent tooth files (Fig. 2). This developmental model with alternate timing and sequential spatial arrangement explains the development of the alternate tooth pattern (Smith, 2003; Smith et al. 2013). It is proposed that the alternate pattern of tooth addition [based on Reif's observations (1978): Fig. 2] is plesiomorphic for the Neoselachii (Fig. 3); as a phylogenetically basal neoselachian developmental pattern that operates in the first stages of the embryonic dentition, it results in alternately timed eruption and shedding. We examined specific taxa in the Squalomorphii and Galeomorphii to see how this model may be altered during development to arrange teeth as a single file tooth pattern in adults, including simultaneous eruption into a cutting blade (Underwood et al. 2016).
In Squatina (Squalomorphii), we have demonstrated that the earliest dentition shows alternate timing (clonal SAT units, Figs 4 and 5) and although tooth size differences are not apparent in the adult (suggesting a shift to a single file pattern), an alternate arrangement appears to be retained in the young adult, indicated by their positions at the proximal jaw margins (Fig. S2C,D). Although well‐separated single files are present in the adult of Chlamydoselachus, similarly rudimentary proximal tooth files in the adult retain an alternate arrangement (Fig. 6F,H); however, we have only scarce data to show that embryos exhibit alternate tooth addition in their early development. A better understanding of how tooth addition timing changed from embryo to adult in these taxa requires gene expression data, including how co‐ordination and alignment of tooth files between upper and lower jaws is programmed.
The Hexanchidae, closely related to Chlamydoselachus (Fig. 3), have a unique and highly distinctive dentition, with tooth morphology very different from that of Chlamydoselachus and Squatina. The lower teeth of adult Hexanchidae are arranged in a single file order, as in the embryo of Hexanchus, but each tooth is wide and abuts the next, so together they form a ‘saw blade’ at the jaw margin (see Smith & Johanson, 2015: fig. 1.7), as in other Squaliformes (Underwood et al. 2016). But unlike the Squaliformes and Squatina (Fig. 3), in Hexanchus this arrangement does not appear to derive from an alternate arrangement in the embryo. However, in hexanchid adults such as Notorhynchus, the most proximal rudimentary teeth do retain a developmental alternate arrangement (Fig. 7D: as in the adults of Squatina and Chlamydoselachus), evidence of an underlying, persistent inherited alternate tooth order.
The mode of tooth replacement in the Lamniformes, a very specialised group of sharks including both macropredators and planktivores with diverse dentitions reflecting their differing trophic roles, has been unclear (Supporting Information Videos S1 and S2; Smith et al. 2013) due to the widely spaced tooth files and high degree of curvature of the jaw cartilages towards the symphysis. They are unique in that the dentition of some species is functional long before birth and is used during intrauterine oophagy and cannibalism, so that the earliest stages of the tooth order are uncertain (e.g. Shimada, 2002; Tomita et al. 2017). In predatory lamniforms the upper dentition is distinctive (Fig. 8F), with tooth files originating in deep proximal and distal bulla, the latter comprising a suite typically of three files. Between these is a raised cartilage bar, which developmentally may relate to a diastema formed by the loss of tooth files (in the Lamniformes), or they may have one or more files of very reduced teeth (Mitsukurina, Carcharias), varying between individuals or between the jaws of the same individual.
We noted that the development of teeth within lamniforms is a two‐stage process, and only the second phase of tooth growth is addressed here. Embryos develop an early suite of teeth (Shimada, 2002; Tomita et al. 2017) that is shed prior to the eruption of the adult‐type teeth (Fig. 8A, white circle). Although the tooth arrangement in the first dental set is as yet unclear (in part due to the rarity of these embryos) we have shown that the tooth size differs in adjacent files and therefore has an alternate timing of tooth addition (Figs 8 and S4). Overall, Isurus and other Lamniformes show an alternate pattern that is modified in early development through loss of tooth files near the diastema in the upper jaw and elsewhere, resulting in portions of the jaw possessing single file tooth replacement (Fig. 3, state 2).
Phylogenetic relationships
Recent molecular phylogenies of extant sharks support the sister group relationship between the major clades Squalomorphii and Galeomorphii, with these forming a sister group to the Batoidea (e.g. Vélez‐Zuazo & Agnarsson, 2011; Fig. 3). Phylogenetically, Synechodus has been resolved as a sister taxon to extant sharks and rays (Klug et al. 2009; Klug, 2010) but is also assigned to the Galeomorphii (Maisey, 2012; Fig. 3). Although teeth of Synechodus are organised in close‐packed files (central cusp labio‐lingually aligned), the tooth bases overlap, reflecting alternate tooth initiation and eruption. An alternate replacement was also demonstrated for the hybodont Acrodus (Hybodontoidea, also resolved as being a sister taxon to extant sharks and rays; Fig. 3). Clearly defined alternate tooth replacement patterns are also present in embryos and adults of both the Galeomorphii (Smith et al. 2013) and Squalomorphii (e.g. Pristiophoridae; Underwood et al. 2016). Along with this, dentitions in the batoids show alternate tooth addition, within both the embryo and adult (Underwood et al. 2015; Fig. 3). These observations suggest that the alternate pattern of tooth addition is plesiomorphic for the Neoselachii as a whole.
Single file tooth replacement in certain dentitions of Squaliformes has been recognised as a derived modification of this alternate pattern during development (Underwood et al. 2016; Fig. 3, state 3). Squatina and Chlamydoselachus show separate tooth files in the adult but retain an alternate pattern of tooth addition, at least proximally (Fig. 3, state 3). Separation of tooth files may have resulted from a fixed number of tooth files (new files not added proximally) combined with jaw growth, or from tooth file loss; nevertheless, these taxa show a markedly different mechanism than that suggested in the Squaliformes (Underwood et al. 2016).
By comparison, in the Hexanchidae, single file tooth replacement is present in both the embryo and adult of Hexanchus, although remnants of an alternate dentition are preserved in files of reduced teeth in adult hexanchids such as Notorhynchus. Isurus and other Lamniformes show a different developmental pattern for replacement, involving modification through loss of whole tooth files near the diastema, resulting in single file tooth replacement limited to this region (e.g. previously alternate files at the same developmental stage are now adjacent to one another).
These modified patterns are uncommon in the neoselachians, representing a derived state acquired from the embryonic alternate dentition, but by different mechanisms: in the Lamniformes by loss of tooth files (Fig. 3, state 2) and within squalomorph clades by independent modification of the alternate differential timing along the jaw (Fig. 3, state 3; see also Strasburg 1963 for dentition modification from an alternate tooth arrangement). These taxa appear to have sufficient developmental plasticity to allow the formation of single file tooth replacement by re‐ordering of tooth production from an embryonic pattern of sequential, alternate timing of tooth initiation. Heterodontiformes, as the most basal taxon in the Galeomorphii, exhibit distinct alternate arrangement in the embryo and retention of this in the adult (Fig. 9).
Conclusions
This study has investigated the initial development of tooth replacement patterns in a number of shark taxa where successional tooth order was previously poorly understood. We speculate that a change in timing of replacement tooth addition, or loss of tooth files, resulted in the shift from embryonic to adult dentitions and loss of the alternate pattern in some taxa. These changes allowed teeth to emerge simultaneously at the jaw margin, forming a continuous cutting edge either as an adaptation to a specific feeding mode or as a functionally driven adaptation.
In addition, we studied tooth replacement series in adults of two extinct species of sharks, representing sister taxa to extant groups. We mapped characters onto a recent phylogeny based on transformation or retention of a developmental process (interpreted for fossil species) into ‘single file’ or ‘alternate file’ ordering of replacement teeth in the adult. In this way we have predicted the basal (alternate) and derived (single file) phylogenetic states and suggested how this evolved through ‘tinkering’ with developmental mechanisms, although by differing methods in the Squalomorphii and the Galeomorphii.
Thus, the combination of fossil and extant phylogenetic data suggests that the alternate tooth pattern is plesiomorphic for the Neoselachii, with modification in adult sharks, although achieved differently in the two major clades. Squalomorphs modified an embryonic alternate tooth replacement pattern using developmental plasticity of timing to generate a single file pattern in the adult, and galeomorphs by loss of tooth files. We postulate that these groups have the developmental plasticity to allow the formation of single file tooth replacement via the re‐ordering of tooth production. This was not a substantial mechanism within the Neoselachii, which otherwise were dominated by alternate patterning of the dentition.
Hence, we propose a developmental‐evolutionary model from the alternate pattern to achieve a single file alignment of replacement teeth, one with co‐equal eruption times at the functional edge of the jaws.
Author contributions
M.M.S. conceived this study. M.M.S., B.C., Z.J. and C.U. generated micro‐CT scan data. J.K. provided data on Chlamydoselachus. All authors contributed to the interpretation of data and the writing of the paper.
Conflict of interests
We declare that we have no conflicts of interest.
Supporting information
Fig. S1. Single file dentition in occlusion in late stage embryo of Squatina californica.
Fig. S2. Single file dentition of Squatina guggenheim small adult.
Fig. S3. Single file dentition of Chlamydoselachus anguineus, adult.
Fig. S4. Dentition of lower jaw of Isurus embryo.
Video S1. Adult Lamna nasus (BMNH2015.3.13 1–3), movie generated from 3D rendered micro‐CT scan. Rotating whole head dissected down to show dentition of teeth only.
Video S2. Adult Lamna nasus (BMNH2015.3.13 1–3), movie generated from 3D‐rendered micro‐CT scan, rotating dissection of teeth organised in a whorl showing order of eruption and developing cusps.
Acknowledgements
We are indebted to Sho Tanaka for rare embryos of Chlamydoselachus that had been cleared and stained with Alcian blue and Alizarin red; to Brian Metscher for preparation of a Chlamydoselachus embryo (Fig. 6A) and for high resolution scanning. We are grateful to Tathyane Teshima (CFD Dental Institute, King's College London) for help with the drawing software. We would like to thank Emma Bernard (Earth Sciences), James MacLaine and Ollie Crimmen [Life Sciences, Natural History Museum, London (NHM)] for access to collections. Amin Garbout and Farah Ahmed provided access to CT‐scanners and computers in the Image and Analysis Centre, NHM. We would also like to thank our reviewers, whose comments helped to improve our paper. This contribution resulted from a research project (2014–2016) supported by NERC Standard grant nos NE/K01434X/1 (to Z.J.), NE/K014293/1 (to C.U.) and NE/K014235/1 (to M.M.S.).
The copyright line for this article was changed on 18 of April 2018 after original online publication
References
- Compagno LJV (1973) Interrelationships of living elasmobranchs In: Interrelationships of Fishes. (eds Greenwood PH, Miles RH, Patterson C.), pp. 15–61. London: Zoological Journal of Linnean Society. [Google Scholar]
- Compagno LJV (1977) Phyletic relationships of living sharks and rays. Am Zool 17, 303–322. [Google Scholar]
- Enault S, Guinot G, Koot M, et al. (2015) Chondrichthyan tooth enameloid: past, present, and future. Zool J Linn Soc 174, 549–570. [Google Scholar]
- Klug S (2010) Monophyly, phylogeny and systematic position of the Synechodontiformes (Chondrichthyes, Neoselachii). Zool Scr 39, 37–49. [Google Scholar]
- Klug S, Kriwet J, Bottcher R, et al. (2009) Skeletal anatomy of the extinct shark Paraorthacodus jurensis (Chondrichthyes; Palaeospinacidae), with comments on synechodontiform and palaeospinacid monophyly. Zool J Linn Soc 157, 107–134. [Google Scholar]
- Lane JA (2010) Morphology of the braincase in the Cretaceous hybodont shark Tribodus limae (Chondrichthyes, Elasmobranchii), based on CT scanning. Am Mus Novit 3681, 1–70. [Google Scholar]
- Maisey JG (1985) Cranial morphology of the fossil elasmobranch Synechodus dubrisiensis . Am Mus Novit 2804, 1–28. [Google Scholar]
- Maisey JG (1987) Cranial anatomy of the Lower Jurassic shark Hybodus reticulatus (Chondrichthyes: Elasmobranchii), with comments on hybodontid systematics. Am Mus Novit 2878, 1–39. [Google Scholar]
- Maisey JG (2012) What is an ‘elasmobranch’? The impact of paleontology in understanding elasmobranch phylogeny and evolution. J Fish Biol 80, 918–951. [DOI] [PubMed] [Google Scholar]
- Martin KJ, Rasch LJ, Cooper RL, et al. (2016) Sox2+ progenitors in sharks link taste development with the evolution of regenerative teeth from denticles. PNAS 113, 14769–14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor G, Caira J, Jensen K, et al. (2012) Elasmobranch phylogeny: a mitochondrial estimate based on 595 species In: Biology of Sharks and their Relatives. 2nd edn (eds Carrier J, Musick J, Heithaus M.), pp. 31–56. Boca Raton: CRC Press. [Google Scholar]
- Nelson JS (2006) Fishes of the World, 4th edn, pp. 601 Hoboken: John Wiley & Sons. [Google Scholar]
- Rasch LJ, Martin KJ, Cooper RL, et al. (2016) An ancient dental gene set governs development and continuous regeneration of teeth in sharks. Dev Biol 415, 347–370. [DOI] [PubMed] [Google Scholar]
- Rees J, Underwood CJ (2006) Hyodont sharks from the Middle Jurassic of the Inner Hebrides, Scotland. Trans R Soc Edinb Earth Sci 96, 351–363. [Google Scholar]
- Reif W‐E (1976) Morphogenesis, pattern formation and function of the dentition of Heterodontus (Selachii). Zoomorphologie 83, 1–47. [Google Scholar]
- Reif W‐E (1978) Shark dentitions: morphogenetic processes and evolution. Neues Jahrb Geol Palaontol Abh 157, 107–115. [Google Scholar]
- Reif W‐E (1980) Development of dentition and dermal skeleton in embryonic Scyliorhinus canicula . J Morphol 166, 275–288. [DOI] [PubMed] [Google Scholar]
- Reif W‐E (1982) Evolution of dermal skeleton and dentition in vertebrates In: Evolutionary Biology. (eds Hecht MK, Wallace B, Prance GT.), pp. 287–368. Boston: Springer. [Google Scholar]
- Shimada K (2002) Teeth of embryos in lamniform sharks (Chondrichthyes: Elasmobranchii). Environ Biol Fishes 63, 309–319. [Google Scholar]
- Smith MM (2003) Vertebrate dentitions at the origin of jaws: when and how pattern evolved. Evol Dev 5, 394–413. [DOI] [PubMed] [Google Scholar]
- Smith MM, Johanson Z (2015) Development and Evolution of the Vertebrate Dentition In: Great Transformations in Vertebrate Evolution. (eds Dial KP, Shubin N, Brainerd EL.), pp. 9–29. Chicago: University Chicago Press. [Google Scholar]
- Smith MM, Fraser GJ, Mistsiadis TA (2009) Dental lamina as source of odontogenic stem cells: evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zool B Mol Dev Evol 312B, 260–280. [DOI] [PubMed] [Google Scholar]
- Smith MM, Johanson Z, Underwood C, et al. (2013) Pattern formation in development of chondrichthyan dentitions: a review of an evolutionary model. Hist Biol 25, 127–142. [Google Scholar]
- Strasburg DW (1963) The diet and dentition of Isistius brasiliensis, with remarks on tooth replacement in other sharks. Copeia 1963, 33–40. [Google Scholar]
- Tomita T, Miyamito K, Kawaguchi A, et al. (2017) Dental ontogeny of a white shark embryo. J Morphol 278, 215–227. [DOI] [PubMed] [Google Scholar]
- Underwood CJ, Mitchell SF, Veltkamp KJ (1999) Shark and ray teeth from the Hauterivian (Lower Cretaceous) of north‐east England. Palaeontology 42, 287–302. [Google Scholar]
- Underwood CJ, Johanson Z, Welten M, et al. (2015) Development and evolution of dentition pattern and tooth order in the skates and rays (Batoidea; Chondrichthyes). PLoS ONE 10, e0122553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood CJ, Johanson Z, Smith MM (2016) Cutting blade dentitions in squaliform sharks form by modification of inherited alternate tooth ordering patterns. R Soc Open Sci 3, 160385 https://doi.org/10.1098/rsos.160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez‐Zuazo X, Agnarsson I (2011) Shark tales: a molecular species‐level phylogeny of sharks (Selachimorpha, Chondrichthyes). Mol Phylogenet Evol 58, 207–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Single file dentition in occlusion in late stage embryo of Squatina californica.
Fig. S2. Single file dentition of Squatina guggenheim small adult.
Fig. S3. Single file dentition of Chlamydoselachus anguineus, adult.
Fig. S4. Dentition of lower jaw of Isurus embryo.
Video S1. Adult Lamna nasus (BMNH2015.3.13 1–3), movie generated from 3D rendered micro‐CT scan. Rotating whole head dissected down to show dentition of teeth only.
Video S2. Adult Lamna nasus (BMNH2015.3.13 1–3), movie generated from 3D‐rendered micro‐CT scan, rotating dissection of teeth organised in a whorl showing order of eruption and developing cusps.


